AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Proposed rule.
SUMMARY:
This proposed rule proposes revisions to the Medicare hospital outpatient prospective payment system (OPPS) and the Medicare ambulatory surgical center (ASC) payment system for CY 2020 based on our continuing experience with these systems. In this proposed rule, we describe the proposed changes to the amounts and factors used to determine the payment rates for Medicare services paid under the OPPS and those paid under the ASC payment system. In addition, this proposed rule would update and refine the requirements for the Hospital Outpatient Quality Reporting (OQR) Program and the ASC Quality Reporting (ASCQR) Program. In addition, in this proposed rule, we are proposing to establish requirements for all hospitals in the United States for making hospital standard charges available to the public; establish a process and requirements for prior authorization for certain covered outpatient department services; revise the conditions for coverage of organ procurement organizations; and revise the regulations to allow grandfathered children's hospitals-within-hospitals to increase the number of beds without resulting in the loss of grandfathered status. We also solicit comments on potential revisions to the laboratory date of service policy under the Clinical Laboratory Fee Schedule. Finally, we solicit comments on an appropriate remedy in litigation involving our OPPS payment policy for 340B-acquired drugs, which would inform future rulemaking in the event of an adverse decision on appeal in that litigation.
DATES:
Comment period: To be assured consideration, comments on this proposed rule must be received at one of the addresses provided in the ADDRESSES section no later than 5 p.m. EST on September 27, 2019.
ADDRESSES:
In commenting, please refer to file code CMS-1717-P when commenting on the issues in this proposed rule. Because of staff and resource limitations, we cannot accept comments by facsimile (FAX) transmission.
Comments, including mass comment submissions, must be submitted in one of the following three ways (please choose only one of the ways listed):
1. Electronically. You may (and we encourage you to) submit electronic comments on this regulation to http://www.regulations.gov. Follow the instructions under the “submit a comment” tab.
2. By regular mail. You may mail written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1717-P, P.O. Box 8013, Baltimore, MD 21244-1850.
Please allow sufficient time for mailed comments to be received before the close of the comment period.
3. By express or overnight mail. You may send written comments via express or overnight mail to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1717-P, Mail Stop C4-26-05, 7500 Security Boulevard, Baltimore, MD 21244-1850.
b. For delivery in Baltimore, MD—Centers for Medicare & Medicaid Services, Department of Health and Human Services, 7500 Security Boulevard, Baltimore, MD 21244-1850.
For information on viewing public comments, we refer readers to the beginning of the SUPPLEMENTARY INFORMATION section.
FOR FURTHER INFORMATION CONTACT:
2-Midnight Rule (Short Inpatient Hospital Stays), contact Lela Strong-Holloway via email Lela.Strong@cms.hhs.gov or at 410-786-3213.
Advisory Panel on Hospital Outpatient Payment (HOP Panel), contact the HOP Panel mailbox at APCPanel@cms.hhs.gov.
Ambulatory Surgical Center (ASC) Payment System, contact Scott Talaga via email Scott.Talaga@cms.hhs.gov or at 410-786-4142.
Ambulatory Surgical Center Quality Reporting (ASCQR) Program Administration, Validation, and Reconsideration Issues, contact Anita Bhatia via email Anita.Bhatia@cms.hhs.gov or at 410-786-7236.
Ambulatory Surgical Center Quality Reporting (ASCQR) Program Measures, contact Vinitha Meyyur via email Vinitha.Meyyur@cms.hhs.gov or at 410-786-8819.
Blood and Blood Products, contact Josh McFeeters via email Joshua.McFeeters@cms.hhs.gov or at 410-786-9732.
Cancer Hospital Payments, contact Scott Talaga via email Scott.Talaga@cms.hhs.gov or at 410-786-4142.
CMS Web Posting of the OPPS and ASC Payment Files, contact Chuck Braver via email Chuck.Braver@cms.hhs.gov or at 410-786-6719.
Control for Unnecessary Increases in Volume of Outpatient Services, contact Elise Barringer via email Elise.Barringer@cms.hhs.gov or at 410-786-9222.
Composite APCs (Low Dose Brachytherapy and Multiple Imaging), contact Elise Barringer via email Elise.Barringer@cms.hhs.gov or at 410-786-9222.
Comprehensive APCs (C-APCs), contact Lela Strong-Holloway via email Lela.Strong@cms.hhs.gov or at 410-786-3213, or Mitali Dayal via email at Mitali.Dayal2@cms.hhs.gov or at 410-786-4329.
CPT and Level II HCPCS Codes, contact Marjorie Baldo via email Marjorie.Baldo@cms.hhs.gov or at 410-786-4617.
Grandfathered Children's Hospitals-within-Hospitals, contact Michele Hudson via email Michele.Hudson@cms.hhs.gov or 410-786-4487.
Hospital Cost Reporting and Chargemaster Comment Solicitation, contact Dr. Terri Postma via email at PriceTransparencyHospitalCharges@cms.hhs.gov.
Hospital Outpatient Quality Reporting (OQR) Program Administration, Validation, and Reconsideration Issues, contact Anita Bhatia via email Anita.Bhatia@cms.hhs.gov or at 410-786-7236.
Hospital Outpatient Quality Reporting (OQR) Program Measures, contact Vinitha Meyyur via email Vinitha.Meyyur@cms.hhs.gov or at 410-786-8819.
Hospital Outpatient Visits (Emergency Department Visits and Critical Care Visits), contact Elise Barringer via email Elise.Barringer@cms.hhs.gov or at 410-786-9222.
Inpatient Only (IPO) Procedures List, contact Lela Strong-Holloway via email Lela.Strong@cms.hhs.gov or at 410-786-3213, or Au'Sha Washington via email at Ausha.Washington@cms.hhs.gov or at 410-786-3736.
New Technology Intraocular Lenses (NTIOLs), contact Scott Talaga via email Scott.Talaga@cms.hhs.gov or at 410-786-4142.
No Cost/Full Credit and Partial Credit Devices, contact Scott Talaga via email Scott.Talaga@cms.hhs.gov or at 410-786-4142.
OPPS Brachytherapy, contact Scott Talaga via email Scott.Talaga@cms.hhs.gov or at 410-786-4142.
OPPS Data (APC Weights, Conversion Factor, Copayments, Cost-to-Charge Ratios (CCRs), Data Claims, Geometric Mean Calculation, Outlier Payments, and Wage Index), contact Erick Chuang via email Erick.Chuang@cms.hhs.gov or at 410-786-1816, Steven Johnson via email Steven.Johnson@cms.hhs.gov or at 410-786-3332, or Scott Talaga via email Scott.Talaga@cms.hhs.gov or at 410-786-4142, or Josh McFeeters via email at Joshua.McFeeters@cms.hhs.gov or at 410-786-9732.
OPPS Drugs, Radiopharmaceuticals, Biologicals, and Biosimilar Products, contact Josh McFeeters via email Joshua.McFeeters@cms.hhs.gov or at 410-786-9732.
OPPS New Technology Procedures/Services, contact the New Technology APC mailbox at NewTechAPCapplications@cms.hhs.gov.
OPPS Packaged Items/Services, contact Lela Strong-Holloway via email Lela.Strong@cms.hhs.gov or at 410-786-3213, or Mitali Dayal via email at Mitali.Dayal2@cms.hhs.gov or at 410-786-4329.
OPPS Pass-Through Devices, contact the Device Pass-Through mailbox at DevicePTapplications@cms.hhs.gov.
OPPS Status Indicators (SI) and Comment Indicators (CI), contact Marina Kushnirova via email Marina.Kushnirova@cms.hhs.gov or at 410-786-2682.
Organ Procurement Organization (OPO) Conditions for Coverage (CfCs), contact Alpha-Banu Wilson via email at AlphaBanu.Wilson@cms.hhs.gov or at 410-786-8687, or Diane Corning via email at Diane.Corning@cms.hhs.gov or at 410-786-8486.
Partial Hospitalization Program (PHP) and Community Mental Health Center (CMHC) Issues, contact the PHP Payment Policy Mailbox at PHPPaymentPolicy@cms.hhs.gov.
Price Transparency of Hospital Standard Charges, contact Dr. Terri Postma or Elizabeth November via email at PriceTransparencyHospitalCharges@cms.hhs.gov.
Prior Authorization Process and Requirements for Certain Hospital Outpatient Department Services, contact Thomas Kessler via email at Thomas.Kessler@cms.hhs.gov or at 410-786-1991.
Quality Measurement Relating to Price Transparency, contact Dr. Reena Duseja or Dr. Terri Postma via email at PriceTransparencyHospitalCharges@cms.hhs.gov
Rural Hospital Payments, contact Josh McFeeters via email at Joshua.McFeeters@cms.hhs.gov or at 410-786-9732.
Skin Substitutes, contact Josh McFeeters via email Joshua.McFeeters@cms.hhs.gov or at 410-786-9732.
Supervision of Outpatient Therapeutic Services in Hospitals and CAHs, contact Josh McFeeters via email Joshua.McFeeters@cms.hhs.gov or at 410-786-9732, or Mitali Dayal via email at Mitali.Dayal2@cms.hhs.gov or at 410-786-4329.
All Other Issues Related to Hospital Outpatient and Ambulatory Surgical Center Payments Not Previously Identified, contact Elise Barringer via email Elise.Barringer@cms.hhs.gov or at 410-786-9222.
SUPPLEMENTARY INFORMATION:
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period on the following website as soon as possible after they have been received: http://www.regulations.gov/. Follow the search instructions on that website to view public comments.
Addenda Available Only Through the Internet on the CMS Website
In the past, a majority of the Addenda referred to in our OPPS/ASC proposed and final rules were published in the Federal Register as part of the annual rulemakings. However, beginning with the CY 2012 OPPS/ASC proposed rule, all of the Addenda no longer appear in the Federal Register as part of the annual OPPS/ASC proposed and final rules to decrease administrative burden and reduce costs associated with publishing lengthy tables. Instead, these Addenda are published and available only on the CMS website. The Addenda relating to the OPPS are available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. The Addenda relating to the ASC payment system are available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
Current Procedural Terminology (CPT) Copyright Notice
Throughout this proposed rule, we use CPT codes and descriptions to refer to a variety of services. We note that CPT codes and descriptions are copyright 2018 American Medical Association. All Rights Reserved. CPT is a registered trademark of the American Medical Association (AMA). Applicable Federal Acquisition Regulations (FAR and Defense Federal Acquisition Regulations (DFAR) apply.
Table of Contents
I. Summary and Background
A. Executive Summary of This Document
B. Legislative and Regulatory Authority for the Hospital OPPS
C. Excluded OPPS Services and Hospitals
D. Prior Rulemaking
E. Advisory Panel on Hospital Outpatient Payment (the HOP Panel or the Panel)
F. Public Comments Received on the CY 2019 OPPS/ASC Final Rule With Comment Period
II. Proposed Updates Affecting OPPS Payments
A. Proposed Recalibration of APC Relative Payment Weights
B. Proposed Conversion Factor Update
C. Proposed Wage Index Changes
D. Proposed Statewide Average Default Cost-to-Charge Ratios (CCRs)
E. Proposed Adjustment for Rural Sole Community Hospitals (SCHs) and Essential Access Community Hospitals (EACHs) Under Section 1833(t)(13)(B) of the Act for CY 2020
F. Proposed Payment Adjustment for Certain Cancer Hospitals for CY 2020
G. Proposed Hospital Outpatient Outlier Payments
H. Proposed Calculation of an Adjusted Medicare Payment From the National Unadjusted Medicare Payment
I. Proposed Beneficiary Copayments
III. Proposed OPPS Ambulatory Payment Classification (APC) Group Policies
A. Proposed OPPS Treatment of New and Revised HCPCS Codes
B. Proposed OPPS Changes—Variations Within APCs
C. Proposed New Technology APCs
D. Proposed APC-Specific Policies
IV. Proposed OPPS Payment for Devices
A. Pass-Through Payments for Devices
B. Proposed Device-Intensive Procedures
V. Proposed OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
A. Proposed OPPS Transitional Pass-Through Payment for Additional Costs of Drugs, Biologicals, and Radiopharmaceuticals
B. Proposed OPPS Payment for Drugs, Biologicals, and Radiopharmaceuticals Without Pass-Through Payment Status
VI. Proposed Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
A. Background
B. Proposed Estimate of Pass-Through Spending
VII. Proposed OPPS Payment for Hospital Outpatient Visits and Critical Care Services
VIII. Proposed Payment for Partial Hospitalization Services
A. Background
B. Proposed PHP APC Update for CY 2020
C. Proposed Outlier Policy for CMHCs
D. Update to PHP Allowable HCPCS Codes
IX. Proposed Procedures That Would Be Paid Only as Inpatient Procedures
A. Background
B. Proposed Changes to the Inpatient Only (IPO) List
X. Proposed Nonrecurring Policy Changes
A. Proposed Changes in the Level of Supervision of Outpatient Therapeutic Services in Hospitals and Critical Access Hospitals (CAHs)
B. Short Inpatient Hospital Stays
C. Method To Control Unnecessary Increases in the Volume of Clinic Visit Services Furnished in Excepted Off-Campus Provider-Based Departments (PBDs)
XI. Proposed CY 2020 OPPS Payment Status and Comment Indicators
A. Proposed CY 2020 OPPS Payment Status Indicator Definitions
B. Proposed CY 2020 Comment Indicator Definitions
XII. MedPAC Recommendations
A. OPPS Payment Rates Update
B. ASC Conversion Factor Update
C. ASC Cost Data
XIII. Proposed Updates to the Ambulatory Surgical Center (ASC) Payment System
A. Background
B. Proposed ASC Treatment of New and Revised Codes
C. Proposed Update to the List of ASC Covered Surgical Procedures and Covered Ancillary Services
D. Proposed Update and Payment for ASC Covered Surgical Procedures and Covered Ancillary Services
E. New Technology Intraocular Lenses (NTIOLs)
F. Proposed ASC Payment and Comment Indicators
G. Proposed Calculation of the ASC Payment Rates and the ASC Conversion Factor
XIV. Requirements for the Hospital Outpatient Quality Reporting (OQR) Program
A. Background
B. Hospital OQR Program Quality Measures
C. Administrative Requirements
D. Form, Manner, and Timing of Data Submitted for the Hospital OQR Program
E. Proposed Payment Reduction for Hospitals That Fail To Meet the Hospital OQR Program Requirements for the CY 2020 Payment Determination
XV. Requirements for the Ambulatory Surgical Center Quality Reporting (ASCQR) Program
A. Background
B. ASCQR Program Quality Measures
C. Administrative Requirements
D. Form, Manner, and Timing of Data Submitted for the ASCQR Program
E. Payment Reduction for ASCs That Fail To Meet the ASCQR Program Requirements
XVI. Proposed Requirements for Hospitals To Make Public a List of Their Standard Charges
A. Introduction and Overview
B. Proposed Definition of “Hospital” and Proposed Special Requirements That Would Apply to Certain Types of Hospitals
C. Proposed Definition of “Items and Services” Provided by Hospitals
D. Proposed Definitions for Types of “Standard Charges”
E. Proposed Requirements for Public Disclosure of All Hospital Standard Charges for All Items and Services
F. Proposed Requirements for Consumer-Friendly Display of the Payer-Specific Negotiated Charges for Selected Shoppable Services
G. Proposed Monitoring and Enforcement of Requirements for Making Standard Charges Public
H. Proposed Appeals Process
XVII. Request for Information (RFI): Quality Measurement Relating to Price Transparency for Improving Beneficiary Access to Provider and Supplier Charge Information
A. Introduction
B. Request for Information
XVIII. Organ Procurement Organizations (OPOs) Conditions for Coverage (CfCs): Proposed Revision of the Definition of “Expected Donation Rate”
A. Background
B. Proposed Revision of the Definition of “Expected Donation Rate”
C. Request for Information Regarding Potential Changes to the Organ Procurement Organization and Transplant Center Regulations
XIX. Clinical Laboratory Fee Schedule: Potential Revisions to the Laboratory Date of Service Policy
A. Background on the Medicare Part B Laboratory Date of Service Policy
B. Medicare DOS Policy and the “14-Day Rule”
C. Billing and Payment for Laboratory Services Under the OPPS
D. ADLTs Under the New Private Payor Rate-Based CLFS
E. Additional Laboratory DOS Policy Exception for the Hospital Outpatient Setting
F. Potential Revisions to Laboratory DOS Policy and Request for Public Comments
XX. Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
A. Background
B. Proposal for a Prior Authorization Process for Certain OPD Services
C. Proposed List of Outpatient Department Services Requiring Prior Authorization
XXI. Comment Solicitation on Cost Reporting, Maintenance of Hospital Chargemasters, and Related Medicare Payment Issues
XXII. Proposed Changes to Requirements for Grandfathered Children's Hospitals-Within-Hospitals (HwHs)
XXIII. Files Available to the Public Via the internet
XXIV. Collection of Information Requirements
A. Statutory Requirement for Solicitation of Comments
B. ICRs for the Hospital OQR Program
C. ICRs for the ASCQR Program
D. ICR for Proposal on Hospital Price Transparency
E. ICRs for Proposed Revision of the Definition of “Expected Donation Rate” for Organ Procurement Organizations
F. ICR for Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
G. Potential Revisions to Laboratory Date of Service (DOS) Policy
H. Total Reduction in Burden Hours and in Costs
XXV. Response to Comments
XXVI. Economic Analyses
A. Statement of Need
B. Overall Impact for the Provisions of This Proposed Rule
C. Detailed Economic Analyses
D. Effects of the Proposals Relating to Price Transparency in Hospital Standard Charges
E. Effects of Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
F. Effects of Proposal Relating to Changes in the Definition of Expected Donation Rate for Organ Procurement Organizations
G. Potential Revisions to the Laboratory Date of Service Policy
H. Effect of Proposed Changes to Requirements for Grandfathered Children's Hospitals-Within-Hospitals (HwHs)
I. Regulatory Review Costs
J. Regulatory Flexibility Act (RFA) Analysis
K. Unfunded Mandates Reform Act Analysis
L. Reducing Regulation and Controlling Regulatory Costs
M. Conclusion
XXVII. Federalism Analysis Regulation Text
I. Summary and Background
A. Executive Summary of This Document
1. Purpose
In this proposed rule, we are proposing to update the payment policies and payment rates for services furnished to Medicare beneficiaries in hospital outpatient departments (HOPDs) and ambulatory surgical centers (ASCs), beginning January 1, 2020. Section 1833(t) of the Social Security Act (the Act) requires us to annually review and update the payment rates for services payable under the Hospital Outpatient Prospective Payment System (OPPS). Specifically, section 1833(t)(9)(A) of the Act requires the Secretary to review certain components of the OPPS not less often than annually, and to revise the groups, the relative payment weights, and the wage and other adjustments that take into account changes in medical practices, changes in technologies, and the addition of new services, new cost data, and other relevant information and factors. In addition, under section 1833(i) of the Act, we annually review and update the ASC payment rates. We describe these and various other statutory authorities in the relevant sections of this proposed rule. In addition, this proposed rule would update and refine the requirements for the Hospital Outpatient Quality Reporting (OQR) Program and the ASC Quality Reporting (ASCQR) Program.
In this proposed rule, we also are proposing to: Establish requirements for all hospitals (including hospitals not paid under the OPPS) in the United States for making hospital standard charges available to the public; establish a process and requirements for prior authorization for certain covered outpatient department services; revise the conditions for coverage for organ procurement organizations; and revise the regulations to allow grandfathered children's hospitals-within-hospitals to increase the number of beds without resulting in the loss of grandfathered status. We also solicit comments on potential revisions to the laboratory date of service policy under the Clinical Laboratory Fee Schedule.
2. Summary of the Major Provisions
- OPPS Update: For CY 2020, we are proposing to increase the payment rates under the OPPS by an Outpatient Department (OPD) fee schedule increase factor of 2.7 percent. This increase factor is based on the proposed hospital inpatient market basket percentage increase of 3.2 percent for inpatient services paid under the hospital inpatient prospective payment system (IPPS), minus the proposed multifactor productivity (MFP) adjustment of 0.5 percentage point. Based on this proposed update, we estimate that total payments to OPPS providers (including beneficiary cost-sharing and estimated changes in enrollment, utilization, and case-mix) for CY 2020 would be approximately $79 billion, an increase of approximately $6 billion compared to estimated CY 2019 OPPS payments.
We are proposing to continue to implement the statutory 2.0 percentage point reduction in payments for hospitals failing to meet the hospital outpatient quality reporting requirements, by applying a reporting factor of 0.980 to the OPPS payments and copayments for all applicable services.
- 2-Midnight Rule (Short Inpatient Hospital Stays): For CY 2020, we are proposing to establish a 1-year exemption from Beneficiary and Family-Centered Care Quality Improvement Organizations (BFCC-QIOs) referrals to Recovery Audit Contractors (RACs) and RAC reviews for “patient status” (that is, site-of-service) for procedures that are removed from the inpatient only (IPO) list under the OPPS beginning on January 1, 2020.
- Comprehensive APCs: For CY 2020, we are proposing to create two new comprehensive APCs (C-APCs). These proposed new C-APCs include the following: C-APC 5182 (Level 2 Vascular Procedures) and proposed C-APC 5461 (Level 1 Neurostimulator and Related Procedures). This proposal would increase the total number of C-APCs to 67.
- Proposed Changes to the Inpatient Only (IPO) List: For CY 2020, we are proposing to remove one procedure from the inpatient only list and we are seeking public comment on the removal of six procedures from the inpatient only (IPO) list.
- Method to Control Unnecessary Increases in the Volume of Clinic Visit Services Furnished in Excepted Off-Campus Provider-Based Departments (PBDs): For CY 2020, we are completing the phase-in of the reduction in payment for the clinic visit services described by HCPCS code G0463 furnished in expected off-campus provider-based departments as a method to control uncessary increases in the volume of this service.
- Device Pass-Through Payment Applications: For CY 2020, we are evaluating seven applications for device pass-through payments and are seeking public comments in this CY 2020 proposed rule on whether these applications meet the criteria for device pass-through payment status.
- Proposed Changes to Substantial Clinical Improvement Criterion: For CY 2020, we are proposing an alternative pathway to the substantial clinical improvement criterion for devices approved under the FDA Breakthrough Devices Program to qualify for device pass-through status beginning with applications received on or after January 1, 2020.
- Cancer Hospital Payment Adjustment: For CY 2020, we are proposing to continue to provide additional payments to cancer hospitals so that a cancer hospital's payment-to-cost ratio (PCR) after the additional payments is equal to the weighted average PCR for the other OPPS hospitals using the most recently submitted or settled cost report data. However, section 16002(b) of the 21st Century Cures Act requires that this weighted average PCR be reduced by 1.0 percentage point. Based on the data and the required 1.0 percentage point reduction, a proposed target PCR of 0.89 will be used to determine the CY 2020 cancer hospital payment adjustment to be paid at cost report settlement. That is, the payment adjustment will be the additional payments needed to result in a PCR equal to 0.89 for each cancer hospital.
- Rural Adjustment: For 2020 and subsequent years, we are continuing the 7.1 percent adjustment to OPPS payments for certain rural SCHs, including essential access community hospitals (EACHs). We intend to continue such 7.1 percent adjustment in the absence of data to suggest a different percentage adjustment should apply.
- 340B -Acquired Drugs: We are proposing to continue to pay ASP−22.5 percent for 340B-acquired drugs including when furnished in nonexcepted off-campus PBDs paid under the PFS. On December 27, 2018, in the case of American Hospital Association et al. v. Azar et al., the United States District Court for the District of Columbia (hereinafter referred to as “the district court”) concluded in the context of reimbursement requests for CY 2018 that the Secretary exceeded his statutory authority by adjusting the Medicare payment rates for drugs acquired under the 340B Program to ASP minus 22.5 percent for that year. CMS respectfully disagreed with the district court's understanding of the scope of CMS' adjustment authority and asked the district court to enter final judgment so as to permit an immediate appeal. On July 10, 2019, the district court granted the government's request and entered final judgment, and the agency does intend to pursue its appeal rights. Nonetheless, CMS is taking the steps necessary to craft an appropriate remedy in the event of an unfavorable decision on appeal. We are soliciting public comments on the appropriate OPPS payment rate for 340B-acquired drugs, including whether a rate of ASP+3 percent could be an appropriate payment amount for these drugs, both for CY 2020 and for purposes of determining the remedy for CYs 2018 and 2019. In addition to comments on the appropriate payment amount for calculating the remedy for CYs 2018 and 2019 and for use for CY 2020, we also seek public comment on how to structure the remedy for CYs 2018 and 2019. This request for public comment includes comments on whether such a remedy should be retrospective in nature (for example, made on a claim-by-claim basis), whether such a remedy could be prospective in nature (for example, an upward adjustment to 340B claims in the future to account for any underpayments in the past), and whether there is some other mechanism that could produce a result equitable to hospitals that do not acquire drugs through the 340B program while respecting the budget neutrality mandate. In the event of an adverse decision on appeal, we would anticipate proposing the specific remedy for CYs 2018 and 2019, and, if necessary, to the CY 2020 rates, in the next available rulemaking vehicle, which is the CY 2021 OPPS/ASC proposed rule. Those proposals will be informed by the comments solicited in this proposed rule.
- ASC Payment Update: For CYs 2019 through 2023, we update the ASC payment system using the hospital market basket update. Using the hospital market basket methodology, for CY 2020, we are proposing to increase payment rates under the ASC payment system by 2.7 percent for ASCs that meet the quality reporting requirements under the ASCQR Program. This proposed increase is based on a proposed hospital market basket of 3.2 percent minus a proposed multifactor productivity adjustment required by the Affordable Care Act of 0.5 percentage point. Based on this proposed update, we estimate that total payments to ASCs (including beneficiary cost-sharing and estimated changes in enrollment, utilization, and case-mix) for CY 2020 would be approximately $4.89 billion, an increase of approximately $200 million compared to estimated CY 2019 Medicare payments.
- Proposed Changes to the List of ASC Covered Surgical Procedures: For CY 2020, we are proposing to add 8 procedures to the ASC list of covered surgical procedures. Additions to the list include a total knee arthroplasty procedure, a mosaicplasty procedure, as well as six coronary intervention procedures. We are soliciting public comments with respect to whether certain other surgical procedures related to the cardiovascular system should be added to the ASC list of covered surgical procedures.
- Proposed Changes to the Level of Supervision of Outpatient Therapeutic Services in Hospitals and Critical Access Hospitals: For CY 2020, we are proposing to change the minimum required level of supervision from direct supervision to general supervision for all hospital outpatient therapeutic services provided by all hospitals and CAHs. This proposal would ensure a standard minimum level of supervision for each hospital outpatient service furnished incident to a physician's service.
- Hospital Outpatient Quality Reporting (OQR) Program: For the Hospital OQR Program, we are proposing to remove OP-33: External Beam Radiotherapy for Bone Metastases for the CY 2022 payment determination and subsequent years.
- Ambulatory Surgical Center Quality Reporting (ASCQR) Program: For the ASCQR Program, we are proposing to adopt one new measure, ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers, beginning with the CY 2024 payment determination and for subsequent years.
Proposed Requirements for Hospitals to Make Public a List of Their Standard Charges: We are proposing to add a new Part 180—Hospital Price Transparency to Title 45 of the Code of Federal Regulations (CFR) which would contain our proposed regulations on price transparency for purposes of section 2718(e) of the PHS Act. In this section, we make proposals related to: (1) A definition of “hospital”; (2) different reporting requirements that would apply to certain hospitals; (3) definitions for two types of “standard charges” (specifically, gross charges and payer-specific negotiated charges) that hospitals would be required to make public, and a request for public comment on other types of standard charges that hospitals should be required to make public; (4) a definition of hospital “items and services” that would include all items and services (including individual items and services and service packages) provided by the hospital to a patient in connection with an inpatient admission or an outpatient department visit; (5) requirements for making public a machine-readable file that contains a hospital's gross charges and payer-specific negotiated charges for all items and services provided by the hospital; (6) requirements for making public payer-specific negotiated charges for select hospital-provided items and services that are “shoppable” and that are displayed in a consumer-friendly manner; (7) monitoring for hospital noncompliance with public disclosure requirements to make public standard charges; (8) actions that would address hospital noncompliance, which include issuing a written warning notice, requesting a corrective action plan, and imposing civil monetary penalties (CMPs) on noncompliant hospitals and publicizing these penalties on a CMS website; and (9) appeals of CMPs.
- Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services: We are proposing a prior authorization process using the authority in section 1833(t)(2)(F) of the Act as a method for controlling unnecessary increases in the volume of the following five categories of services: (1) Blepharoplasty, (2) botulinum toxin injections, (3) panniculectomy, (4) rhinoplasty, and (5) vein ablation.
- Organ Procurement Organizations (OPOs) Conditions for Coverage (CfCs) Proposed Revision of the Definition of “Expected Donation Rate”: We are proposing to revise the definition of “expected donation rate” that is included in the second outcome measure to match the Scientific Registry of Transplant Recipients (SRTR) definition.
We are also proposing to reduce the time period for the second outcome measure and calculate the expected donation rate using 12 out of the 24 months of data (from January 1, 2020 through December 31, 2020) for the 2022 recertification cycle only.
- Request for Information Regarding Potential Changes to the Organ Procurement Organization and Transplant Center Regulations: We are soliciting public comments regarding what revisions may be appropriate for the current OPO CfCs and the current transplant center CoPs. In addition, we are seeking public comments on two potential outcome measures for OPOs.
3. Summary of Costs and Benefits
In sections XXVI. and XXVII. of this proposed rule, we set forth a detailed analysis of the regulatory and federalism impacts that the proposed changes would have on affected entities and beneficiaries. Key estimated impacts are described below.
a. Impacts of All OPPS Proposed Changes
Table 41 in section XXVI. of this proposed rule displays the distributional impact of all the proposed OPPS changes on various groups of hospitals and CMHCs for CY 2020 compared to all estimated OPPS payments in CY 2019. We estimate that the policies in this proposed rule would result in a 2.0 percent overall increase in OPPS payments to providers. We estimate that total OPPS payments for CY 2020, including beneficiary cost-sharing, to the approximately 3,734 facilities paid under the OPPS (including general acute care hospitals, children's hospitals, cancer hospitals, and CMHCs) would increase by approximately $940 million compared to CY 2019 payments, excluding our estimated changes in enrollment, utilization, and case-mix.
We estimated the isolated impact of our proposed OPPS policies on CMHCs because CMHCs are only paid for partial hospitalization services under the OPPS. Continuing the provider-specific structure we adopted beginning in CY 2011, and basing payment fully on the type of provider furnishing the service, we estimate a 3.9 percent increase in CY 2020 payments to CMHCs relative to their CY 2019 payments.
b. Impacts of the Proposed Updated Wage Indexes
We estimate that our proposed update of the wage indexes based on the FY 2020 IPPS proposed rule wage indexes would result in no estimated payment change for urban hospitals under the OPPS and an estimated increase of 0.8 percent for rural hospitals. These proposed wage indexes include the continued implementation of the OMB labor market area delineations based on 2010 Decennial Census data, with updates, as discussed in section II.C. of this proposed rule.
c. Impacts of the Proposed Rural Adjustment and the Cancer Hospital Payment Adjustment
There are no significant impacts of our proposed CY 2020 payment policies for hospitals that are eligible for the rural adjustment or for the cancer hospital payment adjustment. We are not proposing to make any change in policies for determining the rural hospital payment adjustments. While we are proposing to implement the reduction to the cancer hospital payment adjustment required by section 16002 of the 21st Century Cures Act for CY 2020, the target payment-to-cost ratio (PCR) for CY 2020 is 0.89, compared to 0.88 for CY 2019, and therefore has a slight impact on budget neutrality adjustments.
d. Impacts of the Proposed OPD Fee Schedule Increase Factor
For the CY 2020 OPPS/ASC, we are proposing an OPD fee schedule increase factor of 2.7 percent and applying that increase factor to the conversion factor for CY 2020. As a result of the proposed OPD fee schedule increase factor and other budget neutrality adjustments, we estimate that urban hospitals would experience an increase of approximately 2.8 percent and that rural hospitals would experience an increase of 3.0 percent. Classifying hospitals by teaching status, we estimate nonteaching hospitals would experience an increase of 3.0 percent, minor teaching hospitals would experience an increase of 3.1 percent, and major teaching hospitals would experience an increase of 2.3 percent. We also classified hospitals by the type of ownership. We estimate that hospitals with voluntary ownership would experience an increase of 2.7 percent in payments, while hospitals with government ownership would experience an increase of 2.8 percent in payments. We estimate that hospitals with proprietary ownership would an experience an increase of 3.6 percent in payments.
e. Impacts of the Proposed ASC Payment Update
For impact purposes, the surgical procedures on the ASC list of covered procedures are aggregated into surgical specialty groups using CPT and HCPCS code range definitions. The percentage change in estimated total payments by specialty groups under the proposed CY 2020 payment rates, compared to estimated CY 2019 payment rates, generally ranges between an increase of 2 and 5 percent, depending on the service, with some exceptions. We estimate the impact of applying the hospital market basket update to proposed ASC payment rates would increase payments by $100 million under the ASC payment system in CY 2020.
f. Impact of the Proposed Changes to the Hospital OQR Program
Across 3,300 hospitals participating in the Hospital OQR Program, we estimate that our proposed requirements would result in the following changes to costs and burdens related to information collection for the Hospital OQR Program compared to previously adopted requirements: If all proposals are adopted as final, there is a net reduction of one measure reported by hospitals, which would result in a minimal net reduction in burden of $21,379.
g. Impact of the Proposed Changes to the ASCQR Program
Across 3,937 ASCs participating in the ASCQR Program, we estimate that our proposed requirements would not result in changes to costs and burdens related to information collection for the ASCQR Program, compared to previously adopted requirements.
h. Impact of the Proposed Requirements for Hospitals To Make Public a List of Their Standard Charges
We estimate the total annual burden for hospitals to review and post their standard charges to be 12 hours per hospital at $1,017.24 per hospital for a total burden of 72,024 hours (12 hours × 6,002 hospitals) and total cost of $6,105,474 ($1,017.24 × 6,002 hospitals) if our policies, as discussed in section XVI. of this proposed rule are finalized as proposed.
i. Impact of the Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
Across all providers, we estimate that the total burden for year one (6 months) would be 73,647 hours and $2,604,281 (Table 48—Year 1 (6 Month) Private Sector Costs of this proposed rule) for the five categories of services for which we are proposing to require prior authorization. In addition, we estimate that the total annual burden, allotted across all providers, would be 125,242 hours and $4,475,116 per year for the services. An annualized burden is estimated at 108,044 hours and $3,851,504. The annualized burden is based on an average of 3 years, that is, 1 year at the 6-month burden and 2 years at the 12-month burden. This accounts for the time associated with submitting the prior authorization request package and related medical documentation to support Medicare payment of the service(s). Medicare would incur $5,787,055 for the first 6 months (Table 49—Year 1 (6 Month) Estimated Annual Medicare Costs of this proposed rule) and $11,571,179 annually therafter, in additional costs associated with processing the prior authorization requests, as well as education, outreach, and systems. Benefits include decreased unnecessary utilization of these OPD services, and subsequently, reduced improper payments made for claims for these services that do not meet Medicare requirements.
j. Impacts of the Proposed Revision of the Definition of “Expected Donation Rate” for Organ Procurement Organizations
All 58 OPOs are required to meet two out of three outcome measures detailed in the OPO CfC regulations at 42 CFR 486.318(b). We are proposing to revise the definition of “expected donation rate” in the OPO CfCs. This revision would eliminate the potential for confusion in the OPO community due to different definitions of the same term. The proposal would not affect data collection or reporting by the OPTN and SRTR, nor their statistical evaluation of OPO performance. Therefore, it would not result in any quantifiable financial impact.
B. Legislative and Regulatory Authority for the Hospital OPPS
When Title XVIII of the Social Security Act (the Act) was enacted, Medicare payment for hospital outpatient services was based on hospital-specific costs. In an effort to ensure that Medicare and its beneficiaries pay appropriately for services and to encourage more efficient delivery of care, the Congress mandated replacement of the reasonable cost-based payment methodology with a prospective payment system (PPS). The Balanced Budget Act of 1997 (BBA) (Pub. L. 105-33) added section 1833(t) to the Act, authorizing implementation of a PPS for hospital outpatient services. The OPPS was first implemented for services furnished on or after August 1, 2000. Implementing regulations for the OPPS are located at 42 CFR parts 410 and 419.
The Medicare, Medicaid, and SCHIP Balanced Budget Refinement Act of 1999 (BBRA) (Pub. L. 106-113) made major changes in the hospital OPPS. The following Acts made additional changes to the OPPS: The Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act of 2000 (BIPA) (Pub. L. 106-554); the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (Pub. L. 108-173); the Deficit Reduction Act of 2005 (DRA) (Pub. L. 109-171), enacted on February 8, 2006; the Medicare Improvements and Extension Act under Division B of Title I of the Tax Relief and Health Care Act of 2006 (MIEA-TRHCA) (Pub. L. 109-432), enacted on December 20, 2006; the Medicare, Medicaid, and SCHIP Extension Act of 2007 (MMSEA) (Pub. L. 110-173), enacted on December 29, 2007; the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA) (Pub. L. 110-275), enacted on July 15, 2008; the Patient Protection and Affordable Care Act (Pub. L. 111-148), enacted on March 23, 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152), enacted on March 30, 2010 (these two public laws are collectively known as the Affordable Care Act); the Medicare and Medicaid Extenders Act of 2010 (MMEA, Pub. L. 111-309); the Temporary Payroll Tax Cut Continuation Act of 2011 (TPTCCA, Pub. L. 112-78), enacted on December 23, 2011; the Middle Class Tax Relief and Job Creation Act of 2012 (MCTRJCA, Pub. L. 112-96), enacted on February 22, 2012; the American Taxpayer Relief Act of 2012 (Pub. L. 112-240), enacted January 2, 2013; the Pathway for SGR Reform Act of 2013 (Pub. L. 113-67) enacted on December 26, 2013; the Protecting Access to Medicare Act of 2014 (PAMA, Pub. L. 113-93), enacted on March 27, 2014; the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015 (Pub. L. 114-10), enacted April 16, 2015; the Bipartisan Budget Act of 2015 (Pub. L. 114-74), enacted November 2, 2015; the Consolidated Appropriations Act, 2016 (Pub. L. 114-113), enacted on December 18, 2015, the 21st Century Cures Act (Pub. L. 114-255), enacted on December 13, 2016; the Consolidated Appropriations Act, 2018 (Pub. L. 115-141), enacted on March 23, 2018; and the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (Pub. L. 115-271), enacted on October 24, 2018.
Under the OPPS, we generally pay for hospital Part B services on a rate-per-service basis that varies according to the APC group to which the service is assigned. We use the Healthcare Common Procedure Coding System (HCPCS) (which includes certain Current Procedural Terminology (CPT) codes) to identify and group the services within each APC. The OPPS includes payment for most hospital outpatient services, except those identified in section I.C. of this proposed rule. Section 1833(t)(1)(B) of the Act provides for payment under the OPPS for hospital outpatient services designated by the Secretary (which includes partial hospitalization services furnished by CMHCs), and certain inpatient hospital services that are paid under Medicare Part B.
The OPPS rate is an unadjusted national payment amount that includes the Medicare payment and the beneficiary copayment. This rate is divided into a labor-related amount and a nonlabor-related amount. The labor-related amount is adjusted for area wage differences using the hospital inpatient wage index value for the locality in which the hospital or CMHC is located.
All services and items within an APC group are comparable clinically and with respect to resource use, as required by section 1833(t)(2)(B) of the Act. In accordance with section 1833(t)(2)(B) of the Act, subject to certain exceptions, items and services within an APC group cannot be considered comparable with respect to the use of resources if the highest median cost (or mean cost, if elected by the Secretary) for an item or service in the APC group is more than 2 times greater than the lowest median cost (or mean cost, if elected by the Secretary) for an item or service within the same APC group (referred to as the “2 times rule”). In implementing this provision, we generally use the cost of the item or service assigned to an APC group.
For new technology items and services, special payments under the OPPS may be made in one of two ways. Section 1833(t)(6) of the Act provides for temporary additional payments, which we refer to as “transitional pass-through payments,” for at least 2 but not more than 3 years for certain drugs, biological agents, brachytherapy devices used for the treatment of cancer, and categories of other medical devices. For new technology services that are not eligible for transitional pass-through payments, and for which we lack sufficient clinical information and cost data to appropriately assign them to a clinical APC group, we have established special APC groups based on costs, which we refer to as New Technology APCs. These New Technology APCs are designated by cost bands which allow us to provide appropriate and consistent payment for designated new procedures that are not yet reflected in our claims data. Similar to pass-through payments, an assignment to a New Technology APC is temporary; that is, we retain a service within a New Technology APC until we acquire sufficient data to assign it to a clinically appropriate APC group.
C. Excluded OPPS Services and Hospitals
Section 1833(t)(1)(B)(i) of the Act authorizes the Secretary to designate the hospital outpatient services that are paid under the OPPS. While most hospital outpatient services are payable under the OPPS, section 1833(t)(1)(B)(iv) of the Act excludes payment for ambulance, physical and occupational therapy, and speech-language pathology services, for which payment is made under a fee schedule. It also excludes screening mammography, diagnostic mammography, and effective January 1, 2011, an annual wellness visit providing personalized prevention plan services. The Secretary exercises the authority granted under the statute to also exclude from the OPPS certain services that are paid under fee schedules or other payment systems. Such excluded services include, for example, the professional services of physicians and nonphysician practitioners paid under the Medicare Physician Fee Schedule (MPFS); certain laboratory services paid under the Clinical Laboratory Fee Schedule (CLFS); services for beneficiaries with end-stage renal disease (ESRD) that are paid under the ESRD prospective payment system; and services and procedures that require an inpatient stay that are paid under the hospital IPPS. In addition, section 1833(t)(1)(B)(v) of the Act does not include applicable items and services (as defined in subparagraph (A) of paragraph (21)) that are furnished on or after January 1, 2017 by an off-campus outpatient department of a provider (as defined in subparagraph (B) of paragraph (21). We set forth the services that are excluded from payment under the OPPS in regulations at 42 CFR 419.22.
Under § 419.20(b) of the regulations, we specify the types of hospitals that are excluded from payment under the OPPS. These excluded hospitals include:
- Critical access hospitals (CAHs);
- Hospitals located in Maryland and paid under the Maryland All-Payer Model;
- Hospitals located outside of the 50 States, the District of Columbia, and Puerto Rico; and
- Indian Health Service (IHS) hospitals.
D. Prior Rulemaking
On April 7, 2000, we published in the Federal Register a final rule with comment period (65 FR 18434) to implement a prospective payment system for hospital outpatient services. The hospital OPPS was first implemented for services furnished on or after August 1, 2000. Section 1833(t)(9)(A) of the Act requires the Secretary to review certain components of the OPPS, not less often than annually, and to revise the groups, relative payment weights, and the wage and other adjustments that take into account changes in medical practices, changes in technologies, and the addition of new services, new cost data, and other relevant information and factors.
Since initially implementing the OPPS, we have published final rules in the Federal Register annually to implement statutory requirements and changes arising from our continuing experience with this system. These rules can be viewed on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices.html.
E. Advisory Panel on Hospital Outpatient Payment (the HOP Panel or the Panel)
1. Authority of the Panel
Section 1833(t)(9)(A) of the Act, as amended by section 201(h) of Public Law 106-113, and redesignated by section 202(a)(2) of Public Law 106-113, requires that we consult with an external advisory panel of experts to annually review the clinical integrity of the payment groups and their weights under the OPPS. In CY 2000, based on section 1833(t)(9)(A) of the Act, the Secretary established the Advisory Panel on Ambulatory Payment Classification Groups (APC Panel) to fulfill this requirement. In CY 2011, based on section 222 of the Public Health Service Act, which gives discretionary authority to the Secretary to convene advisory councils and committees, the Secretary expanded the panel's scope to include the supervision of hospital outpatient therapeutic services in addition to the APC groups and weights. To reflect this new role of the panel, the Secretary changed the panel's name to the Advisory Panel on Hospital Outpatient Payment (the HOP Panel or the Panel). The HOP Panel is not restricted to using data compiled by CMS, and in conducting its review, it may use data collected or developed by organizations outside the Department.
2. Establishment of the Panel
On November 21, 2000, the Secretary signed the initial charter establishing the Panel, and, at that time, named the APC Panel. This expert panel is composed of appropriate representatives of providers (currently employed full-time, not as consultants, in their respective areas of expertise) who review clinical data and advise CMS about the clinical integrity of the APC groups and their payment weights. Since CY 2012, the Panel also is charged with advising the Secretary on the appropriate level of supervision for individual hospital outpatient therapeutic services. The Panel is technical in nature, and it is governed by the provisions of the Federal Advisory Committee Act (FACA). The current charter specifies, among other requirements, that the Panel—
- May advise on the clinical integrity of Ambulatory Payment Classification (APC) groups and their associated weights;
- May advise on the appropriate supervision level for hospital outpatient services;
- Continues to be technical in nature;
- Is governed by the provisions of the FACA;
- Has a Designated Federal Official (DFO); and
- Is chaired by a Federal Official designated by the Secretary.
The Panel's charter was amended on November 15, 2011, renaming the Panel and expanding the Panel's authority to include supervision of hospital outpatient therapeutic services and to add critical access hospital (CAH) representation to its membership. The Panel's charter was also amended on November 6, 2014 (80 FR 23009), and the number of members was revised from up to 19 to up to 15 members. The Panel's current charter was approved on November 19, 2018, for a 2-year period.
The current Panel membership and other information pertaining to the Panel, including its charter, Federal Register notices, membership, meeting dates, agenda topics, and meeting reports, can be viewed on the CMS website at: https://www.cms.gov/Regulations-and-Guidance/Guidance/FACA/AdvisoryPanelonAmbulatoryPaymentClassificationGroups.html.
3. Panel Meetings and Organizational Structure
The Panel has held many meetings, with the last meeting taking place on August 20, 2018. Prior to each meeting, we publish a notice in the Federal Register to announce the meeting and, when necessary, to solicit nominations for Panel membership, to announce new members, and to announce any other changes of which the public should be aware. Beginning in CY 2017, we have transitioned to one meeting per year (81 FR 31941). The next meeting will take place on August 19-20, 2019. Complete information on the 2019 summer meeting, including information related to meeting presentations and submittals, meeting attendance/admittance, and web streaming of the meeting, can be found in the meeting notice published in the Federal Register on June 5, 2019 (84 FR 26117) and available on the website at: https://www.govinfo.gov/content/pkg/FR-2019-06-05/pdf/2019-11756.pdf. Registration to attend the meeting in person may be made through the CMS website at: https://www.cms.gov/apps/events/event.asp?id=3745.
In addition, the Panel has established an operational structure that, in part, currently includes the use of three subcommittees to facilitate its required review process. The three current subcommittees include the following:
- APC Groups and Status Indicator Assignments Subcommittee, which advises the Panel on the appropriate status indicators to be assigned to HCPCS codes, including but not limited to whether a HCPCS code or a category of codes should be packaged or separately paid, as well as the appropriate APC assignment of HCPCS codes regarding services for which separate payment is made;
- Data Subcommittee, which is responsible for studying the data issues confronting the Panel and for recommending options for resolving them; and
- Visits and Observation Subcommittee, which reviews and makes recommendations to the Panel on all technical issues pertaining to observation services and hospital outpatient visits paid under the OPPS.
Each of these subcommittees was established by a majority vote from the full Panel during a scheduled Panel meeting, and the Panel recommended at the August 20, 2018 meeting that the subcommittees continue. We accepted this recommendation.
Discussions of the other recommendations made by the Panel at the August 20, 2018 Panel meeting, namely CPT codes and a comprehensive APC for autologous hematopoietic stem cell transplantation, OPPS payment for outpatient clinic visits and restrictions to service line expansions, and packaging policies, were discussed in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58827). For discussions of earlier Panel meetings and recommendations, we refer readers to previously published OPPS/ASC proposed and final rules, the CMS website mentioned earlier in this section, and the FACA database at http://facadatabase.gov.
F. Public Comments Received on the CY 2019 OPPS/ASC Final Rule With Comment Period
We received over 540 timely pieces of correspondence on the CY 2019 OPPS/ASC final rule with comment period that appeared in the Federal Register on November 30, 2018 (83 FR 61567), some of which contained comments on the interim APC assignments and/or status indicators of new or replacement Level II HCPCS codes (identified with comment indicator “NI” in OPPS Addendum B, ASC Addendum AA, and ASC Addendum BB to that final rule).
II. Proposed Updates Affecting OPPS Payments
A. Proposed Recalibration of APC Relative Payment Weights
1. Database Construction
a. Database Source and Methodology
Section 1833(t)(9)(A) of the Act requires that the Secretary review not less often than annually and revise the relative payment weights for APCs. In the April 7, 2000 OPPS final rule with comment period (65 FR 18482), we explained in detail how we calculated the relative payment weights that were implemented on August 1, 2000 for each APC group.
In this CY 2020 OPPS/ASC proposed rule, for CY 2020, we are proposing to recalibrate the APC relative payment weights for services furnished on or after January 1, 2020, and before January 1, 2021 (CY 2020), using the same basic methodology that we described in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58827 through 58828), using updated CY 2018 claims data. That is, we are proposing to recalibrate the relative payment weights for each APC based on claims and cost report data for hospital outpatient department (HOPD) services, using the most recent available data to construct a database for calculating APC group weights.
For the purpose of recalibrating the APC proposed relative payment weights for CY 2020, we began with approximately 164 million final action claims (claims for which all disputes and adjustments have been resolved and payment has been made) for HOPD services furnished on or after January 1, 2018, and before January 1, 2019, before applying our exclusionary criteria and other methodological adjustments. After the application of those data processing changes, we used approximately 88 million final action claims to develop the proposed CY 2020 OPPS payment weights. For exact numbers of claims used and additional details on the claims accounting process, we refer readers to the claims accounting narrative under supporting documentation for this CY 2020 OPPS/ASC proposed rule on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
Addendum N to this proposed rule (which is available via the internet on the CMS website) includes the proposed list of bypass codes for CY 2020. The proposed list of bypass codes contains codes that were reported on claims for services in CY 2018 and, therefore, includes codes that were in effect in CY 2018 and used for billing, but were deleted for CY 2019. We retained these deleted bypass codes on the proposed CY 2020 bypass list because these codes existed in CY 2018 and were covered OPD services in that period, and CY 2018 claims data were used to calculate proposed CY 2020 payment rates. Keeping these deleted bypass codes on the bypass list potentially allows us to create more “pseudo” single procedure claims for ratesetting purposes. “Overlap bypass codes” that are members of the proposed multiple imaging composite APCs are identified by asterisks (*) in the third column of Addendum N to this proposed rule. HCPCS codes that we are proposing to add for CY 2020 are identified by asterisks (*) in the fourth column of Addendum N.
Table 1 contains the list of codes that we are proposing to remove from the CY 2020 bypass list.

b. Proposed Calculation and Use of Cost-to-Charge Ratios (CCRs)
For CY 2020, in this CY 2020 OPPS/ASC proposed rule, we are proposing to continue to use the hospital-specific overall ancillary and departmental cost-to-charge ratios (CCRs) to convert charges to estimated costs through application of a revenue code-to-cost center crosswalk. To calculate the APC costs on which the proposed CY 2020 APC payment rates are based, we calculated hospital-specific overall ancillary CCRs and hospital-specific departmental CCRs for each hospital for which we had CY 2018 claims data by comparing these claims data to the most recently available hospital cost reports, which, in most cases, are from CY 2017. For the proposed CY 2020 OPPS payment rates, we used the set of claims processed during CY 2018. We applied the hospital-specific CCR to the hospital's charges at the most detailed level possible, based on a revenue code-to-cost center crosswalk that contains a hierarchy of CCRs used to estimate costs from charges for each revenue code. That crosswalk is available for review and continuous comment on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
To ensure the completeness of the revenue code-to-cost center crosswalk, we reviewed changes to the list of revenue codes for CY 2018 (the year of claims data we used to calculate the proposed CY 2020 OPPS payment rates) and found that the National Uniform Billing Committee (NUBC) did not add any new revenue codes to the NUBC 2018 Data Specifications Manual.
In accordance with our longstanding policy, we calculate CCRs for the standard and nonstandard cost centers accepted by the electronic cost report database. In general, the most detailed level at which we calculate CCRs is the hospital-specific departmental level. For a discussion of the hospital-specific overall ancillary CCR calculation, we refer readers to the CY 2007 OPPS/ASC final rule with comment period (71 FR 67983 through 67985). The calculation of blood costs is a longstanding exception (since the CY 2005 OPPS) to this general methodology for calculation of CCRs used for converting charges to costs on each claim. This exception is discussed in detail in the CY 2007 OPPS/ASC final rule with comment period and discussed further in section II.A.2.a.(1) of this proposed rule.
In the CY 2014 OPPS/ASC final rule with comment period (78 FR 74840 through 74847), we finalized our policy of creating new cost centers and distinct CCRs for implantable devices, magnetic resonance imaging (MRIs), computed tomography (CT) scans, and cardiac catheterization. However, in response to the CY 2014 OPPS/ASC proposed rule, commenters reported that some hospitals currently use an imprecise “square feet” allocation methodology for the costs of large moveable equipment like CT scan and MRI machines. They indicated that while CMS recommended using two alternative allocation methods, “direct assignment” or “dollar value,” as a more accurate methodology for directly assigning equipment costs, industry analysis suggested that approximately only half of the reported cost centers for CT scans and MRIs rely on these preferred methodologies. In response to concerns from commenters, we finalized a policy for the CY 2014 OPPS to remove claims from providers that use a cost allocation method of “square feet” to calculate CCRs used to estimate costs associated with the APCs for CT and MRI (78 FR 74847). Further, we finalized a transitional policy to estimate the imaging APC relative payment weights using only CT and MRI cost data from providers that do not use “square feet” as the cost allocation statistic. We provided that this finalized policy would sunset in 4 years to provide a sufficient time for hospitals to transition to a more accurate cost allocation method and for the related data to be available for ratesetting purposes (78 FR 74847). Therefore, beginning CY 2018, with the sunset of the transition policy, we would estimate the imaging APC relative payment weights using cost data from all providers, regardless of the cost allocation statistic employed. However, in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59228 and 59229), we finalized a policy to extend the transition policy for 1 additional year and continued to remove claims from providers that use a cost allocation method of “square feet” to calculate CT and MRI CCRs for the CY 2018 OPPS.
As we discussed in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59228), some stakeholders had raised concerns regarding using claims from all providers to calculate CT and MRI CCRs, regardless of the cost allocations statistic employed (78 FR 74840 through 74847). Stakeholders noted that providers continue to use the “square feet” cost allocation method and that including claims from such providers would cause significant reductions in the imaging APC payment rates.
Table 2 demonstrates the relative effect on imaging APC payments after removing cost data for providers that report CT and MRI standard cost centers using “square feet” as the cost allocation method by extracting HCRIS data on Worksheet B-1. Table 3 provides statistical values based on the CT and MRI standard cost center CCRs using the different cost allocation methods.

Our analysis shows that since the CY 2014 OPPS in which we established the transition policy, the number of valid MRI CCRs has increased by 17.5 percent to 2,184 providers and the number of valid CT CCRs has increased by 15.1 percent to 2,274 providers. However, as shown in Table 2, nearly all imaging APCs would see an increase in payment rates for CY 2020 if claims from providers that report using the “square feet” cost allocation method were removed. This can be attributed to the generally lower CCR values from providers that use a “square feet” cost allocation method as shown in Table 2.
For the CY 2019 OPPS, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58831), we extended our transition policy for an additional year and removed claims from providers that use a cost allocation method of “square feet” to calculate CCRs used to estimate costs with the APCs for CT and MRI identified in Table 2.
We note that the CT and MRI cost center CCRs have been available for ratesetting since the CY 2014 OPPS in which we established the transition policy. Since the initial 4-year transition, we have extended the transition an additional 2 years to offer provider flexibility in applying cost allocation methodologies for CT and MRI cost centers other than “square feet.” We believe we have provided sufficient time for providers to adopt an alternative cost allocation methodology for CT and MRI cost centers if they intended to do so. However, many providers continue to use the “square feet” cost allocation methodology, which we believe indicates that these providers believe this methodology is a sufficient method for attributing costs to this cost center. Additionally, we generally believe that increasing the amount of claims data available for use in ratesetting improves our ratesetting process. Therefore, we are proposing that, for the CY 2020 OPPS/ASC proposed rule and final rule with comment period, we will use all claims with valid CT and MRI cost center CCRs, including those that use a “square feet” cost allocation method, to estimate costs for the APCs for CT and MRI identified in Table 2. We do not believe another extension is warranted and expect to determine the imaging APC relative payment weights for CY 2020 using cost data from all providers, regardless of the cost allocation method employed.
In addition, as we stated in the CY 2014 OPPS/ASC final rule with comment period (78 FR 74845), we have noted the potential impact the CT and MRI CCRs may have on other payment systems. We understand that payment reductions for imaging services under the OPPS could have significant payment impacts under the Physician Fee Schedule (PFS) where the technical component payment for many imaging services is capped at the OPPS payment amount. We will continue to monitor OPPS imaging payments in the future and consider the potential impacts of payment changes on the PFS and the ASC payment system.
2. Proposed Data Development and Calculation of Costs Used for Ratesetting
In this section of this proposed rule, we discuss the use of claims to calculate the proposed OPPS payment rates for CY 2020. The Hospital OPPS page on the CMS website on which this proposed rule is posted (http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html) provides an accounting of claims used in the development of the proposed payment rates. That accounting provides additional detail regarding the number of claims derived at each stage of the process. In addition, below in this section, we discuss the file of claims that comprises the data set that is available upon payment of an administrative fee under a CMS data use agreement. The CMS website, http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html, includes information about obtaining the “OPPS Limited Data Set,” which now includes the additional variables previously available only in the OPPS Identifiable Data Set, including ICD-10-CM diagnosis codes and revenue code payment amounts. This file is derived from the CY 2018 claims that were used to calculate the proposed payment rates for this CY 2020 OPPS/ASC proposed rule.
Previously, the OPPS established the scaled relative weights, on which payments are based using APC median costs, a process described in the CY 2012 OPPS/ASC final rule with comment period (76 FR 74188). However, as discussed in more detail in section II.A.2.f. of the CY 2013 OPPS/ASC final rule with comment period (77 FR 68259 through 68271), we finalized the use of geometric mean costs to calculate the relative weights on which the CY 2013 OPPS payment rates were based. While this policy changed the cost metric on which the relative payments are based, the data process in general remained the same, under the methodologies that we used to obtain appropriate claims data and accurate cost information in determining estimated service cost. In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue to use geometric mean costs to calculate the proposed relative weights on which the CY 2020 OPPS payment rates are based.
We used the methodology described in sections II.A.2.a. through II.A.2.c. of this proposed rule to calculate the costs we used to establish the proposed relative payment weights used in calculating the proposed OPPS payment rates for CY 2020 shown in Addenda A and B to this proposed rule (which are available via the internet on the CMS website). We refer readers to section II.A.4. of this proposed rule for a discussion of the conversion of APC costs to scaled payment weights.
We note that, under the OPPS, CY 2019 was the first year in which claims data containing lines with the modifier “PN” were available, which indicate nonexcepted items and services furnished and billed by off-campus provider-based departments (PBDs) of hospitals. Because nonexcepted services are not paid under the OPPS, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58832), we finalized a policy to remove those claim lines reported with modifier “PN” from the claims data used in ratesetting for the CY 2019 OPPS and subsequent years. For the CY 2020 OPPS, we will continue to remove these claim lines with modifier “PN” from the ratesetting process.
For details of the claims process used in this proposed rule, we refer readers to the claims accounting narrative under supporting documentation for this CY 2020 OPPS/ASC proposed rule on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
a. Proposed Calculation of Single Procedure APC Criteria-Based Costs
(1) Blood and Blood Products
(a) Methodology
Since the implementation of the OPPS in August 2000, we have made separate payments for blood and blood products through APCs rather than packaging payment for them into payments for the procedures with which they are administered. Hospital payments for the costs of blood and blood products, as well as for the costs of collecting, processing, and storing blood and blood products, are made through the OPPS payments for specific blood product APCs.
In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue to establish payment rates for blood and blood products using our blood-specific CCR methodology, which utilizes actual or simulated CCRs from the most recently available hospital cost reports to convert hospital charges for blood and blood products to costs. This methodology has been our standard ratesetting methodology for blood and blood products since CY 2005. It was developed in response to data analysis indicating that there was a significant difference in CCRs for those hospitals with and without blood-specific cost centers, and past public comments indicating that the former OPPS policy of defaulting to the overall hospital CCR for hospitals not reporting a blood-specific cost center often resulted in an underestimation of the true hospital costs for blood and blood products. Specifically, in order to address the differences in CCRs and to better reflect hospitals' costs, we are proposing to continue to simulate blood CCRs for each hospital that does not report a blood cost center by calculating the ratio of the blood-specific CCRs to hospitals' overall CCRs for those hospitals that do report costs and charges for blood cost centers. We also are proposing to apply this mean ratio to the overall CCRs of hospitals not reporting costs and charges for blood cost centers on their cost reports in order to simulate blood-specific CCRs for those hospitals. We are proposing to calculate the costs upon which the proposed CY 2020 payment rates for blood and blood products are based using the actual blood-specific CCR for hospitals that reported costs and charges for a blood cost center and a hospital-specific, simulated blood-specific CCR for hospitals that did not report costs and charges for a blood cost center.
We continue to believe that the hospital-specific, simulated blood-specific, CCR methodology better responds to the absence of a blood-specific CCR for a hospital than alternative methodologies, such as defaulting to the overall hospital CCR or applying an average blood-specific CCR across hospitals. Because this methodology takes into account the unique charging and cost accounting structure of each hospital, we believe that it yields more accurate estimated costs for these products. We continue to believe that this methodology in CY 2020 would result in costs for blood and blood products that appropriately reflect the relative estimated costs of these products for hospitals without blood cost centers and, therefore, for these blood products in general.
We note that, as discussed in section II.A.2.b.(1). of the CY 2019 OPPS/ASC final rule with comment period (82 FR 58837 through 58843), we defined a comprehensive APC (C-APC) as a classification for the provision of a primary service and all adjunctive services provided to support the delivery of the primary service. Under this policy, we include the costs of blood and blood products when calculating the overall costs of these C-APCs. In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue to apply the blood-specific CCR methodology described in this section when calculating the costs of the blood and blood products that appear on claims with services assigned to the C-APCs. Because the costs of blood and blood products would be reflected in the overall costs of the C-APCs (and, as a result, in the proposed payment rates of the C-APCs), we are proposing to not make separate payments for blood and blood products when they appear on the same claims as services assigned to the C-APCs (we refer readers to the CY 2015 OPPS/ASC final rule with comment period (79 FR 66796)).
We also refer readers to Addendum B to this CY 2020 OPPS/ASC proposed rule (which is available via the internet on the CMS website) for the proposed CY 2020 payment rates for blood and blood products (which are identified with status indicator “R”). For a more detailed discussion of the blood-specific CCR methodology, we refer readers to the CY 2005 OPPS proposed rule (69 FR 50524 through 50525). For a full history of OPPS payment for blood and blood products, we refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66807 through 66810).
(b) Pathogen-Reduced Platelets Payment Rate
In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70322 through 70323), we reiterated that we calculate payment rates for blood and blood products using our blood-specific CCR methodology, which utilizes actual or simulated CCRs from the most recently available hospital cost reports to convert hospital charges for blood and blood products to costs. Because HCPCS code P9072 (Platelets, pheresis, pathogen reduced or rapid bacterial tested, each unit), the predecessor code to HCPCS code P9073 (Platelets, pheresis, pathogen-reduced, each unit), was new for CY 2016, there were no claims data available on the charges and costs for this blood product upon which to apply our blood-specific CCR methodology. Therefore, we established an interim payment rate for HCPCS code P9072 based on a crosswalk to existing blood product HCPCS code P9037 (Platelets, pheresis, leukocytes reduced, irradiated, each unit), which we believed provided the best proxy for the costs of the new blood product. In addition, we stated that once we had claims data for HCPCS code P9072, we would calculate its payment rate using the claims data that should be available for the code beginning in CY 2018, which is our practice for other blood product HCPCS codes for which claims data have been available for 2 years.
We stated in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59233) that, although our standard practice for new codes involves using claims data to set payment rates once claims data become available, we were concerned that there may have been confusion among the provider community about the services that HCPCS code P9072 described. That is, as early as 2016, there were discussions about changing the descriptor for HCPCS code P9072 to include the phrase “or rapid bacterial tested”, which is a less costly technology than pathogen reduction. In addition, effective January 2017, the code descriptor for HCPCS code P9072 was changed to describe rapid bacterial testing of platelets and, effective July 1, 2017, the descriptor for the temporary successor code (HCPCS code Q9988) for HCPCS code P9072 was changed again back to the original descriptor for HCPCS code P9072 that was in place for 2016.
Based on the ongoing discussions involving changes to the original HCPCS code P9072 established in CY 2016, we believed that claims from CY 2016 for pathogen reduced platelets may have potentially reflected certain claims for rapid bacterial testing of platelets. Therefore, we decided to continue to crosswalk the payment amount for services described by HCPCS code P9073 (the successor code to HCPCS code P9072 established January 1, 2018) to the payment amount for services described by HCPCS code P9037 for CY 2018 (82 FR 59232), as had been done previously, to determine the payment rate for services described by HCPCS code P9072. In the CY 2019 OPPS/ASC proposed rule (83 FR 37058), for CY 2019, we discussed that we had reviewed the CY 2017 claims data for the two predecessor codes to HCPCS code P9073 (HCPCS codes P9072 and Q9988), along with the claims data for the CY 2017 temporary code for pathogen test for platelets (HCPCS code Q9987), which describes rapid bacterial testing of platelets. We found that there were over 2,200 claims billed with either HCPCS code P9072 or Q9988 in the CY 2017 claims data available for CY 2019 rulemaking. Accordingly, we believed that there were a sufficient number of claims to calculate a payment rate for HCPCS code P9073 for CY 2019 without using a crosswalk.
We also performed checks to estimate the share of claims that may have been billed for rapid bacterial testing of platelets as compared to the share of claims that may have been billed for pathogen-reduced, pheresis platelets (based on when HCPCS code P9072 was an active procedure code from January 1, 2017 to June 30, 2017). First, we found that the geometric mean cost for pathogen-reduced, pheresis platelets, as reported by HCPCS code Q9988 when billed separately from rapid bacterial testing of platelets, was $453.87, and that over 1,200 claims were billed for services described by HCPCS code Q9988. Next, we found that the geometric mean cost for rapid bacterial testing of platelets, as reported by HCPCS code Q9987 on claims, was $33.44, and there were 59 claims reported for services described by HCPCS code Q9987, of which 3 were separately paid.
These findings implied that almost all of the claims billed for services reported with HCPCS code P9072 were for pathogen-reduced, pheresis platelets. In addition, the geometric mean cost for services described by HCPCS code P9072, which may have contained rapid bacterial testing of platelets claims, was $468.11, which was higher than the geometric mean cost for services described by HCPCS code Q9988 of $453.87, which should not have contained claims for rapid bacterial testing of platelets. Because the geometric mean for services described by HCPCS code Q9987 was only $33.44, it would be expected that if a significant share of claims billed for services described by HCPCS code P9072 were for the rapid bacterial testing of platelets, the geometric mean cost for services described by HCPCS code P9072 would be lower than the geometric mean cost for services described by HCPCS code Q9988. Instead, we found that the geometric mean cost for services described by HCPCS code Q9988 was higher than the geometric mean cost for services described by HCPCS code P9072.
However, we received many comments from providers and stakeholders requesting that we not implement our proposal for CY 2019, and instead that we should once again establish the payment rate for HCPCS code P9073 by performing a crosswalk from the payment amount for services described by HCPCS code P9073 to the payment amount for services described by HCPCS code P9037. The commenters were concerned that the payment rate for HCPCS code P9073 calculated by using claims data for that service was too low. Several commenters believed the claim costs for pathogen-reduced platelets were lower than actual costs because of coding errors by providers, providers who did not use pathogen-reduced platelets when billing the service, and confusion over whether to use the hospital CCR or the blood center CCR to report charges for pathogen-reduced platelets. We considered the comments we received and decided not to finalize our proposal for CY 2019 to calculate the payment rate for services described by HCPCS code P9073 using claims payment history. Instead, for CY 2019, we established the payment rate for services described by HCPCS code P9073 by crosswalking the payment rate for the services described by HCPCS code P9073 from the payment rate for services described by HCPCS code P9037 (83 FR 58834).
For CY 2020 and subsequent years, we are proposing to calculate the payment rate for services described by HCPCS code P9073 by using claims payment history, which is the standard methodology used under the OPPS to calculate payment rates for HCPCS codes with at least 2 years of claims history. Claims for HCPCS code P9073 and its predecessor codes have been billed under the OPPS for over 3 years and we believe providers have had sufficient time to become familiar with the services covered by the procedure code and the appropriate charges and CCRs used to report the service. Also, it has been more than a year and half since the issue in which payment for pathogen-reduced platelets and payment for rapid bacterial testing were combined under the same code was resolved. In our analysis of claims data from CY 2018, we found that approximately 4,700 claims have been billed for services described by HCPCS code P9073 and the estimated payment rate for services described by HCPCS code P9073 based on the claims data is approximately $585. The claims-based payment rate for services described by HCPCS code P9073 is approximately $60 less than the estimated crosswalked payment rate using HCPCS code P9037 of approximately $645. The claims data show that services described by HCPCS code P9073 have been reported regularly by providers during CY 2018 and the payment rate is close to the payment rate of the crosswalked payment rate for services described by HCPCS code P9037. Therefore, we believe that the payment rate for services described by HCPCS code P9073 can be determined using claims data without a crosswalk from the payment rate for services described by HCPCS code P9037.
We refer readers to Addendum B of this proposed rule for the proposed payment rate for services described by HCPCS code P9073 reportable under the OPPS. Addendum B is available via the internet on the CMS website.
(2) Brachytherapy Sources
Section 1833(t)(2)(H) of the Act mandates the creation of additional groups of covered OPD services that classify devices of brachytherapy consisting of a seed or seeds (or radioactive source) (“brachytherapy sources”) separately from other services or groups of services. The statute provides certain criteria for the additional groups. For the history of OPPS payment for brachytherapy sources, we refer readers to prior OPPS final rules, such as the CY 2012 OPPS/ASC final rule with comment period (77 FR 68240 through 68241). As we have stated in prior OPPS updates, we believe that adopting the general OPPS prospective payment methodology for brachytherapy sources is appropriate for a number of reasons (77 FR 68240). The general OPPS methodology uses costs based on claims data to set the relative payment weights for hospital outpatient services. This payment methodology results in more consistent, predictable, and equitable payment amounts per source across hospitals by averaging the extremely high and low values, in contrast to payment based on hospitals' charges adjusted to costs. We believe that the OPPS methodology, as opposed to payment based on hospitals' charges adjusted to cost, also would provide hospitals with incentives for efficiency in the provision of brachytherapy services to Medicare beneficiaries. Moreover, this approach is consistent with our payment methodology for the vast majority of items and services paid under the OPPS. We refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70323 through 70325) for further discussion of the history of OPPS payment for brachytherapy sources.
In this CY 2020 OPPS/ASC proposed rule, for CY 2020, we are proposing to use the costs derived from CY 2018 claims data to set the proposed CY 2020 payment rates for brachytherapy sources because CY 2018 is the year of data we are proposing to use to set the proposed payment rates for most other items and services that would be paid under the CY 2020 OPPS. We are proposing to base the payment rates for brachytherapy sources on the geometric mean unit costs for each source, consistent with the methodology that we are proposing for other items and services paid under the OPPS, as discussed in section II.A.2. of this proposed rule. We also are proposing to continue the other payment policies for brachytherapy sources that we finalized and first implemented in the CY 2010 OPPS/ASC final rule with comment period (74 FR 60537). We are proposing to pay for the stranded and nonstranded not otherwise specified (NOS) codes, HCPCS codes C2698 (Brachytherapy source, stranded, not otherwise specified, per source) and C2699 (Brachytherapy source, non-stranded, not otherwise specified, per source), at a rate equal to the lowest stranded or nonstranded prospective payment rate for such sources, respectively, on a per source basis (as opposed to, for example, a per mCi), which is based on the policy we established in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66785). We also are proposing to continue the policy we first implemented in the CY 2010 OPPS/ASC final rule with comment period (74 FR 60537) regarding payment for new brachytherapy sources for which we have no claims data, based on the same reasons we discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66786; which was delayed until January 1, 2010 by section 142 of Pub. L. 110-275). Specifically, this policy is intended to enable us to assign new HCPCS codes for new brachytherapy sources to their own APCs, with prospective payment rates set based on our consideration of external data and other relevant information regarding the expected costs of the sources to hospitals. The proposed CY 2020 payment rates for brachytherapy sources are included in Addendum B to this proposed rule (which is available via the internet on the CMS website) and are identified with status indicator “U”. For CY 2020, we are proposing to continue to assign status indicator “U” (Brachytherapy Sources, Paid under OPPS; separate APC payment) to HCPCS code C2645 (Brachytherapy planar source, palladium-103, per square millimeter). However, our CY 2018 claims data include two claims with over 9,000 units of HCPCS code C2645. For the CY 2019 OPPS/ASC final rule with comment period, our CY 2017 claims data only included one claim with one unit of HCPCS code C2645. Therefore, we believe the CY 2018 claims data are adequate to establish an APC payment rate for HCPCS code C2645 and to discontinue our use of external data for this brachytherapy source. Specifically, we are proposing to set the proposed CY 2020 payment rate at the geometric mean cost of HCPCS code C2645 based on CY 2018 claims data, which is $1.02 per mm2.
We continue to invite hospitals and other parties to submit recommendations to us for new codes to describe new brachytherapy sources. Such recommendations should be directed to the Division of Outpatient Care, Mail Stop C4-01-26, Centers for Medicare and Medicaid Services, 7500 Security Boulevard, Baltimore, MD 21244. We will continue to add new brachytherapy source codes and descriptors to our systems for payment on a quarterly basis.
b. Proposed Comprehensive APCs (C-APCs) for CY 2020
(1) Background
In the CY 2014 OPPS/ASC final rule with comment period (78 FR 74861 through 74910), we finalized a comprehensive payment policy that packages payment for adjunctive and secondary items, services, and procedures into the most costly primary procedure under the OPPS at the claim level. The policy was finalized in CY 2014, but the effective date was delayed until January 1, 2015, to allow additional time for further analysis, opportunity for public comment, and systems preparation. The comprehensive APC (C-APC) policy was implemented effective January 1, 2015, with modifications and clarifications in response to public comments received regarding specific provisions of the C-APC policy (79 FR 66798 through 66810).
A C-APC is defined as a classification for the provision of a primary service and all adjunctive services provided to support the delivery of the primary service. We established C-APCs as a category broadly for OPPS payment and implemented 25 C-APCs beginning in CY 2015 (79 FR 66809 through 66810). In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70332), we finalized 10 additional C-APCs to be paid under the existing C-APC payment policy and added 1 additional level to both the Orthopedic Surgery and Vascular Procedures clinical families, which increased the total number of C-APCs to 37 for CY 2016. In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79584 through 79585), we finalized another 25 C-APCs for a total of 62 C-APCs. In the CY 2018 OPPS/ASC final rule with comment period, we did not change the total number of C-APCs from 62. In the CY 2019 OPPS/ASC final rule with comment period, we created 3 new C-APCs, increasing the total number to 65 (83 FR 58844 through 58846).
Under our C-APC policy, we designate a service described by a HCPCS code assigned to a C-APC as the primary service when the service is identified by OPPS status indicator “J1”. When such a primary service is reported on a hospital outpatient claim, taking into consideration the few exceptions that are discussed below, we make payment for all other items and services reported on the hospital outpatient claim as being integral, ancillary, supportive, dependent, and adjunctive to the primary service (hereinafter collectively referred to as “adjunctive services”) and representing components of a complete comprehensive service (78 FR 74865 and 79 FR 66799). Payments for adjunctive services are packaged into the payments for the primary services. This results in a single prospective payment for each of the primary, comprehensive services based on the costs of all reported services at the claim level.
Services excluded from the C-APC policy under the OPPS include services that are not covered OPD services, services that cannot by statute be paid for under the OPPS, and services that are required by statute to be separately paid. This includes certain mammography and ambulance services that are not covered OPD services in accordance with section 1833(t)(1)(B)(iv) of the Act; brachytherapy seeds, which also are required by statute to receive separate payment under section 1833(t)(2)(H) of the Act; pass-through payment drugs and devices, which also require separate payment under section 1833(t)(6) of the Act; self-administered drugs (SADs) that are not otherwise packaged as supplies because they are not covered under Medicare Part B under section 1861(s)(2)(B) of the Act; and certain preventive services (78 FR 74865 and 79 FR 66800 through 66801). A list of services excluded from the C-APC policy is included in Addendum J to this proposed rule (which is available via the internet on the CMS website).
The C-APC policy payment methodology set forth in the CY 2014 OPPS/ASC final rule with comment period for the C-APCs and modified and implemented beginning in CY 2015 is summarized as follows (78 FR 74887 and 79 FR 66800):
Basic Methodology. As stated in the CY 2015 OPPS/ASC final rule with comment period, we define the C-APC payment policy as including all covered OPD services on a hospital outpatient claim reporting a primary service that is assigned to status indicator “J1”, excluding services that are not covered OPD services or that cannot by statute be paid for under the OPPS. Services and procedures described by HCPCS codes assigned to status indicator “J1” are assigned to C-APCs based on our usual APC assignment methodology by evaluating the geometric mean costs of the primary service claims to establish resource similarity and the clinical characteristics of each procedure to establish clinical similarity within each APC.
In the CY 2016 OPPS/ASC final rule with comment period, we expanded the C-APC payment methodology to qualifying extended assessment and management encounters through the “Comprehensive Observation Services” C-APC (C-APC 8011). Services within this APC are assigned status indicator “J2”. Specifically, we make a payment through C-APC 8011 for a claim that:
- Does not contain a procedure described by a HCPCS code to which we have assigned status indicator “T” that is reported with a date of service on the same day or 1 day earlier than the date of service associated with services described by HCPCS code G0378;
- Contains 8 or more units of services described by HCPCS code G0378 (Hospital observation services, per hour);
- Contains services provided on the same date of service or 1 day before the date of service for HCPCS code G0378 that are described by one of the following codes: HCPCS code G0379 (Direct admission of patient for hospital observation care) on the same date of service as HCPCS code G0378; CPT code 99281 (Emergency department visit for the evaluation and management of a patient (Level 1)); CPT code 99282 (Emergency department visit for the evaluation and management of a patient (Level 2)); CPT code 99283 (Emergency department visit for the evaluation and management of a patient (Level 3)); CPT code 99284 (Emergency department visit for the evaluation and management of a patient (Level 4)); CPT code 99285 (Emergency department visit for the evaluation and management of a patient (Level 5)) or HCPCS code G0380 (Type B emergency department visit (Level 1)); HCPCS code G0381 (Type B emergency department visit (Level 2)); HCPCS code G0382 (Type B emergency department visit (Level 3)); HCPCS code G0383 (Type B emergency department visit (Level 4)); HCPCS code G0384 (Type B emergency department visit (Level 5)); CPT code 99291 (Critical care, evaluation and management of the critically ill or critically injured patient; first 30-74 minutes); or HCPCS code G0463 (Hospital outpatient clinic visit for assessment and management of a patient); and
- Does not contain services described by a HCPCS code to which we have assigned status indicator “J1”.
The assignment of status indicator “J2” to a specific combination of services performed in combination with each other allows for all other OPPS payable services and items reported on the claim (excluding services that are not covered OPD services or that cannot by statute be paid for under the OPPS) to be deemed adjunctive services representing components of a comprehensive service and resulting in a single prospective payment for the comprehensive service based on the costs of all reported services on the claim (80 FR 70333 through 70336).
Services included under the C-APC payment packaging policy, that is, services that are typically adjunctive to the primary service and provided during the delivery of the comprehensive service, include diagnostic procedures, laboratory tests, and other diagnostic tests and treatments that assist in the delivery of the primary procedure; visits and evaluations performed in association with the procedure; uncoded services and supplies used during the service; durable medical equipment as well as prosthetic and orthotic items and supplies when provided as part of the outpatient service; and any other components reported by HCPCS codes that represent services that are provided during the complete comprehensive service (78 FR 74865 and 79 FR 66800).
In addition, payment for hospital outpatient department services that are similar to therapy services and delivered either by therapists or nontherapists is included as part of the payment for the packaged complete comprehensive service. These services that are provided during the perioperative period are adjunctive services and are deemed not to be therapy services as described in section 1834(k) of the Act, regardless of whether the services are delivered by therapists or other nontherapist health care workers. We have previously noted that therapy services are those provided by therapists under a plan of care in accordance with section 1835(a)(2)(C) and section 1835(a)(2)(D) of the Act and are paid for under section 1834(k) of the Act, subject to annual therapy caps as applicable (78 FR 74867 and 79 FR 66800). However, certain other services similar to therapy services are considered and paid for as hospital outpatient department services. Payment for these nontherapy outpatient department services that are reported with therapy codes and provided with a comprehensive service is included in the payment for the packaged complete comprehensive service. We note that these services, even though they are reported with therapy codes, are hospital outpatient department services and not therapy services. We refer readers to the July 2016 OPPS Change Request 9658 (Transmittal 3523) for further instructions on reporting these services in the context of a C-APC service.
Items included in the packaged payment provided in conjunction with the primary service also include all drugs, biologicals, and radiopharmaceuticals, regardless of cost, except those drugs with pass-through payment status and SADs, unless they function as packaged supplies (78 FR 74868 through 74869 and 74909 and 79 FR 66800). We refer readers to Section 50.2M, Chapter 15, of the Medicare Benefit Policy Manual for a description of our policy on SADs treated as hospital outpatient supplies, including lists of SADs that function as supplies and those that do not function as supplies.
We define each hospital outpatient claim reporting a single unit of a single primary service assigned to status indicator “J1” as a single “J1” unit procedure claim (78 FR 74871 and 79 FR 66801). Line item charges for services included on the C-APC claim are converted to line item costs, which are then summed to develop the estimated APC costs. These claims are then assigned one unit of the service with status indicator “J1” and later used to develop the geometric mean costs for the C-APC relative payment weights. (We note that we use the term “comprehensive” to describe the geometric mean cost of a claim reporting “J1” service(s) or the geometric mean cost of a C-APC, inclusive of all of the items and services included in the C-APC service payment bundle.) Charges for services that would otherwise be separately payable are added to the charges for the primary service. This process differs from our traditional cost accounting methodology only in that all such services on the claim are packaged (except certain services as described above). We apply our standard data trims, which exclude claims with extremely high primary units or extreme costs.
The comprehensive geometric mean costs are used to establish resource similarity and, along with clinical similarity, dictate the assignment of the primary services to the C-APCs. We establish a ranking of each primary service (single unit only) to be assigned to status indicator “J1” according to its comprehensive geometric mean costs. For the minority of claims reporting more than one primary service assigned to status indicator “J1” or units thereof, we identify one “J1” service as the primary service for the claim based on our cost-based ranking of primary services. We then assign these multiple “J1” procedure claims to the C-APC to which the service designated as the primary service is assigned. If the reported “J1” services on a claim map to different C-APCs, we designate the “J1” service assigned to the C-APC with the highest comprehensive geometric mean cost as the primary service for that claim. If the reported multiple “J1” services on a claim map to the same C-APC, we designate the most costly service (at the HCPCS code level) as the primary service for that claim. This process results in initial assignments of claims for the primary services assigned to status indicator “J1” to the most appropriate C-APCs based on both single and multiple procedure claims reporting these services and clinical and resource homogeneity.
Complexity Adjustments. We use complexity adjustments to provide increased payment for certain comprehensive services. We apply a complexity adjustment by promoting qualifying paired “J1” service code combinations or paired code combinations of “J1” services and certain add-on codes (as described further below) from the originating C-APC (the C-APC to which the designated primary service is first assigned) to the next higher paying C-APC in the same clinical family of C-APCs. We apply this type of complexity adjustment when the paired code combination represents a complex, costly form or version of the primary service according to the following criteria:
- Frequency of 25 or more claims reporting the code combination (frequency threshold); and
- Violation of the 2 times rule in the originating C-APC (cost threshold).
These criteria identify paired code combinations that occur commonly and exhibit materially greater resource requirements than the primary service. The CY 2017 OPPS/ASC final rule with comment period (81 FR 79582) included a revision to the complexity adjustment eligibility criteria. Specifically, we finalized a policy to discontinue the requirement that a code combination (that qualifies for a complexity adjustment by satisfying the frequency and cost criteria thresholds described above) also not create a 2 times rule violation in the higher level or receiving APC.
After designating a single primary service for a claim, we evaluate that service in combination with each of the other procedure codes reported on the claim assigned to status indicator “J1” (or certain add-on codes) to determine if there are paired code combinations that meet the complexity adjustment criteria. For a new HCPCS code, we determine initial C-APC assignment and qualification for a complexity adjustment using the best available information, crosswalking the new HCPCS code to a predecessor code(s) when appropriate.
Once we have determined that a particular code combination of “J1” services (or combinations of “J1” services reported in conjunction with certain add-on codes) represents a complex version of the primary service because it is sufficiently costly, frequent, and a subset of the primary comprehensive service overall according to the criteria described above, we promote the claim including the complex version of the primary service as described by the code combination to the next higher cost C-APC within the clinical family, unless the primary service is already assigned to the highest cost APC within the C-APC clinical family or assigned to the only C-APC in a clinical family. We do not create new APCs with a comprehensive geometric mean cost that is higher than the highest geometric mean cost (or only) C-APC in a clinical family just to accommodate potential complexity adjustments. Therefore, the highest payment for any claim including a code combination for services assigned to a C-APC would be the highest paying C-APC in the clinical family (79 FR 66802).
We package payment for all add-on codes into the payment for the C-APC. However, certain primary service add-on combinations may qualify for a complexity adjustment. As noted in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70331), all add-on codes that can be appropriately reported in combination with a base code that describes a primary “J1” service are evaluated for a complexity adjustment.
To determine which combinations of primary service codes reported in conjunction with an add-on code may qualify for a complexity adjustment for CY 2020, in this CY 2020 OPPS/ASC proposed rule, we are proposing to apply the frequency and cost criteria thresholds discussed above, testing claims reporting one unit of a single primary service assigned to status indicator “J1” and any number of units of a single add-on code for the primary “J1” service. If the frequency and cost criteria thresholds for a complexity adjustment are met and reassignment to the next higher cost APC in the clinical family is appropriate (based on meeting the criteria outlined above), we make a complexity adjustment for the code combination; that is, we reassign the primary service code reported in conjunction with the add-on code to the next higher cost C-APC within the same clinical family of C-APCs. As previously stated, we package payment for add-on codes into the C-APC payment rate. If any add-on code reported in conjunction with the “J1” primary service code does not qualify for a complexity adjustment, payment for the add-on service continues to be packaged into the payment for the primary service and is not reassigned to the next higher cost C-APC. We list the proposed complexity adjustments for “J1” and add-on code combinations for CY 2020, along with all of the other proposed complexity adjustments, in Addendum J to this CY 2020 OPPS/ASC proposed rule (which is available via the internet on the CMS website).
Addendum J to this proposed rule includes the cost statistics for each code combination that would qualify for a complexity adjustment (including primary code and add-on code combinations). Addendum J to this proposed rule also contains summary cost statistics for each of the paired code combinations that describe a complex code combination that would qualify for a complexity adjustment and are proposed to be reassigned to the next higher cost C-APC within the clinical family. The combined statistics for all proposed reassigned complex code combinations are represented by an alphanumeric code with the first 4 digits of the designated primary service followed by a letter. For example, the proposed geometric mean cost listed in Addendum J for the code combination described by complexity adjustment assignment 3320R, which is assigned to C-APC 5224 (Level 4 Pacemaker and Similar Procedures), includes all paired code combinations that are proposed to be reassigned to C-APC 5224 when CPT code 33208 is the primary code. Providing the information contained in Addendum J to this proposed rule allows stakeholders the opportunity to better assess the impact associated with the proposed reassignment of claims with each of the paired code combinations eligible for a complexity adjustment.
(2) Proposed Additional C-APCs for CY 2020
For CY 2020 and subsequent years, in this CY 2020 OPPS/ASC proposed rule, we are proposing to continue to apply the C-APC payment policy methodology. We refer readers to the CY 2017 OPPS/ASC final rule with comment period (81 FR 79583) for a discussion of the C-APC payment policy methodology and revisions.
Each year, in accordance with section 1833(t)(9)(A) of the Act, we review and revise the services within each APC group and the APC assignments under the OPPS. As a result of our annual review of the services and the APC assignments under the OPPS, in this proposed rule, we are proposing to add two C-APCs under the existing C-APC payment policy in CY 2020: Proposed C-APC 5182 (Level 2 Vascular Procedures); and proposed C-APC 5461 (Level 1 Neurostimulator and Related Procedures). These APCs were selected to be included in this proposed rule because, similar to other C-APCs, these APCs include primary, comprehensive services, such as major surgical procedures, that are typically reported with other ancillary and adjunctive services. Also, similar to other APCs that have been converted to C-APCs, there are higher APC levels within the clinical family or related clinical family of these APCs that have previously been assigned to a C-APC. Table 4 of this proposed rule lists the proposed C-APCs for CY 2020. All C-APCs are displayed in Addendum J to this proposed rule (which is available via the internet on the CMS website). Addendum J to this proposed rule also contains all of the data related to the C-APC payment policy methodology, including the list of proposed complexity adjustments and other information.
We also are considering developing an episode-of-care for skin substitutes and are interested in comments regarding a future C-APC for procedures using skin substitute products furnished in the hospital outpatient department setting. We note that this comment solicitation is discussed in section V.B.7. of this proposed rule.



(3) Exclusion of Procedures Assigned to New Technology APCs From the C-APC Policy
Services that are assigned to New Technology APCs are typically new procedures that do not have sufficient claims history to establish an accurate payment for the procedures. Beginning in CY 2002, we retain services within New Technology APC groups until we gather sufficient claims data to enable us to assign the service to an appropriate clinical APC. This policy allows us to move a service from a New Technology APC in less than 2 years if sufficient data are available. It also allows us to retain a service in a New Technology APC for more than 2 years if sufficient data upon which to base a decision for reassignment have not been collected (82 FR 59277).
The C-APC payment policy packages payment for adjunctive and secondary items, services, and procedures into the most costly primary procedure under the OPPS at the claim level. Prior to CY 2019 when a procedure assigned to a New Technology APC was included on the claim with a primary procedure, identified by OPPS status indicator “J1”, payment for the new technology service was typically packaged into the payment for the primary procedure. Because the new technology service was not separately paid in this scenario, the overall number of single claims available to determine an appropriate clinical APC for the new service was reduced. This was contrary to the objective of the New Technology APC payment policy, which is to gather sufficient claims data to enable us to assign the service to an appropriate clinical APC.
For example, for CY 2017, there were seven claims generated for HCPCS code 0100T (Placement of a subconjunctival retinal prosthesis receiver and pulse generator, and implantation of intraocular retinal electrode array, with vitrectomy), which involves the use of the Argus® II Retinal Prosthesis System. However, several of these claims were not available for ratesetting because HCPCS code 0100T was reported with a “J1” procedure and, therefore, payment was packaged into the associated C-APC payment. If these services had been separately paid under the OPPS, there would be at least two additional single claims available for ratesetting. As mentioned previously, the purpose of the new technology APC policy is to ensure that there are sufficient claims data for new services, which is particularly important for services with a low volume such as procedures described by HCPCS code 0100T. Another concern is the costs reported for the claims when payment is not packaged for a new technology procedure may not be representative of all of the services included on a claim that is generated, which may also affect our ability to assign the new service to the most appropriate clinical APC.
To address this issue and help ensure that there is sufficient claims data for services assigned to New Technology APCs, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58847), we excluded payment for any procedure that is assigned to a New Technology APC (APCs 1491 through 1599 and APCs 1901 through 1908) from being packaged when included on a claim with a “J1” service assigned to a C-APC. For CY 2020, we are proposing to continue to exclude payment for any procedure that is assigned to a New Technology APC from being packaged when included on a claim with a “J1” service assigned to a C-APC.
Some stakeholders have raised questions about whether the policy established in the CY 2019 OPPS/ASC final rule with comment period would also apply to comprehensive observation services assigned status indicator “J2.” We recognize that the policy described and adopted in the CY 2019 rulemaking may have been ambiguous with respect to this issue. While our intention in the CY 2019 rulemaking was only to exclude payment for services assigned to New Technology APCs from being bundled into the payment for a comprehensive “J1” service, we believe that there may also be some instances in which it would be clinically appropriate to provide a new technology service when providing comprehensive observation services. We would not generally expect that to be the case, because procedures assigned to New Technology APCs typically are new or low-volume surgical procedures, or are specialized tests to diagnosis a specific condition. In addition, it is highly unlikely a general observation procedure would be assigned to a New Technology APC because there are clinical APCs already established under the OPPS to classify general observation procedures. As we stated in the CY 2016 OPPS/ASC final rule with comment period, observation services may not be used for post-operative recovery and, as such, observation services furnished with services assigned to status indicator “T” will always be packaged (80 FR 70334). Therefore, we are proposing that payment for services assigned to a New Technology APC when included on a claim for a service assigned status indicator “J2” assigned to a C-APC will be packaged into the payment for the comprehensive service. Nonetheless, we are seeking public comments on whether it would be clinically appropriate to exclude payment for any New Technology APC procedures from being packaged into the payment for a comprehensive “J2” service starting in CY 2020.
c. Proposed Calculation of Composite APC Criteria-Based Costs
As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66613), we believe it is important that the OPPS enhance incentives for hospitals to provide necessary, high quality care as efficiently as possible. For CY 2008, we developed composite APCs to provide a single payment for groups of services that are typically performed together during a single clinical encounter and that result in the provision of a complete service. Combining payment for multiple, independent services into a single OPPS payment in this way enables hospitals to manage their resources with maximum flexibility by monitoring and adjusting the volume and efficiency of services themselves. An additional advantage to the composite APC model is that we can use data from correctly coded multiple procedure claims to calculate payment rates for the specified combinations of services, rather than relying upon single procedure claims which may be low in volume and/or incorrectly coded. Under the OPPS, we currently have composite policies for mental health services and multiple imaging services. (We note that, in the CY 2018 OPPS/ASC final rule with comment period, we finalized a policy to delete the composite APC 8001 (LDR Prostate Brachytherapy Composite) for CY 2018 and subsequent years.) We refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66611 through 66614 and 66650 through 66652) for a full discussion of the development of the composite APC methodology, and the CY 2012 OPPS/ASC final rule with comment period (76 FR 74163) and the CY 2018 OPPS/ASC final rule with comment period (82 FR 59241 through 59242 and 59246 through 52950) for more recent background.
(1) Mental Health Services Composite APC
In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue our longstanding policy of limiting the aggregate payment for specified less resource-intensive mental health services furnished on the same date to the payment for a day of partial hospitalization services provided by a hospital, which we consider to be the most resource-intensive of all outpatient mental health services. We refer readers to the April 7, 2000 OPPS final rule with comment period (65 FR 18452 through 18455) for the initial discussion of this longstanding policy and the CY 2012 OPPS/ASC final rule with comment period (76 FR 74168) for more recent background.
In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79588 through 79589), we finalized a policy to combine the existing Level 1 and Level 2 hospital-based PHP APCs into a single hospital-based PHP APC, and thereby discontinue APCs 5861 (Level 1—Partial Hospitalization (3 services) for Hospital-Based PHPs) and 5862 (Level—2 Partial Hospitalization (4 or more services) for Hospital-Based PHPs) and replace them with APC 5863 (Partial Hospitalization (3 or more services per day)).
In the CY 2018 OPPS/ASC proposed rule and final rule with comment period (82 FR 33580 through 33581 and 59246 through 59247, respectively), we proposed and finalized the policy for CY 2018 and subsequent years that, when the aggregate payment for specified mental health services provided by one hospital to a single beneficiary on a single date of service, based on the payment rates associated with the APCs for the individual services, exceeds the maximum per diem payment rate for partial hospitalization services provided by a hospital, those specified mental health services will be paid through composite APC 8010 (Mental Health Services Composite). In addition, we set the payment rate for composite APC 8010 for CY 2018 at the same payment rate that will be paid for APC 5863, which is the maximum partial hospitalization per diem payment rate for a hospital, and finalized a policy that the hospital will continue to be paid the payment rate for composite APC 8010. Under this policy, the I/OCE will continue to determine whether to pay for these specified mental health services individually, or to make a single payment at the same payment rate established for APC 5863 for all of the specified mental health services furnished by the hospital on that single date of service. We continue to believe that the costs associated with administering a partial hospitalization program at a hospital represent the most resource intensive of all outpatient mental health services. Therefore, we do not believe that we should pay more for mental health services under the OPPS than the highest partial hospitalization per diem payment rate for hospitals.
In this CY 2020 OPPS/ASC proposed rule, for CY 2020, we are proposing that when the aggregate payment for specified mental health services provided by one hospital to a single beneficiary on a single date of service, based on the payment rates associated with the APCs for the individual services, exceeds the maximum per diem payment rate for partial hospitalization services provided by a hospital, those specified mental health services would be paid through composite APC 8010 for CY 2020. In addition, we are proposing to set the proposed payment rate for composite APC 8010 at the same payment rate that we are proposing for APC 5863, which is the maximum partial hospitalization per diem payment rate for a hospital, and that the hospital continue to be paid the proposed payment rate for composite APC 8010.
(2) Multiple Imaging Composite APCs (APCs 8004, 8005, 8006, 8007, and 8008)
Effective January 1, 2009, we provide a single payment each time a hospital submits a claim for more than one imaging procedure within an imaging family on the same date of service, in order to reflect and promote the efficiencies hospitals can achieve when performing multiple imaging procedures during a single session (73 FR 41448 through 41450). We utilize three imaging families based on imaging modality for purposes of this methodology: (1) Ultrasound; (2) computed tomography (CT) and computed tomographic angiography (CTA); and (3) magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA). The HCPCS codes subject to the multiple imaging composite policy and their respective families are listed in Table 12 of the CY 2014 OPPS/ASC final rule with comment period (78 FR 74920 through 74924).
While there are three imaging families, there are five multiple imaging composite APCs due to the statutory requirement under section 1833(t)(2)(G) of the Act that we differentiate payment for OPPS imaging services provided with and without contrast. While the ultrasound procedures included under the policy do not involve contrast, both CT/CTA and MRI/MRA scans can be provided either with or without contrast. The five multiple imaging composite APCs established in CY 2009 are:
- APC 8004 (Ultrasound Composite);
- APC 8005 (CT and CTA without Contrast Composite);
- APC 8006 (CT and CTA with Contrast Composite);
- APC 8007 (MRI and MRA without Contrast Composite); and
- APC 8008 (MRI and MRA with Contrast Composite).
We define the single imaging session for the “with contrast” composite APCs as having at least one or more imaging procedures from the same family performed with contrast on the same date of service. For example, if the hospital performs an MRI without contrast during the same session as at least one other MRI with contrast, the hospital will receive payment based on the payment rate for APC 8008, the “with contrast” composite APC.
We make a single payment for those imaging procedures that qualify for payment based on the composite APC payment rate, which includes any packaged services furnished on the same date of service. The standard (noncomposite) APC assignments continue to apply for single imaging procedures and multiple imaging procedures performed across families. For a full discussion of the development of the multiple imaging composite APC methodology, we refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68559 through 68569).
In this CY 2020 OPPS/ASC proposed rule, we are proposing, for CY 2020, to continue to pay for all multiple imaging procedures within an imaging family performed on the same date of service using the multiple imaging composite APC payment methodology. We continue to believe that this policy would reflect and promote the efficiencies hospitals can achieve when performing multiple imaging procedures during a single session.
The proposed CY 2020 payment rates for the five multiple imaging composite APCs (APCs 8004, 8005, 8006, 8007, and 8008) are based on proposed geometric mean costs calculated from CY 2018 claims available for the CY 2020 OPPS/ASC proposed rule that qualified for composite payment under the current policy (that is, those claims reporting more than one procedure within the same family on a single date of service). To calculate the proposed geometric mean costs, we used the same methodology that we have used to calculate the geometric mean costs for these composite APCs since CY 2014, as described in the CY 2014 OPPS/ASC final rule with comment period (78 FR 74918). The imaging HCPCS codes referred to as “overlap bypass codes” that we removed from the bypass list for purposes of calculating the proposed multiple imaging composite APC geometric mean costs, in accordance with our established methodology as stated in the CY 2014 OPPS/ASC final rule with comment period (78 FR 74918), are identified by asterisks in Addendum N to this CY 2020 OPPS/ASC proposed rule (which is available via the internet on the CMS website) and are discussed in more detail in section II.A.1.b. of this CY 2020 OPPS/ASC proposed rule.
For this CY 2020 OPPS/ASC proposed rule, we were able to identify approximately 700,000 “single session” claims out of an estimated 4.9 million potential claims for payment through composite APCs from our ratesetting claims data, which represents approximately 14 percent of all eligible claims, to calculate the proposed CY 2020 geometric mean costs for the multiple imaging composite APCs. Table 5 of this CY 2020 OPPS/ASC proposed rule lists the proposed HCPCS codes that would be subject to the multiple imaging composite APC policy and their respective families and approximate composite APC proposed geometric mean costs for CY 2020.



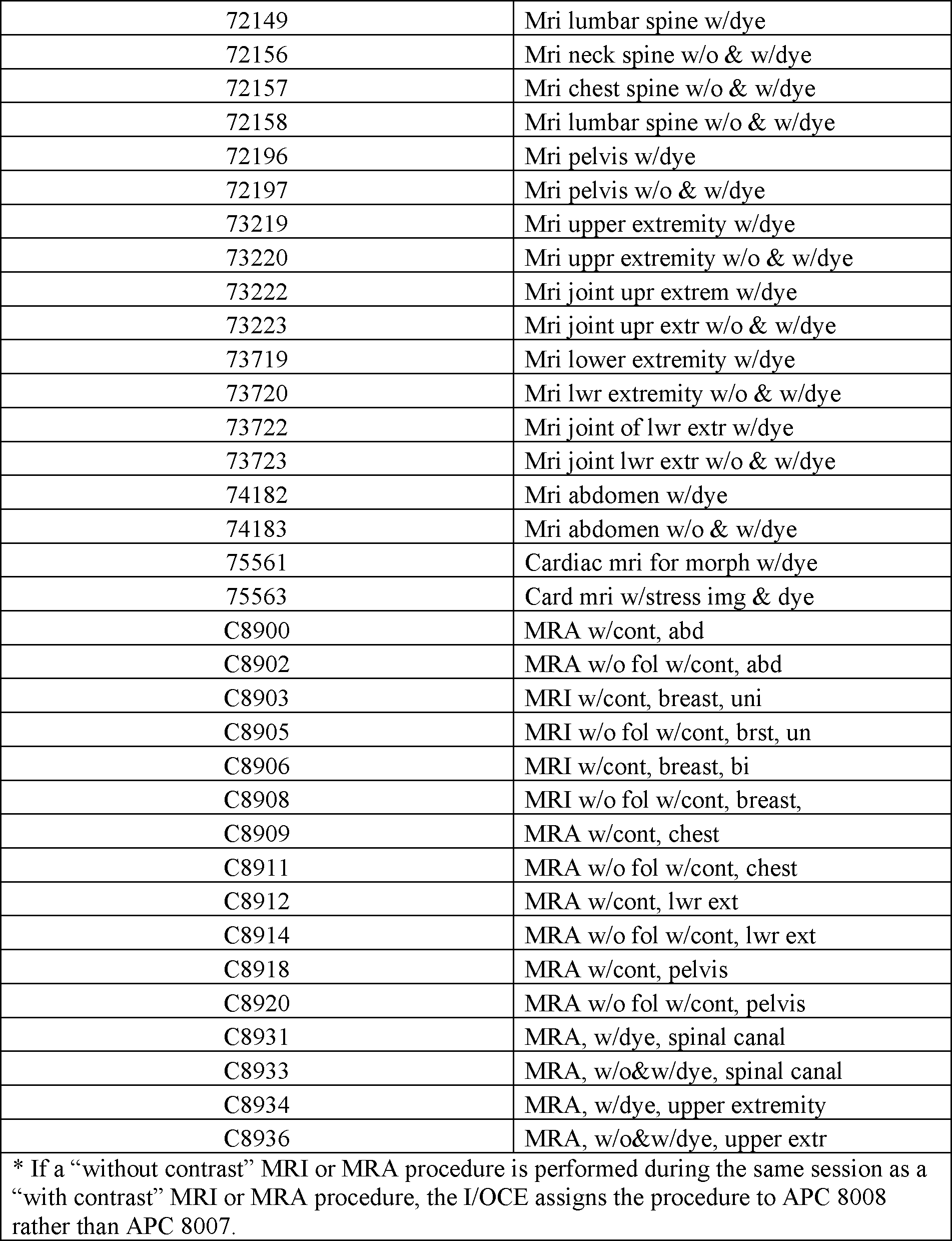
3. Proposed Changes to Packaged Items and Services
a. Background and Rationale for Packaging in the OPPS
Like other prospective payment systems, the OPPS relies on the concept of averaging to establish a payment rate for services. The payment may be more or less than the estimated cost of providing a specific service or a bundle of specific services for a particular beneficiary. The OPPS packages payments for multiple interrelated items and services into a single payment to create incentives for hospitals to furnish services most efficiently and to manage their resources with maximum flexibility. Our packaging policies support our strategic goal of using larger payment bundles in the OPPS to maximize hospitals' incentives to provide care in the most efficient manner. For example, where there are a variety of devices, drugs, items, and supplies that could be used to furnish a service, some of which are more costly than others, packaging encourages hospitals to use the most cost-efficient item that meets the patient's needs, rather than to routinely use a more expensive item, which often occurs if separate payment is provided for the item.
Packaging also encourages hospitals to effectively negotiate with manufacturers and suppliers to reduce the purchase price of items and services or to explore alternative group purchasing arrangements, thereby encouraging the most economical health care delivery. Similarly, packaging encourages hospitals to establish protocols that ensure that necessary services are furnished, while scrutinizing the services ordered by practitioners to maximize the efficient use of hospital resources. Packaging payments into larger payment bundles promotes the predictability and accuracy of payment for services over time. Finally, packaging may reduce the importance of refining service-specific payment because packaged payments include costs associated with higher cost cases requiring many ancillary items and services and lower cost cases requiring fewer ancillary items and services. Because packaging encourages efficiency and is an essential component of a prospective payment system, packaging payments for items and services that are typically integral, ancillary, supportive, dependent, or adjunctive to a primary service has been a fundamental part of the OPPS since its implementation in August 2000. For an extensive discussion of the history and background of the OPPS packaging policy, we refer readers to the CY 2000 OPPS final rule (65 FR 18434), the CY 2008 OPPS/ASC final rule with comment period (72 FR 66580), the CY 2014 OPPS/ASC final rule with comment period (78 FR 74925), the CY 2015 OPPS/ASC final rule with comment period (79 FR 66817), the CY 2016 OPPS/ASC final rule with comment period (80 FR 70343), the CY 2017 OPPS/ASC final rule with comment period (81 FR 79592), the CY 2018 OPPS/ASC final rule with comment period (82 FR 59250), and the CY 2019 OPPS/ASC final rule with comment period (83 FR 58854). As we continue to develop larger payment groups that more broadly reflect services provided in an encounter or episode of care, we have expanded the OPPS packaging policies. Most, but not necessarily all, categories of items and services currently packaged in the OPPS are listed in 42 CFR 419.2(b). Our overarching goal is to make payments for all services under the OPPS more consistent with those of a prospective payment system and less like those of a per-service fee schedule, which pays separately for each coded item. As a part of this effort, we have continued to examine the payment for items and services provided under the OPPS to determine which OPPS services can be packaged to further achieve the objective of advancing the OPPS toward a more prospective payment system.
For CY 2020, we examined the items and services currently provided under the OPPS, reviewing categories of integral, ancillary, supportive, dependent, or adjunctive items and services for which we believe payment would be appropriately packaged into payment for the primary service that they support. Specifically, we examined the HCPCS code definitions (including CPT code descriptors) and outpatient hospital billing patterns to determine whether there were categories of codes for which packaging would be appropriate according to existing OPPS packaging policies or a logical expansion of those existing OPPS packaging policies. In this CY 2020 OPPS/ASC proposed rule, for CY 2020, we are proposing to conditionally package the costs of selected newly identified ancillary services into payment with a primary service where we believe that the packaged item or service is integral, ancillary, supportive, dependent, or adjunctive to the provision of care that was reported by the primary service HCPCS code. Below we discuss the proposed changes to the packaging policies beginning in CY 2020.
b. Packaging Policy for Non-Opioid Pain Management Treatments
(1) Background on OPPS/ASC Non-Opioid Pain Management Packaging Policies
In the CY 2018 OPPS/ASC proposed rule (82 FR 33588), within the framework of existing packaging categories, such as drugs that function as supplies in a surgical procedure or diagnostic test or procedure, we requested stakeholder feedback on common clinical scenarios involving currently packaged items and services described by HCPCS codes that stakeholders believe should not be packaged under the OPPS. We also expressed interest in stakeholder feedback on common clinical scenarios involving separately payable HCPCS codes for which payment would be most appropriately packaged under the OPPS. Commenters who responded to the CY 2018 OPPS/ASC proposed rule expressed a variety of views on packaging under the OPPS. In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59255), we summarized these public comments. The public comments ranged from requests to unpackage most items and services that are either conditionally or unconditionally packaged under the OPPS, including drugs and devices, to specific requests for separate payment for a specific drug or device.
In terms of Exparel® in particular, we received several requests to pay separately for the drug Exparel® rather than packaging payment for it as a surgical supply. We had previously stated that we considered Exparel® to be a drug that functions as a surgical supply because it is indicated for the alleviation of postoperative pain (79 FR 66874 and 66875). We had also stated before that we considered all items related to the surgical outcome and provided during the hospital stay in which the surgery is performed, including postsurgical pain management drugs, to be part of the surgery for purposes of our drug and biological surgical supply packaging policy. (We note that Exparel® is a liposome injection of bupivacaine, an amide local anesthetic, indicated for single-dose infiltration into the surgical site to produce postsurgical analgesia. In 2011, Exparel® was approved by the FDA for single-dose infiltration into the surgical site to provide postsurgical analgesia.[1 2] Exparel® had pass-through payment status from CYs 2012 through 2014 and was separately paid under both the OPPS and the ASC payment system during this 3-year period. Beginning in CY 2015, Exparel® was packaged as a surgical supply under both the OPPS and the ASC payment system.)
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59345), we reiterated our position with regard to payment for Exparel®, stating that we believed that payment for this drug is appropriately packaged with the primary surgical procedure. We also stated in the CY 2018 OPPS/ASC final rule with comment period that CMS would continue to explore and evaluate packaging policies under the OPPS and consider these policies in future rulemaking.
In addition to stakeholder feedback regarding OPPS packaging policies in response to the CY 2018 OPPS/ASC proposed rule, the President's Commission on Combating Drug Addiction and the Opioid Crisis (the Commission) had recommended that CMS examine payment policies for certain drugs that function as a supply, specifically non-opioid pain management treatments. The Commission was established in 2017 to study ways to combat and treat drug abuse, addiction, and the opioid crisis. The Commission's report [3] included a recommendation for CMS to “. . . review and modify ratesetting policies that discourage the use of non-opioid treatments for pain, such as certain bundled payments that make alternative treatment options cost prohibitive for hospitals and doctors, particularly those options for treating immediate postsurgical pain. . . .” [4] With respect to the packaging policy, the Commission's report states that “. . . the current CMS payment policy for `supplies' related to surgical procedures creates unintended incentives to prescribe opioid medications to patients for postsurgical pain instead of administering non-opioid pain medications. Under current policies, CMS provides one all-inclusive bundled payment to hospitals for all `surgical supplies,' which includes hospital administered drug products intended to manage patients' postsurgical pain. This policy results in the hospitals receiving the same fixed fee from Medicare whether the surgeon administers a non-opioid medication or not.” [5] HHS also presented an Opioid Strategy in April 2017 [6] that aims in part to support cutting-edge research and advance the practice of pain management. On October 26, 2017, the President declared the opioid crisis a national public health emergency under Federal law [7] and this declaration was most recently renewed on April 19, 2019.[8]
For the CY 2019 rulemaking, we reviewed available literature with respect to Exparel®, including a briefing document [9] submitted for the FDA Advisory Committee Meeting held February 14-15, 2018, by the manufacturer of Exparel® that notes that “. . . Bupivacaine, the active pharmaceutical ingredient in Exparel®, is a local anesthetic that has been used for infiltration/field block and peripheral nerve block for decades” and that “since its approval, Exparel® has been used extensively, with an estimated 3.5 million patient exposures in the US.” [10] On April 6, 2018, the FDA approved Exparel®'s new indication for use as an interscalene brachial plexus nerve block to produce postsurgical regional analgesia.[11] We stated in the CY 2019 OPPS/ASC proposed rule that, based on our review of currently available OPPS Medicare claims data and public information from the manufacturer of the drug, we did not believe that the OPPS packaging policy had discouraged the use of Exparel® for either of the drug's indications when furnished in the hospital outpatient department setting.
In the CY 2019 OPPS/ASC proposed rule, in response to stakeholder comments on the CY 2018 OPPS/ASC final rule with comment period (82 FR 59345) and in light of the recommendations regarding payment policies for certain drugs, we evaluated the impact of our packaging policy for drugs that function as a supply when used in a surgical procedure on the utilization of these drugs in both the hospital outpatient department and the ASC setting. Our packaging policy is that the costs associated with packaged drugs that function as a supply are included in the ratesetting methodology for the surgical procedures with which they are billed, and the payment rate for the associated procedure reflects the costs of the packaged drugs and other packaged items and services to the extent they are billed with the procedure. In our evaluation, we used currently available data to analyze the utilization patterns associated with specific drugs that function as a supply over a 5-year time period to determine whether this packaging policy reduced the use of these drugs. If the packaging policy discouraged the use of drugs that function as a supply or impeded access to these products, we would expect to see a significant decline in utilization of these drugs over time, although we note that a decline in utilization could also reflect other factors, such as the availability of alternative products.
The results of the evaluation of our packaging policies under the OPPS and the ASC payment system showed decreased utilization for certain drugs that function as a supply in the ASC setting, in comparison to the hospital outpatient department setting. In light of these results, as well as the Commission's recommendation to examine payment policies for non-opioid pain management drugs that function as a supply, we believed it was appropriate to pay separately for evidence-based non-opioid pain management drugs that function as a supply in a surgical procedure in the ASC setting to address the decreased utilization of these drugs and to encourage use of these types of drugs rather than prescription opioids. Therefore, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58855 through 58860), we finalized the proposed policy to unpackage and pay separately at ASP+6 percent for the cost of non-opioid pain management drugs that function as surgical supplies when they are furnished in the ASC setting for CY 2019. We also stated that we would continue to analyze the issue of access to non-opioid alternatives in the hospital outpatient department setting and in the ASC setting as we implemented section 6082 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities (SUPPORT) Act (Pub. L. 115-271) enacted on October 24, 2018 (83 FR 58860 through 58861).
(2) Evaluation and CY 2020 Proposal for Payment for Non-Opioid Alternatives
Section 1833(t)(22)(A)(i) of the Act, as added by section 6082(a) of the SUPPORT Act, states that the Secretary must review payments under the OPPS for opioids and evidence-based non-opioid alternatives for pain management (including drugs and devices, nerve blocks, surgical injections, and neuromodulation) with a goal of ensuring that there are not financial incentives to use opioids instead of non-opioid alternatives. As part of this review, under section 1833(t)(22)(A)(iii) of the Act, the Secretary must consider the extent to which revisions to such payments (such as the creation of additional groups of covered OPD services to separately classify those procedures that utilize opioids and non-opioid alternatives for pain management) would reduce the payment incentives for using opioids instead of non-opioid alternatives for pain management. In conducting this review and considering any revisions, the Secretary must focus on covered OPD services (or groups of services) assigned to C-APCs, APCs that include surgical services, or services determined by the Secretary that generally involve treatment for pain management. If the Secretary identifies revisions to payments pursuant to section 1833(t)(22)(A)(iii) of the Act, section 1833(t)(22)(C) of the Act requires the Secretary to, as determined appropriate, begin making revisions for services furnished on or after January 1, 2020. Any revisions under this paragraph are required to be treated as adjustments for purposes of paragraph (9)(B), which requires any adjustments to be made in a budget neutral manner. Pursuant to these requirements, in our evaluation of whether there are payment incentives for using opioids instead of non-opioid alternatives, for this CY 2020 OPPS/ASC proposed rule, we used currently available data to analyze the payment and utilization patterns associated with specific non-opioid alternatives, including drugs that function as a supply, nerve blocks, and neuromodulation products, to determine whether our packaging policies have reduced the use of non-opioid alternatives. We focused on covered OPD services for this review, including services assigned to C-APCs, surgical APCs, and other pain management services. We believed that if the packaging policy discouraged the use of these non-opioid alternatives or impeded access to these products, we would expect to see a decline in the utilization over time, although we note that a decline in utilization could also reflect other factors, such as the availability of alternative products.
We evaluated continuous peripheral nerve blocks and neuromodulation alternatives to determine if the current packaging policy represented a barrier to access. For each product, we examined the most recently available Medicare claims data. All of the alternatives examined showed consistent or increasing utilization in recent years, with no products showing decreases in utilization.
We also evaluated drugs that function as surgical supplies over a 6-year time period (CYs 2013 through 2018). During our evaluation, we did not observe significant declines in the total number of units used in the hospital outpatient department for a majority of the drugs included in our analysis. In fact, under the OPPS, we observed the opposite effect for several drugs that function as surgical supplies, including Exparel® (HCPCS code C9290). This trend indicates appropriate packaged payments that adequately reflect the cost of the drug and are not prohibiting beneficiary access.
From CYs 2013 through 2018, we found that there was an overall increase in the OPPS Medicare utilization of Exparel® of approximately 491 percent (from 2.3 million units to 13.6 million units) during this 6-year time period. The total number of claims reporting the use of Exparel® increased by 463 percent (from 10,609 claims to 59,724 claims) over this 6-year time period. This increase in utilization continued, even after the expiration of the 3-year pass-through payment status for this drug in 2014, resulting in a 109-percent overall increase in the total number of units used between CYs 2015 and 2018, from 6.5 million units to 13.6 million units. The number of claims reporting the use of Exparel® increased by 112 percent during this time period, from 28,166 claims to 59,724 claims.
The results of our review and evaluation of our claims data do not provide evidence to indicate that the OPPS packaging policy has had the unintended consequence of discouraging the use of non-opioid treatments for postsurgical pain management in the hospital outpatient department. Therefore, based on this data evaluation, we do not believe that changes are necessary under the OPPS for the packaged drug policy for drugs that function as a surgical supply, nerve blocks, surgical injections, and neuromodulation products when used in a surgical procedure in the OPPS setting at this time.
For Exparel®, we reviewed claims data for development of this CY 2020 OPPS/ASC proposed rule and, based on these data and available literature, we concluded that there is no clear evidence that the OPPS packaging policy discourages the use of Exparel® for either of the drug's indications in the hospital outpatient department setting because the use of Exparel® continues to increase in this setting. Accordingly, we continue to believe it is appropriate to package payment for the use of Exparel®, as we do for other postsurgical pain management drugs, when it is furnished in a hospital outpatient department. In addition, our updated review of claims data showed a continued decline in the utilization of Exparel® in the ASC setting, which we believe supports our proposal to continue paying separately for Exparel® in the ASC setting.
Therefore, for CY 2020, we are proposing to continue our policy to pay separately at ASP+6 percent for the cost of non-opioid pain management drugs that function as surgical supplies in the performance of surgical procedures when they are furnished in the ASC setting and continue to package payment for non-opioid pain management drugs that function as surgical supplies in the performance of surgical procedures in the hospital outpatient department setting for CY 2020. However, we are inviting public comments on this proposal and asking the public to provide peer reviewed evidence, if any, to describe existing evidence-based non-opioid pain management therapies used in the outpatient and ASC setting. We are also inviting the public to provide detailed claims-based evidence to document how specific unfavorable utilization trends are due to the financial incentives of the payment systems rather than other factors.
Multiple stakeholders, largely manufacturers of devices and drugs, have requested separate payments for various non-opioid pain management treatments, such as continuous nerve blocks (including a disposable elastomeric pump that delivers non-opioid local anesthetic to a surgical site or nerve), cooled thermal radiofrequency ablation, and local anesthetics designed to reduce postoperative pain for cataract surgery and other procedures. These stakeholders have suggested various mechanisms through which separate payment or a higher-paying APC assignment for the primary service could be made. The stakeholders have offered surveys, reports, studies, and anecdotal evidence of varying degrees to support why the devices, drugs, or services offer an alternative to or a reduction of the need for opioid prescriptions. The majority of these stakeholder offerings have lacked adequate sample size, contained possible conflicts of interest such as studies conducted by employees of device manufacturers, have not been fully published in peer-reviewed literature, or have only provided anecdotal evidence as to how the drug or device could serve as an alternative to, or reduce the need for, opioid prescriptions.
After reviewing the data from stakeholders and Medicare claims data, we have not found compelling evidence to suggest that revisions to our OPPS payment policies for non-opioid pain management alternatives are necessary for CY 2020. Additionally, MedPAC's March 2019 Report to Congress supports CMS' conclusion. Specifically, Chapter 16 of MedPAC's report, titled Mandated Report: Opioids and Alternatives in Hospital Settings—Payments, Incentives, and Medicare Data, concludes that there is no clear indication that Medicare's OPPS provides systematic payment incentives that promote the use of opioid analgesics over non-opioid analgesics.[12] However, we are inviting public comments on whether there are other non-opioid pain management alternatives for which our payment policy should be revised to allow separate payment. We are requesting public comments that provide evidence-based support, such as published peer-reviewed literature, that we could use to determine whether these products help to deter or avoid prescription opioid use and addiction as well as evidence that the current packaged payment for such non-opioid alternatives presents a barrier to access to care and therefore warrants revised, including possibly separate, payment under the OPPS. Evidence that current payment policy provides a payment incentive for using opioids instead of non-opioid alternatives should align with available Medicare claims data.
4. Proposed Calculation of OPPS Scaled Payment Weights
We established a policy in the CY 2013 OPPS/ASC final rule with comment period (77 FR 68283) of using geometric mean-based APC costs to calculate relative payment weights under the OPPS. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 58860 through 58861), we applied this policy and calculated the relative payment weights for each APC for CY 2019 that were shown in Addenda A and B to that final rule with comment period (which were made available via the internet on the CMS website) using the APC costs discussed in sections II.A.1. and II.A.2. of that final rule with comment period. For CY 2020, as we did for CY 2019, we are proposing to continue to apply the policy established in CY 2013 and calculate relative payment weights for each APC for CY 2020 using geometric mean-based APC costs.
For CY 2012 and CY 2013, outpatient clinic visits were assigned to one of five levels of clinic visit APCs, with APC 0606 representing a mid-level clinic visit. In the CY 2014 OPPS/ASC final rule with comment period (78 FR 75036 through 75043), we finalized a policy that created alphanumeric HCPCS code G0463 (Hospital outpatient clinic visit for assessment and management of a patient), representing any and all clinic visits under the OPPS. HCPCS code G0463 was assigned to APC 0634 (Hospital Clinic Visits). We also finalized a policy to use CY 2012 claims data to develop the CY 2014 OPPS payment rates for HCPCS code G0463 based on the total geometric mean cost of the levels one through five CPT E/M codes for clinic visits previously recognized under the OPPS (CPT codes 99201 through 99205 and 99211 through 99215). In addition, we finalized a policy to no longer recognize a distinction between new and established patient clinic visits.
For CY 2016, we deleted APC 0634 and reassigned the outpatient clinic visit HCPCS code G0463 to APC 5012 (Level 2 Examinations and Related Services) (80 FR 70372). For CY 2020, as we did for CY 2019, we are proposing to continue to standardize all of the relative payment weights to APC 5012. We believe that standardizing relative payment weights to the geometric mean of the APC to which HCPCS code G0463 is assigned maintains consistency in calculating unscaled weights that represent the cost of some of the most frequently provided OPPS services. For CY 2020, as we did for CY 2019, we are proposing to assign APC 5012 a relative payment weight of 1.00 and to divide the geometric mean cost of each APC by the geometric mean cost for APC 5012 to derive the unscaled relative payment weight for each APC. The choice of the APC on which to standardize the relative payment weights does not affect payments made under the OPPS because we scale the weights for budget neutrality.
We note that in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59004 through 59015), we discussed our policy, implemented on January 1, 2019, to control for unnecessary increases in the volume of covered outpatient department services by paying for clinic visits furnished at excepted off-campus provider-based department (PBD) at a reduced rate. While the volume associated with these visits is included in the impact model, and thus used in calculating the weight scalar, the policy has a negligible effect on the scalar. Specifically, under this policy, there was no change to the relativity of the OPPS payment weights because the adjustment is made at the payment level rather than in the cost modeling. Further, under this policy, the savings that would result from the change in payments for these clinic visits would not be budget neutral. Therefore, the impact of this policy would generally not be reflected in the budget neutrality adjustments, whether the adjustment is to the OPPS relative weights or to the OPPS conversion factor.
Section 1833(t)(9)(B) of the Act requires that APC reclassification and recalibration changes, wage index changes, and other adjustments be made in a budget neutral manner. Budget neutrality ensures that the estimated aggregate weight under the OPPS for CY 2020 is neither greater than nor less than the estimated aggregate weight that would have been made without the changes. To comply with this requirement concerning the APC changes, we are proposing to compare the estimated aggregate weight using the CY 2019 scaled relative payment weights to the estimated aggregate weight using the proposed CY 2020 unscaled relative payment weights.
For CY 2019, we multiplied the CY 2019 scaled APC relative payment weight applicable to a service paid under the OPPS by the volume of that service from CY 2018 claims to calculate the total relative payment weight for each service. We then added together the total relative payment weight for each of these services in order to calculate an estimated aggregate weight for the year. For CY 2020, we are proposing to apply the same process using the estimated CY 2020 unscaled relative payment weights rather than scaled relative payment weights. We are proposing to calculate the weight scalar by dividing the CY 2019 estimated aggregate weight by the proposed unscaled CY 2020 estimated aggregate weight.
For a detailed discussion of the weight scalar calculation, we refer readers to the OPPS claims accounting document available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. Click on the CY 2020 OPPS proposed rule link and open the claims accounting document link at the bottom of the page.
We are proposing to compare the estimated unscaled relative payment weights in CY 2020 to the estimated total relative payment weights in CY 2019 using CY 2018 claims data, holding all other components of the payment system constant to isolate changes in total weight. Based on this comparison, we are proposing to adjust the calculated CY 2020 unscaled relative payment weights for purposes of budget neutrality. We are proposing to adjust the estimated CY 2020 unscaled relative payment weights by multiplying them by a proposed weight scalar of 1.4401 to ensure that the proposed CY 2020 relative payment weights are scaled to be budget neutral. The proposed CY 2020 relative payment weights listed in Addenda A and B to this proposed rule (which are available via the internet on the CMS website) were scaled and incorporated the recalibration adjustments discussed in sections II.A.1. and II.A.2. of this proposed rule.
Section 1833(t)(14) of the Act provides the payment rates for certain SCODs. Section 1833(t)(14)(H) of the Act provides that additional expenditures resulting from this paragraph shall not be taken into account in establishing the conversion factor, weighting, and other adjustment factors for 2004 and 2005 under paragraph (9), but shall be taken into account for subsequent years. Therefore, the cost of those SCODs (as discussed in section V.B.2. of this proposed rule) is included in the budget neutrality calculations for the CY 2020 OPPS.
B. Proposed Conversion Factor Update
Section 1833(t)(3)(C)(ii) of the Act requires the Secretary to update the conversion factor used to determine the payment rates under the OPPS on an annual basis by applying the OPD fee schedule increase factor. For purposes of section 1833(t)(3)(C)(iv) of the Act, subject to sections 1833(t)(17) and 1833(t)(3)(F) of the Act, the OPD fee schedule increase factor is equal to the hospital inpatient market basket percentage increase applicable to hospital discharges under section 1886(b)(3)(B)(iii) of the Act. In the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19401), consistent with current law, based on IHS Global, Inc.'s fourth quarter 2018 forecast of the FY 2020 market basket increase, the proposed FY 2020 IPPS market basket update is 3.2 percent. However, sections 1833(t)(3)(F) and 1833(t)(3)(G)(v) of the Act, as added by section 3401(i) of the Patient Protection and Affordable Care Act of 2010 (Pub. L. 111-148) and as amended by section 10319(g) of that law and further amended by section 1105(e) of the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152), provide adjustments to the OPD fee schedule increase factor for CY 2020.
Specifically, section 1833(t)(3)(F)(i) of the Act requires that, for 2012 and subsequent years, the OPD fee schedule increase factor under subparagraph (C)(iv) be reduced by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. Section 1886(b)(3)(B)(xi)(II) of the Act defines the productivity adjustment as equal to the 10-year moving average of changes in annual economy-wide, private nonfarm business multifactor productivity (MFP) (as projected by the Secretary for the 10-year period ending with the applicable fiscal year, year, cost reporting period, or other annual period) (the “MFP adjustment”). In the FY 2012 IPPS/LTCH PPS final rule (76 FR 51689 through 51692), we finalized our methodology for calculating and applying the MFP adjustment, and then revised this methodology, as discussed in the FY 2016 IPPS/LTCH PPS final rule (80 FR 49509). According to the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19402), the proposed MFP adjustment for FY 2020 is 0.5 percentage point.
For CY 2020, we are proposing that the MFP adjustment for the CY 2020 OPPS is 0.5 percentage point. We are proposing that if more recent data become subsequently available after the publication of this proposed rule (for example, a more recent estimate of the market basket increase and the MFP adjustment), we would use such updated data, if appropriate, to determine the CY 2020 market basket update and the MFP adjustment, which are components in calculating the OPD fee schedule increase factor under sections 1833(t)(3)(C)(iv) and 1833(t)(3)(F) of the Act, in this CY 2020 OPPS/ASC proposed rule.
We note that section 1833(t)(3)(F) of the Act provides that application of this subparagraph may result in the OPD fee schedule increase factor under section 1833(t)(3)(C)(iv) of the Act being less than 0.0 percent for a year, and may result in OPPS payment rates being less than rates for the preceding year. As described in further detail below, we are proposing to apply an OPD fee schedule increase factor of 2.7 percent for the CY 2020 OPPS (which is 3.2 percent, the proposed estimate of the hospital inpatient market basket percentage increase, less the proposed 0.5 percentage point MFP adjustment).
Hospitals that fail to meet the Hospital OQR Program reporting requirements are subject to an additional reduction of 2.0 percentage points from the OPD fee schedule increase factor adjustment to the conversion factor that would be used to calculate the OPPS payment rates for their services, as required by section 1833(t)(17) of the Act. For further discussion of the Hospital OQR Program, we refer readers to section XIV. of this proposed rule.
We are proposing to amend 42 CFR 419.32(b)(1)(iv)(B) by adding a new paragraph (11) to reflect the requirement in section 1833(t)(3)(F)(i) of the Act that, for CY 2020, we reduce the OPD fee schedule increase factor by the MFP adjustment as determined by CMS.
To set the OPPS conversion factor for CY 2020, we are proposing to increase the CY 2019 conversion factor of $79.490 by 2.7 percent. In accordance with section 1833(t)(9)(B) of the Act, we are proposing further to adjust the conversion factor for CY 2020 to ensure that any revisions made to the wage index and rural adjustment were made on a budget neutral basis. We are proposing to calculate an overall budget neutrality factor of 0.9993 for wage index changes. This adjustment is comprised of a 1.0005 proposed budget neutrality adjustment, using our standard calculation, of comparing proposed total estimated payments from our simulation model using the proposed FY 2020 IPPS wage indexes to those payments using the FY 2019 IPPS wage indexes, as adopted on a calendar year basis for the OPPS as well as a 0.9988 proposed budget neutrality adjustment for the proposed CY 2020 5 percent cap on wage index decreases to ensure that this transition wage index is implemented in a budget neutral manner, consistent with the proposed FY 2020 IPPS wage index policy (84 FR 19398). We believe it is appropriate to ensure that this proposed wage index transition policy (that is, the proposed CY 2020 5 percent cap on wage index decreases) does not increase estimated aggregate payments under the OPPS beyond the payments that would be made without this transition policy. We are proposing to calculate this budget neutrality adjustment by comparing total estimated OPPS payments using the FY 2020 IPPS wage index, adopted on a calendar year basis for the OPPS, where a 5 percent cap on wage index decreases is not applied to total estimated OPPS payments where the 5 percent cap on wage index decreases is applied. These two proposed wage index budget neutrality adjustments would maintain budget neutrality for the proposed CY 2020 OPPS wage index (which, as discussed in section II.C of this proposed rule, would use the FY 2020 IPPS post-reclassified wage index and any adjustments, including without limitation any proposed adjustments finalized under the IPPS to address wage index disparities).
For the CY 2020 OPPS, we are maintaining the current rural adjustment policy, as discussed in section II.E. of this proposed rule. Therefore, the proposed budget neutrality factor for the rural adjustment is 1.0000.
For this CY 2020 OPPS/ASC proposed rule, we are proposing to continue previously established policies for implementing the cancer hospital payment adjustment described in section 1833(t)(18) of the Act, as discussed in section II.F. of this proposed rule. We are proposing to calculate a CY 2020 budget neutrality adjustment factor for the cancer hospital payment adjustment by comparing estimated total CY 2020 payments under section 1833(t) of the Act, including the proposed CY 2020 cancer hospital payment adjustment, to estimated CY 2020 total payments using the CY 2019 final cancer hospital payment adjustment, as required under section 1833(t)(18)(B) of the Act. The proposed CY 2020 estimated payments applying the proposed CY 2020 cancer hospital payment adjustment are the same as estimated payments applying the CY 2019 final cancer hospital payment adjustment. Therefore, we are proposing to apply a budget neutrality adjustment factor of 0.9998 to the conversion factor for the cancer hospital payment adjustment. In accordance with section 16002(b) of the 21st Century Cures Act, we are proposing to apply a budget neutrality factor calculated as if the proposed cancer hospital adjustment target payment-to-cost ratio is 0.90, not the 0.89 target payment-to-cost ratio we are proposing to apply as stated in section II.F. of this proposed rule.
For this CY 2020 OPPS/ASC proposed rule, we estimate that proposed pass-through spending for drugs, biologicals, and devices for CY 2020 would equal approximately $268.8 million, which represents 0.34 percent of total projected CY 2020 OPPS spending. Therefore, the proposed conversion factor would be adjusted by the difference between the 0.14 percent estimate of pass-through spending for CY 2019 and the 0.34 percent estimate of proposed pass-through spending for CY 2020, resulting in a proposed decrease for CY 2020 of 0.20 percent. Proposed estimated payments for outliers would remain at 1.0 percent of total OPPS payments for CY 2020. We estimate for this proposed rule that outlier payments would be 1.03 percent of total OPPS payments in CY 2019; the 1.00 percent for proposed outlier payments in CY 2020 would constitute a 0.03 percent increase in payment in CY 2020 relative to CY 2019.
For this CY 2020 OPPS/ASC proposed rule, we also are proposing that hospitals that fail to meet the reporting requirements of the Hospital OQR Program would continue to be subject to a further reduction of 2.0 percentage points to the OPD fee schedule increase factor. For hospitals that fail to meet the requirements of the Hospital OQR Program, we are proposing to make all other adjustments discussed above, but use a reduced OPD fee schedule update factor of 0.7 percent (that is, the proposed OPD fee schedule increase factor of 2.7 percent further reduced by 2.0 percentage points). This would result in a proposed reduced conversion factor for CY 2020 of $79.770 for hospitals that fail to meet the Hospital OQR Program requirements (a difference of −1.628 in the conversion factor relative to hospitals that meet the requirements).
In summary, for CY 2020, we are proposing to amend § 419.32 by adding a new paragraph (b)(1)(iv)(B)(11) to reflect the reductions to the OPD fee schedule increase factor that are required for CY 2020 to satisfy the statutory requirements of sections 1833(t)(3)(F) and (t)(3)(G)(v) of the Act. We are proposing to use a reduced conversion factor of $79.770 in the calculation of payments for hospitals that fail to meet the Hospital OQR Program requirements (a difference of −1.628 in the conversion factor relative to hospitals that meet the requirements).
For CY 2020, we are proposing to use a conversion factor of $81.398 in the calculation of the national unadjusted payment rates for those items and services for which payment rates are calculated using geometric mean costs; that is, the proposed OPD fee schedule increase factor of 2.7 percent for CY 2020, the required proposed wage index budget neutrality adjustment of approximately 0.9993, the proposed cancer hospital payment adjustment of 0.9998, and the proposed adjustment of −0.20 percentage point of projected OPPS spending for the difference in pass-through spending that resulted in a proposed conversion factor for CY 2020 of $81.398. We refer readers to section XXVI.B. of this proposed rule for a discussion of the estimated effect on the conversion factor of a policy to pay for 340B-acquired drugs at ASP+3 percent, which is a policy on which we solicit comments for potential future rulemaking in the event of an adverse decision on appeal in the ongoing litigation involving our payment policy for 340B-acquired drugs.
C. Proposed Wage Index Changes
Section 1833(t)(2)(D) of the Act requires the Secretary to determine a wage adjustment factor to adjust the portion of payment and coinsurance attributable to labor-related costs for relative differences in labor and labor-related costs across geographic regions in a budget neutral manner (codified at 42 CFR 419.43(a)). This portion of the OPPS payment rate is called the OPPS labor-related share. Budget neutrality is discussed in section II.B. of this proposed rule.
The OPPS labor-related share is 60 percent of the national OPPS payment. This labor-related share is based on a regression analysis that determined that, for all hospitals, approximately 60 percent of the costs of services paid under the OPPS were attributable to wage costs. We confirmed that this labor-related share for outpatient services is appropriate during our regression analysis for the payment adjustment for rural hospitals in the CY 2006 OPPS final rule with comment period (70 FR 68553). In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue this policy for the CY 2020 OPPS. We refer readers to section II.H. of this proposed rule for a description and an example of how the wage index for a particular hospital is used to determine payment for the hospital.
As discussed in the claims accounting narrative included with the supporting documentation for this proposed rule (which is available via the internet on the CMS website), for estimating APC costs, we would standardize 60 percent of estimated claims costs for geographic area wage variation using the same FY 2020 pre-reclassified wage index that CMS is proposing to use under the IPPS to standardize costs. This standardization process removes the effects of differences in area wage levels from the determination of a national unadjusted OPPS payment rate and copayment amount.
Under 42 CFR 419.41(c)(1) and 419.43(c) (published in the OPPS April 7, 2000 final rule with comment period (65 FR 18495 and 18545)), the OPPS adopted the final fiscal year IPPS post-reclassified wage index as the calendar year wage index for adjusting the OPPS standard payment amounts for labor market differences. Therefore, the wage index that applies to a particular acute care, short-stay hospital under the IPPS also applies to that hospital under the OPPS. As initially explained in the September 8, 1998 OPPS proposed rule (63 FR 47576), we believe that using the IPPS wage index as the source of an adjustment factor for the OPPS is reasonable and logical, given the inseparable, subordinate status of the HOPD within the hospital overall. In accordance with section 1886(d)(3)(E) of the Act, the IPPS wage index is updated annually.
The Affordable Care Act contained several provisions affecting the wage index. These provisions were discussed in the CY 2012 OPPS/ASC final rule with comment period (76 FR 74191). Section 10324 of the Affordable Care Act added section 1886(d)(3)(E)(iii)(II) to the Act, which defines a frontier State and amended section 1833(t) of the Act to add paragraph (19), which requires a frontier State wage index floor of 1.00 in certain cases, and states that the frontier State floor shall not be applied in a budget neutral manner. We codified these requirements at § 419.43(c)(2) and (3) of our regulations. For the CY 2020 OPPS, we are proposing to implement this provision in the same manner as we have since CY 2011. Under this policy, the frontier State hospitals would receive a wage index of 1.00 if the otherwise applicable wage index (including reclassification, the rural floor, and rural floor budget neutrality) is less than 1.00. Because the HOPD receives a wage index based on the geographic location of the specific inpatient hospital with which it is associated, the frontier State wage index adjustment applicable for the inpatient hospital also would apply for any associated HOPD. We refer readers to the FY 2011 through FY 2019 IPPS/LTCH PPS final rules for discussions regarding this provision, including our methodology for identifying which areas meet the definition of “frontier States” as provided for in section 1886(d)(3)(E)(iii)(II) of the Act: For FY 2011, 75 FR 50160 through 50161; for FY 2012, 76 FR 51793, 51795, and 51825; for FY 2013, 77 FR 53369 through 53370; for FY 2014, 78 FR 50590 through 50591; for FY 2015, 79 FR 49971; for FY 2016, 80 FR 49498; for FY 2017, 81 FR 56922; for FY 2018, 82 FR 38142; and for FY 2019, 83 FR 41380.
In addition to the changes required by the Affordable Care Act, we note that the proposed FY 2020 IPPS wage indexes continue to reflect a number of adjustments implemented over the past few years, including, but not limited to, reclassification of hospitals to different geographic areas, the rural floor provisions, an adjustment for occupational mix, and an adjustment to the wage index based on commuting patterns of employees (the out-migration adjustment). In addition, we note that, as discussed in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19393 through 19399), we proposed a number of policies under the IPPS to address wage index disparities between high and low wage index value hospitals. In particular, in the FY 2020 IPPS/LTCH PPS proposed rule, we proposed to (1) calculate the rural floor without including the wage data of urban hospitals that have reclassified as rural under section 1886(d)(8)(E) of the Act (as implemented in § 412.103) (84 FR 19396 through 19398); (2) remove the wage data of urban hospitals that have reclassified as rural under § 412.103 from the calculation of “the wage index for rural areas in the State” for purposes of applying section 1886(d)(8)(C)(iii) of the Act (84 FR 19398); (3) increase the wage index values for hospitals with a wage index below the 25th percentile wage index value across all hospitals by half the difference between the otherwise applicable final wage index value for a year for that hospital and the 25th percentile wage index value for that year, and to offset the estimated increase in payments to hospitals with wage index values below the 25th percentile by decreasing the wage index values for hospitals with wage index values above the 75th percentile wage index value across all hospitals (84 FR 19394 through 19396); and (4) apply a 5-percent cap for FY 2020 on any decrease in a hospital's final wage index from the hospital's final wage index in FY 2019, as a proposed transition wage index to help mitigate any significant negative impacts on hospitals (84 FR 19398). In addition, in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19398), we proposed to apply a budget neutrality adjustment to the standardized amount so that our proposed transition wage index for hospitals that may be negatively impacted (described in item (4) above) would be implemented in a budget neutral manner. Furthermore, in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19398 through 19399), we noted that our proposed adjustment relating to the rural floor calculation also would be budget neutral. We refer readers to the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19373 through 19399) for a detailed discussion of all proposed changes to the FY 2020 IPPS wage indexes.
As discussed in the FY 2015 IPPS/LTCH PPS final rule (79 FR 49951 through 49963) and in each subsequent IPPS/LTCH PPS final rule, including the FY 2019 IPPS/LTCH PPS final rule (83 FR 41362), the Office of Management and Budget (OMB) issued revisions to the labor market area delineations on February 28, 2013 (based on 2010 Decennial Census data), that included a number of significant changes, such as new Core Based Statistical Areas (CBSAs), urban counties that became rural, rural counties that became urban, and existing CBSAs that were split apart (OMB Bulletin 13-01). This bulletin can be found at: https://obamawhitehouse.archives.gov/sites/default/files/omb/bulletins/2013/b13-01.pdf. In the FY 2015 IPPS/LTCH PPS final rule (79 FR 49950 through 49985), for purposes of the IPPS, we adopted the use of the OMB statistical area delineations contained in OMB Bulletin No. 13-01, effective October 1, 2014. For purposes of the OPPS, in the CY 2015 OPPS/ASC final rule with comment period (79 FR 66826 through 66828), we adopted the use of the OMB statistical area delineations contained in OMB Bulletin No. 13-01, effective January 1, 2015, beginning with the CY 2015 OPPS wage indexes. In the FY 2017 IPPS/LTCH PPS final rule (81 FR 56913), we adopted revisions to statistical areas contained in OMB Bulletin No. 15-01, issued on July 15, 2015, which provided updates to and superseded OMB Bulletin No. 13-01 that was issued on February 28, 2013. For purposes of the OPPS, in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79598), we adopted the revisions to the OMB statistical area delineations contained in OMB Bulletin No. 15-01, effective January 1, 2017, beginning with the CY 2017 OPPS wage indexes.
On August 15, 2017, OMB issued OMB Bulletin No. 17-01, which provided updates to and superseded OMB Bulletin No. 15-01 that was issued on July 15, 2015. The attachments to OMB Bulletin No. 17-01 provided detailed information on the update to the statistical areas since July 15, 2015, and were based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2014 and July 1, 2015. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 58863 through 58865), we adopted the updates set forth in OMB Bulletin No. 17-01, effective January 1, 2019, beginning with the CY 2019 wage index. We continue to believe that it is important for the OPPS to use the latest labor market area delineations available as soon as is reasonably possible in order to maintain a more accurate and up-to-date payment system that reflects the reality of population shifts and labor market conditions. For a complete discussion of the adoption of the updates set forth in OMB Bulletin No. 17-01, we refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 58864 through 58865).
As we stated in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19374), for the FY 2020 IPPS wage indexes, we would continue to use the OMB delineations that were adopted, beginning with FY 2015 (based on the revised delineations issued in OMB Bulletin No. 13-01) to calculate the area wage indexes, with updates as reflected in OMB Bulletin Nos. 15-01 and 17-01. Similarly, in this CY 2020 OPPS/ASC proposed rule, for the CY 2020 OPPS wage indexes, we would continue to use the OMB delineations that were adopted under the OPPS, beginning with CY 2015 (based on the revised delineations issued in OMB Bulletin No. 13-01) to calculate the area wage indexes, with updates as reflected in OMB Bulletin Nos. 15-01 and 17-01.
CBSAs are made up of one or more constituent counties. Each CBSA and constituent county has its own unique identifying codes. The FY 2018 IPPS/LTCH PPS final rule (82 FR 38130) discussed the two different lists of codes to identify counties: Social Security Administration (SSA) codes and Federal Information Processing Standard (FIPS) codes. Historically, CMS listed and used SSA and FIPS county codes to identify and crosswalk counties to CBSA codes for purposes of the IPPS and OPPS wage indexes. However, the SSA county codes are no longer being maintained and updated, although the FIPS codes continue to be maintained by the U.S. Census Bureau. The Census Bureau's most current statistical area information is derived from ongoing census data received since 2010; the most recent data are from 2015. The Census Bureau maintains a complete list of changes to counties or county equivalent entities on the website at: https://www.census.gov/geo/reference/county-changes.html (which, as of May 6, 2019, migrated to: https://www.census.gov/programs-surveys/geography.html). In the FY 2018 IPPS/LTCH PPS final rule (82 FR 38130), for purposes of crosswalking counties to CBSAs for the IPPS wage index, we finalized our proposal to discontinue the use of the SSA county codes and begin using only the FIPS county codes. Similarly, for the purposes of crosswalking counties to CBSAs for the OPPS wage index, in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59260), we finalized our proposal to discontinue the use of SSA county codes and begin using only the FIPS county codes for the purposes of crosswalking counties to CBSAs for the OPPS wage index. For CY 2020, under the OPPS, we are continuing to use only the FIPS county codes for purposes of crosswalking counties to CBSAs.
In this CY 2020 OPPS/ASC proposed rule, we are proposing to use the FY 2020 hospital IPPS post-reclassified wage index for urban and rural areas as the wage index for the OPPS to determine the wage adjustments for both the OPPS payment rate and the copayment standardized amount for CY 2020. Therefore, any adjustments for the FY 2020 IPPS post-reclassified wage index, including, but not limited to, any proposed policies finalized under the IPPS to address wage index disparities between low and high wage index value hospitals as discussed above and in the FY 2020 IPPS/LTCH PPS proposed rule at 84 FR 19393 through19399, would be reflected in the final CY 2020 OPPS wage index beginning on January 1, 2020. (We refer readers to the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19373 through 19399) and the proposed FY 2020 hospital wage index files posted on the CMS website.) With regard to budget neutrality for the CY 2020 OPPS wage index, we refer readers to section II.B. of this proposed rule. We continue to believe that using the IPPS wage index as the source of an adjustment factor for the OPPS is reasonable and logical, given the inseparable, subordinate status of the HOPD within the hospital overall.
Hospitals that are paid under the OPPS, but not under the IPPS, do not have an assigned hospital wage index under the IPPS. Therefore, for non-IPPS hospitals paid under the OPPS, it is our longstanding policy to assign the wage index that would be applicable if the hospital were paid under the IPPS, based on its geographic location and any applicable wage index adjustments. In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue this policy for CY 2020, and are including a brief summary of the major proposed FY 2020 IPPS wage index policies and adjustments that we are proposing to apply to these hospitals under the OPPS for CY 2020, which we have summarized below. We refer readers to the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19373 through 19399) for a detailed discussion of the proposed changes to the FY 2020 IPPS wage indexes.
It has been our longstanding policy to allow non-IPPS hospitals paid under the OPPS to qualify for the out-migration adjustment if they are located in a section 505 out-migration county (section 505 of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA)). Applying this adjustment is consistent with our policy of adopting IPPS wage index policies for hospitals paid under the OPPS. We note that, because non-IPPS hospitals cannot reclassify, they are eligible for the out-migration wage index adjustment if they are located in a section 505 out-migration county. This is the same out-migration adjustment policy that applies if the hospital were paid under the IPPS. For CY 2020, we are proposing to continue our policy of allowing non-IPPS hospitals paid under the OPPS to qualify for the out-migration adjustment if they are located in a section 505 out-migration county (section 505 of the MMA). In addition, for non-IPPS hospitals paid under the OPPS, we are proposing to apply any proposed policies that are finalized under the IPPS relating to wage index disparities as discussed earlier in this proposed rule and in the FY 2020 IPPS/LTCH PPS proposed rule at 84 FR 19393 through 19399. We also are proposing that the wage index that would apply to non-IPPS hospitals for CY 2020 would include the rural floor adjustment.
For CMHCs, for CY 2020, we are proposing to continue to calculate the wage index by using the post-reclassification IPPS wage index based on the CBSA where the CMHC is located. We also are proposing to apply any proposed policies that are finalized under the IPPS relating to wage index disparities as discussed earlier in this proposed rule and in the FY 2020 IPPS/LTCH PPS proposed rule at 84 FR 19393 through 19399. In addition, we are proposing that the wage index that would apply to CMHCs for CY 2020 would include the rural floor adjustment. Also, we are proposing that the wage index that would apply to CMHCs would not include the out-migration adjustment because that adjustment only applies to hospitals.
Table 4 associated with the FY 2020 IPPS/LTCH PPS proposed rule (available via the internet on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html) identifies counties eligible for the out-migration adjustment. Table 2 associated with the FY 2020 IPPS/LTCH PPS proposed rule (available for download via the website above) identifies IPPS hospitals that would receive the out-migration adjustment for FY 2020. We are including the out-migration adjustment information from Table 2 associated with the FY 2020 IPPS/LTCH PPS proposed rule as Addendum L to this proposed rule with the addition of non-IPPS hospitals that would receive the section 505 out-migration adjustment under this CY 2020 OPPS/ASC proposed rule. Addendum L is available via the internet on the CMS website. We refer readers to the CMS website for the OPPS at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. At this link, readers will find a link to the proposed FY 2020 IPPS wage index tables and Addendum L.
D. Proposed Statewide Average Default Cost-to-Charge Ratios (CCRs)
In addition to using CCRs to estimate costs from charges on claims for ratesetting, CMS uses overall hospital-specific CCRs calculated from the hospital's most recent cost report to determine outlier payments, payments for pass-through devices, and monthly interim transitional corridor payments under the OPPS during the PPS year. For certain hospitals, under the regulations at 42 CFR 419.43(d)(5)(iii), CMS uses the statewide average default CCRs to determine the payments mentioned earlier if it is unable to determine an accurate CCR for a hospital in certain circumstances. This includes hospitals that are new, hospitals that have not accepted assignment of an existing hospital's provider agreement, and hospitals that have not yet submitted a cost report. CMS also uses the statewide average default CCRs to determine payments for hospitals whose CCR falls outside the predetermined ceiling threshold for a valid CCR or for hospitals in which the most recent cost report reflects an all-inclusive rate status (Medicare Claims Processing Manual (Pub. 100-04), Chapter 4, Section 10.11).
We discussed our policy for using default CCRs, including setting the ceiling threshold for a valid CCR, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594 through 68599) in the context of our adoption of an outlier reconciliation policy for cost reports beginning on or after January 1, 2009. For details on our process for calculating the statewide average CCRs, we refer readers to the CY 2020 OPPS proposed rule Claims Accounting Narrative that is posted on the CMS website. In this CY 2020 OPPS/ASC proposed rule, we are proposing to update the default ratios for CY 2020 using the most recent cost report data. We will update these ratios in the final rule if more recent cost report data are available.
Beginning with this CY 2020 proposed rule, we are no longer publishing a table in the Federal Register containing the statewide average CCRs in the annual OPPS proposed rule and final rule. These CCRs with the upper limit will be available for download with each OPPS calendar year proposed rule and final rule on the CMS website. We refer the reader to the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices.html; click on the link on the left of the page titled “Hospital Outpatient Regulations and Notices” and then select the relevant regulation to download the statewide CCRs and upper limit in the downloads section of the web page.
E. Proposed Adjustment for Rural Sole Community Hospitals (SCHs) and Essential Access Community Hospitals (EACHs) Under Section 1833(t)(13)(B) of the Act for CY 2020
In the CY 2006 OPPS final rule with comment period (70 FR 68556), we finalized a payment increase for rural sole community hospitals (SCHs) of 7.1 percent for all services and procedures paid under the OPPS, excluding drugs, biologicals, brachytherapy sources, and devices paid under the pass-through payment policy, in accordance with section 1833(t)(13)(B) of the Act, as added by section 411 of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (Pub. L. 108-173). Section 1833(t)(13) of the Act provided the Secretary the authority to make an adjustment to OPPS payments for rural hospitals, effective January 1, 2006, if justified by a study of the difference in costs by APC between hospitals in rural areas and hospitals in urban areas. Our analysis showed a difference in costs for rural SCHs. Therefore, for the CY 2006 OPPS, we finalized a payment adjustment for rural SCHs of 7.1 percent for all services and procedures paid under the OPPS, excluding separately payable drugs and biologicals, brachytherapy sources, items paid at charges reduced to costs, and devices paid under the pass-through payment policy, in accordance with section 1833(t)(13)(B) of the Act.
In the CY 2007 OPPS/ASC final rule with comment period (71 FR 68010 and 68227), for purposes of receiving this rural adjustment, we revised § 419.43(g) of the regulations to clarify that essential access community hospitals (EACHs) also are eligible to receive the rural SCH adjustment, assuming these entities otherwise meet the rural adjustment criteria. Currently, two hospitals are classified as EACHs, and as of CY 1998, under section 4201(c) of Public Law 105-33, a hospital can no longer become newly classified as an EACH.
This adjustment for rural SCHs is budget neutral and applied before calculating outlier payments and copayments. We stated in the CY 2006 OPPS final rule with comment period (70 FR 68560) that we would not reestablish the adjustment amount on an annual basis, but we may review the adjustment in the future and, if appropriate, would revise the adjustment. We provided the same 7.1 percent adjustment to rural SCHs, including EACHs, again in CYs 2008 through 2019. Further, in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68590), we updated the regulations at § 419.43(g)(4) to specify, in general terms, that items paid at charges adjusted to costs by application of a hospital-specific CCR are excluded from the 7.1 percent payment adjustment.
For the CY 2020 OPPS, we are proposing to continue the current policy of a 7.1 percent payment adjustment that is done in a budget neutral manner for rural SCHs, including EACHs, for all services and procedures paid under the OPPS, excluding separately payable drugs and biologicals, brachytherapy sources, items paid at charges reduced to costs, and devices paid under the pass-through payment policy.
F. Proposed Payment Adjustment for Certain Cancer Hospitals for CY 2020
1. Background
Since the inception of the OPPS, which was authorized by the Balanced Budget Act of 1997 (BBA) (Pub. L. 105-33), Medicare has paid the 11 hospitals that meet the criteria for cancer hospitals identified in section 1886(d)(1)(B)(v) of the Act under the OPPS for covered outpatient hospital services. These cancer hospitals are exempted from payment under the IPPS. With the Medicare, Medicaid and SCHIP Balanced Budget Refinement Act of 1999 (Pub. L. 106-113), Congress established section 1833(t)(7) of the Act, “Transitional Adjustment to Limit Decline in Payment,” to determine OPPS payments to cancer and children's hospitals based on their pre-BBA payment amount (often referred to as “held harmless”).
As required under section 1833(t)(7)(D)(ii) of the Act, a cancer hospital receives the full amount of the difference between payments for covered outpatient services under the OPPS and a “pre-BBA amount.” That is, cancer hospitals are permanently held harmless to their “pre-BBA amount,” and they receive transitional outpatient payments (TOPs) or hold harmless payments to ensure that they do not receive a payment that is lower in amount under the OPPS than the payment amount they would have received before implementation of the OPPS, as set forth in section 1833(t)(7)(F) of the Act. The “pre-BBA amount” is the product of the hospital's reasonable costs for covered outpatient services occurring in the current year and the base payment-to-cost ratio (PCR) for the hospital defined in section 1833(t)(7)(F)(ii) of the Act. The “pre-BBA amount” and the determination of the base PCR are defined at 42 CFR 419.70(f). TOPs are calculated on Worksheet E, Part B, of the Hospital Cost Report or the Hospital Health Care Complex Cost Report (Form CMS-2552-96 or Form CMS-2552-10, respectively), as applicable each year. Section 1833(t)(7)(I) of the Act exempts TOPs from budget neutrality calculations.
Section 3138 of the Affordable Care Act amended section 1833(t) of the Act by adding a new paragraph (18), which instructs the Secretary to conduct a study to determine if, under the OPPS, outpatient costs incurred by cancer hospitals described in section 1886(d)(1)(B)(v) of the Act with respect to APC groups exceed outpatient costs incurred by other hospitals furnishing services under section 1833(t) of the Act, as determined appropriate by the Secretary. Section 1833(t)(18)(A) of the Act requires the Secretary to take into consideration the cost of drugs and biologicals incurred by cancer hospitals and other hospitals. Section 1833(t)(18)(B) of the Act provides that, if the Secretary determines that cancer hospitals' costs are higher than those of other hospitals, the Secretary shall provide an appropriate adjustment under section 1833(t)(2)(E) of the Act to reflect these higher costs. In 2011, after conducting the study required by section 1833(t)(18)(A) of the Act, we determined that outpatient costs incurred by the 11 specified cancer hospitals were greater than the costs incurred by other OPPS hospitals. For a complete discussion regarding the cancer hospital cost study, we refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74200 through 74201).
Based on these findings, we finalized a policy to provide a payment adjustment to the 11 specified cancer hospitals that reflects their higher outpatient costs, as discussed in the CY 2012 OPPS/ASC final rule with comment period (76 FR 74202 through 74206). Specifically, we adopted a policy to provide additional payments to the cancer hospitals so that each cancer hospital's final PCR for services provided in a given calendar year is equal to the weighted average PCR (which we refer to as the “target PCR”) for other hospitals paid under the OPPS. The target PCR is set in advance of the calendar year and is calculated using the most recently submitted or settled cost report data that are available at the time of final rulemaking for the calendar year. The amount of the payment adjustment is made on an aggregate basis at cost report settlement. We note that the changes made by section 1833(t)(18) of the Act do not affect the existing statutory provisions that provide for TOPs for cancer hospitals. The TOPs are assessed, as usual, after all payments, including the cancer hospital payment adjustment, have been made for a cost reporting period. For CYs 2012 and 2013, the target PCR for purposes of the cancer hospital payment adjustment was 0.91. For CY 2014, the target PCR was 0.90. For CY 2015, the target PCR was 0.90. For CY 2016, the target PCR was 0.92, as discussed in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70362 through 70363). For CY 2017, the target PCR was 0.91, as discussed in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79603 through 79604). For CY 2018, the target PCR was 0.88, as discussed in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59265 through 59266). For CY 2019, the target PCR was 0.88, as discussed in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58871 through 58873).
2. Proposed Policy for CY 2020
Section 16002(b) of the 21st Century Cures Act (Pub. L. 114-255) amended section 1833(t)(18) of the Act by adding subparagraph (C), which requires that in applying 42 CFR 419.43(i) (that is, the payment adjustment for certain cancer hospitals) for services furnished on or after January 1, 2018, the target PCR adjustment be reduced by 1.0 percentage point less than what would otherwise apply. Section 16002(b) also provides that, in addition to the percentage reduction, the Secretary may consider making an additional percentage point reduction to the target PCR that takes into account payment rates for applicable items and services described under section 1833(t)(21)(C) of the Act for hospitals that are not cancer hospitals described under section 1886(d)(1)(B)(v) of the Act. Further, in making any budget neutrality adjustment under section 1833(t) of the Act, the Secretary shall not take into account the reduced expenditures that result from application of section 1833(t)(18)(C) of the Act.
For CY 2020, we are proposing to provide additional payments to the 11 specified cancer hospitals so that each cancer hospital's final PCR is equal to the weighted average PCR (or “target PCR”) for the other OPPS hospitals, using the most recent submitted or settled cost report data that were available at the time of the development of this proposed rule, reduced by 1.0 percentage point, to comply with section 16002(b) of the 21st Century Cures Act.
We are not proposing an additional reduction beyond the 1.0 percentage point reduction required by section 16002(b) for CY 2020. To calculate the proposed CY 2020 target PCR, we are using the same extract of cost report data from HCRIS, as discussed in section II.A. of this proposed rule, used to estimate costs for the CY 2020 OPPS. Using these cost report data, we are including data from Worksheet E, Part B, for each hospital, using data from each hospital's most recent cost report, whether as submitted or settled.
We then limited the dataset to the hospitals with CY 2018 claims data that we used to model the impact of the proposed CY 2020 APC relative payment weights (3,770 hospitals) because it is appropriate to use the same set of hospitals that are being used to calibrate the modeled CY 2020 OPPS. The cost report data for the hospitals in this dataset were from cost report periods with fiscal year ends ranging from 2016 to 2018. We then removed the cost report data of the 49 hospitals located in Puerto Rico from our dataset because we did not believe their cost structure reflected the costs of most hospitals paid under the OPPS, and, therefore, their inclusion may bias the calculation of hospital-weighted statistics. We also removed the cost report data of 23 hospitals because these hospitals had cost report data that were not complete (missing aggregate OPPS payments, missing aggregate cost data, or missing both), so that all cost reports in the study would have both the payment and cost data necessary to calculate a PCR for each hospital, leading to a proposed analytic file of 3,539 hospitals with cost report data.
Using this smaller dataset of cost report data, we estimated that, on average, the OPPS payments to other hospitals furnishing services under the OPPS were approximately 90 percent of reasonable cost (weighted average PCR of 0.90). Therefore, after applying the 1.0 percentage point reduction, as required by section 16002(b) of the 21st Century Cures Act, we are proposing that the payment amount associated with the cancer hospital payment adjustment to be determined at cost report settlement would be the additional payment needed to result in a proposed target PCR equal to 0.89 for each cancer hospital.
Table 6 shows the estimated percentage increase in OPPS payments to each cancer hospital for CY 2020, due to the cancer hospital payment adjustment policy. The actual amount of the CY 2020 cancer hospital payment adjustment for each cancer hospital will be determined at cost report settlement and will depend on each hospital's CY 2020 payments and costs. We note that the requirements contained in section 1833(t)(18) of the Act do not affect the existing statutory provisions that provide for TOPs for cancer hospitals. The TOPs will be assessed, as usual, after all payments, including the cancer hospital payment adjustment, have been made for a cost reporting period.

G. Proposed Hospital Outpatient Outlier Payments
1. Background
The OPPS provides outlier payments to hospitals to help mitigate the financial risk associated with high-cost and complex procedures, where a very costly service could present a hospital with significant financial loss. As explained in the CY 2015 OPPS/ASC final rule with comment period (79 FR 66832 through 66834), we set our projected target for aggregate outlier payments at 1.0 percent of the estimated aggregate total payments under the OPPS for the prospective year. Outlier payments are provided on a service-by-service basis when the cost of a service exceeds the APC payment amount multiplier threshold (the APC payment amount multiplied by a certain amount) as well as the APC payment amount plus a fixed-dollar amount threshold (the APC payment plus a certain amount of dollars). In CY 2019, the outlier threshold was met when the hospital's cost of furnishing a service exceeded 1.75 times (the multiplier threshold) the APC payment amount and exceeded the APC payment amount plus $4,825 (the fixed-dollar amount threshold) (83 FR 58874 through 58875). If the cost of a service exceeds both the multiplier threshold and the fixed-dollar threshold, the outlier payment is calculated as 50 percent of the amount by which the cost of furnishing the service exceeds 1.75 times the APC payment amount. Beginning with CY 2009 payments, outlier payments are subject to a reconciliation process similar to the IPPS outlier reconciliation process for cost reports, as discussed in the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594 through 68599).
It has been our policy to report the actual amount of outlier payments as a percent of total spending in the claims being used to model the OPPS. Our estimate of total outlier payments as a percent of total CY 2018 OPPS payments, using CY 2018 claims available for this CY 2020 OPPS/ASC proposed rule, is approximately 1.0 percent of the total aggregated OPPS payments. Therefore, for CY 2018, we estimated that we paid the outlier target of 1.0 percent of total aggregated OPPS payments. Using an updated claims dataset for this CY 2020 OPPS proposed rule, we estimate that we paid approximately 1.03 percent of the total aggregated OPPS payments in outliers for CY 2018.
For this CY 2020 OPPS/ASC proposed rule, using CY 2018 claims data and CY 2019 payment rates, we estimate that the aggregate outlier payments for CY 2019 would be approximately 1.03 percent of the total CY 2019 OPPS payments. We are providing estimated CY 2020 outlier payments for hospitals and CMHCs with claims included in the claims data that we used to model impacts in the Hospital—Specific Impacts—Provider-Specific Data file on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
2. Proposed Outlier Calculation for CY 2020
For CY 2020, we are proposing to continue our policy of estimating outlier payments to be 1.0 percent of the estimated aggregate total payments under the OPPS. We are proposing that a portion of that 1.0 percent, an amount equal to less than 0.01 percent of outlier payments (or 0.0001 percent of total OPPS payments), would be allocated to CMHCs for PHP outlier payments. This is the amount of estimated outlier payments that would result from the proposed CMHC outlier threshold as a proportion of total estimated OPPS outlier payments. As discussed in section VIII.C. of this proposed rule, we are proposing to continue our longstanding policy that if a CMHC's cost for partial hospitalization services, paid under APC 5853 (Partial Hospitalization for CMHCs), exceeds 3.40 times the payment rate for proposed APC 5853, the outlier payment would be calculated as 50 percent of the amount by which the cost exceeds 3.40 times the proposed APC 5853 payment rate.
For further discussion of CMHC outlier payments, we refer readers to section VIII.C. of this proposed rule.
To ensure that the estimated CY 2020 aggregate outlier payments would equal 1.0 percent of estimated aggregate total payments under the OPPS, we are proposing that the hospital outlier threshold be set so that outlier payments would be triggered when a hospital's cost of furnishing a service exceeds 1.75 times the APC payment amount and exceeds the APC payment amount plus $4,950.
We calculated the proposed fixed-dollar threshold of $4,950 using the standard methodology most recently used for CY 2019 (83 FR 58874 through 58875). For purposes of estimating outlier payments for the proposed rule, we are using the hospital-specific overall ancillary CCRs available in the April 2019 update to the Outpatient Provider-Specific File (OPSF). The OPSF contains provider-specific data, such as the most current CCRs, which are maintained by the MACs and used by the OPPS Pricer to pay claims. The claims that we use to model each OPPS update lag by 2 years.
In order to estimate the CY 2020 hospital outlier payments for this proposed rule, we inflate the charges on the CY 2018 claims using the same inflation factor of 1.11189 that we used to estimate the proposed IPPS fixed-dollar outlier threshold for the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19596). We used an inflation factor of 1.05446 to estimate CY 2019 charges from the CY 2018 charges reported on CY 2018 claims. The methodology for determining this charge inflation factor is discussed in the FY 2019 IPPS/LTCH PPS final rule (83 FR 41717 through 41718). As we stated in the CY 2005 OPPS final rule with comment period (69 FR 65845), we believe that the use of these charge inflation factors is appropriate for the OPPS because, with the exception of the inpatient routine service cost centers, hospitals use the same ancillary and outpatient cost centers to capture costs and charges for inpatient and outpatient services.
As noted in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68011), we are concerned that we could systematically overestimate the OPPS hospital outlier threshold if we do not apply a CCR inflation adjustment factor. Therefore, we are proposing to apply the same CCR inflation adjustment factor that we proposed to apply for the FY 2020 IPPS outlier calculation to the CCRs used to simulate the proposed CY 2020 OPPS outlier payments to determine the fixed-dollar threshold. Specifically, for CY 2020, we are proposing to apply an adjustment factor of 0.97517 to the CCRs that were in the April 2019 OPSF to trend them forward from CY 2019 to CY 2020. The methodology for calculating the proposed adjustment is discussed in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19597).
To model hospital outlier payments for the proposed rule, we are applying the overall CCRs from the April 2019 OPSF after adjustment (using the proposed CCR inflation adjustment factor of 0.97517 to approximate CY 2020 CCRs) to charges on CY 2018 claims that were adjusted (using the proposed charge inflation factor of 1.11189 to approximate CY 2020 charges). We simulated aggregated CY 2020 hospital outlier payments using these costs for several different fixed-dollar thresholds, holding the 1.75 multiplier threshold constant and assuming that outlier payments would continue to be made at 50 percent of the amount by which the cost of furnishing the service would exceed 1.75 times the APC payment amount, until the total outlier payments equaled 1.0 percent of aggregated estimated total CY 2020 OPPS payments. We are estimating that a proposed fixed-dollar threshold of $4,950, combined with the proposed multiplier threshold of 1.75 times the APC payment rate, would allocate 1.0 percent of aggregated total OPPS payments to outlier payments. For CMHCs, we are proposing that, if a CMHC's cost for partial hospitalization services, paid under APC 5853, exceeds 3.40 times the payment rate for APC 5853, the outlier payment would be calculated as 50 percent of the amount by which the cost exceeds 3.40 times the APC 5853 payment rate.
Section 1833(t)(17)(A) of the Act, which applies to hospitals, as defined under section 1886(d)(1)(B) of the Act, requires that hospitals that fail to report data required for the quality measures selected by the Secretary, in the form and manner required by the Secretary under section 1833(t)(17)(B) of the Act, incur a 2.0 percentage point reduction to their OPD fee schedule increase factor; that is, the annual payment update factor. The application of a reduced OPD fee schedule increase factor results in reduced national unadjusted payment rates that will apply to certain outpatient items and services furnished by hospitals that are required to report outpatient quality data and that fail to meet the Hospital OQR Program requirements. For hospitals that fail to meet the Hospital OQR Program requirements, we are continuing the policy that we implemented in CY 2010 that the hospitals' costs will be compared to the reduced payments for purposes of outlier eligibility and payment calculation. For more information on the Hospital OQR Program, we referred readers to section XIV. of this proposed rule.
H. Proposed Calculation of an Adjusted Medicare Payment From the National Unadjusted Medicare Payment
The basic methodology for determining prospective payment rates for HOPD services under the OPPS is set forth in existing regulations at 42 CFR part 419, subparts C and D. For this CY 2020 OPPS/ASC proposed rule, the payment rate for most services and procedures for which payment is made under the OPPS is the product of the conversion factor calculated in accordance with section II.B. of this proposed rule and the relative payment weight determined under section II.A. of this proposed rule. Therefore, the proposed national unadjusted payment rate for most APCs contained in Addendum A to this proposed rule (which is available via the internet on the CMS website) and for most HCPCS codes to which separate payment under the OPPS has been assigned in Addendum B to this proposed rule (which is available via the internet on the CMS website) was calculated by multiplying the proposed CY 2020 scaled weight for the APC by the proposed CY 2020 conversion factor.
We note that section 1833(t)(17) of the Act, which applies to hospitals, as defined under section 1886(d)(1)(B) of the Act, requires that hospitals that fail to submit data required to be submitted on quality measures selected by the Secretary, in the form and manner and at a time specified by the Secretary, incur a reduction of 2.0 percentage points to their OPD fee schedule increase factor, that is, the annual payment update factor. The application of a reduced OPD fee schedule increase factor results in reduced national unadjusted payment rates that apply to certain outpatient items and services provided by hospitals that are required to report outpatient quality data and that fail to meet the Hospital OQR Program (formerly referred to as the Hospital Outpatient Quality Data Reporting Program (HOP QDRP)) requirements. For further discussion of the payment reduction for hospitals that fail to meet the requirements of the Hospital OQR Program, we refer readers to section XIV. of this proposed rule.
Below we demonstrate the steps used to determine the APC payments that will be made in a calendar year under the OPPS to a hospital that fulfills the Hospital OQR Program requirements and to a hospital that fails to meet the Hospital OQR Program requirements for a service that has any of the following status indicator assignments: “J1”, “J2”, “P”, “Q1”, “Q2”, “Q3”, “Q4”, “R”, “S”, “T”, “U”, or “V” (as defined in Addendum D1 to this proposed rule, which is available via the internet on the CMS website), in a circumstance in which the multiple procedure discount does not apply, the procedure is not bilateral, and conditionally packaged services (status indicator of “Q1” and “Q2”) qualify for separate payment. We note that, although blood and blood products with status indicator “R” and brachytherapy sources with status indicator “U” are not subject to wage adjustment, they are subject to reduced payments when a hospital fails to meet the Hospital OQR Program requirements.
Individual providers interested in calculating the payment amount that they will receive for a specific service from the national unadjusted payment rates presented in Addenda A and B to this proposed rule (which are available via the internet on the CMS website) should follow the formulas presented in the following steps. For purposes of the payment calculations below, we refer to the national unadjusted payment rate for hospitals that meet the requirements of the Hospital OQR Program as the “full” national unadjusted payment rate. We refer to the national unadjusted payment rate for hospitals that fail to meet the requirements of the Hospital OQR Program as the “reduced” national unadjusted payment rate. The reduced national unadjusted payment rate is calculated by multiplying the reporting ratio of 0.980 times the “full” national unadjusted payment rate. The national unadjusted payment rate used in the calculations below is either the full national unadjusted payment rate or the reduced national unadjusted payment rate, depending on whether the hospital met its Hospital OQR Program requirements in order to receive the full CY 2020 OPPS fee schedule increase factor.
Step 1. Calculate 60 percent (the labor-related portion) of the national unadjusted payment rate. Since the initial implementation of the OPPS, we have used 60 percent to represent our estimate of that portion of costs attributable, on average, to labor. We refer readers to the April 7, 2000 OPPS final rule with comment period (65 FR 18496 through 18497) for a detailed discussion of how we derived this percentage. During our regression analysis for the payment adjustment for rural hospitals in the CY 2006 OPPS final rule with comment period (70 FR 68553), we confirmed that this labor-related share for hospital outpatient services is appropriate.
The formula below is a mathematical representation of Step 1 and identifies the labor-related portion of a specific payment rate for a specific service.
X is the labor-related portion of the national unadjusted payment rate.
X = .60 * (national unadjusted payment rate).
Step 2. Determine the wage index area in which the hospital is located and identify the wage index level that applies to the specific hospital. We note that, under the proposed CY 2020 OPPS policy for continuing to use the OMB labor market area delineations based on the 2010 Decennial Census data for the wage indexes used under the IPPS, a hold harmless policy for the wage index may apply, as discussed in section II.C. of this proposed rule. The wage index values assigned to each area reflect the geographic statistical areas (which are based upon OMB standards) to which hospitals are assigned for FY 2020 under the IPPS, reclassifications through the Medicare Geographic Classification Review Board (MGCRB), section 1886(d)(8)(B) “Lugar” hospitals, reclassifications under section 1886(d)(8)(E) of the Act, as defined in § 412.103 of the regulations, and hospitals designated as urban under section 601(g) of Public Law 98-21. For further discussion of the proposed changes to the FY 2020 IPPS wage indexes, as applied to the CY 2020 OPPS, we refer readers to section II.C. of this proposed rule. We are proposing to continue to apply a wage index floor of 1.00 to frontier States, in accordance with section 10324 of the Affordable Care Act of 2010.
Step 3. Adjust the wage index of hospitals located in certain qualifying counties that have a relatively high percentage of hospital employees who reside in the county, but who work in a different county with a higher wage index, in accordance with section 505 of Public Law 108-173. Addendum L to this proposed rule (which is available via the internet on the CMS website) contains the qualifying counties and the associated wage index increase developed for the proposed FY 2020 IPPS, which are listed in Table 2 associated with the FY 2020 IPPS/LTCH PPS proposed rule and available via the internet on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html. (Click on the link on the left side of the screen titled “FY 2020 IPPS Proposed Rule Home Page” and select “FY 2020 Proposed Rule Tables.”) This step is to be followed only if the hospital is not reclassified or redesignated under section 1886(d)(8) or section 1886(d)(10) of the Act.
Step 4. Multiply the applicable wage index determined under Steps 2 and 3 by the amount determined under Step 1 that represents the labor-related portion of the national unadjusted payment rate.
The formula below is a mathematical representation of Step 4 and adjusts the labor-related portion of the national unadjusted payment rate for the specific service by the wage index.
Xais the labor-related portion of the national unadjusted payment rate (wage adjusted).
Xa = .60 * (national unadjusted payment rate) * applicable wage index.
Step 5. Calculate 40 percent (the nonlabor-related portion) of the national unadjusted payment rate and add that amount to the resulting product of Step 4. The result is the wage index adjusted payment rate for the relevant wage index area.
The formula below is a mathematical representation of Step 5 and calculates the remaining portion of the national payment rate, the amount not attributable to labor, and the adjusted payment for the specific service.
Y is the nonlabor-related portion of the national unadjusted payment rate.
Y = .40 * (national unadjusted payment rate).
Adjusted Medicare Payment = Y + Xa.
Step 6. If a provider is an SCH, as set forth in the regulations at § 412.92, or an EACH, which is considered to be an SCH under section 1886(d)(5)(D)(iii)(III) of the Act, and located in a rural area, as defined in § 412.64(b), or is treated as being located in a rural area under § 412.103, multiply the wage index adjusted payment rate by 1.071 to calculate the total payment.
The formula below is a mathematical representation of Step 6 and applies the rural adjustment for rural SCHs.
Adjusted Medicare Payment (SCH or EACH) = Adjusted Medicare Payment * 1.071.
We are providing examples below of the calculation of both the full and reduced national unadjusted payment rates that will apply to certain outpatient items and services performed by hospitals that meet and that fail to meet the Hospital OQR Program requirements, using the steps outlined above. For purposes of this example, we are using a provider that is located in Brooklyn, New York that is assigned to CBSA 35614. This provider bills one service that is assigned to APC 5071 (Level 1 Excision/Biopsy/Incision and Drainage). The proposed CY 2020 full national unadjusted payment rate for APC 5071 is approximately $617.00. The proposed reduced national unadjusted payment rate for APC 5071 for a hospital that fails to meet the Hospital OQR Program requirements is approximately $604.66. This reduced rate is calculated by multiplying the reporting ratio of 0.980 by the full unadjusted payment rate for APC 5071.
The proposed FY 2020 wage index for a provider located in CBSA 35614 in New York, which includes the proposed adoption of IPPS 2020 wage index policies, is 1.2747. The labor-related portion of the proposed full national unadjusted payment is approximately $471.89 (.60 * $617.00 * 1.2747). The labor-related portion of the proposed reduced national unadjusted payment is approximately $462.46 (.60 * 604.66 * 1.2747). The nonlabor-related portion of the proposed full national unadjusted payment is approximately $246.80 (.40 * $617.00). The nonlabor-related portion of the proposed reduced national unadjusted payment is approximately $241.86. (.40 * $604.66). The sum of the labor-related and nonlabor-related portions of the proposed full national adjusted payment is approximately $718.69 ($471.89. + $246.80). The sum of the portions of the proposed reduced national adjusted payment is approximately $704.32 ($462.46. + $241.86).
I. Proposed Beneficiary Copayments
1. Background
Section 1833(t)(3)(B) of the Act requires the Secretary to set rules for determining the unadjusted copayment amounts to be paid by beneficiaries for covered OPD services. Section 1833(t)(8)(C)(ii) of the Act specifies that the Secretary must reduce the national unadjusted copayment amount for a covered OPD service (or group of such services) furnished in a year in a manner so that the effective copayment rate (determined on a national unadjusted basis) for that service in the year does not exceed a specified percentage. As specified in section 1833(t)(8)(C)(ii)(V) of the Act, the effective copayment rate for a covered OPD service paid under the OPPS in CY 2006, and in calendar years thereafter, shall not exceed 40 percent of the APC payment rate.
Section 1833(t)(3)(B)(ii) of the Act provides that, for a covered OPD service (or group of such services) furnished in a year, the national unadjusted copayment amount cannot be less than 20 percent of the OPD fee schedule amount. However, section 1833(t)(8)(C)(i) of the Act limits the amount of beneficiary copayment that may be collected for a procedure (including items such as drugs and biologicals) performed in a year to the amount of the inpatient hospital deductible for that year.
Section 4104 of the Affordable Care Act eliminated the Medicare Part B coinsurance for preventive services furnished on and after January 1, 2011, that meet certain requirements, including flexible sigmoidoscopies and screening colonoscopies, and waived the Part B deductible for screening colonoscopies that become diagnostic during the procedure. Our discussion of the changes made by the Affordable Care Act with regard to copayments for preventive services furnished on and after January 1, 2011, may be found in section XII.B. of the CY 2011 OPPS/ASC final rule with comment period (75 FR 72013).
2. Proposed OPPS Copayment Policy
For CY 2020, we are proposing to determine copayment amounts for new and revised APCs using the same methodology that we implemented beginning in CY 2004. (We refer readers to the November 7, 2003 OPPS final rule with comment period (68 FR 63458).) In addition, we are proposing to use the same standard rounding principles that we have historically used in instances where the application of our standard copayment methodology would result in a copayment amount that is less than 20 percent and cannot be rounded, under standard rounding principles, to 20 percent. (We refer readers to the CY 2008 OPPS/ASC final rule with comment period (72 FR 66687) in which we discussed our rationale for applying these rounding principles.) The proposed national unadjusted copayment amounts for services payable under the OPPS that would be effective January 1, 2020 are included in Addenda A and B to this proposed rule (which are available via the internet on the CMS website).
As discussed in section XIV.E. of this proposed rule, for CY 2020, the proposed Medicare beneficiary's minimum unadjusted copayment and national unadjusted copayment for a service to which a reduced national unadjusted payment rate applies will equal the product of the reporting ratio and the national unadjusted copayment, or the product of the reporting ratio and the minimum unadjusted copayment, respectively, for the service.
We note that OPPS copayments may increase or decrease each year based on changes in the calculated APC payment rates, due to updated cost report and claims data, and any changes to the OPPS cost modeling process. However, as described in the CY 2004 OPPS final rule with comment period, the development of the copayment methodology generally moves beneficiary copayments closer to 20 percent of OPPS APC payments (68 FR 63458 through 63459).
In the CY 2004 OPPS final rule with comment period (68 FR 63459), we adopted a new methodology to calculate unadjusted copayment amounts in situations including reorganizing APCs, and we finalized the following rules to determine copayment amounts in CY 2004 and subsequent years.
- When an APC group consists solely of HCPCS codes that were not paid under the OPPS the prior year because they were packaged or excluded or are new codes, the unadjusted copayment amount would be 20 percent of the APC payment rate.
- If a new APC that did not exist during the prior year is created and consists of HCPCS codes previously assigned to other APCs, the copayment amount is calculated as the product of the APC payment rate and the lowest coinsurance percentage of the codes comprising the new APC.
- If no codes are added to or removed from an APC and, after recalibration of its relative payment weight, the new payment rate is equal to or greater than the prior year's rate, the copayment amount remains constant (unless the resulting coinsurance percentage is less than 20 percent).
- If no codes are added to or removed from an APC and, after recalibration of its relative payment weight, the new payment rate is less than the prior year's rate, the copayment amount is calculated as the product of the new payment rate and the prior year's coinsurance percentage.
- If HCPCS codes are added to or deleted from an APC and, after recalibrating its relative payment weight, holding its unadjusted copayment amount constant results in a decrease in the coinsurance percentage for the reconfigured APC, the copayment amount would not change (unless retaining the copayment amount would result in a coinsurance rate less than 20 percent).
- If HCPCS codes are added to an APC and, after recalibrating its relative payment weight, holding its unadjusted copayment amount constant results in an increase in the coinsurance percentage for the reconfigured APC, the copayment amount would be calculated as the product of the payment rate of the reconfigured APC and the lowest coinsurance percentage of the codes being added to the reconfigured APC.
We noted in the CY 2004 OPPS final rule with comment period that we would seek to lower the copayment percentage for a service in an APC from the prior year if the copayment percentage was greater than 20 percent. We noted that this principle was consistent with section 1833(t)(8)(C)(ii) of the Act, which accelerates the reduction in the national unadjusted coinsurance rate so that beneficiary liability will eventually equal 20 percent of the OPPS payment rate for all OPPS services to which a copayment applies, and with section 1833(t)(3)(B) of the Act, which achieves a 20-percent copayment percentage when fully phased in and gives the Secretary the authority to set rules for determining copayment amounts for new services. We further noted that the use of this methodology would, in general, reduce the beneficiary coinsurance rate and copayment amount for APCs for which the payment rate changes as the result of the reconfiguration of APCs and/or recalibration of relative payment weights (68 FR 63459).
3. Proposed Calculation of an Adjusted Copayment Amount for an APC Group
Individuals interested in calculating the national copayment liability for a Medicare beneficiary for a given service provided by a hospital that met or failed to meet its Hospital OQR Program requirements should follow the formulas presented in the following steps.
Step 1. Calculate the beneficiary payment percentage for the APC by dividing the APC's national unadjusted copayment by its payment rate. For example, using APC 5071, $617.00 is approximately 20 percent of the proposed full national unadjusted payment rate of $123.40. For APCs with only a minimum unadjusted copayment in Addenda A and B to this proposed rule (which are available via the internet on the CMS website), the beneficiary payment percentage is 20 percent.
The formula below is a mathematical representation of Step 1 and calculates the national copayment as a percentage of national payment for a given service.
B is the beneficiary payment percentage.
B = National unadjusted copayment for APC/national unadjusted payment rate for APC.
Step 2. Calculate the appropriate wage-adjusted payment rate for the APC for the provider in question, as indicated in Steps 2 through 4 under section II.H. of this proposed rule. Calculate the rural adjustment for eligible providers, as indicated in Step 6 under section II.H. of this proposed rule.
Step 3. Multiply the percentage calculated in Step 1 by the payment rate calculated in Step 2. The result is the wage-adjusted copayment amount for the APC.
The formula below is a mathematical representation of Step 3 and applies the beneficiary payment percentage to the adjusted payment rate for a service calculated under section II.H. of this proposed rule, with and without the rural adjustment, to calculate the adjusted beneficiary copayment for a given service.
Wage-adjusted copayment amount for the APC = Adjusted Medicare Payment * B.
Wage-adjusted copayment amount for the APC (SCH or EACH) = (Adjusted Medicare Payment * 1.071) * B.
Step 4. For a hospital that failed to meet its Hospital OQR Program requirements, multiply the copayment calculated in Step 3 by the reporting ratio of 0.980.
The proposed unadjusted copayments for services payable under the OPPS that would be effective January 1, 2020, are shown in Addenda A and B to this proposed rule (which are available via the internet on the CMS website). We note that the proposed national unadjusted payment rates and copayment rates shown in Addenda A and B to this proposed rule reflect the proposed CY 2020 OPD fee schedule increase factor discussed in section II.B. of this proposed rule.
In addition, as noted earlier, section 1833(t)(8)(C)(i) of the Act limits the amount of beneficiary copayment that may be collected for a procedure performed in a year to the amount of the inpatient hospital deductible for that year.
III. Proposed OPPS Ambulatory Payment Classification (APC) Group Policies
A. Proposed OPPS Treatment of New and Revised HCPCS Codes
Payment for OPPS procedures, services, and items are generally based on medical billing codes, specifically, HCPCS codes, that are reported on HOPD claims. The HCPCS is divided into two principal subsystems, referred to as Level I and Level II of the HCPCS. Level I is comprised of CPT (Current Procedural Terminology), a numeric and alphanumeric coding system maintained by the American Medical Association (AMA), and consist of Category I, II, and III CPT codes. Level II, which is maintained by CMS, is a standardized coding system that is used primarily to identify products, supplies, and services not included in the CPT codes. HCPCS codes are used to report surgical procedures, medical services, items, and supplies under the hospital OPPS. Specifically, CMS recognizes the following codes on OPPS claims:
- Category I CPT codes, which describe surgical procedures, diagnostic and therapeutic services, and vaccine codes;
- Category III CPT codes, which describe new and emerging technologies, services, and procedures; and
- Level II HCPCS codes (also known as alphanumeric codes), which are used primarily to identify drugs, devices, ambulance services, durable medical equipment, orthotics, prosthetics, supplies, temporary surgical procedures, and medical services not described by CPT codes.
CPT codes are established by the American Medical Association (AMA) while the Level II HCPCS codes are established by the CMS HCPCS Workgroup. These codes are updated and changed throughout the year. CPT and Level II HCPCS code changes that affect the OPPS are published through the annual rulemaking cycle and through the OPPS quarterly update Change Requests (CRs). Generally, these code changes are effective January 1, April 1, July 1, or October 1. CPT code changes are released by the AMA while Level II HCPCS code changes are released to the public via the CMS HCPCS website. CMS recognizes the release of new CPT and Level II HCPCS codes and makes the codes effective (that is, the codes can be reported on Medicare claims) outside of the formal rulemaking process via OPPS quarterly update CRs. Based on our review, we assign the new codes to interim status indicators (SIs) and APCs. These interim assignments are finalized in the OPPS/ASC final rules. This quarterly process offers hospitals access to codes that more accurately describe items or services furnished and provides payment for these items or services in a timelier manner than if we waited for the annual rulemaking process. We solicit public comments on the new CPT and Level II HCPCS codes and finalize our proposals through our annual rulemaking process.
We note that, under the OPPS, the APC assignment determines the payment rate for an item, procedure, or service. Those items, procedures, or services not paid separately under the hospital OPPS are assigned to appropriate status indicators. Certain payment status indicators provide separate payment while other payment status indicators do not. In section XI. of this proposed rule (Proposed CY 2020 OPPS Payment Status and Comment Indicators), we discuss the various status indicators used under the OPPS. We also provide a complete list of the status indicators and their definitions in Addendum D1 to this CY 2020 OPPS/ASC proposed rule.
1. April 2019 Codes for Which We Are Soliciting Public Comments in This Proposed Rule
For the April 2019 update, there were no new CPT codes. However, eight new Level II HCPCS codes were established and made effective on April 1, 2019. These codes and their long descriptors are listed in Table 7. Through the April 2019 OPPS quarterly update CR (Transmittal 4255, Change Request 11216, dated March 15, 2019), we recognized several new Level II HCPCS codes for separate payment under the OPPS. In this CY 2020 OPPS/ASC proposed rule, we are soliciting public comments on the proposed APC and status indicator assignments for the codes listed Table 7. The proposed status indicator, APC assignment, and payment rate for each HCPCS code can be found in Addendum B to this proposed rule. The complete list of status indicators and corresponding definitions used under the OPPS can be found in Addendum D1 to this proposed rule. These new codes that are effective April 1, 2019 are assigned to comment indicator “NP” in Addendum B to this proposed rule to indicate that the codes are assigned to an interim APC assignment and that comments will be accepted on their interim APC assignments. Also, the complete list of comment indicators and definitions used under the OPPS can be found in Addendum D2 to this proposed rule. We note that OPPS Addendum B, Addendum D1, and Addendum D2 are available via the internet on the CMS website.

2. July 2019 HCPCS Codes for Which We Are Soliciting Public Comments in This Proposed Rule
For the July 2019 update, 58 new codes were established and made effective July 1, 2019. The codes and long descriptors are listed in Table 8. Through the July 2019 OPPS quarterly update CR (Transmittal 4313, Change Request 11318, dated May 24, 2019), we recognized several new codes for separate payment and assigned them to appropriate interim OPPS status indicators and APCs. In this CY 2020 OPPS/ASC proposed rule, we are soliciting public comments on the proposed APC and status indicator assignments for the codes implemented on July 1, 2019, all of which are listed in Table 8. The proposed status indicator, APC assignment, and payment rate for each HCPCS code can be found in Addendum B to this proposed rule. The complete list of status indicators and corresponding definitions used under the OPPS can be found in Addendum D1 to this proposed rule. These new codes that are effective July 1, 2019 are assigned to comment indicator “NP” in Addendum B to this proposed rule to indicate that the codes are assigned to an interim APC assignment and that comments will be accepted on their interim APC assignments. Also, the complete list of comment indicators and definitions used under the OPPS can be found in Addendum D2 to this proposed rule. We note that OPPS Addendum B, Addendum D1, and Addendum D2 are available via the internet on the CMS website.

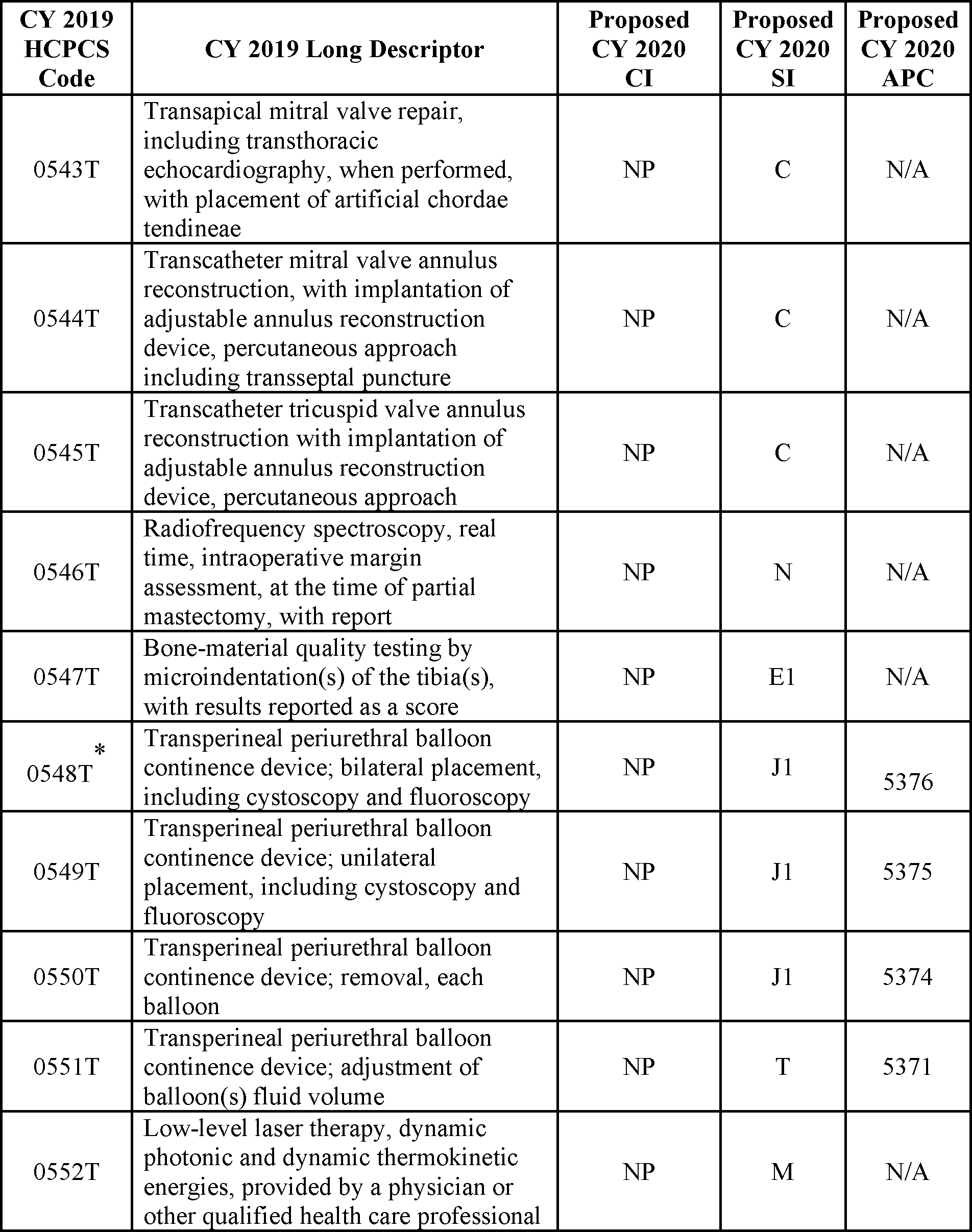







3. October 2019 HCPCS Codes for Which We Will Be Soliciting Public Comments in the CY 2020 OPPS/ASC Final Rule With Comment Period
As has been our practice in the past, we will solicit comments on the new CPT and Level II HCPCS codes that will be effective October 1, 2019 in the CY 2020 OPPS/ASC final rule with comment period, thereby allowing us to finalize the status indicators and APC assignments for the codes in the CY 2021 OPPS/ASC final rule with comment period. The Level II HCPCS codes will be released to the public through the October 2019 OPPS Update CR and the CMS HCPCS website while the CPT codes will be released to the public through the AMA website.
For CY 2020, we are proposing to continue our established policy of assigning comment indicator “NI” in Addendum B to the OPPS/ASC final rule with comment period to those new HCPCS codes that are effective October 1, 2019 to indicate that we are assigning them an interim status indicator, which is subject to public comment. We will be inviting public comments in the CY 2020 OPPS/ASC final rule with comment period on the status indicator and APC assignments, which would then be finalized in the CY 2021 OPPS/ASC final rule with comment period.
4. January 2020 HCPCS Codes
a. New Level II HCPCS Codes for Which We Will Be Soliciting Public Comments in the CY 2020 OPPS/ASC Final Rule With Comment Period
Consistent with past practice, we will solicit comments on the new Level II HCPCS codes that will be effective January 1, 2020 in the CY 2020 OPPS/ASC final rule with comment period, thereby allowing us to finalize the status indicators and APC assignments for the codes in the CY 2021 OPPS/ASC final rule with comment period. Unlike the CPT codes that are effective January 1 and are included in the OPPS/ASC proposed rules, and except for the G-codes listed in Addendum O of this proposed rule, most Level II HCPCS codes are not released until sometime around November to be effective January 1. Because these codes are not available until November, we are unable to include them in the OPPS/ASC proposed rules. Therefore, these Level II HCPCS codes will be released to the public through the CY 2020 OPPS/ASC final rule with comment period, January 2020 OPPS Update CR, and the CMS HCPCS website.
For CY 2020, we are proposing to continue our established policy of assigning comment indicator “NI” in Addendum B to the OPPS/ASC final rule with comment period to the new HCPCS codes that will be effective January 1, 2020 to indicate that we are assigning them an interim status indicator, which is subject to public comment. We will be inviting public comments in the CY 2020 OPPS/ASC final rule with comment period on the status indicator and APC assignments, which would then be finalized in the CY 2021 OPPS/ASC final rule with comment period.
b. CPT Codes for Which We Are Soliciting Public Comments in This Proposed Rule
In the CY 2015 OPPS/ASC final rule with comment period (79 FR 66841 through 66844), we finalized a revised process of assigning APC and status indicators for new and revised Category I and III CPT codes that would be effective January 1. Specifically, for the new/revised CPT codes that we receive in a timely manner from the AMA's CPT Editorial Panel, we finalized our proposal to include the codes that would be effective January 1 in the OPPS/ASC proposed rules, along with proposed APC and status indicator assignments for them, and to finalize the APC and status indicator assignments in the OPPS/ASC final rules beginning with the CY 2016 OPPS update. For those new/revised CPT codes that were received too late for inclusion in the OPPS/ASC proposed rule, we finalized our proposal to establish and use HCPCS G-codes that mirror the predecessor CPT codes and retain the current APC and status indicator assignments for a year until we can propose APC and status indicator assignments in the following year's rulemaking cycle. We note that even if we find that we need to create HCPCS G-codes in place of certain CPT codes for the PFS proposed rule, we do not anticipate that these HCPCS G-codes will always be necessary for OPPS purposes. We will make every effort to include proposed APC and status indicator assignments for all new and revised CPT codes that the AMA makes publicly available in time for us to include them in the proposed rule, and to avoid the resort to HCPCS G-codes and the resulting delay in utilization of the most current CPT codes. Also, we finalized our proposal to make interim APC and status indicator assignments for CPT codes that are not available in time for the proposed rule and that describe wholly new services (such as new technologies or new surgical procedures), solicit public comments, and finalize the specific APC and status indicator assignments for those codes in the following year's final rule.
For the CY 2020 OPPS update, we received the CPT codes that will be effective January 1, 2020 from AMA in time to be included in this proposed rule. The new, revised, and deleted CPT codes can be found in Addendum B to this proposed rule (which is available via the internet on the CMS website). We note that the new and revised CPT codes are assigned to comment indicator “NP” in Addendum B of this proposed rule to indicate that the code is new for the next calendar year or the code is an existing code with substantial revision to its code descriptor in the next calendar year as compared to current calendar year with a proposed APC assignment, and that comments will be accepted on the proposed APC assignment and status indicator.
Further, we note that the CPT code descriptors that appear in Addendum B are short descriptors and do not accurately describe the complete procedure, service, or item described by the CPT code. Therefore, we are including the 5-digit placeholder codes and the long descriptors for the new and revised CY 2020 CPT codes in Addendum O to this proposed rule (which is available via the internet on the CMS website) so that the public can adequately comment on our proposed APCs and status indicator assignments. The 5-digit placeholder codes can be found in Addendum O, specifically under the column labeled “CY 2020 OPPS/ASC Proposed Rule 5-Digit AMA Placeholder Code”. The final CPT code numbers will be included in the CY 2020 OPPS/ASC final rule with comment period.
In summary, we are soliciting public comments on the proposed CY 2020 status indicators and APC assignments for the new and revised CPT codes that will be effective January 1, 2020. Because the CPT codes listed in Addendum B appear with short descriptors only, we list them again in Addendum O to this proposed rule with long descriptors. In addition, we are proposing to finalize the status indicator and APC assignments for these codes (with their final CPT code numbers) in the CY 2020 OPPS/ASC final rule with comment period. The proposed status indicator and APC assignment for these codes can be found in Addendum B to this proposed rule (which is available via the internet on the CMS website).
Finally, in Table 9, we summarize our current process for updating codes through our OPPS quarterly update CRs, seeking public comments, and finalizing the treatment of these codes under the OPPS.

B. Proposed OPPS Changes—Variations Within APCs
1. Background
Section 1833(t)(2)(A) of the Act requires the Secretary to develop a classification system for covered hospital outpatient department services. Section 1833(t)(2)(B) of the Act provides that the Secretary may establish groups of covered OPD services within this classification system, so that services classified within each group are comparable clinically and with respect to the use of resources. In accordance with these provisions, we developed a grouping classification system, referred to as Ambulatory Payment Classifications (APCs), as set forth in regulations at 42 CFR 419.31. We use Level I (also known as CPT codes) and Level II HCPCS codes (also known as alphanumeric codes) to identify and group the services within each APC. The APCs are organized such that each group is homogeneous both clinically and in terms of resource use. Using this classification system, we have established distinct groups of similar services. We also have developed separate APC groups for certain medical devices, drugs, biologicals, therapeutic radiopharmaceuticals, and brachytherapy devices that are not packaged into the payment for the procedure.
We have packaged into the payment for each procedure or service within an APC group the costs associated with those items and services that are typically ancillary and supportive to a primary diagnostic or therapeutic modality and, in those cases, are an integral part of the primary service they support. Therefore, we do not make separate payment for these packaged items or services. In general, packaged items and services include, but are not limited to, the items and services listed in regulations at 42 CFR 419.2(b). A further discussion of packaged services is included in section II.A.3. of this proposed rule.
Under the OPPS, we generally pay for covered hospital outpatient services on a rate-per-service basis, where the service may be reported with one or more HCPCS codes. Payment varies according to the APC group to which the independent service or combination of services is assigned. For CY 2020, we are proposing that each APC relative payment weight represents the hospital cost of the services included in that APC, relative to the hospital cost of the services included in APC 5012 (Clinic Visits and Related Services). The APC relative payment weights are scaled to APC 5012 because it is the hospital clinic visit APC and clinic visits are among the most frequently furnished services in the hospital outpatient setting.
2. Application of the 2 Times Rule
Section 1833(t)(9)(A) of the Act requires the Secretary to review, not less often than annually, and revise the APC groups, the relative payment weights, and the wage and other adjustments described in paragraph (2) to take into account changes in medical practice, changes in technology, the addition of new services, new cost data, and other relevant information and factors. Section 1833(t)(9)(A) of the Act also requires the Secretary to consult with an expert outside advisory panel composed of an appropriate selection of representatives of providers to review (and advise the Secretary concerning) the clinical integrity of the APC groups and the relative payment weights. We note that the HOP Panel recommendations for specific services for the CY 2020 OPPS update will be discussed in the relevant specific sections throughout the CY 2020 OPPS/ASC final rule with comment period.
In addition, section 1833(t)(2) of the Act provides that, subject to certain exceptions, the items and services within an APC group cannot be considered comparable with respect to the use of resources if the highest cost for an item or service in the group is more than 2 times greater than the lowest cost for an item or service within the same group (referred to as the “2 times rule”). The statute authorizes the Secretary to make exceptions to the 2 times rule in unusual cases, such as low-volume items and services (but the Secretary may not make such an exception in the case of a drug or biological that has been designated as an orphan drug under section 526 of the Federal Food, Drug, and Cosmetic Act). In determining the APCs with a 2 times rule violation, we consider only those HCPCS codes that are significant based on the number of claims. We note that, for purposes of identifying significant procedure codes for examination under the 2 times rule, we consider procedure codes that have more than 1,000 single major claims or procedure codes that both have more than 99 single major claims and contribute at least 2 percent of the single major claims used to establish the APC cost to be significant (75 FR 71832). This longstanding definition of when a procedure code is significant for purposes of the 2 times rule was selected because we believe that a subset of 1,000 or fewer claims is negligible within the set of approximately 100 million single procedure or single session claims we use for establishing costs. Similarly, a procedure code for which there are fewer than 99 single claims and that comprises less than 2 percent of the single major claims within an APC will have a negligible impact on the APC cost (75 FR 71832). In this section of this proposed rule, for CY 2020, we are proposing to make exceptions to this limit on the variation of costs within each APC group in unusual cases, such as for certain low-volume items and services.
For the CY 2020 OPPS update, we have identified the APCs with violations of the 2 times rule. Therefore, we are proposing changes to the procedure codes assigned to these APCs in Addendum B to this proposed rule. We note that Addendum B does not appear in the printed version of the Federal Register as part of this CY 2020 OPPS/ASC proposed rule. Rather, it is published and made available via the internet on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. To eliminate a violation of the 2 times rule and improve clinical and resource homogeneity, we are proposing to reassign these procedure codes to new APCs that contain services that are similar with regard to both their clinical and resource characteristics. In many cases, the proposed procedure code reassignments and associated APC reconfigurations for CY 2020 included in this proposed rule are related to changes in costs of services that were observed in the CY 2018 claims data newly available for CY 2020 ratesetting. Addendum B to this CY 2020 OPPS/ASC proposed rule identifies with a comment indicator “CH” those procedure codes for which we are proposing a change to the APC assignment or status indicator, or both, that were initially assigned in the July 1, 2019 OPPS Addendum B Update (available via the internet on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates.html).
3. Proposed APC Exceptions to the 2 Times Rule
Taking into account the APC changes that we are proposing to make for CY 2020, we reviewed all of the APCs to determine which APCs would not meet the requirements of the 2 times rule. We used the following criteria to evaluate whether to propose exceptions to the 2 times rule for affected APCs:
- Resource homogeneity;
- Clinical homogeneity;
- Hospital outpatient setting utilization;
- Frequency of service (volume); and
- Opportunity for upcoding and code fragments.
Based on the CY 2018 claims data available for this CY 2020 proposed rule, we found 18 APCs with violations of the 2 times rule. We applied the criteria as described above to identify the APCs for which we are proposing to make exceptions under the 2 times rule for CY 2020, and found that all of the 18 APCs we identified meet the criteria for an exception to the 2 times rule based on the CY 2018 claims data available for this proposed rule. We did not include in that determination those APCs where a 2 times rule violation was not a relevant concept, such as APC 5401 (Dialysis), which only has two HCPCS codes assigned to it that have a similar geometric mean costs and do not create a 2 time rule violation. Therefore, we have only identified those APCs, including those with criteria-based costs, such as device-dependent CPT/HCPCS codes, with violations of the 2 times rule.
We note that, for cases in which a recommendation by the HOP Panel appears to result in or allow a violation of the 2 times rule, we may accept the HOP Panel's recommendation because those recommendations are based on explicit consideration (that is, a review of the latest OPPS claims data and group discussion of the issue) of resource use, clinical homogeneity, site of service, and the quality of the claims data used to determine the APC payment rates.
Table 10 of this proposed rule lists the 18 APCs that we are proposing to make an exception for under the 2 times rule for CY 2020 based on the criteria cited above and claims data submitted between January 1, 2018, and December 31, 2018, and processed on or before December 31, 2018. For the final rule with comment period, we intend to use claims data for dates of service between January 1, 2018, and December 31, 2018, that were processed on or before June 30, 2019, and updated CCRs, if available. The proposed geometric mean costs for covered hospital outpatient services for these and all other APCs that were used in the development of this proposed rule can be found on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices.html.

C. Proposed New Technology APCs
1. Background
In the CY 2002 OPPS final rule (66 FR 59903), we finalized changes to the time period in which a service can be eligible for payment under a New Technology APC. Beginning in CY 2002, we retain services within New Technology APC groups until we gather sufficient claims data to enable us to assign the service to an appropriate clinical APC. This policy allows us to move a service from a New Technology APC in less than 2 years if sufficient data are available. It also allows us to retain a service in a New Technology APC for more than 2 years if sufficient data upon which to base a decision for reassignment have not been collected.
In the CY 2004 OPPS final rule with comment period (68 FR 63416), we restructured the New Technology APCs to make the cost intervals more consistent across payment levels and refined the cost bands for these APCs to retain two parallel sets of New Technology APCs, one set with a status indicator of “S” (Significant Procedures, Not Discounted when Multiple. Paid under OPPS; separate APC payment) and the other set with a status indicator of “T” (Significant Procedure, Multiple Reduction Applies. Paid under OPPS; separate APC payment). These current New Technology APC configurations allow us to price new technology services more appropriately and consistently.
For CY 2019, there were 52 New Technology APC levels, ranging from the lowest cost band assigned to APC 1491 (New Technology—Level 1A ($0-$10)) through the highest cost band assigned to APC 1908 (New Technology—Level 52 ($145,001-$160,000)). We note that the cost bands for the New Technology APCs, specifically, APCs 1491 through 1599 and 1901 through 1908, vary with increments ranging from $10 to $14,999. These cost bands identify the APCs to which new technology procedures and services with estimated service costs that fall within those cost bands are assigned under the OPPS. Payment for each APC is made at the mid-point of the APC's assigned cost band. For example, payment for New Technology APC 1507 (New Technology—Level 7 ($501-$600)) is made at $550.50.
Under the OPPS, one of our goals is to make payments that are appropriate for the services that are necessary for the treatment of Medicare beneficiaries. The OPPS, like other Medicare payment systems, is budget neutral and increases are limited to the annual hospital inpatient market basket increase adjusted for multifactor productivity. We believe that our payment rates generally reflect the costs that are associated with providing care to Medicare beneficiaries. Furthermore, we believe that our payment rates are adequate to ensure access to services (80 FR 70374).
For many emerging technologies, there is a transitional period during which utilization may be low, often because providers are first learning about the technologies and their clinical utility. Quite often, parties request that Medicare make higher payment amounts under the New Technology APCs for new procedures in that transitional phase. These requests, and their accompanying estimates for expected total patient utilization, often reflect very low rates of patient use of expensive equipment, resulting in high per-use costs for which requesters believe Medicare should make full payment. Medicare does not, and we believe should not, assume responsibility for more than its share of the costs of procedures based on projected utilization for Medicare beneficiaries and does not set its payment rates based on initial projections of low utilization for services that require expensive capital equipment. For the OPPS, we rely on hospitals to make informed business decisions regarding the acquisition of high-cost capital equipment, taking into consideration their knowledge about their entire patient base (Medicare beneficiaries included) and an understanding of Medicare's and other payers' payment policies. (We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68314) for further discussion regarding this payment policy.)
We note that, in a budget neutral system, payments may not fully cover hospitals' costs in a particular circumstance, including those for the purchase and maintenance of capital equipment. We rely on hospitals to make their decisions regarding the acquisition of high-cost equipment with the understanding that the Medicare program must be careful to establish its initial payment rates, including those made through New Technology APCs, for new services that lack hospital claims data based on realistic utilization projections for all such services delivered in cost-efficient hospital outpatient settings. As the OPPS acquires claims data regarding hospital costs associated with new procedures, we regularly examine the claims data and any available new information regarding the clinical aspects of new procedures to confirm that our OPPS payments remain appropriate for procedures as they transition into mainstream medical practice (77 FR 68314). For CY 2020, we are including the proposed payment rates for New Technology APCs 1491 through 1599 and 1901 through 1908 in Addendum A to this CY 2020 OPPS/ASC proposed rule (which is available via the internet on the CMS website).
2. Establishing Payment Rates for Low-Volume New Technology Procedures
Procedures that are assigned to New Technology APCs are typically new procedures that do not have sufficient claims history to establish an accurate payment for the procedures. One of the objectives of establishing New Technology APCs is to generate sufficient claims data for a new procedure so that it can be assigned to an appropriate clinical APC. Some procedures that are assigned to New Technology APCs have very low annual volume, which we consider to be fewer than 100 claims. We consider procedures with fewer than 100 claims annually as low-volume procedures because there is a higher probability that the payment data for a procedure may not have a normal statistical distribution, which could affect the quality of our standard cost methodology that is used to assign services to an APC. In addition, services with fewer than 100 claims per year are not generally considered to be a significant contributor to the APC ratesetting calculations and, therefore, are not included in the assessment of the 2 times rule. As we explained in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58890), we were concerned that the methodology we use to estimate the cost of a procedure under the OPPS by calculating the geometric mean for all separately paid claims for a HCPCS procedure code from the most recent available year of claims data may not generate an accurate estimate of the actual cost of the procedure for these low-volume procedures.
In accordance with section 1833(t)(2)(B) of the Act, services classified within each APC must be comparable clinically and with respect to the use of resources. As described earlier, assigning a procedure to a new technology APC allows us to gather claims data to price the procedure and assign it to the APC with services that use similar resources and are clinically comparable. However, where utilization of services assigned to a New Technology APC is low, it can lead to wide variation in payment rates from year to year, resulting in even lower utilization and potential barriers to access to new technologies, which ultimately limits our ability to assign the service to the appropriate clinical APC. To mitigate these issues, we determined in the CY 2019 OPPS/ASC final rule with comment period that it was appropriate to utilize our equitable adjustment authority at section 1833(t)(2)(E) of the Act to adjust how we determined the costs for low-volume services assigned to New Technology APCs (83 FR 58892 through 58893). We have utilized our equitable adjustment authority at section 1833(t)(2)(E) of the Act, which states that the Secretary shall establish, in a budget neutral manner, other adjustments as determined to be necessary to ensure equitable payments, to estimate an appropriate payment amount for low-volume new technology procedures in the past (82 FR 59281). Although we have used this adjustment authority on a case-by-case basis in the past, we stated in the CY 2019 OPPS/ASC final rule with comment period that we believe it is appropriate to adopt an adjustment for low-volume services assigned to New Technology APCs in order to mitigate the wide payment fluctuations that have occurred for new technology services with fewer than 100 claims and to provide more predictable payment for these services.
For purposes of this adjustment, we stated that we believe that it is appropriate to use up to 4 years of claims data in calculating the applicable payment rate for the prospective year, rather than using solely the most recent available year of claims data, when a service assigned to a New Technology APC has a low annual volume of claims, which, for purposes of this adjustment, we define as fewer than 100 claims annually. We adopted a policy to consider procedures with fewer than 100 claims annually as low-volume procedures because there is a higher probability that the payment data for a procedure may not have a normal statistical distribution, which could affect the quality of our standard cost methodology that is used to assign services to an APC. We explained that we were concerned that the methodology we use to estimate the cost of a procedure under the OPPS by calculating the geometric mean for all separately paid claims for a HCPCS procedure code from the most recent available year of claims data may not generate an accurate estimate of the actual cost of the low-volume procedure. Using multiple years of claims data will potentially allow for more than 100 claims to be used to set the payment rate, which would, in turn, create a more statistically reliable payment rate.
In addition, to better approximate the cost of a low-volume service within a New Technology APC, we stated that we believe using the median or arithmetic mean rather than the geometric mean (which “trims” the costs of certain claims out) could be more appropriate in some circumstances, given the extremely low volume of claims. Low claim volumes increase the impact of “outlier” claims; that is, claims with either a very low or very high payment rate as compared to the average claim, which would have a substantial impact on any statistical methodology used to estimate the most appropriate payment rate for a service. We also explained that we believe having the flexibility to utilize an alternative statistical methodology to calculate the payment rate in the case of low-volume new technology services would help to create a more stable payment rate. Therefore, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58893), we established that, in each of our annual rulemakings, we will seek public comments on which statistical methodology should be used for each low-volume service assigned to a New Technology APC. In the preamble of each annual rulemaking, we stated that we would present the result of each statistical methodology and solicit public comment on which methodology should be used to establish the payment rate for a low-volume new technology service. In addition, we will use our assessment of the resources used to perform a service and guidance from the developer or manufacturer of the service, as well as other stakeholders, to determine the most appropriate payment rate. Once we identify the most appropriate payment rate for a service, we will assign the service to the New Technology APC with the cost band that includes its payment rate.
Accordingly for CY 2020, we are proposing to continue our policy adopted in CY 2019 under which we will utilize our equitable adjustment authority under section 1833(t)(2)(E) of the Act to calculate the geometric mean, arithmetic mean, and median using multiple years of claims data to select the appropriate payment rate for purposes of assigning services with fewer than 100 claims per year to a New Technology APC. Additional details on our policy is available in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58892 through 58893).
3. Procedures Assigned to New Technology APC Groups for CY 2020
As we explained in the CY 2002 OPPS final rule with comment period (66 FR 59902), we generally retain a procedure in the New Technology APC to which it is initially assigned until we have obtained sufficient claims data to justify reassignment of the procedure to a clinically appropriate APC.
In addition, in cases where we find that our initial New Technology APC assignment was based on inaccurate or inadequate information (although it was the best information available at the time), where we obtain new information that was not available at the time of our initial New Technology APC assignment, or where the New Technology APCs are restructured, we may, based on more recent resource utilization information (including claims data) or the availability of refined New Technology APC cost bands, reassign the procedure or service to a different New Technology APC that more appropriately reflects its cost (66 FR 59903).
Consistent with our current policy, for CY 2020, in this proposed rule, we are proposing to retain services within New Technology APC groups until we obtain sufficient claims data to justify reassignment of the service to a clinically appropriate APC. The flexibility associated with this policy allows us to reassign a service from a New Technology APC in less than 2 years if sufficient claims data are available. It also allows us to retain a service in a New Technology APC for more than 2 years if sufficient claims data upon which to base a decision for reassignment have not been obtained (66 FR 59902).
a. Magnetic Resonance-Guided Focused Ultrasound Surgery (MRgFUS) (APCs 1575, 5114, and 5414)
Currently, there are four CPT/HCPCS codes that describe magnetic resonance image-guided, high-intensity focused ultrasound (MRgFUS) procedures, three of which we are proposing to continue to assign to standard APCs, and one that we are proposing to continue to assign to a New Technology APC for CY 2020. These codes include CPT codes 0071T, 0072T, and 0398T, and HCPCS code C9734. CPT codes 0071T and 0072T describe procedures for the treatment of uterine fibroids, CPT code 0398T describes procedures for the treatment of essential tremor, and HCPCS code C9734 describes procedures for pain palliation for metastatic bone cancer.
As shown in Table 11 of this CY 2020 OPPS/ASC proposed rule, and as listed in Addendum B to this CY 2020 OPPS/ASC proposed rule, we are proposing to continue to assign the procedures described by CPT codes 0071T and 0072T to APC 5414 (Level 4 Gynecologic Procedures) for CY 2020. We also are proposing to continue to assign the APC to status indicator “J1” (Hospital Part B services paid through a comprehensive APC). In addition, we are proposing to continue to assign the services described by HCPCS code C9734 (Focused ultrasound ablation/therapeutic intervention, other than uterine leiomyomata, with magnetic resonance (mr) guidance) to APC 5115 (Level 5 Musculoskeletal Procedures) for CY 2020. We also are proposing to continue to assign HCPCS code C9734 to status indicator “J1”. We refer readers to Addendum B to this proposed rule for the proposed payment rates for CPT codes 0071T and 0072T and HCPCS code C9734 under the OPPS. Addendum B is available via the internet on the CMS website.
For the procedure described by CPT code 0398T, we have identified 37 paid claims from CY 2016 through CY 2018 (1 claim in CY 2016, 11 claims in CY 2017, and 25 claims in CY 2018). We note that the procedure described by CPT code 0398T was first assigned to a New Technology APC in CY 2016. Accordingly, there are 3 years of claims data available for the OPPS ratesetting purposes. The payment amounts for the claims vary widely, with a cost of approximately $29,254 for the sole CY 2016 claim, a geometric mean cost of approximately $4,647 for the 11 claims from CY 2017, and a geometric mean cost of approximately $11,716 for the 25 claims from CY 2018. We are concerned about the large fluctuation in the cost of the procedure described by CPT code 0398T from year to year and the relatively small number of claims available to establish a payment rate for the service. To be in accordance with section 1833(t)(2)(B) of the Act, we must establish that services classified within each APC are comparable clinically and with respect to the use of resources.
Therefore, as discussed in section III.C.2. of this proposed rule, we are proposing to apply the policy we adopted in CY 2019, under which we will utilize our equitable adjustment authority under section 1833(t)(2)(E) of the Act to calculate the geometric mean, arithmetic mean, and median costs using multiple years of claims data to select the appropriate payment rate for purposes of assigning CPT code 0398T to a New Technology APC. We believe using this approach to assign CPT code 0398T to a New Technology APC is more likely to yield a payment rate that will be representative of the cost of the procedure described by CPT code 0398T, despite the fluctuating geometric mean costs for the procedure available in the claims data used for this proposed rule. We continue to believe that the situation for the procedure described by CPT code 0398T is unique, given the limited number of claims for the procedure and the high variability for the cost of the claims, which makes it challenging to determine a reliable payment rate.
Our analysis found that the estimated geometric mean cost of the 37 claims was approximately $8,829, the estimated arithmetic mean cost of the claims was approximately $10,021, and the median cost of the claims was approximately $11,985. While the results of using different methodologies range from approximately $8,800 to nearly $12,000, two of the estimates fall within the cost bands of New Technology APC 1575 (New Technology—Level 38 ($10,001-$15,000)), with a proposed payment rate of $12,500.50. Consistent with our policy stated in section III.C.2. of this proposed rule, we are presenting the result of each statistical methodology in this preamble, and we are seeking public comments on which methodology should be used to establish payment for the procedures described by CPT code 0398T. We note that we believe that the median cost estimate is the most appropriate representative cost of the procedure described by CPT code 0398T because it is consistent with the payment rates established for the procedure from CY 2017 to CY 2019 and does not involve any trimming of claims. Calculating the payment rate using either the geometric mean cost or the arithmetic mean cost would involve trimming the one paid claim from CY 2016, because the paid amount for the claim of $29,254 is substantially larger than the amount for any other paid claim reported for the procedure described by CPT code 0398T. The median cost estimate for CPT code 0398T also falls within the same New Technology APC cost band that was used to set the payment rate for CY 2019, which is $12,500.50 for this procedure. Therefore, for purposes of determining the proposed CY 2020 payment rate, we are proposing to estimate the cost for the procedure described by CPT code 0398T by calculating the median cost of the 37 paid claims for the procedures in CY 2016 through CY 2018, and assigning the procedure described by CPT code 0398T to the New Technology APC that includes the estimated cost. Accordingly, we are proposing to maintain the procedure described by CPT code 0398T in APC 1575 (New Technology—Level 38 ($10,001-$15,000)), with a proposed payment rate of $12,500.50 for CY 2020. We refer readers to Addendum B to this proposed rule for the proposed payment rates for all codes reportable under the OPPS. Addendum B is available via the internet on the CMS website.
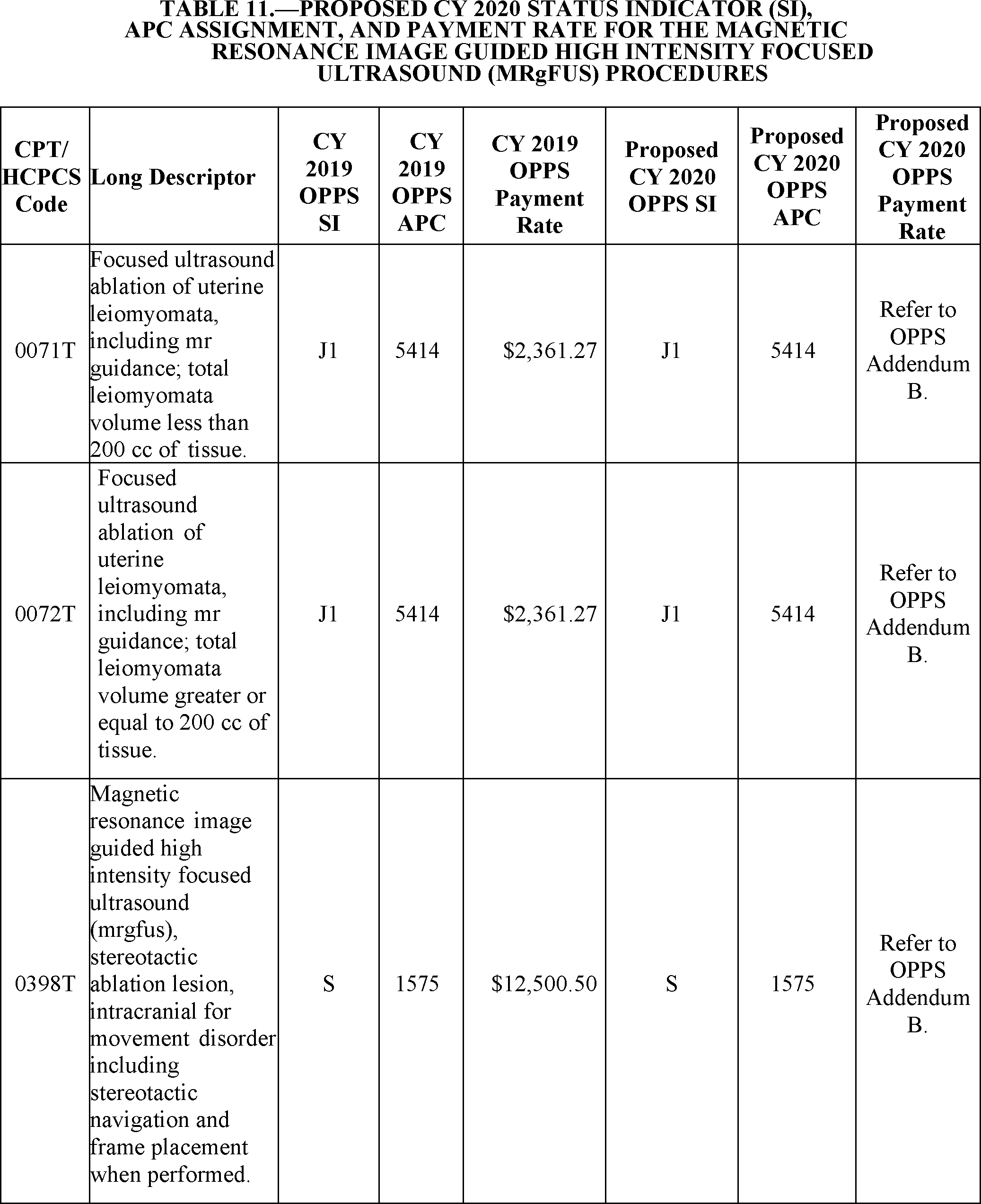

b. Retinal Prosthesis Implant Procedure
CPT code 0100T (Placement of a subconjunctival retinal prosthesis receiver and pulse generator, and implantation of intra-ocular retinal electrode array, with vitrectomy) describes the implantation of a retinal prosthesis, specifically, a procedure involving the use of the Argus® II Retinal Prosthesis System. This first retinal prosthesis was approved by the Food and Drug Administration (FDA) in 2013 for adult patients diagnosed with severe to profound retinitis pigmentosa. Pass-through payment status was granted for the Argus® II device under HCPCS code C1841 (Retinal prosthesis, includes all internal and external components) beginning October 1, 2013, and this status expired on December 31, 2015. We note that after pass-through payment status expires for a medical device, the payment for the device is packaged into the payment for the associated surgical procedure. Consequently, for CY 2016, the device described by HCPCS code C1841 was assigned to OPPS status indicator “N” to indicate that payment for the device is packaged and included in the payment rate for the surgical procedure described by CPT code 0100T. For CY 2016, the procedure described by CPT code 0100T was assigned to New Technology APC 1599, with a payment rate of $95,000, which was the highest paying New Technology APC for that year. This payment included both the surgical procedure (CPT code 0100T) and the use of the Argus® II device (HCPCS code C1841). However, stakeholders (including the device manufacturer and hospitals) believed that the CY 2016 payment rate for the procedure involving the Argus® II System was insufficient to cover the hospital cost of performing the procedure, which includes the cost of the retinal prosthesis at the retail price of approximately $145,000.
For CY 2017, analysis of the CY 2015 OPPS claims data used for the CY 2017 OPPS/ASC final rule with comment period showed 9 single claims (out of 13 total claims) for the procedure described by CPT code 0100T, with a geometric mean cost of approximately $142,003 based on claims submitted between January 1, 2015, through December 31, 2015, and processed through June 30, 2016. Based on the CY 2015 OPPS claims data available for the final rule with comment period and our understanding of the Argus® II procedure, we reassigned the procedure described by CPT code 0100T from New Technology APC 1599 to New Technology APC 1906, with a final payment rate of $150,000.50 for CY 2017. We noted that this payment rate included the cost of both the surgical procedure (CPT code 0100T) and the retinal prosthesis device (HCPCS code C1841).
For CY 2018, the reported cost of the Argus® II procedure based on CY 2016 hospital outpatient claims data for 6 claims used for the CY 2018 OPPS/ASC final rule with comment period was approximately $94,455, which was more than $55,000 less than the payment rate for the procedure in CY 2017, but closer to the CY 2016 payment rate for the procedure. We noted that the costs of the Argus® II procedure are extraordinarily high compared to many other procedures paid under the OPPS. In addition, the number of claims submitted has been very low and has not exceeded 10 claims within a single year. We believed that it is important to mitigate significant payment differences, especially shifts of several tens of thousands of dollars, while also basing payment rates on available cost information and claims data. In CY 2016, the payment rate for the Argus® II procedure was $95,000.50. The payment rate increased to $150,000.50 in CY 2017. For CY 2018, if we had established the payment rate based on updated final rule claims data, the payment rate would have decreased to $95,000.50 for CY 2018, a decrease of $55,000 relative to CY 2017. We were concerned that these large fluctuations in payment could potentially create an access to care issue for the Argus® II procedure, and we wanted to establish a payment rate to mitigate the potential sharp decline in payment from CY 2017 to CY 2018.
In accordance with section 1833(t)(2)(B) of the Act, we must establish that services classified within each APC are comparable clinically and with respect to the use of resources. Therefore, for CY 2018, we used our equitable adjustment authority under section 1833(t)(2)(E) of the Act, which states that the Secretary shall establish, in a budget neutral manner, other adjustments as determined to be necessary to ensure equitable payments, to maintain the payment rate for this procedure, despite the lower geometric mean costs available in the claims data used for the final rule with comment period. For CY 2018, we reassigned the Argus® II procedure to APC 1904 (New Technology—Level 50 ($115,001-$130,000)), which established a payment rate for the Argus® II procedure of $122,500.50, which was the arithmetic mean of the payment rates for the procedure for CY 2016 and CY 2017.
For CY 2019, the reported cost of the Argus® II procedure based on the geometric mean cost of 12 claims from the CY 2017 hospital outpatient claims data was approximately $171,865, which was approximately $49,364 more than the payment rate for the procedure for CY 2018. In the CY 2019 OPPS/ASC final rule with comment period, we continued to note that the costs of the Argus® II procedure are extraordinarily high compared to many other procedures paid under the OPPS (83 FR 58897 through 58898). In addition, the number of claims submitted continued to be very low for the Argus® II procedure. We stated that we continued to believe that it is important to mitigate significant payment fluctuations for a procedure, especially shifts of several tens of thousands of dollars, while also basing payment rates on available cost information and claims data because we are concerned that large decreases in the payment rate could potentially create an access to care issue for the Argus® II procedure. In addition, we indicated that we wanted to establish a payment rate to mitigate the potential sharp increase in payment from CY 2018 to CY 2019, and potentially ensure a more stable payment rate in future years.
In accordance with section 1833(t)(2)(B) of the Act, we must establish that services classified within each APC are comparable clinically and with respect to the use of resources. Therefore, as discussed in section III.C.2. of the CY 2019 OPPS/ASC final rule with comment period (83 FR 58892 through 58893), we used our equitable adjustment authority under section 1833(t)(2)(E) of the Act, which states that the Secretary shall establish, in a budget neutral manner, other adjustments as determined to be necessary to ensure equitable payments, to establish a payment rate that is more representative of the likely cost of the service. We stated that we believed the likely cost of the Argus® II procedure is higher than the geometric mean cost calculated from the claims data used for the CY 2018 OPPS/ASC final rule with comment period but lower than the geometric mean cost calculated from the claims data used for the CY 2019 OPPS/ASC final rule with comment period.
For CY 2019, we analyzed claims data for the Argus® II procedure using 3 years of available data from CY 2015 through CY 2017. These data included claims from the last year that the Argus® II received transitional device pass-through payments (CY 2015) and the first 2 years since device pass-through payment status for the Argus® II expired. We found that the geometric mean cost for the procedure was approximately $145,808, the arithmetic mean cost was approximately $151,367, and the median cost was approximately $151,266. As we do each year, we reviewed claims data regarding hospital costs associated with new procedures. We regularly examine the claims data and any available new information regarding the clinical aspects of new procedures to confirm that OPPS payments remain appropriate for procedures like the Argus® II procedure as they transition into mainstream medical practice (77 FR 68314). We noted that the proposed payment rate included both the surgical procedure (CPT code 0100T) and the use of the Argus® II device (HCPCS code C1841). For CY 2019, the estimated costs using all three potential statistical methods for determining APC assignment under the New Technology low-volume policy fell within the cost band of New Technology APC 1908, which is between $145,001 and $160,000. Therefore, we reassigned the Argus® II procedure (CPT code 0100T) to APC 1908 (New Technology—Level 52 ($145,001-$160,000)), with a payment rate of $152,500.50 for CY 2019.
For CY 2020, the number of reported claims for the Argus® II procedure continues to be very low with a substantial fluctuation in cost from year to year.
The high annual variability of the cost of the Argus® II procedure continues to make it difficult to establish a consistent and stable payment rate for the procedure. In accordance with section 1833(t)(2)(B) of the Act, we are required to establish that services classified within each APC are comparable clinically and with respect to the use of resources. Therefore, for CY 2020, we are proposing to apply the policy we adopted in CY 2019, under which we utilize our equitable adjustment authority under section 1833(t)(2)(E) of the Act to calculate the geometric mean, arithmetic mean, and median costs using multiple years of claims data to select the appropriate payment rate for purposes of assigning the Argus® II procedure (CPT code 0100T) to a New Technology APC.
We identified 35 claims reporting the procedure described by CPT code 0100T for the 4-year period of CY 2015 through CY 2018. We found the geometric mean cost for the procedure described by CPT code 0100T to be approximately $146,059, the arithmetic mean cost to be approximately $152,123, and the median cost to be approximately $151,267. All of the resulting estimates from using the three statistical methodologies fall within the same New Technology APC cost band ($145,001-$160,000), where the Argus® II procedure is assigned for CY 2019. Consistent with our policy stated in section III.C.2. of this proposed rule, we are presenting the result of each statistical methodology in this preamble, and we are seeking public comments on which method should be used to assign procedures described by CPT code 0100T to a New Technology APC. All three potential statistical methodologies used to estimate the cost of the Argus® II procedure fall within the cost band for New Technology APC 1908, with the estimated cost being between $145,001 and $160,000. Accordingly, we are proposing to maintain the assignment of the procedure described by CPT code 0100T in APC 1908 (New Technology—Level 52 ($145,001-$160,000)), with a proposed payment rate of $152,500.50 for CY 2020. We note that the proposed payment rate includes both the surgical procedure (CPT code 0100T) and the use of the Argus® II device (HCPCS code C1841). We refer readers to Addendum B to this proposed rule for the proposed payment rates for all codes reportable under the OPPS. Addendum B is available via the internet on the CMS website.
As we discussed in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58898), the claims data from CY 2017 showed another payment issue with regard to the Argus® II procedure. We found that payment for the Argus® II procedure was sometimes bundled into the payment for another procedure. Therefore in CY 2019, we implemented a policy to exclude payment for all procedures assigned to New Technology APCs from being bundled into the payment for procedures assigned to a C-APC. For CY 2020, we are proposing to continue this policy as described in section II.A.2.b.(3) of this proposed rule. Our proposal would continue to exclude payment for any procedure that is assigned to a New Technology APC from being packaged when included on a claim with a service assigned to status indicator “J1”. While we are not proposing to exclude payment for a procedure assigned to a New Technology APC from being packaged when included on a claim with a service assigned to status indicator “J2”, we are seeking public comments on this issue.
c. Bronchoscopy With Transbronchial Ablation of Lesion(s) by Microwave Energy
Effective January 1, 2019, CMS established HCPCS code C9751 (Bronchoscopy, rigid or flexible, transbronchial ablation of lesion(s) by microwave energy, including fluoroscopic guidance, when performed, with computed tomography acquisition(s) and 3-D rendering, computer-assisted, image-guided navigation, and endobronchial ultrasound (EBUS) guided transtracheal and/or transbronchial sampling (e.g., aspiration[s]/biopsy[ies]) and all mediastinal and/or hilar lymph node stations or structures and therapeutic intervention(s)). This microwave ablation procedure utilizes a flexible catheter to access the lung tumor via a working channel and may be used as an alternative procedure to a percutaneous microwave approach. Based on our review of the New Technology APC application for this service and the service's clinical similarity to existing services paid under the OPPS, we estimated the likely cost of the procedure would be between $8,001 and $8,500. We have not received any claims data for this service. Therefore, we are proposing to continue to assign the procedure described by HCPCS code C9751 to New Technology APC 1571 (New Technology—Level 34 ($8,001-$8,500)), with a proposed payment rate of $8,250.50 for CY 2020. Details regarding HCPCS code C9751 are shown in Table 12.

d. Pathogen Test for Platelets
As stated in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59281), HCPCS code P9100 is used to report any test used to identify bacterial or other pathogen contamination in blood platelets. Currently, there are two rapid bacterial detection tests cleared by the FDA that are described by HCPCS code P9100. According to their instructions for use, rapid bacterial detection tests should be performed on platelets from 72 hours after collection. Currently, certain rapid and culture-based tests can be used to extend the dating for platelets from 5 days to 7 days. Blood banks and transfusion services may test and use 6-day old to 7-day old platelets if the test results are negative for bacterial contamination.
HCPCS code P9100 was assigned in CY 2019 to New Technology APC 1493 (New Technology—Level 1C ($21-$30)), with a payment rate of $25.50. For CY 2020, based on CY 2018 claims data, there are approximately 1,100 claims reported for this service with a geometric mean cost of approximately $32. This geometric mean cost would result in the assignment of the service described by HCPCS code P9100 to a New Technology APC, based on the associated cost band, with a higher payment rate than where the service is currently assigned. Therefore, for CY 2020, we are proposing to reassign the service described by HCPCS code P9100 to New Technology APC 1494 (New Technology—Level 1D ($31-$40)), with a proposed payment rate of $35.50.
e. Fractional Flow Reserve Derived From Computed Tomography (FFRCT)
Fractional Flow Reserve Derived From Computed Tomography (FFRCT), also known by the trade name HeartFlow, is a noninvasive diagnostic service that allows physicians to measure coronary artery disease in a patient through the use of coronary CT scans. The HeartFlow procedure is intended for clinically stable symptomatic patients with coronary artery disease, and, in many cases, may avoid the need for an invasive coronary angiogram procedure. HeartFlow uses a proprietary data analysis process performed at a central facility to develop a three-dimensional image of a patient's coronary arteries, which allows physicians to identify the fractional flow reserve to assess whether or not patients should undergo further invasive testing (that is, a coronary angiogram).
For many procedures in the OPPS, payment for analytics that are performed after the main diagnostic/image procedure are packaged into the payment for the primary procedure. However, in CY 2018, we determined that HeartFlow should receive a separate payment because the procedure is performed by a separate entity (that is, a HeartFlow technician who conducts computer analysis offsite) rather than the provider performing the CT scan. We assigned CPT code 0503T, which describes the analytics performed, to New Technology APC 1516 (New Technology—Level 16 ($1,401-$1,500)), with a payment rate of $1,450.50 based on pricing information provided by the developer of the procedure that indicated the price of the procedure was approximately $1,500.
For CY 2020, based on our analysis of the CY 2018 claims data, we found that over 840 claims had been submitted for payment for HeartFlow during CY 2018. The estimated geometric mean cost of HeartFlow is $788.19, which is over $660 lower that the payment rate for CY 2019 of $1,450.50. Therefore, for CY 2020, we are proposing to reassign the service described by CPT code 0503T in order to adjust the payment rate to better reflect the cost for the service. We are proposing to reassign the service described by CPT code 0503T to New Technology APC 1509 (New Technology—Level 9 ($701-$800)), with a proposed payment rate of $750.50 for CY 2020. We are seeking public comments on this proposal.
D. Proposed APC Specific Policies
1. Intraocular Procedures (APCs 5491 Through 5494)
In prior years, CPT code 0308T (Insertion of ocular telescope prosthesis including removal of crystalline lens or intraocular lens prosthesis) was assigned to the APC 5495 (Level 5 Intraocular Procedures) based on its estimated costs. In addition, its relative payment weight has been based on its median cost under our payment policy for low-volume device-intensive procedures because the APC contained a low volume of claims. The low-volume device-intensive procedures payment policy is discussed in more detail in section III.C.2. of this proposed rule.
In the CY 2019 OPPS/ASC proposed rule, we proposed to reassign CPT code 0308T from APC 5495 to APC 5493 (Level 3 Intraocular Procedures), based on the data for two claims available for ratesetting for the proposed rule, and to delete APC 5495 (83 FR 37096 through 37097). However in the CY 2019 OPPS/ASC final rule with comment period, based on updated data on a single claim available for ratesetting for the final rule, we modified our proposal and reassigned procedure code CPT code 0308T to the APC 5494 (Level 4 Intraocular Procedures) (83 FR 58917 through 58918). We made this change based on the similarity of the estimated cost for the single claim of $12,939.75 compared to that of the APC ($11,427.14). However, this created a discrepancy in payments between the OPPS setting and the ASC setting in which the ASC payments would be higher than the OPPS payments for the same service because of the intersection of the estimated cost for the encounter determined under a comprehensive methodology within the OPPS and the estimated cost determined under the payment methodology for device-intensive services within the ASC payment system.
In reviewing the claims data available for this proposed rule for CY 2020 OPPS ratesetting, we found several claims reporting the procedure described by CPT code 0308T. Based on the claims data, the procedure would have a geometric mean cost of $28,122.51 and a median cost of $19,864.38. These cost statistics are significantly higher than the geometric mean cost of the other procedure assigned to APC 5494, that is, the procedure described by CPT code 67027 (Implant eye drug system), which has a geometric mean cost of $12,296.27. In addition, if we continued to assign the procedure described by CPT code 0308T to APC 5494 (the Level 4 Intraocular Procedures APC), the discrepancy between payments within the OPPS and the ASC payment system would also continue to exist. As a result, we are proposing to reestablish APC 5495 (Level 5 Intraocular Procedures) because we believe that the procedure described by CPT code 0308T would be most appropriately placed in this APC based on its estimated cost. Assignment of the procedure to the Level 5 Intraocular Procedures APC is consistent with its historical placement and would also address the large differential discrepancy in payment for the procedure between the OPPS and the ASC payment system. We note that, based on data available for the proposed rule, the proposed payment rate for this procedure when performed in an ASC, as discussed in more detail in section XIII.D.1.c. of this proposed rule, would be no higher than the OPPS payment rate for this procedure performed in the hospital outpatient setting. We will continue to monitor the volume of claims data available for the procedure for ratesetting purposes.
Therefore, for CY 2020, we are proposing to reestablish APC 5495 (Level 5 Intraocular Procedures) and reassign the procedure described by CPT code 0308T from APC 5494 to APC 5495. Under this proposal, the proposed CY 2020 OPPS payment rate for the service would be established based on its median cost, as discussed in section V.A.5. of this proposed rule, because it is a device-intensive procedure assigned to an APC with fewer than 100 total annual claims within the APC.
2. Musculoskeletal Procedures (APCs 5111 Through 5116)
Prior to the CY 2016 OPPS, payment for musculoskeletal procedures was primarily divided according to anatomy and the type of musculoskeletal procedure. As part of the CY 2016 reorganization to better structure the OPPS payments towards prospective payment packages, we consolidated those individual APCs so that they became a general Musculoskeletal APC series (80 FR 70397 through 70398).
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59300), we continued to apply a six-level structure for the Musculoskeletal APCs because doing so provided an appropriate distinction for resource costs at each level and provided clinical homogeneity. However, we indicated that we would continue to review the structure of these APCs to determine whether additional granularity would be necessary.
In the CY 2019 OPPS proposed rule (83 FR 37096), we recognized that commenters had previously expressed concerns regarding the granularity of the current APC levels and, therefore, requested comment on the establishment of additional levels. Specifically, we solicited comments on the creation of a new APC level between the current Level 5 and Level 6 within the Musculoskeletal APC series. While some commenters provided suggested APC reconfigurations and requests for change to APC assignments, many commenters requested that we maintain the current six-level structure and continue to monitor the claims data as they become available. Therefore, in the CY 2019 OPPS/ASC final rule with comment period, we maintained the six-level APC structure for the Musculoskeletal Procedures APCs (83 FR 58920 through 58921).
Based on the claims data available for this CY 2020 OPPS/ASC proposed rule, we continue to believe that the six-level APC structure for the Musculoskeletal Procedures APC series is appropriate. Therefore, we are proposing to maintain the APC structure for the CY 2020 OPPS update.
We note that this is the first year for which claims data are available for the total knee arthroplasty procedure described by CPT code 27447, which was removed from the inpatient only list in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59382 through 59385). Based on approximately 60,000 hospital outpatient claims reporting the procedure that are available for ratesetting in this proposed rule, the geometric mean cost is approximately $12,472.05, which is similar to the geometric mean cost for APC 5115 (Level 5 Musculoskeletal Procedures) of $11,879.66, and within a range of the lowest geometric mean cost of the significant procedure costs of $9,969.37 and the highest geometric mean cost of the significant procedure costs of $12,894.18. Therefore, we believe that the assignment of the procedure described by CPT code 27447 in the Level 5 Musculoskeletal Procedures APC series remains appropriate and, therefore, we are proposing to continue to assign CPT code 27447 to APC 5115 (Level 5 Musculoskeletal Procedures) for CY 2020.
We also are proposing to remove the procedure described by CPT code 27130 (Total hip arthroplasty) from the CY 2020 OPPS inpatient only list. Based on the estimated costs derived from in the available claims data, as well as the 50th percentile IPPS payment for TKA/THA procedures without major complications or comorbidities (MS-DRG 470) of approximately $11,900 for FY 2020 when the procedure is performed on an inpatient basis, we believe that it is appropriate to assign the procedure described by CPT code 27130 to the Level 5 Musculoskeletal Procedures APC series, which has a geometric mean cost of $11,879.66. Therefore, for CY 2020, we also are proposing to assign the procedure described by CPT code 27130 to APC 5115. We note that we will monitor the claims data reflecting these procedures as they become available. For a more detailed discussion of the procedures that are being proposed to be removed from the inpatient only (IPO) list for CY 2020 under the OPPS, we refer readers to section IX. of this proposed rule.
Table 13 displays the CY 2020 Musculoskeletal Procedures APC series' structure and APC geometric mean costs.

IV. Proposed OPPS Payment for Devices
A. Pass-Through Payment for Devices
1. Beginning Eligibility Date for Device Pass-Through Status and Quarterly Expiration of Device Pass-Through Payments
a. Background
Under section 1833(t)(6)(B)(iii) of the Act, the period for which a device category eligible for transitional pass-through payments under the OPPS can be in effect is at least 2 years but not more than 3 years. Prior to CY 2017, our regulation at 42 CFR 419.66(g) provided that this pass-through payment eligibility period began on the date CMS established a particular transitional pass-through category of devices, and we based the pass-through status expiration date for a device category on the date on which pass-through payment was effective for the category. In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79654), in accordance with section 1833(t)(6)(B)(iii)(II) of the Act, we amended § 419.66(g) to provide that the pass-through eligibility period for a device category begins on the first date on which pass-through payment is made under the OPPS for any medical device described by such category.
In addition, prior to CY 2017, our policy was to propose and finalize the dates for expiration of pass-through status for device categories as part of the OPPS annual update. This means that device pass-through status would expire at the end of a calendar year when at least 2 years of pass-through payments had been made, regardless of the quarter in which the device was approved. In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79655), we changed our policy to allow for quarterly expiration of pass-through payment status for devices, beginning with pass-through devices approved in CY 2017 and subsequent calendar years, to afford a pass-through payment period that is as close to a full 3 years as possible for all pass-through payment devices. We refer readers to the CY 2017 OPPS/ASC final rule with comment period (81 FR 79648 through 79661) for a full discussion of the changes to the device pass-through payment policy. We also have an established policy to package the costs of the devices that are no longer eligible for pass-through payments into the costs of the procedures with which the devices are reported in the claims data used to set the payment rates (67 FR 66763).
b. Expiration of Transitional Pass-Through Payments for Certain Devices
As stated earlier, section 1833(t)(6)(B)(iii) of the Act requires that, under the OPPS, a category of devices be eligible for transitional pass-through payments for at least 2 years, but not more than 3 years. There currently is one device category eligible for pass-through payment: HCPCS code C1822 (Generator, neurostimulator (implantable), high frequency, with rechargeable battery and charging system), which was established effective January 1, 2019. The pass-through payment status of the device category for HCPCS code C2624 will expire on December 31, 2022. Therefore, HCPCS code C2624 will continue to receive device pass-through payments in CY 2020.
2. New Device Pass-Through Applications
a. Background
Section 1833(t)(6) of the Act provides for pass-through payments for devices, and section 1833(t)(6)(B) of the Act requires CMS to use categories in determining the eligibility of devices for pass-through payments. As part of implementing the statute through regulations, we have continued to believe that it is important for hospitals to receive pass-through payments for devices that offer substantial clinical improvement in the treatment of Medicare beneficiaries to facilitate access by beneficiaries to the advantages of the new technology. Conversely, we have noted that the need for additional payments for devices that offer little or no clinical improvement over previously existing devices is less apparent. In such cases, these devices can still be used by hospitals, and hospitals will be paid for them through appropriate APC payment. Moreover, a goal is to target pass-through payments for those devices where cost considerations might be most likely to interfere with patient access (66 FR 55852; 67 FR 66782; and 70 FR 68629). We note that, in section IV.A.4. of this proposed rule, we are proposing an alternative pathway that would grant fast-track device pass-through payment under the OPPS for devices approved under the FDA Breakthrough Device Program for OPPS device pass-through payment applications received on or after January 1, 2020. We refer the reader to section IV.A.4. of this proposed rule for a complete discussion on this proposal.
As specified in regulations at 42 CFR 419.66(b)(1) through (3), to be eligible for transitional pass-through payment under the OPPS, a device must meet the following criteria:
- If required by FDA, the device must have received FDA approval or clearance (except for a device that has received an FDA investigational device exemption (IDE) and has been classified as a Category B device by the FDA), or meet another appropriate FDA exemption; and the pass-through payment application must be submitted within 3 years from the date of the initial FDA approval or clearance, if required, unless there is a documented, verifiable delay in U.S. market availability after FDA approval or clearance is granted, in which case CMS will consider the pass-through payment application if it is submitted within 3 years from the date of market availability;
- The device is determined to be reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body part, as required by section 1862(a)(1)(A) of the Act; and
- The device is an integral part of the service furnished, is used for one patient only, comes in contact with human tissue, and is surgically implanted or inserted (either permanently or temporarily), or applied in or on a wound or other skin lesion.
In addition, according to § 419.66(b)(4), a device is not eligible to be considered for device pass-through payment if it is any of the following: (1) Equipment, an instrument, apparatus, implement, or item of this type for which depreciation and financing expenses are recovered as depreciation assets as defined in Chapter 1 of the Medicare Provider Reimbursement Manual (CMS Pub. 15-1); or (2) a material or supply furnished incident to a service (for example, a suture, customized surgical kit, or clip, other than a radiological site marker).
Separately, we use the following criteria, as set forth under § 419.66(c), to determine whether a new category of pass-through payment devices should be established. The device to be included in the new category must—
- Not be appropriately described by an existing category or by any category previously in effect established for transitional pass-through payments, and was not being paid for as an outpatient service as of December 31, 1996;
- Have an average cost that is not “insignificant” relative to the payment amount for the procedure or service with which the device is associated as determined under § 419.66(d) by demonstrating: (1) The estimated average reasonable costs of devices in the category exceeds 25 percent of the applicable APC payment amount for the service related to the category of devices; (2) the estimated average reasonable cost of the devices in the category exceeds the cost of the device-related portion of the APC payment amount for the related service by at least 25 percent; and (3) the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount for the device exceeds 10 percent of the APC payment amount for the related service (with the exception of brachytherapy and temperature-monitored cryoablation, which are exempt from the cost requirements as specified at § 419.66(c)(3) and (e)); and
- Demonstrate a substantial clinical improvement, that is, substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment.
Beginning in CY 2016, we changed our device pass-through evaluation and determination process. Device pass-through applications are still submitted to CMS through the quarterly subregulatory process, but the applications will be subject to notice-and-comment rulemaking in the next applicable OPPS annual rulemaking cycle. Under this process, all applications that are preliminarily approved upon quarterly review will automatically be included in the next applicable OPPS annual rulemaking cycle, while submitters of applications that are not approved upon quarterly review will have the option of being included in the next applicable OPPS annual rulemaking cycle or withdrawing their application from consideration. Under this notice-and-comment process, applicants may submit new evidence, such as clinical trial results published in a peer-reviewed journal or other materials for consideration during the public comment process for the proposed rule. This process allows those applications that we are able to determine meet all of the criteria for device pass-through payment under the quarterly review process to receive timely pass-through payment status, while still allowing for a transparent, public review process for all applications (80 FR 70417 through 70418).
More details on the requirements for device pass-through payment applications are included on the CMS website in the application form itself at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/passthrough_payment.html, in the “Downloads” section. In addition, CMS is amenable to meeting with applicants or potential applicants to discuss research trial design in advance of any device pass-through application or to discuss application criteria, including the substantial clinical improvement criterion.
b. Applications Received for Device Pass-Through Payment for CY 2020
We received seven complete applications by the March 1, 2019 quarterly deadline, which was the last quarterly deadline for applications to be received in time to be included in this CY 2020 OPPS/ASC proposed rule. We received one of the applications in the second quarter of 2018, three of the applications in the fourth quarter of 2018, and three of the applications in the first quarter of 2019. None of the applications were approved for device pass-through payment during the quarterly review process.
Applications received for the later deadlines for the remaining 2019 quarters (June 1, September 1, and December 1), if any, will be presented in the CY 2021 OPPS/ASC proposed rule. We note that the quarterly application process and requirements have not changed in light of the addition of rulemaking review. Detailed instructions on submission of a quarterly device pass-through payment application are included on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/catapp.pdf. A discussion of the applications received by the March 1, 2019 deadline is presented below.
(1) Surefire® SparkTM Infusion System
TriSalus Life Sciences submitted an application for a new device category for transitional pass-through payment status for the Surefire® SparkTM Infusion System. The Surefire® SparkTM Infusion System is described as a flexible, ultra-thin microcatheter with a self-expanding, nonocclusive one-way microvalve at the distal end. The applicant stated that it has designed the Pressure Enabled Drug DeliveryTM technology of the Surefire® SparkTM Infusion System to overcome intratumoral pressure in solid tumors and improve distribution and penetration of therapy during Transcatheter Arterial Chemoembolization (TACE) procedures. TACE is a minimally invasive, image-guided procedure used to infuse a high dose of chemotherapy into liver tumors. According to the applicant, the pliable, one-way valve at the distal tip of the Surefire® SparkTM Infusion System creates a temporary local increase in pressure during infusion, opening up collapsed vessels in tumors, which enables perfusion and therapy delivery in areas inaccessible to the systemic circulation, a positive hydrostatic pressure gradient, and restores convective flow to enable therapy to penetrate deeper into the tumor. During the TACE procedure, the physician first gains catheter access into the arterial system of the hepatic arteries through a small incision in the groin or the wrist. The applicant stated that the physician then uses real-time fluoroscopic guidance to navigate the Surefire® SparkTM Infusion System into the blood vessels feeding the tumors, infusing the chemotherapy and embolic materials through the Surefire® SparkTM Infusion System until the tumor bed is completely saturated.
With respect to the newness criterion at § 419.66(b)(1), the FDA granted 510(k) premarket clearance as of April 3, 2018. The application for a new device category for transitional pass-through payment status for the Surefire® SparkTM Infusion System was received on November 29, 2018, which is within 3 years of the date of the initial FDA approval or clearance. We are inviting public comments on whether the Surefire® SparkTM Infusion System meets the newness criterion.
With respect to the eligibility criterion at § 419.66(b)(3), according to the applicant, the use of the Surefire® SparkTM Infusion System is integral to the service of providing delivery of chemotherapy into liver tumors, is used for one patient only, comes in contact with human skin, and is applied in or on a wound or other skin lesion. The applicant also claimed the Surefire® SparkTM Infusion System meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or items for which depreciation and financing expenses are recovered, and it is not a supply or material furnished incident to a service. We are inviting public comments on whether the Surefire® SparkTM Infusion System meets the eligibility criteria at § 419.66(b).
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any of the existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. We have identified several existing pass-through payment categories that may be applicable to the Surefire® SparkTM Infusion System. The Surefire® SparkTM Infusion System may be described by HCPCS code C1887 (Catheter, guiding (may include infusion/perfusion capability)). The applicant describes the Surefire® SparkTM Infusion System as a device used in vascular interventional procedures to deliver diagnostic and therapeutic agents in the peripheral vasculatures. The CMS List of Device Category Codes for Present or Previous Pass-Through Payment and Related Definitions describes HCPCS code C1887 as intended for the introduction of interventional/diagnostic devices into the coronary or peripheral vascular systems. The Surefire® SparkTM Infusion System may also be described by HCPCS code C1751 (Catheter, infusion, inserted peripherally, centrally or midline (other than hemodialysis)). The applicant describes the Surefire® SparkTM Infusion System as being inserted through a small incision in the groin or the wrist. We are inviting public comments on this issue.
The second criterion for establishing a device category, at § 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. With respect to this criterion, the applicant submitted four studies to support the claim that their technology represents a substantial clinical improvement over existing technologies. The applicant asserts that the Surefire® SparkTM Infusion System represents a substantial clinical improvement over existing technologies because it offers a treatment option that no other catheters currently available can provide. The manufacturer notes that the self-expanding, nonocclusive, one-way valve can infuse therapy at pressure higher than the baseline mean arterial pressure, and this pressurized delivery opens up collapsed vessels in tumors and enables perfusion and therapy delivery into hypoxic areas of the liver tumors. The applicant also believes that the Surefire® SparkTM Infusion System represents a substantial clinical improvement because the technology has shown improved tumor response rates in hepatocellular carcinoma, as well as a decrease in the rate of disease recurrence and the need for subsequent treatment.
The first pilot study of nine patients being treated for hepatocellular carcinoma, who received infusions via both a conventional end-hole catheter and an antireflux microcatheter, demonstrated statistically significant reductions in downstream distribution of embolic particles with the antireflux catheter and increases in tumor deposition (p<0.05).[13] The second singlecenter retrospective study was conducted with 22 patients treated for hepatocellular carcinoma with the Surefire® SparkTM Infusion System and TACE. As assessed by MRI, there appeared to be overall disease response in 91 percent of patients and 85 percent of lesions and complete response in 32 percent of patients and 54 percent of lesions.[14] In the first study for a case-control series, 19 patients undergoing treatment using SIS-TACE had a statistically significant improvement in disease response rate compared to 19 patients treated with end-hole microcatheters, 78.9 percent compared to 36.8 percent for initial overall response rate (p = 0.008).[15] In the second study, a multi-center registry of 72 patients demonstrated high response rate when compared to historical control at 6 months follow-up.[16]
Based on the information submitted by the applicant, one concern is that large-scale studies with long-term follow-up are limited. Also, the majority of studies presented had a sample size of less than 25 and the highest sample size presented was less than 100 patients. Additionally, patient follow-up occurred mostly within a 3 to 6 month timeframe with few studies occurring beyond this range.
Another concern is that none of the studies presented improvements in mortality with the use of the Surefire® SparkTM Infusion System. Outcomes focused primarily on tumor response rates and lesion size, based upon imaging. Additional data on mortality endpoints would be helpful to fully assess substantial clinical improvement.
We are inviting public comments on whether the Surefire® SparkTM Infusion System meets the substantial clinical improvement criterion.
The third criterion for establishing a device category, at § 419.66(c)(3), requires us to determine that the cost of the device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. The applicant provided the following information in support of the cost significance requirements. The applicant stated that the Surefire® SparkTM Infusion System would be reported with CPT code 37243, which is assigned to APC 5193 (Level 3 Endovascular Procedures). To meet the cost criterion for device pass-through payment status, a device must pass all three tests of the cost criterion for at least one APC. For our calculations, we used APC 5193, which has a CY 2019 payment rate of $9,669.04. Beginning in CY 2017, we calculated the device offset amount at the HCPCS/CPT code level instead of the APC level (81 FR 79657). CPT code 37243 had a device offset amount of $3,894.69 at the time the application was received. According to the applicant, the cost of the Surefire® SparkTM Infusion System is $7,750.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The estimated average reasonable cost of $7,750 for the Surefire® SparkTM Infusion System is 80.2 percent of the applicable APC payment amount for the service related to the category of devices of $9,669.04 ($7,750/$9,669.04 × 100 = 80.2 percent). Therefore, we believe the Surefire® SparkTM Infusion System meets the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of the devices in the category must exceed the cost of the device-related portion of the APC payment amount for the related service by at least 25 percent, which means that the device cost needs to be at least 125 percent of the offset amount (the device-related portion of the APC found on the offset list). The estimated average reasonable cost of $7,750 for the Surefire® SparkTM Infusion System exceeds the cost of the device-related portion of the APC payment amount for the related service of $3,894.69 by 199 percent ($7,750/$3,894.69) × 100 = 198.99 percent). Therefore, we believe that the Surefire® SparkTM Infusion System meets the second cost significance requirement.
The third cost significance requirement, at § 419.66(d)(3), provides that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount for the device must exceed 10 percent of the APC payment amount for the related service. The difference between the estimated average reasonable cost of $7,750 for the SparkTM Infusion System and the portion of the APC payment amount for the device of $3,894.69 exceeds the APC payment amount for the related service of $9,669.04 by 40 percent (($7,750−$3,894.69)/$9,669.04) × 100 = 39.87 percent). Therefore, we believe that the Surefire® SparkTM Infusion System meets the third cost significance requirement.
We are inviting public comments on whether the Surefire® SparkTM Infusion System meets the device pass-through payment criteria discussed in this section, including the cost criterion.
(2) TracPatch
According to the applicant, TracPatch is a wearable device which utilizes an accelerometer, temperature sensor and step counter to allow the surgeon and patient to monitor recovery and help ensure critical milestones are being met. The applicant states that TracPatch utilizes wearable monitoring technology and methods in an effort to enhance the rehabilitation experience for both patients and physicians. Accelerometers are utilized to recognize and record the results when patients perform standard physical therapy exercises, in addition to providing standard step count and high-acceleration events that may indicate a fall. A temperature sensor monitors the skin temperature near the joint.
TracPatch is described by the applicant as a 24/7 remote monitoring wearable device that captures a patient's key daily activities: Such as range of motion progress, exercise compliance, and ambulation. TracPatch is used for pre- and post-operative patient monitoring, patient engagement, data analytics and post-op cost reduction.
According to the applicant, the wearable devices stick on the skin above and below the knee. The wearables are applied before total knee surgery to determine a patient's baseline activity levels, and then again after surgery to allow the patient and surgeon to monitor activity, pain, range of motion and physical therapy. The use of the Bluetooth connectivity allows the device to be paired with any smartphone and the TracPatch cloud allows for unlimited data collection and storage. The applicant states that TracPatch includes a web dashboard and computer application, which permit a health care provider to monitor a patient's recovery in real-time, allowing for immediate care adjustments and the ability for providers and patients to respond to issues that may occur during recovery from surgery.
With respect to the newness criterion at § 419.66(b)(1), the applicant stated that TracPatch does not need FDA clearance because it is a Class I device that would be assigned to a generic category of devices described in title 21 of the Code of Federal Regulations, parts 862 through 892 (21 CFR parts 862 through 892) that do not require FDA clearance. However, the applicant did not identify which category of exempted devices that TracPatch would be assigned. The applicant also stated that TracPatch will be introduced into the market in 2019, which would be within 3 years of the device pass-through payment application for TracPatch that was received in March 2019. We are inviting public comments on whether the TracPatch is exempt from FDA clearance and if the TracPatch meets the newness criterion.
With respect to the eligibility criterion at § 419.66(b)(3), the applicant claimed that the TracPatch is an integral part of monitoring the range of motion for a knee prior to and after total knee arthroplasty, is used for one patient only, and is placed on the skin above and below the knee and secured by Velcro strips. The applicant stated that the device is not surgically implanted or inserted into the patient and is not applied in or on a wound or other skin lesion. We have concerns with the TracPatch's eligibility with respect to the criterion at § 419.66(b)(3) because to be eligible for pass-through payment a device must be surgically implanted or inserted into the patient or applied in a wound or on other skin lesions. In addition, the applicant stated that the TracPatch meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or item for which depreciation and financing expenses are recovered. We have determined that TracPatch is not a material or supply furnished incident to a service. We are inviting public comments on whether the TracPatch meets the eligibility criterion.
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. With respect to the existence of a previous pass-through device category that describes the TracPatch, the applicant suggested a category descriptor of “Real time patient monitoring surface sensor technology for pre and post-op Total Knee Arthroplasty.” We have not identified an existing pass-through payment category that describes the TracPatch, but we welcome public comments on this topic.
The second criterion for establishing a device category, at § 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. The applicant asserted that use of the TracPatch significantly improves clinical outcomes for a patient population because the TracPatch allows both real-time and remote monitoring of the knee after total knee arthroplasty, which allows providers to make care decisions with up-to-the-minute data. The applicant noted that health care providers have instant access to a patient's pre-operative and post-operative data and can adjust care plans based on the data. The applicant stated that physicians will be able to preoperatively monitor patient activity to set a clinical baseline, but surgeons will also be able to monitor how their patients are recovering long after they have been discharged, which the applicant claims will ultimately result in fewer patients being readmitted to the hospital and higher success rates of surgery. The applicant asserted that the use of the TracPatch will result in decreased rate of subsequent diagnostics and therapeutic interventions and physician visits. The applicant also noted that the TracPatch system will allow physicians to monitor their patients in real-time and take corrective actions in a timely manner, which will result in reduced recovery time as well as improved patient outcomes.
Although the applicant presented these claims, the applicant provided no clinical research evidence to support them; only the testimonials from practicing physicians and large hospital systems were presented. The testimonials addressed the benefits of remote data monitoring and stated that the real-time data would provide better information to understand the effectiveness of surgeries performed, according to one provider. However, there were no reference articles submitted to support the claims made in the application and the testimonials nor were any data provided on the clinical effectiveness of the use of the TracPatch. We are concerned that, without clinical data to support the applicant's claims, we do not have sufficient information to determine whether the use of the TracPatch is a substantial clinical improvement over the current methods to monitor recovery from total knee arthroplasty. We are inviting public comments on whether the TracPatch meets the substantial clinical improvement criterion.
The third criterion for establishing a device category, at § 419.66(c)(3), requires us to determine that the cost of device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. With respect to the cost criterion, the applicant stated that the use of the TracPatch would be reported with either CPT code 99453 (Remote monitoring of physiologic parameter(s) (e.g., weight, blood pressure, pulse oximetry, respiratory flow rate), initial; set-up and patient education on use of equipment) or CPT code 99454 (Remote monitoring of physiologic parameter(s) (e.g., weight, blood pressure, pulse oximetry, respiratory flow rate), initial; device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days). CPT code 99453 is assigned to APC 5012 (Clinic Visits and Related Services), with a proposed CY 2020 payment rate of $120.16, and there is no device offset for the procedure. CPT code 99454 is assigned to APC 5741 (Level 1 Electronic Analysis of Devices), with a proposed CY 2020 payment rate of $38.04, and there is no device offset for the procedure. The applicant stated that the cost of the TracPatch device is $3,250.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The cost of $3,250 for the TracPatch exceeds the applicable APC amount for CPT code 99454 of $38.04 by 8,543.64 percent ($3,250/$38.04 × 100 = 8,543.64 percent). Therefore, the TracPatch appears to meet the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of devices in the category must exceed the cost of the device-related portion of the APC payment amount by at least 25 percent, which means the device cost needs to be at least 125 percent of the device offset amount (the device-related portion of the APC found on the offset list). The two procedure codes that would be billed for the use of the TracPatch do not have a device offset amount, which means the TracPatch would appear to meet the second cost significance requirement.
Section 419.66(d)(3), the third cost significance requirement, provides that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount determined to be associated with the device exceeds 10 percent of the APC payment amount for the related service. The difference between the cost of $3,250 for the TracPatch and the portion of the APC payment for the device of $0.00 exceeds 10 percent at 8,543.64 percent (($3,250 − $0.00)/$38.04 × 100 = 8,543.64 percent). Therefore, the TracPatch appears to meet the third cost significance requirement and, therefore, satisfies the cost significance criterion. We are inviting public comments on whether the TracPatch meets the device pass-through payment criteria discussed in this section.
(3) Vagus Nerve Stimulation (VNS) Therapy® System for Treatment Resistant Depression (TRD)
LivaNova USA Inc. submitted an application for the Vagus Nerve Stimulation (VNS) Therapy® System for Treatment Resistant Depression (TRD). According to the applicant, the VNS Therapy® System consists of two implantable components: A programmable electronic pulse generator and a bipolar electrical lead that is connected to the programmable electronic pulse generator. The applicant stated that the surgical procedure to implant the VNS Therapy® System involves subcutaneous implanting of the pulse generator in the intraclavicular region as well as insertion of the bipolar electrical lead which entails wrapping two spiral electrodes around the cervical portion of the left vagus nerve within the carotid sheath.
According to the applicant, following implant and recovery, the physician programs the pulse generator to intermittently stimulate the vagus nerve at a level that balances efficacy and patient tolerability. The pulse generator delivers electrical stimulation via the bipolar electrical lead to the cervical portion of the left vagus nerve within the carotid sheath thereby relaying information to the brain stem modulating structures relevant to depression. Stimulation typically consists of a 30-second period of “on time,” during which the device stimulates at a fixed level of output current, followed by a 5-minute “off time” period of no stimulation.
The applicant states that a hand-held programmer is utilized to program the pulse generator stimulation parameters, including the current charge, pulse width, pulse frequency, and the on/off stimulus time, which is also known as the on/off duty cycle. Initial settings can be adjusted to enhance the tolerability of the device as well as its clinical effects on the patient. The generator runs continuously, but patients can temporarily turn off the device by holding a magnet over it. The generator can also be turned on and off by the programmer.
The applicant states that the VNS Therapy® System provides indirect modulation of brain activity through the stimulation of the vagus nerve. The vagus nerve, the tenth cranial nerve, has parasympathetic outflow that regulates the autonomic (that is, involuntary) functions of heart rate and gastric acid secretion, and also includes the primary functions of sensation from the pharynx, muscles of the vocal cords and swallowing. It is a nerve that carries both sensory and motor information to and from the brain. Importantly, the vagus nerve has influence over widespread brain areas and it is believed that electrical stimulation of the vagus nerve alters various networks of the brain in order to treat psychiatric disease.
With respect to the newness criterion at § 419.66(b)(1), the applicant received FDA clearance for the VNS Therapy® System for TRD through the premarket approval (PMA) process on July 15, 2005, and the VNS Therapy® for TRD device was introduced to the market in September 2005. However, on May 4, 2007, a national coverage determination (NCD 160.18) was released prohibiting Medicare from covering the use of the VNS Therapy® System for TRD. This NCD remained in effect until February 15, 2019, when CMS determined that the VNS Therapy® for TRD could receive payment if the service was performed in CMS-approved coverage with evidence development (CED) studies. Although the VNS Therapy® System for TRD was introduced to the market in September 2005, Medicare has only covered it for slightly more than 11/2 years. However, § 419.66(b)(1) states that a pass-through payment application for a device must be received within 3 years of when the device either received FDA approval or was introduced to the market. The applicant stated that the VNS Therapy® System for TRD was introduced to the market in September 2005, which means the device pass-through payment application would have needed to have been submitted to CMS by September 2008. However, the pass-through application for the device was not received by CMS until March 2019.
In addition, it appears that the neurostimulator device for the VNS Therapy® System for TRD is the same device that has been used since 1997 to treat epilepsy.[17] The applicant stated the following three differences between the two devices: (1) How the device is programmed to treat epilepsy versus TRD; (2) how the external magnets of the device are used for epilepsy treatment as compared to TRD treatment; and (3) that the battery life of the device to treat epilepsy is different than the battery life of the device when treating TRD. However, it is not clear that these differences demonstrate that the actual device used to treat TRD is any different than the device used to treat epilepsy.
Based on the information presented, we are inviting public comments on whether the VNS Therapy® System for TRD meets the newness criterion.
With respect to the eligibility criterion at § 419.66(b)(3), the applicant claimed that the VNS Therapy® System for TRD is an integral part of a procedure to provide adjunctive treatment of chronic or recurrent depression in adult patients that have failed four or more antidepressant treatments. The VNS Therapy® System for TRD is used for one patient only, comes in contact with human tissue, and is surgically implanted or inserted into the patient. In addition, the applicant stated that the VNS Therapy® System for TRD meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or item for which depreciation and financing expenses are recovered. We have determined that the VNS Therapy® for TRD is not a material or supply furnished incident to a service. We are inviting public comments on whether the VNS Therapy® for TRD meets the eligibility criterion.
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. With respect to the existence of a previous pass-through device category that describes the device used for the VNS Therapy® System for TRD, the applicant suggested a category descriptor of “Generator, neurostimulator (implantable), treatment resistant depression, non-rechargeable.” However, the device category represented by HCPCS code C1767 is described as “Generator, neurostimulator (implantable), non-rechargeable,” which appears to encompass the device category descriptor for the VNS Therapy® System for TRD suggested by the applicant. The applicant asserts that the device category descriptor for HCPCS code C1767 is overly broad and noted the establishment of HCPCS code C1823 (Generator, neurostimulator (implantable), nonrechargeable, with transvenous sensing and stimulation leads), effective January 1, 2019, as an example of where a new device category for a nonrechargeable neurostimulation system to treat central sleep apnea was carved out from the broad category described by HCPCS code C1767.
The applicant believes its proposed category for the device for the VNS Therapy® System for TRD should qualify for a similar carve-out. However, HCPCS code C1823 was established due to specific device features which distinguish that device category from HCPCS code C1767. The applicant for the VNS Therapy® System for TRD requested a new device category based on a beneficiary's diagnosis, but OPPS does not differentiate payment by diagnosis. We welcome public comments on whether the proposed device category for the VNS Therapy® for TRD is not described by any existing categories or by any category previously in effect and meets the requirements of § 419.66(c)(1).
The second criterion for establishing a device category, at 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. The applicant stated that the VNS Therapy® System for TRD would be a substantial clinical improvement because it is a treatment option for beneficiaries that have failed four or more antidepressant treatments. Patients with residual depressive symptoms despite treatment may be demonstrating TRD, but a universally accepted definition of TRD has yet to be achieved.[18] The applicant described the VNS Therapy® System for TRD as a treatment option for beneficiaries who have exhausted all other available options to treat depression. The applicant also provided studies to show how beneficial impacts on the quality of life by using the VNS Therapy® System for TRD can be maintained for multiple years. These studies have been fully reviewed and discussed by the CMS Coverage and Analysis Group's (CAG) national coverage determination with coverage with evidence development for VNS therapy for TRD.[19]
We reviewed the studies provided by the applicant to determine if the VNS Therapy® for TRD and its associated device offered a treatment option for patients unresponsive to or ineligible for currently available treatments. Our review also examined whether the VNS Therapy® System for TRD provides a benefit relative to a previously established device category or other available treatment. To show that the VNS Therapy® for TRD provides a relative benefit, the applicant submitted the same studies it had submitted to the CMS CAG in October 2017. These studies had been submitted as a part of a request to reconsider the NCD in place at that time that prohibited Medicare from providing coverage for the VNS Therapy® System for TRD. Therefore, our review focuses on and is consistent with the eight studies discussed in detail in the “Decision Memo for Vagus Nerve Stimulation (VNS) for Treatment Resistant Depression (TRD)” (CAG-00313R2).[20] We also reviewed an additional study submitted by the applicant for this device pass-through application.
The first study was a randomized control trial.[21] The study was a double-blind, randomized, multi-centered study and its goal was to compare the clinical outcomes in patients diagnosed with TRD of three VNS dose response curves with variable output currents and pulse widths, but with the same duty cycle and pulse frequency. Groups were designated high, medium and low dose and a total of 331 patients participated in the study. Enrollment criteria included: Individuals 18 years of age or older with a diagnosis of a chronic (>2 years) or recurrent (≥2 prior episodes) MDD or bipolar disorder and a current diagnosis of MDE as defined by the DSM-4 and determined using the Mini- International Neuropsychiatric Interview; a history of failure to respond to four or more adequate dose/duration of antidepressant treatment trials from at least two different antidepressant treatment categories as documented through medical history and record review; a minimum pre-study and baseline score of 24 on the MADRS, with no greater than a 25-percent decrease between the pre-study and baseline visits; current recipient of at least one antidepressant treatment (medication or ECT); and a stable regimen of all current antidepressant treatments for at least 4 weeks before the baseline visit. Furthermore, patients with bipolar disease had to be receiving a mood stabilizer at baseline. Exclusion criteria included a history of psychotic disorder, a history of rapid cycling bipolar disorder, a current history of bipolar disorder mixed phase, a history of borderline personality disorder, clinically significant suicidal intent at the time of screening, a history of drug/alcohol dependence in the last year, and a previous history of use of VNS. The only study personnel unblinded to the assignment of treatment groups were study programmers at each site and clinical engineers who were employed by the sponsor to monitor the programmers.
Eligible patients were implanted with a VNS Therapy® System for TRD device and then randomized to low, medium or high target settings. The low dose was chosen to deliver active stimulation at the lowest available setting for amplitude of output current with a narrow pulse width (0.25 mA; 130 μs). The high dose was chosen to be consistent with higher levels of stimulation, often seen in the treatment of epilepsy (1.25-1.5mA; 250 μs). The medium dose was chosen to track closely to the high dose, but without overlap (0.5-1.0 mA; 250 μs), potentially providing a better opportunity to demonstrate efficacy versus the low dose.
The study authors reported that in neither the acute nor the long-term phase were there any significant differences in response or remission rates among the treatment groups (response was defined as ≥50 percent improvement from baseline; remission was defined as ≤14 on the Inventory of Depressive Symptomatology Clinician Administered Version (IDS-C)). However, the authors stated that although effect sizes were limited, statistically significant decreases in mean depression scores (based on IDS-C) were observed in all groups. Mean IDS-C scores decreased approximately 15 points from baseline through week 50. The authors concluded that within the limits of this study, the VNS Therapy® System for TRD provided as adjunctive treatment to patients diagnosed with TRD as described above offers significant improvement at study endpoint as compared with baseline and that the effect is durable over 1 year. The authors also stated that higher electrical dose parameters were associated with higher response durability.
The second study by Aaronson et al. was a prospective, multi-center, open label, nonrandomized, longitudinal, naturalistic, observational post marketing FDA surveillance study for which a registry was designed to follow the clinical response and outcome over 5 years of patients with a major depressive disorder (MDD), including those with unipolar or bipolar depression.[22] Patients participating in this study were recruited by physician referral and received treatment as usual (TAU) and VNS or just TAU. Subjects included those who were being evaluated for surgery or anesthesia to undergo VNS implantation, patients who had signed consent forms to receive a VNS device, patients who had scheduled VNS implantation surgery, and patients who had completed participation in a previous study termed the D-21 study [NCT 00305565: Study Comparing Outcomes for Patients With Treatment Resistant Depression Who Receive VNS Therapy at Different Doses].
The VNS arm included 335 patients without prior VNS treatment as well as 159 patients who received VNS treatment in the previous D-21 investigation. The TAU arm contained 301 patients. Eligibility criteria for the study included: Age 18 years or older; a current major depressive disorder diagnosed according to DSM-IV-TR criteria and confirmed by the Mini International Neuropsychiatric Interview of at least 2 years in duration (unipolar or bipolar depression) or a history of at least three depressive episodes including the current major depression episode; and a history of inadequate response to at least four depression treatments (including maintenance pharmacotherapy, psychotherapy and ECT). Maintenance pharmacotherapy was defined as dosage per Physician's Desk Reference labeling for a minimum of 4 weeks. Exclusion criteria included a history of schizophrenia, schizoaffective disorder, other psychotic disorder, current psychosis, history of rapid cycling bipolar disorder and a CGI score <4. Other than the patients from the D-21 study, the individuals in the study had not previously experienced VNS.
All patients (except those who participated in the D-21 study) were allowed to choose the treatment arm of their choice. However, the patients could be assigned to receive the alternate treatment due to various reasons (for example, availability of surgical implantation at a site, failure to receive insurance coverage for the procedure, availability of donated VNS devices, among others). There were no restrictions on concomitant treatments.
Post baseline follow-up visits for all patients were scheduled to occur at 3, 6, 9, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months. During these scheduled visits, data were collected on medical status, need for adjustment of mood disorder therapy and concomitant treatments. Also, various depression scale ratings were collected as well as data concerning mortality and suicidality. Central raters (un-blinded nurses with special training) conducted an assessment of suicidality via telephone after each patient visit.
Propensity scores were used to adjust for imbalance of baseline prognostic factors between treatment arms. The ITT population included those study participants who completed a baseline visit, received their respective treatment and completed at least one post-baseline treatment.
Of the 494 patients in the VNS arm, 300 (61 percent) completed all 5 years of data. It is noted that the D-21 patients rolled over into this study at various time points after implantation. Of the 301 TAU patients, 138 (46 percent) completed all 5 years of data.
Approximately 70 percent of all study participants were female and over 90 percent were Caucasian in both groups. A diagnosis of severe recurrent major depressive disorder was reported in 46 percent of the patients in the VNS arm and 32 percent in the TAU arm. A diagnosis of primary bipolar I or bipolar II disorder was reported in 28 percent of patients in the VNS arm and 24 percent in the TAU arm. Other psychiatric diagnoses included moderate recurrent major depression, moderate single episode major depression, severe recurrent major depression, and severe single episode major depression. Fifty-seven percent of the VNS group and 40 percent of the TAU group had experienced past treatments of ECT.
Of the patients who withdrew early, 40 percent (195) were from the VNS arm and 54 percent (163) were from the TAU arm. The investigators observed that reasons for early withdrawal were similar between the treatment arms. It was also noted that after premature closure of a study site where 48 patients were participating in the TAU arm, most of the patients at that site were either lost to follow up or were dropped from the study for nonadherence.
The primary efficacy measure was a response rate, defined as a decrease of ≥50 percent in baseline Montgomery-Äsberg Depression Rating Scale (MADRS) score at any post-baseline visit during the study. The study authors report a 5-year cumulative response rate of 67.6 percent [95 percent CI = 63.4, 71.7] in the VNS group and 40.9 percent [95 percent CI = 35.4, 47.1] in the TAU group (p <0.001). Also, the authors note that the cumulative percentage of first-time responders in the VNS Therapy® System arm was approximately double that in the TAU arm at all post-baseline points in time through the 5 years of the study. The authors concluded that adjunctive treatment with the use of the VNS Therapy® System device resulted in superior outcomes in both effectiveness and mortality over a 5-year period compared with treatment as usual for patients diagnosed with chronic, severe TRD.
A third study by Conway et al. compared quality of life (QoL) changes associated with treatment using VNS + TAU versus TAU in patients diagnosed with unipolar and bipolar TRD.[23] QoL data were gathered on all patients using the patient reported Quality of Life Enjoyment and Satisfaction Questionnaire Short Form (Q-LES-Q-SF), as well as the clinician reported CGI-I scale.
The data were collected as part of the 5 year registry described in Aaronson et al. (2017), noted above. However, the patient population analyzed was somewhat different, in that patients who rolled over from the previous D-21 study (Aaronson et al., 2017) were excluded so that all subjects had the same follow-up period. Furthermore, patients who were not depressed at baseline according to their MADRS scores, were also excluded. Therefore, the data from 328 patients treated with VNS + TAU and 271 patients treated with TAU were analyzed.
Females comprised 68.6 percent of the VNS + TAU group and 70.8 percent of the TAU group; 97 percent of the VNS + TAU group and 90.8 percent of the TAU group were Caucasian. Major depressive disorder was diagnosed in 70.4 percent of the VNS + TAU group and 78.2 percent of the TAU group. Bipolar I or II disorder (most recent episode depressed) was diagnosed in 29.6 percent of the VNS + TAU group and 21.7 percent of the TAU group.
Paired data analysis (for example, change in Q-LES-Q-SF versus percent change in MADRS score) were matched by assigned visit number; however these assessments for any given month might have taken place on separate visits (visit window was ±45 days until 1 year of follow-up; thereafter ±90 days). The authors report that the time difference between the paired measures was similar between the two groups and was a median of 4 weeks. Missing data were excluded if one component of a paired observation was lacking.
Among the results, the authors reported that on average, there was a comparative QoL advantage observed for the VNS + TAU group as early as 3 months, which was sustained throughout the 5-year study. The VNS + TAU treatment group demonstrated a significantly greater improvement in Q-LES-Q-SF scores than the TAU treatment group for the same percentage drop in MADRS score from baseline. The authors reported a similar pattern when the Clinical Global Impression (CGI) score was used. The authors concluded that adjunctive treatment using the VNS Therapy® System for TRD provided greater and sustained improvements in QoL as compared to TAU alone. Further, TRD patients treated with THE VNS Therapy® System for TRD experienced clinically meaningful QoL improvements even with symptom reduction less than the traditional 50 percent reduction used to describe a “response” to treatment.
The goal of the fourth study by Olin et al. was to characterize all-cause mortality rate and suicide risk in patients diagnosed with TRD who were treated with standard TAU and those treated with VNS + TAU.[24]
The study was an observational, open label, longitudinal, multi-center registry. The registry was a post-market surveillance study required by the FDA as a condition of approval of the TRD indication for VNS therapy to evaluate long-term patient outcomes. Patients were followed for 60 months, until withdrawal from the study, death or study completion.
Patients in the VNS + TAU group had been followed for an average of 3.2 years; those in the TAU group had been followed for 2.1 years. Because baseline characteristics of each group showed areas of imbalance, the use of propensity score modeling was required.
Suicidal ideation was evaluated by a central ratings group using both the Assessment of Suicidality (AOS) [Has the patient made a suicidal gesture or attempt since the last visit; yes or no] and MADRS Item 10, score ≥4, [“Probably better off dead. Suicide thoughts are common, and suicide is considered a possible solution, but without specific plans or intention”]. Among other criteria, eligible patients for the Registry were: Individuals who had been diagnosed with a current MDE according to the DSM-IV-TR criteria; individuals who had been in the current depressive episode for at least 2 years or had experienced at least three lifetime MDEs (including the current episode); individuals who had an inadequate response to four or more adequate antidepressive treatments; and individuals who had a CGI-S of 4 or greater. Exclusion criteria included schizophrenia, schizoaffective disorder, any other psychotic disorder, a history of rapid cycling bipolar disorder, or previous use of VNS.
After completing a screening visit, patients self-selected the treatment course that they believed was the best medical option. However, after the study started, there were some treatment arm changes due to the implementation of a Medicare noncoverage policy and subsequent lack of reimbursement for the VNS procedure. The authors stated that they believed that the majority of individuals who chose VNS + TAU did so as a final alternative when all other treatments failed.
There were 335 patients in the VNS + TAU group and 301 subjects in the TAU group. Average age of all patients was between 48 and 50 years. In the VNS + TAU group, 68.4 percent of patients were female; 96.4 percent were Caucasian. In the TAU group, 70.1 percent of the patients were female; 91 percent were Caucasian. Major depressive disorder was diagnosed in 71.1 percent of the VNS + TAU group and 76.4 percent of the TAU group. Bipolar disorder was diagnosed in 28.9 percent of the VNS + TAU group and 23.6 percent of the TAU group. In the VNS + TAU group, 58.2 percent of patients had a history of ECT; in the TAU group, 45.2 percent had a history of ECT treatment.
The authors found that the standardized all-cause mortality (4.46 [VNS + TAU] versus 8.06 [TAU only] per 1,000 person years) and suicide rates (0.88 [VNS + TAU] versus 1.61 [TAU only] per 1,000 person years) for patients treated with VNS + TAU were approximately half that of the patients treated only with TAU. However, the specific results were not statistically different due to the low mortality rates in both groups. Similar results were noted when stratifying by propensity score quintiles.
However, both groups had a significantly higher rate of suicide relative to the U.S. population; VNS + TAU 5.72 (95 percent CI; 0.07, 31.82) and TAU 9.98 (95 percent CI; 0.13, 55.55). The authors stated that individuals treated with VNS + TAU had a 10 percent—20 percent reduction in the risk of suicidality as compared to individuals treated with TAU alone, as measured by the MADRS Item 10 score. However, when the Assessment of Suicidality was used, no statistical difference was noted between treatment groups.
The authors further noted that the side effects profiles as measured by the Frequency, Intensity and Burden of Side Effects Rating questionnaire demonstrated that the percentage of unacceptable side effects for VNS + TAU was higher than that of TAU; however, this difference lessens over time.
The authors concluded that treatment with adjunctive VNS in this population can potentially lower the risk of all-cause mortality, suicide and suicide attempts.
The fifth study by Berry et al. performed a Bayesian meta-analysis of patient level data from six clinical studies that had been previously performed and supported by the manufacturer of the VNS Therapy® System for TRD device (Cyberonics).[25] The investigations included in the meta-analysis were two single arm studies of VNS + TAU, a randomized trial of VNS + TAU versus TAU, a single arm study of patients receiving only TAU, a randomized trial of VNS + TAU comparing different VNS intensities, and a nonrandomized registry of patients who received either VNS + TAU or TAU.
The MADRS and CGI-I were selected as the primary endpoints for the meta-analysis, though they were not necessarily the primary outcome measures in the individual studies analyzed. Outcomes of interest were response, remission and sustained response based on these scales of disease severity. Response was assessed across five of the six studies using the MADRS and defined as a follow up score of at least a 50 percent reduction compared to baseline score. Response per the CGI Improvement subscale (CGI-I) was defined as a follow-up score of 1—“very much improved” or 2—“much improved.” Remission was assessed using the MADRS (score at follow up <10 points). The study designs of the original investigations included in the meta-analysis necessitated that the TAU group data be limited to two trials for the CGI-I scale and one trial for the MADRS scale.
Because only one of the studies randomized patients to VNS + TAU or TAU groups, the authors used propensity scores to control for potential differences between treatment groups. The researchers calculated propensity scores using standard methods and included the score in mixed effects repeated measures models to account for the fact that the patients in all of the different studies arrived at their assessment points at different points in real time.
In the final analysis, there were 425 TAU patients, and 1,035 VNS + TAU patients. The authors reported that while outcomes for both groups tended to improve, those who were treated with VNS + TAU demonstrated better outcomes over 96 weeks of treatment. The repeated measures analysis showed that, compared to patients who received TAU only, those who received VNS + TAU had lower MADRS scores (mean difference −3.26 points; 95 percent CI: −3.99, −2.54). The odds of a MADRS response in the VNS + TAU group was 3.19 times greater (95 percent CI: 2.12, 4.66) and the odds of a MADRS remission was 4.99 times greater (95 percent CI: 2.93, 7.76) than those individuals who received TAU alone. Similarly, those in the VNS + TAU group had lower CGI-I scores (mean difference of −0.49 points; 95 percent CI: −0.59, −0.39) and had 7 times the odds of a CGI-I response (95 percent CI: 4.63, 10.83) compared to individuals receiving TAU alone. The authors concluded that the Bayesian meta-analysis demonstrated consistent superiority of VNS + TAU as compared to the use of TAU alone. The authors stated that, for patients diagnosed with TRD, treatment using VNS + TAU has greater response and remission rates that are more likely to persist than TAU.
The sixth study was another meta-analysis study, by Cimpianu et al., involving a systematic review that summarized the evidence regarding the use of invasive and noninvasive VNS for the treatment of TRD and other psychiatric disorders.[26] The study authors searched through the PubMed/MEDLINE database (up to September 2016) to identify relevant publications for their review.
The authors noted that very few studies exhibited a double-blind randomized sham controlled design; instead the majority were single blinded, open label observational or cohort investigations. Nonetheless, the text of the review pertaining to invasive VNS in the treatment of depressive disorders focused on those studies that used a randomized double blind design in at least one period (beginning) of a trial. However, of those investigations described, the authors observed that, for the most part, effect sizes were either not reported at all or were not reported in detail.
The authors found that the application of the VNS Therapy® System for TRD received a mixed recommendation in national guidelines. They stated that there is a consensus in the field that further randomized controlled studies, as well as long term naturalistic studies are needed for the future evaluation of the efficacy of VNS for the treatment of depression.
The seventh study was a meta-analysis study as well. Daban et al. performed a systematic review of studies published between 2000 and September 2007, found in the Medline, Psychological Abstracts and Current Content databases, that evaluated the safety and efficacy of VNS therapy in TRD patients.[27] The authors reviewed 6 short-term studies and 12 long-term studies. The measured outcomes consisted of baseline depression severity compared to ratings 2 weeks after implantation and after 3 months in acute and long-term studies and also after 6, 9, 12, and subsequent months in long-term studies. The authors stated their review demonstrated that VNS therapy has been reported to have antidepressant effects in open and long-term studies and that these effects may be sustained. However, they also noted that the evidence base is weak and the only blinded randomized trial was inconclusive, and they suggest more double-blinded, sham-controlled, randomized studies be conducted.
The eighth and final study discussed in the NCD with CED reconsideration decision memo was also a meta-analysis study. This study, by Martin et al., performed a systematic review to determine the efficacy of VNS for the treatment of depression.[28] In order to achieve this goal, a review of the pertinent scientific literature available until December 2010 was conducted. The databases searched were Medline/PubMed, Embase, The Cochrane Controlled Trials Register, Pascal Biomed and CINAL. References found on the web pages of ongoing clinical trials were also examined. Selection criteria included any RCT or pre/post design study, in which depressive symptomatology was measured and the intervention studied was VNS. The outcomes assessed were levels of depression severity as measured by depression symptomatology scales and percentage of responders, defined as subjects whose symptomatology scores demonstrated ≥50 percent change from baseline. The outcomes were analyzed in the short term (≤12 weeks), medium term (>12 and <48 weeks) and long term (>48 weeks).
In their literature search, the authors found only one randomized controlled trial involving VNS for treatment of depression. The primary outcome was a response rate as measured by the Hamilton Depression Rating Scale (HDRS). No statistically significant differences between the active and the placebo group were noted. However, the meta analysis of efficacy for the uncontrolled pre/post studies, showed a significant reduction in HDRS scores and the percentage of responders was 31.8 percent ([23.2 percent-41.8 percent]. p<0.001). To study the cause of this heterogeneity, a meta-regression was performed, which implied that an 84 percent variation in effect size across the studies was explained by baseline severity of depression (p<0.0001). In the uncontrolled pre/post studies that were meta-analyzed, the incidence density of suicide or attempted suicides was practically identical in the studies of the use of VNS and selective serotonin reuptake inhibitors. Therefore, the authors stated that the use of VNS did not appear to provoke suicide conduct any more than treatment with the comparator antidepressant.
The authors concluded that insufficient data exist to describe VNS as an effective treatment for depression. Moreover, they stated that the ability of the uncontrolled studies to show causality is limited and positive outcomes might be caused by placebo effect, regression to the mean, spontaneous remission, differences in patient characteristics or the Hawthorn effect (the alteration of behavior by subjects in a study because they are aware of being observed). They stated that evidence to determine the benefit (or not) of VNS therapy should be based on long-term clinical trials with a control group aimed at monitoring the possible latency involved in the effect of the use of VNS, as well as the associated adverse effects.
The applicant submitted an additional study by Kumar et al. This was an observational study attempting to compare the duration of treatment response for patients that received VNS and treatment as usual (TAU) together as compared to the duration of response for patients receiving only TAU.[29] Data from 271 participants receiving TAU and 328 participants receiving VNS + TAU were analyzed. Response was defined as ≥50 percent decrease in baseline MADRS score at post-baseline visit and was considered retained until the decrease was <40 percent. In the VNS + TAU group, 62.5 percent (205/328) of participants had a first response over 5 years compared with 39.9 percent (108/271) in the TAU group. The time to first response was significantly shorter for VNS + TAU participants than for TAU participants (P<0.01). The authors of the study concluded that combining VNS therapy with TAU for patients having severe TRD leads to a faster response and a greater likelihood of response to treatment as compared to TAU alone. Also, the duration of the treatment response is longer for those receiving VNS + TAU.
With regard to the studies presented, we are concerned that the clinical utility of the VNS Therapy® System for TRD has not been well demonstrated by the applicant. The majority of the studies presented were case series, open labeled, or not randomized. The literature presented did not appear to have comparator arms with current treatment options like Magnetic Stimulation (TMS). We note that the CMS CAG found that all of the studies they reviewed and submitted for this application indicated some positive findings regarding clinical improvement with the use of VNS therapy. However, the CMS CAG also identified significant issues with the studies that either reduced the overall quality and strength of evidence and/or the clinical significance of the outcomes. Nevertheless, some of the published evidence suggests that the use of VNS is a promising treatment for patients diagnosed with TRD, which contributed to CMS CAG's decision to propose coverage with evidence development.
We are inviting public comments on whether the VNS Therapy® System for TRD meets the substantial clinical improvement criterion.
The third criterion for establishing a device category at § 419.66(c)(3) requires us to determine that the cost of the device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. With respect to the cost criterion, the applicant stated that the VNS Therapy® System for TRD would be reported with CPT code 64568 (Incision for implantation of cranial nerve (e.g., vagus nerve) neurostimulator electrode array and pulse generator), which is assigned to APC 5464 (Level 4 Neurostimulator and Related Services). The proposed CY 2020 payment rate for CPT code 64568 is $28,511.24, with a device offset of $24,168.98. The applicant stated that the cost of the VNS Therapy® System for TRD device is $42,000.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The cost of $42,000 for the VNS Therapy® System for TRD device exceeds the applicable APC amount for CPT code 64568 of $28,511.24 by 147.31 percent ($42,000/$28,511.24 × 100 = 147.31 percent). Therefore, the VNS Therapy® System for TRD appears to meet the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of devices in the category must exceed the cost of the device-related portion of the APC payment amount by at least 25 percent, which means the device cost needs to be at least 125 percent of the device offset amount (the device-related portion of the APC found on the offset list). The estimated cost of $42,000 for the VNS Therapy® System for TRD device exceeds the device-related portion of the APC amount for the related service of $24,168.98 by 173.78 percent ($42,000/$24,168.98 × 100 = 173.78 percent). Therefore, the VNS Therapy® System for TRD appears to meet the second cost significance requirement.
Section 419.66(d)(3), the third cost significance requirement, requires that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount determined to be associated with the device exceeds 10 percent of the APC payment amount for the related service. The difference between cost of $42,000 for the VNS Therapy® System for TRD and the portion of the APC payment for the device of $24,168.98 exceeds 10 percent at 62.54 percent (($42,000−$24,168.98)/$28,511.24 × 100 = 62.54 percent). Therefore, the VNS Therapy® System for TRD appears to meet the third cost significance requirement and, therefore, satisfies the cost significance criterion. We are inviting public comments on whether the VNS Therapy® System for TRD meets the device pass-through payment criteria discussed in this section, including the cost criterion.
(4) Optimizer® System
Impulse Dynamics submitted an application for a new device category for transitional pass-through payment status for the Optimizer® System. According to the applicant, the Optimizer® System is an implantable device that delivers Cardiac Contractility Modulation (CCM) therapy for the treatment of patients with moderate to severe chronic heart failure. CCM therapy is intended to treat patients with persistent symptomatic heart failure despite receiving guideline directed medical therapy (GDMT). The applicant stated that the Optimizer System consists of the Optimizer Implantable Pulse Generator (IPG), Optimizer Mini Charger, and Omni II Programmer with Omni Smart Software. Lastly, the applicant stated that the Optimizer® System delivers CCM signals to the myocardium. CCM signals are nonexcitatory electrical signals applied during the cardiac absolute refractory period that, over time, enhance the strength of cardiac muscle contraction.
With respect to the newness criterion at § 419.66(b)(1), the applicant received a Category B-3 Investigational Device Exemption (IDE) from the FDA on April 6, 2017. Subsequently, the applicant received its premarket approval (PMA) application from the FDA on March 21, 2019. We received the application for a new device category for transitional pass-through payment status for the Optimizer® System on February 26, 2019, which is within 3 years of the date of the initial FDA approval or clearance. We are inviting public comments on whether the Optimizer® System meets the newness criterion.
With respect to the eligibility criterion at § 419.66(b)(3), according to the applicant, the Optimizer® System is integral to the CCM therapy service provided, is used for one patient only, comes in contact with human skin, and is applied in or on a wound or other skin lesion. The applicant also stated that the Optimizer® System meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or items for which depreciation and financing expenses are recovered, and it is not a supply or material furnished incident to a service.
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any of the existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. We have not identified an existing pass-through payment category that describes the Optimizer® System.
The second criterion for establishing a device category, at § 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. The applicant stated that the use of CCM significantly improves clinical outcomes for a patient population compared to currently available treatments. With respect to this criterion, the applicant submitted studies that examined the impact of CCM on quality of life, exercise tolerance, hospitalizations, and mortality.
The applicant noted that the use of the Optimizer® System significantly improves clinical outcomes for patients with moderate-to-severe chronic heart failure, and specifically improves exercise tolerance, quality of life, and functional status of patients that are otherwise underserved. The applicant claims that the Optimizer® System fulfills an unmet need because there is currently no therapeutic medical device therapies available for the 70 percent of heart failure patients who have New York Heart Association (NYHA) Class III heart failure, normal QRS duration and reduced ejection fraction (EF).
The applicant presented several studies to support these claims. According to the applicant, the results of a randomized clinical study in which patients with NYHA functional Class III, ambulatory Class IV heart failure despite OMT, an EF from 25-45 percent, or a normal sinus rhythm with QRS duration <130 ms (n=160) were randomized to continued medical therapy (n=86) or CCM with the Optimizer® System (n=74) for 24 weeks showed a statistically significant improvement in the primary endpoint of peak oxygen consumption (pVO2 = 0.84, 95 percent Bayesian credible interval 0.123 to 1.52) compared with the patients who were randomized to continued medical therapy.[30] The secondary endpoint of quality of life, measured by Minnesota Living with Heart Failure Questionnaire (MLWHFQ) (p<0.001), 6-minute hall walk test (p=0.02), and an NYHA function class assessment (p<0.001) were better in the treatment group versus control group. The secondary endpoint of heart failure-related hospitalizations was lowered from 10.8 percent to 2.9 percent (p=0.048). The applicant also reported a registry study of 140 patients with a left ventricular ejection fraction from 25-45 percent receiving CCM therapy with a primary endpoint of comparing observed survival to Seattle Heart Failure Model (SHFM) predicted survival over 3 years of follow-up. All patients implanted with the Optimizer® System at participating centers were offered participation and 72 percent of patients agreed to enroll in the registry. There were improvements in quality of life markers (MLWHFQ) and a 75-percent reduction in heart failure hospitalizations (p<0.0001). Survival at 3 years was similar between the two study arms with CCM at 82.8 percent [73.4 percent-89.1 percent] and SHFM at 76.7 percent (p = 0.16). However, for patients with a left ventricular ejection fraction from 35-45 percent receiving CCM therapy, the 3-year mortality for CCM therapy was significantly better than predicted with 88 percent for CCM compared to 74.7 percent for SHFM (p=0.0463).[31] The applicant presented a randomized, double blind, crossover study of CCM signals with 164 patients with EF ≤35 percent and NYHA Class II (24 percent) or III (76 percent) symptoms who received a CCM pulse generator. After the 6-month treatment period, results indicated statistically significantly improved peak VO2 and MLWHFQ (p=0.03 for each parameter), concluding that CCM signals appear to be safe for patients and that exercise tolerance and quality of life were significantly better while patients were receiving active CCM treatment.[32]
A study was conducted with 68 consecutive heart failure patients with NYHA Class II or III symptoms, QRS duration ≤130 ms, and who had been implanted with a CCM device between May 2002 and July 2013 in Germany. Based upon pre-implant SHFM survival rates, 4.5 years mean follow-up, and an average patient age of 61 years old, the study found lower mortality rates for CCM therapy group with 0 percent at 1 year, 3.5 percent at 2 years, and 14.2 percent at 5 years, compared to 6.1 percent, 11.8 percent, and 27.7 percent predicted by SHFM, respectively (p=0.007). [33] In a study on long-term outcomes, 41 consecutive heart failure patients with left ventricular ejection fraction (EF) <40 percent receiving CCM therapy were compared to a control group of 41 similar heart failure patients and primarily evaluated for all-cause mortality, as well as heart failure hospitalization, cardiovascular death, and a death and heart failure hospitalization composite. After 6 years of follow-up, the results showed that all-cause mortality was lower for the CCM group as compared to the control group (39 percent versus 71 percent respectively, p=0.001), especially among patients with EF ≥25-40 percent with 36 percent for the CCM group versus 80 percent for the control group (p<0.001). Although heart failure hospitalization was similar between the treatment and control cohorts, there was a significantly lower heart failure hospitalization rate for CCM patients with EF ≥25-40 percent (36 percent versus 64 percent respectively, p=0.005).[34] The applicant also presented additional studies [35 36] that presented similar conclusions to the studies discussed above, noting that CCM therapy provided improvements in quality of life, exercise capacity, NYHA class, and mortality rates.
We noted several concerns with the studies presented by the applicant. One concern regarding the evidence for the Optimizer® System involves the mixed mortality outcomes presented. Three studies showed significantly lower mortality rates with the use of CCM compared to controls or predicted mortality. Each of these studies focused on slightly different mortality outcomes, including all-cause mortality, a composite of death and heart failure hospitalization, and cardiac mortality rates from 1 to 5 years. Two studies show mixed results. For the first, 3-year survival was not significant for the overall population, despite a significantly higher survival rate found in a subpopulation. For the second, mortality rates were significant compared to predictions at 1 year, but not 3 years. The final study did not report significance in its overall survival at 2 years. Although the studies and trials presented show improvements in mortality when evaluating CCM therapy with comparators, the studies have small sample sizes and limited timeframes for measuring survival. Additionally, three studies compared observed mortality rates to statistically projected mortality rates. In the two studies with observed mortality rates, the overall improvement in mortality was not significant, despite some significance found in subanalyses. These issues raise concerns about the strength of the conclusions related to the use of CCM therapy improving patient outcomes.
Another concern with the studies presented for the Optimizer® System is that the included study population may not be necessarily representative of the Medicare beneficiary population. Several studies had a predominantly white, male patient population, which could make generalization of study results to a more diverse Medicare population difficult. Additionally, the average age of patients for several studies was under 65 years old, which may also be a limitation in applying these study results to the Medicare population.
Overall, there is a lack of evidence from large trials for the CCM therapy provided by the Optimizer® System. The studies presented had sample sizes fewer than 500 patients. Other limitations include the potential placebo effects and selection bias that may have impacted study results. Only two studies presented were randomized and only one of those two was a double-blinded study. For the remaining studies, no blinding occurred to minimize potential biases, which indicates that patients and researchers knew they were receiving CCM therapy. This is a limitation because observed outcomes may be impacted by the placebo effect. Although most studies matched participants for similar demographics, there could be systematic differences and unmeasured bias between the two groups beyond the similarities addressed in the study that could affect outcomes. The lack of randomization may have implications for the strength of the studies' conclusions.
Based upon the evidence presented, we are inviting public comments on whether the Optimizer® System meets the substantial clinical improvement criterion.
The third criterion for establishing a device category, at § 419.66(c)(3), requires us to determine that the cost of the device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. The applicant provided the following information in support of the cost significance requirements. The applicant stated that the Optimizer® System would be reported with CPT codes 0408T, 0409T, 0410T, 0411T, 0412T, 0413T, 0414T, 0415T, 0416T, 0417T, and 0418T. The associated APCs are APC 5231 (Level 1 ICD and Similar Procedures) and APC 5222 (Level 2 Pacemaker and Similar Procedures). To meet the cost criterion for device pass-through payment status, a device must pass all three tests of the cost criterion for at least one APC. For our calculations, we used APC 5222, which had a CY 2019 payment rate of $7,404.11 at the time the application was received. Beginning in CY 2017, we calculate the device offset amount at the HCPCS/CPT code level instead of the APC level (81 FR 79657). CPT code 0410T had a device offset amount of $2,295.27 at the time the application was received. According to the applicant, the cost of the Optimizer® System was $15,700.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The estimated average reasonable cost of $15,700 for the Optimizer® System exceeds 212 percent of the applicable APC payment amount for the service related to the category of devices of $7,404.11 ($15,700/$7,404.11 × 100 = 212 percent). Therefore, we believe the Optimizer® System meets the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of the devices in the category must exceed the cost of the device-related portion of the APC payment amount for the related service by at least 25 percent, which means that the device cost needs to be at least 125 percent of the offset amount (the device-related portion of the APC found on the offset list). The estimated average reasonable cost of $15,700 for the Optimizer® System exceeds the cost of the device-related portion of the APC payment amount for the related service of $2,295.27 by 684 percent ($15,700/$2,295.27) × 100 = 684 percent. Therefore, we believe that the Optimizer® System meets the second cost significance requirement.
The third cost significance requirement, at § 419.66(d)(3), provides that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount for the device must exceed 10 percent of the APC payment amount for the related service. The difference between the estimated average reasonable cost of $15,700 for the Optimizer® System and the portion of the APC payment amount for the device of $2,295.27 exceeds the APC payment amount for the related service of $7,404.11 by 181 percent (($15,700−$2,295.27)/$7,404.11) × 100 = 181 percent). Therefore, we believe that the Optimizer® System meets the third cost significance requirement.
We are inviting public comments on whether the Optimizer® System meets the device pass-through payment criteria discussed in this section, including the cost criterion for device pass-through payment status.
(5) AquaBeam® System
PROCEPT BioRobotics Corporation submitted an application for a new device category for transitional pass-through payment status for the AquaBeam® System as a resubmission of their CY 2019 application. The AquaBeam® System is intended for the resection and removal of prostate tissue in males suffering from lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH). The applicant stated that this is a very common condition typically occurring in elderly men. The clinical symptoms of this condition can include diminished urinary stream and partial urethral obstruction.[37] According to the applicant, the AquaBeam® system resects the prostate to relieve symptoms of urethral compression. The resection is performed robotically using a high velocity, nonheated sterile saline water jet (in a procedure called Aquablation). The applicant stated that the AquaBeam® System utilizes real-time intra-operative ultrasound guidance to allow the surgeon to precisely plan the surgical resection area of the prostate and then the system delivers Aquablation therapy to accurately resect the obstructive prostate tissue without the use of heat. The materials submitted by the applicant state that the AquaBeam® System consists of a disposable, single-use handpiece as well as other components that are considered capital equipment.
With respect to the newness criterion at § 419.66(b)(1), the FDA granted a De Novo request classifying the AquaBeam® System as a Class II device under section 513(f)(2) of the Federal Food, Drug, and Cosmetic Act on December 21, 2017. The application for a new device category for transitional pass-through payment status for the AquaBeam® System was received on March 1, 2018, which is within 3 years of the date of the initial FDA approval or clearance. We are inviting public comments on whether the AquaBeam® System meets the newness criterion.
With respect to the eligibility criterion at § 419.66(b)(3), according to the applicant, the AquaBeam® System is integral to the service provided, is used for one patient only, comes in contact with human skin, and is applied in or on a wound or other skin lesion. The applicant also claimed the AquaBeam® System meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or items for which depreciation and financing expenses are recovered, and it is not a supply or material furnished incident to a service. However, in the CY 2019 OPPS/ASC proposed and final rules, we cited the CY 2000 OPPS interim final rule with comment period (65 FR 67804 through 67805), where we explained how we interpreted § 419.43(e)(4)(iv). We stated that we consider a device to be surgically implanted or inserted if is surgically inserted or implanted via a natural or surgically created orifice, or inserted or implanted via a surgically created incision. We also stated that we do not consider an item used to cut or otherwise create a surgical opening to be a device that is surgically implanted or inserted. We consider items used to create incisions, such as scalpels, electrocautery units, biopsy apparatuses, or other commonly used operating room instruments, to be supplies or capital equipment, not eligible for transitional pass-through payments. We stated that we believe the function of these items is different and distinct from that of devices that are used for surgical implantation or insertion. Finally, we stated that, generally, we would expect that surgical implantation or insertion of a device occurs after the surgeon uses certain primary tools, supplies, or instruments to create the surgical path or site for implanting the device. In the CY 2006 OPPS final rule with comment period (70 FR 68329 and 68630), we adopted as final our interpretation that surgical insertion or implantation criteria include devices that are surgically inserted or implanted via a natural or surgically created orifice, as well as those devices that are inserted or implanted via a surgically created incision. We reiterated that we maintain all of the other criteria in § 419.66 of the regulations, namely, that we do not consider an item used to cut or otherwise create a surgical opening to be a device that is surgically implanted or inserted.
The applicant resubmitted their application with additional information that they believe supports their stance that the device should be considered eligible under the device pass-through payment eligibility criteria. The applicant stated that the AquaBeam® System's handpiece is temporarily surgically inserted into the urethra via the urinary meatus. The applicant indicated that the AquaBeam® System's handpiece does not create an incision or surgical opening or pathway, but instead ablates prostate tissue. The applicant further stated that the device only cuts the prostatic tissue after being inserted into the prostatic urethra and therefore it should be considered eligible. The applicant also stated that the prostatic urethra tissue is cut because it is at the center of the obstruction in the prostate. Additionally, the applicant explained that to relieve the symptoms of BPH, both the prostatic urethra and prostate tissue encircling the prostatic urethra must be ablated, or cut, to relieve the symptoms of BPH and provide some additional clearance for future swelling or growth of the prostate. The applicant stated that the prostatic urethra tissue is not cut or disturbed to access the prostate tissue underneath, but the removal of the prostatic urethra is a key aspect of treating the obstruction that causes BPH symptoms. Finally, the applicant believes that clinically the distinction between the prostatic urethra tissue and the prostate tissue are not meaningful in the context of a BPH surgical intervention. We are inviting public comments on whether the AquaBeam® System meets the eligibility criteria at § 419.66(b).
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any of the existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. We have not identified an existing pass-through payment category that describes the AquaBeam® System. The applicant proposed a category descriptor for the AquaBeam® System of “Probe, image guided, robotic resection of prostate.” We are inviting public comments on whether the AquaBeam® System meets this criterion.
The second criterion for establishing a device category, at § 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. The applicant stated that the AquaBeam® System provides a substantial clinical improvement as the first autonomous tissue resection robot for the treatment of lower urinary tract symptoms due to BPH. The applicant further provided that the AquaBeam® System is also a substantial clinical improvement because the Aquablation procedure demonstrated superior efficacy and safety for larger prostates (prostates sized 50-80 mL) as compared to transurethral resection of the prostate (TURP). The applicant also believes that the Aquablation procedure would provide better outcomes for patients with large prostates (>80 mL) who may undergo open prostatectomy whereas the open prostatectomy procedure would require a hospital inpatient admission. With respect to this criterion, the applicant submitted several articles that examined the use of a current standard treatment for BPH—transurethral prostatectomy TURP, including complications associated with the procedure and the comparison of the effectiveness of TURP to other modalities used to treat BPH, including holmium laser enucleation of the prostate (HoLEP) [38] and photoselective vaporization (PVP).[39]
The most recent clinical study involving the AquaBeam® System was an accepted manuscript describing a double-blind trial that compared men treated with the AquaBeam® System versus men treated with traditional TURP.[40] This was a multicenter study in 4 countries with 17 sites, 6 of which contributed 5 patients or fewer. Patients were randomized to receive treatment with either the AquaBeam® System or TURP in a two-to-one ratio. With exclusions and dropouts, 117 patients were treated with the AquaBeam® System and 67 patients with TURP. The data on efficacy supported the equivalence of the two procedures based upon noninferiority analysis. The safety data were reported as showing superiority of the AquaBeam® System over TURP, although the data were difficult to track because adverse consequences were combined into categories. The applicant claimed that the International Prostate Symptom Scores (IPPS) were significantly improved in AquaBeam® System patients as compared to TURP patients in men whose prostate was greater the 50 ml in size. The applicant also claimed that the proportion of men with a worsening of sexual function (as shown with a decrease in Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ) score of at least 2 points or a decrease in International Index of Erectile Function (IIEF-5) score of at least 6 points by 6 months) was lower for the Aquablation procedure at 32.9 percent compared to the TURP groups at 52.8 percent.
Although there may be some evidence of the improved safety of the AquaBeam® System over TURP, we believe that the comparison of the AquaBeam® System with TURP does not recognize that there are other treatment modalities available that are likely to have a similar safety profile as the AquaBeam® System. No studies comparing other treatment modalities were cited to show that the AquaBeam® System is a significant improvement over other available procedures.
Based on the evidence submitted with the application, we are concerned that there is a lack of sufficient evidence that the AquaBeam® System provides a substantial clinical improvement over other similar products, particularly in the outpatient setting where large prostates are less likely to be treated. We are inviting public comments on whether the AquaBeam® System meets the substantial clinical improvement criterion.
The third criterion for establishing a device category, at § 419.66(c)(3), requires us to determine that the cost of the device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. The applicant provided the following information in support of the cost significance requirements. The applicant stated that the AquaBeam® System would be reported with CPT code 0421T. CPT code 0421T is assigned to APC 5375 (Level 5 Urology and Related Services). To meet the cost criterion for device pass-through payment status, a device must pass all three tests of the cost criterion for at least one APC. For our calculations, we used APC 5375, which has a CY 2018 payment rate of $3,706.03. Beginning in CY 2017, we calculate the device offset amount at the HCPCS/CPT code level instead of the APC level (81 FR 79657). CPT code 0421T had device offset amount of $0.00 at the time the application was received. According to the applicant, the cost of the handpiece for the AquaBeam® System is $2,500.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The estimated average reasonable cost of $2,500 for the AquaBeam® System exceeds 25 percent of the applicable APC payment amount for the service related to the category of devices of $3,706.03 ($2,500/$3,706.03 × 100 = 67.5 percent). Therefore, we believe the AquaBeam® System meets the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of the devices in the category must exceed the cost of the device-related portion of the APC payment amount for the related service by at least 25 percent, which means that the device cost needs to be at least 125 percent of the offset amount (the device-related portion of the APC found on the offset list). The estimated average reasonable cost of $2,500 for the AquaBeam® System exceeds the cost of the device-related portion of the APC payment amount for the related service of $0.00 by at least 25 percent. Therefore, we believe that the AquaBeam® System meets the second cost significance requirement.
The third cost significance requirement, at § 419.66(d)(3), provides that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount for the device must exceed 10 percent of the APC payment amount for the related service. The difference between the estimated average reasonable cost of $2,500 for the AquaBeam® System and the portion of the APC payment amount for the device of $0.00 exceeds the APC payment amount for the related service of $3,706.03 by 68 percent (($2,500 − $0.00)/$3,706.03 × 100 = 67.5 percent). Therefore, we believe that the AquaBeam® System meets the third cost significance requirement.
We are inviting public comments on whether the AquaBeam® System meets the device pass-through payment criteria discussed in this section, including the cost criterion.
(6) EluviaTM Drug-Eluting Vascular Stent System
Boston Scientific Corporation submitted an application for new technology add-on payments for the EluviaTM Drug-Eluting Vascular Stent System for FY 2020. According to the applicant, the EluviaTM system is a sustained-release drug-eluting stent indicated for improving luminal diameter in the treatment of peripheral artery disease (PAD) with symptomatic de novo or restenotic lesions in the native superficial femoral artery (SFA) and/or the proximal popliteal artery (PPA) with reference vessel diameters (RVD) ranging from 4.0 to 6.0 mm and total lesion lengths up to 190 mm.
The applicant stated that PAD is a circulatory condition in which narrowed arteries reduce blood flow to the limbs, usually in the legs. Symptoms of PAD may include lower extremity pain due to varying degrees of ischemia, claudication which is characterized by pain induced by exercise and relieved with rest. According to the applicant, risk factors for PAD include individuals who are age 70 years old and older; individuals who are between the ages of 50 years old and 69 years old with a history of smoking or diabetes; individuals who are between the ages of 40 years old and 49 years old with diabetes and at least one other risk factor for atherosclerosis; leg symptoms suggestive of claudication with exertion, or ischemic pain at rest; abnormal lower extremity pulse examination; known atherosclerosis at other sites (for example, coronary, carotid, renal artery disease); smoking; hypertension, hyperlipidemia, and homocysteinemia.[41] PAD is primarily caused by atherosclerosis—the buildup of fatty plaque in the arteries. PAD can occur in any blood vessel, but it is more common in the legs than the arms. Approximately 8.5 million people in the United States have PAD, including 12 to 20 percent of individuals who are age 60 years old and older.[42]
Management of the disease is aimed at improving symptoms, improving functional capacity, and preventing amputations and death. Management of patients who have been diagnosed with lower extremity PAD may include medical therapies to reduce the risk for future cardiovascular events related to atherosclerosis, such as myocardial infarction, stroke, and peripheral arterial thrombosis. Such therapies may include antiplatelet therapy, smoking cessation, lipid-lowering therapy, and treatment of diabetes and hypertension. For patients with significant or disabling symptoms unresponsive to lifestyle adjustment and pharmacologic therapy, intervention (percutaneous, surgical) may be needed. Surgical intervention includes angioplasty, a procedure in which a balloon-tip catheter is inserted into the artery and inflated to dilate the narrowed artery lumen. The balloon is then deflated and removed with the catheter. For patients with limb-threatening ischemia (for example, pain while at rest and/or ulceration), revascularization is a priority to reestablish arterial blood flow. According to the applicant, treatment of the SFA is problematic due to multiple issues including high rate of restenosis and significant forces of compression.
The applicant describes the EluviaTM Drug-Eluting Vascular Stent System as a sustained-release drug-eluting self-expanding, nickel titanium alloy (nitinol) mesh stent used to reestablish blood flow to stenotic arteries. According to the applicant, the EluviaTM stent is coated with the drug paclitaxel, which helps prevent the artery from restenosis. The applicant stated that EluviaTM's polymer-based drug delivery system is uniquely designed to sustain the release of paclitaxel beyond 1 year to match the restenotic process in the SFA. According to the applicant, the EluviaTM Drug-Eluting Vascular Stent System is comprised of: (1) The implantable endoprosthesis; and (2) the stent delivery system (SDS). On both the proximal and distal ends of the stent, radiopaque markers made of tantalum increase visibility of the stent to aid in placement. The tri-axial designed delivery system consists of an outer shaft to stabilize the stent delivery system, a middle shaft to protect and constrain the stent, and an inner shaft to provide a guide wire lumen. The delivery system is compatible with 0.035 in (0.89 mm) guide wires. The EluviaTM stent is available in a variety of diameters and lengths. The delivery system is offered in 2 working lengths (75 cm and 130 cm).
With respect to the newness criterion at § 419.66(b)(1), EluviaTM received FDA premarket approval (PMA) on September 18, 2018. The application for a new device category for transitional pass-through payment status for EluviaTM was received on November 15, 2018, which is within 3 years of the date of the initial FDA approval or clearance. We are inviting public comments on whether the EluviaTM Drug-Eluting Vascular Stent System meets the newness criterion. With respect to the eligibility criterion at § 419.66(b)(3), according to the applicant, the EluviaTM Drug-Eluting Vascular Stent System is integral to the service provided, is used for one patient only, comes in contact with human skin, and is applied in or on a wound or other skin lesion. The applicant also claimed that the EluviaTM Drug-Eluting Vascular Stent System meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or items for which depreciation and financing expenses are recovered, and it is not a supply or material furnished incident to a service. We are inviting public comments on whether the EluviaTM Drug-Eluting Vascular Stent System meets the eligibility criterion at § 419.66(b).
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any of the existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. We have not identified an existing pass-through payment category that describes the EluviaTM Drug-Eluting Vascular Stent System. The applicant proposed a category descriptor for the EluviaTM Drug-Eluting Vascular Stent System of “Stent, non-coronary, polymer matrix, minimum 12-month sustained drug release, with delivery system.” We are inviting public comments on this issue.
The second criterion for establishing a device category, at § 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. With respect to this criterion, the applicant submitted several articles that examined the use of a current standard treatment for peripheral artery disease (PAD) with symptomatic de novo or restenotic lesions in the native superficial femoral artery (SFA) and/or proximal popliteal artery (PPA), with claims of substantial clinical improvement in achieving superior primary patency; reducing the rate of subsequent therapeutic interventions; decreasing the number of future hospitalizations or physician visits; reducing hospital readmission rates; reducing the rate of device-related complications; and achieving similar functional outcomes and EQ-5D index values while associated with half the rate of target lesion revascularizations (TLRs) procedures.
The applicant submitted the results of the MAJESTIC study, a single-arm, first-in-human study of the EluviaTM Drug-Eluting Vascular Stent System. The MAJESTIC [43] study is a prospective, multi-center, single-arm, open-label study. According to the applicant, the MAJESTIC study demonstrated long-term treatment durability among patients whose femoropopliteal arteries were treated with the EluviaTM stent. The applicant asserts that the MAJESTIC study demonstrates the sustained impact of the EluviaTM stent on primary patency. The MAJESTIC study enrolled 57 patients who had been diagnosed with symptomatic lower limb ischemia and lesions in the SAF or PPA. Efficacy measures at 2 years included primary patency, defined as duplex ultrasound peak systolic velocity ratio of less than 2.5 and the absence of TLR or bypass. Safety monitoring through 3 years included adverse events and TLR. The 24-month clinic visit was completed by 53 patients; 52 had Doppler ultrasound evaluable by the core laboratory, and 48 patients had radiographs taken for stent fracture analysis. The 3-year follow-up was completed by 54 patients. At 2 years, 90.6 percent (48/53) of the patients had improved by 1 or more Rutherford categories as compared with the pre-procedure level without the need for TLR (when those with TLR were included, 96.2 percent sustained improvement); only 1 patient exhibited a worsening in level, 66.0 percent (35/53) of the patients exhibited no symptoms (Category 0) and 24.5 percent (13/53) had mild claudication (Category 1) at the 24-month visit. Mean ABI improved from 0.73 ± 0.22 at baseline to 1.02 ± 0.20 at 12 months and 0.93 ± 0.26 at 24 months. At 24 months, 79.2 percent (38/48) of the patients had an ABI increase of at least 0.1 compared with baseline or had reached an ABI of at least 0.9. The applicant also noted that at 12 months the Kaplan-Meier estimate of primary patency was 96.4 percent.
With regard to the EluviaTM stent achieving superior primary patency, the applicant submitted the results of the IMPERIAL [44] study in which the EluviaTM stent is compared, head-to-head, to the Zilver® PTX Drug-Eluting stent. The IMPERIAL study is a global, multi-center, randomized controlled trial consisting of 465 subjects. Eligible patients were aged 18 years old or older and had a diagnosis of symptomatic lower-limb ischaemia, defined as Rutherford Category 2, 3, or 4 and stenotic, restenotic (treated with a drug-coated balloon greater than 12 months before the study or standard percutaneous transluminal angioplasty only), or occlusive lesions in the native SFA or PPA, with at least 1 infrapopliteal vessel patent to the ankle or foot. Patients had to have stenosis of 70 percent or more (via angiographic assessment), vessel diameter between 4 mm and 6 mm, and total lesion length between 30 mm and 140 mm.
Patients who had previously stented target lesion/vessels treated with drug-coated balloon less than 12 months prior to randomization/enrollment and patients who had undergone prior surgery of the SFA/PPA in the target limb to treat atherosclerotic disease were excluded from the study. Two concurrent single-group (EluviaTM only) substudies were done: A nonblinded, nonrandomized pharmacokinetic sub-study and a nonblinded, nonrandomized study of patients who had been diagnosed with long lesions (greater than 140 mm in diameter).
The IMPERIAL study is a prospective, multi-center, single-blinded randomized, controlled (RCT) noninferiority trial. Patients were randomized (2:1) to implantation of either a paclitaxel-eluting polymer stent (EluviaTM) or a paclitaxel-coated stent (Zilver® PTX) after the treating physician had successfully crossed the target lesion with a guide wire. The primary endpoints of the study are Major Adverse Events defined as all causes of death through 1 month, Target Limb Major Amputation through 12 months and/or Target Lesion Revascularization (TLR) procedure through 12 months and primary vessel patency at 12 months post-procedure. Secondary endpoints included the Rutherford categorization, Walking Impairment Questionnaire, and EQ-5D assessments at 1 month, 6 months, and 12 months post-procedure. Patient demographic and characteristics were balanced between the EluviaTM stent and Zilver® PTX stent groups.
The applicant noted that lesion characteristics for the patients in the EluviaTM stent versus the Zilver® PTX stent arms were comparable. Clinical follow-up visits related to the study were scheduled for 1 month, 6 months, and 12 months after the procedure, with follow-up planned to continue through 5 years, including clinical visits at 24 months and 5 years and clinical or telephone follow-up at 3 and 4 years.
The applicant asserted that in the IMPERIAL study the EluviaTM stent demonstrated superior primary patency over the Zilver® PTX stent, 86.8 percent versus 77.5 percent, respectively (p=0.0144). The noninferiority primary efficacy endpoint was also met. The applicant asserts that the superior primary patency results at the SFA are noteable because the SFA presents unique challenges with respect to maintaining long-term patency. There are distinct pathological differences between the SFA and coronary arteries. The SFA tends to have higher levels of calcification and chronic total occlusions when compared to coronary arteries. Following an intervention within the SFA, the SFA produces a healing response which often results in restenosis or re-narrowing of the arterial lumen. This cascade of events leading to restenosis starts with inflammation, followed by smooth muscle cell proliferation and matrix formation.[45] Because of the unique mechanical forces in the SFA, this restenotic process of the SFA can continue well beyond 300 days from the initial intervention. Results from the IMPERIAL study showed that primary patency at 12 months, by Kaplan-Meier estimate, was significantly greater for EluviaTM than for Zilver® PTX, 88.5 percent and 79.5 percent, respectively (p=0.0119). According to the applicant, these results are consistent with the 96.4 percent primary patency rate at 12 months in the MAJESTIC study.
The IMPERIAL study included two concurrent single-group (EluviaTM only) substudies: A nonblinded, nonrandomized pharmacokinetic substudy and a nonblinded, nonrandomized study of patients with long lesions (greater than 140 mm in diameter). For the pharmacokinetic sub-study, patients had venous blood drawn before stent implantation and at intervals ranging from 10 minutes to 24 hours post implantation, and again at either 48 hours or 72 hours post implantation. The pharmacokinetics sub-study confirmed that plasma paclitaxel concentrations after EluviaTM stent implantation were well below thresholds associated with toxic effects in studies in patients who had been diagnosed with cancer (0.05 μM or ~43 ng/mL).
The IMPERIAL substudy long lesion subgroup consisted of 50 patients with average lesion length of 162.8 mm that were each treated with two EluviaTM stents. According to the applicant, 12-month outcomes for the long lesion subgroup are 87 percent primary patency and 6.5 percent TLR. According to the applicant, in a separate subgroup analysis of patients 65 years old and older (Medicare population), the primary patency rate in the EluviaTM stent group is 92.6 percent, compared to 75.0 percent for the Zilver® PTX stent group (p=0.0386).
With regard to reducing the rate of subsequent therapeutic interventions, secondary outcomes in the IMPERIAL study included repeat re-intervention on the same lesion, referred to as target lesion revascularization (TLR), over the 12 months following the index procedure. The rate of subsequent interventions, or TLRs, in the EluviaTM stent group was 4.5 percent compared to 9.0 percent in the Zilver® PTX stent group. The applicant asserted that the TLR rate in the EluviaTM stent group represents a substantial reduction in reintervention on the target lesion compared to that of the Zilver® PTX stent group (at a p=0.067 p-value). The Eluvia® stent group clinically driven TLR rates through 12 months following the index procedure were likewise lower for U.S. patients age 65 and older as well as for those with medically treated diabetes (confidential and unpublished as of the date of the device transitional pass-through payment application, data on file with Boston Scientific). In the subgroup of U.S. patients age 65 and older, the rates of TLR were 2.4 percent in the EluviaTM group compared to 3.1 percent in the Zilver® PTX group, and in the subgroup of medically treated diabetes patients, the rates of TLR were 3.7 percent compared to 13.6 percent in the Zilver® PTX group (p=0.0269).
With regard to decreasing the number of future hospitalizations or physician visits, the applicant asserted that the substantial reduction in the lesion revascularization rate led to a reduced need to provide additional intensive care, distinguishing the EluviaTM stent group from the Zilver® PTX stent group. In the IMPERIAL study, the EluviaTM-treated patients required fewer days of re-hospitalization. Patients in the EluviaTM group averaged 13.9 days of rehospitalization for all adverse events compared to 17.7 days of rehospitalization for patients in the Zilver® PTX stent group. Patients in the EluviaTM group were rehospitalized for 2.8 days for TLR/Total Vessel Revascularization (TVR) compared to 7.1 days in the Zilver® PTX stent group. Lastly, patients in the EluviaTM stent group were rehospitalized for 2.7 days for procedure/device-related adverse events compared to 4.5 days from the Zilver® PTX stent group.
Regarding reduction in hospital readmission rates, the applicant asserted that patients treated in the EluviaTM stent group experienced reduced rates of hospital readmission following the index procedure compared to those in the Zilver® PTX stent group. Hospital readmission rates at 12 months were 3.9 percent for the EluviaTM stent group compared to 7.1 percent for the Zilver® PTX stent group. Similar results were noted at 1 and 6 months; 1.0 percent versus 2.6 percent and 2.4 percent versus 3.8 percent, respectively.
With regard to reducing the rate of device-related complications, the applicant asserted that while the rates of adverse events were similar in total between treatment arms in the IMPERIAL study, there were measurable differences in device-related complications. Device-related adverse-events were reported in 8 percent of the patients in the EluviaTM stent group compared to 14 percent of the patients in the Zilver® PTX stent group.
Lastly, the applicant asserted that while functional outcomes appear similar between the EluviaTM and Zilver® PTX stent groups at 12 months, these improvements for the Zilver® PTX stent group are associated with twice as many TLRs to achieve similar EQ-5D index values.[46] Secondary endpoints improved after stent implantation and were generally similar between the groups. At 12 months, of the patients with complete Rutherford assessment data, 241 (86 percent) of the 281 patients in the EluviaTM group and 120 (85 percent) of the 142 patients in the Zilver® PTX group had symptoms reported as Rutherford Category 0 or 1 (none to mild claudication). The mean ankle-brachial index was 1.0 (SD 0.2) in both groups at 12 months (baseline mean ankle-brachial index 0.7 [SD 0.2] for EluviaTM; 0.8 [0.2] for Zilver® PTX), with sustained hemodynamic improvement for approximately 80 percent of the patients in both groups. Walking function improved significantly from baseline to 12 months in both groups, as measured with the Walking Impairment Questionnaire and the 6-minute walk test. In both groups, the majority of patients had sustained improvement in the mobility dimension of the EQ-5D, and approximately half had sustained improvement in the pain or discomfort dimension. No significant between-group differences were observed in the Walking Impairment Questionnaire, 6-minute walk test, or EQ-5D. Secondary endpoint results for the EluviaTM stent and Zilver® PTX stent groups are as follows:

We note that the IMPERIAL study, which showed significant differences in primary patency at 12 months, was designed for noninferiority and not superiority. Therefore, we are concerned that results showing primary patency at 12 months may not be valid given the study design. We also are concerned that the results of a recently published meta-analysis of randomized controlled trials of the risk of death associated with the use of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg, which found that there is increased risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the lower limbs and that further investigations are urgently warranted,[47] although the EluviaTM system was not included in the meta-analysis. We are concerned that the findings from this study indicate that the data suggesting that drug-coated stents are substantially clinically improved are unconfirmed. We are inviting public comments on whether the EluviaTM Drug-Eluting Vascular Stent System meets the substantial clinical improvement criterion, including the implications of the meta-analysis results with respect to a finding of substantial clinical improvement for the EluviaTM system.
We further note that the applicant for the EluviaTM Drug Eluting Vascular Stent System also applied for the IPPS new technology add-on payment (FY 2020 IPPS/LTCH PPS proposed rule; 86 FR 19314). In the FY 2020 IPPS/LTCH PPS proposed rule, we discuss several publicly available comments that also raised concerns relating to substantial clinical improvement. We list several of those concerns below. While the EluviaTM IMPERIAL study does cite a reduced rate of “Subsequent Therapeutic Interventions”, public comments for the IPPS proposed rule note that “Subsequent Therapeutic Interventions” was not further defined in the New Technology Town Hall presentation nor in the IMPERIAL study. The commenters stated that it would appear from the presentation materials, however, that this claim refers specifically to “target lesion revascularizations (TLR)”, which does not appear statistically significant.
With regard to the applicant's assertion that the use of the EluviaTM stent reduces hospital readmission rates, a commenter noted that during the New Technology Town Hall presentation, the presenter noted that the EluviaTM group had a hospital readmission rate at 12 months of 3.9 percent compared to the Zilver® PTX group's rate of 7.1 percent, and that no p-value was included on the slide used for the presentation to offer an assessment of the statistical significance of this difference. The commenter noted that the manufacturer of the EluviaTM stent did not discuss this particular hospital readmission rate data comparison in the main body of the Lancet paper; however, the data could be found in the online appendix and is shown as not statistically significant.
With regards to longer-term data on the Zilver® PTX stent and the EluviaTM stent, the commenter noted that in the commentary in The Lancet paper accompanying the IMPERIAL study, Drs. Salvatore Cassese and Robert Byrne write that a follow-up duration of 12 months is insufficient to assess late failure, which is not infrequently observed. According to Drs. Cassese and Byrne, the preclinical models of restenosis after stenting of peripheral arteries have shown that stents permanently overstretch the arterial wall, thus stimulating persistent neointimal growth, which might cause a catch-up phenomenon and late failure. The Lancet paper noted that, in this regard, data on outcomes beyond 1 year will be important to confirm the durability of the efficacy of the EluviaTM stent.[48] The commenter stated that, at this point in time, very limited longer-term data are available on the use of the EluviaTM stent and that the IMPERIAL study offers only 12-month data, although data out to 3 years have been published from the relatively small 57-patient single-arm MAJESTIC study. The commenter noted that the MAJESTIC study demonstrates a decrease in primary patency from 96.4 percent at 1 year to 83.5 percent at 2 years; and a doubling in TLR rates from 1 year to 2 years (3.6 percent to 7.2 percent) and again from 2 years to 3 years (7.2 percent to 14.7 percent). The commenter stated that this is not inconsistent with Drs. Cassese and Byrne's commentary regarding late failure, and that the relatively small, single-arm design of the study does not lend itself well to direct comparison to other SFA treatment options such as the Zilver® PTX stent.
The commenter also stated that EluviaTM's lack of long-term data contrasts with 5-year data that is available from the Zilver® PTX stent's pivotal 479-patient RCT comparing the use of the Zilver® PTX stent to angioplasty (with a sub-randomization comparing provisional use of Zilver® PTX stenting to bare metal Zilver stenting in patients experiencing an acute failure of percutaneous transluminal angioplasty (PTA)). The commenter believed that these 5-year data demonstrate that the superiority of the use of the Zilver® PTX stent demonstrated at 12 and 24 months is maintained through 5 years compared to PTA and provisional bare metal stenting, and actually increases rather than decreases over time. The commenter also believed that, given that these stent devices are permanent implants and they are used to treat a chronic disease, long-term data are important to fully understand an SFA stent's clinical benefits. The commenter stated that with 5-year data available to support the ongoing safety and effectiveness of the use of the Zilver® PTX stent, but no such corresponding data available for the use of the EluviaTM stent, it seems incongruous to suggest that the use of the EluviaTM stent results in a substantial clinical improvement compared to the Zilver® PTX stent.
The commenter further stated that, in addition to the limited long-term data available for the EluviaTM stent, there is also a lack of clinical data for the use of the EluviaTM stent to confirm the benefit of the device outside of a strictly controlled clinical study population. The commenter stated that, in contrast, the Zilver® PTX stent has demonstrated comparable outcomes across a broad patient population, including a 787 patient study conducted in Europe with 2-year follow-up and a 904-patient study of all-comers (no exclusion criteria) in Japan with 5-year follow-up completed. The commenter believed that, with no corresponding data for the use of the EluviaTM stent in a broad patient population, it seems unreasonable to suggest that the use of the EluviaTM stent results in a substantial clinical improvement compared to the Zilver® PTX stent.
Based on the evidence submitted with the application, we are concerned that there is a lack of sufficient evidence that the EluviaTM Vascular Drug-Eluting Stent System provides a substantial clinical improvement over other similar products. We are inviting public comments on whether EluviaTM Vascular Drug-Eluting Stent System meets the substantial clinical improvement criterion.
The third criterion for establishing a device category, at § 419.66(c)(3), requires us to determine that the cost of the device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. The applicant provided the following information in support of the cost significance requirements. The applicant stated that use of the EluviaTM stent would be reported with CPT code 37226, which is assigned to APC 5193 (Level 3 Endovascular Procedures). To meet the cost criterion for device pass-through payment status, a device must pass all three tests of the cost criterion for at least one APC. For our calculations, we used APC 5193, which has a CY 2019 payment rate of $10,509.72. Beginning in CY 2017, we calculate the device offset amount at the HCPCS/CPT code level instead of the APC level (81 FR 79657). CPT code 37226 had a device offset amount of $4,996.32. According to the applicant, the cost of the EluviaTM Vascular Drug-Eluting Stent System is $1,995 to $2,895 per stent, with each procedure requiring approximately 2.2 stents per procedure at an average device cost of $5,768 per procedure.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The estimated average reasonable cost of the EluviaTM stent exceeds 25 percent of the applicable APC payment amount for the service related to the category of devices of $10,509.72−(($5,768/$10,509.72) × 100 = 55 percent). Therefore, we believe that the EluviaTM Vascular Drug-Eluting Stent System meets the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of the devices in the category must exceed the cost of the device-related portion of the APC payment amount for the related service by at least 25 percent, which means that the device cost needs to be at least 125 percent of the offset amount (the device-related portion of the APC found on the offset list). The estimated average reasonable cost of $5,768 for the EluviaTM stent exceeds the cost of the device-related portion of the APC payment amount for the related service of $4,996.32 by less than 25 percent (($5,768/$4,996.32) × 100 = 115 percent). Therefore, we do not believe that the EluviaTM Vascular Drug-Eluting Stent System meets the second cost significance requirement.
The third cost significance requirement, at § 419.66(d)(3), provides that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount for the device must exceed 10 percent of the APC payment amount for the related service. The difference between the estimated average reasonable cost of $5,768 for the EluviaTM stent and the portion of the APC payment amount for the device of $4,996.32 does not exceed 10 percent of the APC payment amount for the related service of $10,509.72 (($5,768 − $4,996.32)/$10,509.72 × 100 = 7.3 percent). Therefore, we do not believe that the EluviaTM Vascular Drug-Eluting Stent System meets the third cost significance requirement.
We are inviting public comments on whether the EluviaTM Vascular Drug-Eluting Stent System meets the device pass-through payment criteria discussed in this section, including the cost criterion.
(7) AUGMENT® Bone Graft
Wright Medical submitted an application for a new device category for transitional pass-through payment status for the AUGMENT® Bone Graft. The applicant describes AUGMENT® Bone Graft as a device/drug indicated for use as an alternative to autograft in arthrodesis of the ankle and/or hindfoot where the need for supplemental graft material is required. The applicant stated that the product has two components: Recombinant human platelet-derived growth factor-BB (rhPDGF-BB) solution (0.3 mg/mL) and Beta-tricalcium phosphate (β-TCP) granules (1000 − 2000 μm). The two components are combined at the point of use and applied to the surgical site. The beta-TCP provides a porous osteoconductive scaffold for new bone growth and the rhPDGF-BB, which act as an osteoinductive chemo-attractant and mitogen for cells involved in wound healing and through promotion of angiogenesis.
According to the applicant, the AUGMENT® Bone Graft is indicated for use in arthrodesis of the ankle and/or hindfoot due to osteoarthritis, post-traumatic arthritis (PTA), rheumatoid arthritis, psoriatic arthritis, avascular necrosis, joint instability, joint deformity, congenital defect or joint arthropathy as an alternative to autograft in patients needing graft material. Osteoarthritis is the most common joint disease among middle aged and older individuals and has been shown to also have health related mental and physical disabilities, which can be compared to the severity as patients with end-stage hip arthritis.[49] Additionally, post-traumatic arthritis develops after an acute direct trauma to the joint and can cause 12 percent of all osteoarthritis cases.[50] Common causes leading to PTOA include intra-articular fractures and meniscal, ligamentous and chondral injuries.[51] The ankle is cited as the most affected joint, reportedly accounting for 54 to 78 percent of over 300,000 injuries occurring in the USA annually. The applicant stated that autologous bone graft has often been used in ankle arthrodesis. Autologous bone is retrieved from a donor site, which may require an incision separate from the arthrodesis.[52] The applicant stated that, in these procedures, harvested autologous bone graft is implanted to stimulate healing between the bones across a diseased joint. The applicant further stated that the procedures may require the use of synthetic bone substitutes to fill the bony voids or gaps or to serve as an alternative to the autograft where autograft is not feasible. The applicant stated that the AUGMENT® Bone Graft removes the need for autologous retrieval. The applicant noted that during the procedure, the surgeon prepares the joint for the graft application and locates any potential bony defect, then applying and packing the AUGMENT® Bone Graft into the joint defects intended for arthrodesis.
With respect to the newness criterion at § 419.66(b)(1), the FDA granted the AUGMENT® Bone Graft premarket approval on September 1, 2015. The application for a new device category for transitional pass-through payment status for the AUGMENT® Bone Graft was received May 31, 2018, which is within 3 years of the date of the initial FDA approval or clearance. We are inviting public comments on whether the AUGMENT® Bone Graft meets the newness criterion.
With respect to the eligibility criterion at § 419.66(b)(3), according to the applicant, the use of the AUGMENT® Bone Graft is integral to the service provided, is used for one patient only, comes in contact with human skin, and is applied in or on a wound or other skin lesion. The applicant also claimed that the AUGMENT® Bone Graft meets the device eligibility requirements of § 419.66(b)(4) because it is not an instrument, apparatus, implement, or items for which depreciation and financing expenses are recovered, and it is not a supply or material furnished incident to a service.
The criteria for establishing new device categories are specified at § 419.66(c). The first criterion, at § 419.66(c)(1), provides that CMS determines that a device to be included in the category is not appropriately described by any of the existing categories or by any category previously in effect, and was not being paid for as an outpatient service as of December 31, 1996. We have not identified an existing pass-through payment category that describes the AUGMENT® Bone Graft. The applicant proposed a category descriptor for the AUGMENT® of “rhPDGF-BB and β-TCP as an alternative to autograft in arthrodesis of the ankle and/or hindfoot.”
The second criterion for establishing a device category, at § 419.66(c)(2), provides that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. The applicant claims that the AUGMENT® Bone Graft provides a substantial clinical improvement over autograft procedures by reducing pain at the autograft donor site. With respect to this criterion, the applicant submitted data that examined the use of autograft arthrodesis of the ankle and/or hind foot and arthrodesis with the use of the AUGMENT® Bone Graft.
In a randomized, nonblinded, placebo controlled, noninferiority trial of the AUGMENT® Bone Graft versus autologous bone graft, the AUGMENT® arm showed equivalence bone bridging as demonstrated by CT, pain on weight bearing, The American Orthopaedic Foot & Ankle Society Ankle-Hindfoot (AOFAS-AHS) score, and the Foot Function Index to autologous bone graft. The study noted that patients experienced significantly decreased (in fact no) pain due to elimination of the donor site procedure. In the autograft group, at 6 months, 18/142 patients (13 percent) experienced pain >20 mm (of 100 mm) on the Visual Analog Scale (VAS) at the autograft donor site as compared to 0/272 in the AUGMENT® Bone Graft group. At 12 months, 13/142 autograft patients (9 percent) had pain defined as >20 mm VAS as compared to 0/272 AUGMENT® patients.[53] The VAS has patients mark a visual representation of pain on a ruler based scale from 1 to 100. The measured distance (in mm) on the 10-cm line between the “no pain” anchor and the patient's mark represents the level of pain. We are concerned that we are unable to sufficiently determine substantial clinical improvement using the provided data, given that a comparison to alternatives to autologous bone graft, such as the reamer-irrigator-aspirator (RIA) technique were not evaluated. Specifically, the RIA technique has been suggested in a number of studies to be a viable alternative to bone autograft, because autogenous bone graft can be readily obtained without the need for additional incisions, therefore eliminating pain from an incisional site.[54] Another concern is the time period of the study because certain ankle arthrodesis complications such as ankle replacement and repeat arthrodesis can happen more than 2 years after the initial surgery.[55] A long-term study of at least 60 months is currently underway in order to assess long-term safety and efficacy, looking at the following 4 primary outcomes: Bone bridging as demonstrated by CT, pain on weight bearing, The American Orthopaedic Foot & Ankle Society Ankle-Hindfoot (AOFAS-AHS) score, and the Foot Function Index. We believe that this long-term study is necessary for meaningful information about long-term efficacy of the Augment® Bone Graft. Further, there was a notable difference in the infection rate, musculoskeletal and tissue disorders, and pain in extremity for those in the AUGMENT® Bone Graft group. These findings were unfortunately not tested for significance and also were not necessarily focused on relevance to the procedure. Should these be significant and related to the device, these findings would suggest that the adverse outcomes due to the Augment® Bone Graft may outweigh its potential benefits.
We are inviting public comments on whether the AUGMENT® Bone Graft meets the substantial clinical improvement criterion.
The third criterion for establishing a device category, at § 419.66(c)(3), requires us to determine that the cost of the device is not insignificant, as described in § 419.66(d). Section 419.66(d) includes three cost significance criteria that must each be met. The applicant provided the following information in support of the cost significance requirements. The applicant stated that the use of the AUGMENT® Bone Graft would be reported with CPT code 27870 (Arthrodesis, ankle, open), which is assigned to APC 5115 (Level 5 Musculoskeletal Procedures). To meet the cost criterion for device pass-through payment status, a device must pass all three tests of the cost criterion for at least one APC. For our calculations, we used APC 5115, which has a CY 2019 payment rate of $10,122.92. Beginning in CY 2017, we calculate the device offset amount at the HCPCS/CPT code level instead of the APC level (81 FR 79657). CPT code 27870 had a device offset amount of $4,553.29. According to the applicant, the cost of the AUGMENT® Bone Graft is $3,077 per device/drug combination. The applicant further provided a weighted average cost of the graft, accounting for how many procedures required one, two, or three AUGMENT® Bone Graft device/drug kits, equaling a weighted average cost of $6,020.22.
Section 419.66(d)(1), the first cost significance requirement, provides that the estimated average reasonable cost of devices in the category must exceed 25 percent of the applicable APC payment amount for the service related to the category of devices. The estimated average reasonable cost of the AUGMENT® Bone Graft is more than 25 percent of the applicable APC payment amount [56] for the service related to the category of devices of $10,122.92 (($6,020.22/$10,122.92) × 100 = 59 percent)). Therefore, we believe that the AUGMENT® Bone Graft meets the first cost significance requirement.
The second cost significance requirement, at § 419.66(d)(2), provides that the estimated average reasonable cost of the devices in the category must exceed the cost of the device related portion of the APC payment amount for the related service by at least 25 percent, which means that the device cost needs to be at least 125 percent of the offset amount (the device-related portion of the APC found on the offset list). The estimated average reasonable cost of $6,020.22 for AUGMENT® Bone Graft exceeds the cost of the device-related portion of the APC payment amount for the related service of $4,553.29 by at least 25 percent (($6,020.22/$4,553.29) × 100 = 132 percent). Therefore, we have concerns about whether the AUGMENT® Bone Graft meets the second cost significance requirement.
The third cost significance requirement, at § 419.66(d)(3), provides that the difference between the estimated average reasonable cost of the devices in the category and the portion of the APC payment amount for the device must exceed 10 percent of the APC payment amount for the related service. The difference between the estimated average reasonable cost of $6,020.22 for the AUGMENT® Bone Graft and the portion of the APC payment amount for the device of $4,553.29 exceeds the APC payment amount for the related service of $10,122.92 by more than 10 percent (($6,020.22−$4,553.29)/$10,122.92 × 100 = 15 percent). Therefore, we believe that AUGMENT® Bone Graft meets the third cost significance test. We are inviting public comments on whether the AUGMENT® Bone Graft meets the device pass-through payment criteria discussed in this section, including the cost criterion.
3. Request for Information and Potential Revisions to the OPPS Device Pass-Through Substantial Clinical Improvement Criterion in the FY 2020 IPPS/LTCH PPS Proposed Rule
As mentioned earlier, section 1833(t)(6) of the Act provides for pass-through payments for devices, and section 1833(t)(6)(B) of the Act requires CMS to use categories in determining the eligibility of devices for pass-through payments. Separately, the criteria as set forth under § 419.66(c) are used to determine whether a new category of pass-through payment devices should be established. One of these criteria, at § 419.66(c)(2), states that CMS determines that a device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment. CMS considers the totality of the substantial clinical improvement claims and supporting data, as well as public comments, when evaluating this aspect of each application. CMS summarizes each applicant's claim of substantial clinical improvement as part of its discussion of the entire application in the relevant proposed rule, as well as any concerns regarding those claims. In the relevant final rule for the OPPS, CMS responds to public comments and discusses its decision to approve or deny the application for separate transitional pass-through payments.
Over the years, applicants and commenters have indicated that it would be helpful for CMS to provide greater guidance on what constitutes “substantial clinical improvement.” In the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19368 through 19371), we requested information on the substantial clinical improvement criterion for OPPS transitional pass-through payments for devices and stated that we were considering potential revisions to that criterion. In particular, we sought public comments in the FY 2020 IPPS/LTCH PPS proposed rule on the type of additional detail and guidance that the public and applicants for device pass-through transitional payment would find useful (84 FR 19367 to 19369). This request for public comments was intended to be broad in scope and provide a foundation for potential rulemaking in future years. We refer readers to the FY 2020 IPPS/LTCH proposed rule for the full text of this request for information.
In addition to this broad request for public comments for potential rulemaking in future years, in order to respond to stakeholder feedback requesting greater understanding of CMS' approach to evaluating substantial clinical improvement, we also solicited comments from the public in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19369 through 19371) on specific changes or clarifications to the IPPS and OPPS substantial clinical improvement criterion that CMS might consider making in the FY 2020 IPPS/LTCH PPS final rule to provide greater clarity and predictability. We refer readers to the FY 2020 IPPS/LTCH PPS proposed rule for complete details on those potential revisions. We note that any responses to public comments we receive on potential revisions to the OPPS substantial clinical improvement criterion in response to the FY 2020 IPPS/LTCH PPS proposed rule, as well as any revisions that might be adopted, will be included in the CY 2020 OPPS/ASC final rule and will inform future OPPS rulemaking. We further invite public comment on this topic in this rule.
4. Proposed Alternative Pathway to the OPPS Device Pass-Through Substantial Clinical Improvement Criterion for Transformative New Devices
Since 2001 when we first established the substantial clinical improvement criterion, the FDA programs for helping to expedite the development and review of transformative new technologies that are intended to treat serious conditions and address unmet medical needs (referred to as FDA's expedited programs) have continued to evolve in tandem with advances in medical innovations and technology. There is currently one expedited FDA program for devices, the Breakthrough Devices Program. The 21st Century Cures Act (Cures Act) (Pub. L. 144-255) established the Breakthrough Devices Program to expedite the development of, and provide for priority review of, medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions and which meet one of the following four criteria: (1) That represent breakthrough technologies; (2) for which no approved or cleared alternatives exist; (3) that offer significant advantages over existing approved or cleared alternatives, including the potential, compared to existing approved alternatives, to reduce or eliminate the need for hospitalization, improve patient quality of life, facilitate patients' ability to manage their own care (such as through self-directed personal assistance), or establish long-term clinical efficiencies; or (4) the availability of which is in the best interest of patients.
Some stakeholders over the years have requested that devices that receive marketing authorization and are part of an FDA expedited program be deemed as representing a substantial clinical improvement for purposes of OPPS device pass-through status. We understand this request would arguably create administrative efficiency because the commenters currently view the two sets of criteria as the same, overlapping, similar, or otherwise duplicative or unnecessary.
The Administration is committed to addressing barriers to health care innovation and ensuring Medicare beneficiaries have access to critical and life-saving new cures and technologies that improve beneficiary health outcomes. As detailed in the President's FY 2020 Budget (we refer readers to HHS FY 2020 Budget in Brief, Improve Medicare Beneficiary Access to Breakthrough Devices, pp. 84-85), HHS is pursuing several policies that will instill greater transparency and consistency around how Medicare covers and pays for innovative technology.
Therefore, given the FDA programs for helping to expedite the development and review of transformative devices that meet expedited program criteria (that is, new devices that treat serious or life-threatening diseases or conditions for which there is an unmet medical need), we considered whether it would also be appropriate to similarly facilitate access to these transformative new technologies for Medicare beneficiaries taking into consideration that marketing authorization (that is, Premarket Approval (PMA); 510(k) clearance; or the granting of a De Novo classification request) for a product that is the subject of one of FDA's expedited programs could lead to situations where the evidence base for demonstrating substantial clinical improvement in accordance with CMS' current standard has not fully developed at the time of FDA marketing authorization (that is, PMA; 510(k) clearance; the granting of a De Novo classification request) (as applicable). We also considered whether FDA marketing authorization of a product that is part of an FDA expedited program is evidence that the product is sufficiently different from existing products for purposes of newness.
After consideration of these issues, and consistent with the Administration's commitment to addressing barriers to health care innovation and ensuring Medicare beneficiaries have access to critical and life-saving new cures and technologies that improve beneficiary health outcomes, we concluded that it would be appropriate to develop an alternative pathway for transformative medical devices. In situations where a new medical device is part of the Breakthrough Devices Program and has received FDA marketing authorization (that is, the device has received PMA; 510(k) clearance; or the granting of a De Novo classification request), we are proposing an alternative outpatient pass-through pathway to facilitate access to this technology for Medicare beneficiaries beginning with applications received for pass-through payment on or after January 1, 2020.
We continue to believe that hospitals should receive pass-through payments for devices that offer clear clinical improvement and that cost considerations should not interfere with patient access. In light of the criteria applied under the FDA's Breakthrough Devices Program, and because we recognize that the technology may not have a sufficient evidence base to demonstrate substantial clinical improvement at the time of FDA marketing authorization, we are proposing to amend the OPPS device transitional pass-through payment regulations to create an alternative pathway to demonstrating substantial clinical improvement that would enable devices approved under the FDA Breakthrough Devices Program to qualify for our quarterly approval process for device pass-through payment under the OPPS for pass-through payment applications received on or after January 1, 2020. With this proposal, OPPS device pass-through payment applicants approved under the FDA Breakthrough Devices Program would not be evaluated in terms of the current substantial clinical improvement criterion at § 419.66(c)(2) for the purposes of determining device pass-through payment status, but would continue to need to meet the other requirements for pass-through payment status in our regulation at § 419.66. Devices approved under the Breakthrough Devices Program that are approved for OPPS device transitional pass-through payment can be approved through the quarterly process and would be announced through that process (81 FR 79655). Finally, we would include proposals regarding these devices and whether pass-through payment status should continue to apply in the next applicable OPPS rulemaking cycle.
As such, we are proposing to revise paragraph (c)(2) under § 419.66. Under proposed revised paragraph (c)(2), we are proposing to establish an alternative pathway where applications for device pass-through payment status for new medical devices received on or after January 1, 2020 that are a part of FDA's Breakthrough Devices Program and have received FDA marketing authorization (that is, the device has received PMA, 510(k) clearance, or the granting of a De Novo classification request) will not be evaluated for substantial clinical improvement for the purposes of determining device pass-through payment status. Under this proposed alternative pathway, a medical device that has received FDA marketing authorization (that is, has been approved or cleared by, or had a De Novo classification request granted by, the FDA) and that is part of the FDA's Breakthrough Devices Program would still need to meet the eligibility criteria under § 419.66(b), the other criteria for establishing device categories under § 419.66(c), and the cost criterion under § 419.66(d). We note that this proposal aligns with a proposal in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19371 through 19373) and will help achieve the goals of expedited access to innovative therapies and further reduce administrative burden.
B. Proposed Device-Intensive Procedures
1. Background
Under the OPPS, prior to CY 2017, device-intensive status for procedures was determined at the APC level for APCs with a device offset percentage greater than 40 percent (79 FR 66795). Beginning in CY 2017, CMS began determining device-intensive status at the HCPCS code level. In assigning device-intensive status to an APC prior to CY 2017, the device costs of all the procedures within the APC were calculated and the geometric mean device offset of all of the procedures had to exceed 40 percent. Almost all of the procedures assigned to device-intensive APCs utilized devices, and the device costs for the associated HCPCS codes exceeded the 40-percent threshold. The no cost/full credit and partial credit device policy (79 FR 66872 through 66873) applies to device-intensive APCs and is discussed in detail in section IV.B.4. of this proposed rule. A related device policy was the requirement that certain procedures assigned to device-intensive APCs require the reporting of a device code on the claim (80 FR 70422). For further background information on the device-intensive APC policy, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70421 through 70426).
a. HCPCS Code-Level Device-Intensive Determination
As stated earlier, prior to CY 2017, the device-intensive methodology assigned device-intensive status to all procedures requiring the implantation of a device that were assigned to an APC with a device offset greater than 40 percent and, beginning in CY 2015, that met the three criteria listed below. Historically, the device-intensive designation was at the APC level and applied to the applicable procedures within that APC. In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79658), we changed our methodology to assign device-intensive status at the individual HCPCS code level rather than at the APC level. Under this policy, a procedure could be assigned device-intensive status regardless of its APC assignment, and device-intensive APCs were no longer applied under the OPPS or the ASC payment system.
We believe that a HCPCS code-level device offset is, in most cases, a better representation of a procedure's device cost than an APC-wide average device offset based on the average device offset of all of the procedures assigned to an APC. Unlike a device offset calculated at the APC level, which is a weighted average offset for all devices used in all of the procedures assigned to an APC, a HCPCS code-level device offset is calculated using only claims for a single HCPCS code. We believe that this methodological change results in a more accurate representation of the cost attributable to implantation of a high-cost device, which ensures consistent device-intensive designation of procedures with a significant device cost. Further, we believe a HCPCS code-level device offset removes inappropriate device-intensive status for procedures without a significant device cost that are granted such status because of APC assignment.
Under our existing policy, procedures that meet the criteria listed below in section IV.B.1.b. of this proposed rule are identified as device-intensive procedures and are subject to all the policies applicable to procedures assigned device-intensive status under our established methodology, including our policies on device edits and no cost/full credit and partial credit devices discussed in sections IV.B.3. and IV.B.4. of this proposed rule, respectively.
b. Use of the Three Criteria To Designate Device-Intensive Procedures
We clarified our established policy in the CY 2018 OPPS/ASC final rule with comment period (82 FR 52474), where we explained that device-intensive procedures require the implantation of a device and additionally are subject to the following criteria:
- All procedures must involve implantable devices that would be reported if device insertion procedures were performed;
- The required devices must be surgically inserted or implanted devices that remain in the patient's body after the conclusion of the procedure (at least temporarily); and
- The device offset amount must be significant, which is defined as exceeding 40 percent of the procedure's mean cost.
We changed our policy to apply these three criteria to determine whether procedures qualify as device-intensive in the CY 2015 OPPS/ASC final rule with comment period (79 FR 66926), where we stated that we would apply the no cost/full credit and partial credit device policy—which includes the three criteria listed above—to all device-intensive procedures beginning in CY 2015. We reiterated this position in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70424), where we explained that we were finalizing our proposal to continue using the three criteria established in the CY 2007 OPPS/ASC final rule with comment period for determining the APCs to which the CY 2016 device intensive policy will apply. Under the policies we adopted in CYs 2015, 2016, and 2017, all procedures that require the implantation of a device and meet the above criteria are assigned device-intensive status, regardless of their APC placement.
2. Device-Intensive Procedure Policy for CY 2019 and Subsequent Years
As part of CMS' effort to better capture costs for procedures with significant device costs, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58944 through 58948), for CY 2019, we modified our criteria for device-intensive procedures. We had heard from stakeholders that the criteria excluded some procedures that stakeholders believed should qualify as device-intensive procedures. Specifically, we were persuaded by stakeholder arguments that procedures requiring expensive surgically inserted or implanted devices that are not capital equipment should qualify as device-intensive procedures, regardless of whether the device remains in the patient's body after the conclusion of the procedure. We agreed that a broader definition of device-intensive procedures was warranted, and made two modifications to the criteria for CY 2019 (83 FR 58948). First, we allow procedures that involve surgically inserted or implanted, single-use devices that meet the device offset percentage threshold to qualify as device-intensive procedures, regardless of whether the device remains in the patient's body after the conclusion of the procedure. We established this policy because we no longer believe that whether a device remains in the patient's body should affect its designation as a device-intensive procedure, as such devices could, nonetheless, comprise a large portion of the cost of the applicable procedure. Second, we modified our criteria to lower the device offset percentage threshold from 40 percent to 30 percent, to allow a greater number of procedures to qualify as device-intensive. We stated that we believe allowing these additional procedures to qualify for device-intensive status will help ensure these procedures receive more appropriate payment in the ASC setting, which will help encourage the provision of these services in the ASC setting. In addition, we stated that this change would help to ensure that more procedures containing relatively high-cost devices are subject to the device edits, which leads to more correctly coded claims and greater accuracy in our claims data. Specifically, for CY 2019 and subsequent years, we finalized that device-intensive procedures will be subject to the following criteria:
- All procedures must involve implantable devices assigned a CPT or HCPCS code;
- The required devices (including single-use devices) must be surgically inserted or implanted; and
- The device offset amount must be significant, which is defined as exceeding 30 percent of the procedure's mean cost (83 FR 58945).
In addition, to further align the device-intensive policy with the criteria used for device pass-through payment status, we finalized, for CY 2019 and subsequent years, that for purposes of satisfying the device-intensive criteria, a device-intensive procedure must involve a device that:
- Has received FDA marketing authorization, has received an FDA investigational device exemption (IDE), and has been classified as a Category B device by the FDA in accordance with 42 CFR 405.203 through 405.207 and 405.211 through 405.215, or meets another appropriate FDA exemption from premarket review;
- Is an integral part of the service furnished;
- Is used for one patient only;
- Comes in contact with human tissue;
- Is surgically implanted or inserted (either permanently or temporarily); and
- Is not either of the following:
(a) Equipment, an instrument, apparatus, implement, or item of this type for which depreciation and financing expenses are recovered as depreciable assets as defined in Chapter 1 of the Medicare Provider Reimbursement Manual (CMS Pub. 15-1); or
(b) A material or supply furnished incident to a service (for example, a suture, customized surgical kit, scalpel, or clip, other than a radiological site marker) (83 FR 58945).
In addition, for new HCPCS codes describing procedures requiring the implantation of medical devices that do not yet have associated claims data, in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79658), we finalized a policy for CY 2017 to apply device-intensive status with a default device offset set at 41 percent for new HCPCS codes describing procedures requiring the implantation or insertion of a medical device that did not yet have associated claims data until claims data are available to establish the HCPCS code-level device offset for the procedures. This default device offset amount of 41 percent was not calculated from claims data; instead, it was applied as a default until claims data were available upon which to calculate an actual device offset for the new code. The purpose of applying the 41-percent default device offset to new codes that describe procedures that implant or insert medical devices was to ensure ASC access for new procedures until claims data become available.
As discussed in the CY 2019 OPPS/ASC proposed rule and final rule with comment period (83 FR 37108 through 37109 and 58945 through 58946, respectively), in accordance with our policy stated above to lower the device offset percentage threshold for procedures to qualify as device-intensive from greater than 40 percent to greater than 30 percent, for CY 2019 and subsequent years, we modified this policy to apply a 31-percent default device offset to new HCPCS codes describing procedures requiring the implantation of a medical device that do not yet have associated claims data until claims data are available to establish the HCPCS code-level device offset for the procedures. In conjunction with the policy to lower the default device offset from 41 percent to 31 percent, we continued our current policy of, in certain rare instances (for example, in the case of a very expensive implantable device), temporarily assigning a higher offset percentage if warranted by additional information such as pricing data from a device manufacturer (81 FR 79658). Once claims data are available for a new procedure requiring the implantation of a medical device, device-intensive status is applied to the code if the HCPCS code-level device offset is greater than 30 percent, according to our policy of determining device-intensive status by calculating the HCPCS code-level device offset.
In addition, in the CY 2019 OPPS/ASC final rule with comment period, we clarified that since the adoption of our policy in effect as of CY 2018, the associated claims data used for purposes of determining whether or not to apply the default device offset are the associated claims data for either the new HCPCS code or any predecessor code, as described by CPT coding guidance, for the new HCPCS code. Additionally, for CY 2019 and subsequent years, in limited instances where a new HCPCS code does not have a predecessor code as defined by CPT, but describes a procedure that was previously described by an existing code, we use clinical discretion to identify HCPCS codes that are clinically related or similar to the new HCPCS code but are not officially recognized as a predecessor code by CPT, and to use the claims data of the clinically related or similar code(s) for purposes of determining whether or not to apply the default device offset to the new HCPCS code (83 FR 58946). Clinically related and similar procedures for purposes of this policy are procedures that have little or no clinical differences and use the same devices as the new HCPCS code. In addition, clinically related and similar codes for purposes of this policy are codes that either currently or previously describe the procedure described by the new HCPCS code. Under this policy, claims data from clinically related and similar codes are included as associated claims data for a new code, and where an existing HCPCS code is found to be clinically related or similar to a new HCPCS code, we apply the device offset percentage derived from the existing clinically related or similar HCPCS code's claims data to the new HCPCS code for determining the device offset percentage. We stated that we believe that claims data for HCPCS codes describing procedures that have minor differences from the procedures described by new HCPCS codes will provide an accurate depiction of the cost relationship between the procedure and the device(s) that are used, and will be appropriate to use to set a new code's device offset percentage, in the same way that predecessor codes are used. If a new HCPCS code has multiple predecessor codes, the claims data for the predecessor code that has the highest individual HCPCS-level device offset percentage is used to determine whether the new HCPCS code qualifies for device-intensive status. Similarly, in the event that a new HCPCS code does not have a predecessor code but has multiple clinically related or similar codes, the claims data for the clinically related or similar code that has the highest individual HCPCS level device offset percentage is used to determine whether the new HCPCS code qualifies for device-intensive status.
As we indicated in the CY 2019 OPPS/ASC proposed rule and final rule with comment period, additional information for our consideration of an offset percentage higher than the default of 31 percent for new HCPCS codes describing procedures requiring the implantation (or, in some cases, the insertion) of a medical device that do not yet have associated claims data, such as pricing data or invoices from a device manufacturer, should be directed to the Division of Outpatient Care, Mail Stop C4-01-26, Centers for Medicare and Medicaid Services, 7500 Security Boulevard, Baltimore, MD 21244-1850, or electronically at outpatientpps@cms.hhs.gov. Additional information can be submitted prior to issuance of an OPPS/ASC proposed rule or as a public comment in response to an issued OPPS/ASC proposed rule. Device offset percentages will be set in each year's final rule.
For CY 2020, we are not proposing any changes to our device-intensive policy. The full listing of the proposed CY 2020 device-intensive procedures can be found in Addendum P to this CY 2020 OPPS/ASC proposed rule (which is available via the internet on the CMS website).
3. Device Edit Policy
In the CY 2015 OPPS/ASC final rule with comment period (79 FR 66795), we finalized a policy and implemented claims processing edits that require any of the device codes used in the previous device-to-procedure edits to be present on the claim whenever a procedure code assigned to any of the APCs listed in Table 5 of the CY 2015 OPPS/ASC final rule with comment period (the CY 2015 device-dependent APCs) is reported on the claim. In addition, in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70422), we modified our previously existing policy and applied the device coding requirements exclusively to procedures that require the implantation of a device that are assigned to a device-intensive APC. In the CY 2016 OPPS/ASC final rule with comment period, we also finalized our policy that the claims processing edits are such that any device code, when reported on a claim with a procedure assigned to a device-intensive APC (listed in Table 42 of the CY 2016 OPPS/ASC final rule with comment period (80 FR 70422)) will satisfy the edit.
In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79658 through 79659), we changed our policy for CY 2017 and subsequent years to apply the CY 2016 device coding requirements to the newly defined device-intensive procedures. For CY 2017 and subsequent years, we also specified that any device code, when reported on a claim with a device-intensive procedure, will satisfy the edit. In addition, we created HCPCS code C1889 to recognize devices furnished during a device-intensive procedure that are not described by a specific Level II HCPCS Category C-code. Reporting HCPCS code C1889 with a device-intensive procedure will satisfy the edit requiring a device code to be reported on a claim with a device-intensive procedure. In the CY 2019 OPPS/ASC final rule with comment period, we revised the description of HCPCS code C1889 to remove the specific applicability to device-intensive procedures (83 FR 58950). For CY 2019 and subsequent years, the description of HCPCS code C1889 is “Implantable/insertable device, not otherwise classified”.
We are not proposing any changes to this policy for CY 2020.
4. Adjustment to OPPS Payment for No Cost/Full Credit and Partial Credit Devices
a. Background
To ensure equitable OPPS payment when a hospital receives a device without cost or with full credit, in CY 2007, we implemented a policy to reduce the payment for specified device-dependent APCs by the estimated portion of the APC payment attributable to device costs (that is, the device offset) when the hospital receives a specified device at no cost or with full credit (71 FR 68071 through 68077). Hospitals were instructed to report no cost/full credit device cases on the claim using the “FB” modifier on the line with the procedure code in which the no cost/full credit device is used. In cases in which the device is furnished without cost or with full credit, hospitals were instructed to report a token device charge of less than $1.01. In cases in which the device being inserted is an upgrade (either of the same type of device or to a different type of device) with a full credit for the device being replaced, hospitals were instructed to report as the device charge the difference between the hospital's usual charge for the device being implanted and the hospital's usual charge for the device for which it received full credit. In CY 2008, we expanded this payment adjustment policy to include cases in which hospitals receive partial credit of 50 percent or more of the cost of a specified device. Hospitals were instructed to append the “FC” modifier to the procedure code that reports the service provided to furnish the device when they receive a partial credit of 50 percent or more of the cost of the new device. We refer readers to the CY 2008 OPPS/ASC final rule with comment period for more background information on the “FB” and “FC” modifiers payment adjustment policies (72 FR 66743 through 66749).
In the CY 2014 OPPS/ASC final rule with comment period (78 FR 75005 through 75007), beginning in CY 2014, we modified our policy of reducing OPPS payment for specified APCs when a hospital furnishes a specified device without cost or with a full or partial credit. For CY 2013 and prior years, our policy had been to reduce OPPS payment by 100 percent of the device offset amount when a hospital furnishes a specified device without cost or with a full credit and by 50 percent of the device offset amount when the hospital receives partial credit in the amount of 50 percent or more of the cost for the specified device. For CY 2014, we reduced OPPS payment, for the applicable APCs, by the full or partial credit a hospital receives for a replaced device. Specifically, under this modified policy, hospitals are required to report on the claim the amount of the credit in the amount portion for value code “FD” (Credit Received from the Manufacturer for a Replaced Medical Device) when the hospital receives a credit for a replaced device that is 50 percent or greater than the cost of the device. For CY 2014, we also limited the OPPS payment deduction for the applicable APCs to the total amount of the device offset when the “FD” value code appears on a claim. For CY 2015, we continued our policy of reducing OPPS payment for specified APCs when a hospital furnishes a specified device without cost or with a full or partial credit and to use the three criteria established in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68072 through 68077) for determining the APCs to which our CY 2015 policy will apply (79 FR 66872 through 66873). In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70424), we finalized our policy to no longer specify a list of devices to which the OPPS payment adjustment for no cost/full credit and partial credit devices would apply and instead apply this APC payment adjustment to all replaced devices furnished in conjunction with a procedure assigned to a device-intensive APC when the hospital receives a credit for a replaced specified device that is 50 percent or greater than the cost of the device.
b. Policy for No Cost/Full Credit and Partial Credit Devices
In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79659 through 79660), for CY 2017 and subsequent years, we finalized our policy to reduce OPPS payment for device-intensive procedures, by the full or partial credit a provider receives for a replaced device, when a hospital furnishes a specified device without cost or with a full or partial credit. Under our current policy, hospitals continue to be required to report on the claim the amount of the credit in the amount portion for value code “FD” when the hospital receives a credit for a replaced device that is 50 percent or greater than the cost of the device.
We are not proposing any changes to our no cost/full credit and partial credit device policies in this proposed rule.
5. Proposed Payment Policy for Low-Volume Device-Intensive Procedures
In CY 2016, we used our equitable adjustment authority under section 1833(t)(2)(E) of the Act and used the median cost (instead of the geometric mean cost per our standard methodology) to calculate the payment rate for the implantable miniature telescope procedure described by CPT code 0308T (Insertion of ocular telescope prosthesis including removal of crystalline lens or intraocular lens prosthesis), which is the only code assigned to APC 5494 (Level 4 Intraocular Procedures) (80 FR 70388). We noted that, as stated in the CY 2017 OPPS/ASC proposed rule (81 FR 45656), we proposed to reassign the procedure described by CPT code 0308T to APC 5495 (Level 5 Intraocular Procedures) for CY 2017, but it would be the only procedure code assigned to APC 5495. The payment rates for a procedure described by CPT code 0308T (including the predecessor HCPCS code C9732) were $15,551 in CY 2014, $23,084 in CY 2015, and $17,551 in CY 2016. The procedure described by CPT code 0308T is a high-cost device-intensive surgical procedure that has a very low volume of claims (in part because most of the procedures described by CPT code 0308T are performed in ASCs). We believe that the median cost is a more appropriate measure of the central tendency for purposes of calculating the cost and the payment rate for this procedure because the median cost is impacted to a lesser degree than the geometric mean cost by more extreme observations. We stated that, in future rulemaking, we would consider proposing a general policy for the payment rate calculation for very low-volume device-intensive APCs (80 FR 70389).
For CY 2017, we proposed and finalized a payment policy for low-volume device-intensive procedures that is similar to the policy applied to the procedure described by CPT code 0308T in CY 2016. In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79660 through 79661), we established our current policy that the payment rate for any device-intensive procedure that is assigned to a clinical APC with fewer than 100 total claims for all procedures in the APC be calculated using the median cost instead of the geometric mean cost, for the reasons described above for the policy applied to the procedure described by CPT code 0308T in CY 2016. The CY 2018 final rule geometric mean cost for the procedure described by CPT code 0308T (based on 19 claims containing the device HCPCS C-code, in accordance with the device-intensive edit policy) was approximately $21,302, and the median cost was approximately $19,521. The final CY 2018 payment rate (calculated using the median cost) was approximately $17,560.
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 58951), for CY 2019, we continued with our policy of establishing the payment rate for any device-intensive procedure that is assigned to a clinical APC with fewer than 100 total claims for all procedures in the APC based on calculations using the median cost instead of the geometric mean cost. For more information on the specific policy for assignment of low-volume device-intensive procedures for CY 2019, we refer readers to section III.D.13. of the CY 2019 OPPS/ASC final rule with comment period (83 FR 58917 through 58918).
For CY 2020, we are proposing to continue our current policy of establishing the payment rate for any device-intensive procedure that is assigned to a clinical APC with fewer than 100 total claims for all procedures in the APC using the median cost instead of the geometric mean cost. For CY 2020, this policy would apply to CPT code 0308T, which we are proposing to assign to APC 5495 (Level 5 Intraocular Procedures) in this proposed rule. The CY 2020 proposed rule geometric mean cost for the procedure described by CPT code 0308T (based on 7 claims containing the device HCPCS C-code, in accordance with the device-intensive edit policy) is approximately $28,237, and the median cost is approximately $19,270. The proposed CY 2020 payment rate (calculated using the median cost) is approximately $19,740 and can be found in Addendum B to this proposed rule (which is available via the internet on the CMS website).
V. Proposed OPPS Payment Changes for Drugs, Biologicals, and Radiopharmaceuticals
A. Proposed OPPS Transitional Pass-Through Payment for Additional Costs of Drugs, Biologicals, and Radiopharmaceuticals
1. Background
Section 1833(t)(6) of the Act provides for temporary additional payments or “transitional pass-through payments” for certain drugs and biologicals. Throughout this proposed rule, the term “biological” is used because this is the term that appears in section 1861(t) of the Act. A “biological” as used in this proposed rule includes (but is not necessarily limited to) a “biological product” or a “biologic” as defined under section 351 of the Public Health Service Act. As enacted by the Medicare, Medicaid, and SCHIP Balanced Budget Refinement Act of 1999 (BBRA) (Pub. L. 106-113), this pass-through payment provision requires the Secretary to make additional payments to hospitals for: Current orphan drugs for rare disease and conditions, as designated under section 526 of the Federal Food, Drug, and Cosmetic Act; current drugs and biologicals and brachytherapy sources used in cancer therapy; and current radiopharmaceutical drugs and biologicals. “Current” refers to those types of drugs or biologicals mentioned above that are hospital outpatient services under Medicare Part B for which transitional pass-through payment was made on the first date the hospital OPPS was implemented.
Transitional pass-through payments also are provided for certain “new” drugs and biologicals that were not being paid for as an HOPD service as of December 31, 1996 and whose cost is “not insignificant” in relation to the OPPS payments for the procedures or services associated with the new drug or biological. For pass-through payment purposes, radiopharmaceuticals are included as “drugs.” As required by statute, transitional pass-through payments for a drug or biological described in section 1833(t)(6)(C)(i)(II) of the Act can be made for a period of at least 2 years, but not more than 3 years, after the payment was first made for the product as a hospital outpatient service under Medicare Part B. Proposed CY 2020 pass-through drugs and biologicals and their designated APCs are assigned status indicator “G” in Addenda A and B to this proposed rule (which are available via the internet on the CMS website).
Section 1833(t)(6)(D)(i) of the Act specifies that the pass-through payment amount, in the case of a drug or biological, is the amount by which the amount determined under section 1842(o) of the Act for the drug or biological exceeds the portion of the otherwise applicable Medicare OPD fee schedule that the Secretary determines is associated with the drug or biological. The methodology for determining the pass-through payment amount is set forth in regulations at 42 CFR 419.64. These regulations specify that the pass-through payment equals the amount determined under section 1842(o) of the Act minus the portion of the APC payment that CMS determines is associated with the drug or biological.
Section 1847A of the Act establishes the average sales price (ASP) methodology, which is used for payment for drugs and biologicals described in section 1842(o)(1)(C) of the Act furnished on or after January 1, 2005. The ASP methodology, as applied under the OPPS, uses several sources of data as a basis for payment, including the ASP, the wholesale acquisition cost (WAC), and the average wholesale price (AWP). In this proposed rule, the term “ASP methodology” and “ASP-based” are inclusive of all data sources and methodologies described therein. Additional information on the ASP methodology can be found on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html.
The pass-through application and review process for drugs and biologicals is described on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/passthrough_payment.html.
2. Three-Year Transitional Pass-Through Payment Period for All Pass-Through Drugs, Biologicals, and Radiopharmaceuticals and Quarterly Expiration of Pass-Through Status
As required by statute, transitional pass-through payments for a drug or biological described in section 1833(t)(6)(C)(i)(II) of the Act can be made for a period of at least 2 years, but not more than 3 years, after the payment was first made for the product as a hospital outpatient service under Medicare Part B. Our current policy is to accept pass-through applications on a quarterly basis and to begin pass-through payments for newly approved pass-through drugs and biologicals on a quarterly basis through the next available OPPS quarterly update after the approval of a product's pass-through status. However, prior to CY 2017, we expired pass-through status for drugs and biologicals on an annual basis through notice-and-comment rulemaking (74 FR 60480). In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79662), we finalized a policy change, beginning with pass-through drugs and biologicals newly approved in CY 2017 and subsequent calendar years, to allow for a quarterly expiration of pass-through payment status for drugs, biologicals, and radiopharmaceuticals to afford a pass-through payment period that is as close to a full 3 years as possible for all pass-through drugs, biologicals, and radiopharmaceuticals.
This change eliminated the variability of the pass-through payment eligibility period, which previously varied based on when a particular application was initially received. We adopted this change for pass-through approvals beginning on or after CY 2017, to allow, on a prospective basis, for the maximum pass-through payment period for each pass-through drug without exceeding the statutory limit of 3 years. Notice of drugs whose pass-through payment status is ending during the calendar year will continue to be included in the quarterly OPPS Change Request transmittals.
3. Proposed Drugs and Biologicals With Expiring Pass-Through Payment Status in CY 2019
We are proposing that the pass-through payment status of six drugs and biologicals would expire on December 31, 2019 as listed in Table 14. These drugs and biologicals will have received OPPS pass-through payment for 3 years during the period of January 1, 2017 until December 31, 2019.
In accordance with the policy finalized in CY 2017 and described earlier, pass-through payment status for drugs and biologicals newly approved in CY 2017 and subsequent years will expire on a quarterly basis, with a pass-through payment period as close to 3 years as possible. With the exception of those groups of drugs and biologicals that are always packaged when they do not have pass-through payment status (specifically, anesthesia drugs; drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure (including diagnostic radiopharmaceuticals, contrast agents, and stress agents); and drugs and biologicals that function as supplies when used in a surgical procedure), our standard methodology for providing payment for drugs and biologicals with expiring pass-through payment status in an upcoming calendar year is to determine the product's estimated per day cost and compare it with the OPPS drug packaging threshold for that calendar year (which is proposed to be $130 for CY 2020), as discussed further in section V.B.2. of this proposed rule. We are proposing that if the estimated per day cost for the drug or biological is less than or equal to the applicable OPPS drug packaging threshold, we would package payment for the drug or biological into the payment for the associated procedure in the upcoming calendar year. If the estimated per day cost of the drug or biological is greater than the OPPS drug packaging threshold, we are proposing to provide separate payment at the applicable relative ASP-based payment amount (which is proposed at ASP+6 percent for CY 2020, as discussed further in section V.B.3. of this proposed rule).

The proposed packaged or separately payable status of each of these drugs or biologicals is listed in Addendum B to this proposed rule (which is available via the internet on the CMS website).
4. Proposed Drugs, Biologicals, and Radiopharmaceuticals With New or Continuing Pass-Through Payment Status in CY 2020
We are proposing to continue pass-through payment status in CY 2020 for 61 drugs and biologicals. These drugs and biologicals, which were approved for pass-through payment status between April 1, 2017 and April 1, 2019 are listed in Table 15. The APCs and HCPCS codes for these drugs and biologicals approved for pass-through payment status on or after January 1, 2020 are assigned status indicator “G” in Addenda A and B to this proposed rule (which are available via the internet on the CMS website). In addition, there are four drugs and biologicals that have already had 3 years of pass-through payment status but for which pass-through payment status is required to be extended for an additional 2 years, effective October 1, 2018 under section 1833(t)(6)(G) of the Act, as added by section 1301(a)(1)(C) of the Consolidated Appropriations Act of 2018 (Pub. L. 115-141). That means the last 9 months of pass-through status for these drugs will occur in CY 2020. Because of this requirement, these drugs and biologicals are also included in Table 15, which brings the total number of drugs and biologicals with proposed pass-through payment status in CY 2020 to 65.
Section 1833(t)(6)(D)(i) of the Act sets the amount of pass-through payment for pass-through drugs and biologicals (the pass-through payment amount) as the difference between the amount authorized under section 1842(o) of the Act and the portion of the otherwise applicable OPD fee schedule that the Secretary determines is associated with the drug or biological. For CY 2020, we are proposing to continue to pay for pass-through drugs and biologicals at ASP+6 percent, equivalent to the payment rate these drugs and biologicals would receive in the physician's office setting in CY 2020. We are proposing that a $0 pass-through payment amount would be paid for pass-through drugs and biologicals under the CY 2020 OPPS because the difference between the amount authorized under section 1842(o) of the Act, which is proposed at ASP+6 percent, and the portion of the otherwise applicable OPD fee schedule that the Secretary determines is appropriate, which is proposed at ASP+6 percent, is $0.
In the case of policy-packaged drugs (which include the following: Anesthesia drugs; drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure (including contrast agents, diagnostic radiopharmaceuticals, and stress agents); and drugs and biologicals that function as supplies when used in a surgical procedure), we are proposing that their pass-through payment amount would be equal to ASP+6 percent for CY 2020 minus a payment offset for any predecessor drug products contributing to the pass-through payment as described in section V.A.6. of this proposed rule. We are making this proposal because, if not for the pass-through payment status of these policy-packaged products, payment for these products would be packaged into the associated procedure.
We are proposing to continue to update pass-through payment rates on a quarterly basis on the CMS website during CY 2020 if later quarter ASP submissions (or more recent WAC or AWP information, as applicable) indicate that adjustments to the payment rates for these pass-through payment drugs or biologicals are necessary. For a full description of this policy, we refer readers to the CY 2006 OPPS/ASC final rule with comment period (70 FR 68632 through 68635).
For CY 2020, consistent with our CY 2019 policy for diagnostic and therapeutic radiopharmaceuticals, we are proposing to provide payment for both diagnostic and therapeutic radiopharmaceuticals that are granted pass-through payment status based on the ASP methodology. As stated earlier, for purposes of pass-through payment, we consider radiopharmaceuticals to be drugs under the OPPS. Therefore, if a diagnostic or therapeutic radiopharmaceutical receives pass-through payment status during CY 2020, we are proposing to follow the standard ASP methodology to determine the pass-through payment rate that drugs receive under section 1842(o) of the Act, which is proposed at ASP+6 percent. If ASP data are not available for a radiopharmaceutical, we are proposing to provide pass-through payment at WAC+3 percent (consistent with our proposed policy in section V.B.2.b. of this proposed rule), the equivalent payment provided to pass-through payment drugs and biologicals without ASP information. Additional detail and comments on the WAC+3 percent payment policy can be found in section V.B.2.b. of this proposed rule. If WAC information also is not available, we are proposing to provide payment for the pass-through radiopharmaceutical at 95 percent of its most recent AWP.
The drugs and biologicals that we are proposing to continue to have pass-through payment status on or after January 1, 2020 or that have been granted pass-through payment status as of April 2019 are shown in Table 15.





5. Proposed Drugs, Biologicals, and Radiopharmaceuticals With Pass-Through Status as a Result of Section 1301 of the Consolidated Appropriations Act of 2018 (Pub. L. 115-141)
As mentioned earlier, section 1301(a)(1) of the Consolidated Appropriations Act of 2018 (Pub. L. 115-141) amended section 1833(t)(6) of the Act and added a new section 1833(t)(6)(G), which provides that for drugs or biologicals whose period of pass-through payment status ended on December 31, 2017 and for which payment was packaged into a covered hospital outpatient service furnished beginning January 1, 2018, such pass-through payment status shall be extended for a 2-year period beginning on October 1, 2018 through September 30, 2020. There are four products whose period of drug and biological pass-through payment status ended on December 31, 2017 and for which payment would have been packaged beginning January 1, 2018. These products were listed in Table 39 of the CY 2019 OPPS/ASC final rule with comment period (83 FR 58962).
Starting in CY 2019, the HCPCS code Q4172 (PuraPly, and PuraPly Antimicrobial, any type, per square centimeter) was discontinued. In its place, two new HCPCS codes were established—Q4195 (Puraply, per square centimeter) and Q4196 (Puraply am, per square centimeter). Because these HCPCS codes are direct successors to HCPCS code Q4172, the provisions of section 1833(t)(6)(G) of the Act apply to HCPCS codes Q4195 and Q4196, and these codes are listed in Table 16. For CY 2020, we are proposing to continue pass-through payment status for the drugs and biologicals listed in Table 16 of this proposed rule (we note that these drugs and biologicals are also listed in Table 15 of this proposed rule) through September 30, 2020 as required in section 1833(t)(6)(G) of the Act, as added by section 1301(a)(1)(C) of the Consolidated Appropriations Act of 2018. The APCs and HCPCS codes for these drugs and biologicals approved for pass-through payment status are assigned status indicator “G” in Addenda A and B to this proposed rule (which are available via the internet on the CMS website).
We are proposing to continue to update pass-through payment rates for HCPCS codes Q4195 and Q4196 along with the other three drugs and biologicals covered by section 1833(t)(6)(G) of the Act on a quarterly basis on the CMS website during CY 2020 if later quarter ASP submissions (or more recent WAC or AWP information, as applicable) indicate that adjustments to the payment rates for these pass-through drugs or biologicals are necessary. The replacement of HCPCS code Q4172 by HCPCS codes Q4195 and Q4196 means there are five HCPCS codes for drugs and biologicals covered by section 1833(t)(6)(G) of the Act. For a full description of this policy, we refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 58960 through 58962).
The five HCPCS codes for drugs and biologicals that we are proposing would have pass-through payment status for CY 2020 under section 1833(t)(6)(G) of the Act, as added by section 1301(a)(1)(C) of the Consolidated Appropriations Act of 2018, are shown in Table 16. Included as two of the five HCPCS codes for drugs and biologicals with pass-through payment status for CY 2020 are HCPCS codes Q4195 (Puraply, per square centimeter) and Q4196 (Puraply am, per square centimeter). PuraPly and PuraPly AM are skin substitute products that were approved for pass-through payment status on January 1, 2015 through the drug and biological pass-through payment process. Beginning on April 1, 2015, skin substitute products are evaluated for pass-through payment status through the device pass-through payment process. However, we stated in the CY 2015 OPPS/ASC final rule with comment period (79 FR 66887) that skin substitutes that are approved for pass-through payment status as biologicals effective on or before January 1, 2015 would continue to be paid as pass-through biologicals for the duration of their pass-through payment period. Because PuraPly and PuraPly AM were approved for pass-through payment status through the drug and biological pass-through payment pathway, we finalized a policy to consider both PuraPly and PuraPly AM to be drugs or biologicals as described by section 1833(t)(6)(G) of the Act, as added by section 1301(a)(1)(C) of the Consolidated Appropriations Act of 2018, and to be eligible for extended pass-through payment under our proposal for CY 2020 (83 FR 58961 through 58962).

6. Proposed Provisions for Reducing Transitional Pass-Through Payments for Policy-Packaged Drugs, Biologicals, and Radiopharmaceuticals To Offset Costs Packaged Into APC Groups
Under the regulations at 42 CFR 419.2(b), nonpass-through drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure are packaged in the OPPS. This category includes diagnostic radiopharmaceuticals, contrast agents, stress agents, and other diagnostic drugs. Also under 42 CFR 419.2(b), nonpass-through drugs and biologicals that function as supplies in a surgical procedure are packaged in the OPPS. This category includes skin substitutes and other surgical-supply drugs and biologicals. As described earlier, section 1833(t)(6)(D)(i) of the Act specifies that the transitional pass-through payment amount for pass-through drugs and biologicals is the difference between the amount paid under section 1842(o) of the Act and the otherwise applicable OPD fee schedule amount. Because a payment offset is necessary in order to provide an appropriate transitional pass-through payment, we deduct from the pass-through payment for policy-packaged drugs, biologicals, and radiopharmaceuticals an amount reflecting the portion of the APC payment associated with predecessor products in order to ensure no duplicate payment is made. This amount reflecting the portion of the APC payment associated with predecessor products is called the payment offset.
The payment offset policy applies to all policy packaged drugs, biologicals, and radiopharmaceuticals. For a full description of the payment offset policy as applied to diagnostic radiopharmaceuticals, contrast agents, stress agents, and skin substitutes, we refer readers to the discussion in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70430 through 70432). For CY 2020, as we did in CY 2019, we are proposing to continue to apply the same policy packaged offset policy to payment for pass-through diagnostic radiopharmaceuticals, pass-through contrast agents, pass-through stress agents, and pass-through skin substitutes. The proposed APCs to which a payment offset may be applicable for pass-through diagnostic radiopharmaceuticals, pass-through contrast agents, pass-through stress agents, and pass-through skin substitutes are identified in Table 17.

We are proposing to continue to post annually on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Annual-Policy-Files.html a file that contains the APC offset amounts that will be used for that year for purposes of both evaluating cost significance for candidate pass-through payment device categories and drugs and biologicals and establishing any appropriate APC offset amounts. Specifically, the file will continue to provide the amounts and percentages of APC payment associated with packaged implantable devices, policy-packaged drugs, and threshold packaged drugs and biologicals for every OPPS clinical APC.
B. Proposed OPPS Payment for Drugs, Biologicals, and Radiopharmaceuticals Without Pass-Through Payment Status
1. Proposed Criteria for Packaging Payment for Drugs, Biologicals, and Radiopharmaceuticals
a. Proposed Packaging Threshold
In accordance with section 1833(t)(16)(B) of the Act, the threshold for establishing separate APCs for payment of drugs and biologicals was set to $50 per administration during CYs 2005 and 2006. In CY 2007, we used the four quarter moving average Producer Price Index (PPI) levels for Pharmaceutical Preparations (Prescription) to trend the $50 threshold forward from the third quarter of CY 2005 (when the Pub. L. 108-173 mandated threshold became effective) to the third quarter of CY 2007. We then rounded the resulting dollar amount to the nearest $5 increment in order to determine the CY 2007 threshold amount of $55. Using the same methodology as that used in CY 2007 (which is discussed in more detail in the CY 2007 OPPS/ASC final rule with comment period (71 FR 68085 through 68086)), we set the packaging threshold for establishing separate APCs for drugs and biologicals at $125 for CY 2019 (83 FR 58963 through 58964).
Following the CY 2007 methodology, for this CY 2020 OPPS/ASC proposed rule, we used the most recently available four quarter moving average PPI levels to trend the $50 threshold forward from the third quarter of CY 2005 to the third quarter of CY 2020 and rounded the resulting dollar amount ($131.19) to the nearest $5 increment, which yielded a figure of $130. In performing this calculation, we used the most recent forecast of the quarterly index levels for the PPI for Pharmaceuticals for Human Use (Prescription) (Bureau of Labor Statistics series code WPUSI07003) from CMS' Office of the Actuary. For this CY 2020 OPPS/ASC proposed rule, based on these calculations using the CY 2007 OPPS methodology, we are proposing a packaging threshold for CY 2020 of $130.
b. Proposed Packaging of Payment for HCPCS Codes That Describe Certain Drugs, Certain Biologicals, and Therapeutic Radiopharmaceuticals Under the Cost Threshold (“Threshold-Packaged Drugs”)
To determine the proposed CY 2020 packaging status for all nonpass-through drugs and biologicals that are not policy packaged, we calculated, on a HCPCS code-specific basis, the per day cost of all drugs, biologicals, and therapeutic radiopharmaceuticals (collectively called “threshold-packaged” drugs) that had a HCPCS code in CY 2018 and were paid (via packaged or separate payment) under the OPPS. We used data from CY 2018 claims processed before January 1, 2019 for this calculation. However, we did not perform this calculation for those drugs and biologicals with multiple HCPCS codes that include different dosages, as described in section V.B.1.d. of this proposed rule, or for the following policy-packaged items that we are proposing to continue to package in CY 2020: Anesthesia drugs; drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure; and drugs and biologicals that function as supplies when used in a surgical procedure.
In order to calculate the per day costs for drugs, biologicals, and therapeutic radiopharmaceuticals to determine their proposed packaging status in CY 2020, we used the methodology that was described in detail in the CY 2006 OPPS proposed rule (70 FR 42723 through 42724) and finalized in the CY 2006 OPPS final rule with comment period (70 FR 68636 through 68638). For each drug and biological HCPCS code, we used an estimated payment rate of ASP+6 percent (which is the payment rate we are proposing for separately payable drugs and biologicals for CY 2020, as discussed in more detail in section V.B.2.b. of this proposed rule) to calculate the CY 2020 proposed rule per day costs. We used the manufacturer-submitted ASP data from the fourth quarter of CY 2018 (data that were used for payment purposes in the physician's office setting, effective April 1, 2019) to determine the proposed rule per day cost.
As is our standard methodology, for CY 2020, we are proposing to use payment rates based on the ASP data from the first quarter of CY 2019 for budget neutrality estimates, packaging determinations, impact analyses, and completion of Addenda A and B to this proposed rule (which are available via the internet on the CMS website) because these are the most recent data available for use at the time of development of this proposed rule. These data also were the basis for drug payments in the physician's office setting, effective April 1, 2019. For items that did not have an ASP-based payment rate, such as some therapeutic radiopharmaceuticals, we used their mean unit cost derived from the CY 2018 hospital claims data to determine their per day cost.
We are proposing to package items with a per day cost less than or equal to $130, and identify items with a per day cost greater than $130 as separately payable unless they are policy-packaged. Consistent with our past practice, we cross-walked historical OPPS claims data from the CY 2018 HCPCS codes that were reported to the CY 2019 HCPCS codes that we display in Addendum B to this proposed rule (which is available via the internet on the CMS website) for proposed payment in CY 2020.
Our policy during previous cycles of the OPPS has been to use updated ASP and claims data to make final determinations of the packaging status of HCPCS codes for drugs, biologicals, and therapeutic radiopharmaceuticals for the OPPS/ASC final rule with comment period. We note that it is also our policy to make an annual packaging determination for a HCPCS code only when we develop the OPPS/ASC final rule with comment period for the update year. Only HCPCS codes that are identified as separately payable in the final rule with comment period are subject to quarterly updates. For our calculation of per day costs of HCPCS codes for drugs and biologicals in this CY 2020 OPPS/ASC proposed rule, we are proposing to use ASP data from the fourth quarter of CY 2018, which is the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology, effective April 1, 2019, along with updated hospital claims data from CY 2018. We note that we also are proposing to use these data for budget neutrality estimates and impact analyses for this CY 2020 OPPS/ASC proposed rule.
Payment rates for HCPCS codes for separately payable drugs and biologicals included in Addenda A and B for the final rule with comment period will be based on ASP data from the third quarter of CY 2019. These data will be the basis for calculating payment rates for drugs and biologicals in the physician's office setting using the ASP methodology, effective October 1, 2019. These payment rates would then be updated in the January 2020 OPPS update, based on the most recent ASP data to be used for physician's office and OPPS payment as of January 1, 2020. For items that do not currently have an ASP-based payment rate, we are proposing to recalculate their mean unit cost from all of the CY 2018 claims data and updated cost report information available for the CY 2020 final rule with comment period to determine their final per day cost.
Consequently, the packaging status of some HCPCS codes for drugs, biologicals, and therapeutic radiopharmaceuticals in this proposed rule may be different from the same drugs' HCPCS codes' packaging status determined based on the data used for the final rule with comment period. Under such circumstances, we are proposing to continue to follow the established policies initially adopted for the CY 2005 OPPS (69 FR 65780) in order to more equitably pay for those drugs whose costs fluctuate relative to the proposed CY 2020 OPPS drug packaging threshold and the drug's payment status (packaged or separately payable) in CY 2019. These established policies have not changed for many years and are the same as described in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70434). Specifically, for CY 2020, consistent with our historical practice, we are proposing to apply the following policies to these HCPCS codes for drugs, biologicals, and therapeutic radiopharmaceuticals whose relationship to the drug packaging threshold changes based on the updated drug packaging threshold and on the final updated data:
- HCPCS codes for drugs and biologicals that were paid separately in CY 2019 and that are proposed for separate payment in CY 2020, and that then have per day costs equal to or less than the CY 2020 final rule drug packaging threshold, based on the updated ASPs and hospital claims data used for the CY 2020 final rule, would continue to receive separate payment in CY 2020.
- HCPCS codes for drugs and biologicals that were packaged in CY 2019 and that are proposed for separate payment in CY 2020, and that then have per day costs equal to or less than the CY 2020 final rule drug packaging threshold, based on the updated ASPs and hospital claims data used for the CY 2020 final rule, would remain packaged in CY 2020.
- HCPCS codes for drugs and biologicals for which we proposed packaged payment in CY 2020 but that then have per-day costs greater than the CY 2020 final rule drug packaging threshold, based on the updated ASPs and hospital claims data used for the CY 2020 final rule, would receive separate payment in CY 2020.
c. Policy Packaged Drugs, Biologicals, and Radiopharmaceuticals
As mentioned earlier in this section, in the OPPS, we package several categories of drugs, biologicals, and radiopharmaceuticals, regardless of the cost of the products. Because the products are packaged according to the policies in 42 CFR 419.2(b), we refer to these packaged drugs, biologicals, and radiopharmaceuticals as “policy-packaged” drugs, biologicals, and radiopharmaceuticals. These policies are either longstanding or based on longstanding principles and inherent to the OPPS and are as follows:
- Anesthesia, certain drugs, biologicals, and other pharmaceuticals; medical and surgical supplies and equipment; surgical dressings; and devices used for external reduction of fractures and dislocations (§ 419.2(b)(4));
- Intraoperative items and services (§ 419.2(b)(14));
- Drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure (including, but not limited to, diagnostic radiopharmaceuticals, contrast agents, and pharmacologic stress agents) (§ 419.2(b)(15)); and
- Drugs and biologicals that function as supplies when used in a surgical procedure (including, but not limited to, skin substitutes and similar products that aid wound healing and implantable biologicals) (§ 419.2(b)(16)).
The policy at § 419.2(b)(16) is broader than that at § 419.2(b)(14). As we stated in the CY 2015 OPPS/ASC final rule with comment period: “We consider all items related to the surgical outcome and provided during the hospital stay in which the surgery is performed, including postsurgical pain management drugs, to be part of the surgery for purposes of our drug and biological surgical supply packaging policy” (79 FR 66875). The category described by § 419.2(b)(15) is large and includes diagnostic radiopharmaceuticals, contrast agents, stress agents, and some other products. The category described by § 419.2(b)(16) includes skin substitutes and some other products. We believe it is important to reiterate that cost consideration is not a factor when determining whether an item is a surgical supply (79 FR 66875).
d. Proposed Packaging Determination for HCPCS Codes That Describe the Same Drug or Biological but Different Dosages
In the CY 2010 OPPS/ASC final rule with comment period (74 FR 60490 through 60491), we finalized a policy to make a single packaging determination for a drug, rather than an individual HCPCS code, when a drug has multiple HCPCS codes describing different dosages because we believed that adopting the standard HCPCS code-specific packaging determinations for these codes could lead to inappropriate payment incentives for hospitals to report certain HCPCS codes instead of others. We continue to believe that making packaging determinations on a drug-specific basis eliminates payment incentives for hospitals to report certain HCPCS codes for drugs and allows hospitals flexibility in choosing to report all HCPCS codes for different dosages of the same drug or only the lowest dosage HCPCS code. Therefore, we are proposing to continue our policy to make packaging determinations on a drug-specific basis, rather than a HCPCS code-specific basis, for those HCPCS codes that describe the same drug or biological but different dosages in CY 2020.
For CY 2020, in order to propose a packaging determination that is consistent across all HCPCS codes that describe different dosages of the same drug or biological, we aggregated both our CY 2018 claims data and our pricing information at ASP+6 percent across all of the HCPCS codes that describe each distinct drug or biological in order to determine the mean units per day of the drug or biological in terms of the HCPCS code with the lowest dosage descriptor. The following drugs did not have pricing information available for the ASP methodology for this CY 2020 OPPS/ASC proposed rule, and as is our current policy for determining the packaging status of other drugs, we used the mean unit cost available from the CY 2018 claims data to make the proposed packaging determinations for these drugs: HCPCS code J1840 (Injection, kanamycin sulfate, up to 500 mg); HCPCS code J1850 (Injection, kanamycin sulfate, up to 75 mg); HCPCS code J3472 (Injection, hyaluronidase, ovine, preservative free, per 1,000 usp units); HCPCS code J7100 (Infusion, dextran 40, 500 ml); and HCPCS code J7110 (Infusion, dextran 75, 500 ml).
For all other drugs and biologicals that have HCPCS codes describing different doses, we then multiplied the proposed weighted average ASP+6 percent per unit payment amount across all dosage levels of a specific drug or biological by the estimated units per day for all HCPCS codes that describe each drug or biological from our claims data to determine the estimated per day cost of each drug or biological at less than or equal to the proposed CY 2020 drug packaging threshold of $130 (so that all HCPCS codes for the same drug or biological would be packaged) or greater than the proposed CY 2020 drug packaging threshold of $130 (so that all HCPCS codes for the same drug or biological would be separately payable). The proposed packaging status of each drug and biological HCPCS code to which this methodology would apply in CY 2020 is displayed in Table 18.

2. Proposed Payment for Drugs and Biologicals Without Pass-Through Status That Are Not Packaged
a. Proposed Payment for Specified Covered Outpatient Drugs (SCODs) and Other Separately Payable Drugs and Biologicals
Section 1833(t)(14) of the Act defines certain separately payable radiopharmaceuticals, drugs, and biologicals and mandates specific payments for these items. Under section 1833(t)(14)(B)(i) of the Act, a “specified covered outpatient drug” (known as a SCOD) is defined as a covered outpatient drug, as defined in section 1927(k)(2) of the Act, for which a separate APC has been established and that either is a radiopharmaceutical agent or is a drug or biological for which payment was made on a pass-through basis on or before December 31, 2002.
Under section 1833(t)(14)(B)(ii) of the Act, certain drugs and biologicals are designated as exceptions and are not included in the definition of SCODs. These exceptions are—
- A drug or biological for which payment is first made on or after January 1, 2003, under the transitional pass-through payment provision in section 1833(t)(6) of the Act.
- A drug or biological for which a temporary HCPCS code has not been assigned.
- During CYs 2004 and 2005, an orphan drug (as designated by the Secretary).
Section 1833(t)(14)(A)(iii) of the Act requires that payment for SCODs in CY 2006 and subsequent years be equal to the average acquisition cost for the drug for that year as determined by the Secretary, subject to any adjustment for overhead costs and taking into account the hospital acquisition cost survey data collected by the Government Accountability Office (GAO) in CYs 2004 and 2005, and later periodic surveys conducted by the Secretary as set forth in the statute. If hospital acquisition cost data are not available, the law requires that payment be equal to payment rates established under the methodology described in section 1842(o), section 1847A, or section 1847B of the Act, as calculated and adjusted by the Secretary as necessary for purposes of paragraph (14). We refer to this alternative methodology as the “statutory default.” Most physician Part B drugs are paid at ASP+6 percent in accordance with section 1842(o) and section 1847A of the Act.
Section 1833(t)(14)(E)(ii) of the Act provides for an adjustment in OPPS payment rates for SCODs to take into account overhead and related expenses, such as pharmacy services and handling costs. Section 1833(t)(14)(E)(i) of the Act required MedPAC to study pharmacy overhead and related expenses and to make recommendations to the Secretary regarding whether, and if so how, a payment adjustment should be made to compensate hospitals for overhead and related expenses. Section 1833(t)(14)(E)(ii) of the Act authorizes the Secretary to adjust the weights for ambulatory procedure classifications for SCODs to take into account the findings of the MedPAC study.[57]
It has been our policy since CY 2006 to apply the same treatment to all separately payable drugs and biologicals, which include SCODs, and drugs and biologicals that are not SCODs. Therefore, we apply the payment methodology in section 1833(t)(14)(A)(iii) of the Act to SCODs, as required by statute, but we also apply it to separately payable drugs and biologicals that are not SCODs, which is a policy determination rather than a statutory requirement. In this CY 2020 OPPS/ASC proposed rule, we are proposing to apply section 1833(t)(14)(A)(iii)(II) of the Act to all separately payable drugs and biologicals, including SCODs. Although we do not distinguish SCODs in this discussion, we note that we are required to apply section 1833(t)(14)(A)(iii)(II) of the Act to SCODs, but we also are applying this provision to other separately payable drugs and biologicals, consistent with our history of using the same payment methodology for all separately payable drugs and biologicals.
For a detailed discussion of our OPPS drug payment policies from CY 2006 to CY 2012, we refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68383 through 68385). In the CY 2013 OPPS/ASC final rule with comment period (77 FR 68386 through 68389), we first adopted the statutory default policy to pay for separately payable drugs and biologicals at ASP+6 percent based on section 1833(t)(14)(A)(iii)(II) of the Act. We continued this policy of paying for separately payable drugs and biologicals at the statutory default for CYs 2014 through 2019.
b. Proposed CY 2020 Payment Policy
For CY 2020, we are proposing to continue our payment policy that has been in effect since CY 2013 to pay for separately payable drugs and biologicals at ASP+6 percent in accordance with section 1833(t)(14)(A)(iii)(II) of the Act (the statutory default). We are proposing to continue to pay for separately payable nonpass-through drugs acquired with a 340B discount at a rate of ASP minus 22.5 percent, but are also soliciting comments on alternative policies as well as the appropriate remedy for CYs 2018 and 2019 in the event that we do not prevail on appeal in the pending litigation, as discussed in greater detail later in this section. We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59353 through 59371) and the CY 2019 OPPS/ASC final rule with comment period (83 FR 58979 through 58981) for more information about how the payment rate for drugs acquired with a 340B discount was established.
In the case of a drug or biological during an initial sales period in which data on the prices for sales for the drug or biological are not sufficiently available from the manufacturer, section 1847A(c)(4) of the Act permits the Secretary to make payments that are based on WAC. Under section 1833(t)(14)(A)(iii)(II), the amount of payment for a separately payable drug equals the average price for the drug for the year established under, among other authorities, section 1847A of the Act. As explained in greater detail in the CY 2019 PFS final rule, under section 1847A(c)(4), although payments may be based on WAC, unlike section 1847A(b) of the Act (which specifies that payments using ASP or WAC must be made with a 6 percent add-on), section 1847A(c)(4) of the Act does not require that a particular add-on amount be applied to WAC-based pricing for this initial period when ASP data is not available. Consistent with section 1847A(c)(4) of the Act, in the CY 2019 PFS final rule (83 FR 59661 to 59666), we finalized a policy that, effective January 1, 2019, WAC-based payments for Part B drugs made under section 1847A(c)(4) of the Act will utilize a 3-percent add-on in place of the 6-percent add-on that was being used according to our policy in effect as of CY 2018. For the CY 2019 OPPS, we followed the same policy finalized in the CY 2019 PFS final rule (83 FR 59661 to 59666). For the CY 2020 OPPS, we are proposing to continue to utilize a 3 percent add-on instead of a 6-percent add-on for WAC-based drugs pursuant to our authority under section 1833(t)(14)(A)(iii)(II) of the Act, which provides, in part, that the amount of payment for a SCOD is the average price of the drug in the year established under section 1847A of the Act. We also are proposing to apply this provision to non-SCOD separately payable drugs. Because we are proposing to establish the average price for a WAC-based drug under section 1847A of the Act as WAC+3 percent instead of WAC+6 percent, we believe it is appropriate to price separately payable WAC-based drugs at the same amount under the OPPS. We are proposing that, if finalized, our proposal to pay for drugs or biologicals at WAC+3 percent, rather than WAC+6 percent, would apply whenever WAC-based pricing is used for a drug or biological. For drugs and biologicals that would otherwise be subject to a payment reduction because they were acquired under the 340B Program, the 340B Program rate (in this case, WAC minus 22.5 percent) would continue to apply. We refer readers to the CY 2019 PFS final rule (83 FR 59661 to 59666) for additional background on this proposal.
We are proposing that payments for separately payable drugs and biologicals are included in the budget neutrality adjustments, under the requirements in section 1833(t)(9)(B) of the Act. We also are proposing that the budget neutral weight scalar not be applied in determining payments for these separately paid drugs and biologicals.
We note that separately payable drug and biological payment rates listed in Addenda A and B to this proposed rule (available via the internet on the CMS website), which illustrate the proposed CY 2020 payment of ASP+6 percent for separately payable nonpass-through drugs and biologicals and ASP+6 percent for pass-through drugs and biologicals, reflect either ASP information that is the basis for calculating payment rates for drugs and biologicals in the physician's office setting effective April 1, 2019, or WAC, AWP, or mean unit cost from CY 2018 claims data and updated cost report information available for this proposed rule. In general, these published payment rates are not the same as the actual January 2020 payment rates. This is because payment rates for drugs and biologicals with ASP information for January 2020 will be determined through the standard quarterly process where ASP data submitted by manufacturers for the third quarter of CY 2019 (July 1, 2019 through September 30, 2019) will be used to set the payment rates that are released for the quarter beginning in January 2020 near the end of December 2019. In addition, payment rates for drugs and biologicals in Addenda A and B to this proposed rule for which there was no ASP information available for April 2019 are based on mean unit cost in the available CY 2018 claims data. If ASP information becomes available for payment for the quarter beginning in January 2020, we will price payment for these drugs and biologicals based on their newly available ASP information. Finally, there may be drugs and biologicals that have ASP information available for this proposed rule (reflecting April 2019 ASP data) that do not have ASP information available for the quarter beginning in January 2020. These drugs and biologicals would then be paid based on mean unit cost data derived from CY 2018 hospital claims. Therefore, the proposed payment rates listed in Addenda A and B to this proposed rule are not for January 2020 payment purposes and are only illustrative of the CY 2020 OPPS payment methodology using the most recently available information at the time of issuance of this proposed rule.
c. Biosimilar Biological Products
For CY 2016 and CY 2017, we finalized a policy to pay for biosimilar biological products based on the payment allowance of the product as determined under section 1847A of the Act and to subject nonpass-through biosimilar biological products to our annual threshold-packaged policy (for CY 2016, 80 FR 70445 through 70446; and for CY 2017, 81 FR 79674). In the CY 2018 OPPS/ASC proposed rule (82 FR 33630), for CY 2018, we proposed to continue this same payment policy for biosimilar biological products.
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59351), we noted that, with respect to comments we received regarding OPPS payment for biosimilar biological products, in the CY 2018 PFS final rule, CMS finalized a policy to implement separate HCPCS codes for biosimilar biological products. Therefore, consistent with our established OPPS drug, biological, and radiopharmaceutical payment policy, HCPCS coding for biosimilar biological products is based on policy established under the CY 2018 PFS final rule.
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59351), after consideration of the public comments we received, we finalized our proposed payment policy for biosimilar biological products, with the following technical correction: All biosimilar biological products are eligible for pass-through payment and not just the first biosimilar biological product for a reference product. In the CY 2019 OPPS/ASC proposed rule (83 FR 37123), for CY 2019, we proposed to continue the policy in place from CY 2018 to make all biosimilar biological products eligible for pass-through payment and not just the first biosimilar biological product for a reference product.
In addition, in CY 2018, we adopted a policy that biosimilars without pass-through payment status that were acquired under the 340B Program would be paid the ASP of the biosimilar minus 22.5 percent of the reference product's ASP (82 FR 59367). We adopted this policy in the CY 2018 OPPS/ASC final rule with comment period because we believe that biosimilars without pass-through payment status acquired under the 340B Program should be treated in the same manner as other drugs and biologicals acquired through the 340B Program. As noted earlier, biosimilars with pass-through payment status are paid their own ASP+6 percent of the reference product's ASP. Separately payable biosimilars that do not have pass-through payment status and are not acquired under the 340B Program are also paid their own ASP+6 percent of the reference product's ASP. If a biosimilar does not have ASP pricing, but instead has WAC pricing, the WAC pricing add-on of either 3 percent or 6 percent is calculated from the biosimilar's WAC and is not calculated from the WAC price of the reference product.
As noted in the CY 2019 OPPS/ASC proposed rule (83 FR 37123), several stakeholders raised concerns to us that the current payment policy for biosimilars acquired under the 340B Program could unfairly lower the OPPS payment for biosimilars not on pass-through payment status because the payment reduction would be based on the reference product's ASP, which would generally be expected to be priced higher than the biosimilar, thus resulting in a more significant reduction in payment than if the 22.5 percent was calculated based on the biosimilar's ASP. We agreed with stakeholders that the current payment policy could unfairly lower the price of biosimilars without pass-through payment status that are acquired under the 340B Program. In addition, we believed that these changes would better reflect the resources and production costs that biosimilar manufacturers incur. We also believed this approach is more consistent with the payment methodology for 340B-acquired drugs and biologicals, for which the 22.5 percent reduction is calculated based on the drug or biological's ASP, rather than the ASP of another product. In addition, we believed that paying for biosimilars acquired under the 340B Program at ASP minus 22.5 percent of the biosimilar's ASP, rather than 22.5 percent of the reference product's ASP, will more closely approximate hospitals' acquisition costs for these products.
Accordingly, in the CY 2019 OPPS/ASC proposed rule (83 FR 37123), for CY 2019, we proposed changes to our Medicare Part B drug payment methodology for biosimilars acquired under the 340B Program. Specifically, for CY 2019 and subsequent years, in accordance with section 1833(t)(14)(A)(iii)(II) of the Act, we proposed to pay nonpass-through biosimilars acquired under the 340B Program at ASP minus 22.5 percent of the biosimilar's ASP instead of the biosimilar's ASP minus 22.5 percent of the reference product's ASP. This proposal was finalized without modification in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58977).
For CY 2020, we are proposing to continue our policy to make all biosimilar biological products eligible for pass-through payment and not just the first biosimilar biological product for a reference product. We also are proposing to continue our policy to pay nonpass-through biosimilars acquired under the 340B Program at the biosimilar's ASP minus 22.5 percent of the biosimilar's ASP instead of the biosimilar's ASP minus 22.5 percent of the reference product's ASP, in accordance with section 1833(t)(14)(A)(iii)(II) of the Act. In addition, as discussed further below, we are soliciting comments on the appropriate remedy in the event of an adverse decision on appeal in the litigation related to our policy for payment of 340B-acquired drugs and biologicals, and we are specifically soliciting comments here on whether paying for 340B-acquired biosimilars at ASP+3 percent of the reference product's ASP would be an appropriate policy in line with that discussion.
3. Proposed Payment Policy for Therapeutic Radiopharmaceuticals
For CY 2020, we are proposing to continue the payment policy for therapeutic radiopharmaceuticals that began in CY 2010. We pay for separately payable therapeutic radiopharmaceuticals under the ASP methodology adopted for separately payable drugs and biologicals. If ASP information is unavailable for a therapeutic radiopharmaceutical, we base therapeutic radiopharmaceutical payment on mean unit cost data derived from hospital claims. We believe that the rationale outlined in the CY 2010 OPPS/ASC final rule with comment period (74 FR 60524 through 60525) for applying the principles of separately payable drug pricing to therapeutic radiopharmaceuticals continues to be appropriate for nonpass-through, separately payable therapeutic radiopharmaceuticals in CY 2020. Therefore, we are proposing for CY 2020 to pay all nonpass-through, separately payable therapeutic radiopharmaceuticals at ASP+6 percent, based on the statutory default described in section 1833(t)(14)(A)(iii)(II) of the Act. For a full discussion of ASP-based payment for therapeutic radiopharmaceuticals, we refer readers to the CY 2010 OPPS/ASC final rule with comment period (74 FR 60520 through 60521). We also are proposing to rely on CY 2018 mean unit cost data derived from hospital claims data for payment rates for therapeutic radiopharmaceuticals for which ASP data are unavailable and to update the payment rates for separately payable therapeutic radiopharmaceuticals according to our usual process for updating the payment rates for separately payable drugs and biologicals on a quarterly basis if updated ASP information is unavailable. For a complete history of the OPPS payment policy for therapeutic radiopharmaceuticals, we refer readers to the CY 2005 OPPS final rule with comment period (69 FR 65811), the CY 2006 OPPS final rule with comment period (70 FR 68655), and the CY 2010 OPPS/ASC final rule with comment period (74 FR 60524). The proposed CY 2020 payment rates for nonpass-through, separately payable therapeutic radiopharmaceuticals are included in Addenda A and B to this proposed rule (which are available via the internet on the CMS website).
4. Proposed Payment for Blood Clotting Factors
For CY 2019, we provided payment for blood clotting factors under the same methodology as other nonpass-through separately payable drugs and biologicals under the OPPS and continued paying an updated furnishing fee (83 FR 58979). That is, for CY 2019, we provided payment for blood clotting factors under the OPPS at ASP+6 percent, plus an additional payment for the furnishing fee. We note that when blood clotting factors are provided in physicians' offices under Medicare Part B and in other Medicare settings, a furnishing fee is also applied to the payment. The CY 2019 updated furnishing fee was $0.220 per unit.
For CY 2020, we are proposing to pay for blood clotting factors at ASP+6 percent, consistent with our proposed payment policy for other nonpass-through, separately payable drugs and biologicals, and to continue our policy for payment of the furnishing fee using an updated amount. Our policy to pay for a furnishing fee for blood clotting factors under the OPPS is consistent with the methodology applied in the physician's office and in the inpatient hospital setting. These methodologies were first articulated in the CY 2006 OPPS final rule with comment period (70 FR 68661) and later discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66765). The proposed furnishing fee update is based on the percentage increase in the Consumer Price Index (CPI) for medical care for the 12-month period ending with June of the previous year. Because the Bureau of Labor Statistics releases the applicable CPI data after the PFS and OPPS/ASC proposed rules are published, we are not able to include the actual updated furnishing fee in the proposed rules. Therefore, in accordance with our policy, as finalized in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66765), we are proposing to announce the actual figure for the percent change in the applicable CPI and the updated furnishing fee calculated based on that figure through applicable program instructions and posting on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html.
5. Proposed Payment for Nonpass-Through Drugs, Biologicals, and Radiopharmaceuticals With HCPCS Codes But Without OPPS Hospital Claims Data
For CY 2020, we are proposing to continue to use the same payment policy as in CY 2019 for nonpass-through drugs, biologicals, and radiopharmaceuticals with HCPCS codes but without OPPS hospital claims data, which describes how we determine the payment rate for drugs, biologicals, or radiopharmaceuticals without an ASP. For a detailed discussion of the payment policy and methodology, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70442 through 70443). The proposed CY 2020 payment status of each of the nonpass-through drugs, biologicals, and radiopharmaceuticals with HCPCS codes but without OPPS hospital claims data is listed in Addendum B to this proposed rule, which is available via the internet on the CMS website.
The proposed CY 2020 payment status of each of the nonpass-through drugs, biologicals, and radiopharmaceuticals with HCPCS codes but without OPPS hospital claims data is listed in Addendum B to this proposed rule, which is available via the internet on the CMS website.
6. CY 2020 OPPS Payment Methodology for 340B Purchased Drugs
In the CY 2018 OPPS/ASC proposed rule (82 FR 33558 through 33724), we proposed changes to the Medicare Part B drug payment methodology for 340B hospitals. We proposed these changes to better, and more accurately, reflect the resources and acquisition costs that these hospitals incur. We believed that such changes would allow Medicare beneficiaries (and the Medicare program) to pay a more appropriate amount when hospitals participating in the 340B Program furnish drugs to Medicare beneficiaries that are purchased under the 340B Program. Subsequently, in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59369 through 59370), we finalized our proposal and adjusted the payment rate for separately payable drugs and biologicals (other than drugs on pass-through payment status and vaccines) acquired under the 340B Program from average sales price (ASP)+6 percent to ASP minus 22.5 percent. We stated that our goal was to make Medicare payment for separately payable drugs more aligned with the resources expended by hospitals to acquire such drugs, while recognizing the intent of the 340B Program to allow covered entities, including eligible hospitals, to stretch scarce resources in ways that enable hospitals to continue providing access to care for Medicare beneficiaries and other patients. Critical access hospitals are not included in this 340B policy change because they are paid under section 1834(g) of the Act. We also excepted rural sole community hospitals, children's hospitals, and PPS-exempt cancer hospitals from the 340B payment adjustment in CY 2018. In addition, as stated in the CY 2018 OPPS/ASC final rule with comment period, this policy change does not apply to drugs on pass-through payment status, which are required to be paid based on the ASP methodology, or vaccines, which are excluded from the 340B Program.
In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79699 through 79706), we implemented section 603 of the Bipartisan Budget Act of 2015. As a general matter, applicable items and services furnished in certain off-campus outpatient departments of a provider on or after January 1, 2017 are not considered covered outpatient services for purposes of payment under the OPPS and are paid “under the applicable payment system,” which is generally the Physician Fee Schedule (PFS). However, consistent with our policy to pay separately payable, covered outpatient drugs and biologicals acquired under the 340B Program at ASP minus 22.5 percent, rather than ASP+6 percent, when billed by a hospital paid under the OPPS that is not excepted from the payment adjustment, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59015 through 59022), we finalized a policy to pay ASP minus 22.5 percent for 340B-acquired drugs and biologicals furnished in nonexcepted off-campus PBDs paid under the PFS. We adopted this payment policy effective for CY 2019 and for subsequent years.
As discussed in the CY 2019 OPPS/ASC proposed rule (83 FR 37125), another topic that had been brought to our attention since we finalized the payment adjustment for 340B-acquired drugs in the CY 2018 OPPS/ASC final rule with comment period was whether drugs that do not have ASP pricing but instead receive WAC or AWP pricing are subject to the 340B payment adjustment. We did not receive public comments on this topic in response to the CY 2018 OPPS/ASC proposed rule. However, we since heard from stakeholders that there had been some confusion about this issue. We clarified in the CY 2019 proposed rule that the 340B payment adjustment applies to drugs that are priced using either WAC or AWP, and it has been our policy to subject 340B-acquired drugs that use these pricing methodologies to the 340B payment adjustment since the policy was first adopted. The 340B payment adjustment for WAC-priced drugs is WAC minus 22.5 percent and AWP-priced drugs have a payment rate of 69.46 percent of AWP when the 340B payment adjustment is applied. The 69.46 percent of AWP is calculated by first reducing the original 95 percent of AWP price by 6 percent to generate a value that is similar to ASP or WAC with no percentage markup. Then we apply the 22.5 percent reduction to ASP/WAC-similar AWP value to obtain the 69.46 percent of AWP, which is similar to either ASP minus 22.5 percent or WAC minus 22.5 percent. The number of separately payable drugs receiving WAC or AWP pricing that are affected by the 340B payment adjustment is small—consisting of less than 10 percent of all separately payable Medicare Part B drugs in April 2018.
Furthermore, data limitations previously inhibited our ability to identify which drugs were acquired under the 340B Program in the Medicare OPPS claims data. This lack of information within the claims data has limited researchers' and our ability to precisely analyze differences in acquisition cost of 340B and non-340B acquired drugs with Medicare claims data. Accordingly, in the CY 2018 OPPS/ASC proposed rule (82 FR 33633), we stated our intent to establish a modifier, to be effective January 1, 2018, for hospitals to report with separately payable drugs that were not acquired under the 340B Program. Because a significant portion of hospitals paid under the OPPS participate in the 340B Program, we stated our belief that it is appropriate to presume that a separately payable drug reported on an OPPS claim was purchased under the 340B Program, unless the hospital identifies that the drug was not purchased under the 340B Program. We stated in the CY 2018 proposed rule that we intended to provide further details about this modifier in the CY 2018 OPPS/ASC final rule with comment period and/or through subregulatory guidance, including guidance related to billing for dually eligible beneficiaries (that is, beneficiaries covered under Medicare and Medicaid) for whom covered entities do not receive a discount under the 340B Program. As discussed in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59369 through 59370), to effectuate the payment adjustment for 340B-acquired drugs, CMS implemented modifier “JG”, effective January 1, 2018. Hospitals paid under the OPPS, other than a type of hospital excluded from the OPPS (such as critical access hospitals or those hospitals paid under the Maryland waiver), or excepted from the 340B drug payment policy for CY 2018, are required to report modifier “JG” on the same claim line as the drug HCPCS code to identify a 340B-acquired drug. For CY 2018, rural sole community hospitals, children's hospitals and PPS-exempt cancer hospitals are excepted from the 340B payment adjustment. These hospitals are required to report informational modifier “TB” for 340B-acquired drugs, and continue to be paid ASP+6 percent.
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59353 through 59370) for a full discussion and rationale for the CY 2018 policies and use of modifier “JG”.
In the CY 2019 OPPS/ASC proposed rule (83 FR 37125), for CY 2019, we proposed to continue the 340B Program policies that were implemented in CY 2018 with the exception of the way we calculate payment for 340B-acquired biosimilars (that is, we proposed to pay for nonpass-through 340B-acquired biosimilars at ASP minus 22.5 percent of the biosimilar's ASP, rather than of the reference product's ASP). More information on our revised policy for the payment of biosimilars acquired through the 340B Program is available in section V.B.2.c. of the CY 2019 OPPS/ASC final rule with comment period. For CY 2019, we proposed, in accordance with section 1833(t)(14)(A)(iii)(II) of the Act, to pay for separately payable Medicare Part B drugs (assigned status indicator “K”), other than vaccines and drugs on pass-through payment status, that meet the definition of “covered outpatient drug” as defined in section 1927(k) of the Act, that are acquired through the 340B Program at ASP minus 22.5 percent when billed by a hospital paid under the OPPS that is not excepted from the payment adjustment. Medicare Part B drugs or biologicals excluded from the 340B payment adjustment include vaccines (assigned status indicator “F”, “L” or “M”) and drugs with OPPS transitional pass-through payment status (assigned status indicator “G”). As discussed in section V.B.2.c. of the CY 2019 OPPS/ASC proposed rule, we proposed to pay nonpass-through biosimilars acquired under the 340B Program at the biosimilar's ASP minus 22.5 percent of the biosimilar's ASP. We also proposed for CY 2019 that Medicare would continue to pay for drugs or biologicals that were not purchased with a 340B discount at ASP+6 percent.
As stated earlier, to effectuate the payment adjustment for 340B-acquired drugs, CMS implemented modifier “JG”, effective January 1, 2018. For CY 2019, we proposed that hospitals paid under the OPPS, other than a type of hospital excluded from the OPPS, or excepted from the 340B drug payment policy for CY 2018, continue to be required to report modifier “JG” on the same claim line as the drug HCPCS code to identify a 340B-acquired drug. We also proposed for CY 2019 that rural sole community hospitals, children's hospitals, and PPS-exempt cancer hospitals continue to be excepted from the 340B payment adjustment. We proposed for CY 2019 that these hospitals be required to report informational modifier “TB” for 340B-acquired drugs, and continue to be paid ASP+6 percent. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 58981), after consideration of the public comments we received, we finalized our proposals without modification.
Our CY 2018 and 2019 OPPS payment policies for 340B-acquired drugs are the subject of ongoing litigation. On December 27, 2018, in the case of American Hospital Association et al. v. Azar et al., the United States District Court for the District of Columbia (hereinafter referred to as “the district court”) concluded in the context of reimbursement requests for CY 2018 that the Secretary exceeded his statutory authority by adjusting the Medicare payment rates for drugs acquired under the 340B Program to ASP minus 22.5 percent for that year.[58] In that same decision, the district court recognized the “`havoc that piecemeal review of OPPS payment could bring about' in light of the budget neutrality requirement,” and ordered supplemental briefing on the appropriate remedy.[59] On May 6, 2019, after briefing on remedy, the district court issued an opinion that reiterated that the 2018 rate reduction exceeded the Secretary's authority, and declared that the rate reduction for 2019 (which had been finalized since the Court's initial order was entered) also exceeded his authority.[60] Rather than ordering HHS to pay plaintiffs their alleged underpayments, however, the district court recognized that crafting a remedy is “no easy task, given Medicare's complexity,” [61] and initially remanded the issue to HHS to devise an appropriate remedy while also retaining jurisdiction. The district court acknowledged that “if the Secretary were to retroactively raise the 2018 and 2019 340B rates, budget neutrality would require him to retroactively lower the 2018 and 2019 rates for other Medicare Part B products and services.” [62] Id. at 19. “And because HHS has already processed claims under the previous rates, the Secretary would potentially be required to recoup certain payments made to providers; an expensive and time-consuming prospect.” [63]
CMS respectfully disagreed with the district court's understanding of the scope of its adjustment authority and asked the district court to enter final judgment so as to permit an immediate appeal. On July 10, 2019, the district court granted the government's request and entered final judgment, and the agency does intend to pursue its appeal rights. Nonetheless, CMS is taking the steps necessary to craft an appropriate remedy in the event of an unfavorable decision on appeal.
Devising an appropriate remedy requires an opportunity for public input. First, these types of changes to the OPPS must be budget neutral, and reversal of the policy change, which raised rates for non-drug items and services to the tune of an estimated $1.7 billion for 2018 alone, could have a significant economic impact on the approximately 3,900 facilities that are reimbursed for outpatient items and services covered under the OPPS. Second, any remedy is likely to significantly affect beneficiary cost-sharing. The items and services that could be affected by the remedy were provided to millions of different Medicare beneficiaries, who, by statute, are required to pay cost-sharing for such items and services, which is usually 20 percent of the total Medicare payment rate.
CMS is soliciting initial public comment on how to formulate a solution that accounts for all of the complexities that the district court recognized. We intend to use this public input to further inform the steps that are required under the Administrative Procedure Act to provide adequate notice and an opportunity for meaningful comment on our proposed policies, which would entail devising the specific remedy itself, presenting the specific budget neutrality implications of that remedy in the proposed rule, and potentially calculating all the different payment rates under the OPPS for 340B-acquired drugs, as well as all other items and services under the OPPS. (In essence, we would need to provide hospitals with sufficient notice of the impact of the remedy on their rates to enable them to comment meaningfully on the proposed rule.) Our own best practices for preparing notices of proposed rulemaking dictate that we begin policy development in the year before the proposed rule is issued, and that we begin the rule drafting process in the first quarter of each year.
In order to comply with the requirements of the Administrative Procedure Act and our regulatory development process and calendar, we would anticipate proposing the specific remedy for CYs 2018 and 2019, as well as changes to the CY 2020 rates, in the next available rulemaking vehicle, which is the CY 2021 OPPS/ASC proposed rule. Those proposals will be informed by the comments solicited in this proposed rule. Specifically, we are using this proposed rule to solicit comment in advance of next year's rulemaking on approaches to the CY 2018 and 2019 remedy, as well as how best to address CY 2020 rates, so we are poised to propose those policies in the CY 2021 rule if necessary.
Thus, for CY 2020 we are proposing to continue to pay ASP-22.5 percent for 340B-acquired drugs including when furnished in nonexcepted off-campus PBDs paid under the PFS. Our proposal would continue the 340B Program policies that were implemented in CY 2018 with the exception of the way we are calculating payment for 340B-acquired biosimilars, which is discussed in section V.B.2.c. of the CY 2019 OPPS/ASC final rule with comment period, and would continue the policy we finalized in CY 2019 to pay ASP minus 22.5 percent for 340B-acquired drugs and biologicals furnished in nonexcepted off-campus PBDs paid under the PFS.
We also seek public comment on the appropriate OPPS payment rate for 340B-acquired drugs, including whether a rate of ASP+3 percent could be an appropriate remedial payment amount for these drugs, both for CY 2020 and for purposes of determining the remedy for CYs 2018 and 2019. To be sure, this amount would result in payment rates that are well above the actual costs hospitals incur in purchasing 340B drugs, and it is being proposed solely because of the court decision. However, to the extent the courts are limiting the size of the payment reduction the agency can permissibly apply, the agency believes it could be appropriate to apply a payment reduction that is at the upper end of that limit, to the extent it has been or could be clearly defined, given the substantial discounts that hospitals receive through the 340B program. For example, absent further guidance from the Court of Appeals on what it believes is an appropriate “adjustment” amount, CMS could look to the district court's December 27, 2018 opinion, which cites to payment reductions of 0.2 percent and 2.9 percent as “not significant enough” to fall outside of the Secretary's authority to “adjust” ASP.[64] This payment rate would apply to 340B-acquired drugs and biologicals billed by a hospital paid under the OPPS that are not excepted from the payment adjustment and to 340B-acquired drugs and biologicals furnished in nonexcepted off-campus PBDs paid under the PFS. We welcome public comments on payment rates other than ASP+3 percent that commenters believe would be appropriate for purposes of addressing CY 2020 payment as an alternative to our proposal above, as well as for potential future rulemaking related to CY 2018 and 2019 underpayments.
In addition to comments on the appropriate payment amount for calculating the remedy for CYs 2018 and 2019 and for use for CY 2020, we also seek public comment on how to structure the remedy for CYs 2018 and 2019. This request for public comment includes comments on whether such a remedy should be retrospective in nature (for example, made on a claim-by-claim basis), whether such a remedy could be prospective in nature (for example, an upward adjustment to 340B claims in the future to account for any underpayments in the past), and whether there is some other mechanism that could produce a result equitable to hospitals that do not acquire drugs through the 340B program while respecting the budget neutrality mandate.
One potential remedy for alleged underpayments in 2018 and 2019 would involve making additional payments to the parties who have demonstrated harm from the alleged underpayments (which could be defined as hospitals that submitted a claim for drug payment with the “JG” modifier in CYs 2018 and 2019) outside the normal claims process. Under this approach, we would calculate the amount that such hospitals should have been paid and would utilize our Medicare contractors to make one payment to each affected hospital. This approach—one additional payment made to each affected hospital by our contractors—is a different approach than reprocessing each and every claim submitted by plaintiff hospitals for 2018 and 2019. Then, depending on when a final decision is rendered, the Secretary would propose to budget-neutralize those additional expenditures for each of CYs 2018 and 2019. For example, if the Court of Appeals were to render a decision in February of 2020, under such an approach we might propose those additional payments and an appropriate budget neutrality adjustment for each of CYs 2018, 2019, and, if necessary, 2020, in time for the CY 2021 rule. We note that we would need to receive a final decision from the Court of Appeals sufficiently early in CY 2020 (likely by March 1, 2020) to make it potentially possible for us to propose and finalize an appropriate remedy and budget neutrality adjustments in the CY 2021 rulemaking. We solicit public comment on this approach as well as other suggested approaches from commenters.
In considering these potential future proposals, we note that we would rely on our statutory authority under section 1833(t)(14) for determining the OPPS payment rates for drugs and biologicals as well as section 1833(t)(9)(A) of the Act to review certain components of the OPPS not less often than annually and to revise the groups, relative payment weights, and other adjustments. In addition, we note that under section 1833(t)(14)(H) of the Act, any adjustments made by the Secretary to payment rates using the statutory formula outlined in section 1833(t)(14)(A)(iii)(II) of the Act are required to be taken into account under the budget neutrality requirements outlined in section 1833(t)(9)(B) of the Act. We are soliciting public comments on the best, most appropriate way to maintain budget neutrality, either under a retrospective claim-by-claim approach, with a prospective approach, or any other proposed remedy. We also solicit comments on whether, depending on the amount of those additional expenditures, we should consider spreading out the relevant budget neutrality adjustment across multiple years. We would be interested to receive public comment on the advantages and disadvantages of such an approach.
In addition, we are interested in public comments on the best, most appropriate treatment of Medicare beneficiary cost-sharing responsibilities under any proposed remedy. These issues—the statutory budget neutrality requirement and beneficiary cost-sharing—are extremely difficult to balance, and we are interested in stakeholder comments as we continue to review the viability of alternative remedies in the event of an adverse decision from the Court of Appeals.
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59369 through 59370) and the CY 2019 OPPS/ASC final rule with comment period (83 FR 58976 through 58977 and 59015 through 59022) for more detail on the policies implemented in CY 2018 and CY 2019 for drugs acquired through the 340B Program.
7. Proposed High Cost/Low Cost Threshold for Packaged Skin Substitutes
In the CY 2014 OPPS/ASC final rule with comment period (78 FR 74938), we unconditionally packaged skin substitute products into their associated surgical procedures as part of a broader policy to package all drugs and biologicals that function as supplies when used in a surgical procedure. As part of the policy to finalize the packaging of skin substitutes, we also finalized a methodology that divides the skin substitutes into a high cost group and a low cost group, in order to ensure adequate resource homogeneity among APC assignments for the skin substitute application procedures (78 FR 74933).
Skin substitutes assigned to the high cost group are described by HCPCS codes 15271 through 15278. Skin substitutes assigned to the low cost group are described by HCPCS codes C5271 through C5278. Geometric mean costs for the various procedures are calculated using only claims for the skin substitutes that are assigned to each group. Specifically, claims billed with HCPCS code 15271, 15273, 15275, or 15277 are used to calculate the geometric mean costs for procedures assigned to the high cost group, and claims billed with HCPCS code C5271, C5273, C5275, or C5277 are used to calculate the geometric mean costs for procedures assigned to the low cost group (78 FR 74935).
Each of the HCPCS codes described above are assigned to one of the following three skin procedure APCs according to the geometric mean cost for the code: APC 5053 (Level 3 Skin Procedures): HCPCS codes C5271, C5275, and C5277); APC 5054 (Level 4 Skin Procedures): HCPCS codes C5273, 15271, 15275, and 15277); or APC 5055 (Level 5 Skin Procedures): HCPCS code 15273). In CY 2019, the payment rate for APC 5053 (Level 3 Skin Procedures) was $482.89, the payment rate for APC 5054 (Level 4 Skin Procedures) was $1,548.96, and the payment rate for APC 5055 (Level 5 Skin Procedures) was $2,766.13. This information also is available in Addenda A and B of the CY 2019 OPPS/ASC final rule with comment period (which is available via the internet on the CMS website).
We have continued the high cost/low cost categories policy since CY 2014, and we are proposing to continue it for CY 2020. Under this current policy, skin substitutes in the high cost category are reported with the skin substitute application CPT codes, and skin substitutes in the low cost category are reported with the analogous skin substitute HCPCS C-codes. For a discussion of the CY 2014 and CY 2015 methodologies for assigning skin substitutes to either the high cost group or the low cost group, we refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 74932 through 74935) and the CY 2015 OPPS/ASC final rule with comment period (79 FR 66882 through 66885).
For a discussion of the high cost/low cost methodology that was adopted in CY 2016 and has been in effect since then, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70434 through 70435). For CY 2020, consistent with our policy since CY 2016, we are proposing to continue to determine the high cost/low cost status for each skin substitute product based on either a product's geometric mean unit cost (MUC) exceeding the geometric MUC threshold or the product's per day cost (PDC) (the total units of a skin substitute multiplied by the mean unit cost and divided by the total number of days) exceeding the PDC threshold. For CY 2020, as we did for CY 2019, we are proposing to assign each skin substitute that exceeds either the MUC threshold or the PDC threshold to the high cost group. In addition, as described in more detail later in this section, for CY 2020, as we did for CY 2019, we are proposing to assign any skin substitute with a MUC or a PDC that does not exceed either the MUC threshold or the PDC threshold to the low cost group. For CY 2020, we are proposing that any skin substitute product that was assigned to the high cost group in CY 2019 would be assigned to the high cost group for CY 2020, regardless of whether it exceeds or falls below the CY 2020 MUC or PDC threshold. This policy was established in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59346 through 59348).
For this CY 2020 OPPS/ASC proposed rule, consistent with the methodology as established in the CY 2014 through CY 2017 final rules with comment period, we analyzed CY 2018 claims data to calculate the MUC threshold (a weighted average of all skin substitutes' MUCs) and the PDC threshold (a weighted average of all skin substitutes' PDCs). The proposed CY 2020 MUC threshold is $49 per cm2 (rounded to the nearest $1) and the proposed CY 2020 PDC threshold is $789 (rounded to the nearest $1).
For CY 2020, we are proposing to continue to assign skin substitutes with pass-through payment status to the high cost category. We are proposing to assign skin substitutes with pricing information but without claims data to calculate a geometric MUC or PDC to either the high cost or low cost category based on the product's ASP+6 percent payment rate as compared to the MUC threshold. If ASP is not available, we are proposing to use WAC+3 percent to assign a product to either the high cost or low cost category. Finally, if neither ASP nor WAC is available, we would use 95 percent of AWP to assign a skin substitute to either the high cost or low cost category. We are proposing to continue to use WAC+3 percent instead of WAC+6 percent to conform to our proposed policy described in section V.B.2.b. of this proposed rule to establish a payment rate of WAC+3 percent for separately payable drugs and biologicals that do not have ASP data available. New skin substitutes without pricing information would be assigned to the low cost category until pricing information is available to compare to the CY 2020 MUC threshold. For a discussion of our existing policy under which we assign skin substitutes without pricing information to the low cost category until pricing information is available, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70436).
Some skin substitute manufacturers have raised concerns about significant fluctuation in both the MUC threshold and the PDC threshold from year to year. The fluctuation in the thresholds may result in the reassignment of several skin substitutes from the high cost group to the low cost group which, under current payment rates, can be a difference of approximately $1,000 in the payment amount for the same procedure. In addition, these stakeholders were concerned that the inclusion of cost data from skin substitutes with pass-through payment status in the MUC and PDC calculations would artificially inflate the thresholds. Skin substitute stakeholders requested that CMS consider alternatives to the current methodology used to calculate the MUC and PDC thresholds and also requested that CMS consider whether it might be appropriate to establish a new cost group in between the low cost group and the high cost group to allow for assignment of moderately priced skin substitutes to a newly created middle group.
We share the goal of promoting payment stability for skin substitute products and their related procedures as price stability allows hospitals using such products to more easily anticipate future payments associated with these products. We have attempted to limit year-to-year shifts for skin substitute products between the high cost and low cost groups through multiple initiatives implemented since CY 2014, including: Establishing separate skin substitute application procedure codes for low-cost skin substitutes (78 FR 74935); using a skin substitute's MUC calculated from outpatient hospital claims data instead of an average of ASP+6 percent as the primary methodology to assign products to the high cost or low cost group (79 FR 66883); and establishing the PDC threshold as an alternate methodology to assign a skin substitute to the high cost group (80 FR 70434 through 70435).
To allow additional time to evaluate concerns and suggestions from stakeholders about the volatility of the MUC and PDC thresholds, in the CY 2018 OPPS/ASC proposed rule (82 FR 33627), we proposed that a skin substitute that was assigned to the high cost group for CY 2017 would be assigned to the high cost group for CY 2018, even if it does not exceed the CY 2018 MUC or PDC thresholds. We finalized this policy in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59347). We stated in the CY 2018 OPPS/ASC proposed rule that the goal of our proposal to retain the same skin substitute cost group assignments in CY 2018 as in CY 2017 was to maintain similar levels of payment for skin substitute products for CY 2018 while we study our skin substitute payment methodology to determine whether refinement to the existing policies is consistent with our policy goal of providing payment stability for skin substitutes.
We stated in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59347) that we would continue to study issues related to the payment of skin substitutes and take these comments into consideration for future rulemaking. We received many responses to our requests for comments in the CY 2018 OPPS/ASC proposed rule about possible refinements to the existing payment methodology for skin substitutes that would be consistent with our policy goal of providing payment stability for these products. In addition, several stakeholders have made us aware of additional concerns and recommendations since the release of the CY 2018 OPPS/ASC final rule with comment period. As discussed in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58967 through 58968), we identified four potential methodologies that have been raised to us that we encouraged the public to review and provide comments on. We stated in the CY 2019 OPPS/ASC final rule with comment period that we were especially interested in any specific feedback on policy concerns with any of the options presented as they relate to skin substitutes with differing per day or per episode costs and sizes and other factors that may differ among the dozens of skin substitutes currently on the market. We also specified in the CY 2019 OPPS/ASC final rule with comment period that we were interested in any new ideas that are not represented below along with an analysis of how different skin substitute products would fare under such ideas. Finally, we stated that we intend to explore the full array of public comments on these ideas for the CY 2020 rulemaking, and we indicated that we will consider the feedback received in response to our requests for comments in developing proposals for CY 2020.
a. Discussion of CY 2019 Comment Solicitation for Episode-Based Payment and Solicitation of Additional Comments for CY 2020
The methodology that commenters discussed most in response to our comment solicitation in CY 2019 and that stakeholders raised in subsequent meetings we have had with the wound care community has been a lump-sum “episode-based” payment for a wound care episode. Commenters that supported an episode-based payment believe that it would allow health care professionals to choose the best skin substitute to treat a patient's wound and would give providers flexibility with the treatments they administer. These commenters also believe an episode-based payment helps to reduce incentives for providers to use excessive applications of skin substitute products or use higher cost products to generate more payment for the services they furnish. In addition, they believe that episode-based payment could help with innovations with skin substitutes by encouraging the development of products that require fewer applications. These commenters noted that episode-based payment would make wound care payment more predictable for hospitals and provide incentives to manage the cost of care that they furnish. Finally, commenters for an episode-based payment believe that workable quality metrics can be developed to monitor the quality of care administered under the payment methodology and limit excessive applications of skin substitutes.
However, many commenters opposed establishing an episode-based payment. One of the main concerns of commenters who opposed episode-based payment was that wound care is too complex and variable to be covered through such a payment methodology. These commenters stated that every patient and every wound is different; therefore, it would be very challenging to establish a standard episode length for coverage. They noted that it would be too difficult to risk-stratify and specialty-adjust an episode-based payment, given the diversity of patients receiving wound care and their providers who administer treatment, as well as the variety of pathologies covered in treatment. Also, these commenters questioned how episodes would be defined for patients when they are having multiple wounds treated at one time or had another wound develop while the original wound was receiving treatment. These commenters expressed concerns that episode-based payment would be burdensome both operationally and administratively for providers. They believe that CMS will need to create a large number of new APCs and HCPCS codes to account for all of the patient situations that would be covered with an episode-based payment, which would increase burdens on providers. Finally, these commenters had concerns about the impacts of episode-based payment on the usage of higher cost skin substitute products. They believed that a single payment could discourage the use of higher-cost products because of the large variability in the cost of skin substitute products, which could limit innovations for skin substitute products.
The wide array of views on episode-based payment for skin substitute products and the unforeseen issues that may arise from the implementation of such a policy make us reluctant to present a proposal for this CY 2020 proposed rule without more review of the issues involved with episode-based payment. Therefore, we are seeking further comments from stakeholders and other interested parties regarding skin substitute payment policies that could be applied in future years to address concerns about excessive utilization and spending on skin substitute products, while avoiding administrative issues such as establishing additional HCPCS codes to describe different treatment situations. One possible policy construct that we are seeking comments on would be to establish a payment period for skin substitute application services (CPT codes 15271 through 15278 and HCPCS codes C5271 through C5278) between 4 weeks and 12 weeks. Under this option, we could also assign CPT codes 15271, 15273, 15275, and 15277, and HCPCS codes C5271, C5273, C5275, and C5277 to comprehensive APCs with the option for a complexity adjustment that would allow for an increase in the standard APC payment for more resource-intensive cases. Our research has found that most wound care episodes require one to three skin substitute applications. Those cases would likely receive the standard APC payment for the comprehensive procedure. Then the complexity adjustment could be applied for the relatively small number of cases that require more intensive treatments. We look forward to comments from stakeholders and other interested parties on this possible policy construct.
b. Potential Revisions to the OPPS Payment Policy for Skin Substitutes: Comment Solicitation for CY 2020
In addition to possible future rulemaking based on the responses to the comment solicitations in the preceding section, we are considering adopting for CY 2020 another payment methodology that generated significant public comments in response to the CY 2019 comment solicitation. That option would be to eliminate the high cost and low cost categories for skin substitutes and have only one payment category and set of procedure codes for the application of all graft skin substitute products. The only available procedure codes to bill for skin substitute graft procedures would be CPT codes 15271 through 15278. HCPCS codes C5271 through C5278 would be eliminated. Providers would bill CPT codes 15271 through 15278 without having to consider either the MUC or PDC of the graft skin substitute product used in the procedure. There would be only one APC for the graft skin substitute application procedures described by CPT codes 15271 (Skin sub graft trnk/arm/leg), 15273 (Skin sub grft t/arm/lg child), 15275 (Skin sub graft face/nk/hf/g), and 15277 (Skn sub grft f/n/hf/g child). The payment rate would be the geometric mean of all graft skin substitutes procedures for a given CPT code that are covered through the OPPS. For example under the current skin substitute payment policy, there are two procedure codes (CPT code 15271 and HCPCS code C5271) that are reported for the procedure described as “application of skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm; first 25 sq cm or less wound surface area”. The geometric mean cost for CPT code 15271 is currently $1,572.17 and the geometric mean cost for HCPCS code C5271 is $728.28. If this policy option was implemented, only CPT code 15271 would be available in the OPPS, and the geometric mean cost for the procedure code would be $1,465.18.
Commenters that supported this option believe this would remove the incentives for manufacturers to develop and providers to use high cost skin substitute products and lead to the use of lower-cost, quality products. Commenters noted that lower Medicare payments for graft skin substitute procedures would lead to lower copayments for beneficiaries. In addition, commenters believed a single payment category would reduce incentives to apply skin substitute products in excessive amounts. Commenters also believed a single payment category is clinically justified because many studies have shown that no one skin substitute product is superior to another. Finally, supporters of a single payment category believed it will simplify coding for providers and reduce administrative burden.
There were also commenters that raised concerns that a single payment category would not offer providers incentives to furnish quality care and would reduce the use of higher-cost skin substitute products. Eliminating the high cost and low cost payment categories also does not maintain homogeneity among APC assignments for services using skin substitutes according to opponents of the single payment category. Commenters stated that instead of having categories grouped by the relative cost of products, there would be only one category to cover the payment of products with a mean unit cost ranging from less than $1 to over $750. Commenters believed a single payment category would favor inexpensive products, which could limit innovation, and could eliminate all but the most inexpensive products from the market. Finally, opponents of a single payment category believed a single payment category would discourage the treatment of wounds that are difficult and costly to treat.
The responses to the comment solicitation show the potential of a single payment category to reduce the cost of wound care services for graft skin substitute procedures for both beneficiaries and Medicare in general. In addition, a single payment category may help to lower administrative burden for providers. Conversely, we are cognizant of other commenters' concerns that a single payment category may hinder innovation of new graft skin substitute products and cause some products that are currently well-utilized to leave the market. Nonetheless, we are persuaded that a single payment category could potentially provide a more equitable payment for many products used with graft skin substitute procedures, while recognizing that procedures performed with expensive skin substitute products would likely receive substantially lower payment.
We believe a more equitable payment rate for graft skin substitute procedures could substantially reduce the amount Medicare pays for these procedures. We welcome suggestions or other information regarding the possibility of utilizing a single payment category to pay for skin substitute products under the OPPS, and, depending on the information we receive in response to this request, we may consider modifying our skin substitute payment policy in the CY 2020 OPPS/ASC final rule with comment period.
We believe some of the concerns commenters who oppose a single payment category for skin substitute products raised might be mitigated if stakeholders have a period of time to adjust to the changes inherent in establishing a single payment category. We are soliciting public comments that provide additional information about how commenters believe we should transition from the current low cost/high cost payment methodology to a single payment category.
Such suggestions to facilitate the payment transition from a low cost/high cost payment methodology to a single payment category methodology could include, but are not limited to—
- Delaying implementation of a single category payment for 1 or 2 years after the payment methodology is adopted; and
- Gradually lowering the MUC and PDC thresholds over 2 or more years to add more graft skin substitute procedures into the current high cost group until all graft skin substitute procedures are assigned to the high cost group and it becomes a single payment category.
We are seeking commenters' feedback on these ideas, or other approaches, to mitigate challenges that could impact providers, manufacturers, and other stakeholders if we establish a single payment category, which we might include as part of a final skin substitute payment policy that we would adopt in the CY 2020 OPPS/ASC final rule with comment period.
c. Proposals for Packaged Skin Substitutes
To allow stakeholders time to analyze and comment on the issues discussed above, we are proposing for CY 2020 to continue our policy established in CY 2018 to assign skin substitutes to the low cost or high cost group. Specifically, we are proposing to assign a skin substitute with a MUC or a PDC that does not exceed either the MUC threshold or the PDC threshold to the low cost group, unless the product was assigned to the high cost group in CY 2019, in which case we would assign the product to the high cost group for CY 2020, regardless of whether it exceeds the CY 2020 MUC or PDC threshold. We also are proposing to assign to the high cost group any skin substitute product that exceeds the CY 2020 MUC or PDC thresholds and assign to the low cost group any skin substitute product that does not exceed the CY 2020 MUC or PDC thresholds and was not assigned to the high cost group in CY 2019. We are proposing to continue to use payment methodologies including ASP+6 percent and 95 percent of AWP for skin substitute products that have pricing information but do not have claims data to determine if their costs exceed the CY 2020 MUC. In addition, we are proposing to use WAC+3 percent for skin substitute products that do not have ASP pricing information or have claims data to determine if those products' costs exceed the CY 2020 MUC. We are proposing to continue our established policy to assign new skin substitute products without pricing information to the low cost group. We look forward to public comments on our proposals.
Table 19 displays the proposed CY 2020 cost category assignment for each skin substitute product.

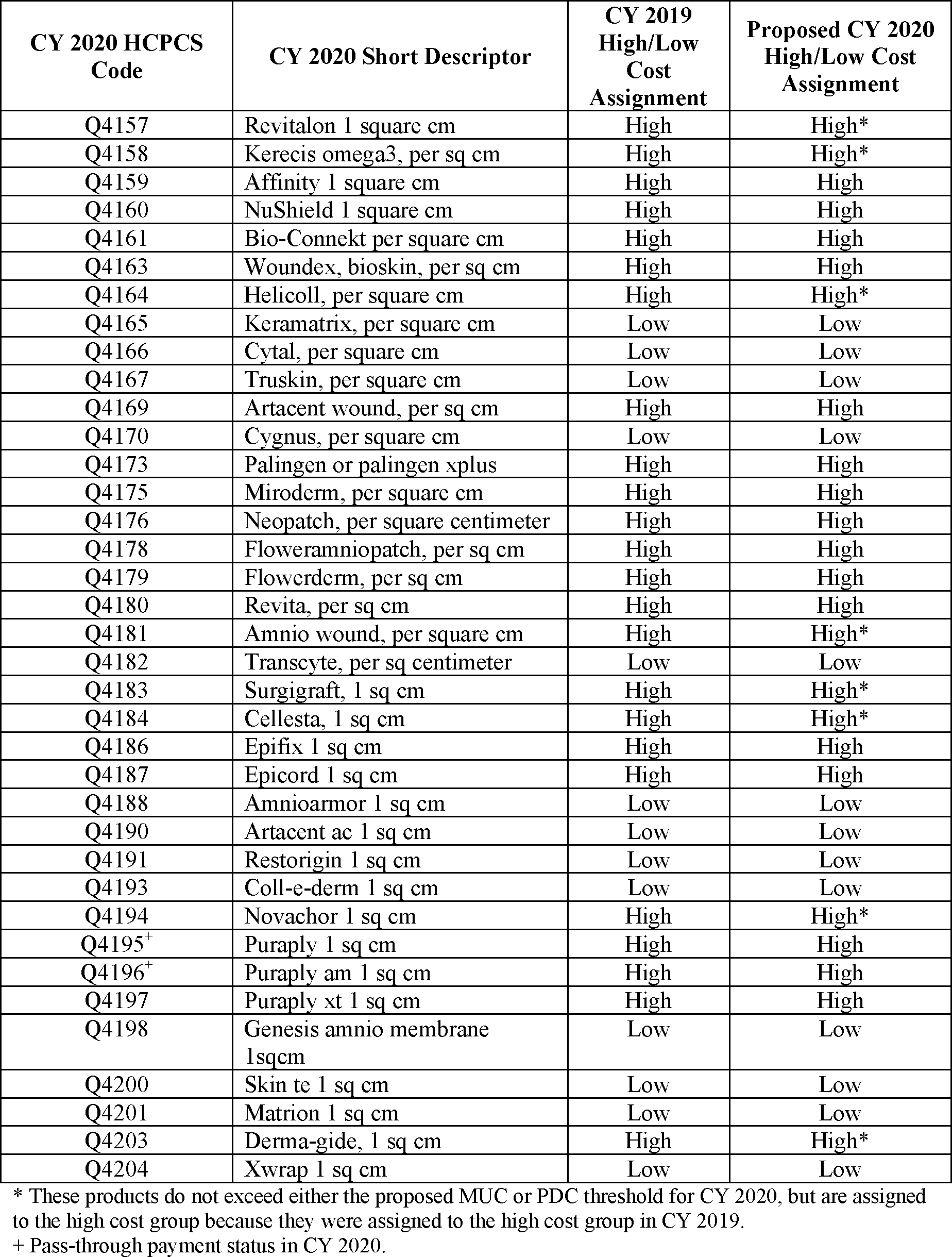
VI. Proposed Estimate of OPPS Transitional Pass-Through Spending for Drugs, Biologicals, Radiopharmaceuticals, and Devices
A. Background
Section 1833(t)(6)(E) of the Act limits the total projected amount of transitional pass-through payments for drugs, biologicals, radiopharmaceuticals, and categories of devices for a given year to an “applicable percentage,” currently not to exceed 2.0 percent of total program payments estimated to be made for all covered services under the OPPS furnished for that year. If we estimate before the beginning of the calendar year that the total amount of pass-through payments in that year would exceed the applicable percentage, section 1833(t)(6)(E)(iii) of the Act requires a uniform prospective reduction in the amount of each of the transitional pass-through payments made in that year to ensure that the limit is not exceeded. We estimate the pass-through spending to determine whether payments exceed the applicable percentage and the appropriate prorata reduction to the conversion factor for the projected level of pass-through spending in the following year to ensure that total estimated pass-through spending for the prospective payment year is budget neutral, as required by section 1833(t)(6)(E) of the Act.
For devices, developing a proposed estimate of pass-through spending in CY 2020 entails estimating spending for two groups of items. The first group of items consists of device categories that are currently eligible for pass-through payment and that will continue to be eligible for pass-through payment in CY 2020. The CY 2008 OPPS/ASC final rule with comment period (72 FR 66778) describes the methodology we have used in previous years to develop the pass-through spending estimate for known device categories continuing into the applicable update year. The second group of items consists of items that we know are newly eligible, or project may be newly eligible, for device pass-through payment in the remaining quarters of CY 2019 or beginning in CY 2020. The sum of the proposed CY 2020 pass-through spending estimates for these two groups of device categories equals the proposed total CY 2020 pass-through spending estimate for device categories with pass-through payment status. We base the device pass-through estimated payments for each device category on the amount of payment as established in section 1833(t)(6)(D)(ii) of the Act, and as outlined in previous rules, including the CY 2014 OPPS/ASC final rule with comment period (78 FR 75034 through 75036). We note that, beginning in CY 2010, the pass-through evaluation process and pass-through payment methodology for implantable biologicals newly approved for pass-through payment beginning on or after January 1, 2010, that are surgically inserted or implanted (through a surgical incision or a natural orifice) use the device pass-through process and payment methodology (74 FR 60476). As has been our past practice (76 FR 74335), in this proposed rule, we are proposing to include an estimate of any implantable biologicals eligible for pass-through payment in our estimate of pass-through spending for devices. Similarly, we finalized a policy in CY 2015 that applications for pass-through payment for skin substitutes and similar products be evaluated using the medical device pass-through process and payment methodology (76 FR 66885 through 66888). Therefore, as we did beginning in CY 2015, for CY 2020, we are also proposing to include an estimate of any skin substitutes and similar products in our estimate of pass-through spending for devices.
For drugs and biologicals eligible for pass-through payment, section 1833(t)(6)(D)(i) of the Act establishes the pass-through payment amount as the amount by which the amount authorized under section 1842(o) of the Act (or, if the drug or biological is covered under a competitive acquisition contract under section 1847B of the Act, an amount determined by the Secretary equal to the average price for the drug or biological for all competitive acquisition areas and year established under such section as calculated and adjusted by the Secretary) exceeds the portion of the otherwise applicable fee schedule amount that the Secretary determines is associated with the drug or biological. Our estimate of drug and biological pass-through payment for CY 2020 for this group of items is $224.1 million, as discussed below, because we are proposing to pay for most nonpass-through separately payable drugs and biologicals under the CY 2020 OPPS at ASP+6 percent with the exception of 340B-acquired separately payable drugs that are paid at ASP minus 22.5 percent, and because we are proposing to pay for CY 2020 pass-through payment drugs and biologicals at ASP+6 percent, as we discuss in section V.A. of this proposed rule. We refer readers to section V.B.6 of this proposed rule where we solicit comments on an appropriate remedy in litigation involving our OPPS payment policy for 340B purchased drugs, which would inform CY 2021 rulemaking in the event of an adverse decision on appeal in that litigation.
Furthermore, payment for certain drugs, specifically diagnostic radiopharmaceuticals and contrast agents without pass-through payment status, is packaged into payment for the associated procedures, and these products will not be separately paid. In addition, we policy-package all nonpass-through drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure and drugs and biologicals that function as supplies when used in a surgical procedure, as discussed in section II.A.3. of this proposed rule. In this proposed rule, we are proposing that all of these policy-packaged drugs and biologicals with pass-through payment status would be paid at ASP+6 percent, like other pass-through drugs and biologicals, for CY 2020. Therefore, our proposed estimate of pass-through payment for policy-packaged drugs and biologicals with pass-through payment status approved prior to CY 2020 is not $0, as discussed below. In section V.A.5. of this proposed rule, we discussed our policy to determine if the costs of certain policy-packaged drugs or biologicals are already packaged into the existing APC structure. If we determine that a policy-packaged drug or biological approved for pass-through payment resembles predecessor drugs or biologicals already included in the costs of the APCs that are associated with the drug receiving pass-through payment, we are proposing to offset the amount of pass-through payment for the policy-packaged drug or biological. For these drugs or biologicals, the APC offset amount is the portion of the APC payment for the specific procedure performed with the pass-through drug or biological, which we refer to as the policy-packaged drug APC offset amount. If we determine that an offset is appropriate for a specific policy-packaged drug or biological receiving pass-through payment, we are proposing to reduce our estimate of pass-through payments for these drugs or biologicals by this amount.
Similar to pass-through spending estimates for devices, the first group of drugs and biologicals requiring a pass-through payment estimate consists of those products that were recently made eligible for pass-through payment and that will continue to be eligible for pass-through payment in CY 2020. The second group contains drugs and biologicals that we know are newly eligible, or project will be newly eligible, in the remaining quarters of CY 2019 or beginning in CY 2020. The sum of the CY 2020 pass-through spending estimates for these two groups of drugs and biologicals equals the total CY 2019 pass-through spending estimate for drugs and biologicals with pass-through payment status.
B. Proposed Estimate of Pass-Through Spending
We are proposing to set the applicable pass-through payment percentage limit at 2.0 percent of the total projected OPPS payments for CY 2020, consistent with section 1833(t)(6)(E)(ii)(II) of the Act and our OPPS policy from CY 2004 through CY 2019 (82 FR 59371 through 59373).
For the first group, consisting of device categories that are currently eligible for pass-through payment and will continue to be eligible for pass-through payment in CY 2020, there is one active category for CY 2020. The active category is described by HCPCS code C1823 (Generator, neurostimulator (implantable), nonrechargeable, with transvenous sensing and stimulation leads). Based on the information from the device manufacturer, we are estimating that 100 devices will receive payment in the OPPS in CY 2019 at an estimated cost of $5,655 per device. Therefore, we are proposing an estimate for the first group of devices of $565,500. In estimating our proposed CY 2020 pass-through spending for device categories in the second group, we included: Device categories that we knew at the time of the development of the proposed rule will be newly eligible for pass-through payment in CY 2020; additional device categories that we estimated could be approved for pass-through status subsequent to the development of the proposed rule and before January 1, 2020; and contingent projections for new device categories established in the second through fourth quarters of CY 2020. For CY 2020, we are proposing to use the general methodology described in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66778), while also taking into account recent OPPS experience in approving new pass-through device categories. For this proposed rule, the proposed estimate of CY 2020 pass-through spending for this second group of device categories is $10 million.
To estimate proposed CY 2020 pass-through spending for drugs and biologicals in the first group, specifically those drugs and biologicals recently made eligible for pass-through payment and continuing on pass-through payment status for at least one quarter in CY 2020, we are proposing to use the most recent Medicare hospital outpatient claims data regarding their utilization, information provided in the respective pass-through applications, historical hospital claims data, pharmaceutical industry information, and clinical information regarding those drugs or biologicals to project the CY 2020 OPPS utilization of the products.
For the known drugs and biologicals (excluding policy-packaged diagnostic radiopharmaceuticals, contrast agents, drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure, and drugs and biologicals that function as supplies when used in a surgical procedure) that will be continuing on pass-through payment status in CY 2020, we estimated the pass-through payment amount as the difference between ASP+6 percent and the payment rate for nonpass-through drugs and biologicals that will be separately paid. Separately payable drugs are paid at a rate of ASP+6 percent with the exception of 340B-acquired drugs that are paid at ASP minus 22.5 percent. Therefore, the payment rate difference between the pass-through payment amount and the nonpass-through payment amount is $224.1 million for this group of drugs. Because payment for policy-packaged drugs and biologicals is packaged if the product was not paid separately due to its pass-through payment status, we are proposing to include in the CY 2020 pass-through estimate the difference between payment for the policy-packaged drug or biological at ASP+6 percent (or WAC+6 percent, or 95 percent of AWP, if ASP or WAC information is not available) and the policy-packaged drug APC offset amount, if we determine that the policy-packaged drug or biological approved for pass-through payment resembles a predecessor drug or biological already included in the costs of the APCs that are associated with the drug receiving pass-through payment, which we estimate for CY 2020 to be $17.0 million. For this proposed rule, using the proposed methodology described above, we calculated a CY 2020 proposed spending estimate for this first group of drugs and biologicals that includes drugs currently on pass-through payment status that would otherwise be separately payable or policy-packaged of approximately $241.1 million.
To estimate proposed CY 2020 pass-through spending for drugs and biologicals in the second group (that is, drugs and biologicals that we knew at the time of development of the proposed rule were newly eligible for pass-through payment in CY 2020, additional drugs and biologicals that we estimated could be approved for pass-through status subsequent to the development of this proposed rule and before January 1, 2020 and projections for new drugs and biologicals that could be initially eligible for pass-through payment in the second through fourth quarters of CY 2020), we are proposing to use utilization estimates from pass-through applicants, pharmaceutical industry data, clinical information, recent trends in the per unit ASPs of hospital outpatient drugs, and projected annual changes in service volume and intensity as our basis for making the CY 2020 pass-through payment estimate. We also are proposing to consider the most recent OPPS experience in approving new pass-through drugs and biologicals. Using our proposed methodology for estimating CY 2020 pass-through payments for this second group of drugs, we calculated a proposed spending estimate for this second group of drugs and biologicals of approximately $17.1 million.
In summary, in accordance with the methodology described earlier in this section, for this proposed rule, we estimate that total pass-through spending for the device categories and the drugs and biologicals that are continuing to receive pass-through payment in CY 2020 and those device categories, drugs, and biologicals that first become eligible for pass-through payment during CY 2020 is approximately $268.8 million (approximately $10.6 million for device categories and approximately $258.2 million for drugs and biologicals) which represents 0.34 percent of total projected OPPS payments for CY 2020 (approximately $80 billion). Therefore, we estimate that pass-through spending in CY 2020 would not amount to 2.0 percent of total projected OPPS CY 2020 program spending.
VII. Proposed OPPS Payment for Hospital Outpatient Visits and Critical Care Services
For CY 2020, we are proposing to continue with our current clinic and emergency department (ED) hospital outpatient visits payment policies. For a description of the current clinic and ED hospital outpatient visits policies, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70448). We also are proposing to continue our payment policy for critical care services for CY 2020. For a description of the current payment policy for critical care services, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70449), and for the history of the payment policy for critical care services, we refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75043). In this proposed rule, we are seeking public comments on any changes to these codes that we should consider for future rulemaking cycles. We continue to encourage commenters to provide the data and analysis necessary to justify any suggested changes.
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59004 through 59015), we adopted a method to control unnecessary increases in the volume of covered outpatient department services under section 1833(t)(2)(F) of the Act by utilizing a Medicare Physician Fee Schedule (PFS)-equivalent payment rate for the hospital outpatient clinic visit (HCPCS code G0463) when it is furnished by excepted off-campus provider-based departments (PBDs). As discussed in section X.D of this proposed rule and the CY 2019 final rule (FR 58818 through 59179), CY 2020 will be the second year of the 2-year transition of this policy, and in CY 2020, these departments will be paid the site-specific PFS rate for the clinic visit service. For a full discussion of this policy, we refer readers to that final rule with comment period.
VIII. Proposed Payment for Partial Hospitalization Services
A. Background
A partial hospitalization program (PHP) is an intensive outpatient program of psychiatric services provided as an alternative to inpatient psychiatric care for individuals who have an acute mental illness, which includes, but is not limited to, conditions such as depression, schizophrenia, and substance use disorders. Section 1861(ff)(1) of the Act defines partial hospitalization services as the items and services described in paragraph (2) prescribed by a physician and provided under a program described in paragraph (3) under the supervision of a physician pursuant to an individualized, written plan of treatment established and periodically reviewed by a physician (in consultation with appropriate staff participating in such program), which sets forth the physician's diagnosis, the type, amount, frequency, and duration of the items and services provided under the plan, and the goals for treatment under the plan. Section 1861(ff)(2) of the Act describes the items and services included in partial hospitalization services. Section 1861(ff)(3)(A) of the Act specifies that a PHP is a program furnished by a hospital to its outpatients or by a community mental health center (CMHC), as a distinct and organized intensive ambulatory treatment service, offering less than 24-hour-daily care, in a location other than an individual's home or inpatient or residential setting. Section 1861(ff)(3)(B) of the Act defines a CMHC for purposes of this benefit.
Section 1833(t)(1)(B)(i) of the Act provides the Secretary with the authority to designate the outpatient department (OPD) services to be covered under the OPPS. The Medicare regulations that implement this provision specify, at 42 CFR 419.21, that payments under the OPPS will be made for partial hospitalization services furnished by CMHCs as well as Medicare Part B services furnished to hospital outpatients designated by the Secretary, which include partial hospitalization services (65 FR 18444 through 18445).
Section 1833(t)(2)(C) of the Act requires the Secretary, in part, to establish relative payment weights for covered OPD services (and any groups of such services described in section 1833(t)(2)(B) of the Act) based on median (or, at the election of the Secretary, mean) hospital costs using data on claims from 1996 and data from the most recent available cost reports. In pertinent part, section 1833(t)(2)(B) of the Act provides that the Secretary may establish groups of covered OPD services, within a classification system developed by the Secretary for covered OPD services, so that services classified within each group are comparable clinically and with respect to the use of resources. In accordance with these provisions, we have developed the PHP APCs. Since a day of care is the unit that defines the structure and scheduling of partial hospitalization services, we established a per diem payment methodology for the PHP APCs, effective for services furnished on or after July 1, 2000 (65 FR 18452 through 18455). Under this methodology, the median per diem costs were used to calculate the relative payment weights for the PHP APCs. Section 1833(t)(9)(A) of the Act requires the Secretary to review, not less often than annually, and revise the groups, the relative payment weights, and the wage and other adjustments described in section 1833(t)(2) of the Act to take into account changes in medical practice, changes in technology, the addition of new services, new cost data, and other relevant information and factors.
We began efforts to strengthen the PHP benefit through extensive data analysis, along with policy and payment changes finalized in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66670 through 66676). In that final rule with comment period, we made two refinements to the methodology for computing the PHP median: The first remapped 10 revenue codes that are common among hospital-based PHP claims to the most appropriate cost centers; and the second refined our methodology for computing the PHP median per diem cost by computing a separate per diem cost for each day rather than for each bill.
In CY 2009, we implemented several regulatory, policy, and payment changes, including a two-tier payment approach for partial hospitalization services under which we paid one amount for days with 3 services under PHP APC 0172 (Level 1 Partial Hospitalization) and a higher amount for days with 4 or more services under PHP APC 0173 (Level 2 Partial Hospitalization) (73 FR 68688 through 68693). We also finalized our policy to deny payment for any PHP claims submitted for days when fewer than 3 units of therapeutic services are provided (73 FR 68694). Additionally, for CY 2009, we revised the regulations at 42 CFR 410.43 to codify existing basic PHP patient eligibility criteria and to add a reference to current physician certification requirements under 42 CFR 424.24 to conform our regulations to our longstanding policy (73 FR 68694 through 68695). We also revised the partial hospitalization benefit to include several coding updates (73 FR 68695 through 68697).
For CY 2010, we retained the two-tier payment approach for partial hospitalization services and used only hospital-based PHP data in computing the PHP APC per diem costs, upon which PHP APC per diem payment rates are based. We used only hospital-based PHP data because we were concerned about further reducing both PHP APC per diem payment rates without knowing the impact of the policy and payment changes we made in CY 2009. Because of the 2-year lag between data collection and rulemaking, the changes we made in CY 2009 were reflected for the first time in the claims data that we used to determine payment rates for the CY 2011 rulemaking (74 FR 60556 through 60559).
In the CY 2011 OPPS/ASC final rule with comment period (75 FR 71994), we established four separate PHP APC per diem payment rates: Two for CMHCs (APC 0172 (for Level 1 services) and APC 0173 (for Level 2 services)) and two for hospital-based PHPs (APC 0175 (for Level 1 services) and APC 0176 (for Level 2 services)), based on each provider type's own unique data. For CY 2011, we also instituted a 2-year transition period for CMHCs to the CMHC APC per diem payment rates based solely on CMHC data. Under the transition methodology, CMHC APCs Level 1 and Level 2 per diem costs were calculated by taking 50 percent of the difference between the CY 2010 final hospital-based PHP median costs and the CY 2011 final CMHC median costs and then adding that number to the CY 2011 final CMHC median costs. A 2-year transition under this methodology moved us in the direction of our goal, which is to pay appropriately for partial hospitalization services based on each provider type's data, while at the same time allowing providers time to adjust their business operations and protect access to care for Medicare beneficiaries. We also stated that we would review and analyze the data during the CY 2012 rulemaking cycle and, based on these analyses, we might further refine the payment mechanism. We refer readers to section X.B. of the CY 2011 OPPS/ASC final rule with comment period (75 FR 71991 through 71994) for a full discussion.
In addition, in accordance with section 1301(b) of the Health Care and Education Reconciliation Act of 2010 (HCERA 2010), we amended the description of a PHP in our regulations to specify that a PHP must be a distinct and organized intensive ambulatory treatment program offering less than 24-hour daily care other than in an individual's home or in an inpatient or residential setting. In accordance with section 1301(a) of HCERA 2010, we revised the definition of a CMHC in the regulations to conform to the revised definition now set forth under section 1861(ff)(3)(B) of the Act (75 FR 71990).
For CY 2012, as discussed in the CY 2012 OPPS/ASC final rule with comment period (76 FR 74348 through 74352), we determined the relative payment weights for partial hospitalization services provided by CMHCs based on data derived solely from CMHCs and the relative payment weights for partial hospitalization services provided by hospital-based PHPs based exclusively on hospital data.
In the CY 2013 OPPS/ASC final rule with comment period, we finalized our proposal to base the relative payment weights that underpin the OPPS APCs, including the four PHP APCs (APCs 0172, 0173, 0175, and 0176), on geometric mean costs rather than on the median costs. We established these four PHP APC per diem payment rates based on geometric mean cost levels calculated using the most recent claims and cost data for each provider type. For a detailed discussion on this policy, we refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68406 through 68412).
In the CY 2014 OPPS/ASC proposed rule (78 FR 43621 through 43622), we solicited comments on possible future initiatives that may help to ensure the long-term stability of PHPs and further improve the accuracy of payment for PHP services, but proposed no changes. In the CY 2014 OPPS/ASC final rule with comment period (78 FR 75050 through 75053), we summarized the comments received on those possible future initiatives. We also continued to apply our established policies to calculate the four PHP APC per diem payment rates based on geometric mean per diem costs using the most recent claims data for each provider type. For a detailed discussion on this policy, we refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75047 through 75050).
In the CY 2015 OPPS/ASC final rule with comment period (79 FR 66902 through 66908), we continued to apply our established policies to calculate the four PHP APC per diem payment rates based on PHP APC geometric mean per diem costs, using the most recent claims and cost data for each provider type.
In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70455 through 70465), we described our extensive analysis of the claims and cost data and ratesetting methodology. We found aberrant data from some hospital-based PHP providers that were not captured using the existing OPPS ±3 standard deviation trims for extreme cost-to-charge ratios (CCRs) and excessive CMHC charges resulting in CMHC geometric mean costs per day that were approximately the same as or more than the daily payment for inpatient psychiatric facility services. Consequently, we implemented a trim to remove hospital-based PHP service days that use a CCR that was greater than 5 to calculate costs for at least one of their component services, and a trim on CMHCs with a geometric mean cost per day that is above or below 2 (±2) standard deviations from the mean. We stated in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70456) that, without using a trimming process, the data from these providers would inappropriately skew the geometric mean per diem cost for Level 2 CMHC services.
In addition, in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70459 through 70460), we corrected a cost inversion that occurred in the final rule data with respect to hospital-based PHP providers. We corrected the cost inversion with an equitable adjustment to the actual geometric mean per diem costs by increasing the Level 2 hospital-based PHP APC geometric mean per diem costs and decreasing the Level 1 hospital-based PHP APC geometric mean per diem costs by the same factor, to result in a percentage difference equal to the average percent difference between the hospital-based Level 1 PHP APC and the Level 2 PHP APC for partial hospitalization services from CY 2013 through CY 2015.
Finally, we renumbered the PHP APCs, which were previously APCs 0172 and 0173 for CMHCs' partial hospitalization Level 1 and Level 2 services, and APCs 0175 and 0176 for hospital-based partial hospitalization Level 1 and Level 2 services to APCs 5851 and 5852 for CMHCs' partial hospitalization Level 1 and Level 2 services, and APCs 5861 and 5862 for hospital-based partial hospitalization Level 1 and Level 2 services, respectively. For a detailed discussion of the PHP ratesetting process, we refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70462 through 70467).
In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79687 through 79691), we continued to apply our established policies to calculate the PHP APC per diem payment rates based on geometric mean per diem costs using the most recent claims and cost data for each provider type. However, we finalized a policy to combine the Level 1 and Level 2 PHP APCs for CMHCs and to combine the Level 1 and Level 2 APCs for hospital-based PHPs because we believed this would best reflect actual geometric mean per diem costs going forward, provide more predictable per diem costs, particularly given the small number of CMHCs, and generate more appropriate payments for these services, for example by avoiding the cost inversions for hospital-based PHPs addressed in the CY 2016 and CY 2017 OPPS/ASC final rules with comment period (80 FR 70459 and 81 FR 79682). We also implemented an 8-percent outlier cap for CMHCs to mitigate potential outlier billing vulnerabilities by limiting the impact of inflated CMHC charges on outlier payments. We stated that we will continue to monitor the trends in outlier payments and consider policy adjustments as necessary.
For a comprehensive description of PHP payment policy, including a detailed methodology for determining PHP per diem amounts, we refer readers to the CY 2016 and CY 2017 OPPS/ASC final rules with comment period (80 FR 70453 through 70455 and 81 FR 79678 through 79680).
In the CYs 2018 and 2019 OPPS/ASC final rules with comment period (82 FR 59373 through 59381, and 83 FR 58983 through 58998, respectively), we continued to apply our established policies to calculate the PHP APC per diem payment rates based on geometric mean per diem costs using the most recent claims and cost data for each provider type. We also continued to designate a portion of the estimated 1.0 percent hospital outpatient outlier threshold specifically for CMHCs, consistent with the percentage of projected payments to CMHCs under the OPPS, excluding outlier payments. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 58997 through 58998), we also included proposed updates to the PHP allowable HCPCS codes. Specifically, we proposed to delete 6 psychological and neuropsychological testing CPT codes, which affect PHPs, and to add 9 new codes as replacements. We refer readers to section VIII.D. of this proposed rule for a discussion of those proposed updates and the applicability for CY 2020.
B. Proposed PHP APC Update for CY 2020
1. Proposed PHP APC Geometric Mean Per Diem Costs
In summary, for CY 2020, we are proposing to use the CY 2020 CMHC geometric mean per diem cost and the CY 2020 hospital-based PHP geometric mean per diem cost, each calculated in accordance with our existing methodology, but with a cost floor equal to the CY 2019 final geometric mean per diem cost for CMHCs of $121.62 and for hospital-based PHPs of $222.76 (83 FR 58991), as the basis for developing the CY 2020 PHP APC per diem rates. As part of this proposal, in the final rule with comment period, we are proposing that we would use the most recent updated claims and cost data to calculate CY 2020 geometric mean per diem costs.
Also, we are proposing to continue to use CMHC APC 5853 (Partial Hospitalization (3 or More Services Per Day)) and hospital-based PHP APC 5863 (Partial Hospitalization (3 or More Services Per Day)). These proposals are discussed in more detail below.
2. Development of the Proposed PHP APC Geometric Mean Per Diem Costs
In preparation for CY 2020 and subsequent years, we followed the PHP ratesetting methodology described in section VIII.B.2. of the CY 2016 OPPS/ASC final rule with comment period (80 FR 70462 through 70466) to calculate the PHP APCs' geometric mean per diem costs and payment rates for APCs 5853 and 5863, incorporating the modifications made in the CY 2017 OPPS/ASC final rule with comment period. As discussed in section VIII.B.1. of the CY 2017 OPPS/ASC final rule with comment period (81 FR 79680 through 79687), the proposed geometric mean per diem cost for hospital-based PHP APC 5863 would be based upon actual hospital-based PHP claims and costs for PHP service days providing 3 or more services. Similarly, the proposed geometric mean per diem cost for CMHC APC 5853 would be based upon actual CMHC claims and costs for CMHC service days providing 3 or more services.
The CMHC or hospital-based PHP APC per diem costs are the provider-type specific costs derived from the most recent claims and cost data. The CMHC or hospital-based PHP APC per diem payment rates are the national unadjusted payment rates calculated from the CMHC or hospital-based PHP APC geometric mean per diem costs, after applying the OPPS budget neutrality adjustments described in section II.A.4. of this proposed rule.
As previously stated, in this CY 2020 OPPS/ASC proposed rule, we applied our established methodologies in calculating the CY 2020 geometric mean per diem costs and payment rates, including the application of a ±2 standard deviation trim on costs per day for CMHCs and a CCR greater than 5 hospital service day trim for hospital-based PHP providers. These two trims were finalized in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70455 through 70462) for CY 2016 and subsequent years.
a. CMHC Data Preparation: Data Trims, Exclusions, and CCR Adjustments
For CY 2020, prior to calculating the geometric mean per diem cost for CMHC APC 5853, we prepared the data by first applying trims and data exclusions, and assessing CCRs as described in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70463 through 70465), so that ratesetting is not skewed by providers with extreme data. Before any trims or exclusions were applied, there were 41 CMHCs in the PHP claims data file. Under the ±2 standard deviation trim policy, we excluded any data from a CMHC for ratesetting purposes when the CMHC's geometric mean cost per day was more than ±2 standard deviations from the geometric mean cost per day for all CMHCs. In applying this trim for CY 2020 ratesetting, no CMHCs had geometric mean costs per day below the trim's lower limit of $21.13 or had geometric mean costs per day above the trim's upper limit of $506.11. Therefore, we did not exclude any CMHCs because of the ±2 standard deviation trim.
In accordance with our PHP ratesetting methodology, we also remove service days with no wage index values, because we use the wage index data to remove the effects of geographic variation in costs prior to APC geometric mean per diem cost calculation (80 FR 70465). For CY 2020, no CMHC was missing wage index data for all of its service days and, therefore, no CMHC was excluded.
In addition to our trims and data exclusions, before calculating the PHP APC geometric mean per diem costs, we also assess CCRs (80 FR 70463). Our longstanding PHP OPPS ratesetting methodology defaults any CMHC CCR greater than 1 to the statewide hospital CCR (80 FR 70457). For CY 2020, there were no CMHCs in the outpatient provider specific file (OPSF) that showed CCRs greater than 1. Therefore, it was not necessary to default any CMHC to its statewide hospital CCR for ratesetting.
In summary, these data preparation steps did not adjust the CCR for any CMHCs shown in the OPSF with a CCR greater than 1 during our ratesetting process. We also did not exclude any CMHCs for other missing data or for failing the ±2 standard deviation trim, resulting in the inclusion of all 41 CMHCs. There were 188 CMHC claims removed during data preparation steps because they either had no PHP-allowable codes or had zero payment days, leaving 10,271 CMHC claims in our CY 2020 proposed rule ratesetting modeling.
After applying all of the above trims, exclusions, and adjustments, we followed the methodology described in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70464 through 70465) and modified in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79687 through 79688, and 79691) to calculate a CMHC APC geometric mean per diem cost.[65] The calculated CY 2020 geometric mean per diem cost for all CMHCs for providing 3 or more services per day (CMHC APC 5853) is $103.42, a decrease from $121.62 calculated last year for CY 2019 ratesetting (83 FR 58986 through 58989).
Due to this fluctuation, we investigated why the calculated CMHC APC geometric mean per diem cost had decreased from the prior year, and found that a single large provider that reported low costs per day was heavily influencing the calculated geometric mean per diem cost. Because this provider had a high number of paid PHP days, and because the CMHC data set is so small (n=41), this provider had a significant influence on the calculated CY 2020 CMHC APC geometric mean per diem cost. In the case of PHPs provided by CMHCs, we note that we have an unusually low number of PHP providers in our ratesetting dataset (41 CMHCs compared to 364 hospital-based PHPs) that provide a small volume of services and, therefore, account for a limited amount of payments, relative to the rest of OPPS payments (total CY 2018 CMHC payments are estimated to be approximately 0.02 percent of all OPPS payments).
We are concerned that a CMHC APC geometric mean per diem cost of $103.42 would not support ongoing access to PHPs in CMHCs. This cost is nearly a 15 percent decrease from the final CY 2019 CMHC geometric mean per diem cost. We believe access to partial hospitalization services and PHPs is better supported when the geometric mean per diem cost does not fluctuate greatly. In addition, while the CMHC APC 5853 is described as providing 3 or more partial hospitalization services per day (81 FR 79680), 95 percent of CMHC paid days in CY 2018 were for providing 4 or more services per day. To be eligible for a PHP, a patient must need at least 20 hours of therapeutic services per week, as evidenced in the patient's plan of care (42 CFR 410.43(c)(1)). To meet those patient needs, most PHP provider paid days are for providing 4 or more services per day (we refer readers to Table 22.—Percentage of PHP Days by Service Unit Frequency of this proposed rule). Therefore, the CMHC APC 5853 is actually heavily weighted to the cost of providing 4 or more services. The per diem costs for CMHC APC 5853 have been calculated as $124.92, $143.22, and $121.62 for CY 2017 (81 FR 79691), CY 2018 (82 FR 59378), and CY 2019 (83 FR 58991), respectively. We do not believe it is likely that the actual cost of providing partial hospitalization services through a PHP by CMHCs has suddenly declined when costs generally increase over time. We are concerned by this fluctuation, which we believe is influenced by data from a single large provider.
Therefore, rather than simply proposing to use the calculated CY 2020 CMHC APC geometric mean per diem cost for CY 2020 ratesetting, we are instead proposing to use the CY 2020 CMHC APC geometric mean per diem cost, calculated in accordance with our existing methodology, but with a cost floor equal to the CY 2019 final geometric mean per diem cost for CMHCs of $121.62 (83 FR 58991), as the basis for developing the CY 2020 CMHC APC per diem rate. As part of this proposal, in the final rule with comment period, we are proposing that we would use the most recent updated claims and cost data to calculate CY 2020 CMHC geometric mean per diem cost. This proposal aligns with our proposal for hospital-based PHPs. We believe using the CY 2019 CMHC geometric mean per diem cost as the floor is appropriate because it is based on very recent CMHC PHP claims and cost data and would help to protect provider access by preventing wide fluctuation in the per diem costs for CMHC APC 5853. Because the calculated amount of $103.42 is less than the final CY 2019 CMHC APC geometric mean per diem cost of $121.62, the inclusion of a cost floor means that the proposed CY 2020 CMHC geometric mean per diem cost at the time of the development of this proposed rule is $121.62. The inclusion of the cost floor would protect CMHCs if the final CY 2020 calculated per diem cost still results in an amount that is less than $121.62. We believe this proposal for CY 2020 ratesetting allows us to use the most recent or very recent CMHC claims and cost reporting data while still protecting provider access. To be clear, this policy would only apply for the CY 2020 ratesetting.
In crafting this proposal, we also considered proposing a 3-year rolling average calculated using the final PHP geometric mean per diem costs, by provider type, from CY 2018 (82 FR 59378), CY 2019 (83 FR 58991), and the calculated CY 2020 geometric mean per diem costs of $103.42 discussed earlier in this section for CMHCs and the calculated CY 2020 geometric mean per diem costs for hospital-based PHPs discussed in section VIII.B.2.b. of this proposed rule. The 3-year rolling averages results in geometric mean per diem cost for CMHCs that would have been $122.75 and for hospital-based PHPs that would have been $209.79. While we believe this option would have avoided the fluctuation in the geometric mean per diem cost and, therefore, supported access to PHPs provided by CMHCs, it would have maintained the fluctuation in the geometric mean per diem costs used to derive the hospital-based PHP APC per diem payment rates. This is further discussed in the hospital-based PHP section VIII.B.2.b. of this proposed rule. In addition, we believe that it is necessary to recalculate the CMHC geometric mean per diem cost for the final rule with comment period using updated claims and cost data, and simply proposing to use a 3-year rolling average for the CMHC geometric mean per diem cost for CY 2020 would not have allowed us to do so. Therefore, we believe that it is more appropriate to propose to use the final CY 2019 geometric mean per diem costs, by provider type, as the cost floor for use with the calculated CY 2020 PHP geometric mean per diem costs, by provider type, because those CY 2019 per diem costs are based on very recent CMHC and hospital-based PHP claims and cost data, are the easiest to understand, and would result in proposed geometric mean per diem costs which would support access for both CMHCs and hospital-based PHPs.
We estimate the aggregate difference in the (prescaled) CMHC geometric mean per diem costs for CY 2020 from proposing the CMHC cost floor amount of $121.62 rather than the calculated CMHC geometric mean per diem cost of $103.42 to be $1.4 million. We refer readers to section XXVI. of this proposed rule for payment impacts, which are budget neutral.
b. Hospital-Based PHP Data Preparation: Data Trims and Exclusions
For this CY 2020 proposed rule, we prepared data consistent with our policies as described in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70463 through 70465) for hospital-based PHP providers, which is similar to that used for CMHCs. The CY 2018 PHP claims included data for 427 hospital-based PHP providers for our calculations in this CY 2020 OPPS/ASC proposed rule.
Consistent with our policies as stated in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70463 through 70465), we prepared the data by applying trims and data exclusions. We applied a trim on hospital service days for hospital-based PHP providers with a CCR greater than 5 at the cost center level. To be clear, the CCR greater than 5 trim is a service day-level trim in contrast to the CMHC ±2 standard deviation trim, which is a provider-level trim. Applying this CCR greater than 5 trim removed affected service days from 1 hospital-based PHP provider with a CCR of 6.944 from our proposed rule ratesetting. However, 100 percent of the service days for this 1 hospital-based PHP provider had at least 1 service associated with a CCR greater than 5, so the trim removed this provider entirely from our proposed rule ratesetting. In addition, 60 hospital-based PHPs were removed for having no PHP costs and, therefore, no days with PHP payment. Two hospital-based PHPs were removed because none of their days included PHP-allowable HCPCS codes. No hospital-based PHPs were removed for missing wage index data, nor were any hospital-based PHPs removed by the OPPS ±3 standard deviation trim on costs per day. (We refer readers to the OPPS Claims Accounting Document, available online at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/CMS-1695-FC-2019-OPPS-FR-Claims-Accounting.pdf.)
Overall, we removed 63 hospital-based PHP providers [(1 with all service days having a CCR greater than 5) + (60 with zero daily costs and no PHP payment) + (2 with no PHP-allowable HCPCS codes)], resulting in 364 (427 total−63 excluded) hospital-based PHP providers in the data used for calculating ratesetting. In addition, 3 hospital-based PHP providers were defaulted to their overall hospital ancillary CCRs due to outlier cost center CCR values.
After completing these data preparation steps, we calculated the CY 2020 geometric mean per diem cost for hospital-based PHP APC 5863 for hospital-based partial hospitalization services by following the methodology described in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70464 through 70465) and modified in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79687 and 79691).[66] The calculated CY 2020 hospital-based PHP APC geometric mean per diem cost for hospital-based PHP providers that provide 3 or more services per service day (hospital-based PHP APC 5863) is $198.53, a decrease from $222.76 calculated last year for CY 2019 ratesetting (83 FR 58989 through 58991).
Due to this fluctuation, we investigated why this calculated hospital-based PHP APC geometric mean per diem cost decreased from the prior year, and found that a single provider with a large number of paid PHP service days had a significant decrease in its cost per day and, therefore, was heavily influencing the data. We are concerned that a hospital-based PHP APC geometric mean per diem cost of $198.53 would not support ongoing access to hospital-based PHPs. This cost is nearly an 11 percent decrease from the final CY 2019 hospital-based PHP geometric mean per diem cost. We believe access is better supported when the geometric mean per diem cost does not fluctuate greatly. In addition, while the hospital-based PHP APC 5863 is described as providing payment for the cost of 3 or more services per day (81 FR 79680), 89 percent of hospital-based PHP paid service days in CY 2018 were for providing 4 or more services per day. To be eligible for a PHP, a patient must need at least 20 hours of therapeutic services per week, as evidenced in the patient's plan of care (42 CFR 410.43(c)(1)). To meet those patient needs, most PHP paid service days provide 4 or more services (we refer readers to Table 22.—Percentage of PHP Days by Service Unit Frequency in this proposed rule). Therefore, the hospital-based PHP APC 5863 is actually heavily weighted to the cost of providing 4 or more services. The per diem costs for hospital-based PHP APC 5863 have been calculated as $213.14, $208.09, and $222.76 for CY 2017 (81 FR 79691), CY 2018 (82 FR 59378), and CY 2019 (83 FR 58991), respectively. We do not believe that it is likely that the cost of providing hospital-based PHP services has suddenly declined when costs generally increase over time. We are concerned by this fluctuation, which we believe is influenced by data from a single large provider that had low service costs per day.
Therefore, rather than proposing the calculated CY 2020 hospital-based PHP APC geometric mean per diem cost, we are instead proposing to use the CY 2020 hospital-based PHP APC geometric mean per diem cost, calculated in accordance with our existing methodology, but with a cost floor equal to the CY 2019 final geometric mean per diem cost for hospital-based PHPs of $222.76 (83 FR 58991), as the basis for developing the CY 2020 hospital-based PHP APC per diem rate. As part of this proposal, in the final rule with comment period, we are proposing that we would use the most recent updated claims and cost data to calculate CY 2020 geometric mean per diem costs. This proposal aligns with our proposal for CMHCs. We believe using the CY 2019 hospital-based PHP per diem cost as the floor is appropriate because it is based on very recent hospital-based PHP claims and cost data and would help to protect provider access by preventing wide fluctuation in the per diem costs for hospital-based APC 5863. Because the calculated amount of $198.53 is less than the final CY 2019 hospital-based PHP APC geometric mean per diem cost of $222.76, the inclusion of a cost floor means that the proposed CY 2020 hospital-based PHP geometric mean per diem cost, as of the time of this proposed rule, is $222.76. The inclusion of the cost floor would protect hospital-based PHPs if the final CY 2020 calculated hospital-based PHP APC geometric mean per diem cost results in an amount that is still less than $222.76. We believe this proposal for CY 2020 ratesetting allows us to use the most recent or very recent hospital-based PHP claims and cost reporting data while still protecting provider access. To be clear, this policy would only apply for the CY 2020 ratesetting.
In crafting this proposal, we also considered proposing a 3-year rolling average calculated using the final PHP geometric mean per diem costs, by provider type, from CY 2018 (82 FR 59378) and CY 2019 (83 FR 58991), and the calculated CY 2020 geometric mean per diem cost of $198.53 discussed earlier in this section for hospital-based PHPs. As discussed previously in this section, the 3-year rolling average per diem cost floor for CMHCs would have been $122.75, but the resulting rolling average per diem cost floor for hospital-based PHPs would have been $209.79. While we believe that this option would have supported access to CMHCs, as discussed previously, it would have resulted in a geometric mean per diem cost for the hospital-based PHP APC which still would have been a decrease from the hospital-based PHP APC geometric mean per diem cost of $222.76 finalized in CY 2019 (83 FR 58991). In addition, we believe that it is necessary to recalculate the hospital-based PHP geometric mean per diem cost for the final rule using updated claims and cost data and simply proposing to use a 3-year rolling average per diem cost floor for the hospital-based PHP APC per diem costs for CY 2020 would not have allowed us to do so. We are concerned that this 3-year rolling average per diem cost would continue to result in a fluctuation in the cost of a hospital providing 4 or more hospital-based PHP services per day. We believe that it is important to support access to partial hospitalization services in both CMHCs and in hospital-based PHPs, and note that hospital-based PHPs provide 80 percent of all paid PHP service days. Therefore, we believe that it is more appropriate to propose to use the final CY 2019 geometric mean per diem costs, by provider type, as the cost floor for use with the calculated CY 2020 PHP geometric mean per diem costs, by provider type, because those CY 2019 per diem costs are based on very recent CMHC and hospital-based PHP claims and cost data, are the easiest to understand, and would result in proposed geometric mean per diem costs which would help to protect provider access by preventing wide fluctuation in the per diem costs for both CMHCs and hospital-based PHPs.
We estimate the aggregate difference in the (prescaled) hospital-based PHP geometric mean per diem costs for CY 2020 from proposing the hospital-based PHP cost floor amount of $222.76 rather than the calculated hospital-based PHP geometric mean per diem cost of $198.53 to be $9.3 million. We refer readers to section XXVI. of this proposed rule for payment impacts, which are budget neutral.
In summary, for CY 2020, we are proposing to use the calculated CY 2020 CMHC geometric mean per diem cost and the calculated CY 2020 hospital-based PHP geometric mean per diem cost, each calculated in accordance with our existing methodology, but with a cost floor equal to the CY 2019 final geometric mean per diem costs as the basis for developing the CY 2020 PHP APC per diem rates. Because the CY 2020 calculated geometric mean per diem costs for these provider types were both less than their respective final CY 2019 APC geometric mean per diem costs, the inclusion of a cost floor in this proposal means that both the proposed CY 2020 CMHC geometric mean per diem cost and the proposed CY 2020 hospital-based PHP geometric mean per diem cost, as of the time of this proposed rule, are $121.62 and $222.76, respectively. As part of this proposal, in the final rule with comment period, we are proposing that we would use the most recent updated claims and cost data to calculate CY 2020 geometric mean per diem costs. The inclusion of a cost floor, which is based on very recent data, would protect providers should the final CY 2020 calculated per diem costs for CMHCs or for hospital-based PHPs result in amounts that are still less than the final CY 2019 CMHC and hospital-based PHP geometric mean per diem costs.
These proposed CY 2020 PHP geometric mean per diem costs are shown in Table 20, and are used to derive the proposed CY 2020 PHP APC per diem rates for CMHCs and hospital-based PHPs. The proposed CY 2020 PHP APC per diem rates are included in Addendum A to this proposed rule (which is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices.html).67

3. PHP Service Utilization Updates
a. Provision of Individual Therapy
In the CY 2016 OPPS/ASC final rule with comment period (81 FR 79684 through 79685), we expressed concern over the low frequency of individual therapy provided to beneficiaries. The CY 2018 claims data used for this CY 2020 proposed rule revealed some changes in the provision of individual therapy compared to CY 2015, CY 2016, and CY 2017 claims data as shown in the Table 21.

As shown in Table 21, the CY 2018 claims show that both CMHCs and hospital-based PHPs have slightly increased the provision of individual therapy on days with 4 or more services, compared to CY 2017 claims. However, on days with 3 services, CMHCs decreased the provision of individual therapy, while hospital-based PHPs provided the same level of individual therapy as in CY 2017.
b. Provision of 3-Service Days
In the CY 2018 OPPS/ASC proposed rule and final rule with comment period (82 FR 33640 and 82 FR 59378), we stated that we are aware that our single-tier payment policy may influence a change in service provision because providers are able to obtain payment that is heavily weighted to the cost of providing 4 or more services when they provide only 3 services. We indicated that we are interested in ensuring that providers furnish an appropriate number of services to beneficiaries enrolled in PHPs. Therefore, with the CY 2017 implementation of CMHC APC 5853 and hospital-based PHP APC 5863 for providing 3 or more PHP services per day, we are continuing to monitor utilization of days with only 3 PHP services.
For this CY 2020 OPPS/ASC proposed rule, we used the CY 2018 claims data. Table 22 shows the utilization findings based on the most recent claims data.

As shown in Table 22, the CY 2018 claims data used for this proposed rule showed that PHPs maintained an appropriately low utilization of 3 service days compared to the 3 prior claim years. Compared to CY 2017, in CY 2018 hospital-based PHPs provided slightly more days with 3 services only, more days with 4 services only, and fewer days with 5 or more services. Compared to CY 2017, in CY 2018 CMHCs decreased their provision of 3 service days, slightly increased their provision of days with 4 services, but have decreased their provision of days with 5 or more services.
The CY 2017 data are the first year of claims data to reflect the change to the single-tier PHP APCs. As we noted in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79685), we will continue to monitor the provision of days with only 3 services, particularly now that the single-tier PHP APCs 5853 and 5863 are established for providing 3 or more services per day for CMHCs and hospital-based PHPs, respectively.
It is important to reiterate our expectation that days with only 3 services are meant to be an exception and not the typical PHP day. In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68694), we clearly stated that we consider the acceptable minimum units of PHP services required in a PHP day to be 3 and explained that it was never our intention that 3 units of service represent the number of services to be provided in a typical PHP day. PHP is furnished in lieu of inpatient psychiatric hospitalization and is intended to be more intensive than a half-day program. We further indicated that a typical PHP day should generally consist of 5 to 6 units of service (73 FR 68689). We explained that days with only 3 units of services may be appropriate to bill in certain limited circumstances, such as when a patient might need to leave early for a medical appointment and, therefore, would be unable to complete a full day of PHP treatment. At that time, we noted that if a PHP were to only provide days with 3 services, it would be difficult for patients to meet the eligibility requirement in 42 CFR 410.43(c)(1) that patients must require a minimum of 20 hours per week of therapeutic services as evidenced in their plan of care (73 FR 68689).
C. Proposed Outlier Policy for CMHCs
In this CY 2020 OPPS/ASC proposed rule, for CY 2020, we are proposing to continue to calculate the CMHC outlier percentage, cutoff point and percentage payment amount, outlier reconciliation, outlier payment cap, and fixed-dollar threshold according to previously established policies. These topics are discussed in more detail below. We refer readers to section II.G. of this proposed rule for our general policies for hospital outpatient outlier payments.
1. Background
As discussed in the CY 2004 OPPS final rule with comment period (68 FR 63469 through 63470), we noted a significant difference in the amount of outlier payments made to hospitals and CMHCs for PHP services. Given the difference in PHP charges between hospitals and CMHCs, we did not believe it was appropriate to make outlier payments to CMHCs using the outlier percentage target amount and threshold established for hospitals. Therefore, beginning in CY 2004, we created a separate outlier policy specific to the estimated costs and OPPS payments provided to CMHCs. We designated a portion of the estimated OPPS outlier threshold specifically for CMHCs, consistent with the percentage of projected payments to CMHCs under the OPPS each year, excluding outlier payments, and established a separate outlier threshold for CMHCs. This separate outlier threshold for CMHCs resulted in $1.8 million in outlier payments to CMHCs in CY 2004 and $0.5 million in outlier payments to CMHCs in CY 2005 (82 FR 59381). In contrast, in CY 2003, more than $30 million was paid to CMHCs in outlier payments (82 FR 59381).
2. CMHC Outlier Percentage
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59267 through 59268), we described the current outlier policy for hospital outpatient payments and CMHCs. We note that we also discussed our outlier policy for CMHCs in more detail in section VIII. C. of that same final rule (82 FR 59381). We set our projected target for all OPPS aggregate outlier payments at 1.0 percent of the estimated aggregate total payments under the OPPS (82 FR 59267). We estimate CMHC per diem payments and outlier payments by using the most recent available utilization and charges from CMHC claims, updated CCRs, and the updated payment rate for APC 5853. For increased transparency, we are providing a more detailed explanation of the existing calculation process for determining the CMHC outlier percentages below. As previously stated, we are proposing to continue to calculate the CMHC outlier percentage according to previously established policies, and we are not proposing any changes to our current methodology for calculating the CMHC outlier percentage for CY 2020. To calculate the CMHC outlier percentage, we follow three steps:
- Step 1: We multiply the OPPS outlier threshold, which is 1.0 percent, by the total estimated OPPS Medicare payments (before outliers) for the prospective year to calculate the estimated total OPPS outlier payments:
(0.01 × Estimated Total OPPS Payments) = Estimated Total OPPS Outlier Payments.
- Step 2: We estimate CMHC outlier payments by taking each provider's estimated costs (based on their allowable charges multiplied by the provider's CCR) minus each provider's estimated CMHC outlier multiplier threshold (we refer readers to section VIII.C.3. of this proposed rule). That threshold is determined by multiplying the provider's estimated paid days by 3.4 times the CMHC PHP APC payment rate. If the provider's costs exceed the threshold, we multiply that excess by 50 percent, as described in section VIII.C.3. of this proposed rule, to determine the estimated outlier payments for that provider. CMHC outlier payments are capped at 8 percent of the provider's estimated total per diem payments (including the beneficiary's copayment), as described in section VIII.C.5. of this proposed rule, so any provider's costs that exceed the CMHC outlier cap would have its payments adjusted downward. After accounting for the CMHC outlier cap, we sum all of the estimated outlier payments to determine the estimated total CMHC outlier payments.
(Each Provider's Estimated Costs—Each Provider's Estimated Multiplier Threshold) = A. If A is greater than 0, then (A × 0.50) = Estimated CMHC Outlier Payment (before cap) = B. If B is greater than (0.08 × Provider's Total Estimated Per Diem Payments), then cap-adjusted B = (0.08 × Provider's Total Estimated Per Diem Payments); otherwise, B = B. Sum (B or cap-adjusted B) for Each Provider = Total CMHC Outlier Payments.
- Step 3: We determine the percentage of all OPPS outlier payments that CMHCs represent by dividing the estimated CMHC outlier payments from Step 2 by the total OPPS outlier payments from Step 1:
(Estimated CMHC Outlier Payments/Total OPPS Outlier Payments).
In CY 2019, we designated approximately 0.01 percent of that estimated 1.0 percent hospital outpatient outlier threshold for CMHCs (83 FR 58996), based on this methodology. In this proposed rule, we are proposing to continue to use the same methodology for CY 2020. Therefore, based on our CY 2020 payment estimates, CMHCs are projected to receive 0.02 percent of total hospital outpatient payments in CY 2020, excluding outlier payments. We are proposing to designate approximately less than 0.01 percent of the estimated 1.0 percent hospital outpatient outlier threshold for CMHCs. This percentage is based upon the formula given in Step 3 above.
3. Cutoff Point and Percentage Payment Amount
As described in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59381), our policy has been to pay CMHCs for outliers if the estimated cost of the day exceeds a cutoff point. In CY 2006, we set the cutoff point for outlier payments at 3.4 times the highest CMHC PHP APC payment rate implemented for that calendar year (70 FR 68551). For CY 2018, the highest CMHC PHP APC payment rate is the payment rate for CMHC PHP APC 5853. In addition, in CY 2002, the final OPPS outlier payment percentage for costs above the multiplier threshold was set at 50 percent (66 FR 59889). In CY 2018, we continued to apply the same 50 percent outlier payment percentage that applies to hospitals to CMHCs and continued to use the existing cutoff point (82 FR 59381). Therefore, for CY 2018, we continued to pay for partial hospitalization services that exceeded 3.4 times the CMHC PHP APC payment rate at 50 percent of the amount of CMHC PHP APC geometric mean per diem costs over the cutoff point. For example, for CY 2018, if a CMHC's cost for partial hospitalization services paid under CMHC PHP APC 5853 exceeds 3.4 times the CY 2018 payment rate for CMHC PHP APC 5853, the outlier payment would be calculated as 50 percent of the amount by which the cost exceeds 3.4 times the CY 2018 payment rate for CMHC PHP APC 5853 [0.50 × (CMHC Cost−(3.4 × APC 5853 rate))].
In this CY 2020 OPPS/ASC proposed rule, for CY 2020, in accordance with our existing policy, we are proposing to continue to pay for partial hospitalization services that exceed 3.4 times the proposed CMHC PHP APC payment rate at 50 percent of the CMHC PHP APC geometric mean per diem costs over the cutoff point. That is, for CY 2020, if a CMHC's cost for partial hospitalization services paid under CMHC PHP APC 5853 exceeds 3.4 times the proposed payment rate for CMHC APC 5853, the outlier payment would be calculated as [0.50 × (CMHC Cost−(3.4 × APC 5853 rate))].
4. Outlier Reconciliation
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68594 through 68599), we established an outlier reconciliation policy to address charging aberrations related to OPPS outlier payments. We addressed vulnerabilities in the OPPS outlier payment system that lead to differences between billed charges and charges included in the overall CCR, which are used to estimate cost and would apply to all hospitals and CMHCs paid under the OPPS. CMS initiated steps to ensure that outlier payments appropriately account for the financial risk when providing an extraordinarily costly and complex service, but are only being made for services that legitimately qualify for the additional payment.
For a comprehensive description of outlier reconciliation, we refer readers to the CY 2019 OPPS/ASC final rules with comment period (83 FR 58874 through 58875 and 81 FR 79678 through 79680).
In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue these policies for partial hospitalization services provided through PHPs for CY 2020. The current outlier reconciliation policy requires that providers whose outlier payments meet a specified threshold (currently $500,000 for hospitals and any outlier payments for CMHCs) and whose overall ancillary CCRs change by plus or minus 10 percentage points or more, are subject to outlier reconciliation, pending approval of the CMS Central Office and Regional Office (73 FR 68596 through 68599). The policy also includes provisions related to CCRs and to calculating the time value of money for reconciled outlier payments due to or due from Medicare, as detailed in the CY 2009 OPPS/ASC final rule with comment period and in the Medicare Claims Processing Manual (73 FR 68595 through 68599 and Medicare Claims Processing internet Only Manual, Chapter 4, Section 10.7.2 and its subsections, available online at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c04.pdf).
5. Outlier Payment Cap
In the CY 2017 OPPS/ASC final rule with comment period, we implemented a CMHC outlier payment cap to be applied at the provider level, such that in any given year, an individual CMHC will receive no more than a set percentage of its CMHC total per diem payments in outlier payments (81 FR 79692 through 79695). We finalized the CMHC outlier payment cap to be set at 8 percent of the CMHC's total per diem payments (81 FR 79694 through 79695). This outlier payment cap only affects CMHCs, it does not affect other provider types (that is, hospital-based PHPs), and is in addition to and separate from the current outlier policy and reconciliation policy in effect. For CY 2019, we continued this policy in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58997).
For CY 2020 and subsequent years, we are proposing to continue to apply the 8 percent CMHC outlier payment cap to the CMHC's total per diem payments.
6. Fixed-Dollar Threshold
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59267 through 59268), for the hospital outpatient outlier payment policy, we set a fixed-dollar threshold in addition to an APC multiplier threshold. Fixed-dollar thresholds are typically used to drive outlier payments for very costly items or services, such as cardiac pacemaker insertions. CMHC PHP APC 5853 is the only APC for which CMHCs may receive payment under the OPPS, and is for providing a defined set of services that are relatively low cost when compared to other OPPS services. Because of the relatively low cost of CMHC services that are used to comprise the structure of CMHC PHP APC 5853, it is not necessary to also impose a fixed-dollar threshold on CMHCs. Therefore, in the CY 2018 OPPS/ASC final rule with comment period, we did not set a fixed-dollar threshold for CMHC outlier payments (82 FR 59381).
In this CY 2020 OPPS/ASC proposed rule, we are proposing to continue this policy for CY 2020.
D. Update to PHP Allowable HCPCS Codes
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 58997 through 58998), we discussed that, during the CY 2019 rulemaking, we received the Category I and III CPT codes from the AMA that were new, revised, and deleted, effective January 1, 2019. This included the deleting of the following psychological and neuropsychological testing CPT codes, which affect PHPs, as of January 1, 2019:
- CPT code 96101 (Psychological testing by psychologist/physician);
- CPT code 96102 (Psychological testing by technician);
- CPT code 96103 (Psychological testing administered by computer);
- CPT code 96118 (Neuropsychological testing by psychologist/physician)
- CPT code 96119 (Neuropsychological testing by technician); and
- CPT code 96120 (Neuropsychological test administered w/computer).
In addition, the AMA added the following psychological and neuropsychological testing CPT codes to replace the deleted codes, as of January 1, 2019:
- CPT code 96130 (Psychological testing evaluation by physician/qualified health care professional; first hour);
- CPT code 93131 (Psychological testing evaluation by physician/qualified health care professional; each additional hour);
- CPT code 96132 (Neuropsychological testing evaluation by physician/qualified health care professional; first hour);
- CPT code 96133 (Neuropsychological testing evaluation by physician/qualified health care professional; each additional hour);
- CPT code 96136 (Psychological/neuropsychological testing by physician/qualified health care professional; first 30 minutes);
- CPT code 96137 (Psychological/neuropsychological testing by physician/qualified health care professional; each additional 30 minutes);
- CPT code 96138 (Psychological/neuropsychological testing by technician; first 30 minutes);
- CPT code 96139 (Psychological/neuropsychological testing by technician; each additional 30 minutes); and
- CPT code 96146 (Psychological/neuropsychological testing; automated result only).
As we proposed, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 58997 through 58998), we included these replacement codes in Addenda B and O. As is our usual practice for including new and revised Category I and III CPT codes under the OPPS, we included interim APC assignments and status indicators for these codes and provided an opportunity under the OPPS for the public to comment on these interim assignments. That is, we included comment indicator “NP” to indicate that the code is new for the next calendar year or the code is an existing code with substantial revision to its code descriptor in the next calendar year as compared to current calendar year with a proposed APC assignment, and that comments will be accepted on the proposed APC and status indicator assignments.
While these interim APC and status indicator assignments under the OPPS were included in Addendum B and Addendum O to the CY 2019 OPPS/ASC proposed rule and final rule with comment period, PHP is a part of the OPPS and PHP providers may not have been aware of those changes because we did not also include these in the PHP discussion presented in the proposed rule. To ensure that PHP providers were aware of the new and replacement codes related to CMHC and hospital-based partial hospitalization programs and had the opportunity to comment on the changes, we utilized a practice similar to the one we use under the OPPS for new Level II HCPCS codes that become effective after the proposed rule is published. Therefore, in the CY 2019 OPPS/ASC final rule with comment period, we proposed to delete the same 6 CPT codes listed above from the PHP-allowable code set for CMHC APC 5853 and hospital-based PHP APC 5863, and replace them with 9 new CPT codes as shown in Table 47 of the final rule with comment period, effective January 1, 2019. We solicited public comments on these proposals and indicated that we will consider the public comments we receive in response to the CY 2019 final rule with comment period and seek to finalize our proposed actions in the CY 2020 OPPS/ASC final rule with comment period.
We also refer readers to section III.A.4. of this proposed rule for a detailed discussion of how we include new and revised Category I and III CPT codes for a related calendar year, assign interim APC and status indicator assignments, and allow for public comments on these interim assignments for finalization in the next calendar year final rule with comment period.
IX. Proposed Procedures That Would Be Paid Only as Inpatient Procedures
A. Background
We refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74352 through 74353) for a full historical discussion of our longstanding policies on how we identify procedures that are typically provided only in an inpatient setting (referred to as the inpatient only (IPO) list) and, therefore, will not be paid by Medicare under the OPPS, and on the criteria that we use to review the IPO list each year to determine whether or not any procedures should be removed from the list. The complete list of proposed codes that describe procedures that would be paid by Medicare in CY 2020 as inpatient only procedures is included as Addendum E to this CY 2020 proposed rule, which is available via the internet on the CMS website.
B. Proposed Changes to the Inpatient Only (IPO) List
1. Methodology for Identifying Appropriate Changes to IPO List
In this proposed rule, for CY 2020, we are proposing to use the same methodology (described in the November 15, 2004 final rule with comment period (69 FR 65834)) of reviewing the current list of procedures on the IPO list to identify any procedures that may be removed from the list. We have established five criteria that are part of this methodology. As noted in the CY 2012 OPPS/ASC final rule with comment period (76 FR 74353), we utilize these criteria when reviewing procedures to determine whether or not they should be removed from the IPO list and assigned to an APC group for payment under the OPPS when provided in the hospital outpatient setting. We note that a procedure is not required to meet all of the established criteria to be removed from the IPO list. The criteria include the following:
1. Most outpatient departments are equipped to provide the services to the Medicare population.
2. The simplest procedure described by the code may be performed in most outpatient departments.
3. The procedure is related to codes that we have already removed from the IPO list.
4. A determination is made that the procedure is being performed in numerous hospitals on an outpatient basis.
5. A determination is made that the procedure can be appropriately and safely performed in an ASC and is on the list of approved ASC procedures or has been proposed by us for addition to the ASC list.
2. Procedures Proposed for Removal From the IPO List
Using the above-listed criteria, for the CY 2020 OPPS, we have identified one procedure described by the following code that we are proposing to remove from the IPO list for CY 2020: CPT code 27130 (Arthroplasty, acetabular and proximal femoral prosthetic replacement (total hip arthroplasty) with or without autograft or allograft). The procedure that we are proposing to remove from the IPO list for CY 2020 and subsequent years, including the CPT/HCPCS code, long descriptor, and the proposed CY 2020 payment indicator is displayed in Table 23 of this proposed rule.
For a number of years, total hip arthroplasty (THA) has been a topic of discussion for removal from the IPO list with both stakeholder support and opposition. Most recently, in the CY 2018 OPPS/ASC proposed rule (82 FR 33644 and 33645), we sought public comment on the possible removal of partial hip arthroplasty (PHA), CPT code 27125 (Hemiarthroplasty, hip, partial (eg, femoral stem prosthesis, bipolar arthroplasty)), and total hip arthroplasty (THA) or total hip replacement, CPT code 27130 (Arthroplasty, acetabular and proximal femoral prosthetic replacement (total hip arthroplasty), with or without autograft or allograft from the IPO list. Both THA and PHA were placed on the original IPO list in the CY 2001 OPPS/ASC final rule with comment period (65 FR 18780).
Among those commenters expressing support in response to the CY 2018 OPPS/ASC proposed rule (which we summarized and responded to in the CY 2018 OPPS/ASC final rule with comment period (82 FR 52527 through 52528) for removal of THA from the IPO list were several surgeons and other stakeholders who believed that, given thorough preoperative screening by medical teams with significant experience and expertise involving hip replacement procedures, the THA procedure could be provided on an outpatient basis for some Medicare beneficiaries. These commenters noted significant success involving same day discharge for patients who met the screening criteria and whose experienced medical teams were able to perform the procedure early enough in the day for the patients to achieve postoperative goals, allowing home discharge by the end of the day. The commenters believed that the benefits of providing the THA procedure on an outpatient basis would lead to significant enhancements in patient well-being, improved efficiency, and cost savings to the Medicare program, including shorter hospital stays resulting in fewer medical complications, improved results, and enhanced patient satisfaction.
We stated in the CY 2018 OPPS/ASC proposed rule that, like most surgical procedures, both PHA and THA need to be tailored to the individual patient's needs. Patients with a relatively low anesthesia risk and without significant comorbidities who have family members at home who can assist them may likely be good candidates for an outpatient PHA or THA procedure. These patients may be determined to also be able to tolerate outpatient rehabilitation in either an outpatient facility or at home postsurgery. On the other hand, patients with multiple medical comorbidities, aside from their osteoarthritis, would more likely require inpatient hospitalization and possibly postacute care in a skilled nursing facility or other facility. Surgeons who discussed outpatient PHA and THA procedures in public comments in response to our CY 2017 OPPS/ASC proposed rule (81 FR 45679) comment solicitation (which we summarized and responded to in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79696)) on the TKA procedure emphasized the importance of careful patient selection and strict protocols to optimize outpatient hip replacement outcomes. These protocols typically manage all aspects of the patient's care, including the at-home preoperative and postoperative environment, anesthesia, pain management, and rehabilitation to maximize rapid recovery, ambulation, and performance of activities of daily living.
Numerous commenters representing a variety of stakeholders, including physicians and other care providers, individual stakeholders, specialty societies, hospital associations, hospital systems, ASCs, device manufacturers, and beneficiaries, responded to our solicitation of comments regarding the removal of PHA and THA from the IPO list (which we summarized and responded to in CY 2018 OPPS/ASC final rule with comment period (82 FR 52527 through 52528)). The comments were diverse and some were similar to the comments we received on our proposal to remove TKA from the IPO list. Some commenters, including hospital systems and associations as well as specialty societies and physicians, stated that it would not be clinically appropriate to remove PHA and THA from the IPO list, indicating that the patient safety profile of outpatient THA and PHA in the non-Medicare population is not well-established. Commenters representing orthopedic surgeons also stated that patients requiring a hemiarthroplasty (PHA) for fragility fractures are by nature higher risk, suffer more extensive comorbidities and require closer monitoring and preoperative optimization; therefore, it would not be medically appropriate to remove the PHA procedure from the IPO list.
Other commenters, including ambulatory surgery centers, physicians, and beneficiaries, supported the removal of PHA and THA from the IPO list. These commenters stated that the procedures were appropriate for certain Medicare beneficiaries and most outpatient departments are equipped to provide THA to some Medicare beneficiaries. They also referenced their own personal successful experiences with outpatient THA.
After reviewing the clinical characteristics of the procedure described by CPT code 27130, considering the public comments described earlier from past rules, additional feedback from stakeholders, and with further consultation with our clinical advisors regarding this procedure, we believe that this procedure meets criterion 2 (the simplest procedure described by the code may be performed in most outpatient departments) and criterion 3 (the procedure is related to codes that we have already removed from the IPO list). As such, we believe that appropriately selected patients could have this procedure performed on an outpatient basis. Therefore, we are proposing to remove THA from the IPO list and to assign the THA procedure (CPT code 27130) to C-APC 5115 with status indicator “J1”. We are seeking public comments on our conclusion that the procedure described by CPT code 27130 meets criteria 2 and 3 and our proposal to assign the procedure to C-APC 5115 with status indicator “J1”. At this time, we are not proposing to remove PHA from the IPO list because we continue to believe that it does not meet the criteria for removal.
3. Solicitation of Public Comments on the Potential Removal of Procedures Described by CPT Codes 22633, 22634, 63265, 63266, 63267, 63268 From the IPO List
Throughout the years, we have received several public comments on additional CPT codes that stakeholders believe fit our criteria and should be removed from the IPO list. In this CY 2020 OPPS/ASC proposed rule, we are seeking public comment on the removal of the following procedures from the IPO list in Table 23.

We have reviewed the clinical characteristics of CPT code 22633 and CPT code 22634 and believe that they are related to codes that we have already removed from the IPO list. Specifically, stakeholders have suggested that CPT codes 22633 and 22634 are related to CPT code 22551(Arthrodesis, anterior interbody, including disc space preparation, discectomy, osteophytectomy and decompression of spinal cord and/or nerve roots; cervical below c2), which is currently performed in the outpatient hospital setting. However, after reviewing the current data available on CPT codes 22633 and 22634, we are concerned that the available data do not provide a large enough sampling of outpatient procedures and do not directly address the criteria for removal from the IPO list. At this time, we are seeking public comments that would provide additional information on the safety of performing CPT codes 22633 and 22634 in the outpatient hospital setting.
In addition, we have reviewed CPT codes 63265, 63266, 63267, and 63268. Over the years, stakeholders have indicated that this series of CPT codes should be considered minimally invasive, arguing that CPT codes 63265, 63266, 63267, and 63268 meet criteria one and two for removal from the IPO list: Most outpatient departments are equipped to provide the services to the Medicare population and the simplest procedure described by the code may be performed in most outpatient departments. At this time, we do not believe that there is sufficient information to demonstrate that CPT codes 63265, 63266, 63267, and 63268 meet the IPO list removal criteria. However, we are seeking public comment on whether CPT codes 63265 through 63268 meet criteria to be removed from the IPO list, including information from commenters to demonstrate that the codes meet these criteria.
Table 24 contains the proposed change that we are proposing to make to the IPO list for CY 2020.

X. Proposed Nonrecurring Policy Changes
A. Proposed Changes in the Level of Supervision of Outpatient Therapeutic Services in Hospitals and Critical Access Hospitals (CAHs)
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59390 through 59391) and in the CY 2009 OPPS/ASC proposed rule and final rule with comment period (73 FR 41518 through 41519 and 73 FR 68702 through 68704, respectively), we clarified that direct supervision is required for hospital outpatient therapeutic services covered and paid by Medicare that are furnished in hospitals as well as in provider-based departments (PBDs) of hospitals, as set forth in the CY 2000 OPPS final rule with comment period (65 FR 18525). In the CY 2010 OPPS/ASC final rule with comment period (74 FR 60575 through 60591), we finalized a technical correction to the title and text of the applicable regulation at 42 CFR 410.27 to clarify that this standard applies in critical access hospitals (CAHs) as well as hospitals. In response to concerns expressed by the hospital community, in particular CAHs and small rural hospitals, that they would have difficulty meeting this standard, on March 15, 2010, we instructed all MACs not to evaluate or enforce the supervision requirements for therapeutic services provided to outpatients in CAHs from January 1, 2010 through December 31, 2010, while the agency revisited the supervision policy during the CY 2011 OPPS/ASC rulemaking cycle.
Due to continued concerns expressed by CAHs and small rural hospitals, we extended this notice of nonenforcement (“enforcement instruction”) as an interim measure for CY 2011, and expanded it to apply to small rural hospitals having 100 or fewer beds (75 FR 72007). We continued to consider the issue further in our annual OPPS notice-and-comment rulemaking, and implemented an independent review process in 2012 to obtain advice from the HOP Panel on this matter (76 FR 74360 through 74371). Under this process used since CY 2012, the HOP Panel considers and advises CMS regarding stakeholder requests for changes in the required minimum level of supervision of individual hospital outpatient therapeutic services. In addition, we extended the enforcement instruction through CY 2012 and CY 2013. For the period of CY 2014 through CY 2017, Congress took legislative action (Pub. L. 113-198, Pub. L. 114-112, Pub. L. 114-255, and Pub. L. 115-123) to extend nonenforcement of the direct supervision requirement for hospital outpatient therapeutic services in CAHs and small rural hospitals having 100 or fewer beds through December 31, 2017. Then in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59391), we reinstated the enforcement instruction providing for the nonenforcement of the direct supervision requirement for hospital outpatient therapeutic services in CAHs and small rural hospitals having 100 or fewer beds through December 31, 2019. The current enforcement instruction is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/Supervision-Moratorium-on-Enforcement-for-CAHs-and-Certain-Small-Rural-Hospitals.pdf.
As discussed in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59390 through 59391), stakeholders have consistently requested that CMS continue the nonenforcement of the direct supervision requirement for hospital outpatient therapeutic services for CAHs and small rural hospitals having 100 or fewer beds. Stakeholders stated that some small rural hospitals and CAHs have insufficient staff available to furnish direct supervision. The primary reason stakeholders cited for this request is the difficulty that CAHs and small rural hospitals have in recruiting physicians and nonphysician practitioners to practice in rural areas. These stakeholders noted that it is particularly difficult to furnish direct supervision for critical specialty services, such as radiation oncology services, that cannot be directly supervised by a hospital emergency department physician or nonphysician practitioner because of the volume of emergency patients or lack of specialty expertise. In addition, we are not aware of any supervision-related complaints from beneficiaries or providers regarding quality of care for services furnished during the several years that the enforcement instruction has been in effect.
The upcoming expiration of the latest enforcement instruction providing for the nonenforcement of the direct supervision requirement for hospital outpatient therapeutic services for CAHs and small rural hospitals having 100 or fewer beds has prompted us to consider whether to change the level of supervision for hospital outpatient therapeutic services for all hospitals and CAHs. The enforcement instructions and legislative actions that have been in place since 2010 have created a two-tiered system of physician supervision requirements for hospital outpatient therapeutic services for providers in the Medicare program, with direct supervision required for most hospital outpatient therapeutic services in most hospital providers, but only general supervision required for most hospital outpatient therapeutic services in CAHs and small rural hospitals with fewer than 100 beds.
However, we have not learned of any data or information from CAHs and small rural hospitals indicating that the quality of outpatient therapeutic services has been affected by requiring only general supervision for these services. It is important to remember that the requirement for general supervision for outpatient therapeutic services does not preclude these hospitals from providing direct supervision for outpatient therapeutic services when the physicians administering the medical procedures decide that it is appropriate to do so. Many outpatient therapeutic services involve a level of complexity and risk such that direct supervision would be warranted even though only general supervision is required.
In addition, CAHs and hospitals in general continue to be subject to conditions of participation (CoPs) that complement the general supervision requirements for hospital outpatient therapeutic services to ensure that the medical services Medicare patients receive are properly supervised. The CoPs for hospitals require Medicare patients to be under the care of a physician (42 CFR 482.12(c)(4))), and for the hospital to “have an organized medical staff that operates under bylaws approved by the governing body, and which is responsible for the quality of medical care provided to patients by the hospital” (42 CFR 482.22). The CoPs for CAHs (42 CFR 485.631(b)(1)(i)) require physicians to provide medical direction for the CAHs' health care activities, consultation for, and medical supervision of the health care staff. The physicians' responsibilities in hospitals and CAHs include supervision of all services performed at those facilities. In addition, physicians must also follow State laws regarding scope of practice. Failure of doctors of medicine or osteopathy to provide adequate supervision in accordance with the hospital and CAH CoPs does not cause payment to be denied for that individual service. However, consistent violations of the CoP supervision requirements can lead to a provider having to establish a corrective action plan to address supervision deficiencies, and if the provider still fails to meet the CoP requirements, the hospital or CAH can be terminated from Medicare participation.
Our experience indicates that Medicare providers will provide a similar quality of hospital outpatient therapeutic services, regardless of whether the minimum level of supervision required under the Medicare program is direct or general. We have come to believe that the direct supervision requirement for hospital outpatient therapeutic services places an additional burden on providers that reduces their flexibility to provide medical care. The issues with increased burden and reduced flexibility to provide medical care have a more significant impact on CAHs and small rural hospitals due to their recruiting and staffing challenges, as we have recognized over the years in providing for nonenforcement of the policy for these hospitals. Larger hospitals and hospitals in urban or suburban areas are less affected by the burden and reduced flexibility of the direct supervision requirement. However, given that the direct supervision requirement has not yet been enforced for CAHs and small rural hospitals, we believe it is time to end what is effectively a two-tiered system of supervision levels for hospital outpatient therapeutic services by proposing a policy that sets an appropriate and uniformly enforceable supervision standard for all hospital outpatient therapeutic services.
Therefore, we are proposing to change the generally applicable minimum required level of supervision for hospital outpatient therapeutic services from direct supervision to general supervision for services furnished by all hospitals and CAHs. General supervision, as defined in our regulation at 42 CFR 410.32(b)(3)(i) means that the procedure is furnished under the physician's overall direction and control, but that the physician's presence is not required during the performance of the procedure. This proposal would ensure a standard minimum level of supervision for each hospital outpatient therapeutic service furnished incident to a physician's service in accordance with the statute. We are proposing to amend the existing regulation at § 410.27(a)(1)(iv) to provide that the default minimum level of supervision for each hospital outpatient therapeutic service is “general.”
We will continue to have the HOP Panel provide advice on the appropriate supervision levels for hospital outpatient services as described in section I.E.2. of this proposed rule. We will also retain the ability to consider a change to the supervision level of an individual hospital outpatient therapeutic service to a level of supervision that is more intensive than general supervision through notice and comment rulemaking. We are seeking public comments on this proposal. Additionally, we are seeking public comments on whether specific types of services, such as chemotherapy administration or radiation therapy, should be excepted from this proposal.
B. Short Inpatient Hospital Stays
1. Background on the 2-Midnight Rule
In the FY 2014 IPPS/LTCH PPS final rule (78 FR 50913 through 50954), we clarified our policy regarding when an inpatient admission is considered reasonable and necessary for purposes of Medicare Part A payment. Under this policy, we established a benchmark providing that surgical procedures, diagnostic tests, and other treatments would be generally considered appropriate for inpatient hospital admission and payment under Medicare Part A when the physician expects the patient to require a stay that crosses at least 2 midnights and admits the patient to the hospital based upon that expectation. Conversely, when a beneficiary enters a hospital for a surgical procedure not designated as an inpatient-only (IPO) procedure as described in 42 CFR 419.22(n), a diagnostic test, or any other treatment, and the physician expects to keep the beneficiary in the hospital for only a limited period of time that does not cross 2 midnights, the services would be generally inappropriate for payment under Medicare Part A, regardless of the hour that the beneficiary came to the hospital or whether the beneficiary used a bed. With respect to services designated under the OPPS as IPO procedures, we explained that because of the intrinsic risks, recovery impacts, or complexities associated with such services, these procedures would continue to be appropriate for inpatient hospital admission and payment under Medicare Part A regardless of the expected length of stay. We also indicated that there might be further “rare and unusual” exceptions to the application of the benchmark, which would be detailed in subregulatory guidance.
2. Current Policy for Medical Review of Inpatient Hospital Admissions Under Medicare Part A
In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70538 through 70549), we revised the previous rare and unusual exceptions policy and finalized a proposal to allow for case-by-case exceptions to the 2-midnight benchmark, whereby Medicare Part A payment may be made for inpatient admissions where the admitting physician does not expect the patient to require hospital care spanning 2 midnights, if the documentation in the medical record supports the physician's determination that the patient nonetheless requires inpatient hospital care.
We note that, in the CY 2016 OPPS/ASC final rule with comment period, we reiterated our position that the 2-midnight benchmark provides clear guidance on when a hospital inpatient admission is appropriate for Medicare Part A payment, while respecting the role of physician judgment. We stated that the following criteria will be relevant to determining whether an inpatient admission with an expected length of stay of less than 2 midnights is nonetheless appropriate for Medicare Part A payment:
- Complex medical factors such as history and comorbidities;
- The severity of signs and symptoms;
- Current medical needs; and
- The risk of an adverse event.
In other words, for purposes of Medicare payment, an inpatient admission is payable under Part A if the documentation in the medical record supports either the admitting physician's reasonable expectation that the patient will require hospital care spanning at least 2 midnights, or the physician's determination based on factors such as those identified above that the patient nonetheless requires care on an inpatient basis. The exceptions for procedures on the IPO list and for “rare and unusual” circumstances designated by CMS as national exceptions were unchanged by the CY 2016 OPPS/ASC final rule with comment period.
As we stated in the CY 2016 OPPS/ASC final rule with comment period, the decision to formally admit a patient to the hospital is subject to medical review. For instance, for cases where the medical record does not support a reasonable expectation of the need for hospital care crossing at least 2 midnights, and for inpatient admissions not related to a surgical procedure specified by Medicare as an IPO procedure under 42 CFR 419.22(n) or for which there was not a national exception, payment of the claim under Medicare Part A is subject to the clinical judgment of the medical reviewer. The medical reviewer's clinical judgment involves the synthesis of all submitted medical record information (for example, progress notes, diagnostic findings, medications, nursing notes, and other supporting documentation) to make a medical review determination on whether the clinical requirements in the relevant policy have been met. In addition, Medicare review contractors must abide by CMS' policies in conducting payment determinations, but are permitted to take into account evidence-based guidelines or commercial utilization tools that may aid such a decision. While Medicare review contractors may continue to use commercial screening tools to help evaluate the inpatient admission decision for purposes of payment under Medicare Part A, such tools are not binding on the hospital, CMS, or its review contractors. This type of information also may be appropriately considered by the physician as part of the complex medical judgment that guides their decision to keep a beneficiary in the hospital and formulation of the expected length of stay.
3. Proposed Change for Medical Review of Certain Inpatient Hospital Admissions Under Medicare Part A for CY 2020 and Subsequent Years
As stated earlier in this section, the procedures on the IPO list of procedures under the OPPS are not subject to the 2-midnight benchmark for purposes of inpatient hospital payment. However, the 2-midnight benchmark is applicable once procedures have been removed from the IPO list. Procedures that are removed from the IPO list are also subject to initial medical reviews of claims for short-stay inpatient admissions conducted by Beneficiary and Family-Centered Care Quality Improvement Organizations (BFCC-QIOs).
BFCC-QIOs may also refer providers to the Recovery Audit Contractors (RACs) for further medical review due to exhibiting persistent noncompliance with Medicare payment policies, including, but not limited to:
- Having high denial rates;
- Consistently failing to adhere to the 2-midnight rule; or
- Failing to improve their performance after QIO educational intervention.
As part of our continued effort to facilitate compliance with our payment policy for inpatient admissions, we are proposing to establish a 1-year exemption from certain medical review activities for procedures removed from the IPO list under the OPPS in CY 2020 and subsequent years. Specifically, we are proposing that procedures that have been removed from the IPO list would not be eligible for referral to RACs for noncompliance with the 2-midnight rule within the first calendar year of their removal from the IPO list. These procedures would not be considered by the BFCC-QIOs in determining whether a provider exhibits persistent noncompliance with the 2-midnight rule for purposes of referral to the RAC nor would these procedures be reviewed by RACs for “patient status.” During this 1-year period, BFCC-QIOs would have the opportunity to review such claims in order to provide education for practitioners and providers regarding compliance with the 2-midnight rule, but claims identified as noncompliant would not be denied with respect to the site-of-service under Medicare Part A. Again, information gathered by the BFCC-QIO when reviewing procedures that are newly removed from the IPO list could be used for educational purposes and would not result in a claim denial during the proposed 1-year exemption period.
We believe that a 1-year exemption from BFCC-QIO referral to RACs and RAC “patient status” review of the setting for procedures removed from the IPO list under the OPPS and performed in the inpatient setting would be an adequate amount of time to allow providers to gain experience with application of the 2-midnight rule to these procedures and the documentation necessary for Part A payment for those patients for which the admitting physician determines that the procedures should be furnished in an inpatient setting. Furthermore, we believe that this 1-year exemption from referrals to RACs, RAC patient status review, and claims denials would be sufficient to allow providers time to update their billing systems and gain experience with respect to newly removed procedures eligible to be paid under either the IPPS or the OPPS, while avoiding potential adverse site-of-service determinations. Nonetheless, we are soliciting public comments regarding the appropriate period of time for this proposed exemption. Commenters may indicate whether and why they believe the proposed 1-year period is appropriate, or whether they believe a longer or shorter exemption period would be more appropriate.
In summary, for CY 2020 and subsequent years, we are proposing to establish a 1-year exemption from site-of-service claim denials, BFCC-QIO referrals to RACs, and RAC reviews for “patient status” (that is, site-of-service) for procedures that are removed from the IPO list under the OPPS beginning on January 1, 2020. We encourage BFCC-QIOs to review these cases for medical necessity in order to educate themselves and the provider community on appropriate documentation for Part A payment when the admitting physician determines that it is medically reasonable and necessary to conduct these procedures on an inpatient basis. We note that we will monitor changes in site-of-service to determine whether changes may be necessary to certain CMS Innovation Center models.
C. Method To Control Unnecessary Increases in the Volume of Clinic Visit Services Furnished in Excepted Off-Campus Provider-Based Departments (PBDs)
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59004 through 59014), we adopted a method to control unnecessary increases in the volume of the clinic visit service furnished in excepted off-campus provider-based departments (PBDs) by removing the payment differential that drives the site-of-service decision and, as a result, unnecessarily increases service volume. We refer readers to the CY 2019 OPPS/ASC final rule with comment period for a detailed discussion of the background, legislative provisions, and the changes in payment policies we developed to address increases in the volume of covered OPD services. Below we discuss the specific policy we finalized in the CY 2019 OPPS/ASC final rule with comment period and its application under the OPPS for CY 2020.
For the CY 2019 OPPS, using our authority under section 1833(t)(2)(F) of the Act to adopt a method to control unnecessary increases in the volume of covered outpatient department services, we applied an amount equal to the site-specific Medicare Physician Fee Schedule (PFS) payment rate for nonexcepted items and services furnished by a nonexcepted off-campus PBD (the PFS payment rate) for the clinic visit service, as described by HCPCS code G0463, when provided at an off-campus PBD excepted from section 1833(t)(21) of the Act (departments that bill the modifier “PO” on claim lines). However, we phased in the application of the reduction in payment for the clinic visit service described by HCPCS code G0463 in the excepted provider-based department setting over 2 years. For CY 2019, the payment reduction was transitioned by applying 50 percent of the total reduction in payment that was applied if these departments were paid the site-specific PFS rate for the clinic visit service. The PFS-equivalent rate was 40 percent of the OPPS payment for CY 2019 (that is, 60 percent less than the OPPS rate). We provided for a 2-year phase-in of this policy under which one-half of the total 60-percent payment reduction (a 30-percent reduction) was applied in CY 2019. These departments are paid approximately 70 percent of the OPPS rate (100 percent of the OPPS rate minus the 30-percent payment reduction that is applied in CY 2019) for the clinic visit service in CY 2019.
For CY 2020, the second year of the 2-year phase-in, we stated that we would apply the total reduction in payment that is applied if these departments (departments that bill the modifier “PO” on claims lines) are paid the site-specific PFS rate for the clinic visit service described by HCPCS code G0463. The proposed PFS-equivalent rate for CY 2020 is 40 percent of the proposed OPPS payment (that is, 60 percent less than the proposed OPPS rate) for CY 2020. Under this policy, departments will be paid approximately 40 percent of the OPPS rate (100 percent of the OPPS rate minus the 60-percent payment reduction that is applied in CY 2020) for the clinic visit service in CY 2020.
In addition, as we stated in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59013), for CY 2020, this policy will be implemented in a non-budget neutral manner. The estimated payment impact of this policy is displayed in Column 5 of Table 44—Estimated Impact of the Proposed CY 2020 Changes for the Hospital Outpatient Prospective Payment System in this CY 2020 OPPS/ASC proposed rule. In order to effectively establish a method for controlling the unnecessary growth in the volume of clinic visits furnished by excepted off-campus PBDs that does not simply reallocate expenditures that are unnecessary within the OPPS, we believe that this method must be adopted in a non-budget neutral manner. The impact associated with this policy is further described in section XXVI. of this CY 2020 OPPS/ASC proposed rule.
XI. Proposed CY 2020 OPPS Payment Status and Comment Indicators
A. Proposed CY 2020 OPPS Payment Status Indicator Definitions
Payment status indicators (SIs) that we assign to HCPCS codes and APCs serve an important role in determining payment for services under the OPPS. They indicate whether a service represented by a HCPCS code is payable under the OPPS or another payment system, and also, whether particular OPPS policies apply to the code.
For CY 2020, we are not proposing to make any changes to the definitions of status indicators that were listed in Addendum D1 to the CY 2019 OPPS/ASC final rule with comment period available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1717-P.html?DLPage=1&DLEntries=10&10DLSort=2DLSortDir=descending.
We are requesting public comments on the proposed definitions of the OPPS status indicators for CY 2020. The complete list of the proposed payment status indicators and their definitions that would apply for CY 2020 is displayed in Addendum D1 to this proposed rule, which is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
The proposed CY 2020 payment status indicator assignments for APCs and HCPCS codes are shown in Addendum A and Addendum B, respectively, to this proposed rule, which are available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
B. Proposed CY 2020 Comment Indicator Definitions
In this proposed rule, we are proposing to use four comment indicators for the CY 2020 OPPS. These comment indicators, “CH”, “NC”, “NI”, and “NP”, are in effect for CY 2019 and we are proposing to continue their use in CY 2020. The proposed CY 2020 OPPS comment indicators are as follows:
- “CH”—Active HCPCS code in current and next calendar year, status indicator and/or APC assignment has changed; or active HCPCS code that will be discontinued at the end of the current calendar year.
- “NC”—New code for the next calendar year or existing code with substantial revision to its code descriptor in the next calendar year, as compared to current calendar year for which we requested comments in the proposed rule, final APC assignment; comments will not be accepted on the final APC assignment for the new code.
- “NI”—New code for the next calendar year or existing code with substantial revision to its code descriptor in the next calendar year, as compared to current calendar year, interim APC assignment; comments will be accepted on the interim APC assignment for the new code.
- “NP”—New code for the next calendar year or existing code with substantial revision to its code descriptor in the next calendar year, as compared to current calendar year, proposed APC assignment; comments will be accepted on the proposed APC assignment for the new code.
The definitions of the proposed OPPS comment indicators for CY 2020 are listed in Addendum D2 to this proposed rule, which is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html.
XII. MedPAC Recommendations
The Medicare Payment Advisory Commission (MedPAC) was established under section 1805 of the Act in large part to advise the U.S. Congress on issues affecting the Medicare program. As required under the statute, MedPAC submits reports to the Congress no later than March and June of each year that present its Medicare payment policy recommendations. The March report typically provides discussion of Medicare payment policy across different payment systems and the June report typically discusses selected Medicare issues. We are including this section of the proposed rule to make stakeholders aware of certain MedPAC recommendations for the OPPS and ASC payment systems as discussed in its March 2019 report.
A. OPPS Payment Rates Update
The March 2019 MedPAC “Report to the Congress: Medicare Payment Policy” recommended that Congress update Medicare OPPS payment rates of 2 percent, with the difference between this and the update amount specified in current law to be used to increase payments in a new suggested Medicare quality program, the “Hospital Value Incentive Program (HVIP).” We refer readers to the March 2019 MedPAC report, which is available for download at www.medpac.gov, for a complete discussion on these recommendations. We appreciate MedPAC's recommendations, but as MedPAC acknowledged in its report, Congress would need to change current law to enable us to implement its recommendations.
B. ASC Conversion Factor Update
In the March 2019 MedPAC “Report to the Congress: Medicare Payment Policy” MedPAC found that, based on its analysis of indicators of payment adequacy, the number of Medicare-certified ASCs had increased, beneficiaries' use of ASCs had increased, and ASC access to capital has been adequate.[68] As a result, for CY 2020, MedPAC stated that payments to ASCs are adequate and recommended that no payment update should be given for 2020 (that is, the update factor would be 0 percent).
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59079), we adopted a policy, which we codified at 42 CFR 416.171(a)(2), to apply the hospital market basket update to ASC payment system rates for an interim period of 5 years. We refer the reader to the CY 2019 OPPS/ASC final rule with comment period for complete details regarding our policy to use the hospital market basket update for the ASC payment system. Therefore, consistent with our policy for the ASC payment system, we are proposing to apply a 2.7 percent MFP-adjusted hospital market basket update factor to the CY 2019 ASC conversion factor for ASCs meeting the quality reporting requirements to determine the CY 2020 ASC payment amounts. See section XIII of this proposed rule for a complete explanation of our relevant policies.
C. ASC Cost Data
MedPAC recommended that Congress require ASCs to report cost data to enable the Commission to examine the growth of ASCs' costs over time and analyze Medicare payments relative to the costs of efficient providers, and that CMS could use ASC cost data to examine whether an existing Medicare price index is an appropriate proxy for ASC costs or an ASC specific market basket should be developed. Further, MedPAC suggested that CMS could limit the scope of the cost reporting system to minimize administrative burden on ASCs and the program.[69]
We recognize that the submission of cost data places additional administrative burden on ASCs. We are interested in methods that would mitigate the burden of reporting costs on ASCs while also collecting enough data to reliably use such data in the determination of ASC costs. We are not proposing any cost reporting requirements for ASCs in this CY 2020 OPPS/ASC proposed rule.
The full March 2019 MedPAC report can be downloaded from MedPAC's website at: http://www.medpac.gov/docs/default-source/reports/mar19_medpac_entirereport_sec.pdf.
XIII. Proposed Updates to the Ambulatory Surgical Center (ASC) Payment System
A. Background
1. Legislative History, Statutory Authority, and Prior Rulemaking for the ASC Payment System
For a detailed discussion of the legislative history and statutory authority related to payments to ASCs under Medicare, we refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74377 through 74378) and the June 12, 1998 proposed rule (63 FR 32291 through 32292). For a discussion of prior rulemaking on the ASC payment system, we refer readers to the CYs 2012, 2013, 2014, 2015, 2016, 2017, 2018 and 2019 OPPS/ASC final rules with comment period (76 FR 74378 through 74379; 77 FR 68434 through 68467; 78 FR 75064 through 75090; 79 FR 66915 through 66940; 80 FR 70474 through 70502; 81 FR 79732 through 79753; 82 FR 59401 through 59424; and 83 FR 59028 through 59080, respectively).
2. Policies Governing Changes to the Lists of Codes and Payment Rates for ASC Covered Surgical Procedures and Covered Ancillary Services
Under 42 CFR 416.2 and 416.166 of the Medicare regulations, subject to certain exclusions, covered surgical procedures in an ASC are surgical procedures that are separately paid under the OPPS, that would not be expected to pose a significant risk to beneficiary safety when performed in an ASC, and for which standard medical practice dictates that the beneficiary would not typically be expected to require active medical monitoring and care at midnight following the procedure (“overnight stay”). We adopted this standard for defining which surgical procedures are covered under the ASC payment system as an indicator of the complexity of the procedure and its appropriateness for Medicare payment in ASCs. We use this standard only for purposes of evaluating procedures to determine whether or not they are appropriate to be furnished to Medicare beneficiaries in ASCs. Historically, we have defined surgical procedures as those described by Category I CPT codes in the surgical range from 10000 through 69999 as well as those Category III CPT codes and Level II HCPCS codes that directly crosswalk or are clinically similar to procedures in the CPT surgical range that we have determined do not pose a significant safety risk, that we would not expect to require an overnight stay when performed in ASCs, and that are separately paid under the OPPS (72 FR 42478).
In the August 2, 2007 final rule (72 FR 42495), we also established our policy to make separate ASC payments for the following ancillary items and services when they are provided integral to ASC covered surgical procedures: (1) Brachytherapy sources; (2) certain implantable items that have pass-through payment status under the OPPS; (3) certain items and services that we designate as contractor-priced, including, but not limited to, procurement of corneal tissue; (4) certain drugs and biologicals for which separate payment is allowed under the OPPS; and (5) certain radiology services for which separate payment is allowed under the OPPS. In the CY 2015 OPPS/ASC final rule with comment period (79 FR 66932 through 66934), we expanded the scope of ASC covered ancillary services to include certain diagnostic tests within the medicine range of CPT codes for which separate payment is allowed under the OPPS when they are provided integral to an ASC covered surgical procedure. Covered ancillary services are specified in § 416.164(b) and, as stated previously, are eligible for separate ASC payment. Payment for ancillary items and services that are not paid separately under the ASC payment system is packaged into the ASC payment for the covered surgical procedure.
We update the lists of, and payment rates for, covered surgical procedures and covered ancillary services in ASCs in conjunction with the annual proposed and final rulemaking process to update the OPPS and the ASC payment system (§ 416.173; 72 FR 42535). We base ASC payment and policies for most covered surgical procedures, drugs, biologicals, and certain other covered ancillary services on the OPPS payment policies, and we use quarterly change requests (CRs) to update services covered under the OPPS. We also provide quarterly update CRs for ASC covered surgical procedures and covered ancillary services throughout the year (January, April, July, and October). We release new and revised Level II HCPCS codes and recognize the release of new and revised CPT codes by the AMA and make these codes effective (that is, the codes are recognized on Medicare claims) via these ASC quarterly update CRs. We recognize the release of new and revised Category III CPT codes in the July and January CRs. These updates implement newly created and revised Level II HCPCS and Category III CPT codes for ASC payments and update the payment rates for separately paid drugs and biologicals based on the most recently submitted ASP data. New and revised Category I CPT codes, except vaccine codes, are released only once a year, and are implemented only through the January quarterly CR update. New and revised Category I CPT vaccine codes are released twice a year and are implemented through the January and July quarterly CR updates. We refer readers to Table 41 in the CY 2012 OPPS/ASC proposed rule for an example of how this process, which we finalized in the CY 2012 OPPS/ASC final rule with comment period, is used to update HCPCS and CPT codes (76 FR 42291; 76 FR 74380 through 74384).
In our annual updates to the ASC list of, and payment rates for, covered surgical procedures and covered ancillary services, we undertake a review of excluded surgical procedures (including all procedures newly proposed for removal from the OPPS inpatient list), new codes, and codes with revised descriptors, to identify any that we believe meet the criteria for designation as ASC covered surgical procedures or covered ancillary services. Updating the lists of ASC covered surgical procedures and covered ancillary services, as well as their payment rates, in association with the annual OPPS rulemaking cycle is particularly important because the OPPS relative payment weights and, in some cases, payment rates, are used as the basis for the payment of many covered surgical procedures and covered ancillary services under the revised ASC payment system. This joint update process ensures that the ASC updates occur in a regular, predictable, and timely manner.
3. Definition of ASC Covered Surgical Procedures
Since the implementation of the ASC prospective payment system, we have historically defined a “surgical” procedure under the payment system as any procedure described within the range of Category I CPT codes that the CPT Editorial Panel of the American Medical Association (AMA) defines as “surgery” (CPT codes 10000 through 69999) (72 FR 42478). We also have included as “surgical,” procedures that are described by Level II HCPCS codes or by Category III CPT codes that directly crosswalk or are clinically similar to procedures in the CPT surgical range that we have determined do not pose a significant safety risk, would not expect to require an overnight stay when performed in an ASC, and that are separately paid under the OPPS (72 FR 42478).
As we noted in the CY 2008 final rule that implemented the revised ASC payment system, using this definition of surgery would exclude from ASC payment certain invasive, “surgery-like” procedures, such as cardiac catheterization or certain radiation treatment services that are assigned codes outside the CPT surgical range (72 FR 42477). We stated in that final rule that we believed continuing to rely on the CPT definition of surgery is administratively straightforward, is logically related to the categorization of services by physician experts who both establish the codes and perform the procedures, and is consistent with a policy to allow ASC payment for all outpatient surgical procedures (72 FR 42477).
However, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59029 through 59030), after consideration of public comments received in response to the CY 2019 proposed rule and earlier OPPS/ASC rulemaking cycles, we revised our definition of a surgical procedure under the ASC payment system. We now define a surgical procedure under the ASC payment system as any procedure described within the range of Category I CPT codes that the CPT Editorial Panel of the American Medical Association (AMA) defines as “surgery” (CPT codes 10000 through 69999) (72 FR 42476), as well as procedures that are described by Level II HCPCS codes or by Category I CPT codes or by Category III CPT codes that directly crosswalk or are clinically similar to procedures in the CPT surgical range that we have determined are not expected to pose a significant risk to beneficiary safety when performed in an ASC, for which standard medical practice dictates that the beneficiary would not typically be expected to require an overnight stay following the procedure, and are separately paid under the OPPS.
B. Proposed ASC Treatment of New and Revised Codes
1. Background on Current Process for Recognizing New and Revised HCPCS Codes
Payment for ASC procedures, services, and items are generally based on medical billing codes, specifically, HCPCS codes, that are reported on ASC claims. The HCPCS is divided into two principal subsystems, referred to as Level I and Level II of the HCPCS. Level I is comprised of CPT (Current Procedural Terminology) codes, a numeric and alphanumeric coding system maintained by the American Medical Association (AMA), and includes Category I, II, and III CPT codes. Level II of the HCPCS, which is maintained by CMS, is a standardized coding system that is used primarily to identify products, supplies, and services not included in the CPT codes. Together, Level I and II HCPCS codes are used to report procedures, services, items, and supplies under the ASC payment system. Specifically, we recognize the following codes on ASC claims:
- Category I CPT codes, which describe surgical procedures, diagnostic and therapeutic services, and vaccine codes;
- Category III CPT codes, which describe new and emerging technologies, services, and procedures; and
- Level II HCPCS codes (also known as alphanumeric codes), which are used primarily to identify drugs, devices, supplies, temporary procedures, and services not described by CPT codes.
We finalized a policy in the August 2, 2007 final rule (72 FR 42533 through 42535) to evaluate each year all new and revised Category I and Category III CPT codes and Level II HCPCS codes that describe surgical procedures, and to make preliminary determinations during the annual OPPS/ASC rulemaking process regarding whether or not they meet the criteria for payment in the ASC setting as covered surgical procedures and, if so, whether or not they are office-based procedures. In addition, we identify new and revised codes as ASC covered ancillary services based upon the final payment policies of the revised ASC payment system. In prior rulemakings, we refer to this process as recognizing new codes. However, this process has always involved the recognition of new and revised codes. We consider revised codes to be new when they have substantial revision to their code descriptors that necessitate a change in the current ASC payment indicator. To clarify, we refer to these codes as new and revised in this CY 2020 OPPS/ASC proposed rule.
We have separated our discussion below based on when the codes are released and whether we are proposing to solicit public comments in this proposed rule (and respond to those comments in the CY 2020 OPPS/ASC final rule with comment period) or whether we will be soliciting public comments in the CY 2020 OPPS/ASC final rule with comment period (and responding to those comments in the CY 2021 OPPS/ASC final rule with comment period).
2. April 2019 HCPCS Codes for Which We Are Soliciting Public Comments in This Proposed Rule
For the April 2019 update, there were no new CPT codes, however, there were several new Level II HCPCS codes. In the April 2019 ASC quarterly update (Transmittal 4263, CR 11232, dated March 22, 2019), we added eight new Level II HCPCS codes to the list of covered ancillary services. Table 25 list the new Level II HCPCS codes that were implemented April 1, 2019, along with their proposed payment indicators for CY 2020. The proposed comment indicators, payment indicators and payment rates, where applicable, for these April codes can be found in Addendum BB to this proposed rule. The list of ASC payment indicators and corresponding definitions can be found in Addendum DD1 to this proposed rule. These new codes that are effective April 1, 2019 are assigned to comment indicator “NP” in Addendum BB to this proposed rule to indicate that the codes are assigned to an interim APC assignment and that comments will be accepted on their interim APC assignments. Also, the list of comment indicators and definitions used under the ASC can be found in Addendum DD2 to this proposed rule. We note that ASC Addendum BB, Addendum DD1, and Addendum DD2 are available via the internet on the CMS website.

We are inviting public comments on these proposed payment indicators and payment rates for the new HCPCS codes that were recognized as ASC ancillary services in April 2019 through the quarterly update CRs, as listed in Table 25. We are proposing to finalize their payment indicators in the CY 2020 OPPS/ASC final rule with comment period.
3. July 2019 HCPCS Codes for Which We Are Soliciting Public Comments in This Proposed Rule
In the July 2019 ASC quarterly update (Transmittal 4076, Change Request 10788, dated June 14, 2019), we added several separately payable Category III CPT and Level II HCPCS codes to the list of covered surgical procedures and ancillary services. Table 26 lists the new HCPCS codes that are effective July 1, 2019. The proposed payment indicators and payment rates for these codes can be found in Addendum AA and Addendum BB to this proposed rule. The list of ASC payment indicators and corresponding definitions can be found in Addendum DD1 to this proposed rule. These new codes that are effective July 1, 2019 are assigned to comment indicator “NP” in Addendum BB to this proposed rule to indicate that the codes are assigned to an interim APC assignment and that comments will be accepted on their interim APC assignments. Also, the list of comment indicators and definitions used under the ASC can be found in Addendum DD2 to this proposed rule. We note that ASC Addendum BB, Addendum DD1, and Addendum DD2 are available via the internet on the CMS website.

In addition, through the July 2019 quarterly update CR, we are also implementing an ASC payment for one new Category III CPT code as an ASC covered ancillary service, effective July 1, 2019. This code is listed in Table 27, along with the proposed comment indicator and payment indicator. The CY 2020 proposed payment rate for this new Category III CPT code can be found in Addendum BB. As noted above, the list of payment indicators and comment indicators used under the ASC can be found in Addendum DD1 and DD2, respectively, of this proposed rule. We note that ASC Addendum BB, Addendum DD1, and Addendum DD2 are available via the internet on the CMS website.

We are inviting public comments on these proposed payment indicators for the new Category III CPT code and Level II HCPCS codes newly recognized as ASC covered surgical procedures or covered ancillary services in July 2019 through the quarterly update CRs, as listed in Tables 25, 26, and 27. We are proposing to finalize the payment indicators in the CY 2020 OPPS/ASC final rule with comment period.
4. October 2019 HCPCS Codes for Which We Will Be Soliciting Public Comments in the CY 2020 OPPS/ASC Final Rule With Comment Period
For CY 2020, consistent with our established policy, we are proposing that the Level II HCPCS codes that will be effective October 1, 2019, would be flagged with comment indicator “NI” in Addendum BB to the CY 2020 OPPS/ASC final rule with comment period to indicate that we have assigned the codes an interim ASC payment status for CY 2020. We will invite public comments in the CY 2020 OPPS/ASC final rule with comment period on the interim payment indicators, which would then be finalized in the CY 2021 OPPS/ASC final rule with comment period.
5. January 2020 HCPCS Codes
a. Level II HCPCS Codes for Which We Will Be Soliciting Public Comments in the CY 2020 OPPS/ASC Final Rule With Comment Period
As has been our practice in the past, we incorporate those new Level II HCPCS codes that are effective January 1 in the final rule with comment period, thereby updating the ASC payment system for the calendar year. We note that unlike the CPT codes that are effective January 1 and are included in the OPPS/ASC proposed rules, and except for the G-codes listed in Addendum O to this proposed rule, most Level II HCPCS codes are not released until sometime around November to be effective January 1. Because these codes are not available until November, we are unable to include them in the OPPS/ASC proposed rules. Therefore, these Level II HCPCS codes will be released to the public through the CY 2020 OPPS/ASC final rule with comment period, January 2020 ASC Update CR, and the CMS HCPCS website.
In addition, for CY 2020, we will propose to continue our established policy of assigning comment indicator “NI” in Addendum AA and Addendum BB to the OPPS/ASC final rule with comment period to the new Level II HCPCS codes that will be effective January 1, 2020 to indicate that we are assigning them an interim payment indicator, which is subject to public comment. We will be inviting public comments in the CY 2020 OPPS/ASC final rule with comment period on the payment indicator assignments, which would then be finalized in the CY 2021 OPPS/ASC final rule with comment period.
b. CPT Codes for Which We Will Be Soliciting Public Comments in This Proposed Rule
For new and revised CPT codes effective January 1, 2020 that were received in time to be included in this proposed rule, we are proposing the appropriate payment indicator assignments, and soliciting public comments on the payment assignments. We will accept comments and finalize the payment indicators in the CY 2020 OPPS/ASC final rule with comment period. For those new/revised CPT codes that are received too late for inclusion in this OPPS/ASC proposed rule, we may either make interim final assignments in the final rule with comment period or possibly use HCPCS G-codes that mirror the predecessor CPT codes and retain the current APC and status indicator assignments for a year until we can propose APC and status indicator assignments in the following year's rulemaking cycle.
For the CY 2020 ASC update, the new and revised Category I and III CPT codes that will be effective on January 1, 2020, can be found in ASC Addendum AA and Addendum BB to this proposed rule (which are available via the internet on the CMS website). The CPT codes are assigned to comment indicator “NP” to indicate that the code is new for the next calendar year or the code is an existing code with substantial revision to its code descriptor in the next calendar year as compared to current calendar year and that comments will be accepted on the proposed payment indicator. Further, we remind readers that the CPT code descriptors that appear in Addendum AA and Addendum BB are short descriptors and do not describe the complete procedure, service, or item described by the CPT code. Therefore, we include the 5-digit placeholder codes and their long descriptors for the new and revised CY 2020 CPT codes in Addendum O to this proposed rule (which is available via the internet on the CMS website) so that the public can comment on our proposed payment indicator assignments. The 5-digit placeholder codes can be found in Addendum O to this proposed rule, specifically under the column labeled “CY 2020 OPPS/ASC Proposed Rule 5-Digit Placeholder Code.” The final CPT code numbers will be included in the CY 2020 OPPS/ASC final rule with comment period where possible.
In summary, we are soliciting public comments on the proposed CY 2020 payment indicators for the new and revised Category I and III CPT codes that will be effective January 1, 2020. Because these codes are listed in Addendum AA and Addendum BB with short descriptors only, we are listing them again in Addendum O with the long descriptors. We are also proposing to finalize the payment indicator for these codes (with their final CPT code numbers) in the CY 2020 OPPS/ASC final rule with comment period. The proposed payment indicator and comment indicator for these codes can be found in Addendum AA and Addendum BB to this proposed rule. The list of ASC payment indicators and corresponding definitions can be found in Addendum DD1 to this proposed rule. The new CPT codes that will be effective January 1, 2020 are assigned to comment indicator “NP” in Addendum AA and Addendum BB to this proposed rule to indicate that the codes are assigned to an interim payment indicator and that comments will be accepted on their interim ASC payment assignments. Also, the list of comment indicators and definitions used under the ASC can be found in Addendum DD2 to this proposed rule. We note that ASC Addendum BB, Addendum DD1, and Addendum DD2 are available via the internet on the CMS website.
Finally, in Table 28, we summarize our process for updating codes through our ASC quarterly update CRs, seeking public comments, and finalizing the treatment of these new codes under the ASC.

C. Proposed Update to the List of ASC Covered Surgical Procedures and Covered Ancillary Services
1. Covered Surgical Procedures
a. Covered Surgical Procedures Designated as Office-Based
(1) Background
In the August 2, 2007 ASC final rule, we finalized our policy to designate as “office-based” those procedures that are added to the ASC Covered Procedures List (CPL) in CY 2008 or later years that we determine are performed predominantly (more than 50 percent of the time) in physicians' offices based on consideration of the most recent available volume and utilization data for each individual procedure code and/or, if appropriate, the clinical characteristics, utilization, and volume of related codes. In that rule, we also finalized our policy to exempt all procedures on the CY 2007 ASC list from application of the office-based classification (72 FR 42512). The procedures that were added to the ASC CPL beginning in CY 2008 that we determined were office-based were identified in Addendum AA to that rule by payment indicator “P2” (Office-based surgical procedure added to ASC list in CY 2008 or later with MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight); “P3” (Office-based surgical procedures added to ASC list in CY 2008 or later with MPFS nonfacility PE RVUs; payment based on MPFS nonfacility PE RVUs); or “R2” (Office-based surgical procedure added to ASC list in CY 2008 or later without MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight), depending on whether we estimated the procedure would be paid according to the standard ASC payment methodology based on its OPPS relative payment weight or at the MPFS nonfacility PE RVU-based amount.
Consistent with our final policy to annually review and update the ASC CPL to include all covered surgical procedures eligible for payment in ASCs, each year we identify covered surgical procedures as either temporarily office-based (these are new procedure codes with little or no utilization data that we have determined are clinically similar to other procedures that are permanently office-based), permanently office-based, or nonoffice-based, after taking into account updated volume and utilization data.
(2) Proposed Changes for CY 2020 to Covered Surgical Procedures Designated as Office-Based
In developing this proposed rule, we followed our policy to annually review and update the covered surgical procedures for which ASC payment is made and to identify new procedures that may be appropriate for ASC payment, including their potential designation as office-based. We reviewed CY 2018 volume and utilization data and the clinical characteristics for all covered surgical procedures that are assigned payment indicator “G2” (Nonoffice-based surgical procedure added in CY 2008 or later; payment based on OPPS relative payment weight) in CY 2018, as well as for those procedures assigned one of the temporary office-based payment indicators, specifically “P2”, “P3”, or “R2” in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59039 through 59040).
As we stated in the CY 2019 final rule with comment period (83 FR 59036), the office-based utilization for CPT codes 36902 and 36905 (dialysis vascular access procedures) was greater than 50 percent. However, we did not designate CPT codes 36902 and 36905 as office-based procedures for CY 2019. These codes became effective January 1, 2017 and CY 2017 was the first year we had claims volume and utilization data for CPT codes 36902 and 36905. We shared commenters' concerns that the available data were not adequate to make a determination that these procedures should be office-based, and believed it was premature to assign office-based payment status to those procedures for CY 2019. For CY 2019, CPT codes 36902 and 36905 were assigned payment indicators of “G2”—Non office-based surgical procedure added in CY 2008 or later; payment based on OPPS relative weight.
In reviewing the CY 2018 volume and utilization data for CPT code 36902 we determined that the procedure was performed more than 50 percent of the time in physicians' offices based on 2018 volume and utilization data.
However, the office-based utilization for CPT code 36902 has fallen from 62 percent based on 2017 data to 52 percent based on 2018 data. In addition, there was a sizeable increase in claims for this service in ASCs—from approximately 14,000 in 2017 to 38,000 in 2018. As previously stated in the CY 2019 OPPS/ASC final rule (83 FR 59036), when we believe that the available data for our review process are inadequate to make a determination that a procedure should be office-based, we either make no change to the procedure's payment status or make the change on a temporary basis, and reevaluate our decision when more data become available for our next evaluation. In light of these changes in utilization and due to the high utilization of this procedure in all settings (over 125,000 claims in 2018), we believe it may be premature to assign office-based payment status to CPT code 36902 at this time.
Therefore, for CY 2020, we are not proposing to designate CPT code 36902 as an office-based procedure and continue to assign CPT code 36902 a payment indicator of “G2”—nonoffice-based surgical procedure paid based on OPPS relative weights.
The CY 2018 volume and utilization data for CPT code 36905 show the procedure was not performed more than 50 percent of the time in physicians' offices. Therefore, we are not considering assigning an office-based designation for CPT code 36905 and the procedure will retain its payment indicator of “G2”—non office-based surgical procedure based on OPPS relative weights.
Our review of the CY 2018 volume and utilization data resulted in our identification of 9 other covered surgical procedures that we believe meet the criteria for designation as permanently office-based. The data indicate that these procedures are performed more than 50 percent of the time in physicians' offices, and we believe that the services are of a level of complexity consistent with other procedures performed routinely in physicians' offices. The CPT codes that we are proposing to permanently designate as office-based for CY 2020 are listed in Table 29.

We also reviewed CY 2018 volume and utilization data and other information for 12 procedures designated as temporarily office-based in Tables 57 and 58 in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59039 through 59040). Of these 12 procedures, there were very few claims in our data and no claims data for 11 procedures described by CPT codes 10005, 10007, 10009, 10011, 11102, 11104, 11106, 65785, 67229, 0402T and 0512T. Consequently, we are proposing to maintain the temporary office-based designations for these 11 CPT codes for CY 2020. We list all of those codes for which we proposed to maintain the temporary office-based designations for CY 2020 in Table 30. The procedures for which the proposed office-based designations for CY 2020 are temporary also are indicated by asterisks in Addendum AA to this proposed rule (which is available via the internet on the CMS website).
The volume and utilization data for the one remaining procedure that has a temporary office-based designation for CY 2019, described by CPT code 38222 (Diagnostic bone marrow; biopsy(ies) and aspiration(s)), are sufficient to indicate that this covered surgical procedures was not performed predominantly in physicians' offices and, therefore, we are proposing to assign a nonoffice-based payment indicator—“G2”—to this code for CY 2020.


For CY 2020, we are proposing to designate 7 new CY 2020 CPT codes for ASC covered surgical procedures as temporarily office-based, as displayed in Table 31. After reviewing the clinical characteristics, utilization, and volume of related procedure codes, we determined that the procedures in Table 30 described by the new CPT codes would be predominantly performed in physicians' offices. We believe the procedure described by CPT codes 93X00 (Duplex scan of arterial inflow and venous outflow for preoperative vessel assessment prior to creation of hemodialysis access; complete bilateral study) and 93X01 (Duplex scan of arterial inflow and venous outflow for preoperative vessel assessment prior to creation of hemodialysis access; complete unilateral study) is clinically similar to HCPCS code G0365 (Vessel mapping of vessels for hemodialysis access (services for preoperative vessel mapping prior to creation of hemodialysis access using an autogenous hemodialysis conduit, including arterial inflow and venous outflow)), which is currently on the list of covered surgical procedures and assigned a proposed payment indicator “R2”—Office-based surgical procedure added to ASC list in CY 2008 or later without MPFS nonfacility PE RVUs; payment based on OPPS relative payment weight—for CY 2020. As such, we are proposing to add CPT codes 93X00 and 93X01 in Table 30 to the list of temporarily office-based covered surgical procedures.
Because we have no utilization data for the procedures specifically described by these new CPT codes, we are proposing to make the office-based designation temporary rather than permanent, and we will reevaluate the procedures when data become available. The procedures for which the proposed office-based designation for CY 2020 is temporary are indicated by asterisks in Addendum AA to this proposed rule (which is available via the internet on the CMS website).

b. Proposed ASC Covered Surgical Procedures To Be Designated as Device-Intensive
(1) Background
We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59040 through 59041), for a summary of our existing policies regarding ASC covered surgical procedures that are designated as device-intensive.
(2) Proposed Changes To List of ASC Covered Surgical Procedures Designated as Device-Intensive for CY 2020
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 590401 through 59043), for CY 2019 we modified our criteria for device-intensive procedures to better capture costs for procedures with significant device costs. We adopted a policy to allow procedures that involve surgically inserted or implanted, high-cost, single-use devices to qualify as device-intensive procedures. In addition, we modified our criteria to lower the device offset percentage threshold from 40 percent to 30 percent. Specifically, for CY 2019 and subsequent years, we adopted a policy that device-intensive procedures would be subject to the following criteria:
- All procedures must involve implantable devices assigned a CPT or HCPCS code;
- The required devices (including single-use devices) must be surgically inserted or implanted; and
- The device offset amount must be significant, which is defined as exceeding 30 percent of the procedure's mean cost. Corresponding to this change in the cost criterion we adopted a policy that the default device offset for new codes that describe procedures that involve the implantation of medical devices will be 31 percent beginning in CY 2019. For new codes describing procedures that are payable when furnished in an ASC involving the implantation of a medical device, we adopted a policy that the default device offset would be applied in the same manner as the policy we adopted in section IV.B.2. of the CY 2019 OPPS/ASC final rule with comment period (83 FR 58944 through 58948). We amended § 416.171(b)(2) of the regulations to reflect these new device criteria.
In addition, as also adopted in section IV.B.2. of that final rule with comment period, to further align the device-intensive policy with the criteria used for device pass-through status, we specified, for CY 2019 and subsequent years, that for purposes of satisfying the device-intensive criteria, a device-intensive procedure must involve a device that:
- Has received FDA marketing authorization, has received an FDA investigational device exemption (IDE) and has been classified as a Category B device by the FDA in accordance with 42 CFR 405.203 through 405.207 and 405.211 through 405.215, or meets another appropriate FDA exemption from premarket review;
- Is an integral part of the service furnished;
- Is used for one patient only;
- Comes in contact with human tissue;
- Is surgically implanted or inserted (either permanently or temporarily); and
- Is not any of the following:
(a) Equipment, an instrument, apparatus, implement, or item of this type for which depreciation and financing expenses are recovered as depreciable assets as defined in Chapter 1 of the Medicare Provider Reimbursement Manual (CMS Pub. 15-1); or
(b) A material or supply furnished incident to a service (for example, a suture, customized surgical kit, scalpel, or clip, other than a radiological site marker).
Based on our modified device-intensive criteria, for CY 2020, we are proposing to update the ASC CPL to indicate procedures that are eligible for payment according to our device-intensive procedure payment methodology, based on the proposed individual HCPCS code device-offset percentages using the CY 2018 OPPS claims and cost report data available for this proposed rule.
The ASC covered surgical procedures that we are proposing to designate as device-intensive, and therefore subject to the device-intensive procedure payment methodology for CY 2020, are assigned payment indicator “J8” and are included in ASC Addendum AA to this proposed rule (which is available via the internet on the CMS website). The CPT code, the CPT code short descriptor, and the proposed CY 2020 ASC payment indicator, and an indication of whether the full credit/partial credit (FB/FC) device adjustment policy would apply because the procedure is designated as device-intensive also are included in Addendum AA to this proposed rule (which is available via the internet on the CMS website). In addition, we note that in our CY 2019 OPPS/ASC proposed rule (83 FR 37158 through 37159), we proposed to apply our device-intensive procedure payment methodology to device-intensive procedures under the ASC payment system only when the device-intensive procedure is furnished with a surgically-inserted or implanted device (including single-used medical devices). We inadvertently omitted language finalizing this policy for CY 2019. For CY 2020 and subsequent calendar years, we are proposing to only apply our device-intensive procedure payment methodology to device-intensive procedures under the ASC payment system when the device-intensive procedure is furnished with a surgically inserted or implanted device (including single use medical devices). The payment rate under the ASC payment system for device-intensive procedures furnished without an implantable or inserted medical device would be calculated by applying the uniform ASC conversion factor to both the device portion and service (non-device) portion of the OPPS relative payment weight for the device-intensive procedure and summing both portions (device and service) to establish the ASC payment rate.
c. Adjustment to ASC Payments for No Cost/Full Credit and Partial Credit Devices
Our ASC payment policy for costly devices implanted in ASCs at no cost/full credit or partial credit, as set forth in § 416.179 of our regulations, is consistent with the OPPS policy that was in effect until CY 2014. Specifically, the OPPS policy that was in effect through CY 2013 provided a reduction in OPPS payment by 100 percent of the device offset amount when a hospital furnishes a specified device without cost or with a full credit and by 50 percent of the device offset amount when the hospital receives partial credit in the amount of 50 percent or more of the cost for the specified device (77 FR 68356 through 68358). The established ASC policy reduces payment to ASCs when a specified device is furnished without cost or with full credit or partial credit for the cost of the device for those ASC covered surgical procedures that are assigned to APCs under the OPPS to which this policy applies. We refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68742 through 68744) for a full discussion of the ASC payment adjustment policy for no cost/full credit and partial credit devices.
In the CY 2019 OPPS/ASC proposed rule (83 FR 37159), we noted that, as discussed in section IV.B. of the CY 2014 OPPS/ASC final rule with comment period (78 FR 75005 through 75006), we finalized our proposal to modify our former policy of reducing OPPS payment for specified APCs when a hospital furnishes a specified device without cost or with a full or partial credit. Formerly, under the OPPS, our policy was to reduce OPPS payment by 100 percent of the device offset amount when a hospital furnished a specified device without cost or with a full credit and by 50 percent of the device offset amount when the hospital received partial credit in the amount of 50 percent or more (but less than 100 percent) of the cost for the specified device. For CY 2014, we finalized our proposal to reduce OPPS payment for applicable APCs by the full or partial credit a provider receives for a replaced device, capped at the device offset amount.
Although we finalized our proposal to modify the policy of reducing payments when a hospital furnishes a specified device without cost or with full or partial credit under the OPPS, in that final rule with comment period (78 FR 75076 through 75080), we finalized our proposal to maintain our ASC policy for reducing payments to ASCs for specified device-intensive procedures when the ASC furnishes a device without cost or with full or partial credit. Unlike the OPPS, there is currently no mechanism within the ASC claims processing system for ASCs to submit to CMS the actual credit received when furnishing a specified device at full or partial credit. Therefore, under the ASC payment system, we finalized our proposal for CY 2014 to continue to reduce ASC payments by 100 percent or 50 percent of the device offset amount when an ASC furnishes a device without cost or with full or partial credit, respectively.
All ASC covered device-intensive procedures are subject to the no cost/full credit and partial credit device adjustment policy. Specifically, when a device-intensive procedure is performed to implant a device that is furnished at no cost or with full credit from the manufacturer, the ASC would append the HCPCS “FB” modifier on the line in the claim with the procedure to implant the device. The contractor would reduce payment to the ASC by the device offset amount that we estimate represents the cost of the device when the necessary device is furnished without cost or with full credit to the ASC. We continue to believe that the reduction of ASC payment in these circumstances is necessary to pay appropriately for the covered surgical procedure furnished by the ASC.
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59043 through 59044), for partial credit, we adopted a policy to reduce the payment for a device-intensive procedure for which the ASC receives partial credit by one-half of the device offset amount that would be applied if a device was provided at no cost or with full credit, if the credit to the ASC is 50 percent or more (but less than 100 percent) of the cost of the new device. The ASC will append the HCPCS “FC” modifier to the HCPCS code for the device-intensive surgical procedure when the facility receives a partial credit of 50 percent or more (but less than 100 percent) of the cost of a device. To report that the ASC received a partial credit of 50 percent or more (but less than 100 percent) of the cost of a new device, ASCs have the option of either: (1) Submitting the claim for the device replacement procedure to their Medicare contractor after the procedure's performance, but prior to manufacturer acknowledgment of credit for the device, and subsequently contacting the contractor regarding a claim adjustment, once the credit determination is made; or (2) holding the claim for the device implantation procedure until a determination is made by the manufacturer on the partial credit and submitting the claim with the “FC” modifier appended to the implantation procedure HCPCS code if the partial credit is 50 percent or more (but less than 100 percent) of the cost of the replacement device. Beneficiary coinsurance would be based on the reduced payment amount. As finalized in the CY 2015 OPPS/ASC final rule with comment period (79 FR 66926), to ensure our policy covers any situation involving a device-intensive procedure where an ASC may receive a device at no cost or receive full credit or partial credit for the device, we apply our “FB”/“FC” modifier policy to all device-intensive procedures.
In this proposed rule, we are not proposing any changes to these policies.
d. Proposed Additions to the List of ASC Covered Surgical Procedures
(1) Proposed Additions to the List of ASC Covered Surgical Procedures for CY 2020
As finalized in section XII.A.3. of the CY 2019 OPPS/ASC final rule with comment period (83 FR 59029 through 59030), we revised our definition of “surgery” for CY 2019 to include certain “surgery-like” procedures that are assigned codes outside the CPT surgical range. For CY 2020 and subsequent years we are proposing to adopt the modified definition we finalized for CY 2019, to include procedures that are described by Category I CPT codes that are not in the surgical range but directly crosswalk or are clinically similar to procedures in the Category I CPT code surgical range that we have determined do not pose a significant safety risk, would not be expected to require an overnight stay when performed in an ASC, and are separately paid under the OPPS. We also are proposing to continue to include in our definition of surgical procedures those procedures described by Category I CPT codes in the surgical range from 10000 through 69999 as well as those Category III CPT codes and Level II HCPCS codes that directly crosswalk or are clinically similar to procedures in the CPT surgical range that we have determined do not pose a significant safety risk, that we would not expect to require an overnight stay when performed in ASCs, and that are separately paid under the OPPS.
We conducted a review of HCPCS codes that currently are paid under the OPPS, but not included on the ASC CPL, and that meet our proposed definition of surgery to determine if changes in technology and/or medical practice affected the clinical appropriateness of these procedures for the ASC setting. Based on this review, we are proposing to update the list of ASC covered surgical procedures by adding a mosiacplasty procedure and three coronary intervention procedures to the list for CY 2020, as shown in Table 32. After reviewing the clinical characteristics of these procedures and consulting with stakeholders and our clinical advisors, we determined that these four procedures are separately paid under the OPPS, would not be expected to pose a significant risk to beneficiary safety when performed in an ASC, and would not be expected to require active medical monitoring and care of the beneficiary at midnight following the procedure. Our regulation at 42 CFR 416.166(c) lists general exclusions from the list of ASC covered surgical procedures based primarily on factors relating to safety, including procedures that generally result in extensive blood loss, require major or prolonged invasion of body cavities, or directly involve major blood vessels. We have assessed each of the proposed added procedures against the regulatory safety criteria and believe that these procedures meet each of the criteria. Although the proposed coronary intervention procedures may involve blood vessels that could be considered major, as stated in the August 2, 2007 ASC final rule (72 FR 42481), we believe the involvement of major blood vessels is best considered in the context of the clinical characteristics of individual procedures, and we do not believe that it is logically or clinically consistent to exclude certain cardiac procedures from the list of ASC covered surgical procedures on the basis of the involvement of major blood vessels, yet continue to provide ASC payment for similar procedures involving major blood vessels that have a history of safe performance in ASCs, such as CPT code 36473 (Mechanicochemical destruction of insufficient vein of arm or leg, accessed through the skin using imaging guidance) and CPT code 37223 (Insertion of stents into groin artery, endovascular, accessed through the skin or open procedure). Based on our review of the clinical characteristics of the procedures and their similarity to other procedures that are currently included on the ASC CPL, we believe these procedures can be safely performed in an ASC. Therefore, we are proposing to include these 3 coronary intervention procedures on the list of ASC covered surgical procedures for CY 2020. We are also proposing to add their respective add-on procedures which are packaged under the ASC payment system.
In the CY 2018 OPPS/ASC proposed rule, we solicited public comments on whether the total knee arthroplasty (TKA) procedure, CPT code 27447 (Arthroplasty, knee, condyle and plateau; medial and lateral compartments with or without patella resurfacing (total knee arthroplasty)), should be added to the ASC CPL. In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59411 through 59412) we noted that some commenters argued that many ASCs are equipped to perform these procedures and orthopedic surgeons in ASCs are increasingly performing these procedures safely and effectively on non-Medicare patients and appropriate Medicare patients. However, other commenters noted that the majority of ASCs were not well-equipped to safely perform TKA procedures on patients and that the majority of Medicare patients are not suitable candidates to receive “overnight” joint arthroplasty procedures in an ASC setting. For CY 2018, we did not finalize adding TKA to the ASC covered surgical procedures list, but noted that we would take the suggestions and recommendations into consideration for future rulemaking.
In this CY 2020 OPPS/ASC proposed rule, we continue to promote site-neutrality, where possible, between the hospital outpatient department and ASC settings. Further, we agree with commenters that there is a small subset of Medicare beneficiaries who may be suitable candidates to receive TKA procedures in an ASC setting based on their clinical characteristics. For example, based on Medicare Advantage encounter data, we estimate over 800 TKA procedures were performed in an ASC on Medicare Advantage enrollees in 2016. We believe that beneficiaries not enrolled in an MA plan should also have the option of choosing to receive the TKA procedure in an ASC setting based on their physicians' determinations.
As we stated in the August 2, 2007 final rule (72 FR 42483 through 42484), we exclude procedures that would otherwise pose a significant safety risk to the typical Medicare beneficiary. However, we believe physicians should continue to play an important role in exercising their clinical judgment when making site-of-service determinations, including for TKA. In light of the information commenters submitted in support of adding TKA to the ASC CPL in response to our CY 2018 public comment solicitation, we are proposing to add TKA to the ASC CPL in CY 2020.
We note that TKA procedures were still predominantly performed in the inpatient hospital setting in CY 2018 (82 percent of the time) based on professional claims data, and we are cognizant of the fact that the majority of beneficiaries may not be suitable candidates to receive TKA in an ASC setting. We believe that appropriate limits are necessary to ensure that Medicare Part B payment will only be made for TKA procedures performed in the ASC setting when that setting is clinically appropriate. Therefore, we are soliciting public comment on the appropriate approach to provide safeguards for Medicare beneficiaries who should not receive the TKA procedure in an ASC setting. Specifically, we are soliciting public comment on methods to ensure beneficiaries receive surgical procedures in the ASC setting only as clinically appropriate. For instance, CMS could issue a new modifier that indicates the physician believes that the beneficiary would not be expected to require active medical monitoring and care at midnight following a particular procedure furnished in the ASC setting. CMS could require that such a modifier be included on the claims line for a surgical procedure performed in an ASC. Alternatively, given the importance of post-operative care in making determinations about whether the ASC is an appropriate setting for a procedure, CMS could require that an ASC has a defined plan of care for each beneficiary following a surgical procedure. We could also establish certain requirements for ASCs that choose to perform certain surgical procedures on Medicare patients, such as requiring an ASC to have a certain amount of experience in performing a procedure before being eligible for payment for performing the procedure under Medicare. We are soliciting comment on these options, and other options, for ensuring that beneficiaries receive surgical procedures, including TKA, that do not pose a significant safety risk when performed in an ASC.
In light of the information we received from commenters in support of adding TKA to the ASC-CPL in response to our comment solicitation in the CY 2018 OPPS/ASC proposed rule, we believe TKA would meet our regulatory requirements established under 42 CFR 416.2 and 416.166(b) for covered surgical procedures in the ASC setting. Therefore, we are proposing to add TKA to the ASC CPL as shown in Table 31 below. Based on the public comments we receive, we will consider appropriate safeguards and limitations for surgical procedures furnished in the ASC setting.
As we stated in the CY 2019 OPPS/ASC proposed rule (83 FR 59054 through 59055), section 1833(i)(1) of the Act requires us, in part, to specify, in consultation with appropriate medical organizations, surgical procedures that are appropriately performed on an inpatient basis in a hospital, but can be safely performed in an ASC, and to review and update the ASC covered surgical procedures list at least every 2 years.
We are also soliciting comment on how CMS should think about the role of the ASC-CPL compared to State regulations and market forces in providing payment for certain surgical procedures in an ASC and whether any modifications should be made to the ASC-CPL. Comments on this topic could help formulate the basis for future policy development regarding how we determine what procedures are payable for Medicare fee-for-service beneficiaries in the ASC setting and maintain the balance between safety and access. Finally, we are soliciting comment on how our proposed additions to the list of ASC covered surgical procedures might affect rural hospitals to the extent rural hospitals rely on providing such procedures.
The procedures that we are proposing to add to the ASC list of covered surgical procedures, including the HCPCS code long descriptors and the proposed CY 2020 payment indicators, are displayed in Table 32.

(2) Comment Solicitation on Coronary Intervention Procedures
For CY 2020, as discussed above, we are proposing to add three coronary intervention procedures (along with the codes describing their respective add-on procedures) that involve major blood vessels that we believe can be safely performed in an ASC setting and would not pose a significant safety risk to beneficiaries if performed in an ASC setting. For this CY 2020 OPPS/ASC proposed rule, in addition to the three coronary intervention procedures we are proposing to add to the ASC CPL, we also reviewed several other coronary intervention procedures. While we do not believe the procedures included in Table 33 meet our criteria for inclusion on the ASC CPL at this time, and we are not proposing to add such procedures to the ASC CPL for CY 2020, we are soliciting public comments on whether stakeholders believe they can be safely performed in an ASC setting and to provide any materials supporting their position. In considering whether or not these procedures should be added to the ASC CPL, we are requesting that commenters provide information and data that specifically address the requirements in our regulations at 42 CFR 416.2 and 416.166. For example, commenters should provide information to support their position as to whether each of these procedures would be expected to pose a significant risk to beneficiary safety when performed in an ASC, whether standard medical practice dictates that the beneficiary would typically be expected to require active medical monitoring and care at midnight following the procedure (“overnight stay”), and whether the procedure would fall under our general exclusions for covered surgical procedures at 42 CFR 416.166(c) (for example, would it generally result in extensive blood loss). We will consider public comments we receive in future rulemaking cycles.


2. Covered Ancillary Services
Consistent with the established ASC payment system policy (72 FR 42497), we are proposing to update the ASC list of covered ancillary services to reflect the payment status for the services under the CY 2020 OPPS. Maintaining consistency with the OPPS may result in proposed changes to ASC payment indicators for some covered ancillary services because of changes that are being proposed under the OPPS for CY 2020. For example, if a covered ancillary service was separately paid under the ASC payment system in CY 2019, but is proposed for packaged status under the CY 2020 OPPS, to maintain consistency with the OPPS, we would also propose to package the ancillary service under the ASC payment system for CY 2020. We are proposing to continue this reconciliation of packaged status for subsequent calendar years. Comment indicator “CH”, which is discussed in section XIII.F. of this proposed rule, is used in Addendum BB to this proposed rule (which is available via the internet on the CMS website) to indicate covered ancillary services for which we are proposing a change in the ASC payment indicator to reflect a proposed change in the OPPS treatment of the service for CY 2020.
All ASC covered ancillary services and their proposed payment indicators for CY 2020 are included in Addendum BB to this proposed rule (which is available via the internet on the CMS website).
D. Proposed Update and Payment for ASC Covered Surgical Procedures and Covered Ancillary Services
1. Proposed ASC Payment for Covered Surgical Procedures
a. Background
Our ASC payment policies for covered surgical procedures under the revised ASC payment system are fully described in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66828 through 66831). Under our established policy, we use the ASC standard ratesetting methodology of multiplying the ASC relative payment weight for the procedure by the ASC conversion factor for that same year to calculate the national unadjusted payment rates for procedures with payment indicators “G2” and “A2”. Payment indicator “A2” was developed to identify procedures that were included on the list of ASC covered surgical procedures in CY 2007 and, therefore, were subject to transitional payment prior to CY 2011. Although the 4-year transitional period has ended and payment indicator “A2” is no longer required to identify surgical procedures subject to transitional payment, we retained payment indicator “A2” because it is used to identify procedures that are exempted from the application of the office-based designation.
The rate calculation established for device-intensive procedures (payment indicator “J8”) is structured so only the service portion of the rate is subject to the ASC standard ratesetting methodology. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59028 through 59080), we updated the CY 2018 ASC payment rates for ASC covered surgical procedures with payment indicators of “A2”, “G2”, and “J8” using CY 2017 data, consistent with the CY 2019 OPPS update. We also updated payment rates for device-intensive procedures to incorporate the CY 2019 OPPS device offset percentages calculated under the standard APC ratesetting methodology, as discussed earlier in this section.
Payment rates for office-based procedures (payment indicators “P2”, “P3”, and “R2”) are the lower of the PFS nonfacility PE RVU-based amount or the amount calculated using the ASC standard rate setting methodology for the procedure. In the CY 2018 OPPS/ASC final rule with comment period, we updated the payment amounts for office-based procedures (payment indicators “P2”, “P3”, and “R2”) using the most recent available MPFS and OPPS data. We compared the estimated CY 2018 rate for each of the office-based procedures, calculated according to the ASC standard rate setting methodology, to the PFS nonfacility PE RVU-based amount to determine which was lower and, therefore, would be the CY 2018 payment rate for the procedure under our final policy for the revised ASC payment system (§ 416.171(d)).
In the CY 2014 OPPS/ASC final rule with comment period (78 FR 75081), we finalized our proposal to calculate the CY 2014 payment rates for ASC covered surgical procedures according to our established methodologies, with the exception of device removal procedures. For CY 2014, we finalized a policy to conditionally package payment for device removal procedures under the OPPS. Under the OPPS, a conditionally packaged procedure (status indicators “Q1” and “Q2”) describes a HCPCS code where the payment is packaged when it is provided with a significant procedure but is separately paid when the service appears on the claim without a significant procedure. Because ASC services always include a covered surgical procedure, HCPCS codes that are conditionally packaged under the OPPS are always packaged (payment indicator “N1”) under the ASC payment system. Under the OPPS, device removal procedures are conditionally packaged and, therefore, would be packaged under the ASC payment system. There would be no Medicare payment made when a device removal procedure is performed in an ASC without another surgical procedure included on the claim; therefore, no Medicare payment would be made if a device was removed but not replaced. To ensure that the ASC payment system provides separate payment for surgical procedures that only involve device removal—conditionally packaged in the OPPS (status indicator “Q2”)—we continued to provide separate payment since CY 2014 and assigned the current ASC payment indicators associated with these procedures.
b. Proposed Update to ASC Covered Surgical Procedure Payment Rates for CY 2020
We are proposing to update ASC payment rates for CY 2020 and subsequent years using the established rate calculation methodologies under § 416.171 and using our definition of device-intensive procedures, as discussed in section XII.C.1.b. of this proposed rule. Because the proposed OPPS relative payment weights are generally based on geometric mean costs, the ASC system would generally use geometric means to determine proposed relative payment weights under the ASC standard methodology. We are proposing to continue to use the amount calculated under the ASC standard ratesetting methodology for procedures assigned payment indicators “A2” and “G2”.
We are proposing to calculate payment rates for office-based procedures (payment indicators “P2”, “P3”, and “R2”) and device-intensive procedures (payment indicator “J8”) according to our established policies and, for device-intensive procedures, using our modified definition of device-intensive procedures, as discussed in section XII.C.1.b. of this proposed rule. Therefore, we are proposing to update the payment amount for the service portion of the device-intensive procedures using the ASC standard rate setting methodology and the payment amount for the device portion based on the proposed CY 2020 OPPS device offset percentages that have been calculated using the standard OPPS APC ratesetting methodology. Payment for office-based procedures would be at the lesser of the proposed CY 2020 MPFS nonfacility PE RVU-based amount or the proposed CY 2020 ASC payment amount calculated according to the ASC standard ratesetting methodology.
As we did for CYs 2014 through 2019, for CY 2020, we are proposing to continue our policy for device removal procedures, such that device removal procedures that are conditionally packaged in the OPPS (status indicators “Q1” and “Q2”) would be assigned the current ASC payment indicators associated with these procedures and would continue to be paid separately under the ASC payment system.
c. Proposed Limit on ASC Payment Rates for Low Volume Device-Intensive Procedures
As stated in section XIII.D.1.b. of this proposed rule, the ASC payment system generally uses OPPS geometric mean costs under the standard methodology to determine proposed relative payment weights under the standard ASC ratesetting methodology. However, for low-volume device-intensive procedures, the proposed relative payment weights are based on median costs, rather than geometric mean costs, as discussed in section IV.B.5. of this proposed rule.
While we believe this policy generally helps to provide more appropriate payment for low-volume device intensive procedures, these procedures can still have data anomalies as a result of the limited data available for these procedures in our ratesetting process. For the Level 5 Intraocular APC, which includes only HCPCS code 0308T (insj ocular telescope prosth), based on the CY 2018 claims data available for this proposed rule, the geometric mean cost and median cost under the standard ASC ratesetting methodology is $67,946.51 and $111,019.30, respectively. As described in section IV.B.5. of this proposed rule, a device-intensive procedure that is assigned to a clinical APC with fewer than 100 total claims for all procedures is considered “low-volume” and the cost of the procedure is based on calculations using the APC's median cost instead of the APC's geometric mean cost. Since this APC meets the criteria for low-volume device-intensive procedure designation, the ASC relative weight would be based on the median cost rather than the geometric mean cost. We note that this median cost for this APC is significantly higher than either the OPPS geometric mean cost or median cost based on the OPPS comprehensive ratesetting methodology, which are $28,122.51 and $19,269.55, respectively. This very large difference in cost calculations between these two settings is largely attributable to the APC's low claims volume and to the comprehensive methodology used under the OPPS which is not utilized in ratesetting under the ASC payment system. The cost calculation for this APC under the ASC payment system is primarily based on charges from one hospital with a significantly higher device cost center cost-to-charge ratio and significantly higher charges when compared to other hospitals providing the procedure.
If the ASC payment system were to base the CY 2020 payment rate for HCPCS code 0308T on the median cost of $111,019.30, the ASC payment rate would be several times greater than the OPPS payment rate for HCPCS code 0308T. We note that the median cost under the OPPS ratesetting methodology based on CY 2018 claims data is closer to the historical average for the median cost of HCPCS code 0308T (approximately $19,000). In addition, given that the outpatient hospital setting is generally considered to have higher costs than the ASC setting and that the payment rates for both settings are based on hospital outpatient cost data, we do not believe there should be a scenario where the payment rate for a low-volume device intensive procedure under the ASC payment system is significantly greater than payment under the OPPS.
Therefore, for CY 2020 and subsequent years, we are proposing to limit the ASC payment rate for low-volume device intensive procedure to a payment rate equal to the OPPS payment rate for that procedure. Under this proposal, where the ASC payment rate based on the standard ASC ratesetting methodology for low volume device-intensive procedures would exceed the rate paid under the OPPS for the same procedure, we are proposing to establish an ASC payment rate for such procedures equal to the OPPS payment rate for the same procedure. In this CY 2020 proposed rule, our proposed policy would only affect HCPCS code 0308T, which has very low claims volume (7 claims used for ratesetting in the OPPS). We are proposing to amend 42 CFR 416.171(b) of the regulations to reflect the proposed new limit on ASC payment rates for low-volume device-intensive procedures. CMS' existing regulation at 42 CFR 416.171(b)(2) requires the payment of the device portion of a device-intensive procedure at an amount derived from the payment rate for the equivalent item under the OPPS using our standard ratesetting methodology. We are proposing to add paragraph (b)(4) to § 416.171 to require that, notwithstanding paragraph (b)(2), low volume device-intensive procedures where the otherwise applicable payment rate calculated based on the standard methodology for device-intensive procedures would exceed the payment rate for the same procedure set under the OPPS, the payment rate for the procedure under the ASC payment system would be equal to the payment rate for the same procedure under the OPPS.
Covered surgical procedures and their proposed payment rates for CY 2020 are listed in Addendum AA to this proposed rule (which is available via the internet on the CMS website).
2. Proposed Payment for Covered Ancillary Services
a. Background
Our payment policies under the ASC payment system for covered ancillary services generally vary according to the particular type of service and its payment policy under the OPPS. Our overall policy provides separate ASC payment for certain ancillary items and services integrally related to the provision of ASC covered surgical procedures that are paid separately under the OPPS and provides packaged ASC payment for other ancillary items and services that are packaged or conditionally packaged (status indicators “N”, “Q1”, and “Q2”) under the OPPS. In the CY 2013 OPPS/ASC rulemaking (77 FR 45169 and 77 FR 68457 through 68458), we further clarified our policy regarding the payment indicator assignment of procedures that are conditionally packaged in the OPPS (status indicators “Q1” and “Q2”). Under the OPPS, a conditionally packaged procedure describes a HCPCS code where the payment is packaged when it is provided with a significant procedure but is separately paid when the service appears on the claim without a significant procedure. Because ASC services always include a surgical procedure, HCPCS codes that are conditionally packaged under the OPPS are generally packaged (payment indictor “N1”) under the ASC payment system (except for device removal procedures, as discussed in section IV. of this proposed rule). Thus, our policy generally aligns ASC payment bundles with those under the OPPS (72 FR 42495). In all cases, in order for those ancillary services also to be paid, ancillary items and services must be provided integral to the performance of ASC covered surgical procedures for which the ASC bills Medicare.
Our ASC payment policies generally provide separate payment for drugs and biologicals that are separately paid under the OPPS at the OPPS rates and package payment for drugs and biologicals for which payment is packaged under the OPPS. However, as discussed in section XIII.D.3. of this proposed rule, below, for CY 2019 we finalized a policy to unpackage and pay separately at ASP + 6 percent for the cost of non-opioid pain management drugs that function as surgical supplies when furnished in the ASC setting, even though payment for these drugs continues to be packaged under the OPPS. We generally pay for separately payable radiology services at the lower of the PFS nonfacility PE RVU-based (or technical component) amount or the rate calculated according to the ASC standard ratesetting methodology (72 FR 42497). However, as finalized in the CY 2011 OPPS/ASC final rule with comment period (75 FR 72050), payment indicators for all nuclear medicine procedures (defined as CPT codes in the range of 78000 through 78999) that are designated as radiology services that are paid separately when provided integral to a surgical procedure on the ASC list are set to “Z2” so that payment is made based on the ASC standard ratesetting methodology rather than the MPFS nonfacility PE RVU amount (“Z3”), regardless of which is lower (42 CFR 416.171(d)(1)).
Similarly, we also finalized our policy to set the payment indicator to “Z2” for radiology services that use contrast agents so that payment for these procedures will be based on the OPPS relative payment weight using the ASC standard ratesetting methodology and, therefore, will include the cost for the contrast agent (42 CFR 416.171(d)(2)).
ASC payment policy for brachytherapy sources mirrors the payment policy under the OPPS. ASCs are paid for brachytherapy sources provided integral to ASC covered surgical procedures at prospective rates adopted under the OPPS or, if OPPS rates are unavailable, at contractor-priced rates (72 FR 42499). Since December 31, 2009, ASCs have been paid for brachytherapy sources provided integral to ASC covered surgical procedures at prospective rates adopted under the OPPS.
Our ASC policies also provide separate payment for: (1) Certain items and services that CMS designates as contractor-priced, including, but not limited to, the procurement of corneal tissue; and (2) certain implantable items that have pass-through payment status under the OPPS. These categories do not have prospectively established ASC payment rates according to ASC payment system policies (72 FR 42502 and 42508 through 42509; 42 CFR 416.164(b)). Under the ASC payment system, we have designated corneal tissue acquisition and hepatitis B vaccines as contractor-priced. Corneal tissue acquisition is contractor-priced based on the invoiced costs for acquiring the corneal tissue for transplantation. Hepatitis B vaccines are contractor-priced based on invoiced costs for the vaccine.
Devices that are eligible for pass-through payment under the OPPS are separately paid under the ASC payment system and are contractor-priced. Under the revised ASC payment system (72 FR 42502), payment for the surgical procedure associated with the pass-through device is made according to our standard methodology for the ASC payment system, based on only the service (non-device) portion of the procedure's OPPS relative payment weight if the APC weight for the procedure includes other packaged device costs. We also refer to this methodology as applying a “device offset” to the ASC payment for the associated surgical procedure. This ensures that duplicate payment is not provided for any portion of an implanted device with OPPS pass-through payment status.
In the CY 2015 OPPS/ASC final rule with comment period (79 FR 66933 through 66934), we finalized that, beginning in CY 2015, certain diagnostic tests within the medicine range of CPT codes for which separate payment is allowed under the OPPS are covered ancillary services when they are integral to an ASC covered surgical procedure. We finalized that diagnostic tests within the medicine range of CPT codes include all Category I CPT codes in the medicine range established by CPT, from 90000 to 99999, and Category III CPT codes and Level II HCPCS codes that describe diagnostic tests that crosswalk or are clinically similar to procedures in the medicine range established by CPT. In the CY 2015 OPPS/ASC final rule with comment period, we also finalized our policy to pay for these tests at the lower of the PFS nonfacility PE RVU-based (or technical component) amount or the rate calculated according to the ASC standard ratesetting methodology (79 FR 66933 through 66934). We finalized that the diagnostic tests for which the payment is based on the ASC standard ratesetting methodology be assigned to payment indicator “Z2” and revised the definition of payment indicator “Z2” to include a reference to diagnostic services and those for which the payment is based on the PFS nonfacility PE RVU-based amount be assigned payment indicator “Z3,” and revised the definition of payment indicator “Z3” to include a reference to diagnostic services.
b. Proposed Payment for Covered Ancillary Services for CY 2020
We are proposing to update the ASC payment rates and to make changes to ASC payment indicators, as necessary, to maintain consistency between the OPPS and ASC payment system regarding the packaged or separately payable status of services and the proposed CY 2020 OPPS and ASC payment rates and subsequent year payment rates. We also are proposing to continue to set the CY 2020 ASC payment rates and subsequent year payment rates for brachytherapy sources and separately payable drugs and biologicals equal to the OPPS payment rates for CY 2020 and subsequent year payment rates.
We note that stakeholders requested that we propose to add CPT code 91040 (Esophageal balloon distension study, diagnostic, with provocation when performed) to the ASC Covered Procedures List (CPL) and ASC list of covered ancillary services as it is integral to the performance of covered surgical procedures such as CPT code 43235 (Esophagogastroduodenoscopy, flexible, transoral; diagnostic, including collection of specimen(s) by brushing or washing, when performed (separate procedure)) and 43239 (Esophagogastroduodenoscopy, flexible, transoral; with biopsy, single or multiple). Based on available data and other information related to CPT code 91040, we do not believe this diagnostic test is integral to the covered surgical procedures of CPT codes 43235 or 43239. Therefore, we are not proposing to add CPT code 91040 as a covered ancillary service.
Covered ancillary services and their proposed payment indicators for CY 2020 are listed in Addendum BB to this proposed rule (which is available via the internet on the CMS website). For those covered ancillary services where the payment rate is the lower of the proposed rates under the ASC standard rate setting methodology and the PFS proposed rates, the proposed payment indicators and rates set forth in this proposed rule are based on a comparison using the proposed PFS rates effective January 1, 2020. For a discussion of the PFS rates, we refer readers to the CY 2020 PFS proposed rule, which will be available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
3. Proposed CY 2020 ASC Packaging Policy for Non-Opioid Pain Management Treatments
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59066 through 59072), we finalized the policy to unpackage and pay separately at ASP+6 percent for the cost of non-opioid pain management drugs that function as surgical supplies when they are furnished in the ASC setting for CY 2019. We also finalized conforming changes to 42 CFR 416.164(a)(4) to exclude non-opioid pain management drugs that function as a supply when used in a surgical procedure from our policy to package payment for drugs and biologicals for which separate payment is not allowed under the OPPS into the ASC payment for the covered surgical procedure. We added a new 42 CFR 416.164(b)(6) to include non-opioid pain management drugs that function as a supply when used in a surgical procedure as covered ancillary services that are integral to a covered surgical procedure. Finally, we finalized a change to 42 CFR 416.171(b)(1) to exclude non-opioid pain management drugs that function as a supply when used in a surgical procedure from our policy to pay for ASC covered ancillary services an amount derived from the payment rate for the equivalent item or service set under the OPPS.
In that final rule with comment period, we noted that we will continue to analyze the issue of access to non-opioid alternatives in the OPPS and ASC settings as we implement section 6082 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT for Patients and Communities Act) (Pub. L. 115-271), enacted on October 24, 2018. We also discussed our policy to unpackage and pay separately at ASP + 6 percent for the cost of non-opioid pain management drugs that function as surgical supplies when furnished in the ASC setting in section II.A.3.b. of the CY 2019 OPPS/ASC final rule with comment period (83 FR 58854 through 58860). As required under Section 6082(b) of the SUPPORT Act, we will continue to review and revise ASC payments for non-opioid alternatives for pain management, as appropriate. For more information on our implementation of section 6082 of the SUPPORT for Patients and Communities Act and related proposals, we refer readers to section II.A.3.b. of this proposed rule.
E. New Technology Intraocular Lenses (NTIOLs)
New Technology Intraocular Lenses (NTIOLs) are intraocular lenses that replace a patient's natural lens that has been removed in cataract surgery and that also meet the requirements listed in 42 CFR 416.195.
1. NTIOL Application Cycle
Our process for reviewing applications to establish new classes of NTIOLs is as follows:
- Applicants submit their NTIOL requests for review to CMS by the annual deadline. For a request to be considered complete, we require submission of the information that is found in the guidance document entitled “Application Process and Information Requirements for Requests for a New Class of New Technology Intraocular Lenses (NTIOLs) or Inclusion of an IOL in an Existing NTIOL Class” posted on the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/NTIOLs.html.
- We announce annually, in the proposed rule updating the ASC and OPPS payment rates for the following calendar year, a list of all requests to establish new NTIOL classes accepted for review during the calendar year in which the proposal is published. In accordance with section 141(b)(3) of Public Law 103-432 and our regulations at 42 CFR 416.185(b), the deadline for receipt of public comments is 30 days following publication of the list of requests in the proposed rule.
- In the final rule updating the ASC and OPPS payment rates for the following calendar year, we—
++ Provide a list of determinations made as a result of our review of all new NTIOL class requests and public comments;
++ When a new NTIOL class is created, identify the predominant characteristic of NTIOLs in that class that sets them apart from other IOLs (including those previously approved as members of other expired or active NTIOL classes) and that is associated with an improved clinical outcome.
++ Set the date of implementation of a payment adjustment in the case of approval of an IOL as a member of a new NTIOL class prospectively as of 30 days after publication of the ASC payment update final rule, consistent with the statutory requirement.
++ Announce the deadline for submitting requests for review of an application for a new NTIOL class for the following calendar year.
2. Requests To Establish New NTIOL Classes for CY 2020
We did not receive any requests for review to establish a new NTIOL class for CY 2020 by March 1, 2019, the due date published in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59072).
3. Payment Adjustment
The current payment adjustment for a 5-year period from the implementation date of a new NTIOL class is $50 per lens. Since implementation of the process for adjustment of payment amounts for NTIOLs in 1999, we have not revised the payment adjustment amount, and we are not proposing to revise the payment adjustment amount for CY 2020.
F. Proposed ASC Payment and Comment Indicators
1. Background
In addition to the payment indicators that we introduced in the August 2, 2007 final rule, we created final comment indicators for the ASC payment system in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66855). We created Addendum DD1 to define ASC payment indicators that we use in Addenda AA and BB to provide payment information regarding covered surgical procedures and covered ancillary services, respectively, under the revised ASC payment system. The ASC payment indicators in Addendum DD1 are intended to capture policy-relevant characteristics of HCPCS codes that may receive packaged or separate payment in ASCs, such as whether they were on the ASC CPL prior to CY 2008; payment designation, such as device-intensive or office-based, and the corresponding ASC payment methodology; and their classification as separately payable ancillary services, including radiology services, brachytherapy sources, OPPS pass-through devices, corneal tissue acquisition services, drugs or biologicals, or NTIOLs.
We also created Addendum DD2 that lists the ASC comment indicators. The ASC comment indicators included in Addenda AA and BB to the proposed rules and final rules with comment period serve to identify, for the revised ASC payment system, the status of a specific HCPCS code and its payment indicator with respect to the timeframe when comments will be accepted. The comment indicator “NI” is used in the OPPS/ASC final rule to indicate new codes for the next calendar year for which the interim payment indicator assigned is subject to comment. The comment indicator “NI” also is assigned to existing codes with substantial revisions to their descriptors such that we consider them to be describing new services, and the interim payment indicator assigned is subject to comment, as discussed in the CY2010 OPPS/ASC final rule with comment period (74 FR 60622).
The comment indicator “NP” is used in the OPPS/ASC proposed rule to indicate new codes for the next calendar year for which the proposed payment indicator assigned is subject to comment. The comment indicator “NP” also is assigned to existing codes with substantial revisions to their descriptors, such that we consider them to be describing new services, and the proposed payment indicator assigned is subject to comment, as discussed in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70497).
The “CH” comment indicator is used in Addenda AA and BB to this proposed rule (which are available via the internet on the CMS website) to indicate that the payment indicator assignment has changed for an active HCPCS code in the current year and the next calendar year, for example if an active HCPCS code is newly recognized as payable in ASCs; or an active HCPCS code is discontinued at the end of the current calendar year. The “CH” comment indicators that are published in the final rule with comment period are provided to alert readers that a change has been made from one calendar year to the next, but do not indicate that the change is subject to comment.
2. Proposed ASC Payment and Comment Indicators for CY 2020
For CY 2020, there are proposed new and revised Category I and III CPT codes as well as new and revised Level II HCPCS codes. Therefore, proposed Category I and III CPT codes that are new and revised for CY 2019 and any new and existing Level II HCPCS codes with substantial revisions to the code descriptors for CY 2020 compared to the CY 2019 descriptors that are included in ASC Addenda AA and BB to this proposed rule are labeled with proposed comment indicator “NP” to indicate that these CPT and Level II HCPCS codes are open for comment as part of this proposed rule. Proposed comment indicator “NP” means a new code for the next calendar year or an existing code with substantial revision to its code descriptor in the next calendar year, as compared to current calendar year; and denotes that comments will be accepted on the proposed ASC payment indicator for the new code.
We will respond to public comments on ASC payment and comment indicators and finalize their ASC assignment in the CY 2020 OPPS/ASC final rule with comment period. We refer readers to Addenda DD1 and DD2 to this proposed rule (which are available via the internet on the CMS website) for the complete list of ASC payment and comment indicators proposed for the CY 2020 update.
G. Proposed Calculation of the ASC Payment Rates and the ASC Conversion Factor
1. Background
In the August 2, 2007 final rule (72 FR 42493), we established our policy to base ASC relative payment weights and payment rates under the revised ASC payment system on APC groups and the OPPS relative payment weights. Consistent with that policy and the requirement at section 1833(i)(2)(D)(ii) of the Act that the revised payment system be implemented so that it would be budget neutral, the initial ASC conversion factor (CY 2008) was calculated so that estimated total Medicare payments under the revised ASC payment system in the first year would be budget neutral to estimated total Medicare payments under the prior (CY 2007) ASC payment system (the ASC conversion factor is multiplied by the relative payment weights calculated for many ASC services in order to establish payment rates). That is, application of the ASC conversion factor was designed to result in aggregate Medicare expenditures under the revised ASC payment system in CY 2008 being equal to aggregate Medicare expenditures that would have occurred in CY 2008 in the absence of the revised system, taking into consideration the cap on ASC payments in CY 2007, as required under section 1833(i)(2)(E) of the Act (72 FR 42522). We adopted a policy to make the system budget neutral in subsequent calendar years (72 FR 42532 through 42533; 42 CFR 416.171(e)).
We note that we consider the term “expenditures” in the context of the budget neutrality requirement under section 1833(i)(2)(D)(ii) of the Act to mean expenditures from the Medicare Part B Trust Fund. We do not consider expenditures to include beneficiary coinsurance and copayments. This distinction was important for the CY 2008 ASC budget neutrality model that considered payments across the OPPS, ASC, and MPFS payment systems. However, because coinsurance is almost always 20 percent for ASC services, this interpretation of expenditures has minimal impact for subsequent budget neutrality adjustments calculated within the revised ASC payment system.
In the CY 2008 OPPS/ASC final rule with comment period (72 FR 66857 through 66858), we set out a step-by-step illustration of the final budget neutrality adjustment calculation based on the methodology finalized in the August 2, 2007 final rule (72 FR 42521 through 42531) and as applied to updated data available for the CY 2008 OPPS/ASC final rule with comment period. The application of that methodology to the data available for the CY 2008 OPPS/ASC final rule with comment period resulted in a budget neutrality adjustment of 0.65.
For CY 2008, we adopted the OPPS relative payment weights as the ASC relative payment weights for most services and, consistent with the final policy, we calculated the CY 2008 ASC payment rates by multiplying the ASC relative payment weights by the final CY 2008 ASC conversion factor of $41.401. For covered office-based surgical procedures, covered ancillary radiology services (excluding covered ancillary radiology services involving certain nuclear medicine procedures or involving the use of contrast agents, as discussed in section XII.D.2. of this proposed rule), and certain diagnostic tests within the medicine range that are covered ancillary services, the established policy is to set the payment rate at the lower of the MPFS unadjusted nonfacility PE RVU-based amount or the amount calculated using the ASC standard ratesetting methodology. Further, as discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66841 through 66843), we also adopted alternative ratesetting methodologies for specific types of services (for example, device-intensive procedures).
As discussed in the August 2, 2007 final rule (72 FR 42517 through 42518) and as codified at § 416.172(c) of the regulations, the revised ASC payment system accounts for geographic wage variation when calculating individual ASC payments by applying the pre-floor and pre-reclassified IPPS hospital wage indexes to the labor-related share, which is 50 percent of the ASC payment amount based on a GAO report of ASC costs using 2004 survey data. Beginning in CY 2008, CMS accounted for geographic wage variation in labor costs when calculating individual ASC payments by applying the pre-floor and pre-reclassified hospital wage index values that CMS calculates for payment under the IPPS, using updated Core Based Statistical Areas (CBSAs) issued by OMB in June 2003.
The reclassification provision in section 1886(d)(10) of the Act is specific to hospitals. We believe that using the most recently available pre-floor and pre-reclassified IPPS hospital wage indexes results in the most appropriate adjustment to the labor portion of ASC costs. We continue to believe that the unadjusted hospital wage indexes, which are updated yearly and are used by many other Medicare payment systems, appropriately account for geographic variation in labor costs for ASCs. Therefore, the wage index for an ASC is the pre-floor and pre-reclassified hospital wage index under the IPPS of the CBSA that maps to the CBSA where the ASC is located.
Generally, OMB issues major revisions to statistical areas every 10 years, based on the results of the decennial census. On February 28, 2013, OMB issued OMB Bulletin No. 13-01, which provides the delineations of all Metropolitan Statistical Areas, Metropolitan Divisions, Micropolitan Statistical Areas, Combined Statistical Areas, and New England City and Town Areas in the United States and Puerto Rico based on the standards published on June 28, 2010 in the Federal Register (75 FR 37246 through 37252) and 2010 Census Bureau data. (A copy of this bulletin may be obtained at: https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2013/b13-01.pdf). In the FY 2015 IPPS/LTCH PPS final rule (79 FR 49951 through 49963), we implemented the use of the CBSA delineations issued by OMB in OMB Bulletin 13-01 for the IPPS hospital wage index beginning in FY 2015.
OMB occasionally issues minor updates and revisions to statistical areas in the years between the decennial censuses. On July 15, 2015, OMB issued OMB Bulletin No. 15-01, which provides updates to and supersedes OMB Bulletin No. 13-01 that was issued on February 28, 2013. OMB Bulletin No. 15-01 made changes that are relevant to the IPPS and ASC wage index. We refer readers to the CY 2017 OPPS/ASC final rule with comment period (81 FR 79750) for a discussion of these changes and our implementation of these revisions. (A copy of this bulletin may be obtained at: https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2015/15-01.pdf).
On August 15, 2017, OMB issued OMB Bulletin No. 17-01, which provided updates to and superseded OMB Bulletin No. 15-01 that was issued on July 15, 2015. We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 58864 through 58865) for a discussion of these changes and our implementation of these revisions. (A copy of this bulletin may be obtained at: https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2017/b-17-01.pdf).
For CY 2020, the proposed CY 2020 ASC wage indexes fully reflect the OMB labor market area delineations (including the revisions to the OMB labor market delineations discussed above, as set forth in OMB Bulletin Nos. 15-01 and 17-01).
We note that, in certain instances, there might be urban or rural areas for which there is no IPPS hospital that has wage index data that could be used to set the wage index for that area. For these areas, our policy has been to use the average of the wage indexes for CBSAs (or metropolitan divisions as applicable) that are contiguous to the area that has no wage index (where “contiguous” is defined as sharing a border). For example, for CY 2014, we applied a proxy wage index based on this methodology to ASCs located in CBSA 25980 (Hinesville-Fort Stewart, GA) and CBSA 08 (Rural Delaware).
When all of the areas contiguous to the urban CBSA of interest are rural and there is no IPPS hospital that has wage index data that could be used to set the wage index for that area, we determine the ASC wage index by calculating the average of all wage indexes for urban areas in the State (75 FR 72058 through 72059). (In other situations, where there are no IPPS hospitals located in a relevant labor market area, we continue our current policy of calculating an urban or rural area's wage index by calculating the average of the wage indexes for CBSAs (or metropolitan divisions where applicable) that are contiguous to the area with no wage index.)
2. Proposed Calculation of the ASC Payment Rates
a. Updating the ASC Relative Payment Weights for CY 2020 and Future Years
We update the ASC relative payment weights each year using the national OPPS relative payment weights (and PFS nonfacility PE RVU-based amounts, as applicable) for that same calendar year and uniformly scale the ASC relative payment weights for each update year to make them budget neutral (72 FR 42533). Consistent with our established policy, we are proposing to scale the CY 2020 relative payment weights for ASCs according to the following method. Holding ASC utilization, the ASC conversion factor, and the mix of services constant from CY 2018, we are proposing to compare the total payment using the CY 2019 ASC relative payment weights with the total payment using the CY 2020 ASC relative payment weights to take into account the changes in the OPPS relative payment weights between CY 2019 and CY 2020. We are proposing to use the ratio of CY 2019 to CY 2020 total payments (the weight scalar) to scale the ASC relative payment weights for CY 2020. The proposed CY 2020 ASC weight scalar is 0.8452 and scaling would apply to the ASC relative payment weights of the covered surgical procedures, covered ancillary radiology services, and certain diagnostic tests within the medicine range of CPT codes, which are covered ancillary services for which the ASC payment rates are based on OPPS relative payment weights.
Scaling would not apply in the case of ASC payment for separately payable covered ancillary services that have a predetermined national payment amount (that is, their national ASC payment amounts are not based on OPPS relative payment weights), such as drugs and biologicals that are separately paid or services that are contractor-priced or paid at reasonable cost in ASCs. Any service with a predetermined national payment amount would be included in the ASC budget neutrality comparison, but scaling of the ASC relative payment weights would not apply to those services. The ASC payment weights for those services without predetermined national payment amounts (that is, those services with national payment amounts that would be based on OPPS relative payment weights) would be scaled to eliminate any difference in the total payment between the current year and the update year.
For any given year's ratesetting, we typically use the most recent full calendar year of claims data to model budget neutrality adjustments. At the time of this proposed rule, we had available 98 percent of CY 2018 ASC claims data.
To create an analytic file to support calculation of the weight scalar and budget neutrality adjustment for the wage index (discussed below), we summarized available CY 2017 ASC claims by ASC and by HCPCS code. We used the National Provider Identifier for the purpose of identifying unique ASCs within the CY 2018 claims data. We used the supplier zip code reported on the claim to associate State, county, and CBSA with each ASC. This file, available to the public as a supporting data file for this proposed rule, is posted on the CMS website at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/ASCPaymentSystem.html.
b. Updating the ASC Conversion Factor
Under the OPPS, we typically apply a budget neutrality adjustment for provider level changes, most notably a change in the wage index values for the upcoming year, to the conversion factor. Consistent with our final ASC payment policy, for the CY 2017 ASC payment system and subsequent years, in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79751 through 79753), we finalized our policy to calculate and apply a budget neutrality adjustment to the ASC conversion factor for supplier level changes in wage index values for the upcoming year, just as the OPPS wage index budget neutrality adjustment is calculated and applied to the OPPS conversion factor. For CY 2020, we calculated the proposed adjustment for the ASC payment system by using the most recent CY 2018 claims data available and estimating the difference in total payment that would be created by introducing the proposed CY 2020 ASC wage indexes. Specifically, holding CY 2018 ASC utilization, service-mix, and the proposed CY 2020 national payment rates after application of the weight scalar constant, we calculated the total adjusted payment using the CY 2019 ASC wage indexes and the total adjusted payment using the proposed CY 2020 ASC wage indexes. We used the 50-percent labor-related share for both total adjusted payment calculations. We then compared the total adjusted payment calculated with the CY 2019 ASC wage indexes to the total adjusted payment calculated with the proposed CY 2020 ASC wage indexes and applied the resulting ratio of 1.0008 (the proposed CY 2020 ASC wage index budget neutrality adjustment) to the CY 2019 ASC conversion factor to calculate the proposed CY 2020 ASC conversion factor.
Section 1833(i)(2)(C)(i) of the Act requires that, if the Secretary has not updated amounts established under the revised ASC payment system in a calendar year, the payment amounts shall be increased by the percentage increase in the Consumer Price Index for all urban consumers (CPI-U), U.S. city average, as estimated by the Secretary for the 12-month period ending with the midpoint of the year involved. The statute does not mandate the adoption of any particular update mechanism, but it requires the payment amounts to be increased by the CPI-U in the absence of any update. Because the Secretary updates the ASC payment amounts annually, we adopted a policy, which we codified at 42 CFR 416.171(a)(2)(ii)), to update the ASC conversion factor using the CPI-U for CY 2010 and subsequent calendar years.
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59075 through 59080), we finalized our proposal to apply the hospital market basket update to ASC payment system rates for an interim period of 5 years (CY 2019 through CY 2023), during which we will assess whether there is a migration of the performance of procedures from the hospital setting to the ASC setting as a result of the use of a hospital market basket update, as well as whether there are any unintended consequences, such as less than expected migration of the performance of procedures from the hospital setting to the ASC setting. In addition, we finalized our proposal to revise our regulations under 42 CFR 416.171(a)(2), which address the annual update to the ASC conversion factor. During this 5-year period, we intend to assess the feasibility of collaborating with stakeholders to collect ASC cost data in a minimally burdensome manner and could propose a plan to collect such information. We refer readers to that final rule for a detailed discussion of the rationale for these policies.
For this proposed rule, the hospital market basket update for CY 2020 is projected to be 3.2 percent, as published in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19402), based on IHS Global Inc.'s (IGI's) 2018 fourth quarter forecast with historical data through the third quarter of 2018.
We finalized the methodology for calculating the MFP adjustment in the CY 2011 PFS final rule with comment period (75 FR 73394 through 73396) and revised it in the CY 2012 PFS final rule with comment period (76 FR 73300 through 73301) and the CY 2016 OPPS/ASC final rule with comment period (80 FR 70500 through 70501). For this proposed rule, the proposed MFP adjustment for CY 2020 is projected to be 0.5 percentage point, as published in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19402) based on IGI's 2018 fourth quarter forecast.
For CY 2020, we are proposing to utilize the hospital market basket update of 3.2 percent minus the MFP adjustment of 0.5 percentage point, resulting in an MFP-adjusted hospital market basket update factor of 2.7 percent for ASCs meeting the quality reporting requirements. Therefore, we are proposing to apply a 2.7 percent MFP-adjusted hospital market basket update factor to the CY 2019 ASC conversion factor for ASCs meeting the quality reporting requirements to determine the CY 2020 ASC payment amounts. The ASCQR Program affected payment rates beginning in CY 2014 and, under this program, there is a 2.0 percentage point reduction to the update factor for ASCs that fail to meet the ASCQR Program requirements. We refer readers to section XIV.E. of the CY 2019 OPPS/ASC final rule with comment period (83 FR 59138 through 59139) and section XIV.E. of this proposed rule for a detailed discussion of our policies regarding payment reduction for ASCs that fail to meet ASCQR Program requirements. We are proposing to utilize the hospital market basket update of 3.2 percent reduced by 2.0 percentage points for ASCs that do not meet the quality reporting requirements and then subtract the 0.5 percentage point MFP adjustment. Therefore, we are proposing to apply a 0.7 percent MFP-adjusted hospital market basket update factor to the CY 2019 ASC conversion factor for ASCs not meeting the quality reporting requirements. We also are proposing that if more recent data are subsequently available (for example, a more recent estimate of the hospital market basket update and MFP), we would use such data, if appropriate, to determine the CY 2020 ASC update for the final rule with comment period.
For CY 2020, we are proposing to adjust the CY 2019 ASC conversion factor ($46.532) by the proposed wage index budget neutrality factor of 1.0008 in addition to the MFP-adjusted hospital market basket update factor of 2.7 percent discussed above, which results in a proposed CY 2020 ASC conversion factor of $47.827 for ASCs meeting the quality reporting requirements. For ASCs not meeting the quality reporting requirements, we are proposing to adjust the CY 2019 ASC conversion factor ($46.532) by the proposed wage index budget neutrality factor of 1.0008 in addition to the quality reporting/MFP-adjusted hospital market basket update factor of 0.7 percent discussed above, which results in a proposed CY 2020 ASC conversion factor of $46.895.
3. Display of Proposed CY 2020 ASC Payment Rates
Addenda AA and BB to this proposed rule (which are available on the CMS website) display the proposed updated ASC payment rates for CY 2020 for covered surgical procedures and covered ancillary services, respectively. For those covered surgical procedures and covered ancillary services where the payment rate is the lower of the proposed rates under the ASC standard ratesetting methodology and the MPFS proposed rates, the proposed payment indicators and rates set forth in this proposed rule are based on a comparison using the proposed PFS rates that would be effective January 1, 2020. For a discussion of the PFS rates, we refer readers to the CY 2020 PFS proposed rule that is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
The proposed payment rates included in these addenda reflect the full ASC payment update and not the reduced payment update used to calculate payment rates for ASCs not meeting the quality reporting requirements under the ASCQR Program. These addenda contain several types of information related to the proposed CY 2020 payment rates. Specifically, in Addendum AA, a “Y” in the column titled “To be Subject to Multiple Procedure Discounting” indicates that the surgical procedure would be subject to the multiple procedure payment reduction policy. As discussed in the CY 2008 OPPS/ASC final rule with comment period (72 FR 66829 through 66830), most covered surgical procedures are subject to a 50-percent reduction in the ASC payment for the lower-paying procedure when more than one procedure is performed in a single operative session.
Display of the comment indicator “CH” in the column titled “Comment Indicator” indicates a change in payment policy for the item or service, including identifying discontinued HCPCS codes, designating items or services newly payable under the ASC payment system, and identifying items or services with changes in the ASC payment indicator for CY 2020. Display of the comment indicator “NI” in the column titled “Comment Indicator” indicates that the code is new (or substantially revised) and that comments will be accepted on the interim payment indicator for the new code. Display of the comment indicator “NP” in the column titled “Comment Indicator” indicates that the code is new (or substantially revised) and that comments will be accepted on the ASC payment indicator for the new code.
The values displayed in the column titled “Proposed CY 2020 Payment Weight” are the proposed relative payment weights for each of the listed services for CY 2020. The proposed relative payment weights for all covered surgical procedures and covered ancillary services where the ASC payment rates are based on OPPS relative payment weights were scaled for budget neutrality. Therefore, scaling was not applied to the device portion of the device-intensive procedures, services that are paid at the MPFS nonfacility PE RVU-based amount, separately payable covered ancillary services that have a predetermined national payment amount, such as drugs and biologicals and brachytherapy sources that are separately paid under the OPPS, or services that are contractor-priced or paid at reasonable cost in ASCs. This includes separate payment for non-opioid pain management drugs.
To derive the proposed CY 2020 payment rate displayed in the “Proposed CY 2020 Payment Rate” column, each ASC payment weight in the “Proposed CY 2020 Payment Weight” column was multiplied by the proposed CY 2020 conversion factor of $47.827. The proposed conversion factor includes a budget neutrality adjustment for changes in the wage index values and the annual update factor as reduced by the productivity adjustment (as discussed in section XIII.G.2.b. of this proposed rule).
In Addendum BB, there are no relative payment weights displayed in the “Proposed CY 2020 Payment Weight” column for items and services with predetermined national payment amounts, such as separately payable drugs and biologicals. The “Proposed CY 2020 Payment” column displays the proposed CY 2020 national unadjusted ASC payment rates for all items and services. The proposed CY 2020 ASC payment rates listed in Addendum BB for separately payable drugs and biologicals are based on ASP data used for payment in physicians' offices in April 2019.
Addendum EE provides the HCPCS codes and short descriptors for surgical procedures that are proposed to be excluded from payment in ASCs for CY 2020.
XIV. Requirements for the Hospital Outpatient Quality Reporting (OQR) Program
A. Background
1. Overview
CMS seeks to promote higher quality and more efficient healthcare for Medicare beneficiaries. Consistent with these goals, CMS has implemented quality reporting programs for multiple care settings including the quality reporting program for hospital outpatient care, known as the Hospital Outpatient Quality Reporting (OQR) Program, formerly known as the Hospital Outpatient Quality Data Reporting Program (HOP QDRP). The Hospital OQR Program is generally aligned with the quality reporting program for hospital inpatient services known as the Hospital Inpatient Quality Reporting (IQR) Program, formerly known as the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) Program.
We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 58820 through 58822) and section I.A.2. of this proposed rule where we discuss our Meaningful Measures Initiative and our approach in evaluating quality program measures.
2. Statutory History of the Hospital OQR Program
We refer readers to the CY 2011 OPPS/ASC final rule with comment period (75 FR 72064 through 72065) for a detailed discussion of the statutory history of the Hospital OQR Program.
3. Regulatory History of the Hospital OQR Program
We refer readers to the CY 2008 through 2019 OPPS/ASC final rules with comment period (72 FR 66860 through 66875; 73 FR 68758 through 68779; 74 FR 60629 through 60656; 75 FR 72064 through 72110; 76 FR 74451 through 74492; 77 FR 68467 through 68492; 78 FR 75090 through 75120; 79 FR 66940 through 66966; 80 FR 70502 through 70526; 81 FR 79753 through 79797; 82 FR 59424 through 59445; and 83 FR 59080 through 59110) for the regulatory history of the Hospital OQR Program. We have codified certain requirements under the Hospital OQR Program at 42 CFR 419.46.
B. Hospital OQR Program Quality Measures
1. Considerations in the Selection of Hospital OQR Program Quality Measures
We refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74458 through 74460) for a detailed discussion of the priorities we consider for the Hospital OQR Program quality measure selection. We are not proposing any changes to these policies in this proposed rule.
2. Retention of Hospital OQR Program Measures Adopted in Previous Payment Determinations
We previously adopted a policy to retain measures from a previous year's Hospital OQR Program measure set for subsequent years' measure sets in the CY 2013 OPPS/ASC final rule with comment period (77 FR 68471) whereby quality measures adopted in a previous year's rulemaking are retained in the Hospital OQR Program for use in subsequent years unless otherwise specified. For more information regarding this policy, we refer readers to that final rule with comment period. We codified this policy at 42 CFR 419.46(h)(1) in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59082).
3. Removal of Quality Measures From the Hospital OQR Program Measure Set
In the CY 2010 OPPS/ASC final rule with comment period (74 FR 60635), we finalized a process to use the regular rulemaking process to remove a measure for circumstances for which we do not believe that continued use of a measure raises specific patient safety concerns.[70] We codified this policy at 42 CFR 419.46(h)(3) in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59082).
a. Considerations in Removing Quality Measures From the Hospital OQR Program
(1) Immediate Removal
In the CY 2010 OPPS/ASC final rule with comment period (74 FR 60634 through 60635), we finalized a process for immediate retirement, which we later termed “removal,” of Hospital OQR Program measures, based on evidence that the continued use of the measure as specified raises patient safety concerns.[71] We codified this policy at 42 CFR 419.46(h)(2) in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59082).
(2) Consideration Factors for Removing Measures
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59083 through 59085), we clarified, finalized, and codified at 42 CFR 419.46(h)(2) and (3) an updated set of factors [72] and policies for determining whether to remove measures from the Hospital OQR Program. We refer readers to that final rule with comment period for a detailed discussion of our policies regarding measure removal. The factors are:
- Factor 1. Measure performance among hospitals is so high and unvarying that meaningful distinctions and improvements in performance can no longer be made (“topped out” measures).
- Factor 2. Performance or improvement on a measure does not result in better patient outcomes.
- Factor 3. A measure does not align with current clinical guidelines or practice.
- Factor 4. The availability of a more broadly applicable (across settings, populations, or conditions) measure for the topic.
- Factor 5. The availability of a measure that is more proximal in time to desired patient outcomes for the particular topic.
- Factor 6. The availability of a measure that is more strongly associated with desired patient outcomes for the particular topic.
- Factor 7. Collection or public reporting of a measure leads to negative unintended consequences other than patient harm.
- Factor 8. The costs associated with a measure outweigh the benefit of its continued use in the program.
b. Proposed Removal of Quality Measure From the Hospital OQR Program Measure Set: OP-33: External Beam Radiotherapy (NQF# 1822)
In this proposed rule, we are proposing to remove one measure from the Hospital OQR Program for the CY 2022 payment determination as discussed below. Specifically, beginning with the CY 2022 payment determination, we are proposing to remove OP-33: External Beam Radiotherapy for Bone Metastases under removal Factor 8, the costs associated with a measure outweigh the benefit of its continued use in the program.
We refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70507 through 70510), where we adopted OP-33: External Beam Radiotherapy (NQF# 1822), beginning with the CY 2018 payment determination and for subsequent years. This measure assesses the “percentage of patients (all-payer) with painful bone metastases and no history of previous radiation who receive EBRT with an acceptable dosing schedule.” [73] We adopted this measure to address the performance gap in External Beam Radiotherapy (EBRT) treatment variation, ensure appropriate use of EBRT, and prevent the overuse of radiation therapy (80 FR 70508).
We believe that removing EBRT from the Hospital OQR Program is appropriate at this time because the costs associated with this measure outweigh the benefit of its continued use in the program (removal Factor 8). The Hospital OQR Program implemented the EBRT measure using “radiation delivery” Current Procedural Terminology (CPT) codes, which are appropriate for hospital-level measurement. We have identified issues with reporting this measure, finding that more questions are received about how to report the EBRT measure than about any other measure in the program. In addition, the measure steward has received feedback on data collection of the measure in the outpatient setting, and has indicated new and significant concerns regarding the “radiation delivery” CPT coding used to report the EBRT measure in the Hospital OQR Program including complicated measure exclusions, sampling concerns, and administrative burden.
“Radiation delivery” CPT codes require complicated measure exclusions, and the use of “radiation delivery” CPT codes causes the administration of EBRT to different anatomic sites to be considered separate cases for this measure. The numerator for this measure includes all patients, regardless of age, with painful bone metastases, and no previous radiation to the same anatomic site who receive EBRT with any of the following recommended fractionation schemes: 30Gy/10fxns, 24Gy/6fxns, 20Gy/5fxns, 8Gy/1fxn. The denominator for this measure includes all patients with painful bone metastases and no previous radiation to the same anatomic site who receive EBRT.[74] As noted above, each anatomic site is considered a different case, and as a result it is necessary to determine when EBRT has been administered to different anatomic sites. This determination is not possible without completing a detailed manual review of the patient's record, creating burden and difficulty in determining which sites and instances of EBRT administration are considered cases and should be included in the denominator for the measure. These challenges in determining which cases are included in the denominator for the measure result in difficulty in determining if sample size requirements for the measure are being met.
Further, current information systems do not automatically calculate the total dose provided, so manual review of patient records by practice staff is also required in order to determine the total dose and fractionation scheme, which in turn is used to determine which cases fall into the numerator for this measure. This manual review of patient records is a labor-intensive process that contributes to burden and difficulty in reporting this measure. As a result, we believe that the complexity of reporting this measure places substantial administrative burden on facilities. This also reflects observations made by the measure steward that implementing the measure in the outpatient setting has proven to be very burdensome, given that facilities have noted confusion regarding when the administration of EBRT to different numbers and locations of bone metastases are considered separate cases. These issues identifying cases have led to questions about sampling and difficulty determining if sample size requirements are met. Additional burdens associated with this measure have come to our attention,—including complicated measure exclusions, sampling concerns, and administrative burden. These challenges cause difficulty in tracking and reporting data for this measure and additional administrative burden, as evidenced by numerous questions about how to report this measure received by CMS and its contractors.
This EBRT measure was also adopted into another CMS quality reporting program, the PCHQR Program (79 FR 50278 through 50279). That program initially used “radiation planning” CPT codes billable at the physician level, but beginning in March 2016, the PCHQR program updated the measure to enable the use of “radiation delivery” CPT codes.[75] In the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19502 through 19503), CMS proposed to remove the measure from the PCHQR Program because the burden associated with the measure outweighs the value of its inclusion in the PCHQR Program. Specifically, the PCHQR Program has proposed to remove the measure because it is overly burdensome and because the measure steward is no longer maintaining the measure. As such, the PCHQR Program stated it can no longer ensure that the measure is in line with clinical guidelines and standards (84 FR 19502 through 19503). We note that while the version of the measure using “radiation planning” CPT codes is less burdensome, Hospital Outpatient Departments (HOPDs) do not have access to physician billing data, and so it is not operationally feasible to use “radiation planning” CPT codes (as opposed to the current “radiation delivery” CPT codes) for the EBRT measure in the Hospital OQR Program.
This measure was originally adopted to address the performance gap in EBRT treatment variation, ensure appropriate use of EBRT, and prevent the overuse of radiation therapy. While we still believe that these goals are important, the benefits of this measure have diminished. Stakeholder feedback has shown that this measure is burdensome and difficult to report. Since the measure steward is no longer maintaining this measure,[76] we no longer believe that we can ensure that the measure is in line with clinical guidelines and standards. Thus, considering these circumstances, we believe the costs associated with this measure outweigh the benefit of its continued use in the program (removal Factor 8).
Therefore, we are proposing to remove the measure beginning with October 2020 encounters used in the CY 2022 payment determination and for subsequent years. We note that in crafting our proposal, we considered removing this measure beginning with the CY 2021 payment determination, but we decided on proposing to delay removal until the CY 2022 payment determination to be sensitive to facilities' planning and operational procedures given that data collection for this measure begins during CY 2019 for the CY 2021 payment determination. We believe that this proposed removal date balances reporting burden, while recognizing that HOPDs must use resources to modify information systems and reporting processes to discontinue reporting the measure.
In summary, we are proposing to remove OP-33: External Beam Radiotherapy for Bone Metastases (NQF #1822) from the Hospital OQR Program beginning with the CY 2022 payment determination and for subsequent years under removal Factor 8.
4. Summary of Proposed Hospital OQR Program Measure Sets for the CY 2022 Payment Determination
We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59099 through 59102) for a summary of the previously finalized Hospital OQR Program measure sets for the CY 2020 and CY 2021 payment determinations and subsequent years.
We are not proposing to add any measures and are proposing to remove one measure for the CY 2022 payment determination for the Hospital OQR Program. The Table 34 summarizes the proposed Hospital OQR Program measure set for the CY 2022 payment determination and subsequent years (including previously adopted measures and excluding one measure proposed for removal in this proposed rule).

5. Hospital OQR Program Measures and Topics for Future Consideration
We are requesting comment on the potential future adoption of four patient safety measures as well as future outcome measures generally.
a. Request for Comment on the Potential Future Adoption of Four Patient Safety Measures
We are seeking comment on the potential future adoption of four patient safety measures for the Hospital OQR Program that were previously adopted for the ASCQR Program: ASC-1: Patient Burn; ASC-2: Patient Fall; ASC-3: Wrong Site, Wrong Side, Wrong Procedure, Wrong Implant; and ASC-4: All-Cause Hospital Transfer/Admission.[77] We refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74497 through 74499), where we adopted these measures (referred to as NQF #0263, NQF #0266, NQF #0267, and NQF #0265 at the time) in the ASCQR Program. We note that data collection for these measures was suspended in the ASCQR Program due to concerns with their data submission method using quality data codes (QDCs) in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59117 through 59123; 59134 through 59135); however, we refer readers to section XV.B.5. of this proposed rule, in which the ASCQR Program is requesting public comment on updating the submission method for these measures in the future. We are requesting public comment on potentially adding these measures with the updated submission method using a CMS online data submission tool, to the Hospital OQR Program in future rulemaking. These measures are currently specified for the ASC setting; we are considering having them specified for the hospital outpatient setting and would seek collaboration with the measure steward if we do so.
We believe these measures could be valuable to the Hospital OQR Program because they would allow us to monitor these types of events and prevent their occurrence to ensure that they remain rare, and because they provide critical data to beneficiaries and further transparency for care provided in the outpatient setting that could be useful in choosing a HOPD. In addition, these measures address an important Meaningful Measure Initiative quality priority, Making Care Safer by Reducing Harm Caused in the Delivery of Care.[78] There has been broad stakeholder support for these measures in the ASC setting; stakeholders believe these measures provide important data for facilities and patients because they are serious and the occurrence of these events should be zero (83 FR 59118). A few commenters noted in the CY 2019 OPPS/ASC final rule with comment period that it would be beneficial to also include these ASCQR Program measures in the Hospital OQR Program in order to provide patients with more meaningful data to compare sites of service (83 FR 59119). The future addition of these measures would further align the Hospital OQR and ASCQR Programs, which would benefit patients because these are two outpatient settings that patients may be interested in comparing, especially if they are able to choose in which of these two settings they receive care.
Although NQF endorsement for these ASC measures was removed (in February 2016 for the All-Cause Hospital Transfer/Admission measure; [79] in May 2016 for the Patient Burn [80] and the Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant [81] measures; and in June 2018 for the Patient Fall measure [82] ), as one commenter pointed out in the CY 2019 OPPS/ASC final rule with comment period, the NQF endorsement of the ASC measures was removed as endorsement was allowed to lapse by the measure steward, not because they failed the endorsement maintenance process (83 FR 59119). If specified for the HOPD setting, we plan to coordinate with the measure steward to seek NQF endorsement for those measures. These measures are discussed in more detail below.
(1) Patient Burn
The ASCQR Patient Burn measure assesses the percentage of admissions experiencing a burn prior to discharge. The numerator for this measure is defined as ASC admissions experiencing a burn prior to discharge and the denominator is defined as all ASC admissions.[83] We believe this measure, if specified for the hospital outpatient setting, would allow HOPDs, Medicare beneficiaries, and other stakeholders to develop a better understanding of the incidence of these events. In the CY 2012 OPPS/ASC final rule with comment period (76 FR 74497 through 74498), we adopted this measure for the ASCQR Program because ASCs serve surgical patients who may face the risk of burns during ambulatory surgical procedures and we believe monitoring patient burns is valuable to patients and other stakeholders. HOPDs also serve surgical patients who may face the risk of burns during outpatient procedures, so we believe this measure would be valuable for the HOPD setting. Further, we have reviewed studies demonstrating the high impact of monitoring patient burns because patient burns are serious reportable events in healthcare [84] and because patient burns are preventable.[85 86]
(2) Patient Fall
The ASCQR Program Patient Fall measure assesses the percentage of admissions experiencing a fall. The numerator for this measure is defined as ASC admissions experiencing a fall within the confines of the ASC and excludes ASC admissions experiencing a fall outside the ASC. The denominator is defined as all ASC admissions and excludes ASC admissions experiencing a fall outside the ASC.[87] We believe this measure, if specified for the hospital outpatient setting, would enable HOPDs to take steps to reduce the risk of falls. In the CY 2012 OPPS/ASC final rule with comment period (76 FR 74498), we adopted this measure for the ASCQR Program because falls, particularly in the elderly, can cause injury and loss of functional status; because the use of anxiolytics, sedatives, and anesthetic agents may put patients undergoing outpatient surgery at increased risk for falls; and because falls in healthcare settings can be prevented through the assessment of risk, care planning, and patient monitoring. These same risks for patient falls are a concern in the HOPD setting. Further, we have reviewed studies demonstrating the high impact of monitoring patient burns because patient falls are serious reportable events in healthcare [88] and because patient falls are preventable.[89]
(3) Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant
The ASCQR Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant measure assesses the percentage of admissions experiencing a wrong site, wrong side, wrong patient, wrong procedure, or wrong implant. The numerator for this measure is defined as ASC admissions experiencing a wrong site, a wrong side, a wrong patient, a wrong procedure, or a wrong implant, and the denominator is defined as all ASC admissions.[90] We believe this measure, if specified for the hospital outpatient setting, would provide important HOPD information about surgeries and procedures performed on the wrong site/side, and wrong patient. In the CY 2012 OPPS/ASC final rule with comment period (76 FR 74498 through 74499), we adopted this measure for the ASCQR Program because surgeries and procedures performed on the wrong site/side, and wrong patient can result in significant impact on patients, including complications, serious disability or death. We also stated that while the prevalence of such serious errors may be rare, such events are considered serious reportable events. These same significant impacts on patients apply for the HOPD setting. Further, we have reviewed studies demonstrating the high impact of monitoring wrong site, wrong side, wrong patient, wrong procedure, wrong implant procedures and surgeries because these types of errors are serious reportable events in healthcare [91] and because these errors are preventable.[92]
(4) All-Cause Hospital Transfer/Admission
The All-Cause Hospital Transfer/Admission measure assesses the rate of admissions requiring a hospital transfer or hospital admission upon discharge. The numerator for this measure is defined as ASC admissions requiring a hospital transfer or hospital admission upon discharge from the ASC and the denominator is defined as all ASC admissions.[93] We believe this measure, if specified for the hospital outpatient setting, would be valuable for HOPDs. In the CY 2012 OPPS/ASC final rule with comment period (76 FR 74499), we adopted this measure for ASCs because the transfer or admission of a surgical patient from an outpatient setting to an acute care setting can be an indication of a complication, serious medical error, or other unplanned negative patient outcome. We also stated that while acute intervention may be necessary in these circumstances, a high rate of such incidents may indicate suboptimal practices or patient selection criteria. These same potential negative patient outcomes apply to the HOPD setting. Further, we have reviewed studies demonstrating the high impact of monitoring patient transfers and admissions because facilities can take steps to prevent and reduce these types of events.[94 95]
b. Future Outcome Measures
In this proposed rule, we are also requesting public comment on future measure topics for the Hospital OQR Program. Specifically, we are requesting public comment on any outcome measures that would be useful to add as well as feedback on any process measures that should be eliminated from the Hospital OQR Program to further our goal of developing a comprehensive set of quality measures for informed decision-making and quality improvement in HOPDs. We are moving towards greater use of outcome measures and away from use of clinical process measures across our Medicare quality reporting programs to better assess the results of care. The current measure set for the Hospital OQR Program includes measures that assess process of care, imaging efficiency patterns, care transitions, ED throughput efficiency, Health Information Technology (health IT) use, care coordination, and patient safety. Measures are of various types, including those of process, structure, outcome, and efficiency. Through future rulemaking, we intend to propose new measures that support our goal of achieving better health care and improved health for Medicare beneficiaries who receive health care in the HOPD setting, while aligning quality measures across the Medicare program to the extent possible.
6. Maintenance of Technical Specifications for Quality Measures
CMS maintains technical specifications for previously adopted Hospital OQR Program measures. These specifications are updated as we modify the Hospital OQR Program measure set. The manuals that contain specifications for the previously adopted measures can be found on the QualityNet website at: https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier2&cid=1196289981244. We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59104 through 59105), where we changed the frequency of the Hospital OQR Program Specifications Manual release beginning with CY 2019 and for subsequent years, such that we will release a manual once every 12 months and release addenda as necessary. We are not proposing any changes to these policies in this proposed rule.
7. Public Display of Quality Measures
We refer readers to the CY 2014 and CY 2017 OPPS/ASC final rules with comment period (78 FR 75092 and 81 FR 79791 respectively) for our previously finalized policies regarding public display of quality measures. In this proposed rule, we are not proposing any changes to our previously finalized public display policies.
C. Administrative Requirements
1. QualityNet Account and Security Administrator
The previously finalized QualityNet security administrator requirements, including setting up a QualityNet account and the associated timelines, are described in the CY 2014 OPPS/ASC final rule with comment period (78 FR 75108 through 75109). We codified these procedural requirements at 42 CFR 419.46(a) in that final rule with comment period. We are not proposing any changes to our requirements for the QualityNet account and security administrator in this proposed rule.
2. Requirements Regarding Participation Status
We refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75108 through 75109), the CY 2016 OPPS/ASC final rule with comment period (80 FR 70519) and the CY 2019 OPPS/ASC final rule with comment period (83 FR 59103 through 59104) for requirements for participation and withdrawal from the Hospital OQR Program. We codified these procedural requirements regarding participation status at 42 CFR 419.46(a) and (b). We are not proposing any changes to our participation status policies in this proposed rule.
D. Form, Manner, and Timing of Data Submitted for the Hospital OQR Program
1. Hospital OQR Program Annual Payment Determinations
In the CY 2014 OPPS/ASC final rule with comment period (78 FR 75110 through 75111) and the CY 2016 OPPS/ASC final rule with comment period (80 FR 70519 through 70520), we specified our data submission deadlines. We codified these submission requirements at 42 CFR 419.46(c).
We refer readers to the CY 2016 OPPS/ASC final rule with comment period (80 FR 70519 through 70520), where we finalized our proposal to shift the quarters upon which the Hospital OQR Program payment determinations are based beginning with the CY 2018 payment determination. The deadlines for the CY 2022 payment determination and subsequent years are illustrated in Table 35.

In the CY 2018 OPPS/ASC final rule with comment period, we finalized a policy to align the initial data submission timeline for all hospitals that did not participate in the previous year's Hospital OQR Program and made conforming revisions at 42 CFR 419.46(c)(3). We are not proposing any changes to these policies in this proposed rule.
2. Requirements for Chart-Abstracted Measures Where Patient-Level Data Are Submitted Directly to CMS for the CY 2022 Payment Determination and Subsequent Years
We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68481 through 68484) for a discussion of the form, manner, and timing for data submission requirements of chart-abstracted measures for the CY 2014 payment determination and subsequent years. In this proposed rule, we are not proposing any changes to our policies regarding the submission of chart-abstracted measure data where patient-level data are submitted directly to CMS.
The following previously finalized Hospital OQR Program chart-abstracted measures will require patient-level data to be submitted for the CY 2022 payment determination and subsequent years:
- OP-2: Fibrinolytic Therapy Received Within 30 Minutes of ED Arrival (NQF #0288);
- OP-3: Median Time to Transfer to Another Facility for Acute Coronary Intervention (NQF #0290);
- OP-18: Median Time from ED Arrival to ED Departure for Discharged ED Patients (NQF #0496); and
- OP-23: Head CT Scan Results for Acute Ischemic Stroke or Hemorrhagic Stroke Patients who Received Head CT Scan Interpretation Within 45 Minutes of ED Arrival (NQF #0661).
3. Claims-Based Measure Data Requirements for the CY 2022 Payment Determination and Subsequent Years
Currently, the following previously finalized Hospital OQR Program claims-based measures are required for the CY 2022 payment determination and subsequent years:
- OP-8: MRI Lumbar Spine for Low Back Pain (NQF #0514);
- OP-10: Abdomen CT—Use of Contrast Material;
- OP-13: Cardiac Imaging for Preoperative Risk Assessment for Non-Cardiac, Low Risk Surgery (NQF #0669);
- OP-32: Facility 7-Day Risk-Standardized Hospital Visit Rate after Outpatient Colonoscopy (NQF #2539);
- OP-35: Admissions and Emergency Department Visits for Patients Receiving Outpatient Chemotherapy; and
- OP-36: Hospital Visits after Hospital Outpatient Surgery (NQF #2687).
We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59106 through 59107), where we established a three-year reporting period for OP-32: Facility 7-Day Risk-Standardized Hospital Visit Rate after Outpatient Colonoscopy beginning with the CY 2020 payment determination and for subsequent years. In that final rule with comment period (83 FR 59136 through 59138), we established a similar policy under the ASCQR Program. In this proposed rule, we are not proposing any changes regarding the submission of claims-based measures.
4. Data Submission Requirements for the OP-37a-e: Outpatient and Ambulatory Surgery Consumer Assessment of Healthcare Providers and Systems (OAS CAHPS) Survey-Based Measures for the CY 2022 Payment Determination and Subsequent Years
We refer readers to the CY 2017 OPPS/ASC final rule with comment period (81 FR 79792 through 79794) for a discussion of the previously finalized requirements related to survey administration and vendors for the OAS CAHPS Survey-based measures. In addition, we refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59432 through 59433), where we finalized a policy to delay implementation of the OP-37a-e OAS CAHPS Survey-based measures beginning with the CY 2020 payment determination (2018 reporting period) until further action in future rulemaking. In this proposed rule, we are not proposing any changes to the previously finalized requirements related to survey administration and vendors for the OAS CAHPS Survey-based measures.
5. Data Submission Requirements for Measures for Data Submitted via a Web-Based Tool for the CY 2022 Payment Determination and Subsequent Years
We refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75112 through 75115) and the CY 2016 OPPS/ASC final rule with comment period (80 FR 70521) and the CMS QualityNet website (https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier2&cid=1205442125082) for a discussion of the requirements for measure data submitted via the CMS QualityNet website for the CY 2017 payment determination and subsequent years. In addition, we refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75097 through 75100) for a discussion of the requirements for measure data submitted via the CDC NHSN website. In this proposed rule, we are not proposing any changes to our policies regarding the submission of measure data submitted via a web-based tool. However, as discussed in section XIV.B.3.b. of this proposed rule, we are proposing to remove OP-33: External Beam Radiotherapy for Bone Metastases beginning with the CY 2022 payment determination and for subsequent years.
If our proposal to remove OP-33 is finalized, the following previously finalized quality measures will require data to be submitted via a web-based tool for the CY 2022 payment determination and subsequent years:
- OP-22: Left Without Being Seen (NQF #0499) (via CMS' QualityNet website);
- OP-29: Endoscopy/Polyp Surveillance: Appropriate Follow-up Interval for Normal Colonoscopy in Average Risk Patients (NQF #0658) (via CMS' QualityNet website); and
- OP-31: Cataracts: Improvement in Patient's Visual Function within 90 Days Following Cataract Surgery (NQF #1536) (via CMS' QualityNet website).
6. Population and Sampling Data Requirements for the CY 2021 Payment Determination and Subsequent Years
We refer readers to the CY 2011 OPPS/ASC final rule with comment period (75 FR 72100 through 72103) and the CY 2012 OPPS/ASC final rule with comment period (76 FR 74482 through 74483) for discussions of our population and sampling requirements. We are not proposing any changes to our population and sampling requirements for chart-abstracted measures in this proposed rule.
7. Hospital OQR Program Validation Requirements
We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68484 through 68487), the CY 2015 OPPS/ASC final rule with comment period (79 FR 66964 through 66965), the CY 2016 OPPS/ASC final rule with comment period (80 FR 70524), and the CY 2018 OPPS/ASC final rule with comment period (82 FR 59441 through 59443), and 42 CFR 419.46(e) for our policies regarding validation. We are not proposing any changes to these policies in this proposed rule.
8. Extraordinary Circumstances Exception (ECE) Process for the CY 2021 Payment Determination and Subsequent Years
We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68489), the CY 2014 OPPS/ASC final rule with comment period (78 FR 75119 through 75120), the CY 2015 OPPS/ASC final rule with comment period (79 FR 66966), the CY 2016 OPPS/ASC final rule with comment period (80 FR 70524), the CY 2017 OPPS/ASC final rule with comment period (81 FR 79795), the CY 2018 OPPS/ASC final rule with comment period (82 FR 59444), and 42 CFR 419.46(d) for a complete discussion of our extraordinary circumstances exception (ECE) process under the Hospital OQR Program. We are not proposing any changes to our ECE policy in this proposed rule.
9. Hospital OQR Program Reconsideration and Appeals Procedures for the CY 2021 Payment Determination and Subsequent Years
We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68487 through 68489), the CY 2014 OPPS/ASC final rule with comment period (78 FR 75118 through 75119), the CY 2016 OPPS/ASC final rule with comment period (80 FR 70524), the CY 2017 OPPS/ASC final rule with comment period (81 FR 79795), and 42 CFR 419.46(f) for our reconsideration and appeals procedures. We are not proposing any changes to our reconsideration and appeals procedures in this proposed rule.
E. Proposed Payment Reduction for Hospitals That Fail To Meet the Hospital OQR Program Requirements for the CY 2020 Payment Determination
1. Background
Section 1833(t)(17) of the Act, which applies to subsection (d) hospitals (as defined under section 1886(d)(1)(B) of the Act), states that hospitals that fail to report data required to be submitted on measures selected by the Secretary, in the form and manner, and at a time, specified by the Secretary will incur a 2.0 percentage point reduction to their Outpatient Department (OPD) fee schedule increase factor; that is, the annual payment update factor. Section 1833(t)(17)(A)(ii) of the Act specifies that any reduction applies only to the payment year involved and will not be taken into account in computing the applicable OPD fee schedule increase factor for a subsequent year.
The application of a reduced OPD fee schedule increase factor results in reduced national unadjusted payment rates that apply to certain outpatient items and services provided by hospitals that are required to report outpatient quality data in order to receive the full payment update factor and that fail to meet the Hospital OQR Program requirements. Hospitals that meet the reporting requirements receive the full OPPS payment update without the reduction. For a more detailed discussion of how this payment reduction was initially implemented, we refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68769 through 68772).
The national unadjusted payment rates for many services paid under the OPPS equal the product of the OPPS conversion factor and the scaled relative payment weight for the APC to which the service is assigned. The OPPS conversion factor, which is updated annually by the OPD fee schedule increase factor, is used to calculate the OPPS payment rate for services with the following status indicators (listed in Addendum B to this proposed rule, which is available via the internet on the CMS website): “J1”, “J2”, “P”, “Q1”, “Q2”, “Q3”, “R”, “S”, “T”, “V”, or “U”. In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79796), we clarified that the reporting ratio does not apply to codes with status indicator “Q4” because services and procedures coded with status indicator “Q4” are either packaged or paid through the Clinical Laboratory Fee Schedule and are never paid separately through the OPPS. Payment for all services assigned to these status indicators will be subject to the reduction of the national unadjusted payment rates for hospitals that fail to meet Hospital OQR Program requirements, with the exception of services assigned to New Technology APCs with assigned status indicator “S” or “`T”. We refer readers to the CY 2009 OPPS/ASC final rule with comment period (73 FR 68770 through 68771) for a discussion of this policy.
The OPD fee schedule increase factor is an input into the OPPS conversion factor, which is used to calculate OPPS payment rates. To reduce the OPD fee schedule increase factor for hospitals that fail to meet reporting requirements, we calculate two conversion factors—a full market basket conversion factor (that is, the full conversion factor), and a reduced market basket conversion factor (that is, the reduced conversion factor). We then calculate a reduction ratio by dividing the reduced conversion factor by the full conversion factor. We refer to this reduction ratio as the “reporting ratio” to indicate that it applies to payment for hospitals that fail to meet their reporting requirements. Applying this reporting ratio to the OPPS payment amounts results in reduced national unadjusted payment rates that are mathematically equivalent to the reduced national unadjusted payment rates that would result if we multiplied the scaled OPPS relative payment weights by the reduced conversion factor. For example, to determine the reduced national unadjusted payment rates that applied to hospitals that failed to meet their quality reporting requirements for the CY 2010 OPPS, we multiplied the final full national unadjusted payment rate found in Addendum B of the CY 2010 OPPS/ASC final rule with comment period by the CY 2010 OPPS final reporting ratio of 0.980 (74 FR 60642).
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68771 through 68772), we established a policy that the Medicare beneficiary's minimum unadjusted copayment and national unadjusted copayment for a service to which a reduced national unadjusted payment rate applies would each equal the product of the reporting ratio and the national unadjusted copayment or the minimum unadjusted copayment, as applicable, for the service. Under this policy, we apply the reporting ratio to both the minimum unadjusted copayment and national unadjusted copayment for services provided by hospitals that receive the payment reduction for failure to meet the Hospital OQR Program reporting requirements. This application of the reporting ratio to the national unadjusted and minimum unadjusted copayments is calculated according to § 419.41 of our regulations, prior to any adjustment for a hospital's failure to meet the quality reporting standards according to § 419.43(h). Beneficiaries and secondary payers thereby share in the reduction of payments to these hospitals.
In the CY 2009 OPPS/ASC final rule with comment period (73 FR 68772), we established the policy that all other applicable adjustments to the OPPS national unadjusted payment rates apply when the OPD fee schedule increase factor is reduced for hospitals that fail to meet the requirements of the Hospital OQR Program. For example, the following standard adjustments apply to the reduced national unadjusted payment rates: The wage index adjustment; the multiple procedure adjustment; the interrupted procedure adjustment; the rural sole community hospital adjustment; and the adjustment for devices furnished with full or partial credit or without cost. Similarly, OPPS outlier payments made for high cost and complex procedures will continue to be made when outlier criteria are met. For hospitals that fail to meet the quality data reporting requirements, the hospitals' costs are compared to the reduced payments for purposes of outlier eligibility and payment calculation. We established this policy in the OPPS beginning in the CY 2010 OPPS/ASC final rule with comment period (74 FR 60642). For a complete discussion of the OPPS outlier calculation and eligibility criteria, we refer readers to section II.G. of this proposed rule.
2. Proposed Reporting Ratio Application and Associated Adjustment Policy for CY 2020
We are proposing to continue our established policy of applying the reduction of the OPD fee schedule increase factor through the use of a reporting ratio for those hospitals that fail to meet the Hospital OQR Program requirements for the full CY 2020 annual payment update factor. For the CY 2020 OPPS, the proposed reporting ratio is 0.980, calculated by dividing the proposed reduced conversion factor of $79.770 by the proposed full conversion factor of $81.398. We are proposing to continue to apply the reporting ratio to all services calculated using the OPPS conversion factor. For the CY 2020 OPPS, we are proposing to apply the reporting ratio, when applicable, to all HCPCS codes to which we have proposed status indicator assignments of “J1”, “J2”, “P”, “Q1”, “Q2”, “Q3”, “R”, “S”, “T”, “V”, and “U” (other than new technology APCs to which we have proposed status indicator assignment of “S” and “T”). We are proposing to continue to exclude services paid under New Technology APCs. We are proposing to continue to apply the reporting ratio to the national unadjusted payment rates and the minimum unadjusted and national unadjusted copayment rates of all applicable services for those hospitals that fail to meet the Hospital OQR Program reporting requirements. We are also proposing to continue to apply all other applicable standard adjustments to the OPPS national unadjusted payment rates for hospitals that fail to meet the requirements of the Hospital OQR Program. Similarly, we are proposing to continue to calculate OPPS outlier eligibility and outlier payment based on the reduced payment rates for those hospitals that fail to meet the reporting requirements.
XV. Requirements for the Ambulatory Surgical Center Quality Reporting (ASCQR) Program
A. Background
1. Overview
We refer readers to section XIV.A.1. of this proposed rule for a general overview of our quality reporting programs and to the CY 2019 OPPS/ASC final rule with comment period (83 FR 58820 through 58822) and section I.A.2. of this proposed rule where we discuss our Meaningful Measures Initiative and our approach in evaluating quality program measures.
2. Statutory History of the ASCQR Program
We refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74492 through 74494) for a detailed discussion of the statutory history of the ASCQR Program.
3. Regulatory History of the ASCQR Program
We seek to promote higher quality and more efficient health care for beneficiaries. This effort is supported by the adoption of widely accepted quality of care measures. We have collaborated with relevant stakeholders to define such measures in most healthcare settings and currently measure some aspect of care for almost all settings of care available to Medicare beneficiaries. These measures assess structural aspects of care, clinical processes, patient experiences with care, and clinical outcomes. We have implemented quality measure reporting programs for multiple healthcare settings. To measure the quality of ASC services and to make such information publicly available, we implemented the ASCQR Program. We refer readers to the CYs 2014 through 2019 OPPS/ASC final rules with comment period (78 FR 75122; 79 FR 66966 through 66987; 80 FR 70526 through 70538; 81 FR 79797 through 79826; 82 FR 59445 through 59476; and 83 FR 59110 through 59139, respectively) for an overview of the regulatory history of the ASCQR Program. We have codified certain requirements under the ASCQR Program at 42 CFR part 16, subpart H (42 CFR 416.300 through 416.330).
B. ASCQR Program Quality Measures
1. Considerations in the Selection of ASCQR Program Quality Measures
We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68493 through 68494) for a detailed discussion of the priorities we consider for ASCQR Program quality measure selection. We are not proposing any changes to these policies in this proposed rule.
2. Policies for Retention and Removal of Quality Measures From the ASCQR Program
a. Retention of Previously Adopted ASCQR Program Measures
We previously finalized a policy that quality measures adopted for an ASCQR Program measure set for a previous payment determination year be retained in the ASCQR Program for measure sets for subsequent payment determination years, except when they are removed, suspended, or replaced as indicated (76 FR 74494 and 74504; 77 FR 68494 through 68495; 78 FR 75122; and 79 FR 66967 through 66969). We are not proposing any changes to this policy in this proposed rule.
b. Removal Factors for ASCQR Program Measures
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59111 through 59115), we clarified, finalized and codified at 42 CFR 416.320 an updated set of factors [96] and the process for removing measures from the ASCQR Program. The factors are:
- Factor 1. Measure performance among ASCs is so high and unvarying that meaningful distinctions and improvements in performance can no longer be made (“topped-out” measures).
- Factor 2. Performance or improvement on a measure does not result in better patient outcomes.
- Factor 3. A measure does not align with current clinical guidelines or practice.
- Factor 4. The availability of a more broadly applicable (across settings, populations, or conditions) measure for the topic.
- Factor 5. The availability of a measure that is more proximal in time to desired patient outcomes for the particular topic.
- Factor 6. The availability of a measure that is more strongly associated with desired patient outcomes for the particular topic.
- Factor 7. Collection or public reporting of a measure leads to negative unintended consequences other than patient harm.
- Factor 8. The costs associated with a measure outweigh the benefit of its continued use in the program.
We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59111 through 59115) for a detailed discussion of our process regarding measure removal.
3. Proposed New Quality Measure for the ASCQR Program Measure Set: Proposal To Adopt ASC-19: Facility-Level 7-Day Hospital Visits After General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357)
In this proposed rule, we are proposing one new quality measure for the ASCQR Program for the CY 2024 payment determination and subsequent years—ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357).
a. Background
Ambulatory surgery in the outpatient setting is common in the United States. Nearly 70 percent of all surgeries in the United States are performed in an outpatient setting with an expanding number and variety of procedures being performed at stand-alone ASCs.[97 98] General surgery procedures are commonly performed at ASCs. Based on an analysis of Medicare fee-for-service (FFS) claims for patients aged 65 years and older, from January 1, 2015 through December 31, 2015, 3,251 ASCs performed 149,468 general surgery procedures. These procedures include abdominal, alimentary tract, breast, skin/soft tissue, wound, and varicose vein stripping procedures. Of the 3,251 ASCs that performed general surgery procedures, 1,157 (35.5 percent) performed at least 25 such procedures during this time period. Because of the large number of general surgery procedures that occur in the ambulatory setting, we believe that adopting ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers in the ASCQR Program will provide beneficiaries with transparent quality data that can be utilized in choosing healthcare facilities.
While ambulatory surgery is considered low risk for complications, there are well-described and potentially preventable adverse events that can occur after ambulatory surgery leading to unplanned care at a hospital, such as emergency department (ED) visits, observation stays, or hospital admissions. These events include uncontrolled pain, urinary retention, infection, bleeding, and venous thromboembolism.[99 100]
Hospital visits following same-day surgery are an important and broadly accepted patient-centered outcome reported in the literature.[101 102 103 104 105 106 107 108] National estimates of hospital visit rates following outpatient surgery vary from 0.5 to 9.0 percent, based on the type of surgery, outcome measured (admissions alone or admissions and ED visits), and length of time between the surgery and the hospital visit.[109 110 111 112 113 114 115 116 117] The frequency of such events also varies among ASCs, suggesting variation in quality of pre-surgical assessment, surgical care, post-surgical care, and the care and support provided to patients post-discharge.[118 119 120 121 122 123 124 125]
We calculated the national unadjusted rate of hospital visits (ED visits, observation stays, or hospital admissions) following any general surgery procedure at an ASC. In a Medicare FFS dataset of claims for services during CY 2015 (January 1, 2015-December 31, 2015), the distribution of unadjusted outcome rates was skewed, suggesting variation in quality of care. Among 1,153 ASCs with at least 25 qualifying general surgery cases in the Medicare FFS CY 2015 dataset, the unadjusted rate of unplanned hospital visits ranged from 0.0 percent to 13.2 percent. These results suggest opportunity for ASCs to improve the quality of care for patients seeking general surgery procedures.
ASCs may be unaware of patients' subsequent unplanned hospital visits given that patients tend to present to the ED or to hospitals unaffiliated with the ASC. In addition, information on the rate of patients' subsequent unplanned hospital visits would provide transparent data to beneficiaries that could be utilized when choosing ambulatory surgery sites of care. Quality measurement of the number of unplanned hospital visits following general surgery procedures performed at ASCs, coupled with transparency through public reporting would make these outcomes more visible to both ASCs and beneficiaries. Therefore, we expect that this would encourage ASCs to incorporate quality improvement activities to reduce the number of unplanned hospital visits and track quality improvement over time.
Therefore, in this proposed rule, we are proposing to adopt ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357) (hereafter referred to as the proposed ASC-19 measure) into the ASCQR Program for the CY 2024 payment determination and subsequent years.
The proposed ASC-19 measure was developed in conjunction with two other measures adopted for the ASCQR Program beginning with the CY 2022 payment determination as finalized in the CY 2018 OPPS/ASC final rule with comment period: ASC-17: Hospital Visits After Orthopedic Ambulatory Surgical Center Procedures (82 FR 59455) and ASC-18: Hospital Visits After Urology Ambulatory Surgical Center Procedures (82 FR 59463). All three measures assess the same patient outcome for care provided in the ASC setting and use the same risk-adjustment methodology. These three measures differ in surgical procedures considered (orthopedic, urological, or general surgery), specific risk variables included, and reporting of the outcome, unplanned hospital visits. The proposed ASC-19 measure reports the outcome as a risk-standardized ratio because the diverse mix of procedures included in the proposed ASC-19 measure can have varying levels of risk of unplanned hospital visits; while the ASC-17 and ASC-18 measures report a risk-standardized rate that reflects clinically specific cohorts with fairly comparable mixes of procedures. We refer readers to section XV.B.3.d. of this proposed rule for a full discussion on the measure outcome calculation.
b. Overview of Measure
The proposed ASC-19 measure is a risk-adjusted outcome measure of acute, unplanned hospital visits within 7 days of a general surgery procedure performed at an ASC among Medicare FFS patients aged 65 years and older. We define an unplanned hospital visit as including an emergency department (ED) visit, observation stay, or unplanned inpatient admission. The measure aligns with the Admissions and Readmissions to Hospitals and Preventable Healthcare Harm Meaningful Measure areas of our Meaningful Measures Initiative.[126] This measure was developed with input from a national Technical Expert Panel (TEP) consisting of patients, surgeons, methodologists, researchers, and providers. We also held a three-week public comment period soliciting stakeholder input on the measure methodology, and publicly posted a summary of the comments received as well as our responses (available in the Downloads section at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/PC-Updates-on-Previous-Comment-Periods.html).
During the measure development public comment period, we received public comment recommending the removal of two specific procedures (CPT 29893 endoscopic plantar and CPT 69222 clean out mastoid cavity) deemed outside the scope of general surgery and to review the cohort procedure list with general surgeons to ensure appropriateness. In response to this feedback, we reviewed the cohort of procedures incorporating feedback from general surgeons and removed 15 individual skin/soft tissue and wound procedure codes from the measure that are outside the scope of general surgery practice. These procedures include those specifically suggested for removal (that is, endoscopic plantar and clean out mastoid cavity) as well as chemical peels, dermabrasions, and nerve procedures.
Section 1890A of the Act requires the Secretary to establish a pre-rulemaking process with respect to the selection of certain categories of quality and efficiency measures. Under section 1890A(a)(2) of the Act, the Secretary must make available to the public by December 1 of each year a list of quality and efficiency measures that the Secretary is considering. The ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers measure was included on a publicly available document entitled “List of Measures under Consideration for December 1, 2017.” [127] The MAP reviewed this measure (MUC17-233) and provided conditional support for rulemaking, pending NQF review and endorsement, with the recognition that this measure assesses an important outcome for patients receiving care at ASCs.[128] The MAP had some concerns about the attribution model of the measure, noting that hospital visits after ASC procedures are relatively rare events and could disproportionately affect low-income or rural ASCs and that the measure may need risk adjustment for social risk factors. At the time of the MAP's review, this measure was still undergoing field testing.
Since the MAP's conditional support,[129] we completed testing for the proposed ASC-19 measure by estimating risk-standardized scores using two full years of Medicare FFS claims data (CYs 2014 and 2015) containing 286,999 procedures. The results showed score variation across ASCs, from a minimum risk-standardized ratio of 0.42 to a maximum of 2.13; the median was 0.97 and the 25th and 75th percentiles were 0.90 and 1.10, respectively. After adjusting for case and procedure mixes of ASCs, these results suggest there are underlying differences in the quality of care and opportunities for quality improvement. The reliability testing found an intraclass correlation coefficient (ICC) score of 0.530, indicating moderate measure score reliability.[130] We considered the face validity of the measure score among TEP members. Among the 14 TEP members, 12 agreed that the measure scores are valid and useful measures of ASC quality of care for general surgery procedures and will provide ASCs with information that can be used to improve their quality of care. Detailed testing results are available in the technical report for this measure, located at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html.
On June 6, 2018, the NQF's Consensus Standards Approval Committee endorsed ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357).[131] The proposed ASC-19 measure is consistent with the information submitted to the NQF and the MAP, supporting its scientific acceptability for use in quality reporting programs. We note that we have made minor annual coding updates to the measure to incorporate changes to the CPT and ICD-10 coding systems and to incorporate clinical input to remove select procedures outside the scope of general surgery as noted above, endoscopic plantar, clean out mastoid cavity, chemical peels, dermabrasions, and nerve procedures. For the current list of codes that define the proposed ASC-19 measure and a description of updates since development, we refer readers to the zip file labeled “Version 1.0 Hospital Visits General Surgery ASC Procedures Measure Technical Report” located at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html.
We believe this proposed measure reflects consensus among stakeholders because it was developed with stakeholder input from a TEP convened by a CMS contractor as well as from the measure development public comment period.[132] During the measure development processes and the MAP meeting, the majority of public commenters supported the measure's focus on assessing patient outcomes after general surgery procedures performed in ASC setting of care. Most commenters supported MAP's conditional support of the measure, noting it should be further developed and NQF-endorsed before implementation in the ASCQR Program. Importantly, the proposed ASC-19 measure addresses the MAP-identified priority measure area of addressing preventable healthcare harm, such as surgical complications, for the ASCQR Program.[133] Therefore, we believe it is appropriate to incorporate this proposed measure into the ASCQR Program measure set because collecting and publicly reporting these data would increase transparency, inform patients and ASCs, and foster quality improvement efforts.
c. Data Sources
The proposed ASC-19 measure is claims-based using Part A and Part B Medicare administrative claims and Medicare enrollment data to calculate the measure.
We are proposing that the data collection period for the proposed ASC-19 measure would be the 2 calendar years ending 2 years prior to the applicable payment determination year. For example, for the CY 2024 payment determination, the data collection period would be CYs 2021 to 2022. Because the measure data are collected via claims, ASCs will not need to submit any additional data directly to CMS. We refer readers to section XV.D.4. of this proposed rule for a more detailed discussion of the requirements for data submitted via claims.
d. Measure Calculation
The measure outcome is all-cause, unplanned hospital visits within 7 days of any general surgery procedure performed at an ASC. For the purposes of this measure, “hospital visits” include emergency department visits, observation stays, and unplanned inpatient admissions. The outcome of hospital visits is limited to 7 days since existing literature suggests that the vast majority of adverse events after outpatient surgery occur within the first 7 days following the surgery.[134 135] When there are two or more qualifying surgical procedures within a 7-day period, the measure considers all procedures as index procedures; however, the timeframe for outcome assessment is defined as the interval between procedures (including the day of the next procedure) and then 7 days after the last procedure.
The facility-level score is a risk-standardized hospital visit ratio (RSHVR), an approach that accounts for the clustering of patients within ASCs and variation in sample size across ASCs. The proposed ASC-19 measure reports the outcome as a risk-standardized ratio because the diverse mix of procedures included in the proposed measure can have varying levels of risk of unplanned hospital visits. The RSHVR is calculated as the ratio of the predicted to the expected number of unplanned hospital visits among ASC patients. For each ASC, the numerator of the ratio is the number of hospital visits predicted for the ASC's patients accounting for its observed rate, the number of the general surgery procedures performed at the ASC, the case-mix, and the surgical complexity mix. The denominator of the ratio is the number of hospital visits expected nationally given the ASC's case-mix and surgical complexity mix. To calculate an ASC's predicted-to-expected (P/E) ratio, the measure uses a two-level hierarchical logistic regression model. The log-odds of the outcome for an index procedure is modeled as a function of the patient demographic, comorbidity, procedure characteristics, and a random ASC-specific intercept. A ratio of less than one indicates the ASC facility's patients were estimated as having fewer post-surgical visits than expected compared to ASCs with similar surgical complexity and patients; and a ratio of greater than one indicates the ASC facility's patients were estimated as having more visits than expected. This approach is analogous to an observed-to-expected ratio, but the method accounts for within-facility correlation of the observed outcome and sample size differences, accommodates the assumption that underlying differences in quality across ASCs lead to systematic differences in outcomes, and is tailored to and appropriate for a publicly reported outcome measure as articulated in published scientific guidelines.[136 137 138] For more information on measure calculations, we refer readers to: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html.
e. Cohort
The patient cohort for the proposed ASC-19 measure includes all Medicare beneficiaries ages 65 and older undergoing outpatient general surgery procedures at an ASC who have 12 prior months of Medicare FFS (Medicare Parts A and B) enrollment. The target group of procedures includes those that: (1) Are routinely performed at ASCs; (2) involve some increased risk of post-surgery hospital visits; and (3) are within the scope of general surgery training. These include the following types of procedures: Abdominal (for example, hernia repair), alimentary tract (for example, hemorrhoid procedures), breast (for example, mastectomies), skin/soft tissue (for example, skin grafting), wound (for example, incision and drainage of skin and subcutaneous tissue), and varicose vein stripping. The proposed ASC-19 measure does not include gastrointestinal endoscopy, endocrine, or vascular procedures, other than varicose vein procedures, because for these procedures, reasons for hospital visits are typically related to patients' underlying comorbidities.
The scope of general surgery overlaps with that of other specialties (for example, vascular surgery and plastic surgery). For this measure, we targeted surgeries that general surgeons are trained to perform with the understanding that other subspecialists may also be performing many of these surgeries at ASCs. Since the type of surgeon performing a particular procedure may vary across ASCs in ways that affect quality, the measure is neutral to surgeons' specialty training.
Procedures included in the measure cohort are on CMS' list of covered ASC procedures.[139] We developed this list to identify surgeries that have a low-to-moderate risk profile. Surgeries on the ASC list of covered procedures do not involve or require major or prolonged invasion of body cavities, extensive blood loss, major blood vessels, or care that is either urgent or life threatening. We annually review and update this list, which includes a transparent public comment submission and review process for addition and/or removal of procedures codes.[140] The current list is accessible in the Downloads section at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/ascpayment/11_addenda_updates.html.
In addition, the measure includes only “major” and “minor” procedures, as indicated by the Medicare Physician Fee Schedule global surgery indicator (GSI) values of 090 and 010, respectively, to focus the measure only on the subset of surgeries on CMS' list of covered ASC procedures that impose a meaningful risk of post-procedure hospital visits. This list of GSI values is publicly available for CY 2015 at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1612-FC.html (download PFS Addenda, Addendum B). Moreover, to identify the subset of ASC procedures within the scope of general surgery, we used the Clinical Classifications Software (CCS) developed by the Agency for Healthcare Research and Quality.[141] We identified and included CCS categories within the scope of general surgery, and only included individual procedures within the CCS categories at the procedure (CPT code) level if they were within the scope of general surgery practice. For more cohort details, we refer readers to the measure technical report located at: https://www.cms.gov/medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html.
To ensure that all patients included under this measure have full data available for outcome assessment, the measure excludes patients who survived at least 7 days following general surgery procedures at an ASC, but were not continuously enrolled in Medicare FFS (Medicare Parts A and B) during the 7 days after surgery. There are no additional patient inclusion or exclusion criteria for the proposed ASC-19 measure. Additional methodology and measure development details are available at: https://www.cms.gov/medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html.
f. Risk Adjustment
The statistical risk-adjustment model includes clinically relevant risk-adjustment variables that are strongly associated with risk of hospital visits within 7 days following ASC general surgery procedures. Accordingly, only comorbidities that convey information about the patient at that time or in the 12 months prior, and not complications that arise during the course of the index procedure, are included in the risk adjustment. The measure risk adjusts for age, 18 comorbidities, procedure type (abdomen vs. alimentary tract vs. breast vs. skin/soft tissue vs. wound vs. varicose vein), a variable for work Relative Value Units (RVUs) to adjust for surgical complexity, and an interaction term of procedure type and surgical complexity.[142]
To select the final set of variables for the risk-adjustment model, candidate risk variables were entered into logistic regression analyses [143] predicting the outcome of hospital visits within 7 days. To develop a parsimonious risk model, non-significant variables were iteratively removed from the model using a stepwise selection approach described by Hosmer and Lemeshow.[144] All variables significant at p<0.05 were retained in the final model. We also tested interaction terms and retained those that were both significant at p<0.05 and demonstrated a clinically plausible relationship to the outcome. Finally, after reviewing TEP and public comments, as well as the statistically selected variables for face validity, we settled upon the model variables. We retained one additional variable (opioid use) for the final risk model because experts advised it was an important risk predictor and expressed a strong preference for including it in the model even though it was not statistically selected. Additional details on risk model development and testing are available in the technical report at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html.
g. Public Reporting
We are proposing that if the proposed ASC-19 measure is adopted, we would publicly report results only for facilities with sufficient case numbers to meet moderate reliability standards.[145] We would determine the case size cutoff for meeting moderate reliability standards by calculating reliability at different case sizes using the ratio of true variance to observed variance during the measure dry run (discussed below).[146] We would provide confidential performance data directly to all facilities including those which do not meet the criteria for sufficient case numbers for reliability considerations so that all facilities can benefit from seeing their measure results and individual patient-level outcomes. We believe that the measure will provide beneficiaries with information about the quality of care for general surgery procedures in the ASC setting. In addition, we believe that these performance data may help ASCs track their patient outcomes and provide information on their cases that facilities can use to improve quality of care.
h. Provision of Facility-Specific Information Prior to Public Reporting
If this proposed measure is finalized, we intend to conduct a dry run before the official data collection period or any public reporting. A dry run is a period of confidential reporting and feedback during which ASCs may review their dry run measure results, and in addition, further familiarize themselves with the measure methodology and ask questions. For the dry run, we intend to use the most current 2-year set of complete claims (usually 12 months prior to the start date) available at the time of dry run. For example, if the dry run began in June 2020, the most current 2-year set of data available would likely be July 2017 to June 2019. Because we use paid, final action Medicare claims, ASCs would not need to submit any additional data for the dry run. The dry run would generate confidential feedback reports for ASCs, including patient-level data indicating whether the patient had a hospital visit and, if so, the type of visit (emergency department visit, observation stay, or unplanned inpatient admission), the admitting facility, and the principal discharge diagnosis. Further, the dry run would enable ASCs to see their dry run measure results prior to the measure being implemented. General information about the dry run as well as confidential facility-specific reports would be made available for ASCs to review on their accounts at: http://www.qualitynet.org. We plan to continue to generate these reports for ASCs after we implement the proposed measure if it is finalized so ASCs can use the information to identify performance gaps and develop quality improvement strategies.
These confidential dry run results are not publicly reported and do not affect payment. We expect the dry run to take approximately one month to conduct, during which facilities would be provided the confidential report and the opportunity to review their performance and provide feedback to us. After the dry run, measure results would have a payment impact and would be publicly reported as discussed above beginning with the CY 2024 payment determination and for subsequent years.
4. Summary of ASCQR Program Quality Measure Set Proposed for the CY 2024 Payment Determination and for Subsequent Years
As discussed above, we are proposing to add one measure beginning with the CY 2024 payment determination and for subsequent years to the ASCQR Program. We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59129 through 59132) for previously finalized ASCQR Program measure sets.
Table 36 summarizes the proposed ASCQR Program measure set for the CY 2024 payment determination and subsequent years (including previously adopted measures).

5. ASCQR Program Measures and Topics for Future Consideration
In this proposed rule, we are considering one topic for future implementation: Updates to the submission method for ASC-1: Patient Burn, ASC-2: Patient Fall, ASC-3: Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant, and ASC-4: All-Cause Hospital Transfer/Admission measures.
ASC-1, ASC-2, ASC-3, and ASC-4 were adopted into the ASCQR Program in the CY 2012 OPPS/ASC final rule with comment period beginning with the CY 2014 payment determination (76 FR 74496 through 74500). These measures were developed by the ASC Quality Collaboration (ASC QC). The ASC QC is a cooperative effort of organizations and companies formed in 2006 with a common interest in ensuring that ASC quality data is measured and reported in a meaningful way.[147] Stakeholders in the ASC QC include ASC corporations, ASC associations, professional societies and accrediting bodies that focus on ASC quality and safety.[148] The ASC QC initiated a process of standardizing ASC quality measure development through evaluation of existing nationally endorsed quality measures to determine which could be directly applied to the outpatient surgery facility setting.[149]
The ASC QC developed and pilot-tested ASC-1, ASC-2, ASC-3, and ASC-4 at the facility-level for feasibility and usability (76 FR 74496). These measures are calculated via quality data codes (QDCs), as described in section XV.D.1. of this proposed rule. ASCs were formerly required to submit the appropriate QDCs on individual Medicare FFS claims billed by the facility (78 FR 75135). In the FY 2013 IPPS/LTCH PPS final rule (77 FR 53640 through 53641), we finalized our policy that the minimum threshold for successful reporting be that at least 50 percent of claims meeting measure specifications contain QDCs. At that time, we believed that 50 percent was a reasonable minimum threshold for the initial implementation years of the ASCQR Program, because ASCs were not yet familiar with how to report quality data under the ASCQR Program and because many ASCs are relatively small and may have needed more time to set up reporting systems (77 FR 53641). We stated in that final rule that we intended to propose to increase this percentage for subsequent years' payment determinations as ASCs become more familiar with reporting requirements for the ASCQR Program. We have assessed this reporting threshold annually and have found that over 78 percent of reporting ASCs report data for at least 90 percent of eligible claims.
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59117 through 59123), we expressed concern that the data submission method for these measures may impact the completeness and accuracy of the data due to the inability of ASCs to correct errors in submitted QDCs that are used to calculate these measures. An ASC that identifies an erroneous or missing QDC is unable to correct or add a QDC if the claim has already been submitted to Medicare and been processed. We also stated that we believe that revising the data submission method for the measures, such as via QualityNet, would address this issue and allow ASCs to correct any data submissions errors, resulting in more complete and accurate data. In that final rule with comment period, we explained that we agree it is important to continue to monitor the types of events included in these measures considering the potential negative impacts to patients' morbidity and mortality, in order to continue to prevent their occurrence and ensure that they remain rare. We acknowledged that these measures provide critical data to beneficiaries and further transparency for care provided in the ASC setting that would be useful in choosing an ASC for care, and that these measures are valuable to the ASC community.
As such, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59117 through 59123; 59134 through 59135), we retained these measures in the ASCQR Program, but suspended their data submission until further action in rulemaking with the goal of updating their data submission method.
In this proposed rule, we are requesting comment about potential future updates to the data submission method for ASC-1: Patient Burn, ASC-2: Patient Fall, ASC-3: Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant, and ASC-4: All-Cause Hospital Transfer/Admission. Specifically, we have considered updating the data submission method to a CMS online data submission tool. We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59473) (and the previous rulemakings cited therein) and 42 CFR 416.310(c)(1) for our requirements regarding data submitted via a CMS online data submission tool. We are currently using the QualityNet website (https://www.qualitynet.org) as our CMS online data submission tool.
To submit measures via an online data submission tool to the QualityNet website, ASCs and any agents submitting data on an ASC's behalf would have to maintain a QualityNet account (42 CFR 416.310(c)(1)). A QualityNet security administrator would be necessary to set up such an account for the purpose of submitting this information (42 CFR 416.310(c)(1)). We believe that using a CMS online data collection tool would address our concern about the ability of ASCs to correct data submission errors because ASCs would simply report their data via the online tool. If data for these measures were submitted via QualityNet, ASCs would still submit claims for reimbursement to CMS, but would not be required to include QDCs. As specified at 42 CFR 416.310(c)(1)(ii), the data collection time period for quality measures for which data are submitted via a CMS online data submission tool is for services furnished during the calendar year 2 years prior to the payment determination year. ASCs would then submit their data for ASC-1, ASC-2, ASC-3, and ASC-4 via QualityNet during the data submission period, January 1 through May 15 in the year prior to the payment determination year. ASCs would be able to submit and modify their data throughout the data submission period and could correct any errors during this period. We are seeking comments on whether updating the data submission method for ASC-1, ASC-2, ASC-3, and ASC-4 to a CMS online data submission tool would be appropriate for these measures in the future.
We are committed to work with stakeholders to ensure the ASCQR Program measure set does not place an inappropriate amount of burden on facilities while addressing and providing information about these types of patient safety, adverse, rare events to patients and other consumers. We recognize that updating the data submission method to a CMS online data submission tool would add some burden to the ASCQR Program due to the additional time for submitting any of these four measures via QualityNet for each payment determination year. Thus, we are also seeking comment about the burden associated with potentially updating the data submission method for ASC-1, ASC-2, ASC-3, and ASC-4 to a CMS online data submission tool (for example, the QualityNet website) in future years.
6. Maintenance of Technical Specifications for Quality Measures
We refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74513 through 74514), where we finalized our proposal to follow the same process for updating the ASCQR Program measures that we adopted for the Hospital OQR Program measures, including the subregulatory process for updating adopted measures. In the CY 2013 OPPS/ASC final rule with comment period (77 FR 68496 through 68497), the CY 2014 OPPS/ASC final rule with comment period (78 FR 75131), and the CY 2015 OPPS/ASC final rule with comment period (79 FR 66981), we provided additional clarification regarding the ASCQR Program policy in the context of the previously finalized Hospital OQR Program policy, including the processes for addressing nonsubstantive and substantive changes to adopted measures. In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70531), we provided clarification regarding our decision to not display the technical specifications for the ASCQR Program on a CMS website, but stated that we will continue to display the technical specifications for the ASCQR Program on the QualityNet website. In addition, our policies regarding the maintenance of technical specifications for the ASCQR Program are codified at 42 CFR 416.325. In this proposed rule, we are not proposing any changes to our policies regarding the maintenance of technical specifications for the ASCQR Program.
7. Public Reporting of ASCQR Program Data
In the CY 2012 OPPS/ASC final rule with comment period (76 FR 74514 through 74515), we finalized a policy to make data that an ASC submitted for the ASCQR Program publicly available on a CMS website after providing an ASC an opportunity to review the data to be made public. In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70531 through 70533), we finalized our policy to publicly display data by the National Provider Identifier (NPI) when the data are submitted by the NPI and to publicly display data by the CCN when the data are submitted by the CCN. In addition, we codified our policies regarding the public reporting of ASCQR Program data at 42 CFR 416.315 (80 FR 70533). In the CY 2017 OPPS/ASC final rule with comment period (81 FR 79819 through 79820), we formalized our current public display practices regarding timing of public display and the preview period by finalizing our proposals to: Publicly display data on the Hospital Compare website, or other CMS website as soon as practicable after measure data have been submitted to CMS; to generally provide ASCs with approximately 30 days to review their data before publicly reporting the data; and to announce the timeframes for each preview period starting with the CY 2018 payment determination on a CMS website and/or on our applicable listservs. In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59455 through 59470), we discussed specific public reporting policies associated with two measures beginning with the CY 2022 payment determination: ASC-17: Hospital Visits after Orthopedic Ambulatory Surgical Center Procedures, and ASC-18: Hospital Visits after Urology Ambulatory Surgical Center Procedures. We are not proposing any changes to our public reporting policies in this proposed rule.
C. Administrative Requirements
1. Requirements Regarding QualityNet Account and Security Administrator
We refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75132 through 75133) for a detailed discussion of the QualityNet security administrator requirements, including setting up a QualityNet account, and the associated timelines, for the CY 2014 payment determination and subsequent years. In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70533), we codified the administrative requirements regarding maintenance of a QualityNet account and security administrator for the ASCQR Program at 42 CFR 416.310(c)(1)(i). We are not proposing any changes to these policies in this proposed rule.
2. Requirements Regarding Participation Status
We refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75133 through 75135) for a complete discussion of the participation status requirements for the CY 2014 payment determination and subsequent years. In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70533 through 70534), we codified these requirements regarding participation status for the ASCQR Program at 42 CFR 416.305. We are not proposing any changes to these policies in this proposed rule.
D. Form, Manner, and Timing of Data Submitted for the ASCQR Program
1. Requirements Regarding Data Processing and Collection Periods for Claims-Based Measures Using Quality Data Codes (QDCs)
We refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75135) for a complete summary of the data processing and collection periods for the claims-based measures using QDCs for the CY 2014 payment determination and subsequent years. In the CY 2016 OPPS/ASC final rule with comment period (80 FR 70534), we codified the requirements regarding data processing and collection periods for claims-based measures using QDCs for the ASCQR Program at 42 CFR 416.310(a)(1) and (2).
We are not proposing any changes to these requirements in this proposed rule. We note that data submission for the following claims-based measures using QDCs was suspended in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59117 through 59123; 59134 through 59135) until further action in rulemaking:
- ASC-1: Patient Burn;
- ASC-2: Patient Fall;
- ASC-3: Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant; and
- ASC-4: Hospital Transfer/Admission.
We also note that we are requesting comment on updating the submission method for the above measures in section XV.B.5. of this proposed rule.
These data processing and collection period requirements will remain in the ASCQR Program for application to any future claims-based measures using QDCs adopted by the ASCQR Program.
2. Minimum Threshold, Minimum Case Volume, and Data Completeness for Claims-Based Measures Using QDCs
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59472) (and the previous rulemakings cited therein), as well as 42 CFR 416.310(a)(3) and 42 CFR 416.305(c) for our policies about minimum threshold, minimum case volume, and data completeness for claims-based measures using QDCs. In this proposed rule, we are not proposing any changes to these policies.
3. Requirements for Data Submitted via an Online Data Submission Tool
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59472) (and the previous rulemakings cited therein) and 42 CFR 416.310(c) for our previously finalized policies for data submitted via an online data submission tool. For more information on data submission using QualityNet, we refer readers to: https://www.qualitynet.org.
a. Requirements for Data Submitted via a Non-CMS Online Data Submission Tool
We refer readers to the CY 2014 OPPS/ASC final rule with comment period (78 FR 75139 through 75140) and the CY 2015 OPPS/ASC final rule with comment period (79 FR 66985 through 66986) for our requirements regarding data submitted via a non-CMS online data submission tool (that is, the CDC NHSN website). We codified our existing policies regarding the data collection time periods for measures involving online data submission and the deadline for data submission via a non-CMS online data submission tool at 42 CFR 416.310(c)(2).
As we noted in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59135), no measures submitted via a non-CMS online data submission tool remain in the ASCQR Program beginning with the CY 2020 payment determination. We are not proposing any changes to our non-CMS online data submission tool reporting requirements; these requirements would apply to any future non-CMS online data submission tool measures adopted in the ASCQR Program.
b. Requirements for Data Submitted via a CMS Online Data Submission Tool
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59473) (and the previous rulemakings cited therein) and 42 CFR 416.310(c)(1) for our requirements regarding data submitted via a CMS online data submission tool. We are currently using the QualityNet website to host our CMS online data submission tool: https://www.qualitynet.org. We note that in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59473), we finalized expanded submission via the CMS online tool to also allow for batch data submission and made corresponding changes to 42 CFR 416.310(c)(1)(i).
In this proposed rule, we are not proposing any changes to this policy. The following previously finalized measures will require data to be submitted via a CMS online data submission tool for the CY 2021 payment determination and subsequent years:
- ASC-9: Endoscopy/Polyp Surveillance: Appropriate Follow-Up Interval for Normal Colonoscopy in Average Risk Patients
- ASC-11: Cataracts: Improvement in Patients' Visual Function within 90 Days Following Cataract Surgery
- ASC-13: Normothermia Outcome
- ASC-14: Unplanned Anterior Vitrectomy
4. Requirements for Non-QDC Based, Claims-Based Measure Data
We are not proposing any changes to our requirements for non-QDC based, claims-based measures in this proposed rule. We refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59136 through 59138, where we established a 3-year reporting period for the previously adopted measure, ASC-12: Facility 7-Day Risk-Standardized Hospital Visit Rate after Outpatient Colonoscopy. In that final rule with comment period (83 FR 59106 through 59107), we established a similar policy under the Hospital OQR Program.
We also note that we are proposing to adopt ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357) in section XV.B.3. of this proposed rule to which these requirements for non-QDC based, claims-based measures would apply if the proposed ASC-19 measure is finalized as proposed.
5. Requirements for Data Submission for ASC-15a-e: Outpatient and Ambulatory Surgery Consumer Assessment of Healthcare Providers and Systems (OAS CAHPS) Survey-Based Measures
We refer readers to the CY 2017 OPPS/ASC final rule with comment period (81 FR 79822 through 79824) for our previously finalized policies regarding survey administration and vendor requirements for the CY 2020 payment determination and subsequent years. In addition, we codified these policies at 42 CFR 416.310(e). However, in the CY 2018 OPPS/ASC final rule with comment period (82 FR 59450 through 59451), we delayed implementation of the ASC-15a-e: OAS CAHPS Survey-based measures beginning with the CY 2020 payment determination (CY 2018 data submission) until further action in future rulemaking, and we refer readers to that discussion for more details. In this proposed rule, we are not proposing any changes to this policy.
6. Extraordinary Circumstances Exception (ECE) Process for the CY 2020 Payment Determination and Subsequent Years
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59474 through 59475) (and the previous rulemakings cited therein) and 42 CFR 416.310(d) for the ASCQR Program's policies for extraordinary circumstance exceptions (ECE) requests.
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59474 through 59475), we: (1) Changed the name of this policy from “extraordinary circumstances extensions or exemption” to “extraordinary circumstances exceptions” for the ASCQR Program, beginning January 1, 2018; and (2) revised 42 CFR 416.310(d) of our regulations to reflect this change. We also clarified that we will strive to complete our review of each request within 90 days of receipt. In this proposed rule, we are not proposing any changes to these policies.
7. ASCQR Program Reconsideration Procedures
We refer readers to the CY 2016 OPPS/ASC final rule with comment period (82 FR 59475) (and the previous rulemakings cited therein) and 42 CFR 416.330 for the ASCQR Program's reconsideration policy. In this proposed rule, we are not proposing any changes to this policy.
E. Payment Reduction for ASCs That Fail To Meet the ASCQR Program Requirements
1. Statutory Background
We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68499) for a detailed discussion of the statutory background regarding payment reductions for ASCs that fail to meet the ASCQR Program requirements.
2. Policy Regarding Reduction to the ASC Payment Rates for ASCs That Fail To Meet the ASCQR Program Requirements for a Payment Determination Year
The national unadjusted payment rates for many services paid under the ASC payment system are equal to the product of the ASC conversion factor and the scaled relative payment weight for the APC to which the service is assigned. For CY 2020, the proposed ASC conversion factor is equal to the conversion factor calculated for the previous year updated by the multifactor productivity (MFP)-adjusted hospital market basket update factor. The MFP adjustment is set forth in section 1833(i)(2)(D)(v) of the Act. The MFP-adjusted hospital market basket update is the annual update for the ASC payment system for a 5-year period (CY 2019 through CY 2023). Under the ASCQR Program in accordance with section 1833(i)(7)(A) of the Act and as discussed in the CY 2013 OPPS/ASC final rule with comment period (77 FR 68499), any annual increase shall be reduced by 2.0 percentage points for ASCs that fail to meet the reporting requirements of the ASCQR Program. This reduction applied beginning with the CY 2014 payment rates (77 FR 68500). For a complete discussion of the calculation of the ASC conversion factor and our finalized proposal to update the ASC payment rates using the inpatient hospital market basket update for CYs 2019 through 2023, we refer readers to the CY 2019 OPPS/ASC final rule with comment period (83 FR 59073 through 59080).
In the CY 2013 OPPS/ASC final rule with comment period (77 FR 68499 through 68500), in order to implement the requirement to reduce the annual update for ASCs that fail to meet the ASCQR Program requirements, we finalized our proposal that we would calculate two conversion factors: A full update conversion factor and an ASCQR Program reduced update conversion factor. We finalized our proposal to calculate the reduced national unadjusted payment rates using the ASCQR Program reduced update conversion factor that would apply to ASCs that fail to meet their quality reporting requirements for that calendar year payment determination. We finalized our proposal that application of the 2.0 percentage point reduction to the annual update may result in the update to the ASC payment system being less than zero prior to the application of the MFP adjustment.
The ASC conversion factor is used to calculate the ASC payment rate for services with the following payment indicators (listed in Addenda AA and BB to the proposed rule, which are available via the internet on the CMS website): “A2”, “G2”, “P2”, “R2” and “Z2”, as well as the service portion of device-intensive procedures identified by “J8” (77 FR 68500). We finalized our proposal that payment for all services assigned the payment indicators listed above would be subject to the reduction of the national unadjusted payment rates for applicable ASCs using the ASCQR Program reduced update conversion factor (77 FR 68500).
The conversion factor is not used to calculate the ASC payment rates for separately payable services that are assigned status indicators other than payment indicators “A2”, “G2”, “J8”, “P2”, “R2” and “Z2.” These services include separately payable drugs and biologicals, pass-through devices that are contractor-priced, brachytherapy sources that are paid based on the OPPS payment rates, and certain office-based procedures, radiology services and diagnostic tests where payment is based on the PFS nonfacility PE RVU-based amount, and a few other specific services that receive cost-based payment (77 FR 68500). As a result, we also finalized our proposal that the ASC payment rates for these services would not be reduced for failure to meet the ASCQR Program requirements because the payment rates for these services are not calculated using the ASC conversion factor and, therefore, not affected by reductions to the annual update (77 FR 68500).
Office-based surgical procedures (generally those performed more than 50 percent of the time in physicians' offices) and separately paid radiology services (excluding covered ancillary radiology services involving certain nuclear medicine procedures or involving the use of contrast agents) are paid at the lesser of the PFS nonfacility PE RVU-based amounts or the amount calculated under the standard ASC ratesetting methodology. Similarly, in the CY 2015 OPPS/ASC final rule with comment period (79 FR 66933 through 66934), we finalized our proposal that payment for certain diagnostic test codes within the medical range of CPT codes for which separate payment is allowed under the OPPS will be at the lower of the PFS nonfacility PE RVU-based (or technical component) amount or the rate calculated according to the standard ASC ratesetting methodology when provided integral to covered ASC surgical procedures. In the CY 2013 OPPS/ASC final rule with comment period (77 FR 68500), we finalized our proposal that the standard ASC ratesetting methodology for this type of comparison would use the ASC conversion factor that has been calculated using the full ASC update adjusted for productivity. This is necessary so that the resulting ASC payment indicator, based on the comparison, assigned to these procedures or services is consistent for each HCPCS code, regardless of whether payment is based on the full update conversion factor or the reduced update conversion factor.
For ASCs that receive the reduced ASC payment for failure to meet the ASCQR Program requirements, we believe that it is both equitable and appropriate that a reduction in the payment for a service should result in proportionately reduced coinsurance liability for beneficiaries (77 FR 68500). Therefore, in the CY 2013 OPPS/ASC final rule with comment period (77 FR 68500), we finalized our proposal that the Medicare beneficiary's national unadjusted coinsurance for a service to which a reduced national unadjusted payment rate applies will be based on the reduced national unadjusted payment rate.
In that final rule with comment period, we finalized our proposal that all other applicable adjustments to the ASC national unadjusted payment rates would apply in those cases when the annual update is reduced for ASCs that fail to meet the requirements of the ASCQR Program (77 FR 68500). For example, the following standard adjustments would apply to the reduced national unadjusted payment rates: The wage index adjustment; the multiple procedure adjustment; the interrupted procedure adjustment; and the adjustment for devices furnished with full or partial credit or without cost (77 FR 68500). We believe that these adjustments continue to be equally applicable to payment for ASCs that do not meet the ASCQR Program requirements (77 FR 68500).
In the CY 2015, CY 2016, CY 2017, CY 2018, and CY 2019 OPPS/ASC final rules with comment period (79 FR 66981 through 66982; 80 FR 70537 through 70538; 81 FR 79825 through 79826; 82 FR 59475 through 59476; and 83 FR 59138 through 59139, respectively), we did not make any other changes to these policies. We are not proposing any changes to these policies for CY 2020 in this proposed rule.
XVI. Proposed Requirements for Hospitals To Make Public a List of Their Standard Charges
A. Introduction and Overview
1. Statutory Basis and Current Guidance
Section 1001 of the Patient Protection and Affordable Care Act (Pub. L. 111-148), as amended by section 10101 of the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152), amended Title XXVII of the Public Health Service Act (the PHS Act), in part, by adding a new section 2718(e). Section 2718 of the PHS Act, entitled “Bringing Down the Cost of Health Care Coverage,” requires each hospital operating within the United States for each year to establish (and update) and make public a list of the hospital's standard charges for items and services provided by the hospital, including for diagnosis-related groups established under section 1886(d)(4) of the Social Security Act (the Act).
In the FY 2015 IPPS/LTCH PPS proposed and final rules (79 FR 28169 and 79 FR 50146, respectively), we reminded hospitals of their obligation to comply with the provisions of section 2718(e) of the PHS Act and provided guidelines for its implementation. At that time, we required hospitals to either make public a list of their standard charges or their policies for allowing the public to view a list of those charges in response to an inquiry. In addition, we stated that we expected hospitals to update the information at least annually, or more often as appropriate, to reflect current charges. We also encouraged hospitals to undertake efforts to engage in consumer-friendly communication of their charges to enable consumers to compare charges for similar services across hospitals and to help consumers understand what their potential financial liability might be for items and services they obtain at the hospital.
In the FY 2019 IPPS/LTCH PPS proposed rule and final rule (83 FR 20164 and 83 FR 41144, respectively), we again reminded hospitals of their obligation to comply with the provisions of section 2718(e) of the PHS Act and updated our guidelines for its implementation. The announced update to our guidelines became effective January 1, 2019, and took one step to further improve the public accessibility of standard charge information. Specifically, we updated our guidelines to require hospitals to make available a list of their current standard charges via the internet in a machine-readable format and to update this information at least annually, or more often as appropriate. We subsequently published two sets of Frequently Asked Questions (FAQs) [150] that provided additional guidance to hospitals, including a FAQ clarifying that while hospitals could choose the format they would use to make public a list of their standard charges, the publicly posted information should represent their standard charges as reflected in the hospital's chargemaster. We also clarified that the requirement applies to all hospitals operating within the United States and to all items and services provided by the hospital.
2. Background
As health care costs continue to rise, health care affordability has become an area of intense focus. Health care spending is projected to consume 20 percent of the economy by 2026.[151] One reason for this upward trajectory in spending is the lack of transparency in health care pricing.[152] Additionally, numerous studies suggest that consumers want greater transparency. For example, a study of high deductible health plan enrollees found that respondents wanted additional health care price information so that they could make more informed decisions about where to seek care based on price.[153] Health economists and other experts state that significant cost containment cannot occur without widespread and sustained transparency in provider prices.[154] We believe there is a direct connection between transparency in hospital standard charge information and having more affordable health care and lower health care coverage costs. We believe health care markets could work more efficiently and provide consumers with higher-value health care if we promote policies that encourage choice and competition.[155] In short, as articulated by the CMS Administrator, we believe that transparency in health care pricing is “critical to enabling patients to become active consumers so that they can lead the drive towards value.” [156]
Many empirical studies have investigated the impact of price transparency on markets, with most research showing that price transparency leads to lower and more uniform prices, consistent with predictions of standard economic theory.[157] Traditional economic analysis suggests that if consumers have better pricing information for health care services, providers would face pressure to lower prices and provide better quality care.[158] Falling prices may, in turn, expand access to health care for consumers.[159]
Presently, however, the information that health care consumers need to make informed decisions based on the prices of health care services is not readily available. The Government Accountability Office (GAO) report (2011), “Health Care Price Transparency: Meaningful Price Information is Difficult for Consumers to Obtain Prior to Receiving Care,” [160] found that opacity in health care prices, coupled with the often wide pricing disparities for particular procedures within the same market, can make it difficult for consumers to understand health care prices and to effectively shop for value. The report references a number of barriers that make it difficult for consumers to obtain price estimates in advance for health care services. Such barriers include the difficulty of predicting health care service needs in advance, a complex billing structure resulting in bills from multiple providers, the variety of insurance benefit structures, and concerns related to the public disclosure of rates negotiated between providers and third party payers. The GAO report goes on to explore various price transparency initiatives, including tools that consumers could use to generate price estimates in advance of receiving a health care service. The report notes that pricing information displayed by tools varies across initiatives, in large part due to limits reported by the initiatives in their access or authority to collect certain necessary price data. According to the GAO report, transparency initiatives were best able to provide reasonable estimates of consumers' complete costs when they had access and integrated pricing data from both providers and insurers.
The concept of making health care provider charges and insurance benefit information available to consumers is not new; some States have required disclosure of pricing information by providers and payers for a number of years. More than half of the States have passed legislation establishing price transparency websites or mandating that health plans, hospitals, or physicians make price information available to consumers.[161] As of early 2012, there were 62 consumer-oriented, State-based health care price comparison websites.[162] Half of these websites were launched after 2006, and most were developed and funded by a State government agency (46.8 percent) or hospital association (38.7 percent).[163] Most websites report prices of inpatient care for medical conditions (72.6 percent) or surgeries (71.0 percent). Information about prices of outpatient services such as diagnostic or screening procedures (37.1 percent), radiology studies (22.6 percent), prescription drugs (14.5 percent), or laboratory tests (9.7 percent) are reported less often.[164]
Since the early 2000s, California-licensed hospitals have been required to submit annually to the State for public posting on a State website: The charge description master (CDM, also known as a “chargemaster”); a list of the hospital's average charges for at least 25 common outpatient procedures, including ancillary services; and the estimated percentage increase in gross revenue due to price changes.[165] The information is required to be submitted in plain language using easily understood terminology.[166] In 2012, Massachusetts began requiring insurers to provide, upon request, the estimated amount insured patients will be responsible to pay for proposed admissions, procedures, or services based upon the information available to the insurer at the time, and also began requiring providers to disclose the charge for the admission, procedure, or service upon request by the patient within 2 working days.[167] Since 2015, Oregon has offered pricing data for the top 100 common hospital outpatient procedures and top 50 common inpatient procedures on its OregonHospitalGuide.org website, which displays the median negotiated amount of the procedure by hospital and includes patient paid amounts such as deductibles and copayments. The data are derived from State-mandated annual hospital claims collection by the State's all payer claims database (APCD) and represent the service package cost for each of the procedures, including ancillary services and elements related to the procedure, with the exception of professional fees which are billed separately.[168] More recently, in 2018, Colorado began requiring hospitals to post the prices of the 50 most used diagnosis-related group (DRG) codes, and the 25 most used outpatient Current Procedural Terminology (CPT) codes or health care services procedure codes with a “plain-English description” of the service, which must be updated at least annually.[169]
Not only have States taken an interest in price transparency, but insurers and self-funded employers have also moved in this direction. For example, some self-funded employers are using price transparency tools to incentivize their employees to make cost-conscious decisions when purchasing health care services. Most large insurers have embedded cost estimation tools into their member websites, and some provide their members with comparative cost and value information, which includes rates that the insurers have negotiated with in-network providers and suppliers.
Research suggests that making such consumer-friendly pricing information available to the public can reduce health care costs for consumers. Specifically, recent research evaluating the impact of New Hampshire's price transparency efforts reveals that providing insured patients with information about prices can have an impact on the out-of-pocket costs paid by consumers for medical imaging procedures, not only by helping users of New Hampshire's website choose lower-cost options, but also by leading to lower prices that benefited all patients, including those in the State that did not use the website.[170]
Despite the growing consumer demand and awareness of the need for health care pricing data, there continues to be a gap in easily accessible pricing information for consumers to use for health care shopping purposes. Specifically, there is inconsistent (and many times nonexistent) availability of provider charge information. We believe this information gap can, in part, be filled by the proposals in this proposed rule which seek to further price transparency by proposing to adopt new requirements under section 2718(e) of the PHS Act, as described below. We believe that ensuring public access to hospital standard charge data will promote and support current and future price transparency efforts. We believe that this, in turn, will enable health care consumers to make more informed decisions, increase market competition, and ultimately drive down the cost of health care services, making them more affordable for all patients.
3. Summary of Stakeholder Engagement
In the FY 2019 IPPS/LTCH PPS proposed rule (83 FR 20548 and 20549) and other Requests for Information (RFIs) published during 2018 (which we will refer to as the 2018 RFIs),[171] we remarked that challenges continue to exist for consumers because of insufficient transparency in pricing information. Therefore, we sought public comment on a variety of questions related to our price transparency efforts, including:
- What types of information would be most beneficial to patients, how can health care providers and suppliers best enable patients to use charge and cost information in their decision-making, and how can CMS and providers help third parties create patient-friendly interfaces with these data?
- Should health care providers and suppliers be required to inform patients how much their out-of- pocket costs for a service will be before those patients are furnished that service? What changes would be needed to support greater transparency around patient obligations for their out-of-pocket costs? What can be done to better inform patients of these obligations? Should health care providers and suppliers play any role in helping to inform patients of what their out-of-pocket obligations will be?
Most of the commenters who responded to the 2018 RFIs supported furthering price transparency efforts, although a few stakeholders opposed efforts to make hospital pricing information available to the public. Reasons stakeholders cited in opposition included, for example: That hospital chargemasters are highly technical documents that frequently identify items and services by the complex payment codes used by hospitals for purposes of billing, instead of terms that consumers can understand; concern that hospital charge data as found in the hospital chargemaster may not be helpful to consumers for determining what they are likely to pay for a service or facility encounter because most consumers have health insurance; concern that some pricing information might be commercially sensitive; and that posting price information without corresponding educational tools might increase patient confusion.
In addition to seeking public input on price transparency issues through the 2018 RFIs, we hosted a series of five listening sessions in the summer and fall of 2018 that were attended by a wide representation of stakeholders, including hospitals, clinicians, payers, tool developers, and consumer and patient advocacy groups. During the listening sessions, several stakeholders applauded our efforts to release public use files on a quarterly basis and stated that they use the information in those files to supplement their algorithms to provide Medicare fee-for-service patients with out-of-pocket pricing information. Price transparency tool developers asserted that machine-readable chargemaster release would provide promising opportunities and support existing efforts for user-friendly tool development, including the development of out-of-pocket comparison cost estimates for self-pay and commercially insured health care consumers. Some stakeholders noted that the most useful pricing information for consumers is information that displays a patient's expected out-of-pocket costs for nonurgent health care services that can be scheduled in advance, also referred to as “shoppable” services.
We appreciate the many detailed comments and suggestions stakeholders have provided us during the past year. In this proposed rule, after taking into consideration our past pricing transparency efforts and stakeholder feedback and our policy objective to make price information more readily available, we are proposing to codify a set of requirements that further implement section 2718(e) of the PHS Act. We believe that the public posting of hospital standard charge information will be useful to health care consumers who need to obtain items and services from a hospital, health care consumers who wish to view hospital prices prior to selecting a hospital, clinicians who use the data at the point of care when making referrals, and other members of the public who may develop consumer-friendly price transparency tools. These proposed requirements represent an important step towards putting health care consumers at the center of their health care and ensuring they have access to the hospital standard charge information they need.
4. Summary of Proposals
Health care consumers continue to lack the meaningful pricing information they need to choose the healthcare services they want and need despite prior requirements for hospitals to publicly post their chargemaster rates online. Therefore, in response to stakeholders and in accordance with President's Executive Order on “Improving Price and Quality Transparency in American Healthcare to Put Patients First” (June 24, 2019), we are proposing an expansion of hospital charge display requirements to include charges and information based on negotiated rates and for common shoppable items and services, in a manner that is consumer-friendly. We believe this will meaningfully inform patients' decision making and allow consumers to compare prices across hospitals. We are also proposing to establish a mechanism for monitoring and the application of penalties for noncompliance.
Specifically, we are proposing to add a new Part 180—Hospital Price Transparency to title 45 of the Code of Federal Regulations (CFR) which would contain our regulations on price transparency for purposes of section 2718(e) of the PHS Act. In our discussions in the sections that follow, we make proposals related to: (1) A definition of “hospital”; (2) different reporting requirements that would apply to certain hospitals; (3) definitions for two types of “standard charges” (specifically, gross charges and payer-specific negotiated charges) that hospitals would be required to make public, and a request for public comment on other types of standard charges that hospitals should be required to make public; (4) a definition of hospital “items and services” that would include all items and services (both itemized and packaged) provided by the hospital to a patient in connection with an inpatient admission or an outpatient department visit; (5) requirements for making public a machine-readable file that contains a hospital's gross charges and payer-specific negotiated charges for all items and services provided by the hospital; (6) requirements for making public payer-specific negotiated charges for select hospital-provided items and services that are “shoppable” and that are displayed and packaged in a consumer-friendly manner; (7) monitoring for hospital noncompliance with public disclosure requirements to make public standard charges; (8) actions that would address hospital noncompliance, which include issuing a written warning notice, requesting a corrective action plan, and imposing civil monetary penalties (CMPs) on noncompliant hospitals and publicizing these penalties on a CMS website; and (9) appeals of CMPs.
We believe that these proposals requiring public release of hospital standard charge information are a necessary and important first step in ensuring transparency in health care prices for consumers, although we recognize that the release of hospital standard charge information is not sufficient by itself to achieve our ultimate goals for price transparency. For example, we know through our stakeholder engagement and research conducted over the past year that consumers of health care services simply want to know where they can get a needed health care service and what that service will cost them out-of-pocket. There are many barriers to achieving this simple desire to make price comparisons for health care services, including that the data necessary for such an analysis are not available to the general public. Necessary data to make price comparisons depends on an individual's circumstances. For example, a self-pay individual may simply want to know the amount a health care provider will accept in cash (or cash equivalent) as payment in full, while an individual with health insurance may want to know the charge negotiated between the health care provider and payer, along with additional individual benefit-specific information such as the amount of cost-sharing, the network status of the health care provider, how much of a deductible has been paid to date, and other information. The proposals in this proposed rule seek to address the barriers related to lack of hospital data by standardizing the release of two types of hospital standard charge information—gross charges and payer-specific negotiated charges.
We believe these proposed policies are an important first step in our efforts to achieve price transparency in health care, and believe our proposed policies should be viewed in the context of the broader price transparency initiative. We are continuing to explore other authorities that the Department can use to further advance our goal of getting patients the information they need to make informed health care decisions.
B. Proposed Definition of “Hospital” and Proposed Special Requirements That Would Apply to Certain Types of Hospitals
1. Proposed Definition of “Hospital”
Section 2718(e) of the PHS Act does not define “hospital.” Initially, we considered proposing to adopt a definition of “hospital” that is used either in other sections of the PHS Act or in the Social Security Act, but we found that no single or combined definition was suitable because those other definitions were applicable to specific programs or Medicare participation and therefore had program-specific requirements that made them too narrow for our purposes. For example, we considered referencing the definition of “hospital” at section 1861(e) of the Social Security Act because that definition is well understood by institutions that participate as hospitals for purposes of Medicare. However, we were concerned that doing so could have had the unintentional effect of limiting the institutions we believe should be covered by section 2718(e) of the PHS Act. Even so, we believe that the licensing requirement described at section 1861(e)(7) of the Social Security Act captures the institutions that we believe should be characterized as hospitals for purposes of this section.
Accordingly, we are proposing to define a “hospital” as an institution in any State in which State or applicable local law provides for the licensing of hospitals, (1) is licensed as a hospital pursuant to such law or (2) is approved, by the agency of such State or locality responsible for licensing hospitals, as meeting the standards established for such licensing (which we propose to codify in new 45 CFR 180.20).
We believe this proposed definition is the best way to ensure that section 2718(e) of the PHS Act applies to each hospital operating within the United States. First, in addition to applying to all Medicare-enrolled hospitals (that, by definition, must be licensed by a State as a hospital, or otherwise approved by the State or local licensing agency as meeting hospital licensing standards), the proposed definition would also capture any institutions that are, in fact, operating as hospitals under State or local law, but might not be considered hospitals for purposes of Medicare participation. As discussed in section XVI.A.2. of this proposed rule, many States have promoted price transparency initiatives and some require institutions they license as hospitals to make certain charges public as a part of those initiatives. Therefore, defining a hospital by its licensure (or by its approval by the State or locality as meeting licensing standards) may carry the advantage of aligning the application of Federal and State price transparency initiatives to the same institutions.
We also are proposing that, for purposes of the definition of “hospital,” a State includes each of the several States, the District of Columbia, Puerto Rico, the Virgin Islands, Guam, American Samoa, and the Northern Mariana Islands. This proposed definition of State would be consistent with how that term is defined under section 2791(d)(14) of the PHS Act. We believe that adopting this definition of “State” for purposes of section 2718(e) of the PHS Act is appropriate because, unlike the other provisions in section 2718 which apply to health insurance issuers, section 2718(e) applies to hospitals. Therefore, it is distinguishable from the approach outlined in the July 2014 letters [172] to the Territories regarding the PHS Act health insurance requirements established or amended by Public Law 111-148 and Public Law 111-152.
Our proposed definition focuses on whether or not the institution is licensed by the State or under applicable local law as a hospital, or is approved, by the agency of such State or locality responsible for licensing hospitals, as meeting the standards established for such licensing. As such, a “hospital” would include each institution that satisfies the definition, regardless of whether that institution is enrolled in Medicare or, if enrolled, regardless of how Medicare designates the institution for its purposes. Thus, the proposed definition would include critical access hospitals (CAHs), inpatient psychiatric facilities (IPFs), sole community hospitals (SCHs), and inpatient rehabilitation facilities (IRFs), which we previously identified in our guidelines as being hospitals for the purposes of section 2718(e),[173] as well as any other type of institution, so long as such institutions are licensed as a hospital (or otherwise approved) as meeting hospital licensing standards.
Finally, we note that the proposed definition of “hospital” would not include entities such as ambulatory surgical centers (ASCs) or other non-hospital sites-of-care from which consumers may seek health care items and services. For example, nonhospital sites may offer ambulatory surgical services, laboratory or imaging services, or other services that are similar or identical to the services offered by hospital outpatient departments. In the interest of increasing opportunities for health care consumers to compare prices for similar services and promoting widespread transparency in health care prices, we encourage non-hospital sites-of-care to make public their lists of standard charges in alignment with these proposed requirements so that consumers can make effective pricing comparisons.
We invite public comments on our proposed definition of “hospital,” which we are proposing to codify at 45 CFR 180.20.
2. Proposed Special Requirements That Would Apply to Certain Hospitals
In sections XVI.E. and XVI.F of this proposed rule, we propose the requirements that most institutions meeting our definition of “hospital” would have to meet in order to comply with section 2718(e) of the PHS Act. However, we are proposing that these requirements would not apply to federally-owned or operated hospitals, including Indian Health Service (IHS) facilities (including Tribally-owned and operated facilities), Veterans Affairs (VA) facilities, and Department of Defense Military Treatment Facilities (MTFs), because, with the exception of some emergency services, these facilities do not provide services to the general public and the established payment rates for services are not subject to negotiation. Instead, each of these facility types is authorized to provide services only to patients who meet specific eligibility criteria. For example, individuals must meet the requirements enumerated at 42 CFR 136.22 through 136.23 to be eligible to receive services from IHS and Tribal facilities. Similarly, under 38 CFR 17.43 through 17.46, Veterans Affairs hospitals provide hospital, domiciliary, and nursing home services to individuals with prior authorization who are discharged or retiring members of the Armed Forces and, upon authorization, beneficiaries of the Public Health Service, Office of Workers' Compensation Programs, and other Federal agencies (38 CFR 17.43). In addition, federally-owned or operated hospitals such as IHS and Tribal facilities [174] impose no cost-sharing, or, in the case of VA hospitals [175] and Department of Defense MTFs,[176] little cost-sharing. With respect to such facilities where there is cost-sharing, the charges are publicized through the Federal Register, Federal websites, or direct communication and therefore known to the populations served by such facilities in advance of receiving health care services. Only emergency services at federally-owned or operated facilities are available to non-eligible individuals. Because these hospitals do not treat the general public, their rates are not subject to negotiation, and the cost sharing obligations for hospital provided services are known to their patients in advance, we believe it is appropriate to establish different requirements that apply to these hospitals. Specifically, we are proposing to deem federally owned or operated hospitals that do not treat the general public (except for emergency services) and whose rates are not subject to negotiation, meet the requirements of section 2718(e) of the PHS Act when their charges for hospital provided services are publicized to their patients in advance (for example, through the Federal Register) (proposed new 45 CFR 180.30(b)).
In addition, as a result of public comments received in response to the 2018 RFIs suggesting that certain hospitals be exempted from having to make public their standard charges, we considered whether it was appropriate to establish different requirements for hospitals located in a rural areas, critical access hospitals (CAHs), or hospitals that are not federally owned or operated but that serve special populations (such as children's hospitals and State psychiatric hospitals). However, because such hospitals are open to the general public, and their charges are generally not made available to the public, we continue to believe there is value in such hospitals making public their standard charges. For example, hospitals may gain market share and enjoy increased patient satisfaction as a result of being transparent with their prices.[177] Moreover, we believe that the proposed requirements are not overly burdensome because hospitals already have these data readily available. Therefore, at this time, we are not proposing different requirements for hospitals located in rural areas, CAHs or hospitals that are not federally owned or operated but that treat special populations. However, we are requesting public comments on whether exceptions to our proposed requirements might be warranted for hospitals (for example, hospitals located in rural areas, CAHs, or hospitals that treat special populations) that are not federally owned or operated, while also ensuring that charges for the services provided by such hospitals are available to the public. Specifically, we recognize that many hospitals are going above and beyond these proposed requirements, for example, by offering patient-friendly price transparency tools that calculate individualized out-of-pocket cost estimates. We seek comment on whether offering such tools could qualify a hospital to be excepted from some of the proposed requirements, for example, the consumer-friendly display requirements discussed in section XVI.F.
C. Proposed Definition of “Items and Services” Provided by Hospitals
Section 2718(e) of the PHS Act requires that hospitals make public a list of the hospital's standard charges for items and services provided by the hospital, including for diagnosis related groups (DRGs). We are proposing that, for purposes of section 2718(e), “items and services” provided by the hospital are all items and services, including individual items and services and service packages, that could be provided by a hospital to a patient in connection with an inpatient admission or an outpatient department visit for which the hospital has established a standard charge. Examples of these items and services include, but are not limited to, supplies, procedures, room and board, use of the facility and other items (generally described as facility fees), services of employed physicians and non-physician practitioners (generally reflected as professional charges), and any other items or services for which a hospital has established a charge.
Our proposed definition includes both individual items and services as well as “service packages” for which a hospital has established a charge. Every hospital maintains a file system known as the chargemaster (or Charge Description Master “CDM”), which contains all billable procedure codes performed at the hospital, along with descriptions of those codes and the hospitals' own list prices. The format and contents of the chargemaster vary from one hospital to the next, although the source codes are derived from common billing code systems (such as the AMA's CPT system). Chargemasters can include tens of thousands of line items, depending on the type of facility, and can be maintained in spreadsheet or database formats.[178] For purposes of section 2718(e) of the PHS Act, we are proposing to define “chargemaster” to mean the list of all individual items and services maintained by a hospital for which the hospital has established a standard charge (at proposed new 45 CFR 180.20). Each individual item or service found on the hospital chargemaster has a corresponding “gross” charge (discussed in more detail in section XVI.D.2). Each individual item or service may also have a corresponding negotiated discount because some hospitals negotiate with third party payers to establish a flat percent discounted rate off the gross charge for each individual item and service listed on the chargemaster; for example, a hospital may negotiate a 50 percent discount off all chargemaster gross rates with a third party payer.
In contrast to the chargemaster or so-called “fee-for-service” price list, hospitals also routinely negotiate rates with third party payers for bundles of services or “service packages” in lieu of charging for each and every imaging study, laboratory test, or alcohol swab found on the chargemaster.[179] Such service packages may have charges established on, for example, the basis of a common procedure or patient characteristic, or may have an established per diem rate that includes all individual items and services furnished during an inpatient stay. Some hospitals present “self-pay package pricing” for prompt same-day payment from health care consumers. The hospital's billing and accounting systems maintain the negotiated charges for service packages which are commonly identified in the hospital's billing system by recognized industry standards and codes. For example, a diagnosis-related group (DRG) system may be used to define a hospital product based on the characteristics of patients receiving similar sets of [itemized] services.[180] Medicare and some commercial insurers have adopted DRG classifications as a method of inpatient hospital payment. Other codes (for example, payer specific codes, CPT or HCPCS codes) are used by hospitals and payers to identify service packages based on procedures.
For purposes of section 2718(e) of the PHS Act, we are proposing to define a “service package” to mean an aggregation of individual items and services into a single service with a single charge (proposed new 45 CFR 180.20). We believe this is appropriate and consistent with section 2718(e) of the PHS Act because we believe its inclusion of DRGs as an item or service in section 2718(e) recognizes that hospital services can be provided and charged for based on the service's individual component parts or as a more inclusive packaged service. While section 2718(e) of the PHS Act specifically includes items and services grouped into DRGs as an example of the items and services for which hospitals must list their standard charges, we believe that our definition of “items and services” should include not just all DRGs (as established under 1886(d)(4) of the Social Security Act) but also all other service packages provided by the hospital, including, for example, service packages the hospital provides in an outpatient setting for which a hospital may have established a standard charge. Therefore, our proposed definition of “items and services” includes both individual items and services and service packages.
We would also include in our proposed definition of “items and services” provided by the hospital the services furnished by physicians and non-physician practitioners who are employed by the hospital. We believe the services the hospital provides through its employed physicians (and non-physician practitioners) are items and services provided by the hospital, because such physicians (and non-physician practitioners) are employed by the hospital specifically so that the hospital can offer such services to the hospital's patients. In addition, the hospital establishes and negotiates the charges for the employed physician and non-physician services. The hospital bills and retains the payment for the professional services of employed physician and non-physician practitioners. We therefore believe it is appropriate for these services to be included in our proposed definition of hospital items and services provided by the hospital under Section 2718(e), and for hospitals to make public the charges for the services of their employed physician and non-physician practitioners.
We also considered including in our proposed definition of items and services the services provided by physicians and non-physician practitioners who are not employed by the hospitals, but who provide services at a hospital location. For example, a procedure performed in a hospital setting may involve anesthesiology services provided by a non-employed physician who has established his or her own charge for the service he or she is providing at a hospital location. These physicians and non-physician practitioners may send a bill that is separate from the hospital bill, or, they may elect to reassign their billing rights to the hospital that will send a single bill that includes both hospital charges and professional service charges. Often, health care consumers are not expecting an additional charge or are otherwise surprised when they receive bills from entities other than the hospital, or when charges for non-employed physicians and non-physician practitioners are higher than expected (for example, when a non-employed physician is out-of-network and the consumer's third party payer declines payment for those services for that reason). We believe that the provision of such additional charge information would be exceptionally valuable to give consumers a more complete picture of the total amount they might be charged in connection with an inpatient admission or an outpatient department visit at a hospital location, potentially helping to address the widely recognized “surprise billing” issue. However, because physicians and non-physician practitioners who are not employed by the hospital are practicing independently, establish their own charges for services, and receive the payment for their services, we do not believe their charges for their services fall within the scope of section 2718(e) as they are not services “provided by the hospital.”
We welcome comment on these proposals.
D. Proposed Definitions for Types of “Standard Charges”
1. Overview and Background
Under our current guidelines regarding section 2718(e) of the PHS Act (as discussed in the FY 2019 IPPS/LTCH PPS proposed rule and final rule (83 FR 20164 and 41144, respectively)), a hospital may choose the format it uses to make public a list of its standard charges, so long as the information represents the hospital's current standard charges as reflected in its chargemaster.
In response to the 2018 RFIs, several commenters, including hospitals and patient advocacy organizations, commented that gross charges as reflected in hospital chargemasters may only apply to a small subset of consumers; for example, those who are self-pay or who are being asked to pay the chargemaster rate because the hospital is not included in the patient's insurance network. Many commenters also noted that the charges listed in a hospital's chargemaster are typically not the amounts that hospitals actually charge to consumers who have health insurance because, for the insured population, hospitals charge amounts reflect discounts to the chargemaster rates that the hospital has negotiated with third party payers. Further, with respect to patients who qualify for financial assistance or who pay in cash, commenters pointed out that some hospitals will charge lower amounts than the rates that appear on the chargemaster. Adding to the complexity, some commenters noted that hospitals often package items and services and charge a single discounted negotiated amount for the packaged service. For example, as discussed in XVI.C. of this proposed rule, instead of itemizing and charging for each individual hospital item or service found on the chargemaster, a hospital may identify a primary common condition or procedure and charge a single negotiated or “cash” amount for the primary common condition or procedure that includes all associated items and services that are necessary for treatment of the common condition or to perform the procedures. We believe that these comments illustrate a fundamental challenge of making health care prices transparent in general, and specifically with respect to the issue of how we should best implement section 2718(e) of the PHS Act; simply put, hospitals do not offer all consumers a single “standard charge” for the items and services they furnish. Rather, the “standard charge” for an item or service (including service packages) varies depending on the circumstances particular to the consumer.
Therefore, we sought public comment through the RFIs issued in 2018 [181] on a definition of “standard charges.” Specifically, we requested information on the following:
- Should “standard charges” be defined to mean: Average or median rates for the items in the chargemaster; average or median rates for groups of services commonly billed together (such as for an MS-DRG), as determined by the hospital based on its billing patterns; or the average discount off the chargemaster amount across all payers, either for each item on the chargemaster or for groups of services commonly billed together?
- Should “standard charges” be defined and reported for both some measure of the average contracted rate and the chargemaster [rate]? Or is the best measure of a hospital's standard charges its chargemaster [rate]?
Commenters responded with a number of suggestions for defining “standard charges” including the following:
- Chargemaster rates.
- Average discount off the chargemaster amount across all payers (for example, an average negotiated rate).
- Actual, estimated, or average out-of-pocket costs to individuals.
- The amount the hospital will accept as payment in full for items and services (without complications) by non-governmental payers and individuals (for example, a negotiated rate).
- Usual and customary charges as defined by the National Council of Insurance Legislators (NCIL). Specifically, the NCIL defines usual and customary as the 80th percentile of physician charges in a geographic region based on an independent unbiased benchmarking charge database.
- Median or average charges for groups of services routinely billed together, such as at the DRG or APC level, or other layman-termed groupings.
- Average median payment rate or average out-of-pocket charges for shoppable services (that is, nonemergent or elective procedures that patients will most likely use).
- Net negotiated charges for health insurance plan networks.
We appreciate the many comments and suggestions on this issue offered by stakeholders. We believe the variety of suggested definitions reflects our assessment that hospitals can have different standard charges for various groups of individuals. In general, for purposes of 2718(e), we believe a standard charge can be identified as a charge that is the regular rate established by the hospital for the items and services provided to a specific group of paying patients. Therefore, we considered what types of standard charges may reflect certain common and identifiable groups of paying patients. After considering the feedback noted above and the various types of standard charges that may exist, we are proposing to define standard charges to mean “gross charges” and “payer-specific negotiated charges,” and to codify this definition in proposed new 45 CFR 180.20. “Gross charges” and “payer-specific negotiated charges” are further defined in sections XVI.D.2. and XVI.D.3., respectively, of this proposed rule. We believe the proposal to define standard charges as gross charges and payer-specific negotiated charges reflects the fact that a hospital's standard charge for an item or service is not typically a single fixed amount, but, rather, depends on factors such as who is being charged for the item or service, and particular circumstances that apply to an identifiable group of people, including, for example, health care consumers that are insured members of third party insurance products and plans that have negotiated a rate on its members' behalf.
We are proposing to define standard charges as “gross charges” and “payer-specific negotiated charges” based on our research and prior stakeholder input. Hospitals would be required to make public these two types of standard charges in the form and manner proposed in sections XVI.E and F. As explained in section XVI.C. of this proposed rule, gross charges found in the chargemaster as well as negotiated charges are both informative and necessary for consumers to understand their potential out-of-pocket cost obligations, but such information is not readily available to consumers. We believe these two specific types of standard charges have the potential to inform two large identifiable groups of health care consumers who do not currently have ready access to hospital charge information, specifically those who have limited power to negotiate charges (for example, self-pay individuals) and those who rely on third party payers to negotiate charges on their behalf. We also believe that these two specific types of standard charges present a limited burden for hospitals to make publicly available, because these charges are already available, maintained, and in use in hospital billing systems. Moreover, we believe these two specific types of standard charges are necessary basic information needed to begin to ensure that consumers have the ability to shop for and compare pricing for health care services. We believe these proposals will help provide information to consumers to help make health care more affordable and drive down the cost of health care coverage.
We acknowledge that the proposed definition of hospital “standard charges” is limited to only two of the many possibilities that exist for defining types of hospital “standard charges,” and we discuss below other potential definitions that we considered, but decided not to propose at this time. We are seeking public input and comment on the alternatives and additional types of standard charges that may be useful to consumers.
2. Proposed Definition of “Gross Charges” as a Type of Standard Charge
As previously noted, in general, for purposes of 2718(e), we believe a standard charge can be identified as a charge that is the regular rate established by the hospital for the items and services provided to a specific group of paying patients. We are proposing that, for purposes of the first type of “standard charge,” a “gross charge” would be defined as the charge for an individual item or service that is reflected on a hospital's chargemaster, absent any discounts (at proposed new 45 CFR 180.20). As we explain in section XVI.C. of this proposed rule, the hospital chargemaster contains a list of all individual items and services the hospital provides. The gross charges reflected in the chargemaster often apply to a specific group of individuals who are self-pay, but do not reflect charges negotiated by third party payers. We also note that the chargemaster does not include charges that the hospital may have negotiated for service packages, such as per diem rates, DRGs or other common payer service packages, and therefore this type of standard charge would not include standard charges for service packages.
We are proposing to require hospitals to make public their gross charges because, in addition to applying to a specific group of individuals, based on research and stakeholder input, we believe gross charges are useful to the general public, necessary to promote price transparency, and necessary to drive down premium and out-of-pocket costs for consumers of health care. For example, studies suggest that the gross charge plays an important role in the negotiation of third party insurance products that are subsequently sold to consumers.[182] Specifically, as hospital executives and others familiar with hospital billing cycles often note, hospitals routinely use gross charges as a starting point for negotiating discounted rates with third party payers, and higher gross charges have been found to be associated with both higher negotiated rates and, in turn, higher premiums and out-of-pocket costs for insured individuals.[183] [184] As such, gross charges are relevant to all consumers, including those with insurance coverage. We believe that requiring transparency of hospital gross charges may drive competition, which might, in turn, have the effect of not only lowering hospital charges for the most vulnerable consumers and those with the least market power to negotiate prices, but also for consumers who have access to charges negotiated on their behalf by a third party payer.
In addition, as a result of stakeholder feedback, we learned that third party developers of consumer price transparency tools can use gross charges in conjunction with additional information (such as an individual's specific insurance and benefit information and quality data) to develop and make available consumer-friendly out-of-pocket cost estimates that allow consumers to compare health care service prices across hospitals and other nonhospital settings of care. Moreover, as previously noted in section XVI.A.2., research suggests that making such consumer-friendly information available to the public has been demonstrated to reduce consumer health care costs. As such, we believe that public access to hospital gross charges is critical to inform all patients (both self-pay and insured) of their choices and drive transparency in prices.
We are proposing to codify the proposed definition of “gross charges” at proposed new 45 CFR 180.20. We are inviting public comments on our proposal to define a type of “standard charge” as a “gross charge” and on our proposed definition of “gross charge.”
3. Proposed Definition of “Payer-Specific Negotiated Charge” as a Type of Standard Charge
As noted in section XVI.D.1, in general, for purposes of 2718(e), we believe a standard charge can be identified based on the regular rate established by the hospital for the items and services provided to a specific group of paying patients. We are proposing that, for purposes of the second type of “standard charge,” a “payer-specific negotiated charge” would be defined as the charge that the hospital has negotiated with a third party payer for an item or service. We are further proposing to define “third party payer” for purposes of section 2718(e) of the PHS Act as an entity that is, by statute, contract, or agreement, legally responsible for payment of a claim for a health care item or service and to codify this definition at proposed new 45 CFR 180.20. As the reference to “third party” suggests, this definition excludes an individual who pays for a health care item or service that he or she receives (such as self-pay patients).
We are proposing to focus on a second type of “standard charge” related to negotiated rates because most consumers (over 90 percent [185] ) rely on a third party payer to cover a portion or all of the cost of health care items and services, including a portion or all of the cost of items and services provided by hospitals (in accordance with the terms and conditions of the third party payer's contract agreement with that consumer). Some third party payers (for example, Medicare fee-for-service or Medicaid fee-for-service) currently make public the maximum rate they pay for a hospital item or service. However, many third party payers do not reveal their negotiated rates, even to individuals on behalf of whom they pay. Additionally, many contracts between third party payers and hospitals contain so-called “gag clauses” that prohibit hospitals from disclosing the rates they have negotiated with third party payers.[186] Because consumers are not generally part of the negotiations or privy to the resulting negotiated rates, consumers often find it difficult to learn in advance of receiving a health care service the rate their third party payers may pay. Having insight into the charges that have been negotiated on one's behalf, however, is necessary for insured health care consumers to determine their potential out-of-pocket obligations prior to receipt of a health care service. For example, if a health care consumer knows that he or she will be responsible for 20 percent of the charges for a hospital service, her or she can compare the charges that the third party negotiated with hospital A and hospital B and, from that, the consumer can determine his or her expected out-of-pocket costs at hospital A versus hospital B.
Knowing a negotiated charge is also important because a growing number of insured health care consumers are finding that some services are more affordable if the consumer chooses to forego insurance and pay out-of-pocket. For example, stakeholders and reports indicate that an increasing number of consumers are discovering that sometimes the providers' cash discount can mean paying lower out-of-pocket costs than paying the out-of-pocket costs calculated after taking a third party payer's higher negotiated rate into account.[187] [188] [189] [190] However, consumers cannot make such determinations without knowing the rate their third party payer has negotiated.
For the reasons discussed above, we agree with commenters that gross charges (as a type of standard charge) are largely applicable to one identifiable group of consumers (for example, self-pay) and are not enough for another large and identifiable group of consumers (for example, those with third party insurance) to know their charges for hospital items. Thus, we are proposing that a type of `standard charge' is the “payer-specific negotiated charge” that would be defined as all charges that the hospital has negotiated with third party payers for an item or service. We decided to focus on negotiated rates rather than all payer rates because charges that are not negotiated (for example, Medicare fee-for-service or Medicaid fee-for-service rates) are often already publicly available.
We recognize that the impact resulting from the release of negotiated rates is largely unknown. While it is clear that such data is necessary for consumers to be able to determine their potential out-of-pocket costs in advance, and we believe the release of such data will help drive down health care costs (as discussed above), some stakeholders have expressed concern with the public display of de-identified negotiated rates which may have the unintended consequence of increasing health care costs of hospital services in highly concentrated markets or as a result of anticompetitive behaviors without additional legislative or regulatory efforts.[191]
Moreover, we recognize that requiring release of all payer-specific negotiated charges for all hospital items and services (both individual items and services as well as service packages) would mean releasing a large amount of data. To get a sense for the number of potential negotiated rates a hospital may have, we conducted an internal analysis of plans in the regulated individual and small group insurance markets under the Patient Protection and Affordable Care Act. Our analysis indicates that the number of products or lines of service per rating area ranges from approximately 1 to 200 in the individual market (averaging nearly 20 products or lines of service in each rating area), while in the small market group, the number ranges from 1 to 400 (averaging nearly 40 products or lines of service in each rating area). Most (if not all) hospitals maintain such data electronically because these data are used routinely for billing, and therefore we believe it presents little burden for a hospital to electronically pull and display these data online in a machine-readable format (as discussed in more detail in section XVI.E). However, we recognize that ensuring display of such a large amount of data in a consumer-friendly manner may pose greater challenges that we address in section (XVI.F).
We note that, in displaying the payer-specific negotiated charges, hospitals would display all negotiated charges, including, for example, charges negotiated with Medicare Advantage (MA) plans because such rates are negotiated. Conversely, hospitals would not include payment rates that are not negotiated, such as rates set by certain health care programs that are directly government-financed, for example, those set by CMS for Medicare fee-for-service. We believe, however, that the display of a non-negotiated rate, for example, display of a Medicare and Medicaid fee-for-service rate for an item or service, in conjunction with the gross charge and the payer-specific negotiated charges for the same item or service could be informative for the public and that nothing in this proposed rule would preclude hospitals from displaying them.
We are proposing to codify the definition of “payer-specific negotiated charge” and “third party payer” at proposed new 45 CFR 180.20. We invite public comments on our proposal to define a type of “standard charge” as a “payer-specific negotiated charge”. Given concerns raised by stakeholders related to release of identifiable negotiated charges, we are seeking public comment on whether and how the release of such specific charge information could result in unintended consequences. We also seek comment on whether and how there may be different methods for making such information available to individuals who seek to understand what their out-of-pocket cost obligations may be in advance of receiving a health care service.
4. Request for Comment on Alternative Definitions for Types of Standard Charges Under Consideration
Although we propose above that two types of charges would be standard charges for purposes of section 2718(e), we are seeking public comments on whether we should instead, or additionally, require the disclosure of other types of charges discussed below as standard charges. We considered alternatives for types of standard charges related to groups of individuals with third party payer coverage and also for types of standard charges that could be useful to groups of individuals who are self-pay.
a. Alternative Types of “Standard Charges” Related to Groups of Individuals With Third Party Payer Coverage
Access to the rate one's third party payer has negotiated on one's behalf can be a challenge. As discussed earlier, we believe that disclosure of negotiated charges will help many consumers with health care coverage know the charge hospitals have negotiated with their third party payers for items and services. However, we understand that the “payer-specific negotiated charge” represents a type of standard charge for some, but not all, groups of individuals with health care coverage; for example, individuals who have third party payer coverage for charges that are not negotiated. Additionally, we recognize concerns that may exist related to the unintended consequence of increased healthcare costs in some geographic regions as a result of disclosure of all negotiated charges. For this reason, we considered several additional or alternative types of “standard charges” that hospitals could be required to make public that would provide estimated or additional information for individuals with health care coverage. Specifically, we considered the following types of “standard charges”:
- Volume driven negotiated charge. As a variant of the definition of the “payer-specific negotiated charge,” we considered defining a type of “standard charge” based on the volume of patients to whom the hospital applies the standard charge. Specifically, we considered defining a type of “standard charge” as the “modal negotiated charge.” The mode of a distribution represents the number that occurs most frequently in a set of numbers. Here, we considered defining “modal negotiated charge” as the most frequently charged rate across all rates the hospital has negotiated with third party payers for an item or service. We believe that this definition could provide a useful and reasonable proxy for payer-specific negotiated charges and decrease burden for the amount of data the hospital would have to make public and display in a consumer-friendly format. While we are not proposing this definition at this time, we are seeking public comment on whether the modal negotiated charge would be as informative to consumers with insurance and whether it should be required as an alternative or in addition to the payer-specific negotiated charges.
- Minimum, median and maximum negotiated charge. We also considered defining a type of “standard charge” as the minimum, median, and maximum negotiated charge. Under this definition, the hospital would be required to make public the lowest, median, and highest charges of the distribution of all negotiated charges across all third party payer plans and products. This information could provide health care consumers with an estimate of what a hospital may charge, because it conveys the range of charges negotiated by all third party payers. Such a definition may also limit the amount of data a hospital would have to make public and package in a consumer-friendly manner which may reduce some burden. It may also relieve some concerns by stakeholders related to the potential for increased healthcare costs in some markets as a result of the disclosure of third party payer negotiated charges.
- All Allowed Charges. We also considered defining a type of “standard charge” as the charges for all items and services for all third party payer plans and products, including charges that are non-negotiated (such as FFS Medicare rates), which we would call “all allowed charges.” This option would require hospitals to provide the broadest set of charge information for all individuals with health insurance coverage because it would have the advantage of including all identified third party payer charges (including third party payer rates that are not negotiated). Additionally, every consumer would have access to charge information specific to their insurance plan. We considered, but are not proposing, this alternative because we believe consumers with non-negotiated health care coverage already have adequate and centralized access to non-negotiated charges for hospital items and services and are largely protected from out-of-pocket costs which may make them less sensitive to price shopping. However, we seek public comment on whether increasing the data hospital would be required to make public would pose a burden, particularly for smaller or rural hospitals that may not keep such data electronically available.
b. Alternative Types of “Standard Charges” Considered for Groups of Individuals That Are Self-Pay
As discussed earlier, hospital gross charge information may be most directly relevant to a large group of self-pay consumers who do not have third party payer insurance coverage or who seek care out-of-network. Such consumers would not need information in additional to hospital gross charges in order to determine their potential out-of-pocket cost obligations. However, stakeholders have indicated that hospitals often offer discounts off the gross charge or make other concessions to individuals who are self-pay. Thus, we considered additional definitions of hospital standard charges that may be relevant to certain subgroups of individuals who are self-pay.
- Discounted Cash Price. We considered defining a type of “standard charge” as the “discounted cash price,” defined as the price the hospital would charge individuals who pay cash (or cash equivalent) for an individual item or service or service package. We considered this alternative definition because there are many consumers who pay in cash (or cash equivalent) for hospital items and services.
The first subgroup of self-pay consumers that could benefit from knowing the discount cash price would be those who are uninsured. The number of uninsured individuals in the United States rose to 27.4 million in 2017.[192] These individuals' need for transparency in hospital charges differs from patients with insurance who generally are otherwise shielded from the full cost of hospitalization and hospital items and services. Uninsured individuals do not have the advantage of having access to a discounted group rate that has been negotiated by a third party payer. Therefore, individuals without insurance may face higher out-of-pocket costs for health care services.
The second subgroup of self-pay consumers who may benefit from knowing the discounted cash price are those who may have some health care coverage but who still bear the full cost of at least certain health care services. For example, these may be individuals who: Have insurance but who go out of network; have exceeded their insurance coverage limits; have high deductible plans but have not yet met their deductible; prefer to pay through a health savings account (HSA) or similar vehicle; or seek noncovered and/or elective items or services.
Many hospitals offer discounts to these groups of individuals, either as a flat percentage discount off the chargemaster rate or the insurer's negotiated rate, while some hospitals offer consumers a cash discount if they pay in full on the day of the service.[193] Other hospitals have developed and offer standardized cash prices for service packages for certain segments of the population who traditionally pay in cash for health care services. Currently, it is difficult for most consumers to determine in advance of receiving a service what discount(s) the hospital may offer an individual because cash and financial need discounts and policies can vary widely among hospitals.
Under this option, we specifically considered an option that would require hospitals to make public the cash discount that would apply for shoppable service packages that would include all ancillary services, similar to our proposals in XVI.F for consumer-friendly display of payer-specific negotiated charges. In this case, the discounted cash price would represent the amount a hospital would accept as payment in full for the shoppable service package from an individual. Such charges could be lower than the rate the hospital negotiates with third party payers because it would not require many of the administrative functions that exist for hospitals to seek payment from third party payers (for example, prior authorization and billing functions). However, we recognize, that many hospitals have not determined or maintain a standard cash discount that would apply uniformly to all self-pay consumers for each of the items and services provided by the hospital or for services packages, unlike they do for negotiated charges. We are seeking comment on this option, specifically, how many shoppable services for which it would be reasonable to require hospitals to develop and maintain and make public a discounted cash price.
- Median Cash Price. Similar to rates hospitals negotiate with third party payers, a hospital may offer a range of cash (or cash equivalent) discounts to various certain groups of self-pay consumers. For example, in addition to other cash discount prices mentioned earlier, many hospitals offer cash discounts on a sliding scale according to financial need. In such instances, as noted above, it may be difficult for a hospital to establish and make public a single standardized cash rate for such groups of consumers. For this reason, we also considered a definition that would take sliding scale cash discounts into account by defining a standard charge as the median cash price. The median cash price would be the midpoint of all cash discounts offered to consumers, including prices for self-pay patients and those qualifying for financial assistance. For uninsured patients who may qualify for financial assistance, the value of making a median cash price public could raise awareness of their available options, including the ability to apply for financial assistance. At this time, we are not proposing to require hospitals to make public their median cash price because we believe such a rate would be less useful to the public than a single standard cash price that the hospital would accept as payment in full as discussed above. However, we continue to consider it and seek public comments on whether this definition would be useful and whether it would enhance our policy goals for improving consumer health care affordability.
E. Proposed Requirements for Public Disclosure of All Hospital Standard Charges for All Items and Services
1. Overview
Section 2718(e) of the PHS Act requires hospitals to make their standard charges public in accordance with guidelines developed by the Secretary. Therefore, in the following sections we make proposals for hospitals to make public their standard charges in two ways: (1) A comprehensive machine-readable file that makes public all standard charge information for all hospital items and services (XVI.E), and (2) a consumer-friendly display of common “shoppable” services derived from the machine-readable file (XVI.F). We believe that these two different methods of making hospital standard charges public is necessary to ensure such data is available to consumers where and when it is needed (for example, via integration into price transparency tools, EHRs, and consumer apps), and also directly available and useful to consumers that search for hospital-specific charge information without use of a developed price transparency tool.
In this section, we make proposals for requirements for hospitals to make public online in a machine-readable file the standard charges (both gross charges and payer-specific negotiated charges) for all items and services (both individual items and services as well as service packages) provided by the hospital. For display of these standard charge data, we are proposing requirements for the file format, the content of the data in the file, and how to ensure the public can easily access and find the file. We believe these data could be of most use to health care consumers indirectly; that is, such data could be used by the public in price transparency tools or integrated into EHRs for purposes of clinical decision-making and referrals.
In section XVI.F. of this proposed rule, we propose requirements for hospitals to make public a limited amount of standard charge data for a limited set of the items and services the hospital provides online in a form and manner that is more user-friendly. Specifically, we are proposing to require hospitals to make public their payer-specific negotiated rates for certain “shoppable” services online in a consumer-friendly format. To do so, we are proposing that the hospital would disply their payer-specific negotiated charges for the primary shoppable service side-by-side with payer-specific negotiated charges for all ancillary items and services the hospital customarily as part of or in conjunction with the primary service. We make additional proposals related to consumer-friendly form, content, and manner of public display of these data. We believe these proposed requirements are responsive to stakeholder feedback and will assist health care consumers by making hospital standard charge information more directly useful and understandable to the public without the use of a developed price transparency tool.
2. Proposed Standardized Data Elements
As discussed in more detail in section XVI.E.3. of this proposed rule, we are proposing that hospitals disclose their list of standard charges for all items and services online in a single digital file that is machine-readable. Without specifying a minimum reporting standard for the machine-readable file, the standard charges data made publicly available by each hospital could vary, making it difficult for consumers to compare items and services. For example, some hospitals currently post a single column of gross charges without any associations to CPT or HCPCS codes or other identifying descriptions of the items and services to which the gross charge applies. A similar example would be a hospital that displays a list of gross charges that is correlated with a list of item numbers that are meaningful to the hospital billing personnel, but a not understandable to the general public. By contrast, some hospitals list their gross charges along with a brief description of the item or service to which each gross charge applies and the corresponding standardized identifying codes (typically HCPCS or CPT codes).
We are concerned that the lack of uniformity leaves the public unable to meaningfully use, understand, and compare standard charge information across hospitals. Therefore, for the first way we are proposing hospital make public their standard charges, which would contain gross charges and payer-specific negotiated charges for all hospital items and services, we are making a proposal to ensure uniformity of the data made publicly available by each hospital. To inform this proposal, we considered what data elements are typically included in a hospital's billing system and which of those elements would result in hospital standard charge data being most transparent, identifiable, meaningful, and comparable.
Based on a review of current State requirements and a sampling of hospitals that are currently making public their charges, we are proposing that hospitals make public a list of each item or service the hospital provides and that the list include the following corresponding information, as applicable, for each item or service:
- Description of each item or service (including both individual items and services and service packages).
- The corresponding gross charge that applies to each individual item or service when provided in, as applicable, the hospital inpatient setting and outpatient department setting.
- The corresponding payer-specific negotiated charge that applies to each item or service (including charges for both individual items and services as well as service packages) when provided in, as applicable, the hospital inpatient setting and outpatient department setting. Each list of payer-specific charges must be clearly associated with the name of the third party payer.
- Any code used by the hospital for purposes of accounting or billing for the item or service, including, but not limited to, the Current Procedural Terminology (CPT) code, Healthcare Common Procedure Coding System (HCPCS) code, Diagnosis-Related Group (DRG), National Drug Code (NDC), or other common payer identifier.
- Revenue code, as applicable.
We are proposing to codify these requirements at proposed new 45 CFR 180.50(b). We believe that these elements are necessary to ensure that the public can compare standard charges for similar or the same items and services provided by different hospitals.
We are proposing that hospitals associate each standard charge with a CPT or HCPCS code, DRG, NDC, or other common payer identifier, as applicable, because hospitals uniformly understand them and commonly use them for billing items and services (including both individual items and services and service packages). We also are proposing that hospitals include item descriptions for each item or service. In the case of items and services that are associated with common billing codes (such as the HCPCS codes), the hospital could use the code's associated short text description.
In addition, based on stakeholder feedback suggesting hospital charge information should include revenue codes to be comparable, we are proposing to require that the hospital include a revenue code where applicable and appropriate. Hospitals use revenue codes to associate items and services to various hospital departments. When a hospital charges differently for the same item or service in a different department, we are proposing that the hospital associate the charge with the department represented by the revenue code, providing the public some additional detail about the charges they may expect for hospital services provided in different hospital departments.
In developing this proposal, we also considered whether the following data elements, which are commonly included in hospital billing systems, might be useful to the public:
- Numeric designation for hospital department.
- General ledger number for accounting purposes.
- Long text description.
- Other identifying elements.
However, we determined that, for various reasons, these data elements may not be as useful as the data elements that we are proposing to require hospitals to make public. For example, data elements such as general ledger numbers are generally relevant to the hospital for accounting purposes but may not add value for the public, while data elements such as alternative code sets (such as ICD-10 codes) or long text descriptions associated with CPT codes, while useful, might be difficult to associate with a single item or service or be otherwise difficult to display in a file that is intended mainly for further computer processing. Because of this, while long text descriptions might benefit health care consumers and be appropriate for the consumer-friendly display of shoppable services (discussed in XVI.F), we believe it may add unnecessary burden for hospitals when such descriptions are not readily electronically available, or when the display of such data is not easily formatted into a machine-readable file. Therefore, we are not proposing to require these additional elements for the machine-readable data file that contains a list of all standard charges for all hospital items and services. We invite public comments on the proposed data elements for standard charge data that hospitals would be required to make public. We also seek public comments on the other data elements that, as we detail above, we considered but are not proposing to require, and on any other standard charge data elements that CMS should consider requiring hospitals to make public.
3. Proposed File Format Requirements
To make public their list of all gross charges and all payer-specific negotiated charges for all hospital items and services, we are proposing to require that hospitals post the charge information in a single digital file that is in a machine-readable format. We are proposing to define a machine-readable format as a digital representation of data or information in a file that can be imported or read into a computer system for further processing. Examples of machine-readable formats include, but are not limited to, .XML, JSON and .CSV formats. A PDF would not meet this definition because the data contained within the PDF file cannot be easily extracted without further processing or formatting. We are proposing to codify these format requirements at proposed new 45 CFR 180.50(c) and the definition of machine-readable at proposed new 45 CFR 180.20. We believe that making public such data in a machine-readable format poses little burden on hospitals because many (if not all) hospitals already keep these data in electronic format in their accounting systems for purposes of, for example, ensuring accurate billing. However, we seek comment on this assumption and the burden associated with transferring hospital charge data into a machine-readable format.
As an alternative, we considered proposing to require that hospitals post their list of all standard charges for all items and services using a single standardized file format, specifically.XML only, because this format is generally easily downloadable and readable for many health care consumers, and it could simplify the ability of price transparency tool developers to access the data. However, we did not want to be overly prescriptive in our requirements for formatting. We are seeking public comments on whether we should require that hospitals use a specific machine-readable format, and if so, which format(s). Specifically, we are seeking public comment on whether we should require hospitals to make all standard charge data for all items and services available as an .XML file only.
In addition, we considered formats that could allow direct public access to hospital standard charge information. For example, through the HHS' outreach on innovation,[194] we have heard ideas from stakeholders about processes involving standards and technologies that could allow public access to hospital standard charge data in real time. Such a process could have a number of benefits for the public and hospitals. Specifically, such a process could ensure the public has access to the most up-to-date standard charge information, rather than waiting for the hospital to update data that is publicly posted in a static digital file. Such technology may require or involve a type of portal or standard(s) in which entities have access to certain nonsensitive data elements or files within the hospital IT system environment, such as the chargemaster, but that otherwise restricts access to sensitive, personal identifying information (PII) commercial, protected health information (PHI), and/or confidential information.
Therefore, we seek public comment from all stakeholders, particularly hospitals and innovative information technology vendors, regarding such technologies or standards that could facilitate public access to real-time updates in a format to make it easier for information to be available when and where consumers want to use it, for example, into applications used by health care consumers or into electronic medical records for point-of-care decision-making and referral opportunities by clinicians. For example, application programming interface (API) standards could be used to facilitate public access to real-time hospital charge information. An API can be thought of as a set of commands, functions, protocols, or tools published by one software developer (“A”) that enable other software developers to create programs (applications or “apps”) that can interact with A's software without needing to know the internal workings of A's software, all while maintaining consumer privacy data standards. This is how API technology enables the seamless user experiences associated with applications familiar from other aspects of many consumers' daily lives, such as travel and personal finance. Standardized, transparent, and procompetitive API technology can similarly benefit consumers of health care services. In the case of “open” APIs, technical and other information required for a third-party application to connect is openly published. More information on API certification criteria and how APIs can be used by patients and health care providers and other entities to exchange electronic information can be found on the website at: https://www.healthit.gov/api-education-module/story_content/external_files/hhs_transcript_module.pdf.
We are specifically seeking public comment on adopting a requirement that hospitals make public their standard charges through an “openly published” (or simply “open”) API through which they would disclose the standard charges and associated data elements discussed in XVI.E.2. of this proposed rule. Being able to access these data through open APIs would allow the health care consumers to use the application of their choice to obtain personalized, actionable health care service price estimates.
An “open API,” for purposes of this comment solicitation, would simply be one for which the technical and other information required for a third-party application to connect to it is openly published. Open API does not imply that any and all applications or application developers would have unfettered access to sensitive information. Rather, an open API's published technical and other information specifically includes what an application developer would need to know to connect to and obtain the data required to be disclosed under this proposed rule. For example, hospitals could use the CMS open source implementation which would facilitate adoption.[195] We also seek public comment on the additional burden that may be associated with a requirement that hospitals make public their standard charges through an open API.
4. Proposed Location and Accessibility Requirements
We have reviewed how hospitals are currently implementing our updated guidelines, which took effect on January 1, 2019, and we are concerned that some charge information made public by hospitals may be difficult for the public to locate. For example, information may be difficult to locate if the public is required to click down several levels in order to find the information. We also are concerned about barriers that could inhibit the public's ability to access the information once located. For example, we are aware that some hospitals require consumers to set up a username and password, or require consumers to submit various types of other information, including, but not limited to, their email address, in order to access the data. We are concerned that these requirements might deter the public from accessing hospital charge information.
Accordingly, we are proposing that a hospital would have discretion to choose the internet location it uses to post its file containing the list of standard charges so long as the file is displayed on a publicly-available web page, it is displayed prominently and clearly identifies the hospital location with which the standard charges information is associated, and the standard charge data are easily accessible, without barriers, and the data can be digitally searched. For purposes of these proposed requirements: (1) “displayed prominently” would mean that the value and purpose of the web page [196] and its content [197] is clearly communicated, there is no reliance on breadcrumbs [198] to help with navigation, and the link to the standard charge file is visually distinguished on the web page; [199] (2) “easily accessible” would mean that standard charge data are presented in a single machine-readable file that is searchable and that the standard charges file posted on a website can be accessed with the fewest number of clicks; [200] and (3) “without barriers” would mean the data can be accessed free of charge, users would not have to input information (such as their name, email address, or other PII) or register to access or use the standard charge data file. We are proposing to codify this requirement at proposed new 45 CFR 180.50(d).
We encourage hospitals to review the HHS Web Standards and Usability Guidelines (available at: https://webstandards.hhs.gov/), which are research-based and are intended to provide best practices over a broad range of web design and digital communications issues.
We also are requesting public comments on an alternative we considered, which would require hospitals to submit a link to the standard charges file to a CMS-specified central website, or submit a link to the standard charge file to CMS that would be made public on a CMS web page. Such a method could allow the public to access standard charge information for their purposes in one centralized location. We believe this could reduce potential confusion about where to find standard charge information and potentially allow standard charge information to be posted alongside CMS hospital quality information. It could also assist in the assessment of hospital compliance with section 2718(e) of the PHS Act. In spite of these possible benefits, we are not now proposing to require hospitals to submit or upload a link to their standard charge information to a CMS-specified centralized website because we believe such an effort could be unnecessarily duplicative of ongoing State and private sector efforts to centralize hospital pricing information and potentially confuse consumers who may reasonably look to a hospital website directly for charge information. However, because we appreciate the advantages of having all data available through a single site, we are considering this alternative and seek public comments. We seek comment on this alternative option, specifically, whether the burden outweighs the advantages.
Finally, we seek public comments on potential additional requirements, including easily-searchable file naming conventions and whether we should specify the website location for posting rather than our current proposal that would permit hospitals some flexibility in choosing an appropriate website. Current instances of machine-readable charge files posted on hospital websites contain variable file types, file names, and locations on each website. Standardizing file name or website location information could provide consumers with a standard pathway to find the information and would provide uniformity, making it easier for potential software to review information on each website. Specific requirements for file naming conventions and locations for posting on websites could also facilitate the monitoring and enforcement of the requirement. Therefore, we are seeking public comments on whether we should propose to adopt these additional requirements or other requirements related to these issues.
5. Proposed Frequency of Updates
The statute requires hospitals to establish, update, and make public their standard charges for each year. Therefore, we are proposing to require hospitals to make public and update their file containing the list of all standard charges for all items and services at least once annually (proposed new 45 CFR 180.50(e)). We recognize that hospital charges may change more frequently and therefore we encourage (but are not requiring) hospitals to update this file more often, as appropriate, so that the public may have access to the most up-to-date charge information. We also recognize that hospitals update their charges at different times during the year and may also have various State price transparency reporting requirements that require updates. For purposes of these requirements, we believe that updates that occur at least once in a 12-month period will satisfy our proposed requirement to update at least once annually and reduce reporting burden for hospitals. In other words, the hospital could make public and update its list of standard charges at any point in time during the year, so long as the update to the charge data occurs no more than 12 months after posting.
We also are proposing to require hospitals to clearly indicate the date of the last update they have made to the standard charge data, with some discretion as to where the date of late update is indicated. For example, if a hospital chooses to make public its list of standard charges in .XML format, the first row of the spreadsheet could indicate the date the file was last updated. The hospital could also indicate the date the file was last updated in text associated with the file on the web page on which it is posted, or could indicate the date in some other way, as long as that date is clearly indicated and associated with the file or location containing the standard charge information.
6. Proposed Requirements for Making Public Separate Files for Different Hospital Locations
We recognize that some hospitals may have different locations operating under a consolidated or single State license, and that different hospital locations may offer different services that have different associated standard charges. To address this circumstance, we are proposing at proposed new 45 CFR 180.50(a)(2) that the proposed requirements for making public the file containing all standard charges for all items and services in this section of this proposed rule would separately apply to each hospital location such that each hospital location would be required to make public a separate identifiable list of standard charges.
F. Proposed Requirements for Consumer-Friendly Display of the Payer-Specific Negotiated Charges for Selected Shoppable Services
1. Background and Overview
We believe that our proposal in section XVI.E. of this proposed rule requiring hospitals to post on the internet a machine-readable file containing a list of all standard charges (both gross charges and payer-specific negotiated charges) for all items and services (both individual items and services and service packages) is a good first step for driving transparency in health care pricing. As noted earlier, we also believe our proposed policy for making these data available in a machine-readable format will help make these data accessible to health care consumers when and where it is needed to make decisions, for example, via integration in price transparency tools or into electronic health record systems. However, as noted by many stakeholders in the 2018 RFIs and listening sessions, such long lists of charges in a file posted online in a machine-readable format may not be immediately or directly useful for many health care consumers, because the amount of data could be overwhelming or not easily understood by consumers. Because of this, we considered ways of requiring or encouraging hospitals to make public standard charges for frequently provided services in a form and manner that is more directly accessible and consumer friendly. In addition to including all their payer-specific negotiated charges for all items and services in the machine-readable file (as described in section XVI.E. of this proposed rule), in the following sections we propose that hospitals must make public their payer-specific negotiated charges for common services for which consumers may have the opportunity to shop.
First, we propose requirements for hospitals to display a list of payer-specific negotiated charges for a set of `shoppable' services. We believe doing so will enable consumers to make comparisons across hospital sites of care. Second, we make proposals intended to ensure the charge information for `shoppable' services are presented in a way that is consumer-friendly. To be consumer-friendly, we believe that the information should be displayed in a way that is understandable to patient (for example, by including plain-language descriptions of the services), that the shoppable service charge is displayed along with charges for ancillary services the hospital customarily provides with the primary shoppable service, and that the consumer can easily search for and find charges for the shoppable services based on the service description, by the code associated with the shoppable service, or by payer.
We believe the proposals related to consumer-friendly display of hospital charge information align with and enhance many ongoing State and hospital efforts. We seek comment from hospitals regarding the extent to which our proposals are duplicative of such ongoing efforts, and how best to ensure consistency of consumer-friendly data display across hospital settings. We further seek comment from consumers regarding their potential engagement with a list of `shoppable' hospital items and services, including whether our proposals provide for a useful amount of data and data elements that allow for actionable comparisons of `shoppable' hospital provided items and services.
2. Proposed Definition of “Shoppable Service”
For purposes of this requirement, a “shoppable service” would be defined as a service package that can be scheduled by a health care consumer in advance. Shoppable services are typically those that are routinely provided in non-urgent situations that do not require immediate action or attention to the patient, thus allowing patients to price shop and schedule a service at a time that is convenient for them. We are proposing this definition because it is consistent with definitions proposed by policy experts or used by researchers who identify a service as `shoppable' if a patient is able to determine where and when they will receive services and can compare charges for multiple providers.[201] Since hospitals may not have insight into whether a particular service is available across multiple providers or where a consumer will ultimately determine where they want to receive a particular services, we have focused our proposed definition on the first aspect, that is, whether or not a service offered by the hospital could be scheduled by the consumer in advance.
Additionally, we are proposing that the charges for such services be displayed as a grouping of related services, meaning that the charge for the shoppable service is displayed along with charges for ancillary items and services the hospital customarily provides as part of or in addition to the primary shoppable service. We are proposing that hospital make public the payer-specific negotiated charge for a shoppable service that is grouped together with charges for associated ancillary services because we believe charge information displayed in such a way is consumer-friendly and patient-focused. In other words, we believe that consumers want to see and shop for healthcare services in the way they experience the service. We are proposing to define an “ancillary service” as an item or service a hospital customarily provides as part of or in conjunction with a shoppable primary service (proposed new 45 CFR 180.20). Ancillary items and services may include laboratory, radiology, drugs, delivery room (including maternity labor room), operating room (including post-anesthesia and postoperative recovery rooms), therapy services (physical, speech, occupational), hospital fees, room and board charges, and charges for employed professional services. Ancillary services may also include other special items and services for which charges are customarily made in addition to a routine service charge. For example, an outpatient procedure may include many services that are provided by the hospital, for example, local and/or global anesthesia, services of employed professionals, supplies, facility and/or ancillary facility fees, imaging services, lab services and pre- and post-op follow up. To the extent that a hospital customarily provides (and bills for) such services as a part of or in conjunction with the primary service, the hospital should group the service charge along with the other payer-specific negotiated charges that are displayed for the shoppable service. We believe such a practice is consumer-friendly by presenting charge information in a way that reflects how the patient experiences the service.
Examples of shoppable services may include certain imaging and laboratory services, medical and surgical procedures, and outpatient clinic visits. The emphasis on shoppable services aligns with various State price transparency efforts and is consistent with stakeholder feedback. We also believe that this emphasis is consistent with research demonstrating that improving price transparency for shoppable services can have an impact on driving down the cost of health care (we refer readers to section XVI.A.2. of this proposed rule). We are proposing to add this definition to our regulations at proposed new 45 CFR 180.20.
3. Proposed Selected Shoppable Services
We are proposing to require hospitals to make public a list of their payer-specific negotiated charges for as many of the 70 shoppable services that we identify in Table 37 below that are provided by the hospital, and as many additional shoppable services selected by the hospital as is necessary for a combined total of at least 300 shoppable services (new § 180.60(a)).
In a study of 2011 claims by autoworkers, researchers identified a set of 350 frequently billed healthcare services that consumers could schedule in advance and for which there was variation in charges across providers.[202] Hospitals that are early adopters of price transparency have suggested that it is possible to initially identify and display good-faith individualized price estimates for at least 350 shoppable health care services identified by primary billing codes (including prices for ancillary services) with more sophisticated price transparency tool developers creating and being able to display individualized pricing estimates for at least 1000 shoppable services. In contrast, most States that require hospital posting of shoppable services range in requiring 25-50 shoppable services, with California being the only State that requires the corresponding charge information to include ancillary services. Since these proposed regulations will apply to all hospitals operating in the United States, some of which may not have any experience in displaying charges for shoppable services, we believe it is reasonable to propose a starting point of at least 300 shoppable services for which hospitals would be required to display payer-specific negotiated charges. We anticipate we would increase this number over time as hospitals become accustomed to displaying charge information to consumers as a grouping of related charges and as such data is more routinely used by consumers.
Moreover, we believe it is reasonable to require a portion of the 300 shoppable services to be CMS-selected in order to ensure standardization that would provide consumers with the ability to compare prices across hospital settings. We further believe it would be prudent to permit hospitals to select a portion of the shoppable services themselves, recognizing that some hospitals may specialize in certain services (for example, specialized procedures) or may serve populations that utilize other shoppable services with more frequency or are more relevant than the ones we have identified for purposes of the CMS-selected services.
The proposed 70 CMS-specified shoppable services are identified by a primary HCPCS, CPT, or DRG code and are in Table 37.



These 70 shoppable services were selected based on an analysis of shoppable services that are currently made public under State price transparency requirements, a review of services that frequently appear in web-based price transparency tools, an analysis of high volume services and high cost procedures derived from External Data Gathering Environment (EDGE) server data [203] ), and a review by CMS medical officers. In other words, we used a combination of quantitative analysis of the EDGE server claims data, a qualitative review of commonly selected services for State and hospital price transparency initiatives and tools, and clinician review to ensure such services could be scheduled in advance in order to identify our list of 70 CMS-selected shoppable services.
In addition to the 70 CMS-selected shoppable services proposed above, we also are proposing that each hospital would select, at minimum, 230 additional shoppable services, identified by a primary HCPCS, CPT, DRG, or other widely used industry code, as applicable, and make publicly available a list of its payer-specific negotiated charges for each of those shoppable services, including the payer-specific negotiated charges for the shoppable service in both the inpatient setting and the outpatient setting, if different. We further propose that hospitals select such services based on the utilization or billing rate of the services in the past year. We believe that enabling hospitals to select most of the shoppable services for which they make their payer-specific negotiated charges available will permit them to tailor their list of shoppable services to their specific patient populations and area of expertise. For example, a children's hospital could select additional shoppable services that are predominantly provided to children.
Although we believe that most hospitals provide the 70 CMS-selected shoppable services which are very common and frequently billed by hospitals, it is possible that some hospitals may not offer all of them (for example, specialty hospitals). Therefore, we are propose that hospitals make public a list of their payer-specific negotiated charges for as many of the 70 shoppable services that we identify in Table 36 that are provided by the hospital, plus as many additional shoppable services as is necessary to reach a total of at least 300 shoppable services.
An alternative option would be for us to propose a larger set of shoppable services and allow hospitals to select up to 70 CMS-selected shoppable services from the larger list for which it would make its payer-specific negotiated charges publicly available. The hospital would then select an additional 230 shoppable services for a total of 300 shoppable services. However, we are not proposing this because we believe most hospitals provide the 70 CMS selected shoppable services and because we have concerns that more discretion will erode our desire to ensure consumers can get hospital charge information for a minimum standardized set of services.
We seek public comments on the 70 CMS-selected shoppable services we identify in Table 36. We are particularly interested in feedback regarding the specific services we have identified as shoppable services and whether other services should be included because they are more common, more shoppable or both. We also are interested in feedback on whether we should require more or less than a total of 300 shoppable services. Specifically, we seek comment from hospitals and consumers on whether a list of 100 shoppable services (or less) is a reasonable starting point. We also are seeking public comment on whether we should identify more specific requirements related to hospital-selected shoppable services; for example, requiring hospitals to select their most frequently billed shoppable services (that are not included in the CMS-specified list).
4. Proposed Required Corresponding Data Elements
We are proposing that the consumer-friendly charge information the hospital makes available to the public online for the CMS and hospital-selected shoppable services must include certain corresponding data elements in order to ensure that consumers understand the hospital's payer-specific negotiated charge for each shoppable service and can use that information to make comparisons across hospitals. Specifically, we are proposing that the consumer-friendly display of payer-specific negotiated charge information contain the following corresponding information for each of the 70 CMS-selected and at least 230 hospital-selected shoppable services:
- A plain-language description of each shoppable service. For example, hospitals would not be required but are invited to review and use the Federal plain language guidelines.[204]
- The payer-specific negotiated charge that applies to each shoppable service. If the hospital does not provide one or more of the CMS-selected shoppable services, the hospital may indicate “N/A” for the corresponding charge or otherwise make it clear that the service is not provided by the hospital. Each payer-specific charge must be clearly associated with the name of the third party payer.
- A list of all the associated ancillary items and services that the hospital provides with the shoppable service, including the payer-specific negotiated charge for each ancillary item or service.
- The location at which each shoppable service is provided by the hospital (for example, Smithville Campus or XYZ Clinic), including whether the payer-specific negotiated charge for the shoppable service applies at that location to the provision of that shoppable service in the inpatient setting or the outpatient department setting or both. If the payer-specific negotiated charge for the shoppable service varies based upon location or whether the hospital provides the shoppable service in the inpatient setting versus the outpatient setting, the hospital would be required to identify each payer-specific negotiated charge.
- Any primary code used by the hospital for purposes of accounting or billing for the shoppable service, including, but not limited to, the Current Procedural Terminology (CPT) code, the Healthcare Common Procedure Coding System (HCPCS) code, the Diagnosis-Related Group (DRG), or other commonly used service billing code.
As discussed in more detail in section XVI.F, we are proposing that hospital make public the payer-specific negotiated charge for a shoppable service in a manner that groups the payer-specific negotiated charge for the primary shoppable service along with charges for associated ancillary services because we believe charge information displayed in such a way is consumer-friendly and patient-focused. In other words, we believe that consumers want to see and shop for healthcare services in the way they experience the service. We recognize that not all hospitals will customarily provide exactly the same ancillary items or services with a primary shoppable service and therefore we believe it is important for hospitals to display a list of which ancillary services are included in conjunction with or as part of the primary shoppable service.
We are proposing to codify these proposed required data elements at proposed new 45 CFR 180.60(b). We are seeking public comments on these data elements and whether there are additional data elements that should be displayed to the public in a consumer-friendly manner. We emphasize that nothing in this proposed rule is meant to inhibit or restrict hospitals from including additional data elements that would improve the ability of health care consumers to understand the hospital's charges for shoppable services, for example, a hospital could choose to display the cash price the hospital would accept as payment in full for the shoppable service from a consumer.
5. Proposals for Format of Display of Consumer-Friendly Information
We are aware that many hospitals are already making public various types of standard charges for shoppable services available online in various formats. For example, some hospitals offer searchable price transparency tools on their website that offer estimated charges (averages or individualized out-of-pocket costs) or may display charges for shoppable services in brochures (both online and offline) that contain self-pay discounted prices for a service package. Because there are a variety of consumer-friendly ways to display charges for hospital services and because we do not want to restrict hospitals from innovating or from having to duplicate efforts, we are not proposing a specific format for making such data public online in a consumer-friendly manner. Specifically, unlike our proposals for the machine-readable list of standard charges for all items and services (discussed in section XVI.E), we are not proposing to require that hospitals make payer-specific charge data public in a single digital file posted online. Instead, we are proposing that hospitals retain flexibility on how best to display the payer-specific negotiated charge data and proposed associated data elements to the public online, so long as the website is easily accessible to the public. We believe this approach would permit some flexibility for hospitals to, for example, post one or more files online with a list of payer-specific charges for the shoppable services and associated data elements, or, for example, to integrate such data into existing price estimate tools.
Additionally, we note that we are not proposing, but are considering, an option that would require hospitals to make these data available in API format. As explained in more detail in section XVI.E.3. of this proposed rule, an API enabled format could allow consumers to access the data by searching for it directly when they do not have a computer by, for example, putting a CPT code in the URL path of the hospital to render in one's mobile phone browser the gross or payer-specific negotiated charge for the service. For example, a consumer searching for the price of a blood test for cholesterol (CPT code 80061) at fictional hospital ABC could look it up by inserting the URL path https://hospitalABC.com/api/80061.
We further recognize not all consumers have access to the internet. Therefore, we are proposing to require hospitals make the data elements proposed in section XVI.F.4. of this proposed rule available in a consumer-friendly manner offline. Specifically, we are proposing that the hospital must provide a paper copy (for example, a brochure or booklet) of the information is available to consumers upon request within 72 hours of the request. We are proposing to codify this provision at proposed new 45 CFR 180.60(c).
6. Proposed Location and Accessibility Requirements
Additionally, we are proposing that hospitals make the data elements proposed in section XVI.F.4. of this proposed rule online in such a way that the payer-specific negotiated charge and associated data elements can be located and accessed easily by consumers.
First, we propose that a hospital would have discretion to select an appropriate internet location it uses to post the standard charge information required under this section (that is, the payer-specific charges for shoppable services and associated data elements). We further propose that the website location be publicly available, that the data be displayed prominently and clearly identifies the hospital location with which the standard charge information is associated, and the standard charge data are easily accessible, without barriers, and the data can be digitally searched. For purposes of these proposed requirements: (1) “displayed prominently” would mean that the value and purpose of the web page [205] and its content [206] is clearly communicated, there is no reliance on breadcrumbs [207] to help with navigation, and the link to the standard charge information is visually distinguished on the web page; [208] (2) “easily accessible” would mean that standard charge data are presented in format that is searchable by service description, billing code, and payer, and that the standard charge data posted on the website can be accessed with the fewest number of clicks; [209] and (3) “without barriers” would mean the data can be accessed free of charge, users would not have to input information (such as their name, email address, or other PII) or register to access or use the standard charge data. We are proposing to codify this requirement at proposed new 45 CFR 180.50(d).
We encourage hospitals to review the HHS Web Standards and Usability Guidelines (available at: https://webstandards.hhs.gov/), which are research-based and are intended to provide best practices over a broad range of web design and digital communications issues.
We seek comment on these proposed location and accessibility requirements and specifically regarding whether there are additional requirements that should be considered to ensure public access to payer-specific negotiated charges for shoppable services.
7. Proposed Frequency of Updates
The statute requires hospitals to establish, update, and make public their standard charges for each year. Therefore, we are proposing to require hospitals to make public and update the standard charge information proposed in section XVI.F.2 at least once annually (proposed new 45 CFR 180.60(e)). We recognize that hospital charges may change more frequently and therefore we encourage (but are not requiring) hospitals to update this file more often, as appropriate, so that the public may have access to the most up-to-date charge information. We also recognize that hospitals update their charges at different times during the year and may also have various State price transparency reporting requirements that require updates. For purposes of these requirements, we believe that updates that occur at least once in a 12-month period will satisfy our proposed requirement to update at least once annually and reduce reporting burden for hospitals. In other words, the hospital could make public and update its list of standard charges at any point in time during the year, so long as the update to the charge data occurs no more than 12 months after posting.
We also are proposing to require hospitals to clearly indicate the date of the last update they have made to the standard charge data, with some discretion as to where the date of late update is indicated.
G. Proposed Monitoring and Enforcement of Requirements for Making Standard Charges Public
1. Background
Section 2718(b)(3) of the PHS Act requires the Secretary to promulgate regulations to enforce the provisions of section 2718 of the PHS Act, and in so doing, the Secretary may provide for appropriate penalties. As such, we are proposing that we may impose penalties on hospitals that fail to make their standard charges public in accordance with the requirements we finalize under section 2718(e) of the PHS Act. In the FY 2019 IPPS/LTCH PPS proposed rule (83 FR 20549), we sought public comments on a variety of issues related to enforcement of the requirement that hospitals make public their standard charges and noted our intent to address enforcement and other actions to ensure compliance in future rulemaking.
We specifically sought comments on the following:
- What is the most appropriate mechanism for CMS to enforce price transparency requirements?
- Should CMS require hospitals to attest to meeting requirements in the provider agreement or elsewhere?
- How should CMS assess hospital compliance?
- Should CMS publicize complaints regarding access to price information or review hospital compliance and post results? What is the most effective way for CMS to publicize information regarding hospitals that fail to comply?
- Should CMS impose civil monetary penalties (CMPs) on hospitals that fail to make standard charges publicly available as required by section 2718(e) of the PHS Act?
- Should CMS use a framework similar to the Federal civil penalties under 45 CFR 158.601 through 158.615, that apply to issuers that fail to report information and pay rebates related to medical loss ratios, as required by sections 2718(a) and (b) of the PHS Act, or would a different framework be more appropriate?
We received a number of comments in response to this RFI. Many commenters agreed that enforcing this requirement under section 2718(e) of the PHS Act would send an important signal that CMS values transparency and ensure that the public has access to hospital charge information. Some commenters suggested that CMS model enforcement after various quality reporting programs, such as the Hospital Inpatient and Outpatient Quality Reporting Programs or the LTCH Quality Reporting Program. Some commenters recommended publicizing noncompliant hospitals or providing a mechanism for the public to file complaints against noncompliant hospitals. Some commenters suggested that CMS propose to make the publication of standard charges a Medicare condition of participation or provider enrollment. However, one commenter indicated that revoking a provider agreement over lack of a website disclosure would be unnecessarily punitive. Other commenters warned that subjecting hospitals violating pricing transparency provisions to compliance actions could pose a challenge, particularly for smaller hospitals, and recommended limiting or deferring compliance actions to a later date. Some commenters agreed that imposing monetary penalties on noncompliant hospitals was appropriate, while other commenters believed that CMS does not have authority to enforce section 2718(e) of the PHS Act and, for that reason, should not adopt penalties for noncompliance.
We agree with commenters who noted that an enforcement regime signals the value we place on price transparency and assurance of public access to hospital standard charges. We interpret section 2718(b)(3) of the PHS Act as authorizing us to enforce the provisions of section 2718(e). Therefore, in this proposed rule, we are proposing to adopt mechanisms to monitor and enforce our requirements for making standard charges public.
2. Proposed Monitoring Methods
Section 2718(e) of the PHS Act requires hospitals to make public their list of standard charges and authorizes the Secretary to promulgate additional criteria that hospitals must satisfy in order to make such charges public. The statute does not prescribe monitoring procedures or the factors we should consider in imposing penalties on hospitals for noncompliance. Based on our experience with the Medicare program and health care marketplace plans, we believe it is important for the public to be informed, and, therefore, for CMS to ensure compliance with this statutory requirement. Therefore, we are proposing to employ methods to monitor and assess hospital compliance with section 2718(e) of the PHS Act, and specifically proposed new 45 CFR 180.40, 180.50, and 180.60.
In general, we are proposing that CMS may use methods to monitor hospital compliance with the requirements under proposed 45 CFR part 180. We anticipate relying predominantly on complaints made to CMS by individuals or entities regarding a hospital's potential noncompliance. Therefore, we are proposing that our monitoring methods may include, but are not limited to, the following, as appropriate:
- CMS' evaluation of complaints made by individuals or entities to CMS.
- CMS review of individuals' or entities' analysis of noncompliance.
As we gain experience with monitoring compliance with the requirements for proposed 45 CFR part 180, based on reports of potential noncompliance, we may consider self-initiating audits of hospitals' websites as a monitoring method. Therefore, we are proposing that our monitoring methods may include CMS audit of hospitals' websites.
We are proposing to set forth these monitoring methods in the regulations at proposed new 45 CFR 180.70.
3. Proposed Actions To Address Hospital Noncompliance With Requirements To Make Public Standard Charges
We are proposing that hospitals that CMS identifies as noncompliant would be notified of their deficiencies and given an opportunity to take corrective action to come into compliance. As discussed in section XVI.G.4. of this proposed rule, for hospitals determined by CMS to be noncompliant with section 2718(e) of the PHS Act that fail to respond to CMS' requests to submit a corrective action plan (CAP) or comply with the requirements of a CAP, we are proposing that we may impose CMPs on hospitals and publicize these penalties on a CMS website.
Should we conclude, based upon the proposed monitoring activities previously described, that a hospital is noncompliant with section 2718(e) of the PHS Act and the requirements of proposed 45 CFR part 180, we are proposing that CMS may take any of the following actions, which generally, but not necessarily, would occur in this order:
- We may provide a written warning notice to the hospital of the specific violation(s).
- We would request a CAP from the hospital if its noncompliance constitutes a material violation of one or more requirements.
- If the hospital fails to respond to CMS' request to submit a CAP or comply with the requirements of a CAP, CMS may impose a CMP on the hospital and publicize the penalty on a CMS website as discussed in section XVI.G.4. of this proposed rule.
Prior to requesting a CAP, or in the case of violations that are deemed nonmaterial violations warranting a CAP, CMS anticipates warning, via written notice, a hospital of noncompliance with one or more of the requirements to make public standard charges (according to section 2718(e) of the PHS Act and the requirements of proposed 45 CFR part 180), and of the need for voluntary corrective action. We would then reevaluate the hospital's compliance with the statutory and proposed regulatory requirements. Should we determine the hospital remains noncompliant and that the noncompliance constitutes a material violation of one or more requirements, we anticipate requiring that the hospital submit a CAP, and there would be increasing consequences for failure to remedy noncompliance.
We are proposing that a material violation may include, but is not limited to, the following:
- A hospital's failure to make public its standard charges required by proposed new 45 CFR 180.40.
- A hospital's failure to make public its standard charges in the form and manner required under to proposed new 45 CFR 180.50 and 180.60.
We are proposing that CMS may request that a hospital submit a CAP, specified in a notice of violation issued by CMS to a hospital. A hospital required to submit a CAP must do so, in the form and manner, and by the deadline, specified in the notice of violation issued by CMS to the hospital and must comply with the requirements of the CAP.
We are proposing that a hospital's CAP must specify elements including, but not limited to, the deficiency or deficiencies that caused noncompliance to occur, the corrective actions or processes the hospital will take to come into compliance with the requirements of 45 CFR part 180, and the timeframe by which the hospital will complete the corrective action. We are proposing that a CAP would be subject to CMS review and approval. We are proposing that after CMS' review and approval of a hospital's CAP, CMS may monitor and evaluate the hospital's compliance with the corrective actions.
We are proposing that a hospital's failure to respond to CMS' request to submit a CAP includes failure to submit a CAP in the form, manner, or by the deadline, specified in a notice of violation issued by CMS to the hospital. We are proposing that a hospital's failure to comply with the requirements of a CAP includes failure to correct violation(s) within the specified timeframes.
We are proposing to set forth in the regulations at proposed new 45 CFR 180.70 the actions CMS may take to address a hospital's noncompliance with the requirements to make public standard charges, and to set forth in proposed new 45 CFR 180.80 the requirements for a CAP, as discussed in this section of this proposed rule.
4. Proposal To Impose Civil Monetary Penalties
We are proposing that we may impose a CMP on a hospital that we identify as noncompliant with the requirements of proposed 45 CFR part 180, and that fails to respond to CMS' request to submit a CAP or comply with the requirements of a CAP as we describe earlier.
We are proposing that we may impose a CMP upon a hospital for a violation of each requirement of proposed 45 CFR part 180. The maximum daily dollar amount for a CMP to which a hospital may be subject would be $300. We are proposing that even if a hospital is in violation of multiple discrete requirements of proposed 45 CFR part 180, the maximum total sum that a single hospital may be assessed per day is $300.
Further, we are proposing to adjust the CMP amount annually by applying the cost-of-living adjustment multiplier determined by OMB for adjusting applicable CMP amounts pursuant to the Federal Civil Penalties Inflation Adjustment Act Improvements Act of 2015. This multiplier, based on the Consumer Price Index for All Urban Consumers (CPI-U), not seasonally adjusted, is applied to the CMPs in 45 CFR 102.3. For instance, the cost-of-living adjustment multiplier for 2018, based on the CPI-U for the month of October 2017, not seasonally adjusted, was 1.02041 (83 FR 51369).
Given the importance of compliance with the price transparency policies, we believe this proposed CMP amount strikes a balance between penalties that are sufficiently harsh to incentivize compliance but not so severe as to be punitive. We reviewed CMP amounts for other CMS programs that require reporting information and we believe our proposed $300 maximum daily dollar amount for a CMP is commensurate with the level of severity of the potential violation, taking into consideration that nondisclosure of standard charges does not rise to the level of harm to the public as other violations (such as safety and quality issues) for which CMS imposes CMPs and, therefore, should remain at a relatively lower level.
We considered applying lower and higher maximum dollar amounts for a CMP for noncompliance with the requirements of proposed 45 CFR part 180. For example, we considered that CMS has imposed $100 per day penalty amounts with respect to other compliance matters, such as where health insurers fail to comply with premium revenue reporting and rebate requirements found at 45 CFR 158.606. The basis for the CMPs under 45 CFR 158.606 is the number of individuals affected. With respect to the disclosure requirements under proposed 45 CFR part 180, where the lack of information could affect an unknown number of consumers and in myriad ways (for example, not just individuals who paid more for items and services), we do not believe it is feasible to utilize a “per person” type basis. We also considered proposing higher maximum daily dollar amounts, such as $400 per day, $500 per day or more.
Further, we considered establishing a cumulative annual total limit for the CMP to which a hospital is subject for noncompliance with proposed 45 CFR part 180. For example, we considered applying a cumulative annual total limit of $100,000 per hospital for each calendar year. However, we are concerned that such an approach could, for example, mitigate the amount of penalty imposed on hospitals that remain noncompliant for multiple years.
If CMS imposes a penalty in accordance with the requirements of proposed 45 CFR part 180, we are proposing that CMS provides a written notice of imposition of a CMP to the hospital via certified mail or another form of traceable carrier. This notice may include, but would not be limited to, the following:
- The basis for the hospital's noncompliance, including, but not limited to, the following: CMS' determination as to which requirement(s) the hospital violated; and the hospital's failure to respond to CMS' request to submit a CAP or comply with the requirements of a CAP.
- CMS' determination as to the effective date for the violation(s). This date would be the latest date of the following:
++ The first day the hospital is required to meet the requirements of proposed 45 CFR part 180.
++ If a hospital previously met the requirements of this part but did not update the information annually as required, the date 12 months after the date of the last annual update specified in information posted by the hospital.
++ A date determined by CMS, such as one resulting from monitoring activities specified in proposed new 45 CFR 180.70, or development of a CAP as specified in proposed new 45 CFR 180.80.
- The amount of the penalty as of the date of the notice.
- A statement that a CMP may continue to be imposed for continuing violation(s).
- Payment instructions.
- Intent to publicize the hospital's noncompliance and CMS' determination to impose a CMP on the hospital for noncompliance with the requirements of proposed 45 CFR part 180 by posting the notice of imposition of a CMP on a CMS website.
- A statement of the hospital's right to a hearing (as described in section XVI.H. of this proposed rule).
- A statement that the hospital's failure to request a hearing within 30 calendar days of the issuance of the notice permits the imposition of the penalty, and any subsequent penalties pursuant to continuing violations, without right of appeal.
Further, in the event that a hospital elects to appeal the penalty, and if the CMP is upheld, in part, by a final and binding decision, we propose that CMS would issue a modified notice of imposition of a CMP.
We are proposing that a hospital must pay a CMP in full within 60 calendar days after the date of the notice of imposition of a CMP from CMS. In the event a hospital requests a hearing (as described in section XVI.H. of this proposed rule), we are proposing that the hospital must pay the amount in full within 60 calendar days after the date of a final and binding decision to uphold, in whole or in part, the CMP. We are also proposing that if the 60th calendar day is a weekend or a Federal holiday, then the timeframe is extended until the end of the next business day.
We also are proposing to publicize, by posting on a CMS website, our notice of imposition of a CMP on a hospital for noncompliance with these requirements, and any subsequently issued notice of imposition of a CMP for continuing violations. In the event that a hospital requests a hearing (as described in section XVI.H. of this proposed rule), we are proposing that CMS would indicate in its posting that the CMP is under review. If the CMP amount is upheld, in whole, by a final and binding decision, we would maintain the posting of the notice of imposition of a CMP on a CMS website. If the CMP is upheld, in part, by a final and binding decision, we would issue a modified notice of imposition of a CMP, and would make this modified notice public on a CMS website. If the CMP is overturned in full by a final and binding decision, we would remove the notice of imposition of a CMP from a CMS website.
In addition, we are proposing that CMS may issue subsequent notice(s) of imposition of a CMP, as described in this section of this proposed rule, that result from the same instance(s) of noncompliance.
We are proposing to set forth in proposed new 45 CFR 180.90 the proposed CMPs for hospitals determined by CMS to be noncompliant with requirements for making standard charges public.
We seek comment on whether the proposed amount of a CMP, in combination with making public on a CMS website our notice of imposition of a CMP, are reasonable and sufficient to ensure hospitals' compliance with the proposed requirements to make public standard charges. We are interested in public comments on our proposed $300 maximum daily dollar amount for a CMP for noncompliance with section 2718(e) of the PHS Act and proposed 45 CFR part 180. In particular, we seek comment on whether we should impose stronger penalties for noncompliance, or whether we should further limit the maximum amount of penalty we would impose on a hospital for a calendar year and the methodology for creating such a limit (for instance through limiting the maximum daily penalty amount, by establishing a cumulative annual total limit on the penalty amount, or both). We seek comment on unintended consequences of the proposed penalties for noncompliance. We also seek commenters' suggestions on whether other penalties should be applied for noncompliance with section 2718(e) of the PHS Act.
H. Proposed Appeals Process
Under section 2718(b)(3) of the PHS Act, we are proposing to impose penalties on hospitals that fail to make their standard charges public in accordance with the requirements we finalize under section 2718(e). We believe it is important to establish a fair administrative process by which a hospital may appeal CMS' decisions to impose penalties under section 2718(b)(3) regarding the hospital's noncompliance with the requirements of section 2718(e) of the PHS Act and the requirements of proposed 45 CFR part 180. Through various Medicare programs, we have gained experience with administrative hearings and other processes to review CMS' determinations.
We are proposing to align the procedures for the appeals process with the procedures established under section 2718(b)(3) of the PHS Act for an issuer to appeal a CMP imposed by HHS for its failure to report information and pay rebates related to medical loss ratios, as required by sections 2718(a) and (b) of the PHS Act, and according to 45 CFR parts 158 and 150. Therefore, we are proposing that a hospital upon which CMS has imposed a penalty under proposed 45 CFR part 180 may appeal that penalty in accordance with 45 CFR part 150, subpart D, except as we have proposed otherwise.
Generally, under this proposed approach, a hospital upon which CMS has imposed a penalty may request a hearing before an Administrative Law Judge (ALJ) of that penalty. The Administrator of CMS, at his or her discretion, may review in whole or in part the ALJ's decision. A hospital against which a final order imposing a CMP is entered may obtain judicial review.
For purposes of applying the appeals procedures at 45 CFR part 150 to appeals of CMPs under proposed 45 CFR part 180, we are proposing the following exceptions to the provisions of 45 CFR part 150:
- Civil money penalty means a civil monetary penalty according to proposed new 45 CFR 180.90.
- Respondent means a hospital that received a notice of imposition of a CMP according to proposed new 45 CFR 180.90(b).
- References to a notice of assessment or proposed assessment, or notice of proposed determination of CMPs, are considered to be references to the notice of imposition of a CMP specified in proposed new 45 CFR 180.90(b).
- Under 45 CFR 150.417(b), in deciding whether the amount of a civil money penalty is reasonable, the ALJ may only consider evidence of record relating to the following:
++ The hospital's posting(s) of its standard charges, if available.
++ Material the hospital timely previously submitted to CMS (including with respect to corrective actions and CAPs).
++ Material CMS used to monitor and assess the hospital's compliance according to proposed new 45 CFR 180.70(a)(2).
- The ALJ's consideration of evidence of acts other than those at issue in the instant case under 45 CFR 150.445(g) does not apply.
We are proposing to set forth in proposed new 45 CFR 180.100 the proposed procedures for a hospital to appeal the CMP imposed by CMS for its noncompliance with the requirements of proposed 45 CFR part 180.
We also are proposing to set forth in proposed new 45 CFR 180.110 the consequences for failure of a hospital to request a hearing. If a hospital does not request a hearing within 30 calendar days of the issuance of the notice of imposition of a CMP described in proposed new 45 CFR 180.90(b), we are proposing that CMS may impose the CMP indicated in such notice and may impose additional penalties pursuant to continuing violations according to proposed new 45 CFR 180.90(f) without right of appeal. We propose that if the 30th calendar day is a weekend or a Federal holiday, then the timeframe is extended until the end of the next business day. We also are proposing that the hospital has no right to appeal a penalty with respect to which it has not requested a hearing in accordance with 45 CFR 150.405, unless the hospital can show good cause, as determined at 45 CFR 150.405(b), for failing to timely exercise its right to a hearing.
Alternatively, we considered and are seeking public comment on following a process for appealing CMPs similar to the approach specified in 42 CFR part 498, subparts D through F. There are differences between the appeals procedures at 42 CFR part 498 compared to 45 CFR part 150. Under the regulations at 42 CFR part 498, for example, either party dissatisfied with a hearing decision by the ALJ may request Departmental Appeals Board review of the ALJ's decision.
XVII. Request for Information (RFI): Quality Measurement Relating to Price Transparency for Improving Beneficiary Access to Provider and Supplier Charge Information
A. Introduction
Last year, we published Requests for Information (RFIs) on price transparency in several Medicare payment rules,[210] including the CY 2019 OPPS/ASC proposed rule (83 FR 37211 and 37212). In the RFIs, we sought public comments on a variety of issues related to making provider and supplier charges for health care services furnished in hospitals more transparent. In general, we encouraged all providers and suppliers of health care services to undertake efforts to engage in consumer-friendly communication of their charges to help patients understand what their potential financial liability might be for services they plan to obtain, and to enable patients to compare charges for similar services. We encouraged providers and suppliers of health services to update this information at least annually, or more often as appropriate, to reflect current charges. We expressed concern that challenges continue to exist for patients due to insufficient price transparency. We also indicated that we are considering potential actions that would be appropriate to further our objective of having providers and suppliers of health care services undertake efforts to engage in consumer-friendly communication of their charges to help patients understand what their potential financial liability might be for services they obtain from them, and to enable patients to compare charges for similar services across providers and suppliers, including when services could be offered in more than one setting, such as a hospital outpatient department or an ambulatory surgical center.
In response to the RFIs, stakeholders consistently indicated support for our efforts to improve transparency in health care pricing. Stakeholders noted that out-of-pocket costs are the most relevant and beneficial information for patients and that such pricing information should be shared with patients along with associated quality of care and outcome data. Some stakeholders suggested that educational efforts would help to increase health care pricing literacy. Stakeholders believed that pricing and quality of care information should be shared with patients in a user-friendly format and be comparable across services and providers, which would allow patients to “shop” for the best value of health care. Multiple stakeholders commented that quality of care and outcome data should be paired with price information to allow patients to make informed decisions about where they could receive their care and to help ensure that consumers do not assume that the high cost of services necessarily equates to higher quality of care. Respondents to the RFIs suggested that quality information could be displayed by health care entities (such as hospitals) in conjunction with the posting of hospital standard charges, integrated electronically with cost and coverage data in electronic health records (EHRs) or regional health information exchanges (HIEs) for use in shared decision making at the point of care, or incorporated into public facing websites and price transparency tools.
Over the years, CMS has made much progress in improving health care quality measurement and making such quality information publicly available through various mechanisms, including public use files (PUFs) on the CMS website. In addition, CMS makes quality of health care information publicly available on the website at https://data.Medicare.gov for a number of different health care providers and suppliers, including hospitals, nursing homes, and physicians. Such data are available for the public and could be used by providers and suppliers of health care and pricing tool developers and integrated into EHRs in the manner identified by respondents to the RFI in the CY 2019 OPPS/ASC proposed rule. In addition, CMS has adopted Medicare quality measures that encourage patient engagement, improve patient experience of care, and create incentives for health care providers and suppliers to help patients understand their treatment choices and the financial implications. For example, starting in 2019, Merit-Based Incentive Payment System eligible clinicians participating in the Quality Payment Program will have the opportunity to receive points in the Improvement Activities performance category for helping patients or their caregivers understand the costs of care and explore different payment options by providing financial counseling (83 FR 60289).
B. Request for Information
To enhance our future efforts to improve policies related to transparency in health care charges, we are interested in stakeholder input on a number of related quality of health care issues, including the following:
1. Improving availability and access to existing quality of health care information for third parties and health care entities to use when developing price transparency tools and when communicating charges for health care services. Stakeholders are invited to submit specific suggestions and comments on the following:
- What type of existing quality of health care information would be most beneficial to patients, and how can health care providers and suppliers best enable patients to use quality of health care information in conjunction with information on charges in their decision making before or at the time a service is sought? For example, would it be feasible to use health care quality information from the Medicare Quality Payment Program (QPP) or the Quality Measures Inventory (QMI)? Could quality of health care information from state-mandated quality reporting initiatives or quality reporting initiatives by nationally recognized accrediting entities, such as the National Committee for Quality Assurance, URAC, the Joint Commission, and the National Quality Forum, be engaged to help patients meaningfully assess quality information at the time care is sought?
- How can CMS help providers, suppliers, and third parties create patient-friendly interfaces with this information? What steps should be taken to ensure that quality outcome and experience of care measure data can be used by providers, suppliers, third party pricing tool developers, and consumers when and where health care decisions are being made? Are there potential strategies CMS should consider to create standardized quality data? We are also interested in comments on the timing of information delivery relative to the referral or event, the form of delivery of the information, and the channels (for instance, verbally by the referring doctor, via a mobile application, and on a website, among others) through which the information could best be delivered.
- Is there value in displaying volume and complications of procedures side by side with charge information for patients? If so, should this information be best displayed at the individual physician level, the group practice level, or the facility level and why?
- Should health care providers and suppliers integrate quality information when informing patients of how much their out-of-pocket costs for services will be before patients are furnished services? How would providers that are not included in certain hospital-based quality initiatives, such as critical access hospitals, integrate quality information? What can be done better to inform patients of quality outcomes and patient experience with various providers and suppliers?
2. Improving incentives and assessing the ability of health care providers and suppliers to communicate and share charge information with patients. Stakeholders are invited to submit specific suggestions and comments on the following:
- Should CMS develop Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) questions to assess how well hospitals and other providers and suppliers communicate and discuss the cost of care with their patients? Example questions could be: “How well did your doctor communicate the expected out-of-pocket cost for your health care services in advance?” “Were you surprised by the amount of out-of-pocket costs you had for a given procedure or hospital stay?”
- Are there existing measures or measure concepts to develop that can help patients when assessing the accuracy of charges that providers and suppliers communicate in advance of a service, including the accuracy of expected out-of-pocket cost information? What indices should be used to assess how well a provider or supplier aggregates charge and quality information for public display?
- Are there Medicare value-based purchasing initiatives that could be improved by developing or implementing additional assessments of how well Medicare providers and suppliers engage and respond to patient inquiries related to cost of care, or how Medicare providers and suppliers engage in shared decision making for future care, including discussions of both charges and quality of referral services?
XVIII. Organ Procurement Organizations (OPOs) Conditions for Coverage (CfCs): Proposed Revision of the Definition of “Expected Donation Rate”
A. Background
1. Organ Procurement Organizations (OPOs)
Organ procurement organizations (OPOs) are vital partners in the procurement, distribution, and transplantation of human organs in a safe and equitable manner for all potential transplant recipients. The role of OPOs is critical to ensuring that the maximum possible number of transplantable human organs is available to seriously ill patients who are on a waiting list for an organ transplant. OPOs are responsible for identifying eligible donors, recovering organs from deceased donors, and complying with all CMS outcome and process performance measures. OPOs also must be a member of, participate in, and abide by the rules and requirements of the Organ Procurement and Transplantation Network (OPTN) that have been approved by the Secretary. The OPTN is a membership organization that links all professionals in the United States organ donation and transplantation system and whose board establishes and maintains transplant policies (which are available on the OPTN website at: https://optn.transplant.hrsa.gov/governance/about-the-optn/). Currently, the United Network for Organ Sharing (UNOS) serves as the OPTN under contract. OPOs are required to report specific information to the OPTN, including the data used to calculate the outcome measures for OPOs.
2. Statutory and Regulatory Provisions
To be an OPO, an entity must meet the applicable requirements of both the Act and the Public Health Service Act (the PHS Act). Section 1138(b) of the Act provides the statutory qualifications and requirements that an OPO must meet in order for organ procurement costs to be paid under the Medicare program or the Medicaid program. Section 1138(b)(1)(A) of the Act specifies that an OPO must operate under a grant made under section 371(a) of the PHS Act or must be certified or recertified by the Secretary as meeting the standards to be a qualified OPO within a certain time period. Congress has provided that payment may be made for organ procurement cost “only if” the OPO meets the performance-related standards prescribed by the Secretary. To receive payment under the Medicare program or the Medicaid program for organ procurement costs, the entity must have an agreement with, or be designated by, the Secretary (section 1138(b)(1)(F) of the Act and 42 CFR 486.304).
Pursuant to section 371(b)(1)(D)(ii)(II) of the PHS Act, the Secretary is required to establish outcome and process performance measures for OPOs to meet based on empirical evidence, obtained through reasonable efforts, of organ donor potential and other related factors in each service area of the qualified OPO. An OPO also must be a member of and abide by the rules and requirements of the OPTN that have been approved by the Secretary (section 1138(b)(1)(D) of the Act). We established Conditions for Coverage (CfCs) for OPOs to be able to receive payments from the Medicare and Medicaid programs at 42 CFR part 486, subpart G, to implement the statutory requirements. These regulations set forth the certification and recertification processes, outcome requirements, and process performance measures for OPOs and were effective on July 31, 2006 (71 FR 30982).
3. HHS Initiatives Related to OPO Services
In 2000, the Secretary's Advisory Committee on Organ Transplantation (ACOT) was established under the general authority of section 222 of the PHS Act, as amended, and implementing regulations under 42 CFR 121.12. A 2012 recommendation by ACOT stated: “The ACOT recognizes that the current CMS and HRSA/OPTN structure creates unnecessary burdens and inconsistent requirements on transplant centers (TCs) and OPOs and that the current system lacks responsiveness to advances in TC and OPO performance metrics. The ACOT recommends that the Secretary direct CMS and HRSA to confer with the OPTN, [Scientific Registry of Transplant Recipients] SRTR, the OPO community, and TC representatives to conduct a comprehensive review of regulatory and other requirements, and to promulgate regulatory and policy changes to requirements for OPOs and TCs that unify mutual goals of increasing organ donation, improving recipient outcomes, and reducing organ wastage and administrative burden on TCs and OPOs. These revisions should include, but not be limited to, improved risk adjustment methodologies for TCs and a statistically sound method for yield measures for OPOs. . . .” [211] We believe that our proposal to harmonize the definitions of “expected donation rate” as discussed below would address this ACOT recommendation. We also believe that the proposal demonstrates responsiveness to advances in OPO metrics and resolves an inconsistency in the OPO requirements for how OPO measures are being determined.
B. Proposed Revision of the Definition of “Expected Donation Rate”
As set forth in 42 CFR 486.328, which specifies the condition for reporting of data, transplant hospitals and OPOs must report data to the OPTN and those data are transmitted on a monthly basis to the SRTR contractor. The OPTN members, including OPOs, are required to submit certain data to the OPTN or SRTR. The OPTN and SRTR collect and analyze the data pursuant to the HRSA mission to increase organ donation and transplantation. Periodically, the data that OPOs must report to the OPTN or the SRTR is revised based on methodologies and clinical practice improvements that enable them to draw more accurate conclusions about donor and organ suitability for transplantation.
The CfCs for OPOs regulations at 42 CFR 486.318(a) and (b) require that an OPO must meet two of the three following outcome measures:
- The OPOs donation rate of eligible donors as a percentage of eligible deaths is no more than 1.5 standard deviations below the mean national donation rate of eligible donors as a percentage of eligible deaths, averaged over the 4 years of the re-certification cycle. Both the numerator and denominator of an individual OPO's donation rate ratio are adjusted by adding a 1 for each donation after cardiac death donor and each donor over the age of 70;
- The observed donation rate is not significantly lower than the expected donation rate for 18 or more months of the 36 months of data used for re-recertification, as calculated by SRTR;
- The OPO data reports, averaged over the 4 years of the re-certification cycle, must meet the rules and requirements of the most current OPTN aggregate donor yield measure.
The expected donation rate used in the second outcome measure is calculated by the SRTR. The CfCs for OPOs at 42 CFR 486.302 defines “expected donation rate” as the donation rate expected for an OPO based on the national experience for OPOs serving similar hospitals and donation service areas (DSAs). This rate is adjusted for the following hospital characteristics: Level I or Level II trauma center; Metropolitan Statistical Area (MSA) size; Metropolitan Statistical (MS) case-mix index; total bed size; number of intensive care unit (ICU) beds; primary service; presence of a neurosurgery unit; and hospital control/ownership.
In 2009, the SRTR modified the definition of “expected donation rate” we used for this outcome measure. The updated SRTR's definition states: “[t]he expected donation rate per 100 eligible deaths is the rate expected for an OPO based on the national experience for OPOs serving similar eligible donor populations and DSAs. This rate is adjusted for the distributions of age, sex, race, and cause of death among eligible deaths.” [212]
To determine the expected donation rate, the SRTR believed that it was important to adjust for characteristics that would allow for isolation of the effects that OPOs' practices were having on donation in that DSA. The SRTR determined that basing the expected donation rate for an OPO on the national experience for OPOs serving similar hospitals and DSAs and then adjusting for hospital characteristics did not take into consideration the eligible donor population in the DSA. The SRTR found that the eligible donor population varies from DSA to DSA across the country and such variations do have an impact on donation rates. Therefore, the SRTR determined that a more precise method to calculate an OPO's expected donation rate would be to base it on the national experience for OPOs serving similar eligible donor populations and DSAs and then adjust for patient characteristics, that is age, sex, race, and cause of death among eligible deaths.
Due to an oversight, CMS did not make a corresponding change to the definition in the CfCs for OPOs at the time that the SRTR made its change. In order to address this issue, we are proposing to change our requirements so that we are consistent with the SRTR's definition for the second outcome measure. Therefore, in this proposed rule, we are proposing to make a change to harmonize the CMS definition with the SRTR definition. We are proposing to make this change at this time in order to clarify the regulatory standard so that we may properly enforce the second outcome measure, eliminate any provider confusion, and further support our goals of accurately and reliably measuring OPO performance.
Specifically, we are proposing to revise the definition to state that the expected donation rate per 100 eligible deaths is the rate expected for an OPO based on the national experience for OPOs serving similar eligible donor populations and DSAs. We are proposing that this rate would be adjusted for the distributions of age, sex, race, and cause of death among eligible deaths.
If we finalize this proposal, this change would take effect on the effective date of the final rule with comment period, which would occur during the 2022 recertification cycle. Because the final regulation change would not be retroactive and, in order to give OPOs adequate time to comply with the change to the definition for “expected donation rate,” we are proposing to change the time period for the observed donation rate for the second outcome measure for the 2022 recertification cycle only. As a result, we also are proposing to revise § 486.318(a)(2), (b)(2), and (c)(1) to reduce the time period for this outcome measure. We are proposing to calculate the expected donation rate using 12 of the 24 months of data following the effective date of the final rule with comment period (using data from January 1, 2020 through December 31, 2020). After the 2022 recertification cycle, and if there are no other changes to the OPO outcome measures, we would assess OPO performance based on 36 months of data.
C. Request for Information Regarding Potential Changes to the Organ Procurement Organization and Transplant Center Regulations
Since the OPO and the transplant center regulations were finalized, we have received substantial feedback from the organ procurement and transplant communities recommending modifications to the current requirements. Therefore, we are considering a comprehensive proposal to update the CfCs for OPOs and possibly the CoPs for transplant centers. We are including transplant centers in this request for information due to the inextricable connection between transplant centers and OPOs. We are seeking public input regarding what revisions may be appropriate for the current CfCs for OPOs that are set forth at 42 CFR 486.301 through 486.360 and the current CoPs for transplant centers that are set forth at 42 CFR 482.68 through 482.104. The CfCs for OPOs set forth the requirements each OPO must meet to be eligible for payment under the Medicare and Medicaid programs. The CoPs for transplant centers set forth the requirements each transplant center must meet to be eligible for payment under the Medicare. In addition, more information on how data regarding OPOs as well as transplant centers are identified and used can be found on the website at: https://www.srtr.org/.
The following are key areas on which we are seeking public input:
- Do the current OPO outcome measures that are set forth at 42 CFR 486.318 accurately and reliably reflect an OPO's performance? If not, please explain.
- What are the impacts or consequences of the current outcome measures on: (1) An OPO's performance; and (2) the availability of transplantable organs?
- What impact, if any, do the certification and decertification processes for OPOs have on organ procurement and transplantation?
- Are there any potential, empirically based outcome measures, other than those currently at § 486.318, that could be used either in addition to, or instead of, the current outcome measures for OPOs? If recommending another outcome measure, what is the empirical evidence for that recommended measure?
- In addition to the outcome measures, are there other indicators of quality that could be used for OPOs in the CfCs? If recommending another quality indicator, why should that indicator be used in the OPOs CfCs and what is the supporting evidence for this indicator?
- Are there any transplant center CoPs that conflict with or should be harmonized with the OPOs CfCs? If yes, identify the specific requirements and how they would harmonize or otherwise modify the requirements.
We also are soliciting public comment on whether the following two potential OPO outcome measures would be valid measures and would be consistent with statutory requirements. We are especially interested in public comments about the validity and reliability of these possible measures.
The first potential measure would be the actual deceased donors as a percentage of inpatient deaths among patients 75 years of age or younger with a cause of death consistent with organ donation. The data on inpatient deaths, including additional related demographic data, would be derived from the CDC Detailed Mortality File and the National Center for Health Statistic's National Vital Statistics Report. We believe that the consistency and quality of this measure could be a significant improvement over the current measures because it relies on independent data to measure true organ donation potential. While this donation rate might include potential donors in the denominator who would never clinically qualify as organ donors, it does so consistently across all OPOs, which provides a reliable comparative performance measure across all OPO DSAs. This outcome measure also would account for: (1) Geographic differences in the manner of deaths across the United States (for example, trauma deaths); (2) geographic differences in the age distribution of deaths; and (3) geographic differences in in-hospital versus out-of-hospital deaths. This measure would reward efforts to maximize total organ procurement and efforts to improve placements of all procured organs.
The second potential measure is the actual organs transplanted as a percentage of inpatient deaths among patients 75 years of age or younger with a cause of death consistent with organ donation. This measure also would reward efforts to maximize total organ procurement and efforts to improve placements of all procured organs.
In addition to public comments on both of these potential outcome measures, we are interested in public comments on appropriate parameters for these measures. How should we determine what percentage indicates that an OPO's performance is acceptable or successful? If commenters cannot recommend a specific percentage, how should we determine what the parameters for the outcome measures should be? We are requesting that commenters explain and include any evidence or data they have to support their comments. We are interested in any comments about what commenters believe would be the benefit or consequences, or perhaps unintended consequences, of using these measures and the potential impact on OPOs, transplant centers, organ donation, and transplant recipients. We are also interested in comments on potential additional compliance burdens on OPOs and transplant centers. Finally, we are seeking comments that demonstrate how revising the OPO outcome measures would benefit or negatively impact patient outcomes, access, and quality of life.
For additional information on these potential outcome measures, we refer readers to the document, Changing Metrics of Organ Procurement Organization Performance in Order to Increase Organ Donation Rates in the United States, published in the American Journal for Transplantations.[213]
We will consider the public comments that we receive from this request for information for future rulemaking and potential revisions to the CfCs for OPOs and the CoPs for transplant centers.
XIX. Clinical Laboratory Fee Schedule: Potential Revisions to the Laboratory Date of Service Policy
A. Background on the Medicare Part B Laboratory Date of Service Policy
The date of service (DOS) is a required data field on all Medicare claims for laboratory services. However, a laboratory service may take place over a period of time—the date the laboratory test is ordered, the date the specimen is collected from the patient, the date the laboratory accesses the specimen, the date the laboratory performs the test, and the date results are produced may occur on different dates. In the final rule on coverage and administrative policies for clinical diagnostic laboratory services published in the Federal Register on November 23, 2001 (66 FR 58791 through 58792), we adopted a policy under which the DOS for clinical diagnostic laboratory services generally is the date the specimen is collected. In that final rule, we also established a policy that the DOS for laboratory tests that use an archived specimen is the date the specimen was obtained from storage (66 FR 58792).
In 2002, we issued Program Memorandum AB-02-134, which permitted contractors discretion in making determinations regarding the length of time a specimen must be stored to be considered “archived.” In response to comments requesting that we issue a national standard to clarify when a stored specimen can be considered “archived,” in the Procedures for Maintaining Code Lists in the Negotiated National Coverage Determinations for Clinical Diagnostic Laboratory Services final notice, published in the Federal Register on February 25, 2005 (70 FR 9357), we defined an “archived” specimen as a specimen that is stored for more than 30 calendar days before testing. Specimens stored for 30 days or less continued to have a DOS of the date the specimen was collected.
B. Medicare DOS Policy and the “14-Day Rule”
In the final rule with comment period entitled, in relevant part, “Revisions to Payment Policies, Five-Year Review of Work Relative Value Units, Changes to the Practice Expense Methodology Under the Physician Fee Schedule, and Other Changes to Payment Under Part B” published in the Federal Register on December 1, 2006 (December 1, 2006 MPFS final rule) (71 FR 69705 through 69706), we added a new § 414.510 in title 42 of the CFR regarding the clinical laboratory DOS requirements and revised our DOS policy for stored specimens. We explained in that MPFS final rule that the DOS of a test may affect payment for the test, especially in situations in which a specimen that is collected while the patient is being treated in a hospital setting (for example, during a surgical procedure) is later used for testing after the patient has been discharged from the hospital. We noted that payment for the test is usually bundled with payment for the hospital service, even when the results of the test did not guide treatment during the hospital stay. To address concerns raised for tests related to cancer recurrence and therapeutic interventions, we finalized modifications to the DOS policy in § 414.510(b)(2)(i) for a test performed on a specimen stored less than or equal to 30 calendar days from the date it was collected (a non-archived specimen), so that the DOS is the date the test was performed (instead of the date of collection) if the following conditions are met:
- The test is ordered by the patient's physician at least 14 days following the date of the patient's discharge from the hospital;
- The specimen was collected while the patient was undergoing a hospital surgical procedure;
- It would be medically inappropriate to have collected the sample other than during the hospital procedure for which the patient was admitted;
- The results of the test do not guide treatment provided during the hospital stay; and
- The test was reasonable and medically necessary for the treatment of an illness.
As we stated in the December 1, 2006 MPFS final rule, we established these five criteria, which we refer to as the “14-day rule,” to distinguish laboratory tests performed as part of posthospital care from the care a beneficiary receives in the hospital. When the 14-day rule applies, laboratory tests are not bundled into the hospital stay, but are instead paid separately under Medicare Part B (as explained in more detail below).
We also revised the DOS requirements for a chemotherapy sensitivity test performed on live tissue. As discussed in the December 1, 2006 MPFS final rule (71 FR 69706), we agreed with commenters that these tests, which are primarily used to determine posthospital chemotherapy care for patients who also require hospital treatment for tumor removal or resection, appear to be unrelated to the hospital treatment in cases where it would be medically inappropriate to collect a test specimen other than at the time of surgery, especially when the specific drugs to be tested are ordered at least 14 days following hospital discharge. As a result, we revised the DOS policy for chemotherapy sensitivity tests, based on our understanding that the results of these tests, even if they were available immediately, would not typically affect the treatment regimen at the hospital. Specifically, we modified the DOS for chemotherapy sensitivity tests performed on live tissue in § 414.510(b)(3) so that the DOS is the date the test was performed if the following conditions are met:
- The decision regarding the specific chemotherapeutic agents to test is made at least 14 days after discharge;
- The specimen was collected while the patient was undergoing a hospital surgical procedure;
- It would be medically inappropriate to have collected the sample other than during the hospital procedure for which the patient was admitted;
- The results of the test do not guide treatment provided during the hospital stay; and
- The test was reasonable and medically necessary for the treatment of an illness.
We explained in the December 1, 2006 MPFS final rule that, for chemotherapy sensitivity tests that meet this DOS policy, Medicare would allow separate payment under Medicare Part B; that is, separate from the payment for hospital services.
C. Billing and Payment for Laboratory Services Under the OPPS
The DOS requirements at 42 CFR 414.510 are used to determine whether a hospital bills Medicare for a clinical diagnostic laboratory test (CDLT) or whether the laboratory performing the test bills Medicare directly. Separate regulations at 42 CFR 410.42(a) and 411.15(m) generally provide that Medicare will not pay for a service furnished to a hospital patient during an encounter by an entity other than the hospital unless the hospital has an arrangement (as defined in 42 CFR 409.3) with that entity to furnish that particular service to its patients, with certain exceptions and exclusions. These regulations, which we refer to as the “under arrangements” provisions in this discussion, require that if the DOS falls during an inpatient or outpatient stay, payment for the laboratory test is usually bundled with the hospital service.
Under our current rules, if a test meets all DOS requirements in § 414.510(b)(2)(i), (b)(3), or (b)(5) (an additional exception finalized in the CY 2018 OPPS/ASC final rule with comment period that we describe later in this section), the DOS is the date the test was performed. In this situation, the laboratory would bill Medicare directly for the test and would be paid under the Clinical Laboratory Fee Schedule (CLFS) directly by Medicare. However, if the test does not meet the DOS requirements in § 414.510(b)(2)(i), (b)(3), or (b)(5), the DOS would be the date the specimen was collected from the patient. In that case, the hospital would bill Medicare for the test and then would pay the laboratory that performed the test, if the laboratory provided the test under arrangement.
In recent rulemakings, we have reviewed appropriate payment under the OPPS for certain diagnostic tests that are not commonly performed by hospitals. In CY 2014, we finalized a policy to package certain CDLTs under the OPPS (78 FR 74939 through 74942 and 42 CFR 419.2(b)(17) and 419.22(l)). In CYs 2016 and 2017, we made some modifications to this policy (80 FR 70348 through 70350; 81 FR 79592 through 79594). Under our current policy, certain CDLTs that are listed on the CLFS are packaged as integral, ancillary, supportive, dependent, or adjunctive to the primary service or services provided in the hospital outpatient setting during the same outpatient encounter and billed on the same claim. Specifically, we conditionally package most CDLTs and only pay separately for a laboratory test when it is: (1) The only service provided to a beneficiary on a claim; (2) considered a preventive service; (3) a molecular pathology test; or (4) an advanced diagnostic laboratory test (ADLT) that meets the criteria of section 1834A(d)(5)(A) of the Act (78 FR 74939 through 74942; 80 FR 70348 through 70350; and 81 FR 79592 through 79594). In the CY 2016 OPPS/ASC final rule with comment period, we excluded all molecular pathology laboratory tests from packaging because we believed these relatively new tests may have a different pattern of clinical use, which may make them generally less tied to a primary service in the hospital outpatient setting than the more common and routine laboratory tests that are packaged.
For similar reasons, in the CY 2017 OPPS/ASC final rule with comment period (81 FR 79592 through 79594), we extended the exclusion to also apply to all ADLTs that meet the criteria of section 1834A(d)(5)(A) of the Act, which we describe below. We stated that we will assign status indicator “A” (Separate payment under the CLFS) to ADLTs once a laboratory test is designated an ADLT under the CLFS. Laboratory tests that are separately payable and are listed on the CLFS are paid at the CLFS payment rates outside the OPPS.
D. ADLTs Under the New Private Payor Rate-Based CLFS
Section 1834A of the Act, as established by section 216(a) of Public Law 113-93, the Protecting Access to Medicare Act of 2014 (PAMA), requires significant changes to how Medicare pays for CDLTs under the CLFS. Section 216(a) of PAMA also establishes a new subcategory of CDLTs known as ADLTs, with separate reporting and payment requirements under section 1834A of the Act. In the CLFS final rule published in the Federal Register on June 23, 2016, entitled “Medicare Program; Medicare Clinical Diagnostic Laboratory Tests Payment System Final Rule” (81 FR 41036), we implemented the requirements of section 1834A of the Act.
As defined in § 414.502, an ADLT is a CDLT covered under Medicare Part B that is offered and furnished only by a single laboratory, and cannot be sold for use by a laboratory other than the single laboratory that designed the test or a successor owner. Also, an ADLT must meet either Criterion (A), which implements section 1834A(d)(5)(A) of the Act, or Criterion (B), which implements section 1834A(d)(5)(B) of the Act, as follows:
- Criterion (A): The test is an analysis of multiple biomarkers of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), or proteins; when combined with an empirically derived algorithm, yields a result that predicts the probability a specific individual patient will develop a certain condition(s) or respond to a particular therapy(ies); provides new clinical diagnostic information that cannot be obtained from any other test or combination of tests; and may include other assays.
Or:
- Criterion (B): The test is cleared or approved by the Food and Drug Administration.
Generally, under the revised CLFS, ADLTs are paid using the same methodology based on the weighted median of private payor rates as other CDLTs. However, updates to ADLT payment rates occur annually instead of every 3 years. The payment methodology for ADLTs is detailed in the June 23, 2016 CLFS final rule (81 FR 41076 through 41083). For additional information regarding ADLTs, we refer readers to the CMS website: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/PAMA-regulations.html.
E. Additional Laboratory DOS Policy Exception for the Hospital Outpatient Setting
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59393 through 59400), we established an additional exception at § 414.510(b)(5) for the hospital outpatient setting so that the DOS for molecular pathology tests and certain ADLTs that are excluded from the OPPS packaging policy is the date the test was performed (instead of the date of specimen collection) if certain conditions are met. Under the exception that we finalized at § 414.510(b)(5), in the case of a molecular pathology test or a test designated by CMS as an ADLT under paragraph (1) of the definition of an ADLT in § 414.502, the DOS of the test must be the date the test was performed only if:
- The test was performed following a hospital outpatient's discharge from the hospital outpatient department;
- The specimen was collected from a hospital outpatient during an encounter (as both are defined in 42 CFR 410.2);
- It was medically appropriate to have collected the sample from the hospital outpatient during the hospital outpatient encounter;
- The results of the test do not guide treatment provided during the hospital outpatient encounter; and
- The test was reasonable and medically necessary for the treatment of an illness.
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59397), we explained that we believed the laboratory DOS policy in effect prior to CY 2018 created administrative complexities for hospitals and laboratories with regard to molecular pathology tests and laboratory tests expected to be designated by CMS as ADLTs that meet the criteria of section 1834A(d)(5)(A) of the Act. We noted that under the laboratory DOS policy in effect prior to CY 2018, if the tests were ordered less than 14 days following a hospital outpatient's discharge from the hospital outpatient department, laboratories generally could not bill Medicare directly for the molecular pathology test or ADLT. In those circumstances, the hospital had to bill Medicare for the test, and the laboratory had to seek payment from the hospital. We noted that commenters informed us that because ADLTs are performed by only a single laboratory and molecular pathology tests are often performed by only a few laboratories, and because hospitals may not have the technical ability to perform these complex tests, the hospital may be reluctant to bill Medicare for a test it would not typically (or never) perform. The commenters also stated that as a result, the hospital might delay ordering the test until at least 14 days after the patient is discharged from the hospital outpatient department, or even cancel the order to avoid the DOS policy, which may restrict a patient's timely access to these tests. In addition, we noted that we had heard from commenters that the laboratory DOS policy in effect prior to CY 2018 may have disproportionately limited access for Medicare beneficiaries under Medicare Parts A and B, because Medicare Advantage plans under Medicare Part C and other private payors allow laboratories to bill directly for tests they perform.
We also recognized that greater consistency between the laboratory DOS rules and the current OPPS packaging policy would be beneficial and would address some of the administrative and billing issues created by the DOS policy in effect prior to CY 2018. We noted that we exclude all molecular pathology tests and ADLTs under section 1834A(d)(5)(A) of the Act from the OPPS packaging policy because we believe these tests may have a different pattern of clinical use, which may make them generally less tied to a primary service in the hospital outpatient setting than the more common and routine laboratory tests that are packaged, and we had already established exceptions to the DOS policy that permit the DOS to be the date of performance for certain tests that we believe are not related to the hospital treatment and are used to determine posthospital care. We stated that we believed a similar exception is justified for the molecular pathology tests and ADLTs excluded from the OPPS packaging policy, which we understood are used to guide and manage the patient's care after the patient is discharged from the hospital outpatient department. We noted that we believed that, like the other tests currently subject to DOS exceptions, these tests can legitimately be distinguished from the care the patient receives in the hospital, and thus we would not be unbundling services that are appropriately associated with hospital treatment. Moreover, we reiterated that these tests are already paid separately outside of the OPPS at CLFS payment rates. Therefore, we agreed with the commenters that the laboratory performing the test should be permitted to bill Medicare directly for these tests, instead of relying on the hospital to bill Medicare on behalf of the laboratory under arrangements.
A list of the specific laboratory tests currently subject to the laboratory DOS exception at § 414.510(b)(5) is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Lab-DOS-Policy.html.
Following publication of the CY 2018 OPPS/ASC final rule with comment period, we issued Change Request (CR) 10419, Transmittal 4000, the claims processing instruction implementing the laboratory DOS exception at § 414.510(b)(5), with an effective date of January 1, 2018 and an implementation date of July 2, 2018. After issuing CR 10419, we heard from stakeholders that many hospitals and laboratories were having administrative difficulties implementing the DOS exception set forth at § 414.510(b)(5). On July 3, 2018, we announced that, for a 6-month period, we would exercise enforcement discretion with respect to the laboratory DOS exception at § 414.510(b)(5). We explained that stakeholder feedback suggested many providers and suppliers would not be able to implement the laboratory DOS exception by the July 2, 2018 implementation date established by CR 10419, and that such entities required additional time to develop the systems changes necessary to enable the performing laboratory to bill for tests subject to the exception. We noted that this enforcement discretion applies to all providers and suppliers with regard to ADLTs and molecular pathology tests subject to the laboratory DOS exception policy, and that during the enforcement discretion period, hospitals may continue to bill for these tests that would otherwise be subject to the laboratory DOS exception.
We then extended the enforcement discretion period for two additional, consecutive 6-month periods, after learning through communications with representatives of providers and suppliers affected by the policy that there are still many entities who will not be able to implement the laboratory DOS exception and will need additional time to come into compliance. The enforcement discretion period is currently in effect until January 2, 2020. The latest enforcement discretion announcement as well as CR 10419, Transmittal 4000 is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Lab-DOS-Policy.html .
During this time, we have continued to gage the industry's readiness to implement the laboratory DOS exception at § 414.510(b)(5). Stakeholders, including representatives of hospitals, have informed us that hospitals, in particular, are having difficulty with developing the systems changes necessary to provide the performing laboratory with the patient's hospital outpatient status, beneficiary demographic information, and insurance information, such as whether the beneficiary is enrolled in original fee-for-service Medicare or a specific Medicare Advantage plan. According to stakeholders, the performing laboratory requires this information so that it can bill Medicare directly for the test instead of seeking payment from the hospital.
In addition, stakeholders, including representatives of laboratories, have noted that some entities performing molecular pathology testing subject to the laboratory DOS exception, such as blood banks and blood centers, may not be enrolled in the Medicare program and may not have established a mechanism to bill Medicare directly. According to these stakeholders, blood banks and blood centers that are not currently enrolled in the Medicare program would need to establish a billing mechanism so that they can bill Medicare directly when the requirements of § 414.510(b)(5) are met. Stakeholders have asserted that establishing a billing mechanism is labor intensive and that blood banks and blood centers currently lack the financial resources and expertise to take on this task.
We also note that protein-based Multianalyte Assays with Algorithmic Analysis (MAAAs) that are not considered molecular pathology tests and are not designated as ADLTs under paragraph (1) of the definition of ADLT in § 414.502, are also conditionally packaged under the OPPS at this time. Several stakeholders have suggested that they believe that the pattern of clinical use of some of these protein-based MAAAs make them relatively unconnected to the primary hospital outpatient service, though they do not currently qualify for the DOS exception at § 414.510(b)(5) solely because they are MAAAs. We note that a protein-based MAAA that is designated by CMS as an ADLT under paragraph (1) of the definition of an ADLT in § 414.502 would be eligible for the DOS exception at § 414.510(b)(5), and we intend to consider policies regarding MAAAs for future rulemaking.
F. Potential Revisions to Laboratory DOS Policy and Request for Public Comments
In response to the implementation concerns raised by stakeholders, we are considering making additional changes to the laboratory DOS policy.
As discussed previously, under the exception that we finalized at § 414.510(b)(5), a molecular pathology test or a test designated by CMS as an ADLT under paragraph (1) of the definition of an ADLT in § 414.502, the DOS of the test must be the date the test was performed only if: (i) The test was performed following a hospital outpatient's discharge from the hospital outpatient department; (ii) the specimen was collected from a hospital outpatient during an encounter (as both are defined in 42 CFR 410.2); (iii) it was medically appropriate to have collected the sample from the hospital outpatient during the hospital outpatient encounter; (iv) the results of the test do not guide treatment provided during the hospital outpatient encounter; and (v) the test was reasonable and medically necessary for the treatment of an illness. When all conditions under the laboratory DOS exception at § 414.510(b)(5) are met, the DOS is the date of test performance, instead of the date of specimen collection, which effectively unbundles the test from the hospital outpatient encounter. As such, the test is not considered a hospital outpatient service for which the hospital must bill Medicare and for which the performing laboratory must seek payment from the hospital, but rather a laboratory test under the CLFS for which the performing laboratory must bill Medicare directly. We are considering three options for potential changes to the laboratory DOS exception at § 414.510(b)(5), and we seek comment on these changes. Specifically, we are seeking comment on:
1. Changing the Test Results Requirement at 42 CFR 414.510(b)(5)(iv);
2. Limiting the Laboratory DOS Exception at 42 CFR 414.510(b)(5) to ADLTs; and/or
3. Excluding Blood Banks and Blood Centers from the Laboratory DOS Exception at 42 CFR 414.510(b)(5).
These potential revisions are discussed below.
1. Changing the Test Results Requirement at 42 CFR 414.510(b)(5)(iv)
Since finalizing the laboratory DOS exception at § 414.510(b)(5), we have continued to review and analyze the factors we use to determine whether a molecular pathology test or Criterion (A) ADLT is unrelated to the hospital treatment and used to determine posthospital care, and therefore should have a DOS that is the date of performance rather than the date of specimen collection. One such factor, in § 414.510(b)(5)(iv), is that the results of the test must not guide treatment provided during the hospital outpatient encounter—meaning, the encounter in which the specimen was collected. We are no longer convinced that the determination as to whether a molecular pathology test or ADLT is separable from a hospital service should be based on whether the test results guide treatment during the specific hospital outpatient encounter in which the specimen was collected. We believe that a molecular pathology test or an ADLT that is performed on a specimen collected during a hospital outpatient encounter, in which the results of the test are intended to guide treatment during a future hospital outpatient encounter, is a hospital service, and therefore should be billed by the hospital that collected the specimen under arrangements, just like if the test does not meet one of the other prongs of § 414.510(b)(5). In contrast, if the results of the test are not intended to guide treatment during a hospital outpatient encounter, and if all other requirements in § 414.510(b)(5) are met, the test is separable from a hospital service and therefore, should be considered a laboratory service and the performing laboratory should bill for the test.
We believe that a test's relationship to a hospital outpatient encounter depends on many factors, including the patient's current diagnosis (or lack of a current diagnosis), the procedure(s) being considered for the patient, the patient's current and previous medical history, and other factors and that the ordering physician would be aware of these beneficiary characteristics. As such, we believe that it should be the role of the ordering physician to determine whether the results of a molecular pathology test or ADLT are or are not intended to guide treatment during a hospital outpatient encounter.
Therefore, we are considering a revision to our current laboratory DOS policy at § 414.510(b)(5)(iv) to specify that the ordering physician would determine whether the results of the ADLT or molecular pathology test are intended to guide treatment provided during a hospital outpatient encounter, if the other four requirements under § 414.510(b)(5) are met. Under this approach, the test would be considered a hospital service unless the ordering physician determines that the test does not guide treatment during a hospital outpatient encounter. If the ordering physician determines that the test results are not intended to guide treatment during the hospital outpatient encounter from which the specimen was collected or during a future hospital outpatient encounter, for purposes of the laboratory DOS exception at § 414.510(b)(5), the DOS service of the test would be the date of test performance. In this situation, the test would not be considered a hospital service and the performing laboratory would be required to bill for the test.
Conversely, if the other four requirements under § 414.510(b)(5) are met, but the ordering physician determines that the results of the laboratory test are intended to guide treatment during a hospital outpatient encounter, the DOS would be the date of specimen collection. As a result, the hospital that collected the specimen would bill for the laboratory test under arrangements and the laboratory would seek payment from the hospital for the test. This potential revision to the laboratory DOS exception at § 414.510(b)(5) would be consistent with our belief that a molecular pathology test or a Criterion (A) ADLT is a hospital service when the results of the test are intended to guide treatment during a hospital outpatient encounter.
We are requesting comments from hospitals, laboratories, physicians and non-physician practitioners, and other interested stakeholders regarding this potential revision to the laboratory DOS exception at § 414.510(b)(5). We are particularly interested in comments regarding our position that when the results of molecular pathology testing and Criterion (A) ADLTs are intended to guide treatment during a future hospital outpatient encounter, the test is a hospital service. We also are interested in receiving public comments regarding the administrative aspects of requiring the ordering physician to determine when the test results are not intended to guide the treatment during a hospital outpatient encounter, as well as the process for the ordering physician to document this decision and provide notification to the hospital that collected the specimen for billing purposes. We note that we would consider finalizing this potential revision to the laboratory DOS policy as a result of our review of the comments received on this topic.
We note that at this time, we are only soliciting comments on potential changes to the laboratory DOS exception at § 414.510(b)(5), and not the 14-day rule DOS exception at § 414.510(b)(2) and the chemotherapy sensitivity test DOS exception at § 414.510(b)(3). These exceptions would continue to include the requirement that the results of the test do not guide treatment provided during the hospital stay, meaning the hospital stay in which the specimen was collected. Although we recognize that the considerations about how a hospital service is determined under § 414.510(b)(5) discussed previously may also be applicable to the 14-day rule DOS exception and chemotherapy sensitivity test DOS exception, we are only considering revisions to the laboratory DOS exception at § 414.510(b)(5) at this time. Because of the administrative issues raised by stakeholders regarding the implementation of the laboratory DOS exception at § 414.510(b)(5), we believe a cautious and incremental approach to making changes to laboratory DOS policy is warranted. As such, any potential changes to the 14-day rule DOS exception at § 414.510(b)(2) and the chemotherapy sensitivity test DOS exception at § 414.510(b)(3) would be addressed in future rulemaking.
2. Limiting the Laboratory DOS Exception at 42 CFR 414.510(b)(5) to ADLTs
As discussed previously in this section, we established a laboratory DOS policy exception for the hospital outpatient setting at § 414.510(b)(5), in part, because of stakeholder concerns that the laboratory DOS policy in effect prior to CY 2018 created beneficiary access issues with regard to molecular pathology tests and laboratory tests expected to be designated by CMS as ADLTs that meet the criteria of section 1834A(d)(5)(A) of the Act. In the CY 2018 OPPS/ASC proposed rule (82 FR 33653), we considered revising the DOS rule to create an exception only for ADLTs that meet the criteria in section 1834A(d)(5)(A) of the Act because ADLTs are offered and furnished only by a single laboratory (as defined in 42 CFR 414.502). We noted that a hospital, or another laboratory that is not the single laboratory (as defined in 42 CFR 414.502), cannot furnish the ADLT, and there may be additional beneficiary concerns for these ADLTs that may not apply to the molecular pathology tests. For example, a hospital may not have an arrangement with the single laboratory that furnishes a particular ADLT, which could lead the hospital to delay the order for the ADLT until 14 days after the patient's discharge to avoid financial risk and thus potentially delay medically necessary care for the beneficiary. We solicited comments as to whether molecular pathology tests present the same concerns of delayed access to medically necessary care as ADLTs, noting that molecular pathology tests are not required to be furnished by a single laboratory and that there may be “kits” for certain molecular pathology tests that a hospital can purchase, allowing the hospital to perform the test. In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59399) we agreed with commenters that limiting the new laboratory DOS exception to include only ADLTs (and not molecular pathology tests) would be inconsistent with the OPPS packaging policy and that relatively few laboratories may perform certain molecular pathology testing. We also acknowledged that hospitals may not currently have the technical expertise or certification requirements necessary to perform molecular pathology testing and therefore must rely on independent laboratories to perform the test. Therefore, we concluded that similar beneficiary access concerns that apply to ADLTs may also apply to molecular pathology tests, and we decided not to limit the exception at 42 CFR 414.510(b)(5) to ADLTs only.
However, after further review of this issue, we no longer believe the same beneficiary access concerns that apply to ADLTs also apply to molecular pathology tests. In particular, unlike ADLTs, molecular pathology tests are not required by statute to be furnished by a single laboratory, so hospital laboratories and independent laboratories are not prevented from performing molecular pathology testing. In addition, we understand that a number of kits have recently been developed and approved by FDA that would allow a hospital to more easily perform some of these molecular pathology tests. As such, we are no longer convinced that molecular pathology tests present the same concerns of delayed access to medically necessary care as ADLTs, which must be performed by a single laboratory. We believe a hospital's laboratory can develop the expertise to perform a molecular pathology test or establish an arrangement with an independent laboratory to perform the test. Therefore, we believe that any incentives that may exist to delay ordering until at least 14 days following a patient's discharge from the hospital outpatient department do not apply to molecular pathology tests.
We recognize that limiting the laboratory DOS exception to ADLTs is not consistent with OPPS packaging policy. As discussed previously in this section of the proposed rule, we exclude all molecular pathology laboratory tests from OPPS packaging because we believe these tests may have a different pattern of clinical use, which may make them generally less tied to a primary service in the hospital outpatient setting than the more common and routine laboratory tests that are packaged (80 FR 70348 through 70350). However, consistency with the OPPS packaging policy only formed part of the basis for the laboratory DOS exception at § 414.510(b)(5). We note that beneficiary access concerns were the primary reason for establishing this laboratory DOS exception and we no longer believe the access concerns are sufficiently compelling for the molecular pathology tests. In light of the billing and enrollment concerns raised by the blood banks and blood centers and administrative issues raised by other stakeholders, CMS believes the policy reasons for removing these tests from the laboratory DOS exception at § 414.510(b)(5) outweigh the difference it creates with the OPPS packaging policy.
Therefore, we are considering a potential revision that would limit the laboratory DOS provisions of § 414.510(b)(5) to tests designated by CMS as an ADLT under paragraph (1) of the definition of an ADLT in § 414.502. Molecular pathology tests would be removed from the provisions of § 414.510(b)(5). However, we note that molecular pathology tests would still be subject to the laboratory DOS provisions of § 414.510(b)(2) and (3).
We are requesting comments on potentially limiting the laboratory DOS exception policy at § 414.510(b)(5) to Criterion (A) ADLTs that have been granted ADLT status by CMS. We note that we would consider finalizing this approach as a result of the public comments received.
3. Excluding Blood Banks and Blood Centers From the Laboratory DOS Exception at 42 CFR 414.510(b)(5)
Following publication of the CY 2018 OPPS/ASC final rule with comment period, stakeholders informed us that blood banks and blood centers perform some of the molecular pathology test codes that are subject to the laboratory DOS exception policy at § 414.510(b)(5). Based on information from stakeholders, it is our understanding that blood banks and centers are entities whose primary function is the collection, storage and dissemination of blood products and are typically accredited by the AABB (formally known as the American Association of Blood Banks). Representatives of blood banks and centers contend that while these entities may perform the same molecular pathology tests that are performed and billed by other laboratories that are not blood banks and centers, the blood banks and centers perform these tests for different reasons. Specifically, they assert that the blood banks and centers perform molecular pathology testing primarily to identify the most compatible blood product for a patient, whereas other laboratories typically provide molecular pathology testing for diagnostic purposes. According to these stakeholders, the patient has already been diagnosed with a specific disease or condition before the blood sample is provided to the blood bank or center, who are then tasked with providing compatible blood products and assessing risks of incompatibility for hospitals. In other words, blood banks and centers perform molecular pathology testing for patients to enable hospitals to prevent adverse conditions associated with blood transfusions, rather than perform molecular pathology testing for diagnostic purposes. Examples of molecular pathology testing performed by blood banks and centers include red blood cell phenotyping, as described by HCPCS code 81403, red blood cell antigen testing as described by HCPCS code 0001U, and platelet antigen testing as described by HCPCS code 81105.
As discussed previously, when a test meets all of the conditions in the current laboratory DOS exception at § 414.510(b)(5), the DOS of the test must be the date the test was performed, and the laboratory that performed the test must bill Medicare directly for the test. This would include circumstances when a laboratory that is a blood bank or blood center performs the test. However, given the different purpose of molecular pathology testing performed by the blood banks and centers, that is, blood compatibility testing, we question whether the molecular pathology testing performed by blood banks and centers is appropriately separable from the hospital stay, given that it typically informs the same patient's treatment during a future hospital stay. We are concerned that our current policy may unbundle molecular testing performed by a blood bank or center for a hospital patient. As such, we believe that molecular pathology testing, when performed by blood banks or centers, is inherently tied to a hospital service because hospitals receive payment for and/or use the blood and/or blood products provided by blood banks and blood centers to treat patients in the hospital setting. Accordingly, we believe that such testing is so connected to the treatment furnished to the patient in the hospital that it must be considered a hospital service and that hospitals should be permitted to bill and receive payment for such testing performed on these blood and/or blood-related products.
Based on our concern and the comments we have received from stakeholders, we are considering a regulatory change that would exclude blood banks and centers from the laboratory DOS exception at § 414.510(b)(5). Under this potential revision, the DOS for laboratory testing performed by blood banks and centers on specimens collected from a hospital outpatient during a hospital outpatient encounter would, depending on the underlying service, be the date of specimen collection. As a result, the hospital would bill for the laboratory test under arrangements and the blood bank or center performing the test would seek payment from the hospital. In addition, for purposes of excluding blood banks and centers from the provisions of § 414.510(b)(5), we would define a blood bank and center as an entity whose primary function is the collection, storage and dissemination of blood products. We believe this potential definition of a blood bank and center describes the primary responsibility of all blood banks and centers, which distinguishes these entities from other laboratory types. In developing a definition of blood banks and centers we are distinguishing blood banks and blood centers from non-blood bank and blood center laboratories that perform the same molecular pathology test codes but for different reasons, that is, for diagnostic purposes rather than for blood compatibility testing.
We are requesting comments from hospitals, blood banks and centers, and other interested stakeholders regarding a potential revision to laboratory DOS policy that would exclude blood banks and centers from the laboratory DOS exception policy at § 414.510(b)(5). We also are requesting specific comments as to how a blood bank and blood center may be defined in the context of this provision, and particularly how to distinguish blood banks and centers from other laboratories. We note that we would consider finalizing a revision to the laboratory DOS policy that excludes blood banks and centers from the provisions of § 414.510(b)(5) as a result of comments received on this topic.
XX. Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
A. Background
As part of its responsibility to protect the Medicare Trust Funds, CMS routinely analyzes data associated with all facets of the Medicare program. This responsibility includes monitoring the total amount or types of claims submitted by providers and suppliers; analyzing the claims data to assess the growth in the number of claims submitted over time (for example, monthly and annually, among other intervals); and conducting comparisons of the data with other relevant data, such as the total number of Medicare beneficiaries served by providers to help ensure the continued appropriateness of payment for services furnished in the hospital outpatient department (OPD).
In line with this responsibility, CMS recently completed an analysis of the volume of covered OPD services furnished and determined that CMS has experienced significant increases in the utilization volume of some of these services. As an initial effort to focus our analysis, we chose to target services that represent procedures that are likely to be cosmetic surgical procedures and/or are directly related to cosmetic surgical procedures that are not covered by Medicare, but may be combined with or masquerading as therapeutic services.[214] However, we also recognized the need to establish baseline measures for comparison purposes, including, but not limited to, the yearly rate-of-increase in the number of OPD claims submitted and the average annual rate-of-increase in Medicare allowed amounts. Our analysis included the review of over 1.1 billion claims related to OPD services during the 11-year period from 2007 through 2017.[215] We note that we determined that the overall rate of OPD claims submitted for payment to the Medicare program increased each year by an average rate of 3.2 percent. This equated to an increase from approximately 90 million OPD claims submitted for payment in 2007 to approximately 118 million claims submitted for payment in 2017. Our analysis also showed an average annual rate-of-increase in the Medicare allowed amount (the amount that Medicare would pay for services regardless of external variables, such as beneficiary plan differences, deductibles, and appeals) of 8.2 percent. We found that the total Medicare allowed amount for the OPD services claims processed in 2007 was approximately $31 billion and increased to $65 billion in 2017, while during this same 11-year period, the average annual increase in the number of Medicare beneficiaries per year was only 1.1 percent. The 8.2 percent increase exceeds the average annual increase of 5.8 percent per year in overall health care spending during that same time period (2007-2017), according to the analysis of the U.S. Bureau of Labor and Statistics Consumer Price Index for medical care.[216]
Upon reviewing specific OPD categories of services in comparison to these figures, we found higher than expected volume increases for several services. Many of these services fall within the following five general categories of services: (1) Blepharoplasty; (2) botulinum toxin injections; (3) panniculectomy; (4) rhinoplasty; and (5) vein ablation.
As discussed in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59004 through 59015), and addressed again in section X.D. of this proposed rule, we have developed many payment policies with the goal in mind of managing the growth in Medicare spending for OPD services, and most recently, to control unnecessary increases in the volume of OPD services using our authority under section 1833(t)(2)(F) of the Act. Section 1833(t)(2)(F) of the Act authorizes CMS to develop a method for controlling unnecessary increases in the volume of covered OPD services. We believe the increases in volume associated with certain covered OPD services described earlier in this section are unnecessary because the data show that the volume of utilization of these services far exceeds what would be expected in light of the average rate-of-increase in the number of Medicare beneficiaries; these procedures are often considered cosmetic and, in those instances, would not be covered by Medicare; and we are unaware of other factors that might contribute to clinically valid increases in volume. Therefore, these above-average increases in volume suggest an increase in unnecessary utilization. As discussed in detail below, we are proposing to use the authority under section 1833(t)(2)(F) of the Act to require prior authorization for certain covered OPD services as a condition of Medicare payment.
B. Proposal for a Prior Authorization Process for Certain OPD Services
We believe a prior authorization process for certain OPD services would ensure that Medicare beneficiaries continue to receive medically necessary care while protecting the Medicare Trust Funds from improper payments, and at the same time keeping the medical necessity documentation requirements unchanged for providers. We believe prior authorization for these services will be an effective method for controlling increases in the volume of these services because we expect that it will reduce the instances in which Medicare pays for these services when they are merely cosmetic and not medically necessary. As a method for controlling unnecessary increases in the volume of certain covered OPD services, we are proposing to use our authority under section 1833(t)(2)(F) of the Act to establish a process through which providers would submit a prior authorization request for a provisional affirmation of coverage before a covered OPD service is furnished to the beneficiary and before the claim is submitted for processing. We are proposing to establish a new subpart I under 42 CFR part 419 to codify the conditions and requirements for the proposed prior authorization for certain covered OPD services to help control unnecessary increases in the volume of covered OPD services. This subpart would establish the conditions of payment for OPD services that require prior authorization; establish the submission requirements for prior authorization requests, including methods for expedited review of prior authorization requests; and provide for suspension of the prior authorization process generally, or for particular services. In order to allow time for providers to better understand this proposed prior authorization process, for CMS to ensure sufficient time is allowed for outreach and education to affected stakeholders, and for contractor operational updates to be in place, we are proposing that this requirement would begin for dates of service on or after July 1, 2020. We note that we are proposing to pattern some of the provisions for prior authorization for covered OPD services after the prior authorization program that we have already established for certain durable medical equipment, prosthetics, and supplies (DMEPOS) under 42 CFR 414.234.
As we noted, CMS routinely analyzes data as part of its oversight of the Medicare program, and our analysis was used as a basis for this proposed rule. Moreover, the Medicare program is continuing to incorporate advancements in health information technology (health IT) into its program operations. This includes improvements in interoperability, the secure electronic transmission of clinical data, and the potential incorporation of artificial intelligence into the claims review process. As these advancements in health IT continue, we are committed to ensuring that these efficiencies and enhancements will be considered, whenever possible, to reduce the burden placed on providers.
As stated earlier, we are proposing to establish a new subpart I under part 419 (containing §§ 419.80 through 419.89 (§§ 419.84 through 419.89 would be reserved)) to codify the following proposed policies for prior authorization for certain covered OPD services.
1. Basis, Scope, and Definitions for Proposed New Subpart I Under Part 419
We are proposing to specify the basis and scope of the proposed subpart under proposed new § 419.80, using section 1833(t)(2)(F) of the Act as our authority to establish the prior authorization process and requirements.
We are proposing to define key terms associated with the proposed prior authorization process for certain covered OPD services under proposed new § 419.81. We are proposing to define “prior authorization” to mean a process through which a request for provisional affirmation of coverage is submitted to CMS or its contractors for review before the service is provided to the beneficiary and before the claim is submitted. We are proposing to define “provisional affirmation” to mean a preliminary finding that a future claim for the service will meet Medicare's coverage, coding, and payment rules. As previously mentioned, we patterned these proposed definitions after the prior authorization process for certain DMEPOS under 42 CFR 414.234. Lastly, we are proposing to define the “list of hospital outpatient department services requiring prior authorization” as the list of outpatient department services CMS publishes in accordance with proposed new § 419.83(a) that require prior authorization as a condition of payment.
2. Prior Authorization as a Method for Controlling Unnecessary Increases in the Volume of Covered Outpatient Services (Proposed New § 419.82)
In proposed new § 419.82(a), we are proposing that, as a condition of Medicare payment, a provider must submit a prior authorization request for services on the list of hospital outpatient department services requiring prior authorization to CMS that meets the requirements of the proposed new § 419.82(c); namely, that the prior authorization request includes all documentation necessary to show that the service meets applicable Medicare coverage, coding, and payment rules, and that the request be submitted before the service is furnished to the beneficiary and before the claim is submitted. We are proposing that claims submitted for services that require prior authorization that have not received a provisional affirmation of coverage from CMS or its contractors would be denied, unless the provider is exempt under § 419.83(c) (proposed new in § 419.82(b)(1)). This would include the denial of any claims associated with the denial of a service listed in proposed § 419.83(a)(1), including services such as anesthesiology services, physician services, and/or facility services. Moreover, we are proposing that even when a provisional affirmation has been received, a claim for services may be denied based on either technical requirements that can only be evaluated after the claim has been submitted for formal processing or information not available at the time the prior authorization request is received (proposed new § 419.82(b)(2)(i) and (ii)).
We are proposing that a provider must submit a prior authorization request for any service on the list of outpatient department services requiring prior authorization that would be published by CMS (proposed new § 419.82(c)). As noted earlier, we are proposing that, in submitting a prior authorization request, the provider must include all relevant documentation necessary to show that the service meets applicable Medicare coverage, coding, and payment rules and that the request be submitted before the service is provided to the beneficiary and before the claim is submitted (proposed new § 419.82(c)(1)(i) and (ii)). We also are proposing that providers have an opportunity to submit prior authorization requests for expedited review when a delay could seriously jeopardize the beneficiary's life, health, or ability to regain maximum function (proposed new § 419.82(c)(2). Documentation that the beneficiary's life, health, or ability to regain maximum function is in serious jeopardy must be submitted with this request.
We are proposing that CMS or its contractor will review a prior authorization request for compliance with applicable Medicare coverage, coding, and payment rules (proposed new § 419.82(d)). If the request meets the applicable Medicare coverage, coding, and payment rules, CMS or its contractor would issue a provisional affirmation to the requesting provider (proposed new § 419.82(d)(1)(i)). If the request does not meet the applicable Medicare coverage, coding, and payment rules, CMS or its contractor would issue a non-affirmation decision to the requesting provider (proposed new § 419.82(d)(1)(ii)). In proposed new § 419.82(d)(iii), we are proposing that CMS or its contractor would issue a decision (affirmative or non-affirmative) within 10 business days.
We are proposing that, if the provider receives a non-affirmation decision, we would allow the provider to resubmit a prior authorization request with any applicable additional relevant documentation. This would include the resubmission of requests for expedited reviews (proposed new § 419.82(e)(1) and (2)).
We are proposing that CMS or its contractor would initiate an expedited review of a prior authorization request when requested by a provider and where CMS or its contractor determines that a delay could seriously jeopardize the beneficiary's life, health or ability to regain maximum function (proposed new § 419.82(d)(2)). Upon making this determination, we are proposing that CMS or its contractor would issue a provisional affirmation or non-affirmation in accordance with proposed new § 419.82(d)(1) using an expedited timeframe of 2 business days.
As part of the requirements for the DMEPOS prior authorization process,[217] under 42 CFR 405.926(t), we specified that a prior authorization request that is non-affirmed is not an initial determination on a claim for payment for services provided and, therefore, would not be appealable. We are proposing to apply this same provision to the OPD services prior authorization process. Therefore, we are proposing to revise § 405.926(t) so that OPD prior authorization requests that are determined non-affirmed also would not be considered an initial determination and, therefore, would not be appealable. However, the provider will still have the opportunity to resubmit a prior authorization request under proposed new § 419.82(e) provided the claim has not yet been submitted and denied.
If a claim is submitted for the services listed in proposed new § 419.83(a)(1) without a provisional affirmation, it will be denied. The claim denial is an initial determination and a redetermination request may be submitted in accordance with 42 CFR 405.940. Consistent with current policy, we also are proposing in proposed new § 419.82(b)(3) that any claims associated with or related to a service listed in proposed new § 419.83(a)(1) for which a claim denial is issued will be denied as well since these services would be unnecessary if the service listed in proposed new § 419.83(a)((1) had not been provided. These associated services include, but are not limited to, services such as anesthesiology services, physician services, and/or facility services. The associated claims would be denied whether a non-affirmation was received for a service listed in proposed new § 419.83(a)(1) or the provider did not request a prior authorization request. A contractor is not required to request medical documentation from the provider who billed the associated claims before making such a denial. We are requesting public comments on whether the requirement in proposed new § 419.82(b)(3) should remain in 42 CFR part 419 or be co-located with the regulatory provisions governing initial determinations located in 42 CFR part 405.
3. Proposed List of Outpatient Department Services That Would Require Prior Authorization (Proposed New § 419.83)
We are proposing that the list of covered OPD services that would require prior authorization are those identified by the CPT codes in Table 38. For ease of review, we are only including the five categories of services within which these CPT codes fall in proposed new § 419.83(a)(1). The five categories of services would be: Blepharoplasty; botulinum toxin injections; panniculectomy; rhinoplasty; and vein ablation. In proposed new § 419.83(a)(2), we are proposing that technical updates, such as corrections or conforming changes to the names of the services or CPT codes, may be made on the CMS web page.
Also, we are proposing that CMS may elect to exempt a provider from the prior authorization process in proposed new § 419.82 upon a provider's demonstration of compliance with Medicare coverage, coding, and payment rules and that this exemption would remain in effect until CMS elects to withdraw the exemption (proposed new § 419.83(c)). We would exempt providers that achieve a prior authorization provisional affirmation threshold of at least 90 percent during a semiannual assessment. We anticipate that an exemption will take approximately 90 calendar days to effectuate. We believe that, by achieving this percentage, the provider would be demonstrating an understanding of the requirements for submitting accurate claims. We do not believe it is necessary for a provider to achieve 100 percent compliance to qualify for an exemption because innocent and sporadic errors could occur that are not deliberate or systematic attempts to submit claims that are not payable. In addition, we propose that we might withdraw an exemption if evidence becomes available based on a review of claims that the provider has begun to submit claims that are not payable based on Medicare's billing, coding or payment requirements. If the rate of nonpayable claims submitted becomes higher than 10 percent during a biannual assessment, we will consider withdrawing exemption. Again, we anticipate that withdrawing the exemption may take approximately 90 calendar days to effectuate.
Moreover, we are proposing that CMS may suspend the outpatient department services prior authorization process requirements generally or for a particular service(s) at any time by issuing notification on CMS' web page (proposed new § 419.83(d)). While we believe this is unlikely to occur, we nonetheless believe it is necessary for us to retain this flexibility in the event of certain circumstances, such as where the cost of the prior authorization program exceeds the savings it generates.
C. Proposed List of Outpatient Department Services Requiring Prior Authorization
As mentioned earlier, we have identified a list of specific services (Table 38) that, based on review and analysis of claims data for the 11-year period from 2007 through 2017, show higher than expected, and therefore, we believe, unnecessary, increases in the volume of service utilization. These services fall within the following five categories: blepharoplasty; botulinum toxin injections; panniculectomy; rhinoplasty; and vein ablation. In making the decision to propose to include the specific services in the proposed list of hospital outpatient department services requiring prior authorization as shown in Table 38, we first considered that these services are most often considered cosmetic and, therefore, are only covered by Medicare in very rare circumstances. We then viewed the current volume of utilization of these services and determined that the utilization far exceeds what would be expected in light of the average rate-of-increase in the number of Medicare beneficiaries. We note that we are unaware of other factors that might contribute to increases in volume of services that indicate that the services are increasingly medically necessary, such as clinical advancements or expanded coverage criteria that would have led to the increases. Below we describe what we believe are the unnecessary increases in volume of each of the categories of services for which we are proposing to require prior authorization:
- Botulinum Toxin Injections: In reviewing CMS data available through the Integrated Data Repository (IDR), we determined that destruction of nerves to muscles of the face via botulinum toxin injections had an overall average annual increase in the number of unique claims of approximately 19.3 percent from 2007 through 2017, with an average annual increase in financial expense to the Medicare program as a result of allowed amounts in service costs and payments of approximately 27.8 percent and an average annual increase in the number of unique patients of approximately 17.9 percent. Based on analysis and comparisons of claims data, these increases in service utilization volume, financial expense, and the number of Medicare patients far exceed the typical baseline rates or trends we identified.
- Panniculectomy: Our analysis of IDR data showed that panniculectomy had an average annual increase in the number of unique claims of approximately 9.2 percent from 2007 through 2017, with an average annual increase in financial expense to the Medicare program as a result of allowed amounts in service costs and payments of approximately 13.9 percent and an average annual increase in the number of unique patients of approximately 9.2 percent. Based on analysis and comparisons of claims data, these increases in service utilization volume, financial expense to the Medicare program, and the number of Medicare patients also far exceed the typical baseline rates or trends we identified (that is, the 9.2 percent average annual increase in the rate of Medicare beneficiaries receiving a panniculectomy is significantly higher than the 1.1 percent average annual increase in the Medicare beneficiaries who received outpatient services over that eleven-year period). Additionally, some panniculectomy services were reported on claims by providers in combination with procedures performed on the patient's chest region, in addition to abdominal procedures.
- Vein Ablation: In reviewing the available data from the IDR, vein ablation had an average annual increase in the number of unique claims of approximately 11.1 percent from 2007 through 2017, with an average annual increase in financial expense to the Medicare program as a result of allowed amounts in service costs and payments of approximately 11.5 percent and an average annual increase in the number of unique patients of approximately 9.5 percent. Based on analysis and comparisons of claims data, these increases in service utilization volume, financial expense to the Medicare program, and the number of Medicare patients also far exceed the typical baseline rates or trends we identified (that is, the 9.5 percent average annual increase in the rate of Medicare beneficiaries receiving vein ablation is significantly higher than the 1.1 percent average annual increase in the Medicare beneficiaries who received outpatient services over that eleven-year period).
- Rhinoplasty: In reviewing available IDR data, rhinoplasty had an average annual increase in the number of unique patients of approximately 1.9 percent. This represents a 64.1 percent increase in comparison to the 1.1 percent rate of increase for unique patients for all OPPS services for that same time period. Even though this category of services includes some procedures that had annual increases in service utilization volume far exceeding what we would expect based on the typical rate, this was not true for all services within the category. One example that did exceed the expected rate was the number of unique claims for the procedure of widening of the nasal passage. This rate increased significantly more than the expected rate and was as much as 34.8 percent from 2016 through 2017.
- Blepharoplasty: In reviewing the IDR data, blepharoplasty, like rhinoplasty, had overall statistics that were similar to the rate increases expected for outpatient services. However, some procedures had annual increases in service utilization volume that far exceeded these expected rates. As an example, the number of unique claims for the procedure of repairing of the upper eyelid muscle to correct drooping or paralysis increased as high as 48.9 percent from 2011 through 2012, which far exceeds the rate we would expect for such a service.
Table 38 lists the specific procedures within the five categories of services that we are proposing for the proposed list of hospital outpatient department services requiring prior authorization.


XXI. Comment Solicitation on Cost Reporting, Maintenance of Hospital Chargemasters, and Related Medicare Payment Issues
The Department is examining the relationship of hospital chargemasters to the Medicare cost report and its use in setting Medicare payment for hospital services in connection with the Department's effort to increase innovation in its programs. For this cause, the Department is seeking public comments, including comments from hospitals and revenue cycle management experts, cost report experts, accounting firms, or others who understand hospital cash flows, on innovative and streamlined methods for establishing hospital payment to the extent permitted by law.
Medicare-certified institutional providers are required to submit an annual cost report to CMS which is used to set prospective payment rates for institutions. The cost report contains provider information such as facility characteristics, utilization data, cost and charges by cost center (in total and for Medicare), Medicare settlement data, and financial statement data.[218] The reported charges are generally those that are derived from the hospital chargemaster. We are seeking public comments on the continued value of the chargemaster charges in setting hospital payment and to other stakeholders, as well as the costs associated with maintaining the chargemaster for purposes of Medicare cost reporting and payment. Further, we are seeking public comments on whether it would be possible to modernize or streamline the Medicare cost reporting process, for example, by replacing it with other processes or if it could be modified in content, methodology, or approach. We also recognize that hospital charge data are used in calculating a number of payments CMS makes to hospitals (for example, in recalibrating relative weights, the calculation of outlier payments, critical access hospital payments, new technology add-on payments, and pretransplant cost reimbursement) and that these charge data may reflect the charges found on the hospital's chargemaster. We are seeking public comments on whether and how the replacement or modification of the chargemaster might affect the submission of data used by CMS to calculate these payments, as well as alternative sources that could be used for the information necessary to calculate these payments. We also are seeking public comments on the decision process, and why the chargemaster might be updated more frequently than on an annual basis and how this more frequent updating could affect costs for patients.
XXII. Proposed Changes to Requirements for Grandfathered Children's Hospitals-Within-Hospitals (HwHs)
Existing regulations at § 412.22(e) define a hospital-within-a-hospital (HwH) as a hospital that occupies space in the same building as another hospital, or in one or more entire buildings located on the same campus as buildings used by another hospital. Existing § 412.22(f) provides for the grandfathering of HwHs that were in existence on or before September 30, 1995, so long as the HwH continues to operate under the same terms and conditions, including the number of beds. Sections 412.22(h) and 412.25(e), relating to satellites of hospitals and hospital units, respectively, excluded from the IPPS, define a satellite facility as a part of a hospital or unit that provides inpatient services in a building also used by another hospital, or in one or more entire buildings located on the same campus as buildings used by another hospital. Sections 412.22(h)(3) and 412.25(e)(3) provide for the grandfathering of excluded hospitals and units that were structured as satellite facilities on September 30, 1999, to the extent that they operate under the same terms and conditions in effect on that date. While these rules initially only applied to LTCHs, in 1997, CMS expanded the scope of these rules to all hospitals excluded from the IPPS (including children's hospitals) because the underlying policy concern of hospitals creating new entities that were separate in name only (essentially operating as units of the hospital) in order to increase Medicare revenue was not unique to LTCHs. For example, we have expressed our concerns that an HwH's “configuration could result in patient admission, treatment, and discharge patterns that are guided more by attempts to maximize Medicare payments than by patient welfare” and that “the unregulated linking of an IPPS hospital and a hospital excluded from the IPPS could lead to two Medicare payments for what was essentially one episode of patient care” (69 FR 48916 and 49191).
In the FY 2018 IPPS/LTCH PPS final rule (82 FR 38292 through 38294), we finalized a change to our HwH regulations at § 412.22(e) to only require, as of October 1, 2017, that IPPS-excluded HwHs that are co-located with IPPS hospitals comply with the separateness and control requirements in those regulations. We adopted this change because we believe that the policy concerns that underlay the previous HwH regulations are sufficiently moderated in situations where IPPS-excluded hospitals are co-located with each other, in large part due to changes that have been made to the way most types of IPPS-excluded hospitals are paid under Medicare. As part of our ongoing efforts to reduce regulatory burdens, we have continued to examine areas in which the rules for co-located entities are no longer necessary. As a result of this examination, we believe that there is no Medicare payment policy rationale for prohibiting grandfathered children's HwHs from increasing their number of beds. Given the low number of Medicare claims submitted by these children's hospitals, which results in a minimal level of Medicare reimbursement to them relative to the payments they receive from other payers, we believe that such a regulatory change would allow these hospitals to address changing community needs for services without any increased incentive for inappropriate patient shifting to maximize Medicare payments. Additionally, we do not believe that allowing grandfathered children's HwHs to increase their bed size would impart an economic advantage to these hospitals relative to other hospitals; however, we invite comment on this area. We are proposing to revise § 412.22(f)(1) and (2) of the regulations to allow a grandfathered children's HwH to increase its number of beds without resulting in the loss of grandfathered status. We are seeking public comment on this proposal. Additionally, we are seeking public comment on whether this proposal could create unintended or inadvertent consequences.
XXIII. Files Available to the Public via the Internet
The Addenda to the OPPS/ASC proposed rules and the final rules with comment period are published and available via the internet on the CMS website. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59154), for CY 2019, we changed the format of the OPPS Addenda A, B, and C, by adding a column entitled “Copayment Capped at the Inpatient Deductible of $1,364.00” where we flag, through use of an asterisk, those items and services with a copayment that is equal to or greater than the inpatient hospital deductible amount for any given year (the copayment amount for a procedure performed in a year cannot exceed the amount of the inpatient hospital deductible established under section 1813(b) of the Act for that year). For CY 2020, we are proposing to retain these columns, updated to reflect the amount of the 2020 inpatient deductible.
To view the Addenda to this proposed rule pertaining to CY 2020 payments under the OPPS, we refer readers to the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices.html; select “1717-P” from the list of regulations. All OPPS Addenda to this proposed rule are contained in the zipped folder entitled “2020 NPRM OPPS Addenda” at the bottom of the page. To view the Addenda to this proposed rule pertaining to CY 2020 payments under the ASC payment system, we refer readers to the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ASCPayment/ASC-Regulations-and-Notices.html; select “1717-P” from the list of regulations. All ASC Addenda to this proposed rule are contained in a zipped folder entitled “Addendum AA, BB, DD1, DD2, and EE.”
XXIV. Collection of Information Requirements
A. Statutory Requirement for Solicitation of Comments
Under the Paperwork Reduction Act of 1995, we are required to provide 60-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the Office of Management and Budget (OMB) for review and approval. In order to fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
In this proposed rule, we are soliciting public comment on each of these issues for the following sections of this document that contain information collection requirements (ICRs).
B. ICRs for the Hospital OQR Program
1. Background
The Hospital OQR Program is generally aligned with the CMS quality reporting program for hospital inpatient services known as the Hospital IQR Program. We refer readers to the CY 2011 through CY 2019 OPPS/ASC final rules with comment periods (75 FR 72111 through 72114; 76 FR 74549 through 74554; 77 FR 68527 through 68532; 78 FR 75170 through 75172; 79 FR 67012 through 67015; 80 FR 70580 through 70582; 81 FR 79862 through 79863; 82 FR 59476 through 59479; and 83 FR 59155 through 59156, respectively) for detailed discussions of Hospital OQR Program information collection requirements we have previously finalized. The information collection requirements associated with the Hospital OQR Program are currently approved under OMB control number 0938-1109 which expires on March 31, 2021. Below we discuss only the changes in burden that would result from the proposed policies in this proposed rule with comment period, if finalized.
In section XIV.B.3.b. of this proposed rule, we are proposing to remove one measure from the Hospital OQR Program for the CY 2022 payment determination; OP-33: External Beam Radiotherapy for Bone Metastases. The reduction in burden associated with this proposal is discussed below.
In the CY 2018 OPPS/ASC final rule with comment period (82 FR 59477), we finalized a proposal to utilize the median hourly wage rate, in accordance with the Bureau of Labor Statistics (BLS), to calculate our burden estimates for the Hospital OQR Program. The BLS describes Medical Records and Health Information Technicians as those responsible for organizing and managing health information data; therefore, we believe it is reasonable to assume that these individuals will be tasked with abstracting clinical data for submission for the Hospital OQR Program. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59156), we utilized a median hourly wage of $18.29 per hour. We note that since then, more recent wage data have become available, and we are updating the wage rate used in these calculations. The more recent data (May 2018) from the Bureau of Labor Statistics reflects a median hourly wage of $19.40 [219] per hour for a Medical Records and Health Information Technician professional. We have finalized a policy to calculate the cost of overhead, including fringe benefits, at 100 percent of the mean hourly wage (82 FR 59477). This is necessarily a rough adjustment, both because fringe benefits and overhead costs vary significantly from employer-to-employer and because methods of estimating these costs vary widely from study-to-study. Nonetheless, we believe that doubling the hourly wage rate ($19.40 × 2 = $38.80) to estimate total cost is a reasonably accurate estimation method and allows for a conservative estimate of hourly costs. This approach is consistent with our previously finalized burden calculation methodology (82 FR 59477). Accordingly, we calculate cost burden to facilities using a wage plus benefits estimate of $38.80 per hour throughout the discussion below for the Hospital OQR Program.
2. Proposed Removal of OP-33 for the CY 2022 Payment Determination and Subsequent Years
In section XIV.B.3.b. of this proposed rule, we are proposing to remove one measure submitted via a web-based tool beginning with the CY 2022 payment determination and for subsequent years: OP-33: External Beam Radiotherapy for Bone Metastases. As we stated in the CY 2016 OPPS/ASC final rule with comment period (80 FR 70582), we estimate that hospitals spend approximately 10 minutes, or 0.167 hours, per measure to report web-based measures. Accordingly, we believe that the proposal to remove OP-33 for the CY 2022 payment determination would reduce burden by 0.167 hours per hospital, resulting in a burden reduction of 551 hours (0.167 hours × 3,300 hospitals) and $21,379 (551 hours × $38.80) across 3,300 hospitals.
C. ICRs for the ASCQR Program
1. Background
We refer readers to the CY 2012 OPPS/ASC final rule with comment period (76 FR 74554), the FY 2013 IPPS/LTCH PPS final rule (77 FR 53672), and the CY 2013, CY 2014, CY 2015, CY 2016, CY 2017, CY 2018, and CY 2019 OPPS/ASC final rules with comment period (77 FR 68532 through 68533; 78 FR 75172 through 75174; 79 FR 67015 through 67016; 80 FR 70582 through 70584; 81 FR 79863 through 79865; 82 FR 59479 through 59481; and 83 FR 59156 through 59157, respectively) for detailed discussions of the ASCQR Program information collection requirements we have previously finalized. The information collection requirements associated with the ASCQR Program are currently approved under OMB control number 0938-1270 which expires on January 31, 2022. As discussed below, there are only nominal changes in burden that would result from the proposed policies in this proposed rule.
2. Proposal To Adopt ASC-19: Facility-Level 7-Day Hospital Visits After General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357)
In section XV.B.3. of this this proposed rule, we are proposing, beginning with the CY 2024 payment determination and for subsequent years, to adopt one measure collected via Medicare claims: ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357). Data used to calculate scores for this measure are collected via Medicare Part A and Part B administrative claims and Medicare enrollment data; therefore, ASCs would not be required to report any additional data. Because this measure does not require ASCs to submit any additional data, we believe there would be only a nominal change in other costs experienced by ASCs associated with this proposal due to having to review and track confidential feedback and reports related to the proposed ASC-19 measure.
D. ICR for Proposal on Hospital Price Transparency
In section XVI. of this proposed rule, we seek to promote price transparency in hospital standard charges so that consumers can be empowered to make more informed decisions about their health care. If finalized, we believe these proposed requirements would represent an important step towards putting consumers at the center of their health care and ensuring they have access to needed information.
We note that hospitals in the United States maintain chargemasters, a list of their gross charges for all individual items and services as part of their standard billing and business practices.[220] Additionally, hospitals maintain electronic data on charges they negotiate with third party payers for hospital items and services as well as service packages. As such, we believe that the burden for making this information publicly available is minimal and estimate only a small burden for each hospital to extract, review, and conform the posting of gross charges and payer-specific negotiated charges for all hospital items and services in the machine-readable format as specified in this proposed rule. In addition, we estimate some burden associated with hospitals making public their payer-specific negotiated charges for a set of at least 300 (70 CMS-specified and at least 230 hospital-selected) shoppable services in a consumer-friendly manner, with flexibility for hospitals to determine the most consumer-friendly format, as discussed in section XVI.F.5. of this proposed rule.
We estimate that this proposed rule applies to 6,002 hospitals operating within the United States under the definition of “hospital” discussed in section XVI.B.1. of this proposed rule. To estimate this number, we subtract 208 federally-owned hospitals from the total number of U.S. hospitals, 6,210 hospitals[221] (6,210 total hospitals—208 federally-owned hospitals).
We estimate the hourly cost for each labor category used in this analysis by referencing Bureau of Labor Statistics report on Occupational Employment and Wages (May 2018 [222] ) in the Table 39. There are many professions involved in any business's processes. Therefore, we use the wages of General and Operations Managers as a proxy for management staff, the wages of Lawyers as a proxy for legal staff, the wages of Network and Computer Systems Administrators as a proxy for information technology (IT) staff, and the wage of Business Operations Specialists as a proxy for other business staff throughout this analysis. Obtaining data on overhead costs is challenging. Overhead costs vary greatly across industries and facility sizes. In addition, the precise cost elements assigned as “indirect” or “overhead” costs, as opposed to direct costs or employee wages, are subject to some interpretation at the facility level. Therefore, we calculate the cost of overhead at 100 percent of the mean hourly wage in line with the Hospital Inpatient Quality Reporting Program and the Hospital Outpatient Quality Reporting Program (81 FR 57260 and 82 FR 59477, respectively).

In order to comply with regulatory updates proposed in this proposed rule, affected hospitals would first need to review the rule. We estimate that this task would take a lawyer on average 1 hour (at $138.68 per hour, which is based on the Bureau of Labor Statistics (BLS) wage for Lawyers (23-1011) [223] ) to perform the initial review, and a general operations manager on average 1 hour (at $119.12 per hour, which is based on the Bureau of Labor Statistics (BLS) wage for General and Operations Managers (11-1021) [224] ) to review and determine compliance requirements. Therefore, we estimate 2 hours per hospital, with a total of 12,004 hours (2 hours × 6,002 hospitals). The cost is $257.80 per hospital (1 hour × $138.68 + 1 hour × $119.12), with a total cost of $1,547,316 ($257.80 × 6,002 hospitals).
After reviewing the rule, hospitals would need to review their policies and business practices in the context of the defined terms and requirements for information collection then determine how to comply. We believe this will require minimal changes for affected hospitals because the standard charge information to be collected is already compiled and maintained as part of hospitals' management practices and electronic accounting and billing systems. Moreover, we are proposing requirements to make payer-specific negotiated rates public for a total of 300 shoppable services (70 CMS-specified and 230 hospital-selected) in a consumer-friendly manner, including listing the charges for associated ancillary services provided by the hospital so that the hospital charge information is more accessible and easier to digest for consumers seeking to obtain pricing information for making decisions about their treatment. We are proposing several definitions and requirements for making data publicly available pertaining to gross charges, negotiated charges and shoppable services at proposed 45 CFR part 180. We estimate it would take a business operations specialist, on average, 8 hours (at $74.00 per hour, which is based on the Bureau of Labor Statistics (BLS) wage for Business Operations Specialists, All Other (13-1199) [225] ) to complete necessary processes and procedures to gather and compile required information and post it to the web in the form and manner specified by this proposed rule. We estimate 8 hours per hospital. The total burden hours are 48,016 hours (8 hours × 6,002 hospitals). The cost is $592.00 per hospital (8 hours × $74.00), with a total cost of $3,553,184 (48,016 hours × $74.00).
We also are proposing several requirements for posting required information at proposed 45 CFR 180.50 and 180.60. These requirements impose form and manner standards for the hospitals as defined in this proposed rule. We estimate that a network and computer system administrator would spend on average 2 hours (at $83.72 per hour, which is based on the Bureau of Labor Statistics (BLS) wage for Network and Computer Systems Administrators (15-1142) [226] ) to meet requirements specified by this proposed rule. Therefore, we estimate 2 hours per hospital. The total burden hours are 12,004 hours (2 hours × 6,002 hospitals). The cost is $167.44 per hospital (2 hours × $83.72), with a total cost of $1,004,975 (12,004 hours × $83.72).
We conclude that the annual burden per hospital should be calculated with all activities performed by four professions combined. We estimate an annual burden assessment to be 12 hours (2 hours + 8 hours + 2 hours) per hospital with a cost of $1,017.24 ($257.80 + $592.00 + $167.44) per hospital. We also estimate a total national burden of 72,024 hours (12 hours × 6,002 hospitals) and total cost of $6,105,474 ($1,017.24 × 6,002 hospitals). (See Table 40.)

E. ICRs for Proposed Revision of the Definition of “Expected Donation Rate” for Organ Procurement Organizations
As described in section XVIII. of this proposed rule, we are proposing to revise the definition of “expected donation rate” in the OPO CfCs. This change would allow OPOs to receive payment for organ donor costs under the Medicare and Medicaid programs using a definition that is consistent with the definition used by the Scientific Registry of Transplant Recipients (SRTR). Because we will be using data from the OPTN and the SRTR in assessing whether OPOs have satisfied the outcome measures of 42 CFR 486.318(b), we are proposing to adopt the definition currently used by the OPTN and SRTR in their statistical evaluation of OPO performance. This proposal would not change the data that are already collected by the OPTN and SRTR, and therefore it will not affect the information collection burden on OPOs.
F. ICR for Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
In section XX. of this proposed rule, we are proposing to establish a prior authorization process for certain hospital outpatient services as a condition for Medicare payment. We are proposing to use our authority under section 1833(t)(2)(F) of the Act, which authorizes CMS to develop a method for controlling unnecessary increases in the volume of covered OPD services, to establish the prior authorization process. We believe a prior authorization process for OPD services would ensure beneficiaries receive medically necessary care while minimizing the risk of improper payments without changing the documentation requirements for providers and, therefore, protect the Medicare Trust fund.
We are proposing that providers would be required to obtain prior authorization from CMS for five groups of services and their related services before the services are provided to Medicare beneficiaries and before the provider could submit claims for payment under Medicare for these services. The five groups of services proposed are: Blepharoplasty, Botulinum Toxin Injections, Panniculectomy, Rhinoplasty, and Vein Ablation. The information collection requirements associated with prior authorization requests for these covered outpatient department services would be the required documentation submitted by providers. We are proposing that a prior authorization request must include all relevant documentation necessary to show that the service meets applicable Medicare coverage, coding, and payment rules and that the request be submitted before the service is provided to the beneficiary and before the claim is submitted for processing. The burden associated with this proposed process is the time and effort necessary for the submitter to locate and obtain the relevant supporting documentation to show that the service meets applicable coverage, coding, and payment rules, and to forward the information to CMS or its contractor (MAC) for review and determination of a provisional affirmation. We expect that this information will generally be maintained by providers within the normal course of business and that this information will be readily available. We estimate that the average time for office clerical activities associated with this task to be 30 minutes, which is equivalent to that for normal prepayment or postpayment medical review. We anticipate that most prior authorization requests would be sent by means other than mail. However, we estimate a cost of $5 per request for mailing medical records. Due to a July start date, the first year of the prior authorization will only include 6 months. Based on calendar year 2017 data, we estimate that for those first 6 months at a minimum there will be 23,309 initial requests mailed during a year. In addition, we estimate there will be 7,650 resubmissions of a request mailed following a non-affirmed decision. Therefore, the total mailing cost is estimated to be $154,799. Based on calendar year 2017 data, we estimate that annually at a minimum there will be 46,618 initial requests mailed during a year. In addition, we estimate there will be 15,299 resubmissions of a request mailed following a non-affirmed decision. Therefore, the total mailing cost is estimated to be $309,584. We also estimate that an additional 3 hours would be required for attending educational meetings and reviewing training documents. While there may be an associated burden on beneficiaries while they wait for the prior authorization decision, we are unable to quantify that burden.
The average labor costs (including 100 percent fringe benefits) used to estimate the costs were calculated using data available from the Bureau of Labor Statistics. Based on the Bureau of Labor Statistics information, we estimate an average hourly rate of $16.63 with a loaded rate of $33.26. Therefore, we estimate that the total burden for the first year (6 months), allotted across all providers, would be 73,647 hours (.5 hours × 103,199 submisions plus 3 hours × 7,349 providers for education). The burden cost for the first year (6 months) is $2,604,281 (73,647 hours × $33.26 plus $154,799 for mailing costs). In addition, we estimate that the total annual burden hours, allotted across all providers, would be 125,242 hours (.5 hours × 206,389 submissions plus 3 hours × 7,349 providers for education). The annual burden cost would be $4,475,116 (125,242 hours × $33.26 plus $309,584 for mailing costs). For the total burden and associated costs, we estimate the annualized burden to be 108,044 hours and $3,851,504 million. The annualized burden is based on an average of 3 years, that is, 1 year at the 6-month burden and 2 years at the 12-month burden. The information collection request is under development and will be submitted to OMB for approval.
G. Potential Revision to Laboratory Date of Service (DOS) Policy
In section XIX. of this proposed rule, we are soliciting comments regarding potential revisions to the laboratory date of service (DOS) provisions at § 414.510(b)(5) for a molecular pathology test or a test designated by CMS as an ADLT under paragraph (1) of the definition of an “advanced diagnostic laboratory test” in § 414.502. The laboratory DOS service policy does not impose any information collection requirements. Consequently, review by the Office of Management and Budget under the authority of the PRA is not required.
H. Total Reduction in Burden Hours and in Costs
The chart below reflects the total burden and associated costs for the provisions included in this proposed rule.

XXV. Response to Comments
Because of the large number of public comments we normally receive on Federal Register documents, we are not able to acknowledge or respond to them individually. We will consider all comments we receive by the date and time specified in the DATES section of this proposed rule, and, when we proceed with a subsequent document(s), we will respond to those comments in the preamble to that document.
XXVI. Economic Analyses
A. Statement of Need
This proposed rule is necessary to make updates to the Medicare hospital OPPS rates. It is necessary to make changes to the payment policies and rates for outpatient services furnished by hospitals and CMHCs in CY 2020. We are required under section 1833(t)(3)(C)(ii) of the Act to update annually the OPPS conversion factor used to determine the payment rates for APCs. We also are required under section 1833(t)(9)(A) of the Act to review, not less often than annually, and revise the groups, the relative payment weights, and the wage and other adjustments described in section 1833(t)(2) of the Act. We must review the clinical integrity of payment groups and relative payment weights at least annually. We are proposing to revise the APC relative payment weights using claims data for services furnished on and after January 1, 2018, through and including December 31, 2018, and processed through December 31, 2018, and updated cost report information.
We note that we are completing the phase-in of our method, as described below, to control unnecessary increases in the volume of covered outpatient department services by paying for clinic visits furnished at off-campus PBDs at an amount equal to the site-specific PFS payment rate for nonexcepted items and services furnished by a nonexcepted off-campus PBD (the PFS payment rate). The site-specific PFS payment rate for clinic visits furnished in excepted off-campus PBDs is the OPPS rate reduced to the amount paid for clinic visits furnished by nonexcepted off-campus PBDs under the PFS, which is 40 percent of the OPPS rate. In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59013 through 59014), we implemented this policy with a 2-year phase-in. In CY 2019, the payment reduction is transitioned by applying 50 percent of the total reduction in payment that would apply if these off-campus PBDs were paid the site-specific PFS payment rate for the clinic visit service. In other words, these excepted off-campus PBDs are paid 70 percent of the OPPS rate for the clinic visit service in CY 2019. In CY 2020, we will complete the transition to paying the PFS-equivalent amount for clinic visits furnished in excepted off-campus PBDs. In other words, these excepted off-campus PBDs will be paid the full reduced payment, or 40 percent of the OPPS rate for the clinic visit service in CY 2020.
This proposed rule also is necessary to make updates to the ASC payment rates for CY 2020, enabling CMS to make changes to payment policies and payment rates for covered surgical procedures and covered ancillary services that are performed in an ASC in CY 2020. Because ASC payment rates are based on the OPPS relative payment weights for most of the procedures performed in ASCs, the ASC payment rates are updated annually to reflect annual changes to the OPPS relative payment weights. In addition, we are required under section 1833(i)(1) of the Act to review and update the list of surgical procedures that can be performed in an ASC, not less frequently than every 2 years.
In the CY 2019 OPPS/ASC final rule with comment period (83 FR 59075 through 59079), we finalized a policy to update the ASC payment system rates using the hospital market basket update instead of the CPI-U for CY 2019 through 2023. We believe that this policy will help stabilize the differential between OPPS payments and ASC payments, given that the CPI-U has been generally lower than the hospital market basket, and encourage the migration of services to lower cost settings as clinically appropriate.
B. Overall Impact for Provisions of This Proposed Rule
We have examined the impacts of this proposed rule, as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96-354), section 1102(b) of the Social Security Act, section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA) (March 22, 1995, Pub. L. 104-4), Executive Order 13132 on Federalism (August 4, 1999), the Congressional Review Act (5 U.S.C. 804(2)), and Executive Order 13771 on Reducing Regulation and Controlling Regulatory Costs (January 30, 2017). This section of this proposed rule contains the impact and other economic analyses for the provisions we are proposing for CY 2020.
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Executive Order 13563 emphasizes the importance of quantifying both costs and benefits, of reducing costs, of harmonizing rules, and of promoting flexibility. This proposed rule has been designated as an economically significant rule under section 3(f)(1) of Executive Order 12866 and a major rule under the Congressional Review Act. Accordingly, this proposed rule has been reviewed by the Office of Management and Budget. We have prepared a regulatory impact analysis that, to the best of our ability, presents the costs and benefits of the provisions of this proposed rule. We are soliciting public comments on the regulatory impact analysis in this proposed rule, and we will address any public comments we receive in the final rule with comment period, as appropriate.
We estimate that the proposed total increase in Federal Government expenditures under the OPPS for CY 2020, compared to CY 2019, due only to the proposed changes to the OPPS in this proposed rule, would be approximately $940 million. Taking into account our estimated changes in enrollment, utilization, and case-mix for CY 2020, we estimate that the OPPS expenditures, including beneficiary cost-sharing, for CY 2020 would be approximately $79.2 billion, which is approximately $6.2 billion higher than estimated OPPS expenditures in CY 2019. We note that these spending estimates include the CY 2020 completion of the phase-in, finalized in CY 2019, to control for unnecessary increases in the volume of covered outpatient department services by paying for clinic visits furnished at excepted off-campus PBDs in CY 2020 at a rate that will be 40 percent of the OPPS rate for a clinic visit service. Because the proposed provisions of the OPPS are part of a proposed rule that is economically significant, as measured by the threshold of an additional $100 million in expenditures in 1 year, we have prepared this regulatory impact analysis that, to the best of our ability, presents its costs and benefits. Table 38 of this proposed rule displays the distributional impact of the proposed CY 2020 changes in OPPS payment to various groups of hospitals and for CMHCs.
As noted in section V.B.5 of this proposed rule, we are proposing for CY 2020 to pay for separately payable drugs and biological products that do not have pass-through payment status and are not acquired under the 340B program at WAC+3 percent instead of WAC+6 percent, if ASP data are unavailable for payment purposes. If WAC data are not available for a drug or biological product, we are proposing to continue our policy to pay separately payable drugs and biological products at 95 percent of the AWP. We note that under our proposed CY 2020 policy, drugs and biologicals that are acquired under the 340B Program would continue to be paid at ASP minus 22.5 percent, WAC minus 22.5 percent, or 69.46 percent of AWP, as applicable.
We note that in the impact tables as displayed in this impact analysis, we have modeled current and prospective payments as if separately payable drugs acquired under the 340B program from hospitals not excepted from the policy are paid in CY 2020 under the OPPS at ASP-22.5 percent. As discussed in more detail in section V.B.6. of this proposed rule, there is ongoing litigation involving our payment policy for 340B-acquired drugs. We are soliciting public comments on the appropriate OPPS payment rate for 340B-acquired drugs, including whether a rate of ASP+3 percent could be an appropriate payment amount for these drugs, both for CY 2020 and for purposes of determining the remedy for CYs 2018 and 2019 in the event of an adverse decision on appeal in that litigation. In addition to comments on the appropriate payment amount for calculating the remedy for CYs 2018 and 2019 and for use for CY 2020, we also seek public comment on how to structure the remedy for CYs 2018 and 2019.
We note that a policy to pay for 340B-acquired drugs and biologicals under the CY 2020 OPPS at an amount of ASP+3 percent would necessitate an accompanying budget neutrality adjustment to the OPPS conversion factor to account for that payment differential. Based on alternative modeling we expect that a policy to pay for 340B-acquired drugs at ASP+3 percent would result in an additional adjustment of 0.9710 to the OPPS conversion factor, with an alternative conversion factor of $79.029, which would result in a reduction of approximately $1.4 billion in payments for non-drug items and services for CY 2020.
We estimate that the proposed update to the conversion factor and other adjustments (not including the effects of outlier payments, the pass-through payment estimates, the application of the frontier State wage adjustment for CY 2020, and the completion of the phase-in to control for unnecessary increases in the volume of covered outpatient department services described in section X.D. of this proposed rule) would increase total OPPS payments by 2.0 percent in CY 2020. The proposed changes to the APC relative payment weights, the proposed changes to the wage indexes, the proposed continuation of a payment adjustment for rural SCHs, including EACHs, and the proposed payment adjustment for cancer hospitals would not increase OPPS payments because these proposed changes to the OPPS are budget neutral. However, these proposed updates would change the distribution of payments within the budget neutral system. We estimate that the total proposed change in payments between CY 2019 and CY 2020, considering all proposed budget neutral payment adjustments, proposed changes in estimated total outlier payments, proposed pass-through payments, the proposed application of the frontier State wage adjustment, and the completion of the phase-in to control unnecessary increases in the volume of outpatient services as described in section X.D. of this proposed rule, in addition to the application of the proposed OPD fee schedule increase factor after all adjustments required by sections 1833(t)(3)(F), 1833(t)(3)(G), and 1833(t)(17) of the Act, would increase total estimated OPPS payments by 2.8 percent.
We estimate the total increase (from proposed changes to the ASC provisions in this proposed rule as well as from enrollment, utilization, and case-mix changes) in Medicare expenditures (not including beneficiary cost-sharing) under the ASC payment system for CY 2020 compared to CY 2019, to be approximately $200 million. Because the proposed provisions for the ASC payment system are part of a proposed rule that is economically significant, as measured by the $100 million threshold, we have prepared a regulatory impact analysis of the proposed changes to the ASC payment system that, to the best of our ability, presents the costs and benefits of this portion of this proposed rule. Tables 42 and 43 of this proposed rule display the redistributive impact of the proposed CY 2020 changes regarding ASC payments, grouped by specialty area and then grouped by procedures with the greatest ASC expenditures, respectively.
C. Detailed Economic Analyses
1. Estimated Effects of Proposed OPPS Changes in This Proposed Rule
a. Limitations of Our Analysis
The distributional impacts presented here are the projected effects of the proposed CY 2020 policy changes on various hospital groups. We post on the CMS website our hospital-specific estimated payments for CY 2020 with the other supporting documentation for this proposed rule. To view the hospital-specific estimates, we refer readers to the CMS website at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. At the website, select “regulations and notices” from the left side of the page and then select “CMS-1717-P” from the list of regulations and notices. The hospital-specific file layout and the hospital-specific file are listed with the other supporting documentation for this proposed rule. We show hospital-specific data only for hospitals whose claims were used for modeling the impacts shown in Table 41. We do not show hospital-specific impacts for hospitals whose claims we were unable to use. We refer readers to section II.A. of this proposed rule for a discussion of the hospitals whose claims we do not use for ratesetting and impact purposes.
We estimate the effects of the proposed individual policy changes by estimating payments per service, while holding all other payment policies constant. We use the best data available, but do not attempt to predict behavioral responses to our proposed policy changes in order to isolate the effects associated with specific policies or updates, but any policy that changes payment could have a behavioral response. In addition, we have not made adjustments for future changes in variables, such as service volume, service-mix, or number of encounters.
b. Estimated Effects of the CY 2020 Completion of Phase-In To Control for Unnecessary Increases in the Volume of Outpatient Services
In section X.D. of this proposed rule, we discuss the CY 2020 completion of the phase-in of our CY 2019 finalized method to control for unnecessary increases in the volume of outpatient department services by paying for clinic visits furnished at an off-campus PBD at an amount equal to the site-specific PFS payment rate for nonexcepted items and services furnished by a nonexcepted off-campus PBD (the PFS payment rate). Specifically, in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59013 through 59014), we finalized our proposal to pay for HCPCS code G0463 (Hospital outpatient clinic visit for assessment and management of a patient) when billed with modifier “PO” at an amount equal to the site-specific PFS payment rate for nonexcepted items and services furnished by a nonexcepted off-campus PBD (the PFS payment rate), with a 2-year transition period. For a discussion of the PFS payment amount for outpatient clinic visits furnished at nonexcepted off-campus PBDs, we refer readers to the CY 2018 PFS final rule with comment period discussion (82 FR 53023 through 53024), as well as the CY 2019 PFS final rule and the CY 2020 PFS proposed rule.
To develop an estimated impact of this policy, we began with CY 2018 outpatient claims data used in ratesetting for the CY 2020 OPPS. We then flagged all claim lines for HCPCS code G0463 that contained modifier “PO” because the presence of this modifier indicates that such claims were billed for services furnished by an off-campus department of a hospital paid under the OPPS. Next, we excluded those that were billed as a component of C-APC 8011 (Comprehensive Observation Services) or packaged into another C-APC because, in those instances, OPPS payment is made for a broader package of services. We then simulated payment for the remaining claim lines as if they were paid at the PFS-equivalent rate. An estimate of the policy that includes the effects of estimated changes in enrollment, utilization, and case-mix based on the FY 2020 President's budget approximates the estimated decrease in total payment under the OPPS at $810 million, with Medicare OPPS payments decreasing by $650 million and beneficiary copayments decreasing by $160 million in CY 2020. This estimate is utilized for the accounting statement displayed in Table 42 of this proposed rule because the impact of this CY 2020 policy, which is not budget neutral, is combined with the impact of the OPD update, which is also not budget neutral, to estimate changes in Medicare spending under the OPPS as a result of the changes proposed in this proposed rule.
We note that our estimates may differ from the actual effect of the proposed policy due to offsetting factors, such as changes in provider behavior. We note that, by removing this payment differential that may influence site-of-service decision-making, we anticipate an associated decrease in the volume of clinic visits provided in the excepted off-campus PBD setting. We note that this estimate could change in the final rule with comment period based on factors such as the availability of updated data.
c. Estimated Effects of Proposed OPPS Changes on Hospitals
Table 41 shows the estimated impact of this proposed rule on hospitals. Historically, the first line of the impact table, which estimates the proposed change in payments to all facilities, has always included cancer and children's hospitals, which are held harmless to their pre-BBA amount. We also include CMHCs in the first line that includes all providers. We include a second line for all hospitals, excluding permanently held harmless hospitals and CMHCs.
We present separate impacts for CMHCs in Table 41, and we discuss them separately below, because CMHCs are paid only for partial hospitalization services under the OPPS and are a different provider type from hospitals. In CY 2020, we are proposing to pay CMHCs for partial hospitalization services under APC 5853 (Partial Hospitalization for CMHCs), and we are proposing to pay hospitals for partial hospitalization services under APC 5863 (Partial Hospitalization for Hospital-Based PHPs).
The estimated increase in the total payments made under the OPPS is determined largely by the increase to the conversion factor under the statutory methodology. The distributional impacts presented do not include assumptions about changes in volume and service-mix. The conversion factor is updated annually by the OPD fee schedule increase factor, as discussed in detail in section II.B. of this proposed rule.
Section 1833(t)(3)(C)(iv) of the Act provides that the OPD fee schedule increase factor is equal to the market basket percentage increase applicable under section 1886(b)(3)(B)(iii) of the Act, which we refer to as the IPPS market basket percentage increase. The proposed IPPS market basket percentage increase for FY 2020 is 3.2 percent. Section 1833(t)(3)(F)(i) of the Act reduces that 3.2 percent by the multifactor productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act, which is proposed to be 0.5 percentage point for FY 2020 (which is also the proposed MFP adjustment for FY 2020 in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19411)), resulting in the proposed OPD fee schedule increase factor of 2.7 percent. We are using the proposed OPD fee schedule increase factor of 2.7 percent in the calculation of the proposed CY 2020 OPPS conversion factor. Section 10324 of the Affordable Care Act, as amended by HCERA, further authorized additional expenditures outside budget neutrality for hospitals in certain frontier States that have a wage index less than 1.0000. The amounts attributable to this frontier State wage index adjustment are incorporated in the CY 2020 estimates in Table 41 of this proposed rule.
To illustrate the impact of the proposed CY 2020 changes, our analysis begins with a baseline simulation model that uses the CY 2019 relative payment weights, the FY 2019 final IPPS wage indexes that include reclassifications, and the final CY 2019 conversion factor. Table 41 shows the estimated redistribution of the proposed increase or decrease in payments for CY 2020 over CY 2019 payments to hospitals and CMHCs as a result of the following factors: The impact of the APC reconfiguration and recalibration changes between CY 2019 and CY 2020 (Column 2); the wage indexes and the provider adjustments (Column 3); the combined impact of all of the proposed changes described in the preceding columns plus the proposed 2.7 percent OPD fee schedule increase factor update to the conversion factor (Column 4); the proposed off-campus PBD clinic visits payment policy (Column 5), and the estimated impact taking into account all proposed payments for CY 2020 relative to all payments for CY 2019, including the impact of proposed changes in estimated outlier payments, and proposed changes to the pass-through payment estimate (Column 6).
We did not model an explicit budget neutrality adjustment for the rural adjustment for SCHs because we are proposing to maintain the current adjustment percentage for CY 2020. Because the proposed updates to the conversion factor (including the proposed update of the OPD fee schedule increase factor), the estimated cost of the proposed rural adjustment, and the estimated cost of projected pass-through payment for CY 2020 are applied uniformly across services, observed redistributions of payments in the impact table for hospitals largely depend on the mix of services furnished by a hospital (for example, how the APCs for the hospital's most frequently furnished services will change), and the impact of the proposed wage index changes on the hospital. However, total payments made under this system and the extent to which this proposed rule will redistribute money during implementation also will depend on changes in volume, practice patterns, and the mix of services billed between CY 2019 and CY 2020 by various groups of hospitals, which CMS cannot forecast.
Overall, we estimate that the proposed rates for CY 2020 would increase Medicare OPPS payments by an estimated 2.0 percent. Removing payments to cancer and children's hospitals because their payments are held harmless to the pre-OPPS ratio between payment and cost and removing payments to CMHCs results in an estimated 2.0 percent increase in Medicare payments to all other hospitals. These estimated payments would not significantly impact other providers.
Column 1: Total Number of Hospitals
The first line in Column 1 in Table 41 shows the total number of facilities (3,734), including designated cancer and children's hospitals and CMHCs, for which we were able to use CY 2018 hospital outpatient and CMHC claims data to model CY 2019 and CY 2020 payments, by classes of hospitals, for CMHCs and for dedicated cancer hospitals. We excluded all hospitals and CMHCs for which we could not plausibly estimate CY 2019 or CY 2020 payment and entities that are not paid under the OPPS. The latter entities include CAHs, all-inclusive hospitals, and hospitals located in Guam, the U.S. Virgin Islands, Northern Mariana Islands, American Samoa, and the State of Maryland. This process is discussed in greater detail in section II.A. of this proposed rule. At this time, we are unable to calculate a DSH variable for hospitals that are not also paid under the IPPS because DSH payments are only made to hospitals paid under the IPPS. Hospitals for which we do not have a DSH variable are grouped separately and generally include freestanding psychiatric hospitals, rehabilitation hospitals, and long-term care hospitals. We show the total number of OPPS hospitals (3,627), excluding the hold-harmless cancer and children's hospitals and CMHCs, on the second line of the table. We excluded cancer and children's hospitals because section 1833(t)(7)(D) of the Act permanently holds harmless cancer hospitals and children's hospitals to their “pre-BBA amount” as specified under the terms of the statute, and therefore, we removed them from our impact analyses. We show the isolated impact on the 41 CMHCs at the bottom of the impact table (Table 41) and discuss that impact separately below.
Column 2: APC Recalibration—All Proposed Changes
Column 2 shows the estimated effect of proposed APC recalibration. Column 2 also reflects any proposed changes in multiple procedure discount patterns or conditional packaging that occur as a result of the proposed changes in the relative magnitude of payment weights. As a result of proposed APC recalibration, we estimate that urban hospitals would experience a 0.1 percent increase, with the impact ranging from an increase of 0.5 percent to no increase, depending on the number of beds. Rural hospitals would experience a decrease of up to 0.8 percent depending on the number of beds. Major teaching hospitals would experience a 0.1 percent decrease.
Column 3: Proposed Wage Indexes and the Effect of the Proposed Provider Adjustments
Column 3 demonstrates the combined budget neutral impact of the proposed APC recalibration; the proposed updates for the wage indexes with the proposed FY 2020 IPPS post-reclassification wage indexes; the proposed rural adjustment; the proposed frontier adjustment, and the proposed cancer hospital payment adjustment. We modeled the independent effect of the proposed budget neutrality adjustments and the proposed OPD fee schedule increase factor by using the relative payment weights and wage indexes for each year, and using a CY 2019 conversion factor that included the OPD fee schedule increase and a budget neutrality adjustment for differences in wage indexes.
Column 3 reflects the independent effects of the proposed updated wage indexes, including the application of budget neutrality for the rural floor policy on a nationwide basis, as well as the CY 2020 proposed changes in wage index policy discussed in section II.C. of this proposed rule. We did not model a budget neutrality adjustment for the rural adjustment for SCHs because we are continuing the rural payment adjustment of 7.1 percent to rural SCHs for CY 2020, as described in section II.E. of this proposed rule. We also modeled a budget neutrality adjustment for the cancer hospital payment adjustment because we are using a proposed payment-to-cost ratio target for the cancer hospital payment adjustment in CY 2020 of .90, which is higher than the ratio that was reported for the CY 2019 OPPS/ASC final rule with comment period (83 FR 58873). We note that, in accordance with section 16002 of the 21st Century Cures Act, we are proposing to apply a budget neutrality factor calculated as if the cancer hospital adjustment target payment-to-cost ratio was 0.90, not the 0.89 target payment-to-cost ratio we are applying in section II.F. of this proposed rule.
We modeled the independent effect of updating the wage indexes by varying only the wage indexes, holding APC relative payment weights, service-mix, and the rural adjustment constant and using the proposed CY 2020 scaled weights and a CY 2019 conversion factor that included a budget neutrality adjustment for the effect of the proposed changes to the wage indexes between CY 2019 and CY 2020.
Column 4: All Proposed Budget Neutrality Changes Combined With the Proposed Market Basket Update
Column 4 demonstrates the combined impact of all of the proposed changes previously described and the proposed update to the conversion factor of 2.7 percent. Overall, these proposed changes would increase payments to urban hospitals by 2.8 percent and to rural hospitals by 3.0 percent. Urban hospitals would receive an increase in line with the 2.8 percent overall increase for all facilities after the update is applied to the proposed budget neutrality adjustments. The increase for classes of rural hospitals would be more variable with sole community hospitals receiving a 3.1 percent increase and other rural hospitals receiving an increase of 3.0 percent.
Column 5: Off-Campus PBD Visits Payment Policy
Column 5 displays the estimated effect of our CY 2020 volume control method, finalized in CY 2019, to pay for clinic visit HCPCS code G0463 (Hospital outpatient clinic visit for assessment and management of a patient) when billed with modifier “PO” by an excepted off-campus PBD at a rate that will be 40 percent of the OPPS rate for a clinic visit service for CY 2020. We note that the numbers provided in this column isolate the estimated effect of this policy adjustment relative to the numerator of Column 4. Therefore, the numbers reported in Column 5 show how much of the difference between the estimates in Column 4 and the estimates in Column 6 are a result of the off-campus PBD visits policy for CY 2020, as finalized in the CY 2019 OPPS/ASC final rule with comment period (83 FR 59013 through 59014).
Column 6: All Proposed Changes for CY 2020
Column 6 depicts the full impact of the proposed CY 2020 policies on each hospital group by including the effect of all proposed changes for CY 2020 and comparing them to all estimated payments in CY 2019. Column 6 shows the combined budget neutral effects of Columns 2 through 3; the proposed OPD fee schedule increase; the effect of the CY 2020 off-campus PBD visits policy finalized in CY 2019, the impact of estimated OPPS outlier payments, as discussed in section II.G. of this proposed rule; the proposed change in the Hospital OQR Program payment reduction for the small number of hospitals in our impact model that failed to meet the reporting requirements (discussed in section XIV. of this proposed rule); and the difference in proposed total OPPS payments dedicated to transitional pass-through payments.
Of those hospitals that failed to meet the Hospital OQR Program reporting requirements for the full CY 2019 update (and assumed, for modeling purposes, to be the same number for CY 2020), we included 23 hospitals in our model because they had both CY 2018 claims data and recent cost report data. We estimate that the cumulative effect of all proposed changes for CY 2020 would increase payments to all facilities by 2.0 percent for CY 2020. We modeled the independent effect of all proposed changes in Column 6 using the final relative payment weights for CY 2019 and the proposed relative payment weights for CY 2020. We used the final conversion factor for CY 2019 of $79.490 and the proposed CY 2020 conversion factor of $81.398 discussed in section II.B. of this proposed rule.
Column 6 contains simulated outlier payments for each year. We used the 1-year charge inflation factor used in the proposed FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19596) of 5.4 percent (1.05446) to increase individual costs on the CY 2018 claims, and we used the most recent overall CCR in the April 2019 Outpatient Provider-Specific File (OPSF) to estimate outlier payments for CY 2019. Using the CY 2018 claims and a 5.4 percent charge inflation factor, we currently estimate that outlier payments for CY 2019, using a multiple threshold of 1.75 and a fixed-dollar threshold of $4,825, would be approximately 1.03 percent of total payments. The estimated current outlier payments of 1.03 percent are incorporated in the comparison in Column 6. We used the same set of claims and a charge inflation factor of 11.2 percent (1.11189) and the CCRs in the April 2019 OPSF, with an adjustment of 0.975167, to reflect relative changes in cost and charge inflation between CY 2018 and CY 2020, to model the proposed CY 2020 outliers at 1.0 percent of estimated total payments using a multiple threshold of 1.75 and a fixed-dollar threshold of $4,950. The charge inflation and CCR inflation factors are discussed in detail in the FY 2020 IPPS/LTCH PPS proposed rule (84 FR 19596 through 19597).
Overall, we estimate that facilities would experience an increase of 2.0 percent under this proposed rule in CY 2020 relative to total spending in CY 2019. This projected increase (shown in Column 6) of Table 38 reflects the proposed 2.7 percent OPD fee schedule increase factor, minus 0.6 percent for the off-campus PBD visits policy, minus 0.2 percent for the proposed change in the pass-through payment estimate between CY 2019 and CY 2020, plus a proposed decrease of 0.03 percent for the difference in estimated outlier payments between CY 2019 (1.03 percent) and CY 2020 (proposed 1.0 percent). We estimate that the combined effect of all proposed changes for CY 2020 would increase payments to urban hospitals by 2.0 percent. Overall, we estimate that rural hospitals would experience a 1.9 percent increase as a result of the combined effects of all the proposed changes for CY 2020.
Among hospitals, by teaching status, we estimate that the impacts resulting from the combined effects of all proposed changes would include an increase of 1.3 percent for major teaching hospitals and an increase of 2.3 percent for nonteaching hospitals. Minor teaching hospitals would experience an estimated increase of 2.1 percent.
In our analysis, we also have categorized hospitals by type of ownership. Based on this analysis, we estimate that voluntary hospitals would experience an increase of 1.8 percent, proprietary hospitals would experience an increase of 3.0 percent, and governmental hospitals would experience an increase of 1.9 percent.




d. Estimated Effects of Proposed OPPS Changes on CMHCs
The last line of Table 41 demonstrates the isolated impact on CMHCs, which furnish only partial hospitalization services under the OPPS. In CY 2019, CMHCs are paid under APC 5853 (Partial Hospitalization (3 or more services) for CMHCs). We modeled the impact of this APC policy assuming CMHCs will continue to provide the same number of days of PHP care as seen in the CY 2018 claims used for ratesetting in this proposed rule. We excluded days with 1 or 2 services because our policy only pays a per diem rate for partial hospitalization when 3 or more qualifying services are provided to the beneficiary. We estimate that CMHCs would experience an overall 3.9 percent increase in payments from CY 2019 (shown in Column 6). We note that this includes the trimming methodology as well as the proposed CY 2020 floor on geometric mean costs used for developing the PHP payment rates described in section VIII.B. of this proposed rule. The CY 2020 proposal to establish a floor based on geometric mean costs, rather than based on a predetermined payment rate, makes the OPPS budget neutrality adjustments for both the weight scaler and the conversion factor applicable.
Column 3 shows that the estimated impact of adopting the proposed FY 2020 wage index values would result in an increase of 0.4 percent to CMHCs. Column 4 shows that combining this proposed OPD fee schedule increase factor, along with proposed changes in APC policy for CY 2020 and the proposed FY 2020 wage index updates, would result in an estimated increase of 4.1 percent. Column 5 shows that the off-campus PBD clinic visits payment policy has no estimated effect on CMHCs. Column 6 shows that adding the proposed changes in outlier and pass-through payments would result in a total 3.9 percent increase in payment for CMHCs. This reflects all proposed changes for CMHCs for CY 2020.
e. Estimated Effect of Proposed OPPS Changes on Beneficiaries
For services for which the beneficiary pays a copayment of 20 percent of the payment rate, the beneficiary's payment would increase for services for which the OPPS payments would rise and would decrease for services for which the OPPS payments would fall. For further discussion on the calculation of the national unadjusted copayments and minimum unadjusted copayments, we refer readers to section II.I. of this proposed rule. In all cases, section 1833(t)(8)(C)(i) of the Act limits beneficiary liability for copayment for a procedure performed in a year to the hospital inpatient deductible for the applicable year.
We estimate that the aggregate beneficiary coinsurance percentage would be 18.2 percent for all services paid under the OPPS in CY 2020. The estimated aggregate beneficiary coinsurance reflects general system adjustments, including the proposed CY 2020 comprehensive APC payment policy discussed in section II.A.2.b. of this proposed rule.
f. Estimated Effects of Proposed OPPS Changes on Other Providers
The relative payment weights and payment amounts established under the OPPS affect the payments made to ASCs, as discussed in section XIII of this proposed rule. No types of providers or suppliers other than hospitals, CMHCs, and ASCs would be affected by the proposed changes in this proposed rule.
g. Estimated Effects of Proposed OPPS Changes on the Medicare and Medicaid Programs
The effect on the Medicare program is expected to be an increase of $940 million in program payments for OPPS services furnished in CY 2020. The effect on the Medicaid program is expected to be limited to copayments that Medicaid may make on behalf of Medicaid recipients who are also Medicare beneficiaries. We estimate that the proposed changes in this proposed rule would increase these Medicaid beneficiary payments by approximately $45 million in CY 2020. Currently, there are approximately 10 million dual-eligible beneficiaries, which represents approximately one third of Medicare Part B fee-for-service beneficiaries. The impact on Medicaid was determined by taking one-third of the beneficiary cost-sharing impact. The national average split of Medicaid payments is 57 percent Federal payments and 43 percent State payments. Therefore, for the estimated $45 million Medicaid increase, approximately $25 million would be from the Federal Government and $20 million would be from State government.
h. Alternative OPPS Policies Considered
Alternatives to the OPPS changes we are proposing and the reasons for our selected alternatives are discussed throughout this proposed rule.
- Alternatives Considered for the Methodology for Assigning Skin Substitutes to High or Low Cost Groups
We refer readers to section V.B.7. of this proposed rule for a discussion of our policy to assign any skin substitute product that was assigned to the high cost group in CY 2019 to the high cost group in CY 2020, regardless of whether the product's mean unit cost (MUC) or the product's per day cost (PDC) exceeds or falls below the overall CY 2020 MUC or PDC threshold. We will continue to assign products that exceed either the overall CY 2020 MUC or PDC threshold to the high cost group. We also considered, but are not proposing, reinstating our methodology from CY 2017 and assigning skin substitutes to the high cost group based on whether an individual product's MUC or PDC exceeded the overall CY 2020 MUC or PDC threshold based on calculations done for either the proposed rule or the final rule with comment period.
- Alternatives Considered for the Methodology for Payment for Non-Opioid Pain Management Treatments
We refer readers to sections II.A.3.b. and XIII.D.3. of this proposed rule and the CY 2019 OPPS/ASC final rule with comment period (83 FR 58860) for a discussion of our change in the packaging policy for certain drugs when administered in the ASC setting and policy of providing separate payment for non-opioid pain management drugs that function as a supply when used in a surgical procedure when the procedure is performed in an ASC. In those sections of the CY 2019 OPPS/ASC final rule with comment period, we discuss the comments we received on whether we should pay separately for other non-opioid treatments for pain under the OPPS and the ASC payment system. In the CY 2019 OPPS/ASC final rule with comment period, we also discuss the comments we received on an alternative policy that would use our equitable adjustment authority under section 1833(t)(2)(E) of the Act to establish an incentive payment for non-opioid alternatives that would apply to drugs and devices under the OPPS that are not currently separately paid, are supported by evidence that demonstrates such drugs and devices are effective at treating acute or chronic pain, and would result in decreased use of prescription opioid drugs and any associated opioid addiction, when furnished in the hospital outpatient setting.
- Alternatives Considered for the Proposed Changes in the Level of Supervision of Outpatient Therapeutic Services in Hospitals and Critical Access Hospitals (CAHs)
We refer readers to section X.A. of this proposed rule for a discussion of our proposal to change the minimum required default level of supervision from direct supervision to general supervision for all hospital outpatient therapeutic services provided by all hospitals and CAHs. We also considered, but are not proposing, reevaluation of the level of physician supervision for cardiac rehabilitation services to determine whether we should propose to change the supervision level from direct supervision to general supervision. Under this alternative, direct supervision would remain the minimum required default level for most hospital outpatient therapeutic services with the exception of those services that have been evaluated by the HOP Panel and received a change in supervision level based on those recommendations.
2. Estimated Effects of Proposed CY 2020 ASC Payment System Changes
Most ASC payment rates are calculated by multiplying the ASC conversion factor by the ASC relative payment weight. As discussed fully in section XIII. of this proposed rule, we are proposing to set the CY 2020 ASC relative payment weights by scaling the proposed CY 2020 OPPS relative payment weights by the proposed ASC scalar of 0.8452. The estimated effects of the proposed updated relative payment weights on payment rates are varied and are reflected in the estimated payments displayed in Tables 39 and 40 below.
Beginning in CY 2011, section 3401 of the Affordable Care Act requires that the annual update to the ASC payment system (which we are proposing will be the hospital market basket for CY 2020) after application of any quality reporting reduction be reduced by a productivity adjustment. The Affordable Care Act defines the productivity adjustment to be equal to the 10-year moving average of changes in annual economy-wide private nonfarm business multifactor productivity (MFP) (as projected by the Secretary for the 10-year period, ending with the applicable fiscal year, year, cost reporting period, or other annual period). For ASCs that fail to meet their quality reporting requirements, the CY 2020 payment determinations will be based on the application of a 2.0 percentage point reduction to the annual update factor, which we are proposing will be the hospital market basket for CY 2020. We calculated the proposed CY 2020 ASC conversion factor by adjusting the CY 2019 ASC conversion factor by 1.0008 to account for changes in the pre-floor and pre-reclassified hospital wage indexes between CY 2019 and CY 2020 and by applying the proposed CY 2020 MFP-adjusted hospital market basket update factor of 2.7 percent (projected hospital market basket update of 3.2 percent minus a projected productivity adjustment proposed to be 0.5 percentage point). The proposed CY 2020 ASC conversion factor is $47.827 for ASCs that successfully meet the quality reporting requirements.
a. Limitations of Our Analysis
Presented here are the projected effects of the proposed changes for CY 2020 on Medicare payment to ASCs. A key limitation of our analysis is our inability to predict changes in ASC service-mix between CY 2018 and CY 2020 with precision. We believe the net effect on Medicare expenditures resulting from the proposed CY 2020 changes would be small in the aggregate for all ASCs. However, such changes may have differential effects across surgical specialty groups, as ASCs continue to adjust to the payment rates based on the policies of the revised ASC payment system. We are unable to accurately project such changes at a disaggregated level. Clearly, individual ASCs would experience changes in payment that differ from the aggregated estimated impacts presented below.
b. Estimated Effects of Proposed ASC Payment System Policies on ASCs
Some ASCs are multispecialty facilities that perform a wide range of surgical procedures from excision of lesions to hernia repair to cataract extraction; others focus on a single specialty and perform only a limited range of surgical procedures, such as eye, digestive system, or orthopedic procedures. The combined effect on an individual ASC of the proposed update to the CY 2020 payments would depend on a number of factors, including, but not limited to, the mix of services the ASC provides, the volume of specific services provided by the ASC, the percentage of its patients who are Medicare beneficiaries, and the extent to which an ASC provides different services in the coming year. The following discussion presents tables that display estimates of the impact of the proposed CY 2020 updates to the ASC payment system on Medicare payments to ASCs, assuming the same mix of services, as reflected in our CY 2018 claims data. Table 39 depicts the estimated aggregate percent change in payment by surgical specialty or ancillary items and services group by comparing estimated CY 2019 payments to estimated proposed CY 2020 payments, and Table 40 shows a comparison of estimated CY 2019 payments to estimated proposed CY 2020 payments for procedures that we estimate would receive the most Medicare payment in CY 2019.
In Table 39, we have aggregated the surgical HCPCS codes by specialty group, grouped all HCPCS codes for covered ancillary items and services into a single group, and then estimated the effect on aggregated payment for surgical specialty and ancillary items and services groups. The groups are sorted for display in descending order by estimated Medicare program payment to ASCs. The following is an explanation of the information presented in Table 42.
- Column 1—Surgical Specialty or Ancillary Items and Services Group indicates the surgical specialty into which ASC procedures are grouped and the ancillary items and services group which includes all HCPCS codes for covered ancillary items and services. To group surgical procedures by surgical specialty, we used the CPT code range definitions and Level II HCPCS codes and Category III CPT codes, as appropriate, to account for all surgical procedures to which the Medicare program payments are attributed.
- Column 2—Estimated CY 2019 ASC Payments were calculated using CY 2018 ASC utilization data (the most recent full year of ASC utilization) and CY 2019 ASC payment rates. The surgical specialty and ancillary items and services groups are displayed in descending order based on estimated CY 2019 ASC payments.
- Column 3—Estimated CY 2020 Percent Change is the aggregate percentage increase or decrease in Medicare program payment to ASCs for each surgical specialty or ancillary items and services group that is attributable to proposed updates to ASC payment rates for CY 2020 compared to CY 2019.
As shown in Table 39, for the six specialty groups that account for the most ASC utilization and spending, we estimate that the proposed update to ASC payment rates for CY 2020 would result in a 3-percent increase in aggregate payment amounts for eye and ocular adnexa procedures, a 3-percent increase in aggregate payment amounts for nervous system procedures, 1-percent increase in aggregate payment amounts for digestive system procedures, a 2-percent increase in aggregate payment amounts for musculoskeletal system procedures, a 2-percent increase in aggregate payment amounts for genitourinary system procedures, and a 5-percent increase in aggregate payment amounts for cardiovascular system procedures. We note that these changes can be a result of different factors, including updated data, payment weight changes, and proposed changes in policy. In general, spending in each of these categories of services is increasing due to the 2.7 percent proposed payment rate update. After the payment rate update is accounted for, aggregate payment increases or decreases for a category of services can be higher or lower than a 2.7-percent increase, depending on if payment weights in the OPPS APCs that correspond to the applicable services increased or decreased or if the most recent data show an increase or a decrease in the volume of services performed in an ASC for a category. For example, we estimate a 3-percent increase in proposed aggregate eye and ocular adnexa procedure payments due to an increase in hospital reported costs for the primary payment grouping for this category under the OPPS. This increases the payment weights for eye and ocular adnexa procedure payments and, overall, is further increased by the proposed 2.7 percent ASC rate update for these procedures. For estimated changes for selected procedures, we refer readers to Table 40 provided later in this section.
Also displayed in Table 42 is a separate estimate of Medicare ASC payments for the group of separately payable covered ancillary items and services. The payment estimates for the covered surgical procedures include the costs of packaged ancillary items and services. We estimate that aggregate payments for these items and services would increase by 5 percent for CY 2020. This is largely attributed to the drug packaging policies adopted under the OPPS and ASC payment system.

Table 43 shows the estimated impact of the proposed updates to the revised ASC payment system on aggregate ASC payments for selected surgical procedures during CY 2020. The table displays 30 of the procedures receiving the greatest estimated CY 2019 aggregate Medicare payments to ASCs. The HCPCS codes are sorted in descending order by estimated CY 2019 program payment.
- Column 1—CPT/HCPCS code.
- Column 2—Short Descriptor of the HCPCS code.
- Column 3—Estimated CY 2019 ASC Payments were calculated using CY 2018 ASC utilization (the most recent full year of ASC utilization) and the CY 2019 ASC payment rates. The estimated CY 2019 payments are expressed in millions of dollars.
- Column 4—Estimated CY 2020 Percent Change reflects the percent differences between the estimated ASC payment for CY 2019 and the estimated payment for CY 2020 based on the proposed update.

c. Estimated Effects of Proposed ASC Payment System Policies on Beneficiaries
We estimate that the proposed CY 2020 update to the ASC payment system would be generally positive (that is, result in lower cost-sharing) for beneficiaries with respect to the new procedures we are proposing to add to the ASC list of covered surgical procedures and for those we are proposing to designate as office-based for CY 2020. For example, using 2018 utilization data and proposed CY 2020 OPPS and ASC payment rates, we estimate that if 5 percent of coronary intervention procedures migrate from the hospital outpatient setting to the ASC setting as a result of this proposed policy, Medicare payments would be reduced by approximately $15 million in CY 2020 and total beneficiary copayments would decline by approximately $3 million in CY 2020. First, other than certain preventive services where coinsurance and the Part B deductible is waived to comply with sections 1833(a)(1) and (b) of the Act, the ASC coinsurance rate for all procedures is 20 percent. This contrasts with procedures performed in HOPDs under the OPPS, where the beneficiary is responsible for copayments that range from 20 percent to 40 percent of the procedure payment (other than for certain preventive services), although the majority of HOPD procedures have a 20-percent copayment. Second, in almost all cases, the ASC payment rates under the ASC payment system are lower than payment rates for the same procedures under the OPPS. Therefore, the beneficiary coinsurance amount under the ASC payment system will almost always be less than the OPPS copayment amount for the same services. (The only exceptions would be if the ASC coinsurance amount exceeds the hospital inpatient deductible. The statute requires that copayment amounts under the OPPS not exceed the hospital inpatient deductible.) Beneficiary coinsurance for services migrating from physicians' offices to ASCs may decrease or increase under the ASC payment system, depending on the particular service and the relative payment amounts under the MPFS compared to the ASC. While the ASC payment system bases most of its payment rates on hospital cost data used to set OPPS relative payment weights, services that are performed a majority of the time in a physician office are generally paid the lesser of the ASC amount according to the standard ASC ratesetting methodology or at the nonfacility practice expense based amount payable under the PFS. For those additional procedures that we are proposing to designate as office-based in CY 2020, the beneficiary coinsurance amount under the ASC payment system generally would be no greater than the beneficiary coinsurance under the PFS because the coinsurance under both payment systems generally is 20 percent (except for certain preventive services where the coinsurance is waived under both payment systems).
3. Accounting Statements and Tables
As required by OMB Circular A-4 (available on the Office of Management and Budget website at: https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/assets/OMB/circulars/a004/a-4.html), we have prepared accounting statements to illustrate the impacts of the proposed OPPS and ASC changes in this proposed rule. The first accounting statement, Table 44, illustrates the classification of expenditures for the CY 2020 estimated hospital OPPS incurred benefit impacts associated with the proposed CY 2020 OPD fee schedule increase. This $940 million in additional Medicare spending estimate includes the $1.6 billion in additional Medicare spending associated with updating the CY 2019 OPPS payment rates by the hospital market basket update for CY 2020, offset by the $650 million in Medicare savings associated with the CY 2020 completion of phase-in finalized in CY 2019 to pay for clinic visits furnished at off-campus PBDs at a PFS-equivalent rate. In addition, we estimate that proposed OPPS changes in this proposed rule would increase copayments that Medicaid may make on behalf of Medicaid recipients who are also Medicare beneficiaries by approximately $45 million in CY 2020. The second accounting statement, Table 45, illustrates the classification of expenditures associated with the proposed 2.7 percent CY 2020 update to the ASC payment system, based on the provisions of this proposed rule and the baseline spending estimates for ASCs. Both tables classify most estimated impacts as transfers. The estimated costs of ICR Burden and Regulatory Familiarization are included in Table 46.


4. Effects of Proposed Changes in Requirements for the Hospital OQR Program
a. Background
We refer readers to the CY 2018 OPPS/ASC final rule with comment period (82 FR 59492 through 59494), for the previously estimated effects of changes to the Hospital OQR Program for the CY 2018, CY 2019, and CY 2020 payment determinations. Of the approximately 3,300 hospitals that met eligibility requirements for the CY 2019 payment determination, we determined that 14 hospitals did not meet the requirements to receive the full OPD fee schedule increase factor. In this proposed rule, we are not proposing to add any quality measures to the Hospital OQR Program measure set for the CY 2021 or CY 2022 payment determinations. However, we are proposing to remove one measure from the program measure set, as discussed in section XIV.B.3.b. of this proposed rule. We do not believe that this proposed policy would increase the number of hospitals that do not receive a full annual payment update for the CY 2021 or CY 2022 payment determinations.
b. Estimated Effects of Proposed Removal of OP-33 for the CY 2022 Payment Determination and Subsequent Years
In section XIV.B.3.b. of this proposed rule, we are proposing to remove OP-33: External Beam Radiotherapy for Bone Metastases beginning with the CY 2022 payment determination and for subsequent years. As discussed in section XXVI.B.2. of this proposed rule, we anticipate a burden reduction of 551 hours and $21,379 associated with the removal of OP-33 for the CY 2022 payment determination. In addition to burden associated with information collection however, we also anticipate that hospitals would experience a general burden and cost reduction associated with this proposal stemming from no longer having to implement, review, track, and maintain program requirements associated with this measure.
5. Effects of Proposed Requirements for the ASCQR Program
a. Background
In section XV. of this proposed rule, we discuss our proposed policies affecting the ASCQR Program. For the CY 2019 payment determination, of the 6,393 ASCs that met eligibility requirements for the ASCQR Program, 203 ASCs did not meet the requirements to receive the full annual payment update. In section XV.B.3. of this proposed rule, we are proposing to adopt ASC-19: Facility-Level 7-Day Hospital Visits After General Surgery Procedures Performed at Ambulatory Surgical Centers to the ASCQR Program measure set for the CY 2024 payment determination and subsequent years. We do not believe that adoption of the proposed ASC-19 measure would cause any ASCs to fail to meet the ASCQR Program requirements. Therefore, we do not believe that our proposal would increase the number of ASCs that do not receive a full annual payment update for the CY 2024 payment determination. Below we discuss only the effects that would result from the provisions proposed in this proposed rule.
b. Estimated Effects of the Proposal To Adopt ASC-19: Facility-Level 7-Day Hospital Visits After General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357)
In section XV.B.3. of this proposed rule, we are proposing, beginning with the CY 2024 payment determination and for subsequent years, to adopt one measure: ASC-19: Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers (NQF #3357). As discussed in section XXVI.C.2. of this proposed rule, data used to calculate scores for this proposed measure are collected via Medicare Part A and Part B administrative claims and Medicare enrollment data. Therefore, ASCs would not be required to report any additional data. Because this change does not affect ASCQR Program participation requirements or data reporting requirements, we do not expect this proposed measure to change the information collection burden and would only nominally affect other costs experienced by ASCs due to having to review and track confidential feedback and reports related to the proposed ASC-19 measure.
D. Effects of the Proposals Relating to Price Transparency in Hospital Standard Charges
1. Background
In section XVI. of this proposed rule, we are proposing to adopt requirements that would implement section 2718(e) of the Public Health Service Act, which requires that each hospital operating within the United States, for each year, establish (and update) and make public (in accordance with guidelines developed by the Secretary) a list of the hospital's standard charges for items and services provided by the hospital, including for diagnosis-related groups established under section 1886(d)(4) of the Act.
In the FY 2015 IPPS/LTCH PPS proposed and final rules (79 FR 28169 and 79 FR 50146, respectively), we reminded hospitals of their obligation to comply with the provisions of section 2718(e) of the PHS Act and provided guidelines for its implementation. At that time, we required hospitals to either make public a list of their standard charges or their policies for allowing the public to view a list of those charges in response to an inquiry. In addition, we stated that we expected hospitals to update the information at least annually, or more often as appropriate, to reflect current charges. We also encouraged hospitals to undertake efforts to engage in consumer-friendly communication of their charges to enable consumers to compare charges for similar services across hospitals and to help consumers understand what their potential financial liability might be for items and services they obtain at the hospital.
In the FY 2019 IPPS/LTCH PPS proposed rule and final rule (83 FR 20164 and 83 FR 41144, respectively), we again reminded hospitals of their obligation to comply with the provisions of section 2718(e) of the PHS Act and updated our guidelines for its implementation. The announced update to our guidelines became effective January 1, 2019, and took one step to further improve the public accessibility of standard charge information. Specifically, we updated our guidelines to require hospitals to make available a list of their current standard charges via the internet in a machine-readable format and to update this information at least annually, or more often as appropriate.
However, we continue to have concerns that health care consumers lack the meaningful pricing information they need to choose the healthcare services they want and need. Therefore, in response to stakeholders and in accordance with Executive Order on Improving Price and Quality Transparency in American Healthcare to Put Patients First (June 24, 2019), we are proposing that hospitals make public their standard charges in two ways: (1) By publicly posting standard charge information, including gross charges and payer-specific negotiated rates and for all items and services, online in a machine-readable format, and (2) by making make public payer-specific negotiated rates for at least 300 shoppable services in a manner that is consumer-friendly that will meaningfully inform patients' decision making and allow consumers to compare prices across hospitals. To be consumer-friendly, the charge information for shoppable services must be displayed along with the charge information for all associated ancillary services, and the data must be easily accessible by the consumer and searchable. We are also proposing to establish a mechanism for monitoring and the application of penalties for noncompliance.
2. Estimated Burden on Hospitals
We estimate the total annual burden for hospitals to review and make public all gross and payer-specific negotiated charges for all items and services in a machine-readable format, and payer-specific negotiated charges for at least 300 shoppable services in a consumer-friendly format, to be 12 hours per hospital at $1,017.24 per hospital for a total burden of 72,024 hours (12 hours × 6,002 hospitals) and total cost of $6,105,474 ($1,017.24 × 6,002 hospitals).
We believe the burden is minimal for several reasons. First, this proposed rule is based on existing statutory requirements for hospitals to make public a list of standard charges (specifically, gross charges), which we stated in the FY 2019 IPPS/LTCH PPS final rule must be displayed in a machine readable format beginning January 1, 2019. Second, most (if not all) hospitals actively review, update, and maintain all gross charges and payer-specific negotiated charges in electronic format in hospital billing systems. Third, we have sampled hospital and state websites to see how hospitals are responding to current chargemaster posting guidance and we find that hospitals appear to be easily complying. Additionally, hospital executives and hospital finance experts have indicated that pulling already electronically available data out of hospital accounting and billing systems is very low burden. For all these reasons, we anticipate little additional burden for hospitals to meet the proposed requirement for making public gross charges and payer-specific negotiated charges online in a machine-readable format, other than the time accounted for below to review these proposed rules to ensure compliance.
As a result, the bulk of this burden estimate (8 hours) applies to the newly proposed requirements related to making public payer-specific negotiated charges in a consumer-friendly format for at least 300 shoppable items and services. In this estimate, we have accounted for activities associated with identifying hospital-selected shoppable services and for displaying payer-specific negotiated charges grouped along with the payer-specific charges for associated ancillary items and services the hospital customarily provides as part of or in conjunction with the primary service. We believe that hospitals will require this time to analyze their claims data to generate a list of hospital-selected shoppable services, to analyze claims data to determine the ancillary services that should be grouped with each shoppable service, and to display standard charges in a consumer-friendly manner in accordance with the proposed requirements at section XIV.F.2 of this proposed rule. We note that we have proposed that hospitals have flexibility to determine the format for making charges for shoppable services available to the public, recognizing that many hospitals may already be doing so in consumer-friendly online price estimator tools. We further note that most of the impact would likely occur in the first year and that updating such data annually would become more routine and automated over time.
As noted above, we believe this is an accurate estimate of burden because maintaining a set of negotiated charge data is part of normal operations for hospitals in order to work with payers and bill patients, and hospitals can readily access billing records to determine which services are commonly billed together to develop a total cost for the service package. Extracting from this data set should be a simple statistical command or formula in either MS Office applications or various database software, and it imposes minimal burden for hospitals' operations staff. We believe that our proposed accessibility requirements will ensure the hospital data can be easily found by consumers and therefore have not included any burden estimates for additional public outreach and education, but seek public comment on whether to further consider a burden for this factor.
We believe that adding to or shifting the number of CMS-specific vs the number of hospital selected shoppable items and services would not alter this burden estimate. In total, we believe that additional burden for our proposals to make public the hospital's standard charges in the form and manner proposed would be, on average, 12 hours per hospital at $1,017.24 per hospital for hospitals in the United States.
3. Limitations of Our Analysis
It would be difficult for us to conduct a detailed quantitative analysis given the lack of studies at the national level on the regulatory impact of making price transparency information publicly available. Since we cannot produce a detailed quantitative analysis, we have developed a qualitative discussion for this regulatory impact analysis, drawing from the experiences of States that have enacted price transparency legislation and the use of price transparency tools in the private health care market. We have taken an approach that assesses potential directional impact of these proposed requirements (that is, increasing versus decreasing health care costs, increasing or decreasing likelihood of certain consumer or insurer behaviors) rather than attempting more specific estimates due to the lack of empirical data. We believe there are many benefits with this regulation, particularly for consumers who have the right to know the hospital services before they commit to them, and to be able to shop for the best value. We also discuss potential unintended consequences as a result of these proposals.
4. Estimated Effects on Private Sector
We believe that by requiring hospitals to make public their standard changes (both gross charges and payer-specific negotiated rates) for all hospital items and services (including individual items and services and service packages), our proposals will release data necessary to better understand how the level of price dispersion in various health care markets impacts health care spending and consumer out-of-pocket costs. As noted in section XVI. of this proposed rule, the negotiated charges for various procedures vary widely within and across geographic regions in the United States.[227] Some factors associated with the level of hospital price dispersion in a geographic area are the hospital's size, health care demand, labor costs, and technology, although it was the hospital's market power (level of competition) that was most positively associated with high price dispersion.[228 229] Cooper found that variation in prices across hospital referral regions is the primary driver of variation in spending per enrollee for those privately insured, while the quantity of care provided across hospital referral regions is the primary driver of variation in spending per beneficiary for Medicare.[230] One major barrier to fully understanding health care price variation (and understanding the impact of transparency of health care pricing in general) is the lack of availability of negotiated charges to researchers and the public.[231] Our proposals would make hospital charge information available which would generate a better understanding of (1) hospital price dispersion, and (2) the relationship between hospital price dispersion and health care spending. Additionally, we believe understanding these relationships through release of pricing data could lead to downward price pressure on hospitals and reductions in overall spending.
We recognize the potential concern that hospitals may attempt to present a more favorable or discounted view of their payer-specific negotiated rates for the limited set of shoppable services, while potentially increasing charges for other items and services (for example, non-shoppable services). However, we believe that this risk will be mitigated by the requirement to post gross charges and the payer-specific negotiated charges for all items and services (including individual items and services and service packages) in a machine-readable format.
In addition to this possibility, we acknowledge there could be an impact in the commercial insurance market. A few studies have examined insurer competition in relation to negotiated hospital prices,[232] or price transparency and markups in health care.[233] We also realize that it takes time for markets to react to public disclosure of payer-specific negotiated rates, and its dynamic could vary state by state. We invite comments on the potential impact of disclosure of payer-specific negotiated charges on commercial insurers.
We believe that price transparency initiatives may reduce overall costs and price dispersion. In their comprehensive analysis of the impact of regulations across more than 30 States requiring public access to the prices of hospital procedures, Christensen et al. found that regulations lowered the price of shoppable procedures such as hip replacements by approximately five percent overall compared to prices for non-shoppable procedures such as appendectomies. They further found that half of the observed price reduction in charges was due to hospitals lowering their prices to remain competitive. This was particularly true for high priced hospitals and for hospitals in competitive urban areas.[234] Research has also indicated that price transparency initiatives can decrease prices paid by consumers and insurers. One study found that following the introduction of a State-run website providing out-of-pocket costs for a subset of shoppable outpatient services reduced the charges for these procedures by approximately 5 percent for consumers, in part by shifting demand to lower cost providers.[235] In addition, the study found that insurers over time experienced a 4-percent reduction in administrative costs for imaging services, following the introduction of the site.
Based on our analysis of comments from stakeholders on the 2018 RFIs, we do not believe the economic effects will vary significantly between rural and critical access markets and larger or consolidated health care markets. However, we seek comment on whether there could be unforeseen effects of the proposals that may differentially impact markets with small, rural, or CAH hospitals.
Another possibility is that transparency in payer-specific negotiated charges may narrow the dispersion of prices in a market, meaning that knowledge of payer-specific charges may not only result in lowering prices for payers currently paying rates above the median, but could also increase prices for payers that are currently paying rates below the median. Making payer-specific negotiated prices public could risk disrupting the ability for certain payers to extract aggressive discounts in the future, especially from providers in markets with limited competition. For example, a hospital providing an aggressive discount to a particular payer may become motivated to withdraw such discount to avoid divulging such information to other payers with whom they contract.
Several studies of mandated price transparency in non-healthcare commodity markets have shown suppliers can use the information to their advantage in maximizing the prices they can charge in markets with limited competition or where commodities are not easily transferable across geographies. Although there are no definitive conclusions on the effects of price transparency on markets one study found that it can either increase or decrease prices depending on the strength of the bargainers and the size of the market.[236] While price transparency gives buyers and sellers important information about the value of items and services, the effect may result in price increases by changing the incentives for buyers and sellers may also enable traders to observe deviations from collusive practices. Allowing weaker bargainers to see prices negotiated by stronger bargainers will change incentives facing buyers and sellers, and can lead to price increases. We seek comment from stakeholders and the public as to whether they believe these types of potential drawbacks are legitimate risks in their market and, if so, whether the potential benefits of making transparent all negotiated prices outweigh the risks outlined above. If commenters believe these are risks, we further request input on what policies could mitigate these risks. If commenters believe the risks are not worth the benefits, we request further input on whether publishing only the minimum, median, and maximum negotiated rates (an alternative considered in this proposed rule) would improve the benefit-risk profile.
In the absence of a national model, we looked to two States that have previously enacted price transparency laws, California and New Hampshire. California enacted a requirement for hospitals to post their charge description master in 2004, and in 2003, New Hampshire created an all-payer claims database, later publishing the data in 2007 in a statewide, web-based price transparency comparison tool.
Studies assessing the impact of the New Hampshire State law have found that the efforts focused on the wide variation of provider prices, which in turn created opportunities for new benefit design that incentivized consumer choice of lower costs providers and sites of service.[237]
In California, the link between hospital chargemaster data and patient cost was validated through a 10-year study of the chargemaster data which found that each dollar in a hospital's list price was associated with an additional 15 cents in payment to a hospital for privately insured patients (versus publicly insured patients).[238]
This effort to improve the availability of charge data can open up the possibility to States to further regulate hospital charges—examples seen in both California and New Hampshire that took further legislative action to reduce price dispersion, reduce surprise billing and to place limits on charges for the uninsured and for out-of-network providers.
As noted earlier, we lack data to quantify the effects of our proposals along these dimensions, and we are seeking public comments on these impacts. In addition, we acknowledge that we may not have considered all areas in which the proposed rule may have effects, and we are seeking public comments on impacts of the proposals in areas we have not discussed here.
5. Estimated Effects on Consumers
In addition to economic effects described above, consumers may feel more satisfied with their care when they are empowered to make decisions about their treatment. A recent survey [239] indicated a strong desire for price transparency and openness. Eighty-eight percent of the population polled, demanded improved transparency with their total financial responsibility, including copays and deductibles. Another study suggests that improving a patient's financial experience served as the biggest area to improve overall customer satisfaction.[240] According to a 2011 GAO report, transparent health care price information may help consumers anticipate their health care costs, reduce the possibility of unexpected expenses, and make more informed choices about their care, including for both shoppable services as defined in this rule and other hospital items and services in both outpatient and inpatient settings.[241] We considered the likelihood of patients would shifting from seeking services from lower cost non-hospital sites such as ASCs, advanced radiology centers, or stand-alone labs as a result of this proposed rule and seek public comment on this potential effect.
A large part of the literature on consumer use of price information comes from studies of price transparency tools, particularly those offered by third party payers and for shoppable services. Some studies of consumer use of price information through web-based tools, such as those offered by self-insured employers or plans, indicate that they may help consumers save money on shoppable services. One study examined consumer use of an employer-sponsored, private price transparency tool and its impact on claims payments for three common medical services: laboratory tests; advanced imaging services; and clinician office visits.[242] That study found that those who used the tool had lower claims payments by approximately 14 percent for laboratory tests; 13 percent for advanced imaging services; and approximately 1 percent for office visits compared to those who did not use the tool. Another study found that those employed by a large corporation who used a price transparency tool were able to reduce their costs by 10 to 17 percent compared to nonusers.[243] Those using the tool mainly searched for information on shoppable services and also tended to have more limited insurance coverage. However, one study of the use of price transparency tools by consumers with an employer-based, high deductible health plan found that consumers' likely perception that higher price is a proxy for higher quality care may lead them to select higher-cost options.[244] This study found a spending drop between 11.8 and 13.8 percent occurring across the spectrum of health care service categories at the health plan level; the majority of spending reductions were due to consumer quantity reductions across a broad range of services, including both high and low value care. Another study of the use of price transparency tools by consumers found that only 10 percent of consumers who were offered a tool with price information utilized it, and that there was a slight relative increase in their out-of-pocket health spending on outpatient services respective to the patient group that was not offered the tool.[245]
Although we do not propose to require that hospitals develop a price comparison tool, we encourage innovation in this area by making standard charges available in a machine-readable format to third-party tool developers as well as the general public. The use of a third-party tool would enhance public access to pricing data, but we do not believe the absence of one would cause confusion among consumers on how to use the available standard charge data made public by the hospital because we are also proposing requirements for hospitals to make public their payer-specific charges for a set of shoppable services in a consumer-friendly manner. A large part of consumer buy-in may depend on providers' willingness and ability to make public, and to have conversations with consumers about, their standard charge data to allow for price comparison and decisions about upcoming medical treatment. As consumers' health care costs continue to rise, clinicians are in a unique position to discuss the financial impacts of health care decisions with their patients. A paper by Chernew et al. found that patients will often choose services based on clinician referral rather than consideration of cost.[246] We believe that if the requirements of this proposed rule are finalized, the pricing information made available would help ensure that clinicians have relevant pricing data to counsel patients on financial options. A systematic review found that clinicians and their patients believe communication about health care costs is important and that they have the potential to influence health and financial outcomes, but that discussions between clinicians and patients about costs are not common.[247] We did find evidence that physicians were open to having these conversations, and that they were occurring more frequently, but providers have also identified the need for price information as a barrier to discussing costs with patients.[248 249] In addition, a literature review of 18 studies measuring the effects of charge display on cost and practice patterns found that having prospective access to prices for radiology and laboratory services changed physician's ordering behavior, and in 7 of the 9 studies on cost reported statistically significant cost reduction when charges were displayed.[250]
6. Alternatives Considered
This proposed rule aims to make price information more readily available to the public in a manner that is consumer friendly. We considered a number of alternative approaches to maximize the value and accessibility of these data to consumers. For example, proposals to require release of hospital standard charge data in an API format, as discussed in section XVI.E.3. We also considered other types of “standard charges” that could be useful to consumers in section XVI.D.4. For example, in addition to or instead of the requirement to disclose gross charges and payer-specific charges, we sought comment on whether we should consider a definition of `standard charge' to be a volume-driven negotiated charge, the minimum/median/maximum negotiated charge, all allowed charges. Such charges could be relevant to specific groups of individuals, particularly those with health insurance coverage. We also seek comment on a definition of `standard charge' that might be relevant to subgroups of individuals who are self-pay, specifically, types of standard charges representing the discounted cash price for a service package, or the median cash price.
Under these alternative definitions of `standard charges', hospitals would employ statistical command or formulas to sort, extract or calculate the rates for all items and services and the list of shoppable services or service packages. We do not believe the burden associated with these alternative requirements would vary significantly, other than to account for extra analysis and statistical steps involved to calculate or extract the rates from the hospital's electronic accounting and billing system. Ultimately, however, we determined that most of these options would simply limit the usefulness of hospital charge data for consumers and that our current proposals for the disclosure of gross charges and payer-specific negotiated charges provide greater transparency and better encourage innovation from third party vendors.
We also considered, but are not proposing, hospitals would display a set of at least 100 shoppable services with their payer-specific negotiated rates (instead of at least 300 shoppable services as currently proposed). As we discussed in section XVI.F.3, some states require hospitals to make public shoppable service packages that include ancillary service. Other hospitals have developed price estimators that take ancillary services into account. We understand that developing consumer-friendly shoppable service packages can be a challenge. With that in mind, we believe that reducing the number of required shoppable services would have a small impact on hospitals' burden, mainly due to a reduced number an analyses that would have to be performed as described in more detail above. We estimate that the burden for hospitals to display payer-specific negotiated charges for 30 selected shoppable services rather than 230 would result in a reduction of 2 hours of operations. However, we assess that this decrease of 2 hours in burden does not outweigh the decrease in benefit of price transparency for consumers.
E. Effects of Proposed Prior Authorization Process and Requirements for Certain Hospital Outpatient Department (OPD) Services
1. Overall Impact
As discussed in section XX. of this proposed rule, we are proposing to developing a new prior authorization process and requirements for certain hospital outpatient department (OPD) services. This proposal would use our authority in section 1833(t)(2)(f) of the Act to require provisional affirmation of coverage as a condition of Medicare payment unless the provider is exempt. This proposed new requirement for prior authorization of certain covered OPD services aims to reduce the unnecessary increases in volume of certain covered hospital outpatient department services.
We believe there are a number of factors that may contribute to the potential growth assumed in the estimate presented below. For example, as the provider community acclimates to using prior authorization as part of their billing practice, there may be greater systemic or other processing efficiencies to allow more extensive implementation.
The overall economic impact of this proposal on the health care sector is dependent on the number of claims affected. Table 47, Overall Economic Impact to the Health Sector, lists an estimate for the overall economic impact to the health sector for the services combined. The values populating this table were obtained from the cost reflected in Table 48, Annual Private Sector Costs, and Table 49, Estimated Annual Medicare Costs. Together, Tables 48 and 49 combine to convey the overall economic impact to the health sector, which is illustrated in Table 47. It should be noted that due to a July start date, year one will include only 6 months of prior authorization requests.
Based on the estimate, the overall economic impact of this proposal is approximately $8.4 million in the first year based on 6 months. The 5-year impact is approximately $71.8 million, and the 10-year impact is approximately $152 million. The 5 and 10 year impacts account for year one including only 6 months. Additional administrative paperwork costs to private sector providers and an increase in Medicare spending to conduct reviews combine to create the financial impact. However, this impact is offset by some savings. We believe there are likely to be other benefits and cost savings that result from the proposed OPD service prior authorization requirement. However, many of those benefits are difficult to quantify. For instance, we expect to see savings in the form of reduced unnecessary utilization, fraud, waste, and abuse, including a reduction in improper Medicare fee-for-service payments (we note that not all improper payments are fraudulent). We are soliciting public comments on the potential increased costs and benefits associated with this proposed provision.

The definition of small entity in the RFA includes nonprofit organizations. According to the RFA's use of the term, most suppliers and providers are small entities. Likewise, the vast majority of physician and nurse practitioner (NP) practices are considered small businesses according to the SBA's size standards total revenues of $10 million or less in any 1 year. While the economic costs and benefits of this proposal are substantial in the aggregate, the economic impact on individual entities would be relatively small. We estimate that 90 to 95 percent of providers who provide these services are small entities under the RFA definition. The rationale behind requiring prior authorization is to control unnecessary increases in the volume of covered OPD services. The impact on these providers could be significant; if finalized, the proposal would change the billing practices of providers. We believe that the purpose of the statute and this proposal is to avoid unnecessary utilization of OPD services. Therefore, we do not view decreased revenues from OPD services subject to unnecessary utilization by providers to be a condition that we must mitigate. We believe that the effect would be minimal on providers who are compliant with Medicare coverage and payment rules and requirements. This proposal would offer an additional protection to a provider's cash flow as the provider would know in advance if the Medicare requirements are met.
2. Anticipated Specific Cost Effects
a. Private Sector Costs
We do not believe that this proposal would significantly affect the number of legitimate claims submitted for these services. However, we do expect a decrease in the overall amount paid for OPD services resulting from a reduction in unnecessary utilization of the services requiring prior authorization.
As described previously in this proposed rule, we have identified a list of specific services that, based on review and analysis of claims data, show higher than expected, and therefore we believe unnecessary, increases in the volume of service utilization. In making the decision to propose to include the specific services in the proposed list of hospital outpatient department services requiring prior authorization, we first considered that these services are considered cosmetic and, therefore, are only covered by Medicare in very rare circumstances. We then viewed the current volume of utilization of these services and determined that the utilization far exceeded what would be expected.
We have developed a proposed list of potential OPD services categories for inclusion in the OPD services prior authorization process—blepharoplasty; botulin toxin injections; panniculectomy; rhinoplasty; vein ablation, and their related services. The list includes services from each of five categories that have demonstrated an unnecessary increase in volume and can serve some cosmetic purpose and/or are being claimed as therapeutic services.
We estimate that the private sector's per-case time burden attributed to submitting documentation and associated clerical activities in support of a prior authorization request is equivalent to that of submitting documentation and clerical activities associated for prepayment review, which is 0.5 hours. We apply this time burden estimate to initial submissions and resubmissions.

b. Medicare Costs
Medicare would incur additional costs associated with processing the proposed prior authorization requests. We use the range of potentially affected cases (submissions and resubmissions) and multiply it by $50, the estimated cost to review each request. The cost also includes other elements such as appeals, education and outreach, and system changes.

c. Estimated Beneficiary Costs
We expect a reduction in the utilization of Medicare OPD services when such utilization does not comply with one or more of Medicare's coverage, coding, and payment rules. While there may be an associated burden on beneficiaries while they wait for the prior authorization decision, we are unable to quantify that burden. Although the proposal is designed to permit utilization that is medically necessary, OPD services that are not medically necessary may still provide convenience or usefulness for beneficiaries; any rule-induced loss of such convenience or usefulness constitutes a cost of the rule that we lack data to quantify. Additionally, beneficiaries may have out-of-pocket costs for those services that are determined not to comply with Medicare requirements and thus, are not eligible for Medicare payment. We lack the data to quantify these costs as well.
3. Estimated Benefits
There would be quantifiable benefits for this proposal because we expect a reduction in the unnecessary utilization of those Medicare OPD services subject to prior authorization. It is difficult to project the decrease in unnecessary utilization. However, we would closely monitor utilization and billing practices. The expected benefits would include a changed billing practice that also enhances the coordination of care for the beneficiary. For example, requiring prior authorization for certain OPD services ensures that the primary care practitioner recommending the service and the facility collaborate more closely to provide the most appropriate OPD services to meet the needs of the beneficiary. The practitioner recommending the service evaluates the beneficiary to determine his or her condition and what services are needed and medically necessary. This requires the facility to collaborate closely with the practitioner early on in the process to ensure the services are truly necessary and met all requirements and the documentation is complete and correct. Improper payments made because the practitioner did not evaluate the patient or the patient does not meet the Medicare requirements, would likely be reduced by the requirement that a provider submit clinical documentation created by as part of its prior authorization request.
F. Effects of Proposal Relating to Changes in the Definition of Expected Donation Rate for Organ Procurement Organizations
In section XVIII. of this proposed rule, we are proposing to revise the definition of “expected donation rate” in the CfCs for OPOs. This proposed change would allow OPOs to receive payment for organ donor costs under the Medicare and Medicaid programs using a definition that is consistent with the definition used by the Scientific Registry of Transplant Recipients (SRTR).
All 58 OPOs are required to meet two out of three outcome measures detailed in the CfCs for OPOs regulations at 42 CFR 486.318(b). The second outcome measure relies on the aforementioned definition, and therefore all OPOs would be affected by the proposed change. This revision would eliminate the potential for confusion in the OPO community due to different definitions of the same term. However, it would not affect data collection or reporting by OPTNs and SRTRs, nor their statistical evaluation of OPO performance, and therefore it would not result in any quantifiable impact.
G. Potential Revisions to the Laboratory Date of Service Policy
In section XIX of this proposed rule, we solicit comments on potential revisions to the laboratory date of service policy exception at 42 CFR 414.510(b)(5) for molecular pathology tests and tests designated by CMS as an ADLT under paragraph (1) of the definition of advanced diagnostic laboratory test in § 414.502. Because these tests are excluded from our packaging policy under the OPPS, and are paid at the applicable rate for the laboratory test under the CLFS, regardless of whether the hospital or the performing laboratory bills Medicare for the test, any aspect of this discussion will not result in net costs or savings to the Medicare program. Accordingly, the discussion in section XIX. of this proposed rule is not reflected in Table 41 in the regulatory impact analysis under section XXVI.C.1. of this proposed rule.
H. Effect of Proposed Changes to Requirements for Grandfathered Children's Hospitals-Within-Hospitals (HwHs)
In section XXII. of this proposed rule, we discuss our proposal to revise the regulations to allow grandfathered children's HwHs to increased beds while maintaining their grandfathered status. This proposed policy change would allow providers to address changing community needs for services without any increased incentive for inappropriate patient shifting to maximize Medicare payments given the low Medicare utilization in children's hospitals. Based on the best available information, there are currently very few grandfathered children's HwHs (3 or less). For these reasons, we estimate any impact on Medicare expenditures as a result of this proposal would be negligible. On average there are approximately 50 Medicare discharges per year from children's hospitals at an average cost of approximately $33,000 per discharge. There are two possible sources for an increase, if any, in Medicare discharges at grandfathered children's hospitals as a result of our proposal: Either the discharges would have been treated at another children's hospital or the cases would have been treated at an IPPS hospital. In either case given the few number of Medicare discharges at children's hospitals, the impact of this proposal on Medicare spending is negligible.
I. Regulatory Review Costs
If regulations impose administrative costs on private entities, such as the time needed to read and interpret a rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review a rule, we assume that the number of commenters on the CY 2019 OPPS/ASC proposed rule (2,990) will be the number of reviewers of this proposed rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this proposed rule. It is possible that not all commenters will review this proposed rule in detail, and it is also possible that some reviewers will choose not to comment on this proposed rule. Nonetheless, we believe that the number of commenters on the CY 2019 OPPS/ASC proposed rule will be a fair estimate of the number of reviewers of this proposed rule. We welcome any comments on the approach in estimating the number of entities that will review this proposed rule. We also recognize that different types of entities are, in many cases, affected by mutually exclusive sections of this proposed rule and the final rule with comment period, and, therefore, for the purposes of our estimate, we assume that each reviewer reads approximately 50 percent of the rule. In this proposed rule, we are seeking public comments.
Using the wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing this rule is $109.36 per hour, including overhead and fringe benefits (https://www.bls.gov/oes/current/oes_nat.htm). Assuming an average reading speed, we estimate that it will take approximately 8 hours for the staff to review half of this proposed rule. For each facility that reviews this proposed rule, the estimated cost is $874.88 (8 hours × $109.36). Therefore, we estimate that the total cost of reviewing this proposed rule is $2,615,891.20 ($874.88 × 2,990 reviewers).
J. Regulatory Flexibility Act (RFA) Analysis
The RFA requires agencies to analyze options for regulatory relief of small entities, if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, we estimate that most hospitals, ASCs and CMHCs are small entities as that term is used in the RFA. For purposes of the RFA, most hospitals are considered small businesses according to the Small Business Administration's size standards with total revenues of $38.5 million or less in any single year or by the hospital's not-for-profit status. Most ASCs and most CMHCs are considered small businesses with total revenues of $15 million or less in any single year. For details, we refer readers to the Small Business Administration's “Table of Size Standards” at: http://www.sba.gov/content/table-small-business-size-standards.
In addition, section 1102(b) of the Act requires us to prepare a regulatory impact analysis if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 603 of the RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a metropolitan statistical area and has 100 or fewer beds. We estimate that this proposed rule would increase payments to small rural hospitals by less than 3 percent; therefore, it should not have a significant impact on approximately 612 small rural hospitals. We note that the estimated payment impact for any category of small entity will depend on both the services that they provide as well as the payment policies and/or payment systems that may apply to them. Therefore, the most applicable estimated impact may be based on the specialty, provider type, or payment system.
We do not believe proposals related to requirements for hospitals to make public their standard charges would have a significant economic impact on small rural hospitals operations or their market positions. As indicated in section XX.VI.D. in this proposed rule, the total annual burden for making public standard charges is minimal on the operations of hospitals, including small rural hospitals. Moreover, small rural hospitals often are situated in a less competitive health care market and studies have indicated that the pricing transparency impact tends to be minimal when the provide competition is weak, which is a representative characteristic of rural healthcare markets.[251] Therefore, we believe this proposed rule imposes minimal operational and/or economic impacts on small rural hospitals.
The analysis above, together with the remainder of this preamble, provides a regulatory flexibility analysis and a regulatory impact analysis.
K. Unfunded Mandates Reform Act Analysis
Section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA) also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. That threshold level is currently approximately $154 million. This proposed rule does not mandate any requirements for State, local, or tribal governments, or for the private sector.
L. Reducing Regulation and Controlling Regulatory Costs
Executive Order 13771, titled Reducing Regulation and Controlling Regulatory Costs, was issued on January 30, 2017. It has been determined that this proposed rule, if finalized, would be a regulatory action for the purposes of Executive Order 13771. We estimate that this proposed rule would generate $7.85 million in annualized cost at a 7-percent discount rate, discounted relative to 2016, over a perpetual time horizon.
M. Conclusion
The changes we are proposing to make in this proposed rule would affect all classes of hospitals paid under the OPPS and would affect both CMHCs and ASCs. We estimate that most classes of hospitals paid under the OPPS would experience a modest increase or a minimal decrease in payment for services furnished under the OPPS in CY 2020. Table 41 demonstrates the estimated distributional impact of the OPPS budget neutrality requirements that would result in a 2.0 percent increase in payments for all services paid under the OPPS in CY 2020, after considering all of the proposed changes to APC reconfiguration and recalibration, as well as the proposed OPD fee schedule increase factor, proposed wage index changes, including the proposed frontier State wage index adjustment, estimated payment for outliers, the finalized off-campus provider-based department clinic visits payment policy, and proposed changes to the pass-through payment estimate. However, some classes of providers that are paid under the OPPS would experience more significant gains or losses in OPPS payments in CY 2020.
The proposed updates to the ASC payment system for CY 2020 would affect each of the approximately 5,600 ASCs currently approved for participation in the Medicare program. The effect on an individual ASC would depend on its mix of patients, the proportion of the ASC's patients who are Medicare beneficiaries, the degree to which the payments for the procedures offered by the ASC are changed under the ASC payment system, and the extent to which the ASC provides a different set of procedures in the coming year. Table 42 demonstrates the estimated distributional impact among ASC surgical specialties of the proposed MFP-adjusted hospital market basket update factor of 2.7 percent for CY 2020.
XXVII. Federalism Analysis
Executive Order 13132 establishes certain requirements that an agency must meet when it promulgates a proposed rule (and subsequent final rule) that imposes substantial direct costs on State and local governments, preempts State law, or otherwise has federalism implications. We have examined the OPPS and ASC provisions included in this proposed rule in accordance with Executive Order 13132, Federalism, and have determined that they will not have a substantial direct effect on State, local or tribal governments, preempt State law, or otherwise have a federalism implication. As reflected in Table 41 of this proposed rule, we estimate that OPPS payments to governmental hospitals (including State and local governmental hospitals) would increase by 1.9 percent under this proposed rule. While we do not know the number of ASCs or CMHCs with government ownership, we anticipate that it is small. The analyses we have provided in this section of this proposed rule, in conjunction with the remainder of this document, demonstrate that this proposed rule is consistent with the regulatory philosophy and principles identified in Executive Order 12866, the RFA, and section 1102(b) of the Act.
This proposed rule would affect payments to a substantial number of small rural hospitals and a small number of rural ASCs, as well as other classes of hospitals, CMHCs, and ASCs, and some effects may be significant.
List of Subjects
42 CFR Part 405
- Administrative practice and procedure
- Health facilities
- Health professions
- Kidney diseases
- Medical devices
- Medicare
- Reporting and recordkeeping requirements
- Rural areas
- X-rays
42 CFR Part 410
- Diseases
- Health facilities
- Health professions
- Laboratories
- Medicare
- Reporting and recordkeeping requirements
- Rural areas
- X-rays
42 CFR Part 412
- Administrative practice and procedure
- Health facilities
- Medicare
- Puerto Rico
- Reporting and recordkeeping requirements
42 CFR Part 416
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 419
- Hospitals
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 486
- Definitions
- Medicare
- Organ procurement
45 CFR Part 180
- Definitions
- Hospitals
- Reporting and recordkeeping requirements
For reasons stated in the preamble of this document, the Centers for Medicare & Medicaid Services proposes to amend 42 CFR chapter IV as set forth below:
PART 405—FEDERAL HEALTH INSURANCE FOR THE AGED AND DISABLED
1. The authority citation for part 405 continues to read as follows:
2. Section 405.926 is amended by revising paragraph (t) to read as follows:
(t) A contractor's prior authorization determination with regard to—
(1) Durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS)); and
(2) Hospital outpatient department (OPD) services.
PART 410—SUPPLEMENTARY MEDICAL INSURANCE (SMI) BENEFITS
3. The authority citation for part 410 continues to read as follows:
4. Section 410.27 is amended by revising paragraph (a)(1)(iv) to read as follows:
(a) * * *
(1) * * *
(iv) Under the general supervision (or other level of supervision as specified by CMS for the particular service) of a physician or a nonphysician practitioner as specified in paragraph (g) of this section, subject to the following requirements:
(A) For services furnished in the hospital or CAH, or in an outpatient department of the hospital or CAH, both on and off-campus, as defined in § 413.65 of this subchapter, general supervision as defined in § 410.32(b)(3)(i).
(B) Certain therapeutic services and supplies may be assigned either direct supervision or personal supervision. For purposes of this section, direct supervision means that the physician or nonphysician practitioner must be immediately available to furnish assistance and direction throughout the performance of the procedure. It does not mean that the physician or nonphysician practitioner must be present in the room when the procedure is performed. And personal supervision means the definition specified at § 410.32(b)(3)(iii);
(C) Nonphysician practitioners may provide the required supervision of services that they may personally furnish in accordance with State law and all additional requirements, including those specified in §§ 410.71, 410.73, 410.74, 410.75, 410.76, and 410.77;
(D) For pulmonary rehabilitation, cardiac rehabilitation, and intensive cardiac rehabilitation services, direct supervision must be furnished by a doctor of medicine or a doctor of osteopathy, as specified in §§ 410.47 and 410.49, respectively; and
PART 412—PROSPECTIVE PAYMENT SYSTEMS FOR INPATIENT HOSPITAL SERVICES
5. The authority citation for part 412 is revised to read as follows:
6. Section 412.22 is amended by revising paragraphs (f)(1) and (2) to read as follows:
(f) * * *
(1) Continues to operate under the same terms and conditions, including the number of beds, unless the hospital is a children's hospital as defined at § 412.23(d), and square footage considered to be part of the hospital for purposes of Medicare participation and payment in effect on September 30, 1995; or
(2) In the case of a hospital that changes the terms and conditions under which it operates after September 30, 1995, but before October 1, 2003, continues to operate under the same terms and conditions, including the number of beds, unless the hospital is a children's hospital as defined at § 412.23(d), and square footage considered to be part of the hospital for purposes of Medicare participation and payment in effect on September 30, 2003.
PART 416—AMBULATORY SURGICAL SERVICES
7. The authority citation for part 416 continues to read as follows:
8. Section 416.171 is amended by adding paragraph (b)(4) to read as follows:
(b) * * *
(4) Notwithstanding paragraph (b)(2) of this section, low volume device-intensive procedures where the otherwise applicable payment rate calculated based on the standard methodology for device intensive procedures described in this paragraph (b) would exceed the payment rate for the equivalent service set under the payment system established under part 419 of this subchapter, for which the payment rate will be set at an amount equal to the amount under that payment system.
PART 419—PROSPECTIVE PAYMENT SYSTEM FOR HOSPITAL OUTPATIENT DEPARTMENT SERVICES
9. The authority citation for part 419 continues to read as follows:
10. Section 419.32 is amended by adding paragraph (b)(1)(iv)(B)( 11) to read as follows:
(b) * * *
(1) * * *
(iv) * * *
(B) * * *
(11) For calendar year 2020, a multifactor productivity adjustment (as determined by CMS) and 0.75 percentage point.
11. Section 419.66 is amended by revising paragraph (c)(2) to read as follows:
(c) * * *
(2) CMS determines either of the following:
(i) The device to be included in the category has demonstrated that it will substantially improve the diagnosis or treatment of an illness or injury or improve the functioning of a malformed body part compared to the benefits of a device or devices in a previously established category or other available treatment; or
(ii) For applications received on or after January 1, 2020, as an alternative pathway to paragraph (c)(2)(i) of this section, the device has received FDA marketing authorization and is part of the FDA's Breakthrough Devices Program.
12. Subpart I, consisting of §§ 419.80 through 419.89, is added to read as follows:
SUBPART I—PRIOR AUTHORIZATION FOR OUTPATIENT DEPARTMENT SERVICES
- 419.80
- Basis and scope of this subpart.
- 419.81
- Definitions.
- 419.82
- Prior authorization for certain covered hospital outpatient department services.
- 419.83
- List of hospital outpatient department services requiring prior authorization.
- 419.84-419.89
- [Reserved]
(a) Basis. The provisions in this subpart are issued under the authority of section 1833(t)(2)(F) of the Act, which authorizes the Secretary to develop a method for controlling unnecessary increases in the volume of covered hospital outpatient department services.
(b) Scope. This subpart specifies the process and requirements for prior authorization for certain hospital outpatient department services as a condition of Medicare payment.
As used in this subpart, unless otherwise specified, the following definitions apply:
List of hospital outpatient department services requiring prior authorization means the list of hospital outpatient department services described in § 419.83(a) that CMS adopts in accordance with § 419.83(b) that require prior authorization as a condition of Medicare payment.
Prior authorization means the process through which a request for provisional affirmation of coverage is submitted to CMS or its contractors for review before the service is provided to the beneficiary and before the claim is submitted for processing.
Provisional affirmation means a preliminary finding that a future claim meets the Medicare coverage, coding, and payment rules in chapter IV of this title or in Title XVIII of the Social Security Act.
(a) Prior authorization as condition of payment. As a condition of Medicare payment for the services in the categories of services on the list of hospital outpatient department services requiring prior authorization as specified in § 419.83(a), a provider must submit to CMS or its contractors a prior authorization request in accordance with the requirements of paragraph (c) of this section.
(b) Denial of claim. (1) CMS or its contractors will deny a claim for a service that requires prior authorization if the provider has not received a provisional affirmation of coverage on the claim from CMS or its contractor unless the provider is exempt under § 419.83(c).
(2) CMS or its contractor may deny a claim that has received a provisional affirmation based on either of the following:
(i) Technical requirements that can only be evaluated after the claim has been submitted for formal processing; or
(ii) Information not available at the time of a prior authorization request.
(3) CMS or its contractor may deny claims for services related to services on the list of hospital outpatient department services for which the provider has received a denial.
(c) Submission of prior authorization request. A provider must submit to CMS or its contractor a prior authorization request for any service on the list of outpatient department services requiring prior authorization.
(1) Prior authorization request requirements. A prior authorization request must—
(i) Include all documentation necessary to show that the service meets applicable Medicare coverage, coding, and payment rules in chapter IV of this title or in Title XVIII of the Social Security Act.
(ii) Be submitted before the service is provided to the beneficiary and before the claim is submitted.
(2) Request for expedited review. A provider may submit a request for expedited review of a prior authorization request. The request for expedited review must comply with the requirements in paragraphs (c)(1)(i) and (ii) of this section and include documentation showing that the processing of the prior authorization request must be expedited due to the beneficiary's life, health, or ability to regain maximum function being in serious jeopardy.
(d) Reviews—(1) Review of prior authorization request. Upon receipt of a prior authorization request, CMS or its contractor will review the request for compliance with applicable Medicare coverage, coding, and payment rules in chapter IV of this title or in Title XVIII of the Social Security Act.
(i) CMS or its contractor will issue a provisional affirmation to the provider if it is determined that applicable Medicare coverage, coding, and payment rules in chapter IV of this title or in Title XVIII of the Social Security Act are met.
(ii) CMS or its contractor will issue a non-affirmation to the provider if it is determined that applicable Medicare coverage, coding, and payment rules in chapter IV of this title or in Title XVIII of the Social Security Act are not met.
(iii) The provisional affirmation or non-affirmation will be issued within 10 business days of receipt of the prior authorization request.
(2) Review of expedited review request. Upon receipt of a request for expedited review, CMS or its contractor will complete an expedited review of the prior authorization request if it is determined that a delay could seriously jeopardize the beneficiary's life, health, or ability to regain maximum function, and issue a provisional affirmation or non-affirmation decision in accordance with paragraph (d)(1) of this section within 2 business days of the expedited review request.
(e) Resubmission. (1) A provider may resubmit a prior authorization request, upon receipt of a non-affirmation, consistent with the requirements in paragraph (c)(1) of this section.
(2) A provider may resubmit a request for expedited review consistent with the requirements in paragraph (c)(1) of this section.
(a) Service categories for the list of hospital outpatient department services requiring prior authorization. (1) The following service categories comprise the list of hospital outpatient department services requiring prior authorization:
(i) Blepharoplasty.
(ii) Botulinum toxin injections.
(iii) Panniculectomy.
(iv) Rhinoplasty.
(v) Vein ablation.
(2) Technical updates to the list of services, such as changes to the name of the service or CPT code, will be published on the CMS website.
(b) Adoption of the list of services. CMS will adopt the list of hospital outpatient department service categories requiring prior authorization and any updates or geographic restrictions through formal notice-and-comment rulemaking.
(c) Exemptions. CMS may elect to exempt a provider from the prior authorization process in § 419.82 upon a provider's demonstration of compliance with Medicare coverage, coding, and payment rules in chapter IV of this title or in Title XVIII of the Social Security Act through such prior authorization process. An exemption will remain in effect until CMS elects to withdraw the exemption.
(d) Suspension of prior authorization process or services. CMS may suspend the outpatient department services prior authorization process requirements generally or for a particular service(s) at any time by issuing notification on the CMS website.
PART 486—CONDITIONS FOR COVERAGE OF SPECIALIZED SERVICES FURNISHED BY SUPPLIERS
13. The authority citation for part 486 is revised to read as follows:
14. Section 486.302 is amended by revising the definition of “Expected donation rate” to read as follows:
Expected donation rate means the expected donation rate per 100 eligible deaths that is the rate expected for an OPO based on the national experience for OPOs serving similar eligible donor populations and donation service areas. This rate is adjusted for the distributions of age, sex, race, and cause of death among eligible deaths.
15. Section 486.318 is amended by revising paragraphs (a)(2), (b)(2), and (c)(1) to read as follows:
(a) * * *
(2) The observed donation rate is not significantly lower than the expected donation rate for 18 or more months of the 36 months of data used for recertification, as calculated by the SRTR. For the 2022 recertification cycle, the observed donation rate is not significantly lower than the expected donation rate for 12 of the 24 months between January 1, 2020 and December 31, 2021, as calculated by the SRTR.
(b) * * *
(2) The observed donation rate is not significantly lower than the expected donation rate for 18 or more months of the 36 months of data used for recertification, as calculated by the SRTR. For the 2022 recertification cycle, the observed donation rate is not significantly lower than the expected donation rate for 12 of the 24 months between January 1, 2020 and December 31, 2021, as calculated by the SRTR.
(c) * * *
(1) Except as specified in paragraph (b)(2) of this section, an OPO's performance on the outcome measures is based on 36 months of data, beginning with January 1 of the first full year of the recertification cycle and ending 36 months later on December 31, 7 months prior to the end of the recertification cycle.
For reasons stated in the preamble of this document, under the authority 5 U.S.C. 301 and 42 U.S.C. 300gg-18, the Department of Health and Human Services proposes to amend title 45, subtitle A of the Code of Federal Regulations as set forth below:
16. Subchapter E, consisting of parts 180 through 199, is added to read as follows:
Title 45—Public Welfare
Subtitle A—Department of Health and Human Services
SUBCHAPTER E—PRICE TRANSPARENCY
181-199 [RESERVED]
PART 180—HOSPITAL PRICE TRANSPARENCY
- 180.10
- Basis and scope.
- 180.20
- Definitions.
- 180.30
- Applicability.
- 180.40
- General requirements.
- 180.50
- Requirements for making public hospital standard charges for all items and services.
- 180.60
- Requirements for making public payer-specific negotiated charges for selected shoppable services.
- 180.70
- Monitoring and enforcement.
- 180.80
- Corrective action plans.
- 180.90
- Civil monetary penalties.
- 180.100
- Appeal of penalty.
- 180.110
- Failure to request a hearing.
Subpart A—General Provisions
This part implements section 2718(e) of the Public Health Service (PHS) Act, which requires each hospital operating within the United States, for each year, to establish, update, and make public a list of the hospital's standard charges for items and services provided by the hospital, including for diagnosis-related groups established under section 1886(d)(4) of the Social Security Act. This part also implements section 2718(b)(3) of the PHS Act, to the extent that section authorizes CMS to promulgate regulations for enforcing section 2718(e).
The following definitions apply to this part, unless specified otherwise:
Ancillary service means an item or service a hospital customarily provides as part of or in conjunction with a shoppable primary service.
Chargemaster (Charge Description Master or CDM) means the list of all individual items and services maintained by a hospital for which the hospital has established a charge.
Gross charge means the charge for an individual item or service that is reflected on a hospital's chargemaster, absent any discounts.
Hospital means an institution in any State in which State or applicable local law provides for the licensing of hospitals, that is licensed as a hospital pursuant to such law or is approved, by the agency of such State or locality responsible for licensing hospitals, as meeting the standards established for such licensing. For purposes of this definition, a State includes each of the several States, the District of Columbia, Puerto Rico, the Virgin Islands, Guam, American Samoa, and the Northern Mariana Islands.
Items and services means all items and services, including individual items and services and service packages, that could be provided by a hospital to a patient in connection with an inpatient admission or an outpatient department visit for which the hospital has established a standard charge. Examples include, but are not limited to, supplies, procedures, room and board, use of the facility and other items (generally described as facility fees), services of employed physicians and non-physician practitioners (generally reflected as professional charges), and any other items or services for which a hospital has established a standard charge.
Machine-readable format means a digital representation of data or information in a file that can be imported or read into a computer system for further processing. Examples of machine-readable formats include, but are not limited to, .XML, JSON and .CSV formats.
Payer-specific negotiated charge means the charge that a hospital has negotiated with a third party payer for an item or service.
Service package means an aggregation of individual items and services into a single service with a single charge.
Shoppable service means a service package that can be scheduled by a health care consumer in advance.
Standard charge means the regular rate established by the hospital for an item or and service provided to a specific group of paying patients
Third party payer means an entity that is, by statute, contract, or agreement, legally responsible for payment of a claim for a health care item or service.
(a) General applicability. Except as provided in paragraph (b) of this section, the requirements of this part apply to hospitals as defined at § 180.20.
(b) Exception. Federally owned or operated hospitals are deemed by CMS to be in compliance with the requirements of this part including but not limited to:
(1) Federally owned hospital facilities, including hospitals operated by the U.S. Department of Veterans Affairs and Military Treatment Facilities operated by the U.S. Department of Defense.
(2) Hospitals operated by an Indian Health Program as defined in section 4(12) of the Indian Health Care Improvement Act.
(c) Online availability. Unless otherwise stated, hospital charge information must be made public electronically via the internet.
Subpart B—Public Disclosure Requirements
A hospital must make public the following:
(a) A machine-readable file containing a list of all standard charges for all items and services as provided in § 180.50.
(b) A consumer-friendly list of payer-specific negotiated charges for a limited set of shoppable services as provided in § 180.60.
(a) General rules. (1) A hospital must establish, update, and make public a list of all standard charges for all items and services online in the form and manner specified in this section.
(2) Each hospital location operating under a single hospital license (or approval) that has a different set of standard charges than the other location(s) operating under the same hospital license (or approval) must separately meet the requirements of this section.
(b) Required data elements. A hospital must include all of the following corresponding data elements in its list of standard charges, as applicable:
(1) Description of each item or service provided by the hospital.
(2) Gross charge that applies to each individual item or service when provided in, as applicable, the hospital inpatient setting and outpatient department setting.
(3) Payer-specific negotiated charge that applies to each item or service when provided in, as applicable, the hospital inpatient setting and outpatient department setting. Each list of payer-specific charges must be clearly associated with the name of the third party payer.
(4) Any code used by the hospital for purposes of accounting or billing for the item or service, including, but not limited to, the Current Procedural Terminology (CPT) code, the Healthcare Common Procedure Coding System (HCPCS) code, the Diagnosis Related Group (DRG), the National Drug Code (NDC), or other common payer identifier.
(5) Revenue codes, as applicable.
(c) Format. The information described in paragraph (b) of this section must be published in a single digital file that is in a machine-readable format.
(d) Location and accessibility. (1) A hospital may select a publicly available website for purposes of making public the standard charge information required under this section.
(2) The standard charge information must be displayed in a prominent manner and clearly identified with the hospital location with which the standard charge information is associated.
(3) The hospital must ensure that the standard charge data is easily accessible, without barriers, including but not limited to ensuring the data is accessible:
(i) Free of charge,
(ii) Without having to establish a user account or password; and
(iii) Without having to submit personal identifying information (PII).
(4) The digital file and standard charge information contained in it must be digitally searchable.
(e) Frequency of updates. The hospital must update the standard charge information described in paragraph (b) of this section at least once annually. The hospital must clearly indicate the date that the standard charge data was most recently updated, either within the file itself or otherwise clearly associated with the file.
(a) General rules. (1) A hospitals must make public its payer-specific negotiated charges for as many of the 70 CMS-selected shoppable services that are provided by the hospital, and as many additional hospital-selected shoppable services as is necessary for a combined total of at least 300 shoppable services.
(2) [Reserved]
(b) Required data elements. A hospital must include, as applicable, all of the following corresponding data elements when displaying its payer-specific negotiated charges for the shoppable services selected under paragraph (a) of this section:
(1) A plain-language description of each shoppable service.
(2) The payer-specific negotiated charge that applies to each shoppable service. For shoppable services not provided by the hospital, the charge must be indicated as “N/A”. Each payer-specific charge must be clearly associated with the name of the third party payer.
(3) A list of all associated ancillary items and services that the hospital provides with the shoppable service, including the payer-specific negotiated charge for each ancillary item or service.
(4) The location at which the shoppable service is provided, including whether the payer-specific negotiated charge for the shoppable service applies at that location to the provision of that shoppable service in the inpatient setting or the outpatient department setting or both.
(5) Any primary code used by the hospital for purposes of accounting or billing for the shoppable service, including, as applicable, the Current Procedural Terminology (CPT) code, the Healthcare Common Procedure Coding System (HCPCS) code, the Diagnosis Related Group (DRG), or other common service billing code.
(c) Format. (1) A hospital has discretion to choose a format for making public the information described in paragraph (b) of this section online.
(2) The hospital must make the information described in paragraph (b) of this section in written format upon request within 72 hours of the request.
(d) Location and accessibility of online data. (1) A hospital has discretion to select an appropriate publicly available internet location for purposes of making public the standard charge information required under this section.
(2) The standard charge information must be displayed in a prominent manner that identifies the hospital location with which the standard charge information is associated.
(3) The standard charge data must be easily accessible, without barriers, including but not limited to ensuring the data is:
(i) Free of charge.
(ii) Accessible without having to register or establish a user account or password.
(iii) Accessible without having to submit personal identifying information (PII).
(iv) Searchable by service description, billing code, and payer.
(e) Frequency. The hospital must update the standard charge information described in paragraph (b) of this section at least once annually. The hospital must clearly indicate the date that the information was most recently updated.
Subpart C—Monitoring and Penalties for Noncompliance
(a) Monitoring. (1) CMS evaluates whether a hospital has complied with the requirements under §§ 180.40, 180.50, and 180.60.
(2) CMS may use methods to monitor and assess hospital compliance with the requirements under this part, including, but not limited to, the following, as appropriate:
(i) CMS' evaluation of complaints made by individuals or entities to CMS.
(ii) CMS review of individuals' or entities' analysis of noncompliance.
(iii) CMS audit of hospitals' websites.
(b) Actions to address hospital noncompliance. If CMS concludes that the hospital is noncompliant with one or more of the requirements of § 180.40, § 180.50, or § 180.60, CMS may take any of the following actions, which generally, but not necessarily, will occur in the following order:
(1) Provide a written warning notice to the hospital of the specific violation(s).
(2) Request a corrective action plan from the hospital if its noncompliance constitutes a material violation of one or more requirements, according to § 180.80.
(3) Impose a civil monetary penalty on the hospital and publicize the penalty on a CMS website according to § 180.90 if the hospital fails to respond to CMS' request to submit a corrective action plan or comply with the requirements of a corrective action plan.
(a) Material violations requiring a corrective action plan. CMS determines if a hospital's noncompliance with the requirements of this part constitutes material violation(s) requiring a corrective action plan. A material violation may include, but is not limited to, the following:
(1) A hospital's failure to make public its standard charges required by § 180.40.
(2) A hospital's failure to make public its standard charges in the form and manner required under §§ 180.50 and 180.60.
(b) Notice of violation. CMS may request that a hospital submit a corrective action plan, specified in a notice of violation issued by CMS to a hospital.
(c) Compliance with corrective action plan requests and corrective actions. (1) A hospital required to submit a corrective action plan must do so, in the form and manner, and by the deadline, specified in the notice of violation issued by CMS to the hospital and must comply with the requirements of the corrective action plan.
(2) A hospital's corrective action plan must specify elements including, but not limited to:
(i) The deficiency or deficiencies that caused noncompliance to occur.
(ii) The corrective actions or processes the hospital will take to come into compliance with the requirements of this part.
(iii) The timeframe by which the hospital will complete the corrective action.
(3) A corrective action plan is subject to CMS review and approval.
(4) After CMS' review and approval of a hospital's corrective action plan, CMS may monitor and evaluate the hospital's compliance with the corrective actions.
(d) Noncompliance with corrective action plan requests and requirements. (1) A hospital's failure to respond to CMS' request to submit a corrective action plan includes failure to submit a corrective action plan in the form, manner, or by the deadline, specified in a notice of violation issued by CMS to the hospital.
(2) A hospital's failure to comply with the requirements of a corrective action plan includes failure to correct violation(s) within the specified timeframes.
(a) Basis for imposing civil monetary penalties. CMS may impose a civil monetary penalty on a hospital identified as noncompliant according to § 180.70, and that fails to respond to CMS' request to submit a corrective action plan or comply with the requirements of a corrective action plan as described in § 180.80(d).
(b) Notice of imposition of a civil monetary penalty. (1) If CMS imposes a penalty in accordance with this part, CMS provides a written notice of imposition of a civil monetary penalty to the hospital via certified mail or another form of traceable carrier.
(2) This notice to the hospital may include, but is not limited to, the following:
(i) The basis for the hospital's noncompliance, including, but not limited to, the following:
(A) CMS' determination as to which requirement(s) the hospital has violated.
(B) The hospital's failure to respond to CMS' request to submit a corrective action plan or comply with the requirements of a corrective action plan, as described in § 180.80(d).
(ii) CMS' determination as to the effective date for the violation(s). This date is the latest date of the following:
(A) The first day the hospital is required to meet the requirements of this part.
(B) If a hospital previously met the requirements of this part but did not update the information annually as required, the date 12 months after the date of the last annual update specified in information posted by the hospital.
(C) A date determined by CMS, such as one resulting from monitoring activities specified in § 180.70, or development of a corrective action plan as specified in § 180.80.
(iii) The amount of the penalty as of the date of the notice.
(iv) A statement that a civil monetary penalty may continue to be imposed for continuing violation(s).
(v) Payment instructions.
(vi) Intent to publicize the hospital's noncompliance and CMS' determination to impose a civil monetary penalty on the hospital for noncompliance with the requirements of this part by posting the notice of imposition of a civil monetary penalty on a CMS website.
(vii) A statement of the hospital's right to a hearing according to subpart D of this part.
(viii) A statement that the hospital's failure to request a hearing within 30 calendar days of the issuance of the notice permits the imposition of the penalty, and any subsequent penalties pursuant to continuing violations, without right of appeal in accordance with § 180.110.
(3) If the civil monetary penalty is upheld, in part, by a final and binding decision according to subpart D of this part, CMS will issue a modified notice of imposition of a civil monetary penalty.
(c) Amount of the civil monetary penalty. (1) CMS may impose a civil monetary penalty upon a hospital for a violation of each requirement of this part.
(2) The maximum daily dollar amount for a civil monetary penalty to which a hospital may be subject is $300. Even if the hospital is in violation of multiple discrete requirements of this part, the maximum total sum that a single hospital may be assessed per day is $300.
(3) The amount of the civil monetary penalty will be adjusted annually using the multiplier determined by OMB for annually adjusting civil monetary penalty amounts under part 102 of this title.
(d) Timing of payment of civil monetary penalty. (1) A hospital must pay the civil monetary penalty in full within 60 calendar days after the date of the notice of imposition of a civil monetary penalty from CMS under paragraph (b) of this section.
(2) In the event a hospital requests a hearing, pursuant to subpart D of this part, the hospital must pay the amount in full within 60 calendar days after the date of a final and binding decision, according to subpart D of this part, to uphold, in whole or in part, the civil monetary penalty.
(3) If the 60th calendar day described paragraphs (d)(1) and (2) of this section is a weekend or a Federal holiday, then the timeframe is extended until the end of the next business day.
(e) Posting of notice. (1) CMS will post the notice of imposition of a civil monetary penalty described in paragraphs (b) and (f) of this section on a CMS website.
(2) In the event that a hospital elects to request a hearing, pursuant to subpart D of this part:
(i) CMS will indicate in its posting, under paragraph (e)(1) of this section, that the civil monetary penalty is under review.
(ii) If the civil monetary penalty is upheld, in whole, by a final and binding decision according to subpart D of this part, CMS will maintain the posting of the notice of imposition of a civil monetary penalty on a CMS website.
(iii) If the civil monetary penalty is upheld, in part, by a final and binding decision according to subpart D of this part, CMS will issue a modified notice of imposition of a civil monetary penalty according to paragraph (b)(3) of this section, and will make this modified notice public on a CMS website.
(iv) If the civil monetary penalty is overturned in full by a final and binding decision according to subpart D of this part, CMS will remove the notice of imposition of a civil monetary penalty from a CMS website.
(f) Continuing violations. CMS may issue subsequent notice(s) of imposition of a civil monetary penalty, according to paragraph (b) of this section, that result from the same instance(s) of noncompliance.
Subpart D—Appeals of Civil Monetary Penalties
(a) A hospital upon which CMS has imposed a penalty under this part may appeal that penalty in accordance with subpart D of part 150 of this title, except as specified in paragraph (b) of this section.
(b) For purposes of applying subpart D of part 150 of this title to appeals of civil monetary penalties under this part:
(1) Civil money penalty means a civil monetary penalty according to § 180.90.
(2) Respondent means a hospital that received a notice of imposition of a civil monetary penalty according to § 180.90(b).
(3) References to a notice of assessment or proposed assessment, or notice of proposed determination of civil monetary penalties, are considered to be references to the notice of imposition of a civil monetary penalty specified in § 180.90(b).
(4) Under § 150.417(b) of this title, in deciding whether the amount of a civil money penalty is reasonable, the ALJ may only consider evidence of record relating to the following:
(i) The hospital's posting(s) of its standard charges, if available.
(ii) Material the hospital timely previously submitted to CMS (including with respect to corrective actions and corrective action plans).
(iii) Material CMS used to monitor and assess the hospital's compliance according to § 180.70(a)(2).
(5) The ALJ's consideration of evidence of acts other than those at issue in the instant case under § 150.445(g) of this title does not apply.
(a) If a hospital does not request a hearing within 30 calendar days of the issuance of the notice of imposition of a civil monetary penalty described in § 180.90(b), CMS may impose the civil monetary penalty indicated in such notice and may impose additional penalties pursuant to continuing violations according to § 180.90(f) without right of appeal in accordance with this part.
(1) If the 30th calendar day described paragraph (a) of this section is a weekend or a Federal holiday, then the timeframe is extended until the end of the next business day.
(2) [Reserved]
(b) The hospital has no right to appeal a penalty with respect to which it has not requested a hearing in accordance with § 150.405 of this title, unless the hospital can show good cause, as determined at § 150.405(b) of this title, for failing to timely exercise its right to a hearing.
181-199 [RESERVED]
Dated: June 21, 2019.
Seema Verma,
Administrator, Centers for Medicare and Medicaid Services.
Dated: June 24, 2019.
Alex M Azar II,
Secretary, Department of Health and Human Services.
Footnotes
1. 2011 product label available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022496s000lbl.pdf.
2. 2011 FDA approval letter available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022496Orig1s000Approv.pdf.
Back to Citation3. President's Commission on Combating Drug Addiction and the Opioid Crisis, Report (2017). Available at: https://www.whitehouse.gov/sites/whitehouse.gov/files/images/Final_Report_Draft_11-1-2017.pdf.
Back to Citation4. Ibid, at page 57, Recommendation 19.
Back to Citation5. Ibid.
Back to Citation6. Available at: https://www.hhs.gov/about/leadership/secretary/speeches/2017-speeches/secretary-price-announces-hhs-strategy-for-fighting-opioid-crisis/index.html.
Back to Citation7. Available at: https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html.
Back to Citation8. Available at: https://www.phe.gov/emergency/news/healthactions/phe/Pages/default.aspx.
Back to Citation9. Food and Drug Administration, Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee Briefing Document (2018). Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM596314.pdf.
Back to Citation10. Ibid, page 9.
Back to Citation11. 2018 updated product label available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022496s009lbledt.pdf.
Back to Citation12. Available at: http://www.medpac.gov/-documents-/reports.
Back to Citation13. Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. Impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: A pilot study. J Vasc Interv Radiol. 2015 May;26(5):660-9.
Back to Citation14. Kim AY, Frantz S, Krishnan P, DeMulder D, Caridi T, Lynskey GE, et al. (2017) Short-term imaging response after drug-eluting embolic trans- arterial chemoembolization delivered with the Surefire Infusion System® for the treatment of hepatocellular carcinoma. PloS one 12.9 (2017): e0183861.
Back to Citation15. N Apseloff, J Keung, T Caridi, D Buckley, G Lynskey, A Kim. Case-control evaluation of endhole microcatheter versus Surefire Infusion System for use during transarterial chemoembolization for hepatocellular carcinoma. Conference abstract presented at 2017 Society of Intervention Radiology Annual Congress, March 8, 2017.
Back to Citation16. Kapoor B, Contreras F, Katz M, Arepally A, Fischman A, Rose S, Kim A, Ferraro J. Surefire Infusion System (SIS) hepatocellular carcinoma registry study interim results: A multicenter study of the safety, feasibility, and outcomes of the SIS expandable-tip microcatheter in DEB-TACE.
Conference abstract presented at 2018 Society of Intervention Radiology Annual Congress, March 19, 2017.
Back to Citation17. Current Behavioral Neuroscience Reports. 2014 Jun; 1(2): 64-73.
Back to Citation18. “Decision Memo for Vagus Nerve Stimulation (VNS) for Treatment Resistant Depression (TRD) (CAG-00313R2).” Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=292&NCDId=230&ncdver=2&IsPopup=y&bc=AAAAAAAAQAAA&.
Back to Citation19. Ibid.
Back to Citation20. Ibid.
Back to Citation21. Aaronson ST, Carpenter LL, Conway CR, et al. Vagus Nerve Stimulation Therapy Randomized to Different Amounts of Electrical Charge for Treatment-Resistant Depression: Acute and Chronic Effects. Brain Stimul. 2013; 6(4):631-40.
Back to Citation22. Aaronson ST, Sears P, Ruvuna F, et al.: A 5-Year Observational Study of Patients With Treatment—Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am J Psychiatry. 2017; 174(7):640-648.
Back to Citation23. Conway CR, Kumar A, Xiong W, et al. Chronic Vagus Nerve Stimulation Significantly Improves Quality of Life in Treatment-Resistant Major Depression. J Clin Psychiatry. 2018; 79:e1-e7.
Back to Citation24. Olin B, Jayewardene AK, Bunker M, Moreno F. Mortality and Suicide Risk in Treatment-Resistant Depression: An Created on 04/12/2019. Page 19 of 49 Observational Study of the Long-Term Impact of Intervention. PLOS ONE. 2012.
Back to Citation25. Berry SM, Broglio K, Bunker M, Jayewardene A, Olin B, Rush AJ. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med Devices (Auckl). 2013.
Back to Citation26. Cimpianu C, Strube W, Falkai P, Palm U, Hasan A. Vagus nerve stimulation in psychiatry: A systematic review of the available evidence. J Neural Transm (Vienna). 2017.
Back to Citation27. Daban C., Martinez-Aran A., Cruz N., Vieta E. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J Affect Disord. 2008; 110(1-2):1-15.
Back to Citation28. Martin JLR and Martin-Sanchez E. Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: Variable results based on study designs. Eur Psychiatry. 2012; 27(3).
Back to Citation29. Kumar A, Bunker M, Aaronson S, Conway C, Rothschild A, Mordenti G, Rush A. Durability of symptomatic responses obtained with adjunctive vagus nerve stimulation in treatment-resistant depression. Neuropsychiatr Dis Treat. 2019:15 457-468.
Back to Citation30. Abraham, W.T., Kuck, K.H., Goldsmith, R.L., Lindenfeld, J., Reddy, V.Y., Carson, P.E., & Wiegn, P. (2018). A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC: Heart Failure, 6(10), 874-883.
Back to Citation31. Anker, S.D., Borggrefe, M., Neuser, H., Ohlow, M. A., Röger, S., Goette, A., & Rousso, B. Cardiac contractility modulation improves long-term survival and hospitalizations in heart failure with reduced ejection fraction. Eur J Heart Fail. 2019 Jan 16. doi: 10.1002/ejhf.1374. [Epub ahead of print]
Back to Citation32. Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M, Pieske B, Misier AR, Curnis A, Bocker D, Remppis A, Kautzner J, Stuhlinger M, Leclerq C, Taborsky M, Frigerio M, Parides M, Burkhoff D and Hindricks G. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29:1019-28.
Back to Citation33. Kloppe A, Lawo T, Mijic D, et al. Long-term survival with Cardiac Contractility Modulation in patients with NYHA II or III symptoms and normal QRS duration. Int J Cardiol. 2016 Apr 15;209:291-5.
Back to Citation34. Liu M, Fang F, Luo XX, Shlomo BH, Burkhoff D, Chan JY, Chan CP, Cheung L, Rousso B, Gutterman D, Yu CM. Improvement of long-term survival by cardiac contractility modulation in heart failure patients: A case-control study. Int J Cardiol. 2016 Mar 1;206:122-6.
Back to Citation35. Müller D, Remppis A, Schauerte P, et al. Clinical effects of long-term cardiac contractility modulation (CCM) in subjects with heart failure caused by left ventricular systolic dysfunction. Clin Res. Cardiol. 2017 Nov 1;106(11):893-904.
36. Kuschyk J, Roeger S, Schneider R, et al. Efficacy and survival in patients with cardiac contractility modulation: Long-term single center experience in 81 patients. Int J Cardiol. 2015;183C:76-81.
Back to Citation37. Chungtai B. Forde JC. Thomas DDM et al. Benign Prostatic Hyperplasia. Nature Reviews Disease Primers 2 (2016) article 16031.
Back to Citation38. Montorsi, F. et al.: Holmium Laser Enucleation Versus Transurethral Resection of The Prostate: Results from A 2-Center, Prospective, Randomized Trial In Patients With Obstructive Benign Prostatic Hyperplasia. J. Urol. 172, 1926-1929 (2004).
Back to Citation39. Bachmann A, et al.: 180-W XPS GreenLight laser vaporisation versus transurethral resection of the prostate for the treatment of benign prostatic obstruction: 6-month safety and efficacy results of a European Multicentre Randomised Trial—the GOLIATH study. Eur Urol, 2014;65(5):931-42.
Back to Citation40. Gilling P. Barber M. Anderson P et al.: WATER—A Double-Blind Randomized Controlled Trial of Aquablation vs Transurethal Resection of the Prostate in Benign Prostatic Hyperplasia. J Urol. Accepted December 29, 2017 doi 10.1016/j.juro.2017.12.065.
Back to Citation41. Neschis, David G. & MD, Golden, M., “Clinical features and diagnosis of lower extremity peripheral artery disease.” Available at: https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-lower-extremity-peripheral-artery-disease.
Back to Citation42. Centers for Disease Control and Prevention, “Peripheral Arterial Disease (PAD) Fact Sheet,” 2018, Available at: https://www.cdc.gov/DHDSP/data_statistics/fact_sheets/fs_PAD.htm.
Back to Citation43. Müller-Hülsbeck, S., et al., “Long-Term Results from the MAJESTIC Trial of the Eluvia Paclitaxel-Eluting Stent for Femoropopliteal Treatment: 3-Year Follow-up,” Cardiovasc Intervent Radiol, December 2017, vol. 40(12), pp. 1832-1838.
Back to Citation44. Gray, W.A., et al., “A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): A randomised, non-inferiority trial,” Lancet, September 24, 2018.
Back to Citation45. Forrester, J.S., Fishbein, M., Helfant, R., Fagin, J., “A paradigm for restenosis based on cell biology: Clues for the development of new preventive therapies,” J Am Coll Cardiol, March 1, 1991, vol. 17(3), pp. 758-69.
Back to Citation46. Gray, W.A., Keirse, K., Soga, Y., et al., “A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomized, non-inferiority trial,” Lancet, 2018. Available at: http://dx.doi.org/10.1016/S0140-6736(18)32262-1.
Back to Citation47. Katsanos, K., et al., “Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials,” JAHA, vol. 7(24).
Back to Citation48. Cassese, S., & Byrne, R.E., “Endovascular stenting in femoropopliteal arteries,” The Lancet, 2018, vol. 392(10157), pp. 1491-1493.
Back to Citation49. Greaser M, Ellington JK. 2014. “Ankle arthritis.” Journal of Arthritis, 3:129. doi:10.4172/2167-7921.1000129.
Back to Citation50. Punzi, Leonardo et al. 2016. “Post-traumatic arthritis: overview on pathogenic mechanisms and role of inflammation.” Rheumatic & Musculoskeletal Diseases. RMD open, 2 (2), e000279. doi:10.1136/rmdopen-2016-000279.
Back to Citation51. Ibid.
Back to Citation52. Lareau, Craig R. et al. 2015.”Does autogenous bone graft work? A logistic regression analysis of data from 159 papers in the foot and ankle literature.” Foot and Ankle Surgery. 21(3): 150-59.
Back to Citation53. DiGiovanni CW, Lin SS, Baumbauer JF, et al. 2013. “Recombinant Human Platelet-Derived Growth Factor-BB and Beta-Tricalcium Phosphate (rhPDGF-BB/b-TCP): An Alternative to Autogenous Bone Graft.” J Bone Joint Surg Am., 95: 1184-92.
Back to Citation54. Herscovici, D., Scaduto, J.M. 2012. “Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis.” The Journal of Bone & Joint Surgery. 94-B: 75-9.
Back to Citation55. Stavrakis, AL., SooHoo, NF. 2016. “Trends in complication rates following ankle arthrodesis and total ankle replacement.” The Journal of Bone & Joint Surgery. JBJS 1453-1458.
Back to Citation56. Due to the timing of the application, the AUGMENT® Bone Graft cost values were calculated using the 2018 proposed rule data.
Back to Citation57. Medicare Payment Advisory Committee. June 2005 Report to the Congress. Chapter 6: Payment for pharmacy handling costs in hospital outpatient departments. Available at: http://www.medpac.gov/docs/default-source/reports/June05_ch6.pdf?sfvrsn=0.
Back to Citation58. American Hosp. Ass'n, et al. v. Azar, et al., No. 1:18-cv-2084 (D.D.C. Dec. 27, 2018).
Back to Citation59. Id. at 35 (quoting Amgen, Inc. v. Smith, 357 F.3d 103, 112 (D.C. Cir. 2004) (citations omitted)).
Back to Citation60. See May 6, 2019 Memorandum Opinion, Granting in Part Plaintiffs' Motion for a Permanent Injunction; Remanding the 2018 and 2019 OPPS Rules to HHS at 10-12.
Back to Citation61. Id. at 13.
Back to Citation62. Id. at 19.
Back to Citation63. Id. (citing Declaration of Elizabeth Richter).
Back to Citation64. 348 F. Supp. 3d 62, 81 (D.D.C. 2018) (citing to payment reductions of 0.2 percent and 2.9 percent that other decisions have recognized as being within the agency's adjustment authority for Medicare rates under the inpatient prospective payment system).
Back to Citation65. Each revenue code on the CMHC claim must have a HCPCS code and charge associated with it. We multiply each claim service line's charges by the CMHC's overall CCR from the OPSF (or statewide CCR, where the overall CCR was greater than 1) to estimate CMHC costs. Only the claims service lines containing PHP allowable HCPCS codes and PHP allowable revenue codes from the CMHC claims remaining after trimming are retained for CMHC cost determination. The costs, payments, and service units for all service lines occurring on the same service date, by the same provider, and for the same beneficiary are summed. CMHC service days must have 3 or more services provided to be assigned to CMHC APC 5853. The proposed geometric mean per diem cost for CMHC APC 5853 is calculated by taking the n th root of the product of n numbers, for days where 3 or more services were provided. CMHC service days with costs ±3 standard deviations from the geometric mean costs within APC 5853 are deleted and removed from modeling. The remaining PHP service days are used to calculate the proposed geometric mean per diem cost for each PHP APC by taking the n th root of the product of n numbers for days where 3 or more services were provided.
Back to Citation66. Each revenue code on the hospital-based PHP claim must have a HCPCS code and charge associated with it. We multiply each claim service line's charges by the hospital's department-level CCR; in CY 2020 and subsequent years, that CCR is determined by using the PHP-only revenue-code-to-cost-center crosswalk. Only the claims service lines containing PHP-allowable HCPCS codes and PHP-allowable revenue codes from the hospital-based PHP claims remaining after trimming are retained for hospital-based PHP cost determination. The costs, payments, and service units for all service lines occurring on the same service date, by the same provider, and for the same beneficiary are summed. Hospital-based PHP service days must have 3 or more services provided to be assigned to hospital-based PHP APC 5863. The proposed geometric mean per diem cost for hospital-based PHP APC 5863 is calculated by taking the n th root of the product of n numbers, for days where 3 or more services were provided. Hospital-based PHP service days with costs ±3 standard deviations from the geometric mean costs within APC 5863 are deleted and removed from modeling. The remaining hospital-based PHP service days are used to calculate the proposed geometric mean per diem cost for hospital-based PHP APC 5863.
Back to Citation67. As discussed in section II.A. of this CY 2020 OPPS/ASC proposed rule, proposed OPPS APC geometric mean per diem costs (including proposed PHP APC geometric mean per diem costs) are divided by the proposed geometric mean per diem costs for APC 5012 (Clinic Visits and Related Services) to calculate each PHP APC's unscaled relative payment weight. An unscaled relative payment weight is one that is not yet adjusted for budget neutrality. Budget neutrality is required under section 1833(t)(9)(B) of the Act, and ensures that the estimated aggregate weight under the OPPS for a calendar year is neither greater than nor less than the estimated aggregate weight that would have been made without the changes. To adjust for budget neutrality (that is, to scale the weights), we compare the estimated aggregated weight using the scaled relative payment weights from the previous calendar year at issue. We refer readers to the ratesetting procedures described in Part 2 of the OPPS Claims Accounting narrative and in section II. of this proposed rule for more information on scaling the weights, and for details on the final steps of the process that lead to proposed PHP APC per diem payment rates. The OPPS Claims Accounting narrative is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices.html.
Back to Citation68. Medicare Payment Advisory Committee. March 2019 Report to the Congress. Chapter 5: Ambulatory surgical center services. Available at: http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch5_sec.pdf?sfvrsn=0.
Back to Citation69. Medicare Payment Advisory Committee. March 2019 Report to the Congress. Chapter 5: Ambulatory surgical center services. Available at: http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch5_sec.pdf?sfvrsn=0.
Back to Citation70. We initially referred to this process as “retirement” of a measure in the 2010 OPPS/ASC proposed rule, but later changed it to “removal” during final rulemaking.
Back to Citation71. We refer readers to the CY 2013 OPPS/ASC final rule with comment period (77 FR 68472 through 68473) for a discussion of our reasons for changing the term “retirement” to “removal” in the Hospital OQR Program.
Back to Citation72. We note that we previously referred to these factors as “criteria” (for example, 77 FR 68472 through 68473); we now use the term “factors” in order to align the Hospital OQR Program terminology with the terminology we use in other CMS quality reporting and pay-for-performance (value-based purchasing) programs.
Back to Citation73. 80 FR 70508.
Back to Citation74. National Quality Forum. NQF #1822 External Beam Radiotherapy for Bone Metastases. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=70374.
Back to Citation75. QualityNet. 2018 EBRT Measure Information Form. Available at: https://www.qualitynet.org/dcs/ContentServer?cid=1228774479863&pagename=QnetPublic%2FPage%2FQnetTier4&c=Page.
Back to Citation76. See language about measure steward no longer maintaining this measure in the FY 2020 IPPS/LTCH PPS proposed rule at 84 FR 19502 through 19503.
Back to Citation77. ASCQR Specifications Manual, discussing these measures, available at: http://qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier2&cid=1228772475754.
Back to Citation78. Centers for Medicare & Medicaid Services. Meaningful Measures Hub. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/MMF/General-info-Sub-Page.html.
Back to Citation79. National Quality Forum. 0265 All-Cause Hospital Transfer/Admission. Available at: http://www.qualityforum.org/QPS/0265.
Back to Citation80. National Quality Forum. 0263 Patient Burn. Available at: http://www.qualityforum.org/QPS/0263.
Back to Citation81. National Quality Forum. 0267 Wrong Site, Wrong Side, Wrong Patient, Wrong Procedure, Wrong Implant. Available at: http://www.qualityforum.org/QPS/0267.
Back to Citation82. National Quality Forum. 0266 Patient Fall. Available at: http://www.qualityforum.org/QPS/0266.
Back to Citation83. ASC Quality Collaboration. Quality measures developed and tested by the ASC Quality Collaboration. Available at: http://ascquality.org/documents/2019-Summary-ASC-QC-Measures.pdf.
Back to Citation84. National Quality Forum. Serious Reportable Events in Healthcare 2006 Update. Washington, DC: NQF, 2007. Available at: https://www.qualityforum.org/Publications/2007/03/Serious_Reportable_Events_in_Healthcare%E2%80%932006_Update.aspx.
Back to Citation85. ECRI Institute. New clinical guide to surgical fire prevention. Health Devices 2009 Oct;38(10):314-32.
86. 170. National Fire Protection Association (NFPA). NFPA 99: Standard for health care facilities. Quincy (MA): NFPA; 2005.
Back to Citation87. ASC Quality Collaboration. Quality measures developed and tested by the ASC Quality Collaboration. Available at: http://ascquality.org/documents/2019-Summary-ASC-QC-Measures.pdf.
Back to Citation88. National Quality Forum. Serious Reportable Events in Healthcare—2006 Update: A Consensus Report. March 2007. Available at: https://www.qualityforum.org/Publications/2007/03/Serious_Reportable_Events_in_Healthcare%E2%80%932006_Update.aspx.
Back to Citation89. Boushon B, Nielsen G, Quigley P, Rutherford P, Taylor J, Shannon D. Transforming Care at the Bedside How-to Guide: Reducing Patient Injuries from Falls. Cambridge, MA: Institute for Healthcare Improvement; 2008.
Back to Citation90. ASC Quality Collaboration. Quality measures developed and tested by the ASC Quality Collaboration. Available at: http://ascquality.org/documents/2019-Summary-ASC-QC-Measures.pdf.
Back to Citation91. National Quality Forum. Serious Reportable Events in Healthcare—2006 Update: A Consensus Report. March 2007. Available at: https://www.qualityforum.org/Publications/2007/03/Serious_Reportable_Events_in_Healthcare%E2%80%932006_Update.aspx.
Back to Citation92. American College of Obstetricians and Gynecologists. ACOG committee opinion #464: patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786-790.
Back to Citation93. ASC Quality Collaboration. Quality measures developed and tested by the ASC Quality Collaboration. Available at: http://ascquality.org/documents/2019-Summary-ASC-QC-Measures.pdf.
Back to Citation94. Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. J Clin Anesth. 2002 Aug; 14(5):349-53.
95. Junger A, Klasen J, Benson M, Sciuk G, Hartmann B, Sticher J, Hempelmann G. Factors determining length of stay of surgical day-case patients. Eur J Anaesthesiol. 2001 May;18(5):314-21.
Back to Citation96. We note that we previously referred to these factors as “criteria” (for example, 79 FR 66967 through 66969); we now use the term “factors” in order to align the ASCQR Program terminology with the terminology we use in other CMS quality reporting and pay-for-performance (value-based purchasing) programs.
Back to Citation97. Cullen KA, Hall MJ, Golosinskiy A, Statistics NCfH. Ambulatory surgery in the United States, 2006. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2009.
98. Medicare Payment Advisory Committee. Report for the Congress: Medicare Payment Policy, March 2019. http://medpac.gov/docs/default-source/reports/mar19_medpac_entirereport_sec.pdf?sfvrsn=0. Accessed May 24, 2019.
Back to Citation99. Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. Journal of clinical anesthesia. 2002;14(5):349-353.
100. Bain J, Kelly H, Snadden D, Staines H. Day surgery in Scotland: patient satisfaction and outcomes. Quality in Health Care. 1999;8(2):86-91.
Back to Citation101. Majholm BB. Is day surgery safe? A Danish multicentre study of morbidity after 57,709 day surgery procedures. Acta anaesthesiologica Scandinavica. 2012;56(3):323-331.
102. Whippey A, Kostandoff G, Paul J, Ma J, Thabane L, Ma HK. Predictors of unanticipated admission following ambulatory surgery: a retrospective case-control study. Canadian Journal of Anesthesia/Journal canadien d'anesthésie. 2013;60(7):675-683.
103. Fleisher LA, Pasternak LR, Herbert R, Anderson GF. Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67-72.
104. Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. Journal of clinical anesthesia. 2002;14(5):349-353.
105. Hollingsworth JMJM. Surgical quality among Medicare beneficiaries undergoing outpatient urological surgery. The Journal of urology. 2012;188(4):1274-1278.
106. Bain J, Kelly H, Snadden D, Staines H. Day surgery in Scotland: patient satisfaction and outcomes. Quality in Health Care. 1999;8(2):86-91.
107. Fortier J, Chung F, Su J. Unanticipated admission after ambulatory surgery—a prospective study. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 1998;45(7):612-619.
108. Aldwinckle R, Montgomery J. Unplanned admission rates and postdischarge complications in patients over the age of 70 following day case surgery. Anaesthesia. 2004;59(1):57-59.
Back to Citation109. Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. Journal of clinical anesthesia. 2002;14(5):349-353.
110. Hollingsworth JMJM. Surgical quality among Medicare beneficiaries undergoing outpatient urological surgery. The Journal of urology. 2012;188(4):1274-1278.
111. Baugh RR. Safety of outpatient surgery for obstructive sleep apnea. Otolaryngology—head and neck surgery. 2013;148(5):867-872.
112. Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: Demographics and perioperative outcomes. The Laryngoscope. 2010;120(3):635-638.
113. Bhattacharyya NN. Unplanned revisits and readmissions after ambulatory sinonasal surgery. The Laryngoscope. 2014;124(9):1983-1987.
114. Bhattacharyya NN. Revisits and postoperative hemorrhage after adult tonsillectomy. The Laryngoscope. 2014;124(7):1554-1556.
115. Hansen DG, Abbott LE, Johnson RM, Fox JP. Variation in hospital-based acute care within 30 days of outpatient plastic surgery. Plastic and reconstructive surgery (1963). 2014;134(3):370e-378e.
116. Mahboubi HH. Ambulatory laryngopharyngeal surgery: evaluation of the national survey of ambulatory surgery. JAMA otolaryngology— head & neck surgery. 2013;139(1):28-31.
117. Orosco RKRK. Ambulatory thyroidectomy: a multistate study of revisits and complications. Otolaryngology—head and neck surgery. 2015;152(6):1017-1023.
Back to Citation118. Baugh RR. Safety of outpatient surgery for obstructive sleep apnea. Otolaryngology—head and neck surgery. 2013;148(5):867-872.
119. Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: Demographics and perioperative outcomes. The Laryngoscope. 2010;120(3):635-638.
120. Bhattacharyya NN. Unplanned revisits and readmissions after ambulatory sinonasal surgery. The Laryngoscope. 2014;124(9):1983-1987.
121. Bhattacharyya NN. Revisits and postoperative hemorrhage after adult tonsillectomy. The Laryngoscope. 2014;124(7):1554-1556.
122. Hansen DG, Abbott LE, Johnson RM, Fox JP. Variation in hospital-based acute care within 30 days of outpatient plastic surgery. Plastic and reconstructive surgery (1963). 2014;134(3):370e-378e.
123. Mahboubi HH. Ambulatory laryngopharyngeal surgery: evaluation of the national survey of ambulatory surgery. JAMA otolaryngology— head & neck surgery. 2013;139(1):28-31.
124. Menachemi. Quality of care differs by patient characteristics: outcome disparities after ambulatory surgical procedures. American journal of medical quality. 2007;22(6):395-401.
125. Orosco RKRK. Ambulatory thyroidectomy: a multistate study of revisits and complications. Otolaryngology—head and neck surgery. 2015;152(6):1017-1023.
Back to Citation126. 83 FR 58820 through 58822.
Back to Citation127. National Quality Forum. List of Measures under Consideration for December 1, 2017. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=86526.
Back to Citation128. National Quality Forum. MAP 2018 Considerations for Implementing Measures: Hospitals—Final Report. Available at: http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=87096.
Back to Citation129. Ibid.
Back to Citation130. Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33(1):159-174.
Back to Citation131. National Quality Forum. Facility-Level 7-Day Hospital Visits after General Surgery Procedures Performed at Ambulatory Surgical Centers. Available at: http://www.qualityforum.org/QPS/3357.
Back to Citation132. National Quality Forum. “MAP 2018 Considerations for Implementing Measures in Federal Programs: Hospitals.” Report. 2017. Available at: http://www.qualityforum.org/map/ under “Hospitals—Final Report.”
Back to Citation133. The Centers for Medicare and Medicaid Services Center for Clinical Standards and Quality. “2018 Measures under Consideration List: Program-Specific Measure Needs and Priorities”. Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Downloads/2018-CMS-Measurement-Priorities-and-Needs.pdf. Accessed February 28, 2019.
Back to Citation134. Fleisher LA, Pasternak LR, Herbert R, Anderson GF. Inpatient hospital admission and death after outpatient surgery in elderly patients: Importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67-72.
135. Mattila K, Toivonen J, Janhunen L, Rosenberg PH, Hynynen M. Postdischarge symptoms after ambulatory surgery: First-week incidence, intensity, and risk factors. Anesthesia and analgesia. 2005;101(6):1643-1650.
Back to Citation136. Krumholz HM, Brindis RG, Brush JE, et al. Standards for Statistical Models Used for Public Reporting of Health Outcomes An American Heart Association Scientific Statement From the Quality of Care and Outcomes Research Interdisciplinary Writing Group: Cosponsored by the Council on Epidemiology and Prevention and the Stroke Council Endorsed by the American College of Cardiology Foundation. Circulation. 2006;113(3):456-462.
137. Normand S-LT, Shahian DM. Statistical and clinical aspects of hospital outcomes profiling. Statistical Science. 2007;22(2):206-226.
138. National Quality Forum. Measure Evaluation Criteria and Guidance for Evaluating Measures for Endorsement. 2015. Available at: http://www.qualityforum.org/Measuring_Performance/Submitting_Standards/2015_Measure_Evaluation_Criteria.aspx. Accessed July 26, 2016.
Back to Citation139. Centers for Medicare and Medicaid Services. “Ambulatory Surgical Center (ASC) Payment: Addenda Updates.” Available at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/ascpayment/11_addenda_updates.html.
Back to Citation140. Ibid.
Back to Citation141. Healthcare Cost and Utilization Project. Clinical Classifications Software for Services and Procedures. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp.
Back to Citation142. S. Coberly. The Basics; Relative Value Units (RVUs). National Health Policy Forum. January 12, 2015. Available at: http://www.nhpf.org/library/the-basics/Basics_RVUs_01-12-15.pdf.
Back to Citation143. Hosmer DW, Lemeshow S. Introduction to the logistic regression model. Applied Logistic Regression, Second Edition. 2000:1-30.
Back to Citation144. Hosmer DW, Lemeshow S. Introduction to the logistic regression model. Applied Logistic Regression, Second Edition. 2000:1-30.
Back to Citation145. Ibid.
Back to Citation146. Snijders TA, Bosker RJ. Multilevel Analysis: An introduction to basic and advanced multilevel modeling. SAGE Publications. 2000. London.
Back to Citation147. ASC Quality Collaboration. ASC Quality Measures Implementation Guide Version 6.1 March 2019. Available at: http://ascquality.org/documents/ASC-QC-Implementation-Guide-6.1-March-2019.pdf.
Back to Citation148. Ibid.
Back to Citation149. Ibid.
Back to Citation150. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Downloads/FAQs-Req-Hospital-Public-List-Standard-Charges.pdf and https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ProspMedicareFeeSvcPmtGen/Downloads/Additional-Frequently-Asked-Questions-Regarding-Requirements-for-Hospitals-To-Make-Public-a-List-of-Their-Standard-Charges-via-the-internet.pdf.
Back to Citation151. CMS. National Health Expenditures Projections, 2018-2027: Forecast Summary. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/ForecastSummary.pdf.
Back to Citation152. Scheurer, D. Lack of Transparency Plagues U.S. Health Care System. The Hospitalist. 2013 May; 2013(5). Available at: https://www.the-hospitalist.org/hospitalist/article/125866/health-policy/lack-transparency-plagues-us-health-care-system. Bees, J. Survey Snapshot: Is Transparency the Answer to Rising Health Care Costs? New England Journal of Medicine Catalyst. March 20, 2019. Available at: https://catalyst.nejm.org/health-care-cost-transparency-answer/. Wetzell, S. Transparency: A Needed Step Towards Health Care Affordability. American Health Policy Institute. March, 2014. Available at: http://www.americanhealthpolicy.org/Content/documents/resources/Transparency%20Study%201%20-%20The%20Need%20for%20Health%20Care%20Transparency.pdf. Robert Wood Johnson Foundation. How Price Transparency Can Control the Cost of Health Care. March 1, 2016. Available at: https://www.rwjf.org/en/library/research/2016/03/how-price-transparency-controls-health-care-cost.html.
Back to Citation153. Sinaiko, A.D., Mehrotra, A., & Sood, N. (2016). Cost-Sharing Obligations, High-Deductible Health Plan Growth, and Shopping for Health Care: Enrollees with Skin in the Game. JAMA internal medicine, 176(3), 395-397. doi:10.1001/jamainternmed.2015.7554.
Back to Citation154. Boynton A, and Robinson, JC. Appropriate Use of Reference Pricing Can Increase Value. July 7, 2015. Available at: https://www.healthaffairs.org/do/10.1377/hblog20150707.049155/full/.
Back to Citation155. Azar, A.M., Mnuchin, S.T., and Acosta, A. “Reforming America's Healthcare System Through Choice and Competition.” December 3, 2018. Available at: https://www.hhs.gov/sites/default/files/Reforming-Americas-Healthcare-System-Through-Choice-and-Competition.pdf.
Back to Citation156. Bresnick J. Verma: Price Transparency Rule a “First Step” for Consumerism. January 11, 2019. Available at: https://healthpayerintelligence.com/news/verma-price-transparency-rule-a-first-step-for-consumerism.
Back to Citation157. Congressional Research Service Report to Congress: Does Price Transparency Improve Market Efficiency? Implications of Empirical Evidence in Other Markets for the Healthcare Sector, July 24, 2007.
Back to Citation158. Ibid.
Back to Citation159. Ibid.
Back to Citation160. Available at: https://www.gao.gov/products/GAO-11-791.
Back to Citation161. Desai S, Hatfield LA, Hicks AL, et al. Association Between Availability of a Price Transparency Tool and Outpatient Spending. JAMA. 2016;315(17):1874-1881. Available at: https://jamanetwork.com/journals/jama/fullarticle/2518264.
Back to Citation162. Kullgren JT, Duey KA, Werner RM. A census of state health care price transparency websites. JAMA. 2013;309(23):2437-2438. Available at: https://jamanetwork.com/journals/jama/fullarticle/1697957.
Back to Citation163. Ibid.
Back to Citation164. Ibid.
Back to Citation165. Available at: https://oshpd.ca.gov/data-and-reports/cost-transparency/hospital-chargemasters/2018-chargemasters/.
Back to Citation166. Jenkins K. CMS Price Transparency Push Trails State Initiatives. The National Law Review. February 8, 2019. Available at: https://www.natlawreview.com/article/cms-price-transparency-push-trails-state-initiatives.
Back to Citation167. Ibid.
Back to Citation168. Available at: http://oregonhospitalguide.org/ and http://oregonhospitalguide.org/understanding-the-data/procedure-costs.html.
Back to Citation169. Jenkins K. CMS Price Transparency Push Trails State Initiatives. The National Law Review. February 8, 2019. Available at: https://www.natlawreview.com/article/cms-price-transparency-push-trails-state-initiatives.
Back to Citation170. Brown, ZY. What would happen if hospitals openly shared their prices? The Conversation. January 30, 3019. Available at: https://theconversation.com/what-would-happen-if-hospitals-openly-shared-their-prices-110352. And http://www-personal.umich.edu/~zachb/zbrown_empirical_model_price_transparency.pdf.
Back to Citation171. FY 2019 IPPS/LTCH PPS proposed rule (83 FR 20164); CY 2019 Home Health proposed rule (83 FR 32473); CY 2020 ESRD PPS proposed rule (83 FR 34394); CY 2020 PFS proposed rule (83 FR 36009); and CY 2019 OPPS/ASC proposed rule (83 FR 37211).
Back to Citation172. The July 2014 letters are available at: https://www.cms.gov/CCIIO/Resources/Letters/index.html#Health%20Market%20Reforms.
Back to Citation173. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ProspMedicareFeeSvcPmtGen/Downloads/Additional-Frequently-Asked-Questions-Regarding-Requirements-for-Hospitals-To-Make-Public-a-List-of-Their-Standard-Charges-via-the-internet.pdf.
Back to Citation174. Section 1680r(b) of the Indian Health Care Improvement Act (25 U.S.C. 1680r).
Back to Citation175. VA cost-sharing information available at: https://www.va.gov/HEALTHBENEFITS/cost/copays.asp.
Back to Citation176. MTF cost-sharing information available at: https://tricare.mil/Costs/Compare and https://comptroller.defense.gov/Portals/45/documents/rates/fy2019/2019_ia.pdf.
Back to Citation177. Hammer, David C. “Adapting customer service to consumer-directed health care: by implementing new tools that provide greater transparency in billing, hospitals can decrease collection costs while improving consumer satisfaction.” Healthcare Financial Management, Sept. 2006, p. 118+. Academic OneFile, Accessed 8 July 2019. https://www.mckesson.com/documents/providers/hfma---adapting-customer-service-to-consumer-directed-health-care/.
Back to Citation178. https://www.healthaffairs.org/doi/10.1377/hlthaff.25.1.45.
Back to Citation179. https://www.healthaffairs.org/doi/10.1377/hlthaff.25.1.81.
Back to Citation180. https://repository.library.georgetown.edu/handle/10822/556896.
Back to Citation181. FY 2019 IPPS/LTCH PPS proposed rule (83 FR 20164); CY 2019 Home Health proposed rule (83 FR 32473); CY 2020 ESRD PPS proposed rule (83 FR 34394); CY 2020 PFS proposed rule (83 FR 36009); and CY 2019 OPPS/ASC proposed rule (83 FR 37211).
Back to Citation182. Bai G and Anderson GF. Market Power: Price Variation Among Commercial Insurers for Hospital Services. Health Affairs. Oct 2018; 37(10): 1615-1622. Available at: https://www.healthaffairs.org/doi/10.1377/hlthaff.2018.0567.
Back to Citation183. Bai G and Anderson GF. Extreme Markup: The Fifty US Hospitals With The Highest Charge-To-Cost Ratios. Health Affairs. Jun 2015; 34(6): 922-928. Available at: https://www.healthaffairs.org/doi/10.1377/hlthaff.2014.1414.
184. Batty M and Ippolito B. Mystery of The Chargemaster: Examining The Role Of Hospital List Prices in What Patients Actually Pay. Health Affairs. April 2017; 36(4): 689-696. Available at: https://www.healthaffairs.org/doi/10.1377/hlthaff.2016.0986.
Back to Citation185. https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-264.pdf.
Back to Citation186. https://pdfs.semanticscholar.org/f604/1a0484c65c593525d0c07e040cf655697f2d.pdf.
Back to Citation187. Beck, M. How to Cut Your Health-Care Bill: Pay Cash. The Wall Street Journal. February 15, 2016. Available at: https://www.wsj.com/articles/how-to-cut-your-health-care-bill-pay-cash-1455592277.
188. Rosato, D. How Paying Your Doctor in Cash Could Save You Money. Consumer Reports. May 4, 2018. Available at: https://www.consumerreports.org/healthcare-costs/how-paying-your-doctor-in-cash-could-save-you-money/.
189. Terhune, C. Many hospitals, doctors offer cash discount for medical bills. Los Angeles Times. March 27, 2012. Available at: https://www.latimes.com/business/healthcare/la-fi-medical-prices-20120527-story.html.
190. https://khn.org/news/an-arm-and-a-leg-can-you-shop-around-for-a-lower-priced-mri/.
Back to Citation191. https://pdfs.semanticscholar.org/f604/1a0484c65c593525d0c07e040cf655697f2d.pdf.
Back to Citation192. Kaiser Family Foundation. Available at: https://www.kff.org/uninsured/press-release/the-number-of-uninsured-people-rose-in-2017-reversing-some-of-the-coverage-gains-under-the-affordable-care-act/.
Back to Citation193. Beck, M. How to Cut Your Health-Care Bill: Pay Cash. The Wall Street Journal. February 15, 2016. Available at: https://www.wsj.com/articles/how-to-cut-your-health-care-bill-pay-cash-1455592277.
Back to Citation194. https://www.hhs.gov/cto/initiatives/index.html.
Back to Citation195. https://developer.cms.gov/.
Back to Citation196. https://webstandards.hhs.gov/guidelines/49.
Back to Citation197. Nielsen, J. (2003, November 10). The ten most violated homepage design guidelines. Alertbox. Available at: http://www.useit.com/alertbox/20031110.html.
Back to Citation198. https://webstandards.hhs.gov/guidelines/78.
Back to Citation199. https://webstandards.hhs.gov/guidelines/88.
Back to Citation200. https://webstandards.hhs.gov/guidelines/181.
Back to Citation201. White, Chapin and Eguchi, Megan. Reference Pricing: A Small Piece of the Health Care Price and Quality Puzzle. National Institute for Health Care Reform Research Brief Number 18 (2014). https://nihcr.org/wp-content/uploads/2016/07/Research_Brief_No._18.pdf.
Back to Citation202. https://nihcr.org/wp-content/uploads/2016/07/Research_Brief_No._18.pdf.
Back to Citation203. Consistent with 45 CFR 153.700, in States where HHS is operating the risk adjustment program, issuers must submit enrollment, claims, and encounter data for risk adjustment-covered plans in the individual and small group markets through the External Data Gathering Environment (EDGE) servers. Issuers upload enrollee, pharmaceutical claim, medical claim, and supplemental diagnosis information from their systems to an issuer-owned and controlled EDGE server.
Back to Citation204. found here: https://plainlanguage.gov/guidelines/.
Back to Citation205. https://webstandards.hhs.gov/guidelines/49.
Back to Citation206. Nielsen, J. (2003, November 10). The ten most violated homepage design guidelines. Alertbox. Available at: http://www.useit.com/alertbox/20031110.html.
Back to Citation207. https://webstandards.hhs.gov/guidelines/78.
Back to Citation208. https://webstandards.hhs.gov/guidelines/88.
Back to Citation209. https://webstandards.hhs.gov/guidelines/181.
Back to Citation210. FY 2019 IPPS/LTCH PPS proposed rule (83 FR 20164); CY 2019 Home Health proposed rule (83 FR 32473); CY 2020 ESRD PPS proposed rule (83 FR 34394); CY 2020 PFS proposed rule (83 FR 36009); and CY 2019 OPPS/ASC proposed rule (83 FR 37211).
Back to Citation211. Available at: https://www.organdonor.gov/about-dot/acot/acotrecs55.html.
Back to Citation212. Available at: https://www.srtr.org/about-the-data/technical-methods-for-the-opo-specific-reports/.
Back to Citation213. Changing Metrics of Organ Procurement Organization Performance in order to Increase Organ Donation Rates in the United States, Am J Transplant, 2017 Dec; 17(12): 3183-3192. doi: 10.1111/ajt. 14391. Epub 2017 Jul20.
Back to Citation214. Medicare Benefit Policy Manual. Internet Only. Publication 100-02, Chapter 16, § 120.
Back to Citation215. The data reviewed are maintained in the CMS Integrated Data Repository (IDR). The IDR is a high-volume data warehouse integrating Medicare Parts A, B, C, and D, and DME claims, beneficiary and provider data sources, along with ancillary data such as contract information and risk scores. Additional information is available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/IDR/index.html.
Back to Citation216. The 5.8 percent average increase per year in overall health care spending was arrived at using data publicly available on the Bureau of Labor and Statistics web page, located at: https://www.bls.gov/cpi/factsheets/medical-care.htm.
Back to Citation217. 80 FR 81674 (December 30, 2015).
Back to Citation218. https://www.cms.gov/research-statistics-data-and-systems/downloadable-public-use-files/cost-reports/.
Back to Citation219. Occupational Employment and Wages, May 2018. Available at: https://www.bls.gov/ooh/healthcare/medical-records-and-health-information-technicians.htm. Accessed May 7, 2019.
Back to Citation220. Batty, M., & Ippolito, B. (2017). Mystery of the chargemaster: Examining the role of hospital list prices in what patients actually pay. Health Affairs, 36 (4), 689-696. Available at: https://www.healthaffairs.org/doi/10.1377/hlthaff.2016.0986.
Back to Citation221. American Hospital Association. Fast Facts on U.S. Hospitals, 2019. Available at: https://www.aha.org/statistics/fast-facts-us-hospitals.
Back to Citation222. Bureau of Labor Statistics. National Occupational Employment and Wage Estimates, May 2018. Available at: http://www.bls.gov/oes/2018/may/oes_nat.htm.
Back to Citation223. Bureau of Labor Statistics. Occupational Employment and Wage Estimates, May 2018: 23-1011 Lawyers. Available at: https://www.bls.gov/oes/current/oes231011.htm.
Back to Citation224. Bureau of Labor Statistics. Occupational Employment and Wage Estimates, May 2018: 11-1021 General and Operations Managers. Available at: https://www.bls.gov/oes/current/oes111021.htm.
Back to Citation225. Bureau of Labor Statistics. Occupational Employment and Wages, May 2018: 13-1199 Business Operations Specialist, All Other. Available at: https://www.bls.gov/oes/current/oes131199.htm.
Back to Citation226. Bureau of Labor Statistics. Occupational Employment and Wages, May 2018: 15-1142 Network and Computer System Administrators. Available at: https://www.bls.gov/oes/current/oes151142.htm.
Back to Citation227. Kennedy K., et al. Health Care Cost Institute. Past the Price Index: Exploring Actual Prices Paid for Specific Services by Metro Area. Healthy Marketplace Index. April 30, 2019. Available at: https://www.healthcostinstitute.org/blog/entry/hmi-2019-service-prices.
Back to Citation228. Cooper Z., et al. The Price Ain't Right? Hospital Prices and Health Spending on the Privately Insured. The Quarterly Journal of Economics. December 2015. Available at: https://pdfs.semanticscholar.org/cb9c/f90786cc39ddac6d88f3ba1074a7c2d5f0a5.pdf.
229. Bai, G., & Anderson, G.F. (2018). Market Power: Price Variation Among Commercial Insurers For Hospital Services. Health Affairs, 37(10), 1615-1622. Available at: https://www.healthaffairs.org/doi/10.1377/hlthaff.2018.0567.
Back to Citation230. Cooper Z., et al. The Price Ain't Right? Hospital Prices and Health Spending on the Privately Insured. The Quarterly Journal of Economics. December 2015. Available at: https://pdfs.semanticscholar.org/cb9c/f90786cc39ddac6d88f3ba1074a7c2d5f0a5.pdf.
Back to Citation231. Ibid.
Back to Citation232. Ho K. & Lee R.S. Insurer Competition and Negotiated Hospital Prices. August 2013. https://pdfs.semanticscholar.org/b6e9/11d7e171d3074b473439f93d377f4a4202bf.pdf
Back to Citation233. Brown Z.Y. An Empirical Model of Price Transparency and Markups in Health Care. August 2018. http://www-personal.umich.edu/~zachb/zbrown_empirical_model_price_transparency.pdf.
Back to Citation234. Christensen H.B., Floyd E., and Maffett M. “The Effects of Price Transparency Regulation on Prices in the Healthcare Industry.” Available at: https://www.bakerinstitute.org/media/files/event/01ce2e80/HPF-paper-AHEC-Floyd.pdf.
Back to Citation235. Brown Z.Y. 2018. “Equilibrium Effects of Health Care Price Information,” Review of Economics and Statistics. Available at: https://www.mitpressjournals.org/doi/abs/10.1162/rest_a_00765.
Back to Citation236. Congressional Research Service Report for Congress: Does Price Transparency Improve Market Efficiency? Implications of Empirical Evidence in Other Markets for the Health Sector. July 24, 2007. Available at: https://fas.org/sgp/crs/secrecy/RL34101.pdf.
Back to Citation237. Tu H, and Gourevitch R. California HealthCare Foundation and Robert Wood Johnson Foundation. Moving Markets, Lessons from the New Hampshire Price Transparency Experiment. April 2014. Available at: https://www.chcf.org/wp-content/uploads/2017/12/PDF-MovingMarketsNewHampshire.pdf.
Back to Citation238. Barry M, and Ippolito B. Mystery Of The Chargemaster: Examining The Role Of Hospital List Prices In What Patients Actually Pay. Health Affairs. April, 2017. Available at: https://www.healthaffairs.org/doi/10.1377/hlthaff.2016.0986.
Back to Citation239. See Gruessner V. Consumer Satisfaction Dips When Payers Lack Price Transparency. Private Payers New s (October 3, 2016). Available at: https://healthpayerintelligence.com/news/consumer-satisfaction-dips-when-payers-lack-price-transparency.
Back to Citation240. Experian Health, Improve the healthcare financial journey. Patient Engagement (June 21, 2018). Available at: https://www.experian.com/blogs/healthcare/2018/06/healthcare-financial-journey/.
Back to Citation241. Government Accountability Office. September 2011. Health Care Price Transparency: Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Available at: https://www.gao.gov/assets/590/585400.pdf.
Back to Citation242. Whaley C., et al. “Association Between Availability of Health Service Prices and Payments for These Services.” JAMA. 2014;312(16):1670-1676. Available at https://jamanetwork.com/journals/jama/fullarticle/1917438.
Back to Citation243. Lieber EMJ. “Does It Pay to Know Prices in Health Care?” American Economic Journal. 2017, 9(1): 154-179. Available at: https://pubs.aeaweb.org/doi/pdfplus/10.1257/pol.20150124.
Back to Citation244. Brot-Goldberg ZC, et al. What Does a Deductible Do? The Impact of Cost-Sharing on Health Care Prices, Quantities, and Spending Dynamics. Cambridge, MA: National Bureau of Economic Research; Working Paper, October 2015. Available at: https://www.nber.org/papers/w21632.pdf.
Back to Citation245. Desai S, et al. Association between availability of a price transparency tool and outpatient spending. JAMA. 2016;315(17):1874-1881. doi:10.1001/jama.2016.4288.
Back to Citation246. Chernew M, et al. “Are Health Care Services Shoppable? Evidence from the Consumption of Lower-Limb MRI Scans”. National Bureau of Economic Research, Working Paper No. 24869. Issued July 2018, revised January 2019. Available at: https://www.nber.org/papers/w24869.
Back to Citation247. Meluch AL, & Oglesby WH. (2015). Physician-patient communication regarding patients' healthcare costs in the US: A systematic review of the literature. Journal of Communication in Healthcare, 8(2), 151-160. Available at: https://www.tandfonline.com/doi/full/10.1179/1753807615Y.0000000010?scroll=top&needAccess=true.
Back to Citation248. Schiavoni KH, et al. How Primary Care Physicians Integrate Price Information into Clinical Decision-Making, J Gen Intern Medicine. 2017 January; 32(1): 81-87. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5215149/.
249. Alexander GC, et al. Barriers to Patient-physician Communication About Out-of-pocket Costs, J Gen Intern Med. 2004 August; 19(8): 856-860. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1492500/.
Back to Citation250. Goetz C, et al. The effect of charge display on cost of care and physician practice behaviors: A systematic review, Journal Gen Intern Med. 2015 Jun; 30(6):835-42. Available at: https://www.ncbi.nlm.nih.gov/pubmed/25691240.
Back to Citation251. Tu, Ha. Impact of HealthCare Price Transparency on Price Variation: The New Hampshire Experience. Center for Studying Health System Change Issue Brief, November, 2009. and Christiansen, Hans et al. The Effects of Price Transparency Regulation on Prices in the Healthcare Industry, The Baker Institute, 2013.
Back to CitationBILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
[FR Doc. 2019-16107 Filed 7-29-19; 4:15 pm]
BILLING CODE 4120-01-P
