AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Final rule.
SUMMARY:
This final rule updates the prospective payment rates, the outlier threshold, and the wage index for Medicare inpatient hospital services provided by Inpatient Psychiatric Facilities (IPFs), which include psychiatric hospitals and excluded psychiatric units of an inpatient prospective payment system hospital or critical access hospital. Additionally, this final rule revises and rebases the IPF market basket to reflect a 2016 base year and removes the IPF Prospective Payment System (PPS) 1-year lag of the wage index data. Finally, this final rule implements updates to the Inpatient Psychiatric Facilities Quality Reporting Program. These changes will be effective for IPF discharges beginning during the fiscal year (FY) from October 1, 2019 through September 30, 2020 (FY 2020).
DATES:
These regulations are effective on October 1, 2019.
FOR FURTHER INFORMATION CONTACT:
The IPF Payment Policy mailbox at IPFPaymentPolicy@cms.hhs.gov for general information.
Mollie Knight, (410) 786-7948 or Hudson Osgood, (410) 786-7897, for information regarding the market basket rebasing, update, or the labor related share.
Theresa Bean, (410) 786-2287 or James Hardesty, (410) 786-2629, for information regarding the regulatory impact analysis.
James Poyer, (410) 786-2261 or Jeffrey Buck, (410) 786-0407, for information regarding the inpatient psychiatric facility quality reporting program.
SUPPLEMENTARY INFORMATION:
Availability of Certain Tables Exclusively Through the Internet on the CMS Website
Addendum A to this final rule summarizes the FY 2020 IPF PPS payment rates, outlier threshold, cost of living adjustment factors for Alaska and Hawaii, national and upper limit cost-to-charge ratios, and adjustment factors. In addition, the B Addenda to this final rule show the complete listing of ICD-10 Clinical Modification (CM) and Procedure Coding System codes underlying the Code First table (Addendum B-1), the FY 2020 IPF PPS comorbidity adjustment (Addenda B-2 and B-3), and electroconvulsive therapy (ECT) procedure codes (Addendum B-4). The A and B addenda are available online at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html.
Tables setting forth the FY 2020 Wage Index for Urban Areas Based on Core-Based Statistical Area (CBSA) Labor Market Areas and the FY 2020 Wage Index Based on CBSA Labor Market Areas for Rural Areas are available exclusively through the internet, on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/IPFPPS/WageIndex.html. In addition, Addendum C to this final rule is a provider-level file of the effects of the change to the wage index methodology, and is available at the same CMS website address.
I. Executive Summary
A. Purpose
This final rule updates the prospective payment rates, the outlier threshold, and the wage index for Medicare inpatient hospital services provided by Inpatient Psychiatric Facilities (IPFs) for discharges occurring during the Fiscal Year (FY) beginning October 1, 2019 through September 30, 2020. Additionally, this final rule rebases and revises the IPF market basket to reflect a 2016 base year and uses the concurrent hospital wage data as the basis of the IPF wage index rather than using the prior year's Inpatient Prospective Payment System (IPPS) hospital wage data. Finally, this final rule updates the Inpatient Psychiatric Facility Quality Reporting (IPFQR) Program.
B. Summary of the Major Provisions
1. Inpatient Psychiatric Facilities Prospective Payment System (IPF PPS)
In this final rule we:
- Rebase and revise the IPF market basket to reflect a 2016 base year: Since the IPF PPS inception, the market basket used to update IPF PPS payments has been periodically rebased and revised to reflect more recent data on IPF cost structures. We last rebased and revised the market basket applicable to IPFs in the FY 2016 IPF PPS rule (80 FR 46656 through 46679), when we adopted a 2012-based IPF-specific market basket.
- Adjust the 2016-based IPF market basket update (2.9 percent) by a reduction for economy-wide productivity (0.4 percentage point) as required by section 1886(s)(2)(A)(i) of the Social Security Act (the Act). We further reduced the 2016-based IPF market basket update by 0.75 percentage point as required by section 1886(s)(2)(A)(ii) of the Act, resulting in an IPF payment rate update of 1.75 percent for FY 2020.
- Made technical rate setting changes: The IPF PPS payment rates are adjusted annually for inflation, as well as statutory and other policy factors. We updated:
++ The IPF PPS federal per diem base rate from $782.78 to $798.55.
++ The IPF PPS federal per diem base rate for providers who failed to report quality data to $782.85.
++ The Electroconvulsive therapy (ECT) payment per treatment from $337.00 to $343.79.
++ The ECT payment per treatment for providers who failed to report quality data to $337.03.
++ The labor-related share from 74.8 percent to 76.9 percent.
++ The core-based statistical area (CBSA) rural and urban wage indices for FY 2020, using the FY 2020 pre-floor, pre-reclassified IPPS hospital wage index data and OMB designations from OMB Bulletin 17-01.
++ The wage index budget-neutrality factor to 1.0026.
++ The fixed dollar loss threshold amount from $12,865 to $14,960 to maintain estimated outlier payments at 2 percent of total estimated aggregate IPF PPS payments.
- Eliminate the 1-year lag in the wage index data: We aligned the IPF wage index data with the concurrent IPPS wage index data by removing the 1-year lag of the pre-floor, pre-reclassified IPPS hospital wage index upon which the IPF wage index is based.
2. Inpatient Psychiatric Facilities Quality Reporting (IPFQR) Program
We updated the IPFQR Program by adding a new measure for the program.
C. Summary of Impacts
| Provision description | Total transfers & cost reductions |
|---|---|
| FY 2020 IPF PPS payment update | The overall economic impact of this final rule is an estimated $65 million in increased payments to IPFs during FY 2020. |
| Updated quality reporting program (IPFQR) Program requirements | $0. |
II. Background
A. Overview of the Legislative Requirements of the IPF PPS
Section 124 of the Medicare, Medicaid, and State Children's Health Insurance Program Balanced Budget Refinement Act of 1999 (BBRA) (Pub. L. 106-113) required the establishment and implementation of an IPF PPS. Specifically, section 124 of the BBRA mandated that the Secretary of the Department of Health and Human Services (the Secretary) develop a per diem PPS for inpatient hospital services furnished in psychiatric hospitals and excluded psychiatric units including an adequate patient classification system that reflects the differences in patient resource use and costs among psychiatric hospitals and excluded psychiatric units. “Excluded psychiatric unit” means a psychiatric unit in an IPPS hospital that is excluded from the IPPS, or a psychiatric unit in a Critical Access Hospital (CAH) that is excluded from the CAH payment system. These excluded psychiatric units would be paid under the IPF PPS.
Section 405(g)(2) of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (Pub. L. 108-173) extended the IPF PPS to psychiatric distinct part units of CAHs.
Sections 3401(f) and 10322 of the Patient Protection and Affordable Care Act (Pub. L. 111-148) as amended by section 10319(e) of that Act and by section 1105(d) of the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152) (hereafter referred to jointly as “the Affordable Care Act”) added subsection (s) to section 1886 of the Act.
Section 1886(s)(1) of the Act titled “Reference to Establishment and Implementation of System,” refers to section 124 of the BBRA, which relates to the establishment of the IPF PPS.
Section 1886(s)(2)(A)(i) of the Act requires the application of the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act to the IPF PPS for the rate year (RY) beginning in 2012 (that is, a RY that coincides with a FY) and each subsequent RY. As noted in our FY 2019 IPF PPS final rule with comment period, published in the Federal Register on August 6, 2018 (83 FR 38576 through 38620), for the RY beginning in 2018, the productivity adjustment currently in place is equal to 0.8 percentage point.
Section 1886(s)(2)(A)(ii) of the Act requires the application of an “other adjustment” that reduces any update to an IPF PPS base rate by a percentage point amount specified in section 1886(s)(3) of the Act for the RY beginning in 2010 through the RY beginning in 2019. As noted in the FY 2019 IPF PPS final rule, for the RY beginning in 2018, section 1886(s)(3)(E) of the Act requires that the other adjustment reduction currently in place be equal to 0.75 percentage point.
Sections 1886(s)(4)(A)-(D) of the Act require that for RY 2014 and each subsequent RY, IPFs that fail to report required quality data with respect to such a RY will have their annual update to a standard federal rate for discharges reduced by 2.0 percentage points. This may result in an annual update being less than 0.0 for a RY, and may result in payment rates for the upcoming RY being less than such payment rates for the preceding RY. Any reduction for failure to report required quality data will apply only to the RY involved, and the Secretary will not take into account such reduction in computing the payment amount for a subsequent RY. (See section II.C of this final rule for an explanation of the IPF PPS RY.) More information about the specifics of the current IPFQR Program is available in the FY 2019 IFP PPS and Quality Reporting Updates for Fiscal Year Beginning October 1, 2018 final rule (83 FR 38589 through 38608).
To implement and periodically update these provisions, we have published various proposed and final rules and notices in the Federal Register. For more information regarding these documents, see the Center for Medicare & Medicaid (CMS) website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/index.html?redirect=/InpatientPsychFacilPPS/.
B. Overview of the IPF PPS
The November 2004 IPF PPS final rule (69 FR 66922) established the IPF PPS, as required by section 124 of the BBRA and codified at 42 CFR part 412, subpart N. The November 2004 IPF PPS final rule set forth the federal per diem base rate for the implementation year (the 18-month period from January 1, 2005 through June 30, 2006), and provided payment for the inpatient operating and capital costs to IPFs for covered psychiatric services they furnish (that is, routine, ancillary, and capital costs, but not costs of approved educational activities, bad debts, and other services or items that are outside the scope of the IPF PPS). Covered psychiatric services include services for which benefits are provided under the fee-for-service Part A (Hospital Insurance Program) of the Medicare program.
The IPF PPS established the federal per diem base rate for each patient day in an IPF derived from the national average daily routine operating, ancillary, and capital costs in IPFs in FY 2002. The average per diem cost was updated to the midpoint of the first year under the IPF PPS, standardized to account for the overall positive effects of the IPF PPS payment adjustments, and adjusted for budget-neutrality.
The federal per diem payment under the IPF PPS is comprised of the federal per diem base rate described previously and certain patient- and facility-level payment adjustments for characteristics that were found in the regression analysis to be associated with statistically significant per diem cost differences, with statistical significance defined as p less than 0.05.
The patient-level adjustments include age, Diagnosis-Related Group (DRG) assignment, and comorbidities; additionally, there are adjustments to reflect higher per diem costs at the beginning of a patient's IPF stay and lower costs for later days of the stay. Facility-level adjustments include adjustments for the IPF's wage index, rural location, teaching status, a cost-of-living adjustment for IPFs located in Alaska and Hawaii, and an adjustment for the presence of a qualifying emergency department (ED).
The IPF PPS provides additional payment policies for outlier cases, interrupted stays, and a per treatment payment for patients who undergo electroconvulsive therapy (ECT). During the IPF PPS mandatory 3-year transition period, stop-loss payments were also provided; however, since the transition ended as of January 1, 2008, these payments are no longer available.
A complete discussion of the regression analysis that established the IPF PPS adjustment factors can be found in the November 2004 IPF PPS final rule (69 FR 66933 through 66936).
C. Annual Requirements for Updating the IPF PPS
Section 124 of the BBRA did not specify an annual rate update strategy for the IPF PPS and was broadly written to give the Secretary discretion in establishing an update methodology. Therefore, in the November 2004 IPF PPS final rule, we implemented the IPF PPS using the following update strategy:
- Calculate the final federal per diem base rate to be budget-neutral for the 18-month period of January 1, 2005 through June 30, 2006.
- Use a July 1 through June 30 annual update cycle.
- Allow the IPF PPS first update to be effective for discharges on or after July 1, 2006 through June 30, 2007.
In RY 2012, we proposed and finalized switching the IPF PPS payment rate update from a RY that begins on July 1 and ends on June 30, to one that coincides with the federal FY that begins October 1 and ends on September 30. In order to transition from one timeframe to another, the RY 2012 IPF PPS covered a 15-month period from July 1, 2011 through September 30, 2012. Therefore, the IPF RY has been equivalent to the October 1 through September 30 federal FY since RY 2013. For further discussion of the 15-month market basket update for RY 2012 and changing the payment rate update period to coincide with a FY period, we refer readers to the RY 2012 IPF PPS proposed rule (76 FR 4998) and the RY 2012 IPF PPS final rule (76 FR 26432).
In November 2004, we implemented the IPF PPS in a final rule that published on November 15, 2004 in the Federal Register (69 FR 66922). In developing the IPF PPS, and to ensure that the IPF PPS is able to account adequately for each IPF's case-mix, we performed an extensive regression analysis of the relationship between the per diem costs and certain patient and facility characteristics to determine those characteristics associated with statistically significant cost differences on a per diem basis. That regression analysis is described in detail in our November 28, 2003 IPF proposed rule (68 FR 66923; 66928 through 66933) and our November 15, 2004 IPF final rule (69 FR 66933 through 66960). For characteristics with statistically significant cost differences, we used the regression coefficients of those variables to determine the size of the corresponding payment adjustments.
In that final rule, we explained the reasons for delaying an update to the adjustment factors, derived from the regression analysis, including waiting until we have IPF PPS data that yields as much information as possible regarding the patient-level characteristics of the population that each IPF serves. We indicated that we did not intend to update the regression analysis and the patient-level and facility-level adjustments until we complete that analysis. Until that analysis is complete, we stated our intention to publish a notice in the Federal Register each spring to update the IPF PPS (69 FR 66966).
On May 6, 2011, we published a final rule in the Federal Register titled, “Inpatient Psychiatric Facilities Prospective Payment System—Update for Rate Year Beginning July 1, 2011 (RY 2012)” (76 FR 26432), which changed the payment rate update period to a RY that coincides with a FY update. Therefore, final rules are now published in the Federal Register in the summer to be effective on October 1. When proposing changes in IPF payment policy, a proposed rule would be issued in the spring, and the final rule in the summer to be effective on October 1. For a detailed list of updates to the IPF PPS, we refer readers to our regulations at 412.428.
The most recent IPF PPS annual update was published in a final rule on August 6, 2018 in the Federal Register titled, “Medicare Program; FY 2019 Inpatient Psychiatric Facilities Prospective Payment System and Quality Reporting Updates” (83 FR 38576), which updated the IPF PPS payment rates for FY 2019. That final rule updated the IPF PPS federal per diem base rates that were published in the FY 2018 IPF PPS Rate Update final rule (82 FR 36771) in accordance with our established policies.
III. Provisions of the FY 2020 IPF PPS Final Rule and Responses to Comments
On April 23, 2019 we published the FY 2020 IPF PPS proposed rule (84 FR 16948). We received 24 comments on the FY 2020 IPF PPS proposed rule, with some commenters addressing multiple issues. We received 4 comments on payment policy issues, 19 comments on quality issues, and 6 comments that were outside of the scope of the proposed rule.
A. Rebasing and Revising of the Market Basket for the IPF PPS
1. Background
Originally, the input price index used to develop the IPF PPS was the Excluded Hospital with Capital market basket. This market basket was based on 1997 Medicare cost reports for Medicare-participating inpatient rehabilitation facilities (IRFs), IPFs, long-term care hospitals (LTCHs), cancer hospitals, and children's hospitals. Although “market basket” technically describes the mix of goods and services used in providing health care at a given point in time, this term is also commonly used to denote the input price index (that is, cost category weights and price proxies) derived from that market basket. Accordingly, the term “market basket,” as used in this document, refers to an input price index.
Since the IPF PPS inception, the market basket used to update IPF PPS payments has been rebased and revised to reflect more recent data on IPF cost structures. We last rebased and revised the market basket applicable to the IPF PPS in the FY 2016 IPF PPS final rule (80 FR 46656 through 46679), where we adopted a 2012-based IPF market basket. The 2012-based IPF market basket used Medicare cost report data for both Medicare-participating freestanding psychiatric hospitals and hospital-based psychiatric units. References to the historical market baskets used to update IPF PPS payments are listed in the FY 2016 IPF PPS final rule (80 FR 46656). For the FY 2020 IPF PPS proposed rule, we proposed to rebase and revise the IPF market basket to reflect a 2016 base year.
2. Overview of the 2016-Based IPF Market Basket
The proposed 2016-based IPF market basket is a fixed-weight, Laspeyres-type price index. A Laspeyres price index measures the change in price, over time, of the same mix of goods and services purchased in the base period. Any changes in the quantity or mix of goods and services (that is, intensity) purchased over time relative to a base period are not measured.
The index itself is constructed in three steps. First, a base period is selected (for the proposed IPF market basket, the base period is 2016) and total base period expenditures are estimated for a set of mutually exclusive and exhaustive spending categories. Each category is calculated as a proportion of total costs. These proportions are called cost or expenditure weights. Second, each expenditure category is matched to an appropriate price or wage variable, referred to as a price proxy. In nearly every instance, these price proxies are derived from publicly available statistical series that are published on a consistent schedule (preferably at least on a quarterly basis). Finally, the expenditure weight for each cost category is multiplied by the level of its respective price proxy. The sum of these products (that is, the expenditure weights multiplied by their price levels) for all cost categories yields the composite index level of the market basket in a given period. Repeating this step for other periods produces a series of market basket levels over time. Dividing an index level for a given period by an index level for an earlier period produces a rate of growth in the input price index over that timeframe.
As noted, the market basket is described as a fixed-weight index because it represents the change in price over time of a constant mix (quantity and intensity) of goods and services needed to furnish IPF services. The effects on total expenditures resulting from changes in the mix of goods and services purchased after the base period are not measured. For example, an IPF hiring more nurses after the base period to accommodate the needs of patients will increase the volume of goods and services purchased by the IPF, but would not be factored into the price change measured by a fixed-weight IPF market basket. Only when the index is rebased will changes in the quantity and intensity be captured, with those changes being reflected in the cost weights. Therefore, we rebase the market basket periodically so that the cost weights reflect recent changes in the mix of goods and services that IPFs purchase to furnish inpatient care between base periods.
3. Creating an IPF-Specific Market Basket
As discussed in the FY 2016 final rule (80 FR 46656 through 46679), the 2012-based IPF market basket reflects the Medicare cost reports for both freestanding and hospital-based facilities. Previous market baskets, such as the 2008-based rehabilitation, psychiatric, and long-term care (RPL) market basket, were calculated using Medicare cost report data for freestanding facilities only. We used only freestanding facilities due to concerns regarding our ability to incorporate Medicare cost report data for hospital-based providers. After research on the available Medicare cost report data, we concluded that Medicare cost report data for both freestanding IPFs and hospital-based IPFs can be used to calculate the major market basket cost weights for a stand-alone IPF market basket. In the FY 2016 IPF PPS final rule (80 FR 46656 through 46679), we finalized a detailed methodology to derive market basket cost weights using Medicare cost report data for both freestanding IPFs and hospital-based IPFs.
For the FY 2020 proposed rule, we proposed to rebase and revise the 2012-based IPF market basket to a 2016 base year reflecting both freestanding IPFs and hospital-based IPFs. In section III.A.3.a., “Development of Cost Categories and Weights,” we provide a detailed description of our proposed methodology used to develop the 2016-based IPF market basket.
a. Development of Cost Categories and Weights
i. Medicare Cost Reports
We proposed a 2016-based IPF market basket that consists of seven major cost categories and a residual derived from the 2016 Medicare cost reports (CMS Form 2552-10 effective for cost reports beginning on or after May 1, 2010) for freestanding and hospital-based IPFs. CMS Form 2552-10 was also used to derive the major cost categories in the 2012-based IPF market basket. The seven cost categories are Wages and Salaries, Employee Benefits, Contract Labor, Pharmaceuticals, Professional Liability Insurance (PLI), Home Office Contract Labor, and Capital. The 2012-based IPF market basket did not have a Home Office Contract Labor cost category. The residual “All Other” category reflects all remaining costs not captured in the seven cost categories. The 2016 cost reports include providers whose cost reporting period beginning date is on or between October 1, 2015 and September 30, 2016. We proposed to select 2016 as the base year because we believe that the Medicare cost reports for this year represent the most recent, complete set of Medicare cost report data available at the time of rulemaking.
Similar to the Medicare cost report data used to develop the 2012-based IPF market basket, the Medicare cost report data for 2016 show large differences between some providers' Medicare length of stay (LOS) and total facility LOS. Our goal has always been to measure cost weights that are reflective of case mix and practice patterns associated with providing services to Medicare beneficiaries. Therefore, we proposed to limit our selection of Medicare cost reports used in the 2016-based IPF market basket to those facilities that had a Medicare LOS within a comparable range of their total facility average LOS. The Medicare average LOS for freestanding IPFs is calculated from data reported on line 14 of Worksheet S-3, part I. The Medicare average LOS for hospital-based IPFs is calculated from data reported on line 16 of Worksheet S-3, part I. To derive the proposed 2016-based IPF market basket, for those IPFs with an average facility LOS of greater than or equal to 15 days, we proposed to include IPFs where the Medicare LOS is within 50 percent (higher or lower) of the average facility LOS. For those IPFs whose average facility LOS is less than 15 days, we proposed to include IPFs where the Medicare LOS is within 95 percent (higher or lower) of the facility LOS. We proposed to apply this LOS edit to the data for IPFs to exclude providers that serve a population whose LOS would indicate that the patients served are not consistent with a LOS of a typical Medicare patient. This is the same LOS edit applied to the 2012-based IPF market basket.
Applying these trims to the approximate 1,600 total cost reports (freestanding and hospital-based) resulted in roughly 1,500 IPF Medicare cost reports with an average Medicare LOS of 12 days, average facility LOS of 9 days, and Medicare utilization (as measured by Medicare inpatient IPF days as a percentage of total facility days) of 26 percent. Providers excluded from the proposed 2016-based IPF market basket (about 130 Medicare cost reports) had an average Medicare LOS of 25 days, average facility LOS of 55 days, and a Medicare utilization of 4 percent. Of those excluded, about 70 percent of these were freestanding providers; on the other hand, freestanding providers represent about 30 percent of all IPFs. We note that seventy percent of those excluded from the 2012-based IPF market basket using this LOS edit were also freestanding providers.
Using the post-LOS set of 2016 Medicare cost reports, we calculated costs for the seven major cost categories (Wages and Salaries, Employee Benefits, Contract Labor, Professional Liability Insurance, Pharmaceuticals, Home Office Contract Labor, and Capital). For comparison, the 2012-based IPF market basket utilized the Bureau of Economic Analysis Benchmark Input-Output data to derive the Home Office Contract Labor cost weight rather than the Medicare cost report data. A more detailed discussion of this methodological change is provided.
Similar to the 2012-based IPF market basket major cost weights, the proposed 2016-based IPF market basket cost weights reflect Medicare allowable costs (routine, ancillary, and capital costs) that are eligible for inclusion under the IPF PPS payments. We proposed to define Medicare allowable costs for freestanding IPFs as Worksheet B, part I, column 26, lines 30 through 35, 50 through 76 (excluding 52 and 75), 90 through 91, and 93. For hospital-based IPFs, we proposed that total Medicare allowable costs be equal to total costs for the IPF inpatient unit after the allocation of overhead costs (Worksheet B, part I, column 26, line 40) and a portion of total ancillary costs (Worksheet B, part I, column 26, lines 50 through 76 (excluding 52 and 75), 90 through 91, and 93). We proposed to calculate the portion of ancillary costs attributable to the hospital-based IPF for a given ancillary cost center by multiplying total facility ancillary costs for the specific cost center (as reported on Worksheet B, part I, column 26) by the ratio of IPF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3 for IPF subproviders) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3 for all Inpatient Prospective Payment System (IPPS), Skilled Nursing Facility (SNF), IRF, and IPF). This is the same methodology used for the 2012-based IPF market basket.
We provide a description of the proposed methodologies used to derive costs for the seven major cost categories.
Wages and Salaries Costs
For freestanding IPFs, we proposed that Wages and Salaries costs be derived as the sum of routine inpatient salaries, ancillary salaries, and a proportion of overhead (or general service cost centers in the MCR) salaries as reported on Worksheet A, column 1. Since overhead salary costs are attributable to the entire IPF, we only include the proportion attributable to the Medicare allowable cost centers. We proposed to estimate the proportion of overhead salaries that are attributed to Medicare allowable costs centers by multiplying the ratio of Medicare allowable salaries (Worksheet A, column 1, lines 50 through 76 (excluding 52 and 75), 90 through 91, and 93) to total salaries (Worksheet A, column 1, line 200) times total overhead salaries (Worksheet A, column 1, lines 4 through 18). This is the same methodology used in the 2012-based IPF market basket.
We proposed that Wages and Salaries costs for hospital-based IPFs are derived by summing inpatient routine salary costs, ancillary salaries, overhead salary costs attributable to the IPF inpatient unit, and a portion of overhead salary costs attributable to the ancillary departments.
We proposed to calculate hospital-based inpatient routine salary costs using Worksheet A, column 1, line 40.
We proposed to calculate hospital-based ancillary salary costs for a specific cost center (Worksheet A, column 1, lines 50 through 76 (excluding 52 and 75), 90 through 91, and 93) using salary costs from Worksheet A, column 1 multiplied by the ratio of IPF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3 for IPF subproviders) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3 for IPPS, SNF, IRF, and IPF).
We proposed to calculate the hospital-based overhead salaries attributable to the IPF inpatient unit by first calculating total noncapital overhead costs (Worksheet B, part I, columns 4-18, line 40 less Worksheet B, part II, columns 4-18) for each ancillary department. We then multiplied total noncapital overhead costs by the ratio of total facility overhead salaries (as reported on Worksheet A, column 1, lines 4-18) to total facility noncapital overhead costs (as reported on Worksheet A, column 1 and 2, lines 4-18).
We proposed to calculate the hospital-based portion of overhead salaries attributable to each ancillary department by first calculating total noncapital overhead costs attributable to each specific ancillary department (Worksheet B, part I, columns 4-18 less Worksheet B, part II, columns 4-18). We then identified the portion of these noncapital overhead costs attributable to Wages and Salaries by multiplying these costs by the ratio of total facility overhead salaries (as reported on Worksheet A, column 1, lines 4-18) to total overhead costs (as reported on Worksheet A, column 1 & 2, lines 4-18). Finally, we identified the portion of these overhead salaries for each ancillary department that is attributable to the hospital-based IPF by multiplying by the ratio of IPF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3 for hospital-based IPFs) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3 for all IPPS, SNF, IRF, and IPF).
This is the same Wages and Salaries Costs methodology used to derive the 2012-based IPF market basket.
Employee Benefits Costs
Effective with the implementation of CMS Form 2552-10, we began collecting Employee Benefits and Contract Labor data on Worksheet S-3, part V.
For 2016 Medicare cost report data, the majority of providers did not report data on Worksheet S-3, part V. One (1) percent of freestanding IPFs and roughly 40 percent of hospital-based IPFs reported data on Worksheet S-3, part V. Again, we continue to encourage all providers to report these data on the Medicare cost report.
For freestanding IPFs, we proposed Employee Benefits costs were equal to the data reported on Worksheet S-3, part V, column 2, line 2. We note that while not required to do so, freestanding IPFs also may report Employee Benefits data on Worksheet S-3, part II, which is applicable to only IPPS providers. For those freestanding IPFs that reported Worksheet S-3, part II data, but not Worksheet S-3, part V, we proposed to use the sum of Worksheet S-3, part II lines 17, 18, 20, and 22 to derive Employee Benefits costs. This proposed method allowed us to obtain data from more than 20 freestanding IPFs (roughly 5 percent of all freestanding IPFs) than if we were to only use Worksheet S-3, part V data as done for the 2012-based IPF market basket.
For hospital-based IPFs, we proposed to calculate total benefit costs as the sum of inpatient unit benefit costs, a portion of ancillary benefits, and a portion of overhead benefits attributable to the routine inpatient unit and a portion of overhead benefits attributable to the ancillary departments.
We proposed hospital-based inpatient unit benefit costs be equal to Worksheet S-3 part V, column 2, line 3.
We proposed the hospital-based portion of ancillary benefit costs be equal to hospital-based ancillary salaries times the ratio of total facility benefits to total facility salaries.
We proposed that the hospital-based portion of overhead benefits attributable to the routine inpatient unit and ancillary departments be calculated by multiplying ancillary salaries for the hospital-based IPF and overhead salaries attributable to the hospital-based IPF (determined in the derivation of hospital-based IPF Wages and Salaries costs as described) by the ratio of total facility benefits to total facility salaries. Total facility benefits is equal to the sum of Worksheet S-3, part II, column 4, lines 17-25 and total facility salaries is equal to Worksheet S-3, part II, column 4, line 1.
Contract Labor Costs
Contract Labor costs are primarily associated with direct patient care services. Contract Labor costs are exclusive of Home Office Contract Labor costs. Contract labor costs for other services such as accounting, billing, and legal are calculated separately using other government data sources as described in section III.A.3.a.iii of this final rule. To derive contract labor costs using Worksheet S-3, part V data, for freestanding IPFs, we proposed Contract Labor costs be equal to Worksheet S-3, part V, column 1, line 2. As we noted for Employee Benefits, freestanding IPFs also may report Contract Labor data on Worksheet S-3, part II, which is applicable to only IPPS providers. For those freestanding IPFs that reported Worksheet S-3, part II data, but not Worksheet S-3, part V, we proposed to use the sum of Worksheet S-3, part II lines 11 and 13 to derive Contract Labor costs. For the 2012-based IPF market basket, we only used data from Worksheet S-3, part V, column 1, line 2 to derive the Contract Labor costs for freestanding IPFs.
For hospital-based IPFs, we proposed that Contract Labor costs be equal to Worksheet S-3, part V, column 1, line 3. Reporting of this data continues to be somewhat limited; therefore, we continue to encourage all providers to report these data on the Medicare cost report.
Pharmaceuticals Costs
For freestanding IPFs, we proposed to calculate pharmaceuticals costs using non-salary costs reported on Worksheet A, column 7 less Worksheet A, column 1 for the pharmacy cost center (line 15) and drugs charged to patients cost center (line 73).
For hospital-based IPFs, we proposed to calculate pharmaceuticals costs as the sum of a portion of the non-salary pharmacy costs and a portion of the non-salary drugs charged to patient costs reported for the total facility.
We proposed that hospital-based non-salary pharmacy costs attributable to the hospital-based IPF are calculated by multiplying total pharmacy costs attributable to the hospital-based IPF (as reported on Worksheet B, part I, column 15, line 40) by the ratio of total non-salary pharmacy costs (Worksheet A, column 2, line 15) to total pharmacy costs (sum of Worksheet A, column 1 and 2 for line 15) for the total facility.
We proposed that hospital-based non-salary drugs charged to patient costs attributable to the hospital-based IPF are calculated by multiplying total non-salary drugs charged to patient costs (Worksheet B, part I, column 0, line 73 plus Worksheet B, part I, column 15, line 73 less Worksheet A, column 1, line 73) for the total facility by the ratio of Medicare drugs charged to patient ancillary costs for the IPF unit (as reported on Worksheet D-3 for IPF subproviders, column 3, line 73) to total Medicare drugs charged to patients ancillary costs for the total facility (equal to the sum of Worksheet D-3, column 3, line 73, for all IPPS, SNF, IRF, and IPF).
This is the same Pharmaceuticals Costs methodology used to derive the 2012-based IPF market basket.
Professional Liability Insurance (PLI) Costs
For freestanding IPFs, we proposed that PLI costs (often referred to as malpractice costs) are equal to premiums, paid losses and self-insurance costs reported on Worksheet S-2, part I, columns 1 through 3, line 118.
For hospital-based IPFs, we proposed to assume that the PLI weight for the total facility is similar to the hospital-based IPF unit since the only data reported on this worksheet is for the entire facility. Therefore, hospital-based IPF PLI costs were equal to total facility PLI (as reported on Worksheet S-2, part I, columns 1 through 3, line 118) divided by total facility costs (as reported on Worksheet A, columns 1 and 2, line 200) times hospital-based IPF Medicare allowable total costs. Our assumption is that the same proportion of expenses are used among each unit of the hospital.
This is the same methodology used to derive the 2012-based IPF market basket.
Home Office/Related Organization Contract Labor Costs
For the 2016-based IPF market basket, we proposed to determine the home office/related organization contract labor costs using Medicare cost report data. This is a different methodology compared to the 2012-based IPF market basket. We believe this proposed methodology is an improvement as it is based on the data directly submitted by providers on the Medicare cost report. It is also consistent with the methodology we adopted when we rebased and revised the 2014-based IPPS market basket (52 FR 38159).
For hospital-based IPFs, we proposed to calculate the home office contract labor cost weight using data reported on Worksheet S-3, part II, column 4, lines 14, 1401, 1402, 2550, and 2551 and total facility costs (Worksheet B, part 1, column 26, line 202). We proposed to use total facility costs as the denominator for calculating the home office contract labor cost weight as these expenses reported on Worksheet S-3, part II reflect the entire hospital facility. Our assumption is that the same proportion of expenses are used among each unit of the hospital. Similar to the other market basket costs weights, we proposed to trim the Home Office Contract Labor cost weight to remove outliers. Since not all hospital-based IPFs will have home office contract labor costs, we proposed to trim the top one percent of the Home Office Contract Labor cost weight. This is the same trimming methodology used to calculate the Home Office Contract Labor cost weight in the 2016-based IPPS market basket. Using this proposed methodology, we calculate a Home Office Contract Labor cost weight for hospital-based IPFs of 3.7 percent. We discuss the trimming methodology for the other major cost categories in the “Final Major Cost Category Computation” in section ii. of this final rule.
Freestanding IPFs are not required to complete Worksheet S-3, part II. Therefore, to estimate the Home Office Contract Labor cost weight, we proposed the following methodology:
(1) Using hospital-based IPFs with a home office and also passing the one percent trim as described, we calculate the ratio of the Home Office Contract Labor cost weight to the Medicare allowable nonsalary, noncapital cost weight (Medicare allowable nonsalary, noncapital costs as a percent of total Medicare allowable costs).
(2) We identify freestanding IPFs that report a home office on Worksheet S-2, part I, line 140—roughly 85 percent. We proposed to calculate a Home Office Contract Labor cost weight for these freestanding IPFs by multiplying the ratio calculated in Step (1) by the Medicare allowable nonsalary, noncapital cost weight for those freestanding IPFs with a home office.
(3) We then calculated the freestanding IPF cost weight by multiplying the Home Office Contract Labor cost weight in step (2) by the total Medicare allowable costs for IPFs with a home office as a percent of total Medicare allowable costs for all freestanding IPFs.
To calculate the Home Office Contract Labor cost weight, we proposed to weight together the freestanding Home Office Contract Labor cost weight (3.0 percent) and the hospital-based Home Office Contract Labor cost weight (3.7 percent) using total Medicare allowable costs. The resulting overall cost weight for Home Office was 3.5 percent (3.0 percent × 37 percent + 3.7 percent × 63 percent).
For the 2012-based IPF market basket, we calculated the Home Office Contract Labor cost weight using the Bureau of Economic Analysis Input-Output expense data for North American Industry Classification System (NAICS) code 55, Management of Companies and Enterprises using the methodology described in section III.A.3.a.iii (Derivation of the Detailed Operating Cost Weights) of this final rule.
Capital Costs
For freestanding IPFs, we proposed capital costs to be equal to Medicare allowable capital costs as reported on Worksheet B, part II, column 26, lines 30 through 35, 50 through 76 (excluding 52 and 75), 90 through 91, and 93. This is the same methodology used for the 2012-based IPF market basket.
For hospital-based IPFs, we proposed capital costs to be equal to IPF inpatient capital costs (as reported on Worksheet B, part II, column 26, line 40) and a portion of IPF ancillary capital costs. We calculated the portion of ancillary capital costs attributable to the hospital-based IPF for a given cost center by multiplying total facility ancillary capital costs for the specific ancillary cost center (as reported on Worksheet B, part II, column 26) by the ratio of IPF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3 for IPF subproviders) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3 for all IPPS, SNF, IRF, and IPF). This is the same methodology used for the 2012-based IPF market basket.
ii. Final Major Cost Category Computation
After we derived costs for the seven major cost categories for each provider using the Medicare cost report data as described, we proposed to trim the data for outliers. The proposed trimming methodology for the Home Office Contract Labor cost weight is slightly different than the proposed trimming methodology for the other six cost categories. For the Wages and Salaries, Employee Benefits, Contract Labor, Pharmaceuticals, Professional Liability Insurance, and Capital cost weights, we first divided the costs for each of these six categories by total Medicare allowable costs calculated for the provider to obtain cost weights for the universe of IPF providers. Next, we applied a mutually exclusive top and bottom 5 percent trim for each cost weight to remove outliers. After the outliers have been removed, we summed the costs for each category across all remaining providers. We then divided this by the sum of total Medicare allowable costs across all remaining providers to obtain a cost weight for the proposed 2016-based IPF market basket for the given category.
Finally, we calculated the residual “All Other” cost weight that reflects all remaining costs that are not captured in the seven cost categories listed. We did not receive any comments on the derivation of the major cost weights. In this final rule, we are finalizing our methodology for deriving the major cost weights as we proposed.
Table 1 presents the major cost categories and weights calculated from the Medicare cost reports for the 2016-based IPF market basket as well as for the 2012-based IPF market basket.
| Major cost categories | Final 2016- based IPF market basket (percent) | 2012-based IPF market basket (percent) |
|---|---|---|
| Wages and Salaries | 51.2 | 51.0 |
| Employee Benefits | 13.5 | 13.1 |
| Contract Labor | 1.3 | 1.3 |
| Professional Liability Insurance (Malpractice) | 0.9 | 1.1 |
| Pharmaceuticals | 4.7 | 4.8 |
| Home Office/Related Organization Contract Labor | 3.5 | n/a |
| Capital | 7.1 | 7.0 |
| “All Other” Residual | 17.9 | 21.6 |
| Note: Total may not sum to 100 due to rounding. | ||
As we did for the 2012-based IPF market basket, we proposed to allocate the Contract Labor cost weight to the Wages and Salaries and Employee Benefits cost weights based on their relative proportions under the assumption that contract labor costs are comprised of both wages and salaries and employee benefits. The Contract Labor allocation proportion for Wages and Salaries is equal to the Wages and Salaries cost weight as a percent of the sum of the Wages and Salaries cost weight and the Employee Benefits cost weight. For the proposed rule, this rounded percentage was 79 percent; therefore, we proposed to allocate 79 percent of the Contract Labor cost weight to the Wages and Salaries cost weight and 21 percent to the Employee Benefits cost weight. The 2012-based IPF market basket percentage was 80 percent. We did not receive any comments on the allocation of the Contract Labor cost weight.
Table 2 shows the Wages and Salaries and Employee Benefit cost weights after Contract Labor cost weight allocation for both the 2016-based IPF market basket and 2012-based IPF market basket.
| Major cost categories | Final 2016- based IPF market basket | 2012-Based IPF market basket |
|---|---|---|
| Wages and Salaries | 52.2 | 52.1 |
| Employee Benefits | 13.8 | 13.4 |
iii. Derivation of the Detailed Operating Cost Weights
To further divide the “All Other” residual cost weight estimated from the 2016 Medicare Cost Report data into more detailed cost categories, we proposed to use the 2012 Benchmark Input-Output (I-O) “Use Tables/Before Redefinitions/Purchaser Value” for NAICS 622000 Hospitals, published by the Bureau of Economic Analysis (BEA). These data, publicly available at http://www.bea.gov/industry/io_annual.htm, are the most recent data available at the time of rulemaking. For the 2012-based IPF market basket, we used the 2007 Benchmark I-O data.
The BEA Benchmark I-O data are scheduled for publication every five years. The 2012 Benchmark I-O data are derived from the 2012 Economic Census and are the building blocks for BEA's economic accounts. They represent the most comprehensive and complete set of data on the economic processes or mechanisms by which output is produced and distributed.[1] BEA also produces Annual I-O estimates; however, while based on a similar methodology, these estimates reflect less comprehensive and less detailed data sources and are subject to revision when benchmark data becomes available. Instead of using the less detailed Annual I-O data, we proposed to inflate the 2012 Benchmark I-O data forward to 2016 by applying the annual price changes from the respective price proxies to the appropriate market basket cost categories obtained from the 2012 Benchmark I-O data. We then proposed to calculate the cost shares that each cost category represents of the inflated 2016 data. These resulting 2016 cost shares were applied to the “All Other” residual cost weight to obtain the proposed detailed cost weights for the 2016-based IPF market basket. For example, the cost for Food: Direct Purchases represents 5.0 percent of the sum of the “All Other” 2016 Benchmark I-O Hospital Expenditures inflated to 2016. Therefore, the Food: Direct Purchases cost weight represents 5.0 percent of the 2016-based IPF market basket's “All Other” cost category (17.9 percent), yielding a “final” Food: Direct Purchases cost weight of 0.9 percent in the proposed 2016-based IPF market basket (0.05 * 17.9 percent = 0.9 percent).
Using this methodology, we proposed to derive seventeen detailed IPF market basket cost category weights from the proposed 2016-based IPF market basket residual cost weight (17.9 percent). These categories were: (1) Electricity, (2) Fuel, Oil, and Gasoline, (3) Food: Direct Purchases, (4) Food: Contract Services, (5) Chemicals, (6) Medical Instruments, (7) Rubber & Plastics, (8) Paper and Printing Products, (9) Miscellaneous Products, (10) Professional Fees: Labor-related, (11) Administrative and Facilities Support Services, (12) Installation, Maintenance, and Repair, (13) All Other Labor-related Services, (14) Professional Fees: Nonlabor-related, (15) Financial Services, (16) Telephone Services, and (17) All Other Nonlabor-related Services. We note that for the 2012-based IPF market basket, we had a Water and Sewerage cost weight. For the proposed 2016-based IPF market basket, we proposed to include Water and Sewerage in the Electricity cost weight due to the small amount of costs in this category.
We did not receive any comments on the derivation of the detailed operating cost weights. In this final rule, we are finalizing our methodology for deriving the detailed operating cost weights as we proposed.
iv. Derivation of the Detailed Capital Cost Weights
As described in section III.A.3.a.i. of this final rule, we proposed a Capital-Related cost weight of 7.1 percent as obtained from the 2016 Medicare cost reports for freestanding and hospital-based IPF providers. We proposed to further separate this total Capital-Related cost weight into more detailed cost categories. Using 2016 Medicare cost reports, we were able to group Capital-Related costs into the following categories: Depreciation, Interest, Lease, and Other Capital-Related costs. For each of these categories, we proposed to determine separately for hospital-based IPFs and freestanding IPFs what proportion of total capital-related costs the category represent.
For freestanding IPFs, we proposed to derive the proportions for Depreciation, Interest, Lease, and Other Capital-related costs using the data reported by the IPF on Worksheet A-7, which is the same methodology used for the 2012-based IPF market basket.
For hospital-based IPFs, data for these four categories were not reported separately for the subprovider; therefore, we proposed to derive these proportions using data reported on Worksheet A-7 for the total facility. We are assuming the cost shares for the overall hospital are representative for the hospital-based subprovider IPF unit. For example, if depreciation costs make up 60 percent of total capital costs for the entire facility, we believe it was reasonable to assume that the hospital-based IPF will also have a 60 percent proportion because it is a subprovider unit contained within the total facility. This is the same methodology used for the 2012-based IPF market basket.
In order to combine each detailed capital cost weight for freestanding and hospital-based IPFs into a single capital cost weight for the 2016-based IPF market basket, we proposed to weight together the shares for each of the categories (Depreciation, Interest, Lease, and Other Capital-related costs) based on the share of total capital costs each provider type represents of the total capital costs for all IPFs for 2016. Applying this methodology results in proportions of total capital-related costs for Depreciation, Interest, Lease and Other Capital-related costs that are representative of the universe of IPF providers. This is the same methodology used for the 2012-based IPF market basket.
Next, we proposed to allocate lease costs across each of the remaining detailed capital-related cost categories as done in the 2012-based IPF market basket. This resulted in three primary capital-related cost categories in the 2016-based IPF market basket: Depreciation, Interest, and Other Capital-Related costs. As done in the 2012-based IPF market basket, lease costs are unique in that they are not broken out as a separate cost category in the 2016-based IPF market basket, but rather we proposed to proportionally distribute these costs among the cost categories of Depreciation, Interest, and Other Capital-Related, reflecting the assumption that the underlying cost structure of leases is similar to that of capital-related costs in general. As done under the 2012-based IPF market basket, we proposed to assume that 10 percent of the lease costs as a proportion of total capital-related costs represents overhead and assign those costs to the Other Capital-Related cost category accordingly. We proposed to distribute the remaining lease costs proportionally across the three cost categories (Depreciation, Interest, and Other Capital-Related) based on the proportion that these categories comprise of the sum of the Depreciation, Interest, and Other Capital-related cost categories (excluding lease expenses). This is the same methodology used for the 2012-based IPF market basket. The allocation of these lease expenses are shown in Table 3.
Finally, we proposed to further divide the Depreciation and Interest cost categories. We proposed to separate Depreciation into the following two categories: (1) Building and Fixed Equipment; and (2) Movable Equipment; and proposed to separate Interest into the following two categories: (1) Government/Nonprofit; and (2) For-profit.
To disaggregate the Depreciation cost weight, we determined the percent of total Depreciation costs for IPFs that is attributable to Building and Fixed Equipment, which we hereafter refer to as the “fixed percentage.” For the 2016-based IPF market basket, we proposed to use slightly different methods to obtain the fixed percentages for hospital-based IPFs compared to freestanding IPFs.
For freestanding IPFs, we proposed to use depreciation data from Worksheet A-7 of the 2016 Medicare cost reports. However, for hospital-based IPFs, we determined that the fixed percentage for the entire facility may not be representative of the IPF subprovider unit due to the entire facility likely employing more sophisticated movable assets that are not utilized by the hospital-based IPF. Therefore, for hospital-based IPFs, we proposed to calculate a fixed percentage using: (1) Building and fixture capital costs allocated to the subprovider unit as reported on Worksheet B, part I line 40; and (2) building and fixture capital costs for the top five ancillary cost centers utilized by hospital-based IPFs. We proposed to then weight these two fixed percentages (inpatient and ancillary) using the proportion that each capital cost type represents of total capital costs in the proposed 2016-based IPF market basket. We then proposed to weight the fixed percentages for hospital-based and freestanding IPFs together using the proportion of total capital costs each provider type represents. For both freestanding and hospital-based IPFs, this is the same methodology used for the 2012-based IPF market basket.
To disaggregate the Interest cost weight, we determined the percent of total interest costs for IPFs that were attributable to government and nonprofit facilities, the “nonprofit percentage.” For the 2016-based IPF market basket, we proposed to use interest costs data from Worksheet A-7 for both freestanding and hospital-based IPFs. We then determined the percent of total interest costs that are attributed to government and nonprofit IPFs separately for hospital-based and freestanding IPFs and weight the nonprofit percentages for hospital-based and freestanding IPFs together using the proportion of total capital costs each provider type represents. This is the same methodology used for the 2012-based IPF market basket.
We did not receive public comments on the derivation of the detailed capital cost weights. In this final rule, we are finalizing our methodology for deriving the detailed capital cost weights as we proposed. Table 3 provides the detailed capital cost share composition of the 2016-based IPF market basket. These detailed capital cost share composition percentages are applied to the total Capital-Related cost weight of 7.1 percent determined in section III.A.3.a.i. of this final rule.
| Capital cost share composition before lease expense allocation (percent) | Capital cost share composition after lease expense allocation (percent) | |
|---|---|---|
| Depreciation | 60 | 74 |
| Building and Fixed Equipment | 43 | 52 |
| Movable Equipment | 18 | 22 |
| Interest | 13 | 16 |
| Government/Nonprofit | 10 | 12 |
| For Profit | 3 | 4 |
| Lease | 20 | n/a |
| Other | 7 | 10 |
| Note: Detail may not add to total due to rounding. | ||
v. 2016-Based IPF Market Basket Cost Categories and Weights
Table 4 shows the cost categories and weights for the final 2016-based IPF market basket and the 2012-based IPF market basket.


b. Selection of Price Proxies
After developing the cost weights for the proposed 2016-based IPF market basket, we selected the most appropriate wage and price proxies currently available to represent the rate of price change for each expenditure category. For the majority of the cost weights, we based the price proxies on Bureau of Labor Statistics (BLS) data and grouped them into one of the following BLS categories:
- Employment Cost Indexes. Employment Cost Indexes (ECIs) measure the rate of change in employment wage rates and employer costs for employee benefits per hour worked. These indexes are fixed-weight indexes and strictly measure the change in wage rates and employee benefits per hour. ECIs are superior to Average Hourly Earnings (AHE) as price proxies for input price indexes because they are not affected by shifts in occupation or industry mix, and because they measure pure price change and are available by both occupational group and by industry. The industry ECIs are based on the NAICS and the occupational ECIs are based on the Standard Occupational Classification System (SOC).
- Producer Price Indexes. Producer Price Indexes (PPIs) measure price changes for goods sold in other than retail markets. PPIs are used when the purchases of goods or services are made at the wholesale level.
- Consumer Price Indexes. Consumer Price Indexes (CPIs) measure change in the prices of final goods and services bought by consumers. CPIs are only used when the purchases are similar to those of retail consumers rather than purchases at the wholesale level, or if no appropriate PPIs are available.
We evaluated the price proxies using the criteria of reliability, timeliness, availability, and relevance:
- Reliability. Reliability indicates that the index is based on valid statistical methods and has low sampling variability. Widely accepted statistical methods ensure that the data were collected and aggregated in a way that can be replicated. Low sampling variability is desirable because it indicates that the sample reflects the typical members of the population. (Sampling variability is variation from the true population parameter that occurs by chance because only a sample was surveyed rather than the entire population.)
- Timeliness. Timeliness implies that the proxy is published regularly, preferably at least once a quarter. The market baskets are updated quarterly and, therefore, it is important for the underlying price proxies to be up-to-date, reflecting the most recent data available. We believe that using proxies that are published regularly (at least quarterly, whenever possible) helps to ensure that we are using the most recent data available to update the market basket. We strive to use publications that are disseminated frequently, because we believe that this is an optimal way to stay abreast of the most current data available.
- Availability. Availability means that the proxy is publicly available. We prefer that our proxies are publicly available because this will help ensure that our market basket updates are as transparent to the public as possible. In addition, this enables the public to be able to obtain the price proxy data on a regular basis.
- Relevance. Relevance means that the proxy is applicable and representative of the cost category weight to which it is applied. The CPIs, PPIs, and ECIs that we selected meet these criteria. Therefore, we believe that they continue to be the best measure of price changes for the cost categories to which they would be applied.
Table 12 lists all price proxies that we proposed to use for the 2016-based IPF market basket. A detailed explanation of the price proxies we proposed for each cost category weight is provided.
i. Price Proxies for the Operating Portion of the 2016-Based IPF Market Basket
Wages and Salaries
There is not a published wage proxy that we believe represents the occupational distribution of workers in IPFs. To measure wage price growth in the proposed 2016-based IPF market basket, we proposed to apply a proxy blend based on six occupational subcategories within the Wages and Salaries category, which would reflect the IPF occupational mix, as done for the 2012-based IPF market basket.
We proposed to use the National Industry-Specific Occupational Employment and Wage estimates for NAICS 622200, Psychiatric & Substance Abuse Hospitals, published by the Bureau of Labor Statistics Office of Occupational Employment Statistics (OES), as the data source for the wage cost shares in the wage proxy blend. We proposed to use May 2016 OES data. Detailed information on the methodology for the national industry-specific occupational employment and wage estimates survey can be found at http://www.bls.gov/oes/current/oes_tec.htm. For the 2012-based IPF market basket, we used May 2012 OES data.
Based on the OES data, there are six wage subcategories: Management; NonHealth Professional and Technical; Health Professional and Technical; Health Service; NonHealth Service; and Clerical. Table 5 lists the 2016 occupational assignments for the six wage subcategories; these are the same occupational groups used in the 2012-based IPF market basket.
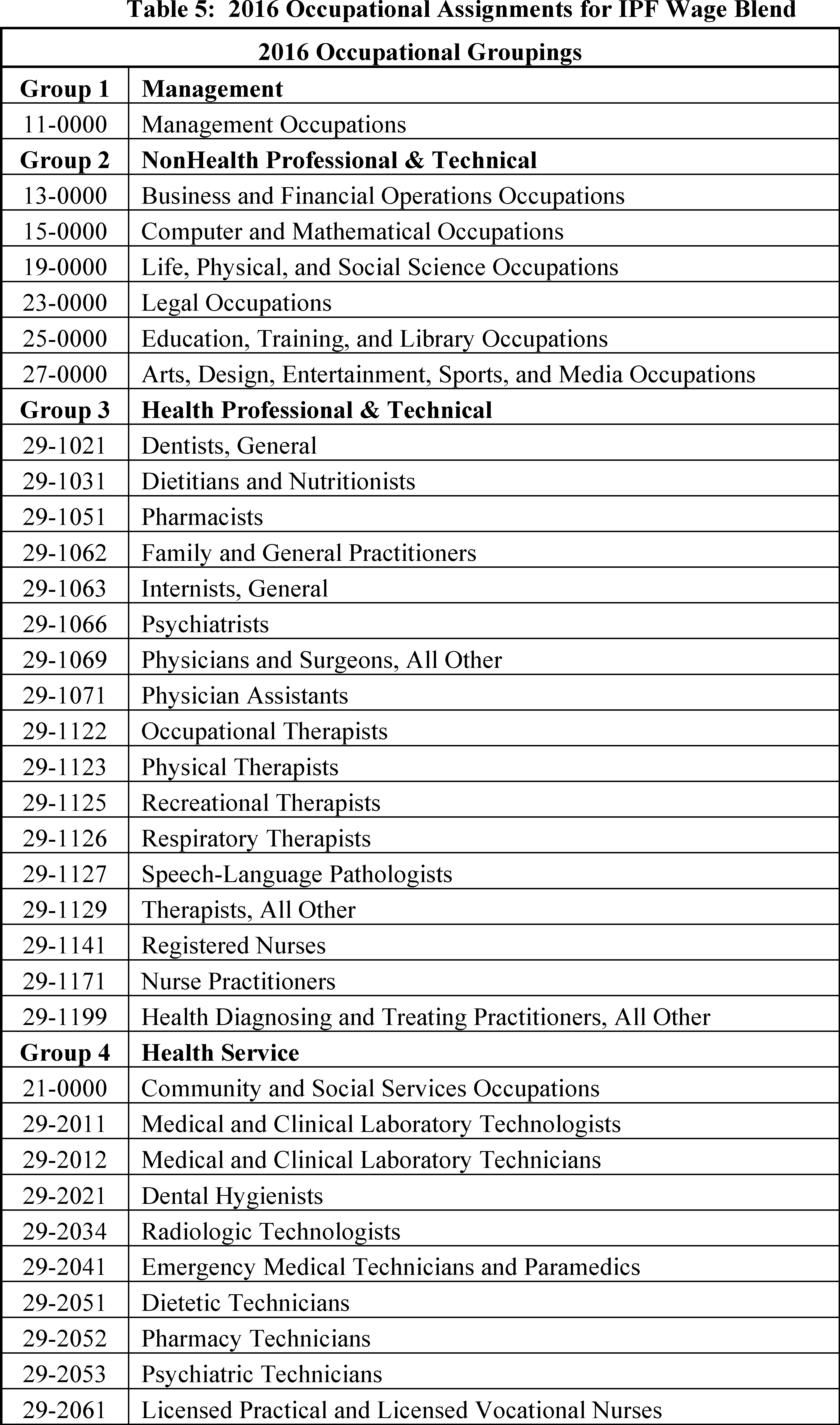
Total expenditures by occupation (that is, occupational assignment) were calculated by taking the OES number of employees multiplied by the OES annual average salary. These expenditures were aggregated based on the six groups in Table 5. We next calculated the proportion of each group's expenditures relative to the total expenditures of all six groups. These proportions, listed in Table 6, represent the weights used in the wage proxy blend. We then proposed to use the published wage proxies in Table 6 for each of the six groups (that is, wage subcategories) as we believe these six price proxies are the most technically appropriate indices available to measure the price growth of the Wages and Salaries cost category. These are the same price proxies used in the 2012-based IPF market basket. We did not receive any public comments on the 2016-based IPF wage price proxy. In this final rule, we are finalizing the 2016-based IPF wage price proxy as proposed.

A comparison of the yearly changes from FY 2017 to FY 2020 for the 2016-based IPF wage blend and the 2012-based IPF wage blend is shown in Table 7. The average annual growth rate is the same for both price proxies over 2017-2020.
| 2017 | 2018 | 2019 | 2020 | Average 2017-2020 | |
|---|---|---|---|---|---|
| 2016-based IPF Final Wage Proxy Blend | 2.4 | 2.6 | 3.0 | 3.2 | 2.8 |
| 2012-based IPF Wage Proxy Blend | 2.4 | 2.6 | 3.0 | 3.2 | 2.8 |
| **Source: IHS Global Inc., 2nd Quarter 2019 forecast with historical data through 1st Quarter 2019. | |||||
Benefits
To measure benefits price growth in the 2016-based IPF market basket, we proposed to apply a benefits proxy blend based on the same six subcategories and the same six blend weights for the wage proxy blend. These subcategories and blend weights are listed in Table 8.
The benefit ECIs, listed in Table 8, are not publically available. Therefore, an “ECIs for Total Benefits” is calculated using publically available “ECIs for Total Compensation” for each subcategory and the relative importance of wages within that subcategory's total compensation. This is the same benefits ECI methodology that we implemented in our 2012-based IPF market basket as well as used in the IPPS, SNF, HHA, RPL, LTCH, and ESRD market baskets. We believe that the six price proxies listed in Table 8 are the most technically appropriate indices to measure the price growth of the Benefits cost category in the proposed 2016-based IPF market basket. We did not receive any public comments on the 2016-based IPF benefit price proxy. In this final rule, we are finalizing the 2016-based IPF benefit price proxy as proposed.
| Wage subcategory | 2016-based benefit blend weight (percent) | 2012-based benefit blend weight (percent) | Price proxy |
|---|---|---|---|
| Health Service | 36.3 | 36.2 | ECI for Total Benefits for All Civilian workers in Healthcare and Social Assistance. |
| Health Professional and Technical | 34.9 | 33.5 | ECI for Total Benefits for All Civilian workers in Hospitals. |
| NonHealth Service | 8.9 | 9.2 | ECI for Total Benefits for Private Industry workers in Service Occupations. |
| NonHealth Professional and Technical | 7.0 | 7.3 | ECI for Total Benefits for Private Industry workers in Professional, Scientific, and Technical Services. |
| Management | 6.8 | 7.1 | ECI for Total Benefits for Private Industry workers in Management, Business, and Financial. |
| Clerical | 6.1 | 6.7 | ECI for Total Benefits for Private Industry workers in Office and Administrative Support. |
| Total | 100.0 | 100.0 |
A comparison of the yearly changes from FY 2017 to FY 2020 for the 2016-based IPF benefit proxy blend and the 2012-based IPF benefit proxy is shown in Table 9. The average annual growth rate is the same for both price proxies over 2017-2020.
| 2017 | 2018 | 2019 | 2020 | Average 2017-2020 | |
|---|---|---|---|---|---|
| 2016-based IPF Final Benefit Proxy Blend | 1.9 | 2.1 | 2.5 | 3.0 | 2.4 |
| 2012-based IPF Benefit Proxy Blend | 1.9 | 2.1 | 2.5 | 3.0 | 2.4 |
| Source: IHS Global Inc., 2nd Quarter 2019 forecast with historical data through 1st Quarter 2019. | |||||
Electricity
We proposed to continue to use the PPI Commodity Index for Commercial Electric Power (BLS series code WPU0542) to measure the price growth of this cost category. This is the same price proxy used in the 2012-based IPF market basket.
Fuel, Oil, and Gasoline
Similar to the 2012-based IPF market basket, for the 2016-based IPF market basket, we proposed to use a blend of the PPI for Petroleum Refineries and the PPI Commodity for Natural Gas. Our analysis of the BEA's 2012 Benchmark I-O data (use table before redefinitions, purchaser's value for NAICS 622000 [Hospitals]) shows that Petroleum Refineries expenses accounts for approximately 90 percent and Natural Gas accounts for approximately 10 percent of Hospitals (NAICS 622000) total Fuel, Oil, and Gasoline expenses. Therefore, we proposed to use a blend of 90 percent of the PPI for Petroleum Refineries (BLS series code PCU324110324110) and 10 percent of the PPI Commodity Index for Natural Gas (BLS series code WPU0531) as the price proxy for this cost category. The 2012-based IPF market basket used a 70/30 blend of these price proxies, reflecting the 2007 I-O data. We believe that these two price proxies continue to be the most technically appropriate indices available to measure the price growth of the Fuel, Oil, and Gasoline cost category in the proposed 2016-based IPF market basket.
Professional Liability Insurance
We proposed to continue to use the CMS Hospital Professional Liability Index to measure changes in professional liability insurance (PLI) premiums. To generate this index, we collect commercial insurance premiums for a fixed level of coverage while holding non-price factors constant (such as a change in the level of coverage). This is the same proxy used in the 2012-based IPF market basket.
Pharmaceuticals
We proposed to continue to use the PPI for Pharmaceuticals for Human Use, Prescription (BLS series code WPUSI07003) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Food: Direct Purchases
We proposed to continue to use the PPI for Processed Foods and Feeds (BLS series code WPU02) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Food: Contract Purchases
We proposed to continue to use the CPI for Food Away From Home (BLS series code CUUR0000SEFV) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Chemicals
Similar to the 2012-based IPF market basket, we proposed to use a four part blended PPI as the proxy for the chemical cost category in the proposed 2016-based IPF market basket. The proposed blend is composed of the PPI for Industrial Gas Manufacturing Primary Products (BLS series code PCU325120325120P), the PPI for Other Basic Inorganic Chemical Manufacturing (BLS series code PCU32518-32518-), the PPI for Other Basic Organic Chemical Manufacturing (BLS series code PCU32519-32519-), and the PPI for Other Miscellaneous Chemical Product Manufacturing (BLS series code PCU325998325998).
We note that the four part blended PPI used in the 2012-based IPF market basket is composed of the PPI for Industrial Gas Manufacturing (BLS series code PCU325120325120P), the PPI for Other Basic Inorganic Chemical Manufacturing (BLS series code PCU32518-32518-), the PPI for Other Basic Organic Chemical Manufacturing (BLS series code PCU32519-32519-), and the PPI for Soap and Cleaning Compound Manufacturing (BLS series code PCU32561-32561-).
We proposed to derive the weights for the PPIs using the 2012 Benchmark I-O data. The 2012-based IPF market basket used the 2007 Benchmark I-O data to derive the weights for the four PPIs.
Table 10 shows the weights for each of the four PPIs used to create proposed blended Chemical proxy for the 2016-based IPF market basket compared to the 2012-based IPF market basket blended Chemical proxy.
| Name | Final 2016-based IPF weights (percent) | 2012-based IPF weights (percent) | NAICS |
|---|---|---|---|
| PPI for Industrial Gas Manufacturing | 19 | 32 | 325120 |
| PPI for Other Basic Inorganic Chemical Manufacturing | 13 | 17 | 325180 |
| PPI for Other Basic Organic Chemical Manufacturing | 60 | 45 | 325190 |
| PPI for Soap and Cleaning Compound Manufacturing | n/a | 6 | 325610 |
| PPI for Other Miscellaneous Chemical Product Manufacturing | 8 | n/a | 325998 |
Medical Instruments
We proposed to continue to use a blend of two PPIs for the Medical Instruments cost category. The 2012 Benchmark I-O data shows an approximate 57/43 split between Surgical and Medical Instruments and Medical and Surgical Appliances and Supplies for this cost category. Therefore, we proposed a blend composed of 57 percent of the commodity-based PPI for Surgical and Medical Instruments (BLS series code WPU1562) and 43 percent of the commodity-based PPI for Medical and Surgical Appliances and Supplies (BLS series code WPU1563). The 2012-based IPF market basket used a 50/50 blend of these PPIs based on the 2007 Benchmark I-O data.
Rubber and Plastics
We proposed to continue to use the PPI for Rubber and Plastic Products (BLS series code WPU07) to measure price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Paper and Printing Products
We proposed to continue to use the PPI for Converted Paper and Paperboard Products (BLS series code WPU0915) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Miscellaneous Products
We proposed to continue to use the PPI for Finished Goods Less Food and Energy (BLS series code WPUFD4131) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Professional Fees: Labor-Related
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Professional and Related (BLS series code CIU2010000120000I) to measure the price growth of this category. This is the same proxy used in the 2012-based IPF market basket.
Administrative and Facilities Support Services
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Office and Administrative Support (BLS series code CIU2010000220000I) to measure the price growth of this category. This is the same proxy used in the 2012-based IPF market basket.
Installation, Maintenance, and Repair
We proposed to continue to use the ECI for Total Compensation for Civilian workers in Installation, Maintenance, and Repair (BLS series code CIU1010000430000I) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
All Other: Labor-Related Services
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Service Occupations (BLS series code CIU2010000300000I) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Professional Fees: Nonlabor-Related
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Professional and Related (BLS series code CIU2010000120000I) to measure the price growth of this category. This is the same proxy used in the 2012-based IPF market basket.
Financial Services
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Financial Activities (BLS series code CIU201520A000000I) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
Telephone Services
We proposed to continue to use the CPI for Telephone Services (BLS series code CUUR0000SEED) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket.
All Other: Nonlabor-Related Services
We proposed to continue to use the CPI for All Items Less Food and Energy (BLS series code CUUR0000SA0L1E) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IPF market basket. We did not receive any public comments on the 2016-based IPF price proxies. In this final rule, we are finalizing the 2016-based IPF price proxies as proposed.
ii. Price Proxies for the Capital Portion of the Proposed 2016-Based IPF Market Basket
Capital Price Proxies Prior to Vintage Weighting
We proposed to continue to use the same price proxies for the capital-related cost categories as were applied in the 2012-based IPF market basket, which are provided and described in Table 12. Specifically, we proposed to proxy:
- Depreciation: Building and Fixed Equipment cost category by BEA's Chained Price Index for Nonresidential Construction for Hospitals and Special Care Facilities (BEA Table 5.4.4. Price Indexes for Private Fixed Investment in Structures by Type).
- Depreciation: Movable Equipment cost category by the PPI for Machinery and Equipment (BLS series code WPU11).
- Nonprofit Interest cost category by the average yield on domestic municipal bonds (Bond Buyer 20-bond index).
- For-profit Interest cost category by the average yield on Moody's Aaa bonds (Federal Reserve).
- Other Capital-Related cost category by the CPI-U for Rent of Primary Residence (BLS series code CUUS0000SEHA).
We believe these are the most appropriate proxies for IPF capital-related costs that meet our selection criteria of relevance, timeliness, availability, and reliability. We also proposed to continue to vintage weight the capital price proxies for Depreciation and Interest in order to capture the long-term consumption of capital. This vintage weighting method is similar to the method used for the 2012-based IPF market basket and is described in the section labeled Vintage Weights for Price Proxies.
Vintage Weights for Price Proxies
Because capital is acquired and paid for over time, capital-related expenses in any given year are determined by both past and present purchases of physical and financial capital. The vintage-weighted capital-related portion of the proposed 2016-based IPF market basket is intended to capture the long-term consumption of capital, using vintage weights for depreciation (physical capital) and interest (financial capital). These vintage weights reflect the proportion of capital-related purchases attributable to each year of the expected life of building and fixed equipment, movable equipment, and interest. We proposed to use vintage weights to compute vintage-weighted price changes associated with depreciation and interest expenses.
Capital-related costs are inherently complicated and are determined by complex capital-related purchasing decisions, over time, based on such factors as interest rates and debt financing. In addition, capital is depreciated over time instead of being consumed in the same period it is purchased. By accounting for the vintage nature of capital, we are able to provide an accurate and stable annual measure of price changes. Annual non-vintage price changes for capital are unstable due to the volatility of interest rate changes and, therefore, do not reflect the actual annual price changes for IPF capital-related costs. The capital-related component of the proposed 2016-based IPF market basket reflects the underlying stability of the capital-related acquisition process.
The methodology used to calculate the vintage weights for the 2016-based IPF market basket is the same as that used for the 2012-based IPF market basket with the only difference being the inclusion of more recent data. To calculate the vintage weights for depreciation and interest expenses, we first needed a time series of capital-related purchases for building and fixed equipment and movable equipment. We found no single source that provides an appropriate time series of capital-related purchases by hospitals for all of the listed components of capital purchases. The early Medicare cost reports did not have sufficient capital-related data to meet this need. Data we obtained from the American Hospital Association (AHA) do not include annual capital-related purchases. However, the AHA provided a consistent database of total expenses back to 1963. Consequently, we proposed to use data from the AHA Panel Survey and the AHA Annual Survey to obtain a time series of total expenses for hospitals. We then proposed to use data from the AHA Panel Survey supplemented with the ratio of depreciation to total hospital expenses obtained from the Medicare cost reports to derive a trend of annual depreciation expenses for 1963 through 2016. We proposed to separate these depreciation expenses into annual amounts of building and fixed equipment depreciation and movable equipment depreciation as previously determined. From these annual depreciation amounts we derived annual end-of-year book values for building and fixed equipment and movable equipment using the expected life for each type of asset category. While data are not available that are specific to IPFs, we believe this information for all hospitals serves as a reasonable alternative for the pattern of depreciation for IPFs.
To continue to calculate the vintage weights for depreciation and interest expenses, we also needed the expected lives for Building and Fixed Equipment, Movable Equipment, and Interest for the proposed 2016-based IPF market basket. We proposed to calculate the expected lives using Medicare cost report data from freestanding and hospital-based IPFs. The expected life of any asset can be determined by dividing the value of the asset (excluding fully depreciated assets) by its current year depreciation amount. This calculation yields the estimated expected life of an asset if the rates of depreciation were to continue at current year levels, assuming straight-line depreciation. We proposed to determine the expected life of building and fixed equipment separately for hospital-based IPFs and freestanding IPFs and weight these expected lives using the percent of total capital costs each provider type represents. We proposed to apply a similar method for movable equipment. Using these proposed methods, we determined the average expected life of building and fixed equipment to be equal to 22 years, and the average expected life of movable equipment to be equal to 11 years. For the expected life of interest, we believe vintage weights for interest should represent the average expected life of building and fixed equipment because, based on previous research described in the FY 1997 IPPS final rule (61 FR 46198), the expected life of hospital debt instruments and the expected life of buildings and fixed equipment are similar. We note that for the 2012-based IPF market basket the expected life of building and fixed equipment is 23 years and the expected life of movable equipment is 11 years.
Multiplying these expected lives by the annual depreciation amounts results in annual year-end asset costs for building and fixed equipment and movable equipment. We then calculated a time series, beginning in 1964, of annual capital purchases by subtracting the previous year's asset costs from the current year's asset costs.
For the building and fixed equipment and movable equipment vintage weights, we proposed to use the real annual capital-related purchase amounts for each asset type to capture the actual amount of the physical acquisition, net of the effect of price inflation. These real annual capital-related purchase amounts are produced by deflating the nominal annual purchase amount by the associated price proxy as provided. For the interest vintage weights, we proposed to use the total nominal annual capital-related purchase amounts to capture the value of the debt instrument (including, but not limited to, mortgages and bonds). Using these capital-related purchase time series specific to each asset type, we proposed to calculate the vintage weights for building and fixed equipment, for movable equipment, and for interest.
The vintage weights for each asset type are deemed to represent the average purchase pattern of the asset over its expected life (in the case of building and fixed equipment and interest, 22 years, and in the case of movable equipment, 11 years). For each asset type, we used the time series of annual capital-related purchase amounts available from 2016 back to 1964. These data allow us to derive thirty-two 22-year periods of capital-related purchases for building and fixed equipment and interest, and forty-two 11-year periods of capital-related purchases for movable equipment. For each 22-year period for building and fixed equipment and interest, or 11-year period for movable equipment, we calculated annual vintage weights by dividing the capital-related purchase amount in any given year by the total amount of purchases over the entire 22-year or 11-year period. This calculation is done for each year in the 22-year or 11-year period and for each of the periods for which we have data. We then calculated the average vintage weight for a given year of the expected life by taking the average of these vintage weights across the multiple periods of data. We did not receive any public comments on the methodology used to derive the vintage weights. In this final rule, we are finalizing the 2016-based IPF market basket vintage weights as proposed. Table 11 presents the vintage weights for the capital-related portion of the 2016-based IPF market basket and the 2012-based IPF market basket.
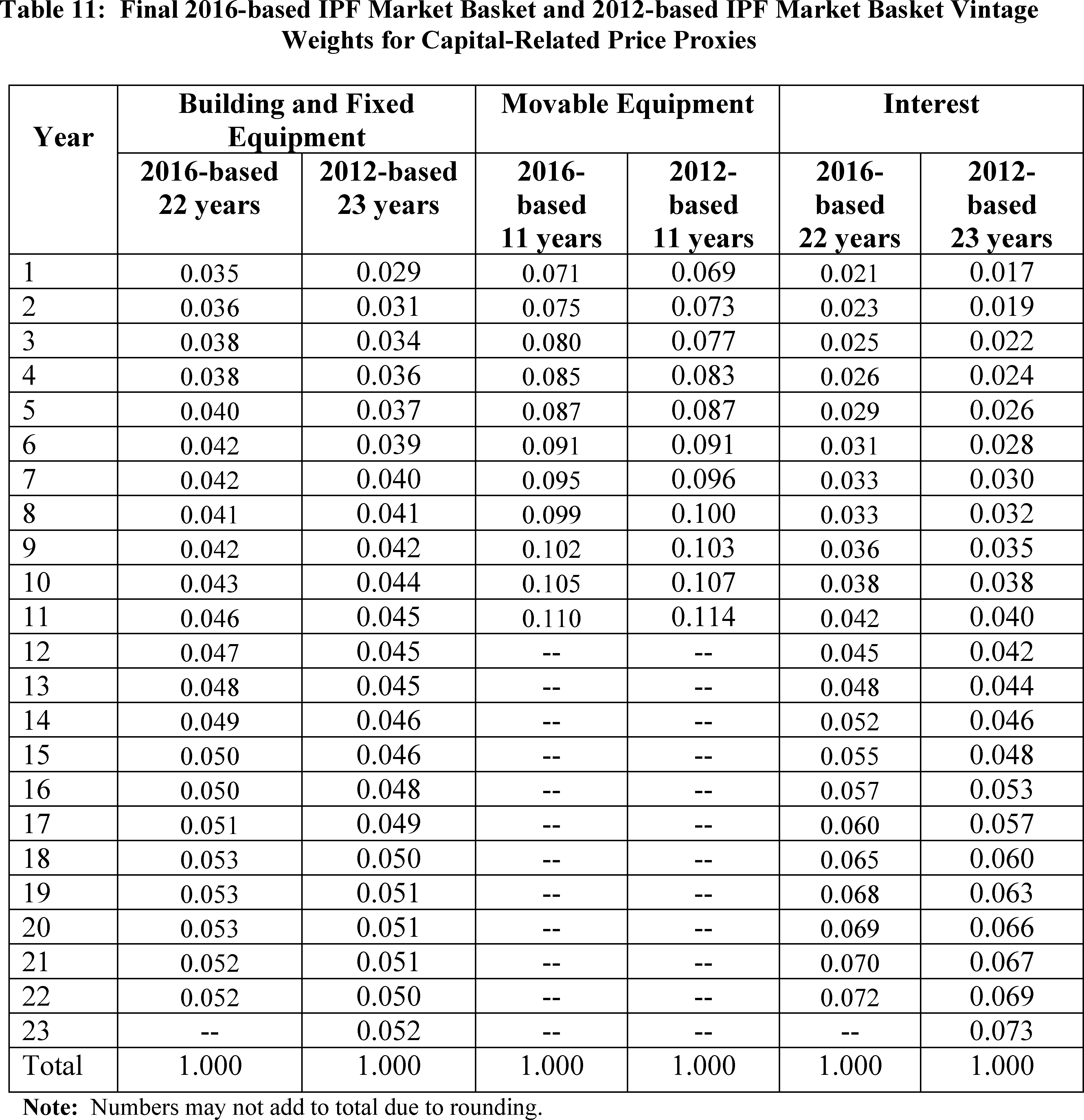
The process of creating vintage-weighted price proxies requires applying the vintage weights to the price proxy index where the last applied vintage weight in Table 11 is applied to the most recent data point. We have provided on the CMS website an example of how the vintage weighting price proxies are calculated, using example vintage weights and example price indices. The example can be found at the following link: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketResearch.html in the zip file titled “Weight Calculations as described in the IPPS FY 2010 Proposed Rule.”
iii. Summary of Price Proxies of the Final 2016-Based IPF Market Basket
Table 12 shows both the operating and capital price proxies for the 2016-based IPF market basket.
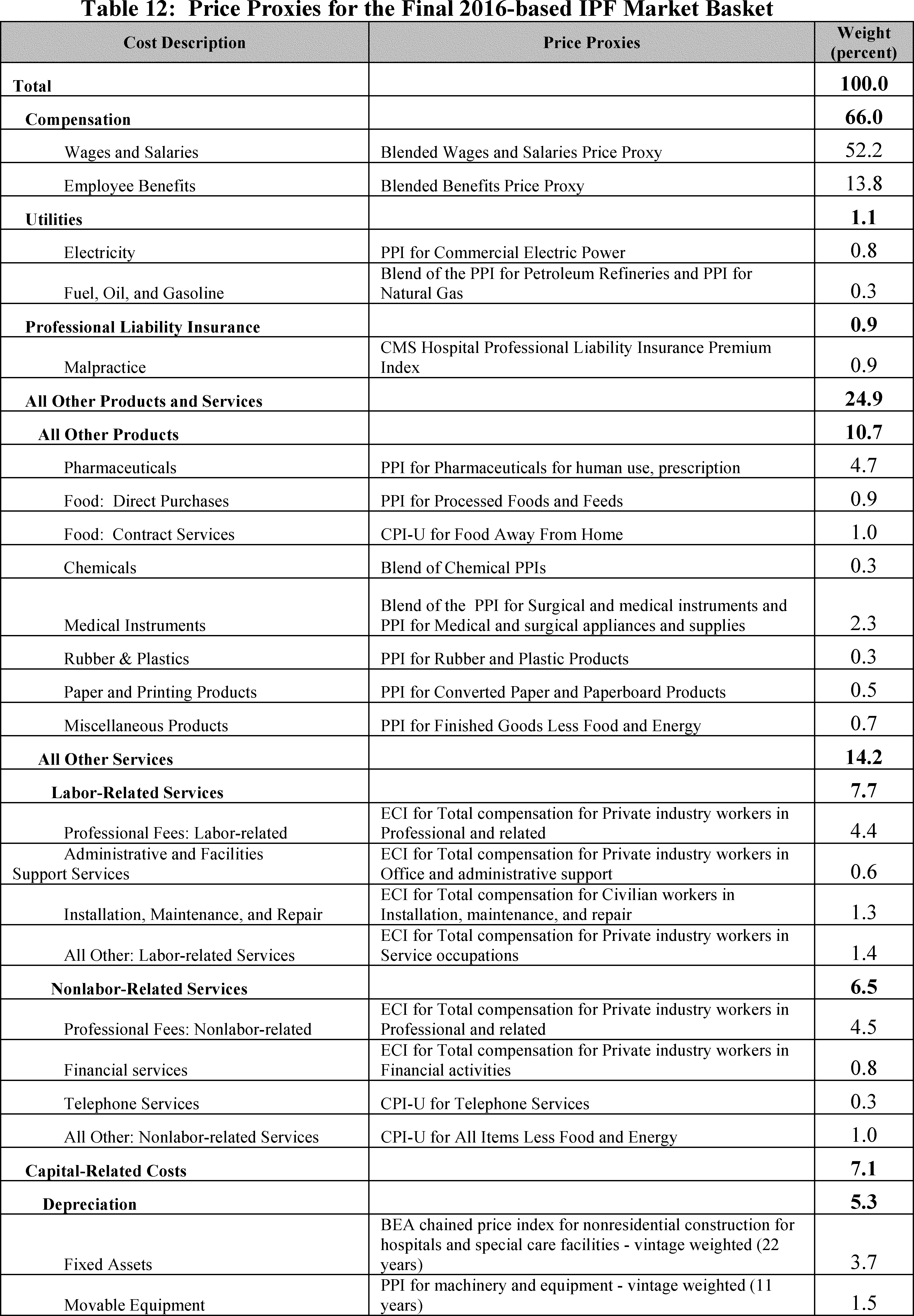

Comment: One commenter supported the proposal to rebase and revise the IPF market basket to reflect a 2016 base year from a 2012 base year—as this ensures the most recent cost data is utilized.
Response: We appreciate the commenter's support.
Final Decision: After careful consideration of public comments, we are finalizing the 2016-based IPF market basket as proposed.
4. FY 2020 Market Basket Update
For FY 2020 (that is, beginning October 1, 2019 and ending September 30, 2020), we proposed to use an estimate of the 2016-based IPF market basket increase factor to update the IPF PPS base payment rate. Consistent with historical practice, we estimate the market basket update for the IPF PPS based on IHS Global Inc.'s (IGI) forecast. IGI is a nationally recognized economic and financial forecasting firm that contracts with CMS to forecast the components of the market baskets and multifactor productivity (MFP). In the FY 2020 IPF proposed rule, we proposed a FY 2020 IPF market basket increase of 3.1 percent based on IGI's fourth quarter 2018 forecast with historical data through third quarter 2018. In the FY 2020 proposed rule, we also proposed that if more recent data are subsequently available (for example, a more recent estimate of the market basket and MFP adjustment) we would use such data, to determine the FY 2020 update in the final rule.
Table 13 compares the final 2016-based IPF market basket and the 2012-based IPF market basket percent changes using the most recent estimate based on IGI's second quarter 2019 forecast with historical data through the first quarter of 2019. The projected 2016-based IPF market basket increase factor for FY 2020 is 2.9 percent. For comparison, the current 2012-based IPF market basket is also projected to increase by 2.9 percent in FY 2020 based on IGI's second quarter 2019 forecast.
| Fiscal year (FY) | Final 2016- based IPF market basket index percent change | 2012-based IPF market basket index percent change |
|---|---|---|
| Historical data: | ||
| FY 2015 | 1.9 | 1.8 |
| FY 2016 | 1.9 | 1.9 |
| FY 2017 | 2.4 | 2.5 |
| FY 2018 | 2.6 | 2.6 |
| Average 2015-2018 | 2.2 | 2.2 |
| Forecast: | ||
| FY 2019 | 2.6 | 2.7 |
| FY 2020 | 2.9 | 2.9 |
| FY 2021 | 3.1 | 3.2 |
| FY 2022 | 3.1 | 3.1 |
| Average 2019-2022 | 2.9 | 3.0 |
| Note: These market basket percent changes do not include any further adjustments as may be statutorily required. Source: IHS Global Inc. 2nd quarter 2019 forecast. | ||
5. Productivity Adjustment
Section 1886(s)(2)(A)(i) of the Act requires the application of the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act to the IPF PPS for the RY beginning in 2012 (that is, a RY that coincides with a FY) and each subsequent RY. The statute defines the productivity adjustment to be equal to the 10-year moving average of changes in annual economy-wide private nonfarm business multifactor productivity (MFP) (as projected by the Secretary for the 10-year period ending with the applicable FY, year, cost reporting period, or other annual period) (the “MFP adjustment”). The BLS publishes the official measure of private non-farm business MFP. We refer readers to the BLS website at http://www.bls.gov/mfp for the BLS historical published MFP data.
MFP is derived by subtracting the contribution of labor and capital inputs growth from output growth. The projections of the components of MFP are currently produced by IGI, a nationally recognized economic forecasting firm with which CMS contracts to forecast the components of the market baskets and MFP. For more information on the productivity adjustment, we refer reader to the discussion in the FY 2016 IPF PPS final rule (80 FR 46675).
For the FY 2020 final rule, using IGI's second quarter 2019 forecast, the MFP adjustment for FY 2020 (the 10-year moving average of MFP for the period ending FY 2020) is projected to be 0.4 percent. Thus, in accordance with section 1886(s)(2)(A)(i) of the Act, we base the FY 2020 market basket update, which is used to determine the applicable percentage increase for the IPF payments, on the most recent estimate of the 2016-based IPF market basket (currently estimated to be 2.9 percent based on IGI's second quarter 2019 forecast). We then reduce this percentage increase of 2.9 percent by the current estimate of the MFP adjustment for FY 2020 of 0.4 percentage point (the 10-year moving average of MFP for the period ending FY 2020 based on IGI's second quarter 2019 forecast) yielding a productivity-adjusted IPF market basket update of 2.5 percent. In addition, for FY 2020 the 2016-based IPF PPS market basket update is further reduced by 0.75 percentage point as required by sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act. This results in a FY 2020 IPF PPS payment rate update of 1.75 percent (2.9−0.4−0.75 = 1.75 percent).
6. Labor-Related Share for FY 2020
Due to variations in geographic wage levels and other labor-related costs, we believe that payment rates under the IPF PPS should continue to be adjusted by a geographic wage index, which would apply to the labor-related portion of the Federal per diem base rate (hereafter referred to as the labor-related share). The labor-related share is determined by identifying the national average proportion of total costs that are related to, influenced by, or vary with the local labor market. We proposed to continue to classify a cost category as labor-related if the costs are labor intensive and vary with the local labor market.
We proposed to include in the labor-related share the sum of the relative importance of the following cost categories: Wages and Salaries, Employee Benefits, Professional Fees: Labor-related, Administrative and Facilities Support Services, Installation, Maintenance, and Repair, All Other: Labor-related Services, and a portion of the Capital-Related cost weight from the proposed 2016-based IPF market basket. These are the same categories as the 2012-based IPF market basket.
Similar to the 2012-based IPF market basket, the 2016-based IPF market basket includes two cost categories for nonmedical Professional fees (including but not limited to, expenses for legal, accounting, and engineering services). These are Professional Fees: Labor-related and Professional Fees: Nonlabor-related. For the 2016-based IPF market basket, we proposed to estimate the labor-related percentage of non-medical professional fees (and assign these expenses to the Professional Fees: Labor-related services cost category) based on the same method that was used to determine the labor-related percentage of professional fees in the 2012-based IPF market basket.
As done in the 2012-based IPF market basket, we proposed to determine the proportion of legal, accounting and auditing, engineering, and management consulting services that meet our definition of labor-related services based on a survey of hospitals conducted by CMS in 2008. We notified the public of our intent to conduct this survey on December 9, 2005 (70 FR 73250) and did not receive any public comments in response to the notice (71 FR 8588). A discussion of the composition of the survey and post-stratification can be found in the FY 2010 IPPS/LTCH PPS final rule (74 FR 43850 through 43856). Based on the weighted results of the survey, we determined that hospitals purchase, on average, the following portions of contracted professional services outside of their local labor market:
- 34 percent of accounting and auditing services.
- 30 percent of engineering services.
- 33 percent of legal services.
- 42 percent of management consulting services.
We proposed to apply each of these percentages to the respective 2012 Benchmark I-O cost category underlying the professional fees cost category to determine the Professional Fees: Nonlabor-related costs. The Professional Fees: Labor-related costs were determined to be the difference between the total costs for each Benchmark I-O category and the Professional Fees: Nonlabor-related costs. This is the same methodology that we used to separate the 2012-based IPF market basket professional fees category into Professional Fees: Labor-related and Professional Fees: Nonlabor-related cost categories.
In the 2016-based IPF market basket, nonmedical professional fees that were subject to allocation based on these survey results represent 3.6 percent of total costs (and are limited to those fees related to Accounting & Auditing, Legal, Engineering, and Management Consulting services). Based on our survey results, we proposed to apportion 2.3 percentage points of the 3.6 percentage point figure into the Professional Fees: Labor-related share cost category and designate the remaining 1.3 percentage point into the Professional Fees: Nonlabor-related cost category.
In addition to the professional services listed, for the 2016-based IPF market basket, we proposed to allocate a proportion of the Home Office Contract Labor cost weight, calculated using the Medicare cost reports, into the Professional Fees: Labor-related and Professional Fees: Nonlabor-related cost categories. We proposed to classify these expenses as labor-related and nonlabor-related as many facilities are not located in the same geographic area as their home office and, therefore, do not meet our definition for the labor-related share that requires the services to be purchased in the local labor market.
Similar to the 2012-based IPF market basket, we proposed for the 2016-based IPF market basket to use the Medicare cost reports for both freestanding IPF providers and hospital-based IPF providers to determine the home office labor-related percentages. The Medicare cost report requires a hospital to report information regarding their home office provider. Using information on the Medicare cost report, we then compare the location of the IPF with the location of the IPF's home office. We proposed to classify an IPF with a home office located in their respective labor market if the IPF and its home office are located in the same Metropolitan Statistical Area (MSA). We then determined the proportion of the Home Office Contract Labor cost weight that should be allocated to the labor-related share based on the percent of total Medicare allowable costs for those IPFs that had home offices located in their respective local labor markets of total Medicare allowable costs for IPFs with a home office. We determined an IPF's and its home office's MSA using their ZIP code information from the Medicare cost report. Using this methodology, we determined that 46 percent of IPFs' Medicare allowable costs were for home offices located in their respective local labor markets. Therefore, we proposed to allocate 46 percent of the Home Office Contract Labor cost weight (1.6 percentage points = 3.5 percent times 46 percent) to the Professional Fees: Labor-related cost weight and 54 percent of the Home Office Contract Labor cost weight to the Professional Fees: Nonlabor-related cost weight (1.9 percentage points = 3.5 percent times 54 percent). For the 2012-based IPF market basket, we used a similar methodology but we relied on provider counts rather than total Medicare allowable costs to determine the labor-related percentage.
In summary, based on the two allocations mentioned earlier, we apportioned percentage points of the professional fees and home office/related organization contract labor cost weights into the Professional Fees: Labor-Related cost category. This amount was added to the portion of professional fees that we already identified as labor-related using the I-O data such as contracted advertising and marketing costs (approximately 0.5 percentage point of total costs) resulting in a Professional Fees: Labor-Related cost weight of 4.4 percent.
As stated, we proposed to include in the labor-related share the sum of the relative importance of Wages and Salaries, Employee Benefits, Professional Fees: Labor-Related, Administrative and Facilities Support Services, Installation, Maintenance, and Repair, All Other: Labor-related Services, and a portion of the Capital-Related cost weight from the proposed 2016-based IPF market basket. The relative importance reflects the different rates of price change for these cost categories between the base year (2016) and FY 2020. Based on IHS Global Inc. 4th quarter 2018 forecast of the proposed 2016-based IPF market basket, we proposed a total labor-related share for FY 2020 of 76.8 percent (the sum of 73.7 percent for the operating cost and 3.1 percent for the labor-related share of Capital).
Comment: One commenter opposed the increase in the labor-related share from 74.8 percent to 76.8 percent stating it would negatively impact any facility with a wage index below 1.0. The growing disparity in wage indices places facilities in low wage areas at a significant disadvantage, and this proposal will further increase that disparity. They encouraged CMS to maintain the FY 2019 labor-related share in FY 2020.
Response: For FY 2020, we proposed the FY 2020 labor-related share to be equal to the sum of the relative importance of shares of the following proposed 2016-based IPF market basket cost categories: Wages and Salaries, Employee Benefits, Professional Fees: Labor-Related, Administrative and Facilities Support Services, Installation, Maintenance, and Repair, All Other: Labor-related Services, and a portion of the Capital-Related cost weight. The FY 2019 labor-related share reflected the sum of the relative importance shares of the same categories using the 2012-based IPF market basket.
The increase in the labor-related share from FY 2019 to FY 2020 is mostly a result of the rebasing and revising of the IPF market basket to reflect more recent data. Of the 2.0-percentage point difference between the FY 2020 labor-related share using the proposed 2016-based IPF market basket and the labor share used in FY 2019, 1.9 percentage point is from rebasing the market basket. The detailed factors contributing to the difference are: 0.6 percentage point is due to an increase in the Compensation and Capital cost weights as a result of incorporating the 2016 MCR data, 0.3 percentage point is due to revising the starting point of the calculation of the relative importance from 2012 to 2016, 0.3 percentage point is due to the use of MCR data to calculate the Home Office Contract Labor cost weight (a portion of which is included in the Professional Fees: Labor-related services cost weight), and the remaining 0.7 percentage point is due to the incorporation of the 2012 Benchmark I-O data, primarily stemming from an increase in the Professional Fees: Labor-related cost weight.
We appreciate the commenter's concern over the increase in the labor-related share; however, we believe it is technically appropriate to use the 2016-based IPF market basket to determine the labor-related share for FY 2020 as it is based on more recent data regarding price pressures and cost structure of IPFs. Our policy to use the most recent market basket to determine the labor-related share is a policy we have regularly adopted for the IPF PPS as well as for other PPSs including but not limited to the IPPS, the Inpatient Rehabilitation Facility PPS, and the Long-term care hospital PPS.
Final Decision: After careful consideration of comments, in this final rule, we are finalizing the 2016-based IPF market basket labor-related share cost weights as proposed.
Based on IHS Global Inc. 2nd quarter 2019 forecast of the 2016-based IPF market basket, the sum of the FY 2020 relative importance for Wages and Salaries, Employee Benefits, Professional Fees: Labor-related, Administrative and Facilities Support Services, Installation Maintenance & Repair Services, and All Other: Labor-related Services is 73.8 percent. The portion of Capital costs that is influenced by the local labor market is estimated to be 46 percent, which is the same percentage applied to the 2012-based IPF market basket. Since the relative importance for Capital is 6.8 percent of the 2016-based IPF market basket in FY 2020, we took 46 percent of 6.8 percent to determine the proposed labor-related share of Capital for FY 2020 of 3.1 percent. Therefore, we are finalizing a total labor-related share for FY 2020 of 76.9 percent (the sum of 73.8 percent for the operating cost and 3.1 percent for the labor-related share of Capital).
Table 14 shows the FY 2020 labor-related share using the final 2016-based IPF market basket relative importance and the FY 2019 labor-related share using the 2012-based IPF market basket.

B. Updates to the IPF PPS Rates for FY Beginning October 1, 2019
The IPF PPS is based on a standardized federal per diem base rate calculated from the IPF average per diem costs and adjusted for budget-neutrality in the implementation year. The federal per diem base rate is used as the standard payment per day under the IPF PPS and is adjusted by the patient-level and facility-level adjustments that are applicable to the IPF stay. A detailed explanation of how we calculated the average per diem cost appears in the November 2004 IPF PPS final rule (69 FR 66926).
1. Determining the Standardized Budget-Neutral Federal Per Diem Base Rate
Section 124(a)(1) of the BBRA required that we implement the IPF PPS in a budget-neutral manner. In other words, the amount of total payments under the IPF PPS, including any payment adjustments, must be projected to be equal to the amount of total payments that would have been made if the IPF PPS were not implemented. Therefore, we calculated the budget-neutrality factor by setting the total estimated IPF PPS payments to be equal to the total estimated payments that would have been made under the Tax Equity and Fiscal Responsibility Act of 1982 (TEFRA) (Pub. L. 97-248) methodology had the IPF PPS not been implemented. A step-by-step description of the methodology used to estimate payments under the TEFRA payment system appears in the November 2004 IPF PPS Final rule (69 FR 66926).
Under the IPF PPS methodology, we calculated the final federal per diem base rate to be budget-neutral during the IPF PPS implementation period (that is, the 18-month period from January 1, 2005 through June 30, 2006) using a July 1 update cycle. We updated the average cost per day to the midpoint of the IPF PPS implementation period (October 1, 2005), and this amount was used in the payment model to establish the budget-neutrality adjustment.
Next, we standardized the IPF PPS federal per diem base rate to account for the overall positive effects of the IPF PPS payment adjustment factors by dividing total estimated payments under the TEFRA payment system by estimated payments under the IPF PPS. Additional information concerning this standardization can be found in the November 2004 IPF PPS final rule (69 FR 66932) and the RY 2006 IPF PPS final rule (71 FR 27045). We then reduced the standardized federal per diem base rate to account for the outlier policy, the stop loss provision, and anticipated behavioral changes. A complete discussion of how we calculated each component of the budget-neutrality adjustment appears in the November 2004 IPF PPS final rule (69 FR 66932 through 66933) and in the RY 2007 IPF PPS final rule (71 FR 27044 through 27046). The final standardized budget-neutral federal per diem base rate established for cost reporting periods beginning on or after January 1, 2005 was calculated to be $575.95.
The federal per diem base rate has been updated in accordance with applicable statutory requirements and § 412.428 through publication of annual notices or proposed and final rules. A detailed discussion on the standardized budget-neutral federal per diem base rate and the electroconvulsive therapy (ECT) payment per treatment appears in the FY 2014 IPF PPS update notice (78 FR 46738 through 46740). These documents are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/index.html.
IPFs must include a valid procedure code for ECT services provided to IPF beneficiaries in order to bill for ECT services, as described in our Medicare Claims Processing Manual, Chapter 3, Section 190.7.3 (available at https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf.) There were no changes to the ECT procedure codes used on IPF claims as a result of the proposed update to the ICD-10-PCS code set for FY 2020. Addendum B-4 to this final rule shows the ECT procedure codes for FY 2020 and is available on our website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html.
2. Update of the Federal Per Diem Base Rate and Electroconvulsive Therapy Payment Per Treatment
The current (FY 2019) federal per diem base rate is $782.78 and the ECT payment per treatment is $337.00. For the FY 2020 federal per diem base rate, we applied the payment rate update of 1.75 percent (that is, the 2016-based IPF market basket increase for FY 2020 of 2.9 percent less the productivity adjustment of 0.4 percentage point, and further reduced by the 0.75 percentage point required under section 1886(s)(3)(E) of the Act), and the wage index budget-neutrality factor of 1.0026 (as discussed in section III.D.1.f of this final rule) to the FY 2019 federal per diem base rate of $782.78, yielding a federal per diem base rate of $798.55 for FY 2020. Similarly, we applied the 1.75 percent payment rate update and the 1.0026 wage index budget-neutrality factor to the FY 2019 ECT payment per treatment of $337.00, yielding an ECT payment per treatment of $343.79 for FY 2020.
Section 1886(s)(4)(A)(i) of the Act requires that for RY 2014 and each subsequent RY, in the case of an IPF that fails to report required quality data with respect to such rate year, the Secretary will reduce any annual update to a standard federal rate for discharges during the RY by 2.0 percentage points. Therefore, we are applying a 2.0 percentage point reduction to the federal per diem base rate and the ECT payment per treatment as follows:
- For IPFs that fail requirements under the Inpatient Psychiatric Facilities Quality Reporting (IPFQR) Program, we applied a −0.25 percent payment rate update (that is, the IPF market basket increase for FY 2020 of 2.9 percent less the productivity adjustment of 0.4 percentage point, further reduced by the 0.75 percentage point for an update of 1.75 percent, and further reduced by 2 percentage points in accordance with section 1886(s)(4)(A)(ii) of the Act, which results in a negative update percentage) and the wage index budget-neutrality factor of 1.0026 to the FY 2019 federal per diem base rate of $782.78, yielding a federal per diem base rate of $782.85 for FY 2020.
- For IPFs that fail to meet requirements under the IPFQR Program, we applied the −0.25 percent annual payment rate update and the 1.0026 wage index budget-neutrality factor to the FY 2019 ECT payment per treatment of $337.00, yielding an ECT payment per treatment of $337.03 for FY 2020.
C. Updates to the IPF PPS Patient-Level Adjustment Factors
1. Overview of the IPF PPS Adjustment Factors
The IPF PPS payment adjustments were derived from a regression analysis of 100 percent of the FY 2002 Medicare Provider and Analysis Review (MedPAR) data file, which contained 483,038 cases. For a more detailed description of the data file used for the regression analysis, see the November 2004 IPF PPS final rule (69 FR 66935 through 66936). We are finalizing our proposal to continue to use the existing regression-derived adjustment factors established in 2005 for FY 2020. However, we have used more recent claims data to simulate payments to finalize the outlier fixed dollar loss threshold amount and to assess the impact of the IPF PPS updates.
2. IPF PPS Patient-Level Adjustments
The IPF PPS includes payment adjustments for the following patient-level characteristics: Medicare Severity Diagnosis Related Groups (MS-DRGs) assignment of the patient's principal diagnosis, selected comorbidities, patient age, and the variable per diem adjustments.
a. Update to MS-DRG Assignment
We believe it is important to maintain for IPFs the same diagnostic coding and Diagnosis Related Group (DRG) classification used under the Inpatient Prospective Payment System (IPPS) for providing psychiatric care. For this reason, when the IPF PPS was implemented for cost reporting periods beginning on or after January 1, 2005, we adopted the same diagnostic code set (ICD-9-CM) and DRG patient classification system (MS-DRGs) that were utilized at the time under the IPPS. In the RY 2009 IPF PPS notice (73 FR 25709), we discussed CMS' effort to better recognize resource use and the severity of illness among patients. CMS adopted the new MS-DRGs for the IPPS in the FY 2008 IPPS final rule with comment period (72 FR 47130). In the RY 2009 IPF PPS notice (73 FR 25716), we provided a crosswalk to reflect changes that were made under the IPF PPS to adopt the new MS-DRGs. For a detailed description of the mapping changes from the original DRG adjustment categories to the current MS-DRG adjustment categories, we refer readers to the RY 2009 IPF PPS notice (73 FR 25714).
The IPF PPS includes payment adjustments for designated psychiatric DRGs assigned to the claim based on the patient's principal diagnosis. The DRG adjustment factors were expressed relative to the most frequently reported psychiatric DRG in FY 2002, that is, DRG 430 (psychoses). The coefficient values and adjustment factors were derived from the regression analysis discussed in detail in the November 28, 2003 IPF proposed rule (68 FR 66923; 66928 through 66933) and the November 15, 2004 IPF final rule (69 FR 66933 through 66960). Mapping the DRGs to the MS-DRGs resulted in the current 17 IPF MS-DRGs, instead of the original 15 DRGs, for which the IPF PPS provides an adjustment. For FY 2020, we did not propose any changes to the IPF MS-DRG adjustment factors but are finalizing our proposal to maintain the existing IPF MS-DRG adjustment factors.
In the FY 2015 IPF PPS final rule published August 6, 2014 in the Federal Register titled, “Inpatient Psychiatric Facilities Prospective Payment System—Update for FY Beginning October 1, 2014 (FY 2015)” (79 FR 45945 through 45947), we finalized conversions of the ICD-9-CM-based MS-DRGs to ICD-10-CM/PCS-based MS-DRGs, which were implemented on October 1, 2015. Further information on the ICD-10-CM/PCS MS-DRG conversion project can be found on the CMS ICD-10-CM website at https://www.cms.gov/Medicare/Coding/ICD10/ICD-10-MS-DRG-Conversion-Project.html.
For FY 2020, we are finalizing our proposal to continue to make the existing payment adjustment for psychiatric diagnoses that group to one of the existing 17 IPF MS-DRGs listed in Addendum A. Addendum A is available on our website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html. Psychiatric principal diagnoses that do not group to one of the 17 designated MS-DRGs will still receive the federal per diem base rate and all other applicable adjustments, but the payment will not include an MS-DRG adjustment.
The diagnoses for each IPF MS-DRG will be updated as of October 1, 2019, using the final IPPS FY 2020 ICD-10-CM/PCS code sets. The FY 2020 IPPS final rule includes tables of the final changes to the ICD-10-CM/PCS code sets which underlie the FY 2020 IPF MS-DRGs. Both the FY 2020 IPPS final rule and the tables of proposed changes to the ICD-10-CM/PCS code sets which underlie the FY 2020 MS-DRGs are available on the IPPS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
Code First
As discussed in the ICD-10-CM Official Guidelines for Coding and Reporting, certain conditions have both an underlying etiology and multiple body system manifestations due to the underlying etiology. For such conditions, the ICD-10-CM has a coding convention that requires the underlying condition be sequenced first followed by the manifestation. Wherever such a combination exists, there is a “use additional code” note at the etiology code, and a “code first” note at the manifestation code. These instructional notes indicate the proper sequencing order of the codes (etiology followed by manifestation). In accordance with the ICD-10-CM Official Guidelines for Coding and Reporting, when a primary (psychiatric) diagnosis code has a “code first” note, the provider would follow the instructions in the ICD-10-CM text. The submitted claim goes through the CMS processing system, which will identify the primary diagnosis code as non-psychiatric and search the secondary codes for a psychiatric code to assign a DRG code for adjustment. The system will continue to search the secondary codes for those that are appropriate for comorbidity adjustment.
For more information on the code first policy, see our November 2004 IPF PPS final rule (69 FR 66945) and see sections I.A.13 and I.B.7 of the FY 2019 ICD-10-CM Coding Guidelines, available at https://www.cdc.gov/nchs/icd/data/10cmguidelines-FY2019-final.pdf. In the FY 2015 IPF PPS final rule, we provided a code first table for reference that highlights the same or similar manifestation codes where the code first instructions apply in ICD-10-CM that were present in ICD-9-CM (79 FR 46009). In FY 2018 and FY 2019, there were no changes to the final ICD-10-CM/PCS codes in the IPF Code First table. For FY 2020, there continue to be no changes to the ICD-10-CM/PCS codes in the proposed IPF Code First table. The final FY 2020 Code First table is shown in Addendum B-1 on our website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html.
b. Payment for Comorbid Conditions
The intent of the comorbidity adjustments is to recognize the increased costs associated with comorbid conditions by providing additional payments for certain existing medical or psychiatric conditions that are expensive to treat. In our RY 2012 IPF PPS final rule (76 FR 26451 through 26452), we explained that the IPF PPS includes 17 comorbidity categories and identified the new, revised, and deleted ICD-9-CM diagnosis codes that generate a comorbid condition payment adjustment under the IPF PPS for RY 2012 (76 FR 26451).
Comorbidities are specific patient conditions that are secondary to the patient's principal diagnosis and that require treatment during the stay. Diagnoses that relate to an earlier episode of care and have no bearing on the current hospital stay are excluded and must not be reported on IPF claims. Comorbid conditions must exist at the time of admission or develop subsequently, and affect the treatment received, length of stay (LOS), or both treatment and LOS.
For each claim, an IPF may receive only one comorbidity adjustment within a comorbidity category, but it may receive an adjustment for more than one comorbidity category. Current billing instructions for discharge claims, on or after October 1, 2015, require IPFs to enter the complete ICD-10-CM codes for up to 24 additional diagnoses if they co-exist at the time of admission, or develop subsequently and impact the treatment provided.
The comorbidity adjustments were determined based on the regression analysis using the diagnoses reported by IPFs in FY 2002. The principal diagnoses were used to establish the DRG adjustments and were not accounted for in establishing the comorbidity category adjustments, except where ICD-9-CM code first instructions applied. In a code first situation, the submitted claim goes through the CMS processing system, which will identify the principal diagnosis code as non-psychiatric and search the secondary codes for a psychiatric code to assign an MS-DRG code for adjustment. The system will continue to search the secondary codes for those that are appropriate for comorbidity adjustment.
As noted previously, it is our policy to maintain the same diagnostic coding set for IPFs that is used under the IPPS for providing the same psychiatric care. The 17 comorbidity categories formerly defined using ICD-9-CM codes were converted to ICD-10-CM/PCS in our FY 2015 IPF PPS final rule (79 FR 45947 through 45955). The goal for converting the comorbidity categories is referred to as replication, meaning that the payment adjustment for a given patient encounter is the same after ICD-10-CM implementation as it would be if the same record had been coded in ICD-9-CM and submitted prior to ICD-10-CM/PCS implementation on October 1, 2015. All conversion efforts were made with the intent of achieving this goal. For FY 2020, we are finalizing our proposal to continue to use the same comorbidity adjustment factors in effect in FY 2019, which are found in Addendum A, available on our website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html.
We have updated the ICD-10-CM/PCS codes which are associated with the existing IPF PPS comorbidity categories, based upon the final FY 2020 update to the ICD-10-CM/PCS code set. The final FY 2020 ICD-10-CM/PCS updates include 4 ICD-10-CM diagnosis codes added to the Poisoning comorbidity category and 88 ICD-10-PCS codes added to the Oncology Procedures comorbidity category. In addition, 3 ICD-10-PCS codes were deleted from the Oncology Procedures comorbidity category. These updates are detailed in Addenda B-2 and B-3 of this final rule, which are available on our website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html.
In accordance with the policy established in the FY 2015 IPF PPS final rule (79 FR 45949 through 45952), we reviewed all new FY 2020 ICD-10-CM codes to remove codes that were site “unspecified” in terms of laterality from the FY 2020 ICD-10-CM/PCS codes in instances where more specific codes are available. As we stated in the FY 2015 IPF PPS final rule, we believe that specific diagnosis codes that narrowly identify anatomical sites where disease, injury, or a condition exists should be used when coding patients' diagnoses whenever these codes are available. We finalized that we would remove site “unspecified” codes from the IPF PPS ICD-10-CM/PCS codes in instances when laterality codes (site specified codes) are available, as the clinician should be able to identify a more specific diagnosis based on clinical assessment at the medical encounter. None of the proposed additions to the FY 2020 ICD-10-CM/PCS codes were site “unspecified” by laterality, therefore we are not removing any of the new codes.
c. Patient Age Adjustments
As explained in the November 2004 IPF PPS final rule (69 FR 66922), we analyzed the impact of age on per diem cost by examining the age variable (range of ages) for payment adjustments. In general, we found that the cost per day increases with age. The older age groups are more costly than the under 45 age group, the differences in per diem cost increase for each successive age group, and the differences are statistically significant. For FY 2020, we are finalizing our proposal to continue to use the patient age adjustments currently in effect in FY 2019, as shown in Addendum A of this rule (see https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html).
d. Variable Per Diem Adjustments
We explained in the November 2004 IPF PPS final rule (69 FR 66946) that the regression analysis indicated that per diem cost declines as the length of stay (LOS) increases. The variable per diem adjustments to the federal per diem base rate account for ancillary and administrative costs that occur disproportionately in the first days after admission to an IPF. As discussed in the November 2004 IPF PPS final rule, we used a regression analysis to estimate the average differences in per diem cost among stays of different lengths (69 FR 66947 to 66950). As a result of this analysis, we established variable per diem adjustments that begin on day 1 and decline gradually until day 21 of a patient's stay. For day 22 and thereafter, the variable per diem adjustment remains the same each day for the remainder of the stay. However, the adjustment applied to day 1 depends upon whether the IPF has a qualifying ED. If an IPF has a qualifying ED, it receives a 1.31 adjustment factor for day 1 of each stay. If an IPF does not have a qualifying ED, it receives a 1.19 adjustment factor for day 1 of the stay. The ED adjustment is explained in more detail in section III.D.4 of this rule.
For FY 2020, we are finalizing our proposal to continue to use the variable per diem adjustment factors currently in effect, as shown in Addendum A of this rule (available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html). A complete discussion of the variable per diem adjustments appears in the November 2004 IPF PPS final rule (69 FR 66946).
D. Updates to the IPF PPS Facility-Level Adjustments
The IPF PPS includes facility-level adjustments for the wage index, IPFs located in rural areas, teaching IPFs, cost of living adjustments for IPFs located in Alaska and Hawaii, and IPFs with a qualifying ED.
1. Wage Index Adjustment
a. Background
As discussed in the RY 2007 IPF PPS final rule (71 FR 27061), RY 2009 IPF PPS (73 FR 25719) and the RY 2010 IPF PPS notices (74 FR 20373), in order to provide an adjustment for geographic wage levels, the labor-related portion of an IPF's payment is adjusted using an appropriate wage index. Currently, an IPF's geographic wage index value is determined based on the actual location of the IPF in an urban or rural area, as defined in § 412.64(b)(1)(ii)(A) and (C).
b. Change to the IPF Wage Index Methodology
Due to the variation in costs and because of the differences in geographic wage levels, in the November 15, 2004 IPF PPS final rule, we required that payment rates under the IPF PPS be adjusted by a geographic wage index. We proposed and finalized a policy to use the unadjusted, pre-floor, pre-reclassified IPPS hospital wage index to account for geographic differences in IPF labor costs. We implemented use of the pre-floor, pre-reclassified IPPS hospital wage data to compute the IPF wage index since there was not an IPF-specific wage index available. We believe that IPFs generally compete in the same labor market as IPPS hospitals so the pre-floor, pre-reclassified IPPS hospital wage data should be reflective of labor costs of IPFs. We believe this pre-floor, pre-reclassified IPPS hospital wage index to be the best available data to use as proxy for an IPF specific wage index. As discussed in the rate year (RY) 2007 IPF PPS final rule (71 FR 27061 through 27067), under the IPF PPS, the wage index is calculated using the IPPS wage index for the labor market area in which the IPF is located, without taking into account geographic reclassifications, floors, and other adjustments made to the wage index under the IPPS. For a complete description of these IPPS wage index adjustments, we refer readers to the FY 2019 IPPS/LTCH PPS final rule (83 FR 41362 through 41390). Our wage index policy was put into regulation at 412.424(a)(2), and requires us to use the best Medicare data available to estimate costs per day, including an appropriate wage index to adjust for wage differences.
When the IPF PPS was implemented in the November 15, 2004 IPF PPS final rule, with an effective date of January 1, 2005, the pre-floor, pre-reclassified IPPS hospital wage index that was available at the time was the FY 2005 pre-floor, pre-reclassified IPPS hospital wage index. Historically, the IPF wage index for a given RY has used the pre-floor, pre-reclassified IPPS hospital wage index from the prior fiscal year as its basis. This has been due in part to the pre-floor, pre-reclassified IPPS hospital wage index data that were available during the IPF rulemaking cycle, where an annual IPF notice or IPF final rule was usually published in early May. This publication timeframe was relatively early compared to other Medicare payment rules because the IPF PPS follows an RY, which was defined in the implementation of the IPF PPS as the 12-month period from July 1 to June 30 (69 FR 66927). Therefore the best available data at the time the IPF PPS was implemented was the pre-floor, pre-reclassified IPPS hospital wage index from the prior fiscal year (for example, the RY 2006 IPF wage index was based on the FY 2005 pre-floor, pre-reclassified IPPS hospital wage index).
In the RY 2012 IPF PPS final rule, we changed the reporting year timeframe for IPFs from a RY to the FY, which begins October 1 and ends September 30 (76 FR 26434 through 26435). In that FY 2012 IPF PPS final rule, we continued our established policy of using the pre-floor, pre-reclassified IPPS hospital wage index from the prior year (that is, from FY 2011) as the basis for the FY 2012 IPF wage index. This policy of basing a wage index on the prior year's pre-floor, pre-reclassified IPPS hospital wage index has been followed by other Medicare payment systems, such as hospice and inpatient rehabilitation facilities. By continuing with our established policy, we remained consistent with other Medicare payment systems.
We proposed to change the IPF wage index methodology to align the IPF PPS wage index with the same wage data timeframe used by the IPPS for FY 2020 and subsequent years. Specifically, we proposed to use the pre-floor, pre-reclassified IPPS hospital wage index from the fiscal year concurrent with the IPF fiscal year as the basis for the IPF wage index. For example, the FY 2020 IPF wage index would be based on the FY 2020 pre-floor, pre-reclassified IPPS hospital wage index rather than on the FY 2019 pre-floor, pre-reclassified IPPS hospital wage index.
We explained in the proposed rule (84 FR 16973), that using the concurrent pre-floor, pre-reclassified IPPS hospital wage index would result in the most up-to-date wage data being the basis for the IPF wage index. It would also result in more consistency and parity in the wage index methodology used by other Medicare payment systems. The Medicare SNF PPS already uses the concurrent IPPS hospital wage index data as the basis for the SNF PPS wage index. Thus, the wage adjusted Medicare payments of various provider types would be based upon wage index data from the same timeframe. CMS proposed similar policies to use the concurrent pre-floor, pre-reclassified IPPS hospital wage index data in other Medicare payment systems, such as hospice and inpatient rehabilitation facilities.
For FY 2020, we also proposed to continue use the pre-floor, pre-reclassified IPPS hospital wage index as the basis for the IPF wage index.
We received 1 comment on our proposal to align the IPF wage index data timeframe with that of the IPPS, by using the concurrent pre-floor, pre-reclassified IPPS hospital wage index as the basis for the IPF wage index for FY 2020 and subsequent years.
Comment: A commenter wrote that he was not opposed to the proposal to eliminate the 1-year lag in the wage index data, but had issues with the data itself. The commenter was opposed to using the FY 2020 IPPS wage index data file discussed in the FY 2020 IPPS proposed rule because the data excluded several hospitals which had wage data based upon regional rather than local labor market rates. The commenter felt this exclusion was inappropriate and that it would negatively affect certain IPFs.
Response: We appreciate the comment, however, we are finalizing our proposal to use the concurrent pre-floor, pre-reclassified IPPS hospital wage index as the basis for IPF wage index for FY 2020 and subsequent years. For FY 2020, we are also finalizing our proposal to continue to use the pre-floor, pre-reclassified IPPS hospital wage index as the basis for the IPF wage index. We believe it is the best available data to use as a proxy for an IPF wage index. This pre-floor, pre-reclassified IPPS hospital wage index is also the most appropriate wage index as IPFs compete in the same labor market as IPPS hospitals; this wage index best reflects the variation in local labor costs of IPFs in the various geographic areas using the most recent IPPS hospital wage data (data from hospital cost reports for the cost reporting period beginning during FY 2016) without any geographic reclassifications, floors, or other adjustments. We will apply the FY 2020 IPF wage index to payments beginning October 1, 2019.
We identified a slight error in the proposed rule wage index values after the FY 2020 IPF PPS proposed rule was published. A programming error caused the data for all providers in a single county to be included twice, which affected the national average hourly rate, and therefore affected nearly all wage index values. We have changed the programming logic so this error cannot occur again. In addition, we corrected the classification of one provider in North Carolina that was erroneously identified as being in an urban CBSA. We also standardized our procedures for rounding, to ensure consistency. The correction to the NPRM wage index data was not completed until after the comment period closed on June 17, 2019. This final rule reflects the corrected and updated wage index data.
We are finalizing this change to the IPF wage index methodology to implement it in a budget-neutral manner, so that total IPF payments will not be affected. However, as shown in Table 15, there will be distributional effects. Table 15 compares the estimated payments calculated using the FY 2020 IPF wage index based on the IPPS hospital wage index data from the prior fiscal year (the current methodology) with the estimated payments calculated using the FY 2020 IPF wage index based on concurrent IPPS hospital wage index data (the proposed change in methodology which we are finalizing). Due to budget neutrality, the effect on total estimated FY 2020 IPF payments is zero. Table 15 shows that urban IPFs are estimated to experience a smaller increase in payments by finalizing the proposed methodology (0.03 percent increase) compared to if we had maintained the current methodology (0.09 percent increase). Rural IPFs are estimated to have a smaller decrease in estimated payments by finalizing the proposed methodology (0.20 percent decrease) compared to if we had maintained the current methodology (0.54 percent decrease).

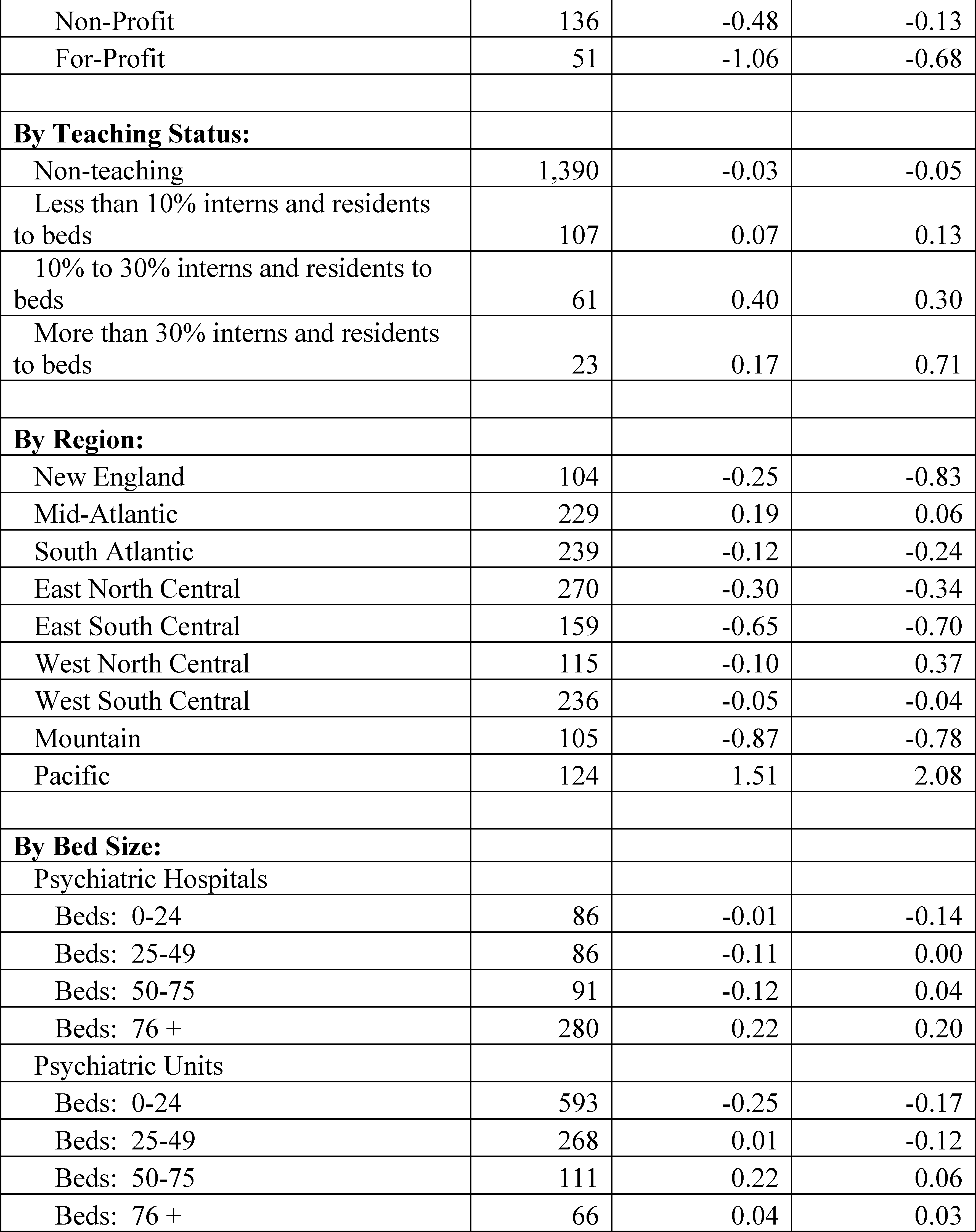
To provide additional information to IPFs about the effect of implementing this change in the IPF wage index methodology on estimated payments, we have also posted a provider-level table of effects (Addendum C) on the CMS website, available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/WageIndex.html.
We are applying the IPF wage index adjustment to the labor-related share of the national base rate or ECT payment per treatment. The labor-related share of the national rate and ECT payment per treatment will change from 74.8 percent in FY 2019 to 76.9 percent in FY 2020. This percentage reflects the labor-related share of the 2016-based IPF market basket for FY 2020 (see section III.A.6 of this rule).
c. Office of Management and Budget Bulletins
OMB publishes bulletins regarding CBSA changes, including changes to CBSA numbers and titles. In the RY 2007 IPF PPS final rule (71 FR 27061 through 27067), we adopted the changes discussed in the OMB Bulletin No. 03-04 (June 6, 2003), which announced revised definitions for MSAs, and the creation of Micropolitan Statistical Areas and Combined Statistical Areas. In adopting the OMB CBSA geographic designations in RY 2007, we did not provide a separate transition for the CBSA-based wage index since the IPF PPS was already in a transition period from TEFRA payments to PPS payments.
In the RY 2009 IPF PPS notice, we incorporated the CBSA nomenclature changes published in the most recent OMB bulletin that applied to the IPPS hospital wage index used to determine the current IPF wage index and stated that we expected to continue to do the same for all the OMB CBSA nomenclature changes in future IPF PPS rules and notices, as necessary (73 FR 25721). The OMB bulletins may be accessed online at https://www.whitehouse.gov/omb/information-for-agencies/bulletins/.
In accordance with our established methodology, we have historically adopted any CBSA changes that are published in the OMB bulletin that corresponds with the IPPS hospital wage index used to determine the IPF wage index. For the FY 2015 IPF wage index, we used the FY 2014 pre-floor, pre-reclassified IPPS hospital wage index to adjust the IPF PPS payments. On February 28, 2013, OMB issued OMB Bulletin No. 13-01, which established revised delineations for MSAs, Micropolitan Statistical Areas, and Combined Statistical Areas in the United States and Puerto Rico based on the 2000 Census, and provided guidance on the use of the delineations of these statistical areas. A copy of this bulletin may be obtained at https://www.whitehouse.gov/omb/information-for-agencies/bulletins/.
Because the FY 2014 pre-floor, pre-reclassified IPPS hospital wage index did not reflect the statistical area revisions set forth in OMB Bulletin 13-01, the FY 2015 IPF PPS wage index, which was based on the FY 2014 pre-floor, pre-reclassified IPPS hospital wage index, did not reflect OMB's new area delineations based on the 2010 Census. According to OMB, “[t]his bulletin provides the delineations of all Metropolitan Statistical Areas, Metropolitan Divisions, Micropolitan Statistical Areas, Combined Statistical Areas, and New England City and Town Areas in the United States and Puerto Rico based on the standards published on June 28, 2010, in the Federal Register (75 FR 37246 through 37252) and Census Bureau data.” These OMB Bulletin changes are reflected in the FY 2015 pre-floor, pre-reclassified IPPS hospital wage index, upon which the FY 2016 IPF wage index was based. We adopted these new OMB CBSA delineations in the FY 2016 IPF wage index and subsequent IPF wage indexes.
Generally, OMB issues major revisions to statistical areas every 10 years, based on the results of the decennial census. However, OMB occasionally issues minor updates and revisions to statistical areas in the years between the decennial censuses. On July 15, 2015, OMB issued OMB Bulletin No. 15-01, which provided minor updates to, and superseded, OMB Bulletin No. 13-01 that was issued on February 28, 2013. The attachment to OMB Bulletin No. 15-01 provides detailed information on the update to statistical areas since February 28, 2013. The updates provided in the attachment to OMB Bulletin No. 15-01 are based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2012 and July 1, 2013. The complete list of statistical areas incorporating these changes is provided in OMB Bulletin No. 15-01. A copy of this bulletin may be obtained at https://www.whitehouse.gov/omb/information-for-agencies/bulletins/. OMB Bulletin No. 15-01 establishes revised delineations for the Nation's Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas. The bulletin also provides delineations of Metropolitan Divisions as well as delineations of New England City and Town Areas.
In accordance with our longstanding policy, the IPF PPS continues to use the latest labor market area delineations available as soon as is reasonably possible to maintain a more accurate and up-to-date payment system that reflects the reality of population shifts and labor market conditions. As discussed in the FY 2017 IPPS/LTCH PPS final rule (81 FR 56913), the updated labor market area definitions from OMB Bulletin 15-01 were implemented under the IPPS beginning on October 1, 2016 (FY 2017). Therefore, we implemented these revisions for the IPF PPS beginning October 1, 2017 (FY 2018), consistent with our historical practice of modeling IPF PPS adoption of the labor market area delineations after IPPS adoption of these delineations (historically the IPF wage index has been based upon the pre-floor, pre-reclassified IPPS hospital wage index from the prior year).
On August 15, 2017, OMB announced in OMB Bulletin No. 17-01 that one Micropolitan Statistical Area now qualifies as a Metropolitan Statistical Area. The new urban CBSA is as follows:
- Twin Falls, Idaho (CBSA 46300).
This CBSA is comprised of the principal city of Twin Falls, Idaho in Jerome County, Idaho and Twin Falls County, Idaho. Prior to this redesignation, Jerome County and Twin Falls County, Idaho were classified as rural. The OMB bulletin is available on the OMB website at https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2017/b-17-01.pdf.
With the change made by OMB Bulletin No. 17-01, these two counties are now designated as urban, and any IPFs in those areas will change their status from being rural to being urban. We adopted these new OMB designations in FY 2020 as they are included in the FY 2020 pre-floor, pre-reclassified IPPS hospital wage index upon which the FY 2020 IPF wage index is proposed to be based. That is, the FY 2020 pre-floor, pre-reclassified IPPS hospital wage index, which is the basis of the final FY 2020 IPF wage index, will include this new OMB designation.
Therefore, the 17 percent IPF rural adjustment will cease for IPF providers in these two counties. Currently, there is a single IPF in new CBSA 46300, which will lose its 17 percent rural adjustment as a result of being re-designated as urban. However, the FY 2020 IPF wage index value for CBSA 46300 is 0.8291, which is 3.5 percent higher than the rural wage index value for Idaho (0.8009). As such, the loss of the 17 percent IPF wage index adjustment will be mitigated in part by the increase in the wage index value when changing from the rural Idaho wage index value to the urban CBSA 46300 wage index value. Given that the loss of the rural adjustment will be mitigated in part by the increase in wage index value, and that only a single IPF is affected by this change, we do not believe it is necessary to transition this provider from its rural to newly urban status.
Thus, we are finalizing our proposal to adopt this new OMB designation in the proposed IPF wage index for FY 2020 and for subsequent fiscal years. The FY 2020 IPF wage index already includes the OMB delineations that were adopted in prior fiscal years. The FY 2020 IPF wage index (including the CBSA update from OMB Bulletin No. 17-01) is located on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/WageIndex.html.
d. Solicitation of Public Comments on the IPF Wage Index
Historically, we have calculated the IPF PPS wage index values using unadjusted wage index values from another provider setting. Stakeholders have occasionally commented on certain aspects of the IPF PPS wage index values and their impact on payments. We solicited comments on concerns stakeholders may have regarding the wage index used to adjust IPF PPS payments and suggestions for possible updates and improvements to the geographic adjustment of IPF PPS payments. We did not receive any comments.
e. Adjustment for Rural Location
In the November 2004 IPF PPS final rule, we provided a 17 percent payment adjustment for IPFs located in a rural area. This adjustment was based on the regression analysis, which indicated that the per diem cost of rural facilities was 17 percent higher than that of urban facilities after accounting for the influence of the other variables included in the regression. This 17 percent adjustment has been part of the IPF PPS each year since the inception of the IPF PPS. For FY 2020, we are finalizing our proposal to continue to apply a 17 percent payment adjustment for IPFs located in a rural area as defined at § 412.64(b)(1)(ii)(C). A complete discussion of the adjustment for rural locations appears in the November 2004 IPF PPS final rule (69 FR 66954).
f. Budget Neutrality Adjustment
Changes to the wage index are made in a budget-neutral manner so that updates do not increase expenditures. Therefore, for FY 2020, we are finalizing our proposal to continue to apply a budget-neutrality adjustment in accordance with our existing budget-neutrality policy. This policy requires us to update the wage index in such a way that total estimated payments to IPFs for FY 2020 are the same with or without the changes (that is, in a budget-neutral manner) by applying a budget neutrality factor to the IPF PPS rates. We use the following steps to ensure that the rates reflect the update to the wage indexes (based on the FY 2016 hospital cost report data) and the labor-related share in a budget-neutral manner:
Step 1. Simulate estimated IPF PPS payments, using the FY 2019 IPF wage index values (available on the CMS website) and labor-related share (as published in the FY 2019 IPF PPS final rule (83 FR 38579)).
Step 2. Simulate estimated IPF PPS payments using the FY 2020 IPF wage index values (available on the CMS website) and FY 2020 labor-related share (based on the latest available data as discussed previously).
Step 3. Divide the amount calculated in step 1 by the amount calculated in step 2. The resulting quotient is the FY 2020 budget-neutral wage adjustment factor of 1.0026.
Step 4. Apply the FY 2020 budget-neutral wage adjustment factor from step 3 to the FY 2019 IPF PPS federal per diem base rate after the application of the market basket update described in section III.A.4 of this rule, to determine the FY 2020 IPF PPS federal per diem base rate.
2. Teaching Adjustment
In the November 2004 IPF PPS final rule, we implemented regulations at § 412.424(d)(1)(iii) to establish a facility-level adjustment for IPFs that are, or are part of, teaching hospitals. The teaching adjustment accounts for the higher indirect operating costs experienced by hospitals that participate in graduate medical education (GME) programs. The payment adjustments are made based on the ratio of the number of full-time equivalent (FTE) interns and residents training in the IPF and the IPF's average daily census (ADC).
Medicare makes direct GME payments (for direct costs such as resident and teaching physician salaries, and other direct teaching costs) to all teaching hospitals including those paid under a PPS, and those paid under the TEFRA rate-of-increase limits. These direct GME payments are made separately from payments for hospital operating costs and are not part of the IPF PPS. The direct GME payments do not address the estimated higher indirect operating costs teaching hospitals may face.
The results of the regression analysis of FY 2002 IPF data established the basis for the payment adjustments included in the November 2004 IPF PPS final rule. The results showed that the indirect teaching cost variable is significant in explaining the higher costs of IPFs that have teaching programs. We calculated the teaching adjustment based on the IPF's “teaching variable,” which is (1 + (the number of FTE residents training in the IPF/the IPF's ADC)). The teaching variable is then raised to 0.5150 power to result in the teaching adjustment. This formula is subject to the limitations on the number of FTE residents, which are described later in this section of this rule.
We established the teaching adjustment in a manner that limited the incentives for IPFs to add FTE residents for the purpose of increasing their teaching adjustment. We imposed a cap on the number of FTE residents that may be counted for purposes of calculating the teaching adjustment. The cap limits the number of FTE residents that teaching IPFs may count for the purpose of calculating the IPF PPS teaching adjustment, not the number of residents teaching institutions can hire or train. We calculated the number of FTE residents that trained in the IPF during a “base year” and used that FTE resident number as the cap. An IPF's FTE resident cap is ultimately determined based on the final settlement of the IPF's most recent cost report filed before November 15, 2004 (publication date of the IPF PPS final rule). A complete discussion of the temporary adjustment to the FTE cap to reflect residents added due to hospital closure and by residency program appears in the RY 2012 IPF PPS proposed rule (76 FR 5018 through 5020) and the RY 2012 IPF PPS final rule (76 FR 26453 through 26456).
In the regression analysis, the logarithm of the teaching variable had a coefficient value of 0.5150. We converted this cost effect to a teaching payment adjustment by treating the regression coefficient as an exponent and raising the teaching variable to a power equal to the coefficient value. We note that the coefficient value of 0.5150 is based on the regression analysis holding all other components of the payment system constant. A complete discussion of how the teaching adjustment was calculated appears in the November 2004 IPF PPS final rule (69 FR 66954 through 66957) and the RY 2009 IPF PPS notice (73 FR 25721). As with other adjustment factors derived through the regression analysis, we do not plan to rerun the teaching adjustment factors in the regression analysis until we more fully analyze IPF PPS data as part of the IPF PPS refinement we discuss in section IV of this rule. Therefore, in this FY 2020 final rule, we are finalizing our proposal to continue to retain the coefficient value of 0.5150 for the teaching adjustment to the federal per diem base rate.
3. Cost of Living Adjustment for IPFs Located in Alaska and Hawaii
The IPF PPS includes a payment adjustment for IPFs located in Alaska and Hawaii based upon the area in which the IPF is located. As we explained in the November 2004 IPF PPS final rule, the FY 2002 data demonstrated that IPFs in Alaska and Hawaii had per diem costs that were disproportionately higher than other IPFs. Other Medicare prospective payment systems (for example: The IPPS and LTCH PPS) adopted a COLA to account for the cost differential of care furnished in Alaska and Hawaii.
We analyzed the effect of applying a COLA to payments for IPFs located in Alaska and Hawaii. The results of our analysis demonstrated that a COLA for IPFs located in Alaska and Hawaii would improve payment equity for these facilities. As a result of this analysis, we provided a COLA in the November 2004 IPF PPS final rule.
A COLA for IPFs located in Alaska and Hawaii is made by multiplying the non-labor-related portion of the federal per diem base rate by the applicable COLA factor based on the COLA area in which the IPF is located.
The COLA factors through 2009 were published by the Office of Personnel Management (OPM), and the OPM memo showing the 2009 COLA factors is available at https://www.chcoc.gov/content/nonforeign-area-retirement-equity-assurance-act.
We note that the COLA areas for Alaska are not defined by county as are the COLA areas for Hawaii. In 5 CFR 591.207, the OPM established the following COLA areas:
- City of Anchorage, and 80-kilometer (50-mile) radius by road, as measured from the federal courthouse.
- City of Fairbanks, and 80-kilometer (50-mile) radius by road, as measured from the federal courthouse.
- City of Juneau, and 80-kilometer (50-mile) radius by road, as measured from the federal courthouse.
- Rest of the State of Alaska.
As stated in the November 2004 IPF PPS final rule, we update the COLA factors according to updates established by the OPM. However, sections 1911 through 1919 of the Nonforeign Area Retirement Equity Assurance Act, as contained in subtitle B of title XIX of the National Defense Authorization Act (NDAA) for FY 2010 (Pub. L. 111-84, October 28, 2009), transitions the Alaska and Hawaii COLAs to locality pay. Under section 1914 of NDAA, locality pay was phased in over a 3-year period beginning in January 2010, with COLA rates frozen as of the date of enactment, October 28, 2009, and then proportionately reduced to reflect the phase-in of locality pay.
When we published the proposed COLA factors in the RY 2012 IPF PPS proposed rule (76 FR 4998), we inadvertently selected the FY 2010 COLA rates, which had been reduced to account for the phase-in of locality pay. We did not intend to propose the reduced COLA rates because that would have understated the adjustment. Since the 2009 COLA rates did not reflect the phase-in of locality pay, we finalized the FY 2009 COLA rates for RY 2010 through RY 2014.
In the FY 2013 IPPS/LTCH final rule (77 FR 53700 through 53701), we established a new methodology to update the COLA factors for Alaska and Hawaii, and adopted this methodology for the IPF PPS in the FY 2015 IPF final rule (79 FR 45958 through 45960). We adopted this new COLA methodology for the IPF PPS because IPFs are hospitals with a similar mix of commodities and services. We think it is appropriate to have a consistent policy approach with that of other hospitals in Alaska and Hawaii. Therefore, the IPF COLAs for FY 2015 through FY 2017 were the same as those applied under the IPPS in those years. As finalized in the FY 2013 IPPS/LTCH PPS final rule (77 FR 53700 and 53701), the COLA updates are determined every 4 years, when the IPPS market basket labor-related share is updated. Because the labor-related share of the IPPS market basket was updated for FY 2018, the COLA factors were updated in FY 2018 IPPS/LTCH rulemaking (82 FR 38529). As such, we also updated the IPF PPS COLA factors for FY 2018 (82 FR 36780 through 36782) to reflect the updated COLA factors finalized in the FY 2018 IPPS/LTCH rulemaking. We are finalizing our proposal to continue to apply the same COLA factors in FY 2020 that were used in FY 2018 and FY 2019.
| Area | FY 2015 through FY 2017 | FY 2018 through FY 2020 |
|---|---|---|
| Alaska: | ||
| City of Anchorage and 80-kilometer (50-mile) radius by road | 1.23 | 1.25 |
| City of Fairbanks and 80-kilometer (50-mile) radius by road | 1.23 | 1.25 |
| City of Juneau and 80-kilometer (50-mile) radius by road | 1.23 | 1.25 |
| Rest of Alaska | 1.25 | 1.25 |
| Hawaii: | ||
| City and County of Honolulu | 1.25 | 1.25 |
| County of Hawaii | 1.19 | 1.21 |
| County of Kauai | 1.25 | 1.25 |
| County of Maui and County of Kalawao | 1.25 | 1.25 |
The IPF PPS COLA factors for FY 2020 are also shown in Addendum A to this final rule, available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientPsychFacilPPS/tools.html.
4. Adjustment for IPFs with a Qualifying Emergency Department (ED)
The IPF PPS includes a facility-level adjustment for IPFs with qualifying EDs. We provide an adjustment to the federal per diem base rate to account for the costs associated with maintaining a full-service ED. The adjustment is intended to account for ED costs incurred by a psychiatric hospital with a qualifying ED or an excluded psychiatric unit of an IPPS hospital or a CAH, for preadmission services otherwise payable under the Medicare Hospital Outpatient Prospective Payment System (OPPS), furnished to a beneficiary on the date of the beneficiary's admission to the hospital and during the day immediately preceding the date of admission to the IPF (see § 413.40(c)(2)), and the overhead cost of maintaining the ED. This payment is a facility-level adjustment that applies to all IPF admissions (with one exception which we described), regardless of whether a particular patient receives preadmission services in the hospital's ED.
The ED adjustment is incorporated into the variable per diem adjustment for the first day of each stay for IPFs with a qualifying ED. Those IPFs with a qualifying ED receive an adjustment factor of 1.31 as the variable per diem adjustment for day 1 of each patient stay. If an IPF does not have a qualifying ED, it receives an adjustment factor of 1.19 as the variable per diem adjustment for day 1 of each patient stay.
The ED adjustment is made on every qualifying claim except as described in this section of the proposed rule. As specified in § 412.424(d)(1)(v)(B), the ED adjustment is not made when a patient is discharged from an IPPS hospital or CAH and admitted to the same IPPS hospital's or CAH's excluded psychiatric unit. We clarified in the November 2004 IPF PPS final rule (69 FR 66960) that an ED adjustment is not made in this case because the costs associated with ED services are reflected in the DRG payment to the IPPS hospital or through the reasonable cost payment made to the CAH.
Therefore, when patients are discharged from an IPPS hospital or CAH and admitted to the same hospital's or CAH's excluded psychiatric unit, the IPF receives the 1.19 adjustment factor as the variable per diem adjustment for the first day of the patient's stay in the IPF. For FY 2020, we are finalizing our proposal to continue to retain the 1.31 adjustment factor for IPFs with qualifying EDs. A complete discussion of the steps involved in the calculation of the ED adjustment factor in our November 2004 IPF PPS final rule (69 FR 66959 through 66960) and the RY 2007 IPF PPS final rule (71 FR 27070 through 27072).
E. Other Payment Adjustments and Policies
1. Outlier Payment Overview
The IPF PPS includes an outlier adjustment to promote access to IPF care for those patients who require expensive care and to limit the financial risk of IPFs treating unusually costly patients. In the November 2004 IPF PPS final rule, we implemented regulations at § 412.424(d)(3)(i) to provide a per-case payment for IPF stays that are extraordinarily costly. Providing additional payments to IPFs for extremely costly cases strongly improves the accuracy of the IPF PPS in determining resource costs at the patient and facility level. These additional payments reduce the financial losses that would otherwise be incurred in treating patients who require more costly care, and therefore, reduce the incentives for IPFs to under-serve these patients. We make outlier payments for discharges in which an IPF's estimated total cost for a case exceeds a fixed dollar loss threshold amount (multiplied by the IPF's facility-level adjustments) plus the federal per diem payment amount for the case.
In instances when the case qualifies for an outlier payment, we pay 80 percent of the difference between the estimated cost for the case and the adjusted threshold amount for days 1 through 9 of the stay (consistent with the median LOS for IPFs in FY 2002), and 60 percent of the difference for day 10 and thereafter. The adjusted threshold amount is equal to the outlier threshold amount adjusted for wage area, teaching status, rural area, and the COLA adjustment (if applicable), plus the amount of the Medicare IPF payment for the case. We established the 80 percent and 60 percent loss sharing ratios because we were concerned that a single ratio established at 80 percent (like other Medicare PPSs) might provide an incentive under the IPF per diem payment system to increase LOS in order to receive additional payments.
After establishing the loss sharing ratios, we determined the current fixed dollar loss threshold amount through payment simulations designed to compute a dollar loss beyond which payments are estimated to meet the 2 percent outlier spending target. Each year when we update the IPF PPS, we simulate payments using the latest available data to compute the fixed dollar loss threshold so that outlier payments represent 2 percent of total estimated IPF PPS payments.
2. Update to the Outlier Fixed Dollar Loss Threshold Amount
In accordance with the update methodology described in § 412.428(d), we updated the fixed dollar loss threshold amount used under the IPF PPS outlier policy. Based on the regression analysis and payment simulations used to develop the IPF PPS, we established a 2 percent outlier policy, which strikes an appropriate balance between protecting IPFs from extraordinarily costly cases while ensuring the adequacy of the federal per diem base rate for all other cases that are not outlier cases.
Based on an analysis of the latest available data (the March 2019 update of FY 2018 IPF claims) and rate increases, we believe it is necessary to update the fixed dollar loss threshold amount to maintain an outlier percentage that equals 2 percent of total estimated IPF PPS payments. We are updating the IPF outlier threshold amount for FY 2020 using FY 2018 claims data and the same methodology that we used to set the initial outlier threshold amount in the RY 2007 IPF PPS final rule (71 FR 27072 and 27073), which is also the same methodology that we used to update the outlier threshold amounts for years 2008 through 2019. Based on an analysis of these updated data, we estimate that IPF outlier payments as a percentage of total estimated payments are approximately 2.23 percent in FY 2019. Therefore, we are finalizing our proposal to update the outlier threshold amount to $14,960 to maintain estimated outlier payments at 2 percent of total estimated aggregate IPF payments for FY 2020. This final rule update is an increase from the FY 2019 threshold of $12,865.
We received one comment on our proposed update to the outlier threshold.
Comment: A commenter was concerned that the 13.4 percent proposed increase in the outlier threshold was too steep to implement in a single year, and suggested that when an increase in the outlier threshold is necessary, it should be limited to no more than 5 percent in any given year.
Response: The outlier fixed dollar threshold amount is calculated by simulating aggregate payments and using an iterative process to determine a threshold that results in outlier payments being equal to 2 percent of total payments under the simulation. To determine the IPF outlier threshold amount for FY 2020 we estimated the FY 2020 IPF PPS aggregate and outlier payments using the most recent claims available (March 2019 update of the FY 2018 MedPAR claims) and the FY 2020 final payment rates. The outlier threshold was varied in this simulation until estimated outlier payments equaled 2 percent of estimated aggregate payments. Based on the regression analysis and payment simulations used to develop the IPF PPS, we established a 2 percent outlier policy in our November 2004 IPF PPS final rule (69 FR 66960 through 66962), which strikes an appropriate balance between protecting IPFs from extraordinarily costly cases while ensuring the adequacy of the federal per diem base rate for all other cases that are not outlier cases. This outlier fixed dollar loss threshold update methodology is based on longstanding IPF payment policy and is described in detail in the RY 2007 IPF PPS final rule (71 FR 27072 and 27073). To continue to maintain this established 2 percent outlier policy, for this final rule we must raise the IPF PPS outlier fixed dollar threshold amount from $12,865 to $14,960. If the fixed dollar threshold amount increase was limited to 5 percent for FY 2020 as suggested by the commenter we would not meet the established 2 percent outlier policy. Our IPF PPS outlier policy limiting outlier payments to a defined percentage of total payments is consistent with the outlier policies in other Medicare payment systems.
3. Update to IPF Cost-to-Charge Ratio Ceilings
Under the IPF PPS, an outlier payment is made if an IPF's cost for a stay exceeds a fixed dollar loss threshold amount plus the IPF PPS amount. In order to establish an IPF's cost for a particular case, we multiply the IPF's reported charges on the discharge bill by its overall cost-to-charge ratio (CCR). This approach to determining an IPF's cost is consistent with the approach used under the IPPS and other PPSs. In the FY 2004 IPPS final rule (68 FR 34494), we implemented changes to the IPPS policy used to determine CCRs for IPPS hospitals, because we became aware that payment vulnerabilities resulted in inappropriate outlier payments. Under the IPPS, we established a statistical measure of accuracy for CCRs to ensure that aberrant CCR data did not result in inappropriate outlier payments.
As we indicated in the November 2004 IPF PPS final rule (69 FR 66961), we believe that the IPF outlier policy is susceptible to the same payment vulnerabilities as the IPPS; therefore, we adopted a method to ensure the statistical accuracy of CCRs under the IPF PPS. Specifically, we adopted the following procedure in the November 2004 IPF PPS final rule:
- Calculated two national ceilings, one for IPFs located in rural areas and one for IPFs located in urban areas.
- Computed the ceilings by first calculating the national average and the standard deviation of the CCR for both urban and rural IPFs using the most recent CCRs entered in the most recent Provider Specific File available.
For FY 2020, we are finalizing our proposal to continue to follow this methodology.
To determine the rural and urban ceilings, we multiplied each of the standard deviations by 3 and added the result to the appropriate national CCR average (either rural or urban). The upper threshold CCR for IPFs in FY 2020 is 2.0239 for rural IPFs, and 1.7263 for urban IPFs, based on CBSA-based geographic designations. If an IPF's CCR is above the applicable ceiling, the ratio is considered statistically inaccurate, and we assign the appropriate national (either rural or urban) median CCR to the IPF.
We apply the national median CCRs to the following situations:
- New IPFs that have not yet submitted their first Medicare cost report. We continue to use these national median CCRs until the facility's actual CCR can be computed using the first tentatively or final settled cost report.
- IPFs whose overall CCR is in excess of three standard deviations above the corresponding national geometric mean (that is, above the ceiling).
- Other IPFs for which the Medicare Administrative Contractor (MAC) obtains inaccurate or incomplete data with which to calculate a CCR.
We are finalizing our proposal to continue to update the FY 2020 national median and ceiling CCRs for urban and rural IPFs based on the CCRs entered in the latest available IPF PPS Provider Specific File. Specifically, for FY 2020, to be used in each of the three situations listed previously, using the most recent CCRs entered in the CY 2019 Provider Specific File, we provide an estimated national median CCR of 0.5720 for rural IPFs and a national median CCR of 0.4370 for urban IPFs. These calculations are based on the IPF's location (either urban or rural) using the CBSA-based geographic designations. A complete discussion regarding the national median CCRs appears in the November 2004 IPF PPS final rule (69 FR 66961 through 66964).
IV. Update on IPF PPS Refinements
For RY 2012, we identified several areas of concern for future refinement, and we invited comments on these issues in the RY 2012 IPF PPS proposed and final rules. For further discussion of these issues and to review the public comments, we refer readers to the RY 2012 IPF PPS proposed rule (76 FR 4998) and final rule (76 FR 26432).
We have delayed making refinements to the IPF PPS until we have completed a thorough analysis of IPF PPS data on which to base those refinements. Specifically, we will delay updating the adjustment factors derived from the regression analysis until we have IPF PPS data that include as much information as possible regarding the patient-level characteristics of the population that each IPF serves. We have begun and will continue the necessary analysis to better understand IPF industry practices so that we may refine the IPF PPS in the future, as appropriate. Our preliminary analysis has also revealed variation in cost and claim data, particularly related to labor costs, drugs costs, and laboratory services. Some providers have very low labor costs, or very low or missing drug or laboratory costs or charges, relative to other providers. As we noted in the FY 2016 IPF PPS final rule (80 FR 46693 through 46694), our preliminary analysis of 2012 to 2013 IPF data found that over 20 percent of IPF stays reported no ancillary costs, such as laboratory and drug costs, in their cost reports, or laboratory or drug charges on their claims. Because we expect that most patients requiring hospitalization for active psychiatric treatment will need drugs and laboratory services, we again remind providers that the IPF PPS federal per diem base rate includes the cost of all ancillary services, including drugs and laboratory services.
On November 17, 2017, we issued Transmittal 12, which made changes to the hospital cost report form CMS-2552-10 (OMB No. 0938-0050), and included the requirement that cost reports from psychiatric hospitals include certain ancillary costs, or the cost report will be rejected. On January 30, 2018, we issued Transmittal 13, which changed the implementation date for Transmittal 12 to be for cost reporting periods ending on or after September 30, 2017. For details, we refer readers to see these Transmittals, which are available on the CMS website at https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/index.html. CMS suspended the requirement that cost reports from psychiatric hospitals include certain ancillary costs effective April 27, 2018, in order to consider excluding all-inclusive rate providers from this requirement. CMS issued Transmittal 15 on October 19, 2018, reinstating the requirement that cost reports from psychiatric hospitals, except all-inclusive rate providers, include certain ancillary costs.
We only pay the IPF for services furnished to a Medicare beneficiary who is an inpatient of that IPF (except for certain professional services), and payments are considered to be payments in full for all inpatient hospital services provided directly or under arrangement (see 42 CFR 412.404(d)), as specified in 42 CFR 409.10.
We will continue to analyze data from claims and cost reports that do not include ancillary charges or costs, and will be sharing our findings with CMS Office of the Center for Program Integrity and CMS Office of Financial Management for further investigation, as the results warrant. Our refinement analysis is dependent on recent precise data for costs, including ancillary costs. We will continue to collect these data and analyze them for both timeliness and accuracy with the expectation that these data will be used in a future refinement. It is currently our intent to explore refinements to the adjustments in future rulemaking. Since we did not propose refinements, for FY 2020 we will continue to use the existing adjustment factors.
V. Inpatient Psychiatric Facilities Quality Reporting (IPFQR) Program
A. Background and Statutory Authority
We refer readers to the FY 2019 IPF PPS final rule (83 FR 38589) for a discussion of the background and statutory authority [2] of the IPFQR Program.
B. Covered Entities
In the FY 2013 IPPS/LTCH-PPS final rule (77 FR 53645), we established that the IPFQR Program's quality reporting requirements cover those psychiatric hospitals and psychiatric units paid under Medicare's IPF PPS (§ 412.404(b)). Generally, psychiatric hospitals and psychiatric units within acute care and critical access hospitals that treat Medicare patients are paid under the IPF PPS. Consistent with previous regulations, we continue to use the term IPF to refer to both inpatient psychiatric hospitals and psychiatric units. This usage follows the terminology in our IPF PPS regulations at § 412.402. For more information on covered entities, we refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53645).
C. Previously Finalized Measures and Administrative Procedures
The current IPFQR Program includes 13 measures. For more information on these measures, we refer readers to the following final rules:
- The FY 2013 IPPS/LTCH PPS final rule (77 FR 53646 through 53652);
- The FY 2014 IPPS/LTCH PPS final rule (78 FR 50889 through 50897);
- The FY 2015 IPF PPS final rule (79 FR 45963 through 45975);
- The FY 2016 IPF PPS final rule (80 FR 46695 through 46714);
- The FY 2017 IPPS/LTCH PPS final rule (81 FR 57238 through 57247); and
- The FY 2019 IPF PPS final rule (83 FR 38590 through 38606).
For more information on previously adopted procedural requirements, we refer readers to the following rules:
- The FY 2013 IPPS/LTCH PPS final rule (77 FR 53653 through 53660);
- The FY 2014 IPPS/LTCH PPS final rule (78 FR 50897 through 50903;
- The FY 2015 IPF PPS final rule (79 FR 45975 through 45978);
- The FY 2016 IPF PPS final rule (80 FR 46715 through 46719);
- The FY 2017 IPPS/LTCH PPS final rule (81 FR 57248 through 57249);
- The FY 2018 IPPS/LTCH PPS final rule (82 FR 38471 through 38474); and
- The FY 2019 IPF PPS final rule (83 FR 38606 through 38608).
D. IPFQR Program Measures
1. Measure Selection Process
Before being proposed for inclusion in the IPFQR Program, measures are placed on a list of measures under consideration (MUC), which is published annually by December 1 on behalf of CMS by the National Quality Forum (NQF). Following publication on the MUC list, the Measure Applications Partnership (MAP), a multi-stakeholder group convened by the NQF, reviews the measures under consideration for the IPFQR Program, among other Federal programs, and provides input on those measures to the Secretary. We considered the input and recommendations provided by the MAP in selecting all measures for the IPFQR Program. Further details concerning the input and recommendations from the MAP for the measure proposed in the FY 2020 IPF PPS Proposed rule (Medication Continuation Following Inpatient Psychiatric Discharge, NQF #3205) are provided in Section V.D.3.
2. Removal or Retention of IPFQR Program Measures
a. Background
In the FY 2018 IPPS/LTCH PPS final rule (82 FR 38463 through 38465), we finalized our proposals to adopt considerations for removing or retaining measures within the IPFQR Program and criteria for determining when a measure is “topped out.” In the FY 2019 IPF PPS final rule (83 FR 38591 through 38593), we added one additional measure removal factor. We are not proposing any changes to these removal factors, topped-out criteria, or retention factors and refer readers to the FY 2018 IPPS/LTCH PPS final rule (82 FR 38463 through 38465) and the FY 2019 IPF PPS final rule (83 FR 38591 through 38593) for more information. We will continue to retain measures from each previous year's IPFQR Program measure set for subsequent years' measure sets, except when we specifically propose to remove or replace a measure. We will continue to use the notice-and-comment rulemaking process to propose measures for removal or replacement, as we described upon adopting these factors in the 2018 IPPS/LTCH PPS final rule (82 FR 38464 through 38465).
b. Application of Considerations for Removal and Retention to Current Measure Set
In the FY 2018 IPPS/LTCH PPS final rule, we noted that several commenters requested that we evaluate the current measures in the IPFQR Program using the removal and retention factors that we finalized in that rule (82 FR 38464). Following this evaluation, we proposed to remove eight measures from the IPFQR Program in the FY 2019 IPF PPS proposed rule (83 FR 21118 through 21123) for the FY 2020 program year and subsequent years. In the FY 2019 IPF PPS final rule (83 FR 38593 through 38604) we finalized removal of five of these measures. In our evaluation of the IPFQR Program measure set subsequent to publication of the FY 2019 IPF PPS final rule, we have not identified additional measures to which our measure removal factors apply. Therefore, we are not proposing to remove any additional measures at this time.
The previously finalized number of measures for the FY 2021 payment determination and subsequent years totals 13.

3. Proposed New Quality Measure for the FY 2021 Payment Determination and Subsequent Years—Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205)
a. Background
Medication continuation is important for patients discharged from the inpatient psychiatric setting with major depressive disorder (MDD), schizophrenia, or bipolar disorder because of significant negative outcomes associated with non-adherence to medication regimens. For example, patients with MDD who do not remain on prescribed medications are more likely to have negative health outcomes such as relapse and readmission, decreased quality of life, and increased healthcare costs.[3 4] Patients with schizophrenia who do not adhere to their medication regimen are more likely to be hospitalized, use emergency psychiatric services, be arrested, be victims of crimes, and consume alcohol or drugs compared to those who adhere to their medication regimen.[5] Patients with bipolar disorder who do not adhere to their medications have increased suicide risk.[6] For these reasons, guidelines from the American Psychiatric Association (APA) and the Department of Veterans Affairs/Department of Defense (VA/DoD), which are based on extensive literature, recommend pharmacotherapy as the primary form of treatment for patients with these conditions.[7 8 9 10 11]
Furthermore, we believe that there are factors external to the IPF that influence filling prescriptions post-discharge in the psychiatric population. While it may not be possible to achieve complete post-discharge compliance with pharmacotherapy, there is evidence that improvements to the quality of care provided by IPFs, including discharge processes, can help to increase medication continuation rates.[12 13 14 15 16] These interventions include patient education, enhanced therapeutic relationships, shared decision-making, and text-message reminders, with multidimensional approaches resulting in the best outcomes.
We proposed to adopt the Medication Continuation Following Inpatient Psychiatric Discharge measure (NQF #3205) for the FY 2020 payment determination and subsequent years in the FY 2018 IPPS/LTCH PPS proposed rule (82 FR 20122 through 20126) to address this important clinical topic. In the FY 2018 IPPS/LTCH PPS final rule (82 FR 38465 through 38470), we did not finalize adoption of the Medication Continuation Following Inpatient Psychiatric Discharge measure (NQF #3205), because we recognized that this measure may place undue burden on facilities that were updating processes to account for previously adopted measures despite being calculated from claims data, which should not require additional information collection burden. We did not want to place undue burden on facilities, especially small, rural facilities, and we wished to accommodate the need for facilities to develop and implement innovative efforts, such as updating their processes and clinical workflows, for this measure.
At that time, we stated that we would consider proposing this measure again in future rulemaking. We note that since the FY 2018 IPPS/LTCH PPS final rule, we have removed five measures from the IPFQR Program (83 FR 38593 through 38602), reducing burden on IPFs by approximately 546,000 hours and $20 million (83 FR38610 through 38611), and IPFs have had an additional 2 years to familiarize themselves with the remaining IPFQR Program measure set and to update processes and clinical workflows accordingly. Therefore, we believe that it is now appropriate to propose this measure for the IPFQR Program again.
Since the FY 2018 IPPS/LTCH PPS final rule, we have not made any changes to the Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) measure's specifications. However, we have taken steps to improve upon the suitability of this measure for the IPFQR Program. First, we considered recommendations and comments received on the Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) measure from the FY 2018 IPPS/LTCH PPS final rule (82 FR 38468 through 38470). We provide more detail about these comments.
Second, since the FY 2018 IPPS/LTCH PPS final rule, we have provided additional information about this measure to the MAP and to the NQF, including reliability and validity testing. The measure was subsequently endorsed by NQF. We continue to believe that this measure evaluates a process with a demonstrated quality gap, because in testing this measure, we found that the range of performance between the 10th percentile and the 80th percentile facility performance was between 67 percent and 88 percent. We found that if all facilities had at least the median rate then 16,000 additional Medicare beneficiaries would fill prescriptions for an evidence-based medication to manage their condition following discharge.[17] Furthermore, we believe this measure has the potential to benefit patients by encouraging facilities to adopt interventions to improve post discharge medication continuation rates with no additional reporting burden to IPFs.
In response to our proposal in the FY 2018 IPPS/LTCH PPS proposed rule, many comments focused on the potential undue burden of the measure given the fact that many facilities were still updating processes to account for previously adopted measures (82 FR 38469). Between the FY 2018 IPPS/LTCH PPS final rule and this proposed rule, we have not adopted any new measures into the program. We believe that IPFs no longer need to update processes to account for previously adopted measures because they have had 2 years to complete all such updates. Therefore, we believe that there is less burden associated with the IPFQR program than when we proposed to adopt this measure in the FY 2018 IPPS/LTCH PPS proposed rule.
Some commenters also expressed concern that patients may experience barriers to filling prescriptions that are beyond the control of IPFs (82 FR 38469 through 38470). While we believe that there are factors external to an IPF that influence filling prescriptions after a patient is discharged, as the methodology report for the measure indicates,[18] IPFs can also undertake interventions to improve the likelihood of a patient's medication continuation post-discharge.
In response to comments that the affected population may be too small to report meaningful data because it is limited to Medicare patients enrolled in Parts A, B, and D (82 FR 38469 through 38470), we note that the NQF found this measure to be valid and reliable,[19] indicating that the size of the population is sufficient to report meaningful data. These commenters additionally expressed that because the measure is limited to Medicare patients enrolled in Parts A, B, and D, there may not be a performance gap because these patients do not experience the same access barriers as other inpatient psychiatric populations. However, we note that in their endorsement review of the measure, the NQF found that there was evidence of a performance gap in the quality area that was addressed by the measure even though the measure is limited to patients enrolled in Medicare A, B, and D.[20]
Finally, in response to comments that the measure had not completed full endorsement review by NQF (82 FR 38469), the measure is now fully endorsed by the NQF as discussed in more detail in Section B of this rule. Further, in its review of the measure for endorsement, the NQF standing committee agreed that there is evidence that lack of adherence to medication leads to relapse and negative outcomes and that claims data related to medication adherence are directly correlated to outcomes.[21]
b. Overview of Measure
The Medication Continuation Following Inpatient Psychiatric Discharge measure (NQF #3205) assesses whether patients admitted to IPFs with diagnoses of MDD, schizophrenia, or bipolar disorder filled at least one evidence-based medication prior to discharge or during the post-discharge period. As detailed in the following discussion, the NQF endorsed this measure on June 28, 2017. For more information about this measure, we refer readers to the measure specifications in the measure technical report https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip) or the measure's NQF page (https://www.qualityforum.org/QPS/3205).
In compliance with section 1890A(a)(2) of the Act, this measure was included in a publicly available document: “List of Measures under Consideration for December 1, 2016” (https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Downloads/Measures-under-Consideration-List-for-2016.pdf). The MAP Hospital Workgroup concluded in its December 2016 meeting that the measure addressed a critical quality objective, was evidence-based, and would contribute to efficient use of resources.[22] One Workgroup member commented that it was appropriate to hold IPFs accountable for patients filling a prescription for an evidence-based medication post-discharge.
The MAP Hospital Workgroup classified the measure as “Refine and Resubmit Prior to Rulemaking.” [23] The measure received this classification because the MAP recommended that measure testing be completed to demonstrate reliability and validity at the facility level in the hospital setting and that the measure be submitted to NQF for review and endorsement.[24] The MAP also requested additional details on the measure, such as: (1) The definition of medication dispensation; (2) how the facility would know whether the medication was dispensed; and (3) how the measure would be impacted if Medicare Part D coverage is optional.[25] The methodology report for the measure (https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/Downloads/Measures-under-Consideration-List-for-2016.pdf) that we finalized, includes the results of reliability and validity testing, and additional measure updates that occurred after the MAP review. This newest methodology report also provides the additional details requested by the MAP at the December 2016 meeting. This includes the specific medication list, which is based on APA and VA/DoD practice guidelines for each medication [26 27 28 29 30] and information about how facilities can help patients fill prescriptions for medications to ensure that the facility knows that the prescription has been filled. Additionally, the methodology report provides details about measure performance among patients with Part D and the performance gap for this patient population.
This measure was submitted to NQF for endorsement on December 16, 2016. Consistent with the recommendation from the December 2016 MAP meeting that testing for reliability and validity should be completed, in Spring 2017 we refined our NQF submission by providing the complete results of all testing for NQF's review of the measure for endorsement. The measure received NQF endorsement on June 28, 2017.[31]
This measure supports the CMS Meaningful Measure Area “promote effective prevention and treatment of chronic disease,” which includes the meaningful measure area of “prevention, treatment, and management of mental health.” The measure would also complement the portfolio of facility-level measures in the IPFQR Program that assess the transition from the inpatient to outpatient setting: Follow-Up After Hospitalization for Mental Illness; Thirty-day All Cause Unplanned Readmission Following Psychiatric Hospitalization in an Inpatient Psychiatric Facility; Transition Record with Specified Elements Received by Discharged Patients; and Timely Transmission of Transition Record.
c. Data Sources
The proposed Medication Continuation Following Inpatient Psychiatric Discharge measure (NQF #3205) uses Medicare fee-for-service (FFS) claims to identify whether patients admitted to IPFs with diagnoses of MDD, schizophrenia, or bipolar disorder filled at least one evidence-based medication such that they would have medication for use post-discharge. The performance period for this measure is 24 months. For example, for the FY 2021 payment determination, the performance period will include discharges between July 1, 2017 and June 30, 2019.[32]
d. Measure Calculation
The numerator for the measure includes discharges for patients with a principal diagnosis of MDD, schizophrenia, or bipolar disorder in the denominator who were dispensed at least one evidence-based outpatient medication within 2 days prior to discharge through 30 days post-discharge. The denominator for the measure includes Medicare fee-for-service (FFS) beneficiaries with Part D coverage aged 18 years and older discharged to home or home health care from an IPF with a principal diagnosis of MDD, schizophrenia, or bipolar disorder. The denominator excludes discharges for patients who:
- Received Electroconvulsive Therapy (ECT) during the inpatient stay or 30 day post-discharge period;
- Received Transcranial Magnetic Stimulation (TMS) during the inpatient stay or follow-up;
- Were pregnant during the inpatient stay;
- Had a secondary diagnosis of delirium; or
- Had a principal diagnosis of schizophrenia with a secondary diagnosis of dementia.
For more information about the development of the measure, including rationale for the 2 day prior to 30 day post-discharge period and the denominator exclusions, we refer readers to the measure technical report (https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip).
We invited public comment on our proposal to adopt the Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) measure for the FY 2021 payment determination and subsequent years as discussed.
Comment: Several commenters expressed support for the Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) measure specifically noting that it is an NQF-endorsed measure that addresses an important clinical topic with a demonstrated quality gap. Several of these commenters noted that the measure will help facilities identify interventions for post-discharge medication compliance, thereby improving care transitions. Some commenters further expressed that the measure aligns with the goal of not increasing provider burden.
Response: We thank these commenters for their support.
Comment: Some commenters recommended that CMS not adopt the Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) measure because this measure imposes burden on facilities.
Response: We do not believe that this measure imposes any data reporting burden on facilities because it is calculated by CMS using data submitted on Medicare Parts A, B, and D claims. We acknowledge that to improve performance on this measure there may be costs or burden associated with updating clinical workflows to improve discharge planning and counseling on the importance of medication continuation. However, because of the severity of the negative health outcomes associated with medication discontinuation for this patient population, we believe that these updates are part of providing high quality inpatient psychiatric care.
Comment: Some commenters recommended that CMS not adopt Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) because they believe that restricting the denominator to patients who have Medicare Parts A, B, and D coverage makes the population size too small to be meaningful.
Response: During measure testing, the denominator was restricted to patients who have Medicare Parts A, B, and D coverage during measure testing and results showed that the majority of providers met the 75 case minimum threshold required to obtain an overall reliability score of at least 0.7, which is the minimum acceptable reliability rating. Furthermore, the NQF standing committee evaluated this when considering the measure for endorsement and determined that the measure meets their scientific acceptability criteria.[33]
Comment: Some commenters recommended that CMS not adopt this measure because they believe that the measure assesses patient behavior (that is, filling prescriptions) as opposed to provider quality and therefore does not produce data that will help consumers select facilities.
Response: We recognize that there are factors external to the IPF that influence filling prescriptions post-discharge in the psychiatric population. While it may not be possible to achieve complete post-discharge compliance with pharmacotherapy, there is evidence that improvements to the quality of care for patients in the IPF setting, including the discharge processes, can help to increase medication continuation rates.[34 35 36 37 38] These interventions include patient education, enhanced therapeutic relationships, shared decision-making, and text-message reminders, with multidimensional approaches resulting in the best outcomes. We note that in testing the measure, the measure developer found a median score of 79.6% and an approximate 21-percentage point difference between the 10th and 90th percentiles. This means that in the 10th percentile facilities, depending on their condition, 60.0 to 63.9 percent of patients (with Medicare Parts A, B, and D) fill prescriptions for evidence-based medications, whereas in the 90th percentile facilities 89.7 to 95.5 percent of such patients fill prescriptions for evidence-based medications.[39] We believe that this performance gap, coupled with the ability of facilities to provide interventions to improve medication continuation, indicate that the measure does provide meaningful information about the quality of care provided to patients.
Comment: Several commenters recommended that CMS not adopt the Medication Continuation Following Inpatient Discharge (NQF #3205) measure because these commenters believe prescription fills do not actually reflect medication adherence.
Response: While we agree with commenters that it is possible that patients may fill prescriptions and then not take the medication, or take it incorrectly, we believe that the measure is a good indicator of patient adherence to medication regimens. The NQF Standing Committee for Behavioral Health evaluated the potential for patients to fill their prescriptions but not be adherent to the medication regimen during their review of the measure and found that most studies related to adverse events for medication non-compliance used the filling of a prescription as a proxy for medication adherence,[40] which aligns with this measure's methodology.
Comment: One commenter recommended that CMS not adopt this measure because facilities cannot internally track performance on this measure and therefore cannot identify performance gaps that require interventions.
Response: We believe that this measure will help facilities identify performance gaps that require interventions by making this data available to facilities. We also note that the American Psychiatric Association's (APA's) and Department of Veterans Affairs and Department of Defense (VA/DoD) practice guidelines for depressive disorder, bipolar disorder, and schizophrenia provide strategies for facilities to implement to help patients fill prescriptions prior to discharge so that the facility can track whether the prescription has been filled.[41 42 43 44 45]
Comment: Several commenters expressed the belief that this measure is not appropriate for the inpatient psychiatric setting and suggested that this or a similar measure be considered for the outpatient setting instead because these commenters believe that outpatient providers have more influence on patients' post-discharge care.
Response: We agree with the commenters that outpatient providers do have more influence on a patient's post-discharge care in the long term; however this measure is specified to address the short term period immediately following discharge from the IPF prior to the patient's follow-up with an outpatient provider (which, according to data collected through the Follow-Up After Hospitalization for Mental Illness (NQF #0576) measure, will be more than 30 days post-discharge nearly half of all patients).[46] Therefore, we do not agree that this measure would be more appropriate for the outpatient setting. This measure addresses care provided during the discharge planning phase of care, which occurs within the IPF to facilitate a safe care transition until the patient can be seen by an outpatient provider. We note that the period immediately following discharge from a psychiatric hospital is a high-risk period for patients, and has been linked to an increased risk of adverse outcomes, including suicide.[47 48] We believe it is vital that patients have continuity of pharmacotherapy consistent with the prescriptions provided by their inpatient providers until they can develop a long-term care plan with their outpatient providers
Comment: One commenter expressed concern that because this measure's patient population has Medicare Parts A, B, and D coverage, these patients do not experience the same barriers to access experienced by patients without similar health insurance coverage and therefore the measure may not provide meaningful data.
Response: We agree that the patients included in the measure may not experience the same barriers to access to medications that some other patients encounter because they have insurance and low-income Medicare patients qualify for additional support to help pay for medications. However, as previously noted, in the measure technical report,[49] the claims data used for analysis and testing of this measure demonstrated ample opportunity for improvement in medication continuation rates for patients with Medicare Parts A, B, and D, with median medication continuation rates of 79% and a variation of 21 percentage points between the 10th and 90th percentile facilities. Further, considering that the Medicare population may have lower barriers to access, we would expect to see higher medication continuation rates and less variation in performance across facilities.
In addition, we note that while the measure denominator includes only patients with Medicare Parts A, B, and D, all patients can benefit from the evidence-based interventions that facilities may implement to improve medication adherence.
Comment: One commenter requested clarification of how CMS will assess prescription refills for patients who do not have Part D.
Response: We note that the denominator of this measure is restricted to patients who have Medicare Parts A, B, and D coverage. Therefore, we will not assess prescription refills for patients who do not have Part D coverage because they are not in the measure's patient population.
Comment: One commenter expressed concern that the measure will not capture medication continuity for patients who filled 90-day supplies prior to admission.
Response: During measure testing, we found that the number of patients who filled a 90-day prescription in the 90 days prior to admission was small. Specifically, 5.5 percent of those with major depressive disorder had a 90-day prescription at some point in the 90 days prior to admission, 2.8 percent of those with bipolar disorder had such a prescription, and 1.2 percent of those with schizophrenia had such a prescription. Furthermore, we believe that medications are often adjusted during the inpatient stay, and patients may need to fill a new prescription following discharge even if they have medications at home. Therefore, we believe that the patient population with appropriate pharmacotherapy due to 90-day prescriptions prior to admission is very small and does not necessitate any changes to the measure specifications.
Comment: One commenter expressed concern that 2 days prior to discharge is too brief a period and recommended expanding the period to 5 days prior to discharge.
Response: When we developed and tested this measure, we found that most outpatient medications filled during the inpatient stay are filled one day prior to discharge.[50] In consulting with clinical experts, we found that discharge planning, including filling prescriptions, could start as early as two days prior to discharge. These experts unanimously agreed to extend the follow-up period to include two days prior to discharge.[51] Because most medications filled during the stay are filled one day prior to discharge and discharge planning typically starts two days prior to discharge we believe that this measure period is appropriate.
Comment: Several commenters requested clarification of whether the data would be publicly reported annually or every two years because the measure has a two year performance period. These commenters further expressed concern that if data is reported annually the data may misrepresent facilities with recent improvement.
Response: The IPFQR Program publicly displays all measure data annually (78 FR 50897 through 50898 and 81 FR 57248 through 57249). For this measure we will post the data annually using a two-year performance period, similar to our reporting of the Thirty-Day All-Cause Unplanned Readmission Following Psychiatric Hospitalization in an Inpatient Psychiatric Facility (NQF #2860) measure. As an example, for both measures the intended performance period for FY 2021 reporting is July 1, 2017 through June 30, 2019. For FY 2022 reporting the performance period is July 1, 2018 through June 30, 2020. We note that these periods do overlap; however we believe that facilities with recent improvement will be distinguishable because their scores will show year-over-year improvement.
Comment: One commenter expressed concern that facilities without outpatient pharmacies may be at a performance disadvantage because they cannot ensure that patients fill prescriptions prior to discharge.
Response: We believe that many of the interventions to improve performance on this measure (for example, patient education at discharge, therapeutic alliance, text message reminders, etc.) are applicable to all facilities, regardless of whether they have an outpatient pharmacy on premises. Furthermore, we note that the practice guidelines for these conditions provide strategies for facilities to implement to help patients fill prescriptions prior to discharge so that the facility can track whether the prescription has been filled.[52 53 54 55 56]
Comment: One commenter requested that CMS provide guidance on what medications are considered evidence-based medications for these conditions.
Response: The measure technical report available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip has a detailed list of medications for each condition. As part of routine measure maintenance, we will evaluate and update this list on a recurrent basis.
Final Rule Action: After consideration of the public comments, we are finalizing as proposed the adoption of the Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205) measure for the FY 2021 payment determination and subsequent years.
4. Summary of Previously Finalized and Newly Proposed Measures for the FY 2021 Payment Determination and Subsequent Years
The previously finalized number of measures for the FY 2021 payment determination and subsequent years totals 13. In this final rule, we are adopting one additional measure for the FY 2021 payment determination and subsequent years which, brings the total to 14, as shown in table 18.

5. Possible IPFQR Program Measures and Topics for Future Consideration
As we have previously indicated in the FY 2015 IPF PPS final rule (79 FR 45974 through 45975), we seek to develop a comprehensive set of quality measures to be available for widespread use for informed decision-making and quality improvement in the IPF setting. In the FY 2020 IPF PPS proposed rule, we sought public comments on possible new measures or new measure topics. We welcomed all comments but expressed particular interest in comments on future adoption of one or more measures of patient experience of care based on a consumer survey, especially such as the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Survey, and potential future measures and topics as part of CMS' Meaningful Measures Framework.
a. Future Adoption a Patient Experience of Care Survey
In past assessments of the IPFQR Program Measure Set, we identified Patient Experience of Care as a measure gap area for this program (78 FR 50897, 79 FR 45964 through 45965, and 83 FR 38596 through 38597), which is consistent with input from past public comment (77 FR 53653). When we adopted the “Assessment of Patient Experience of Care Measure” for the FY 2016 payment determination and subsequent years, we noted that in addition to serving as an indicator of quality within IPFs, information gathered through the collection of this measure would be helpful in developing a standardized survey as a successor to the measure (79 FR 45964). When we removed the Assessment of Patient Experience of Care measure from the IPFQR Program, we stated we believe that we have now collected sufficient information to inform development of a patient experience of care measure (83 FR 38596).
At that time, several commenters expressed support for ensuring that patients have an opportunity to express their perspectives on their experience of receiving care at an IPF (83 FR 38597). Our analysis of the FY 2018 payment determination data (that is, data that represents facility assessment of patient experience of care as of December 31, 2016) collected under the Assessment of Patient Experience of Care measure shows that approximately one third of facilities use the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey [57] to assess patient experience of care. This is more than the portion of facilities using any other survey.
We sought public comment on how such providers have implemented the survey in their facilities, on whether they use the entire HCAHPS survey, or a subset of the survey questions; and if a subset, which specific questions they use. Additionally, we sought public comment on other potential surveys that commenters believe would be appropriate to adopt for the IPFQR Program. We intend to use this information to inform future development and testing of a survey-based patient experience of care measure (or measures) for the inpatient psychiatric patient population.
Comment: Many commenters supported future adoption of a patient experience of care survey. Several of these commenters expressed concern about the potential adoption of the HCAHPS survey for this patient population, specifically noting that this survey does not include some of the unique aspects of inpatient psychiatric care including group therapy, non-physician providers, and involuntary admissions. Some commenters observed that while most IPFs use a patient experience of care survey, there is not one survey used predominantly across settings and recommended that CMS partner with providers to either develop a minimally burdensome survey or to establish a core set of questions that should be included, therefore allowing provider flexibility to ask additional questions. These commenters believe that a custom developed survey would better address the needs of the patient population and would be preferable for providers than having to switch from a setting specific survey to a survey not designed for this setting. One commenter recommended that adoption of a patient experience of care measure should be done incrementally through a voluntary data collection period to ensure feasibility of collection prior to mandatory data submission. Several commenters also noted that the HCAHPS survey modalities (phone or mail post-discharge) may limit participation and recommended additional survey modalities for this potentially more transient patient population. One commenter expressed concern that a patient experience of care measure could be misinterpreted as the current state of care when the data has been collected in the past.
Response: We thank these commenters for their input and will consider these suggestions and concerns as we seek to develop or select an appropriate patient experience of care survey for the IPF setting.
b. Other Future Measures
In the FY 2020 IPF PPS proposed rule, we also sought feedback and suggestions for future measures and topics for the IPFQR Program that align with CMS's Meaningful Measures Framework (FY 2019 IPF PPS final rule, 83 FR 38590 through 38591).
Comment: One commenter recommended that CMS collaborate with providers to identify measure concepts and develop measures appropriate to the setting. Several commenters provided recommendations for future measure considerations; specifically measures that assess:
- Facility use of a standardized assessment of patient outcomes between admission and discharge;
- Family and caregiver engagement;
- Clinical improvement outcomes;
- Patient empowerment;
- Safety planning for patients with suicidal ideation;
- Discharge and transitions of care;
- Access to care; and
- Inpatient assaults and violence.
Response: We thank these commenters for their suggestions and will consider these concepts as we continue to develop a measure set that meets the specific needs of IPFs and inpatient psychiatric patients and their families.
E. Public Display and Review Requirements
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53653 through 53654), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50897 through 50898), and the FY 2017 IPPS/LTCH PPS final rule (81 FR 57248 through 57249) for discussion of our previously finalized public display and review requirements. We did not propose any changes to these requirements.
F. Form, Manner, and Timing of Quality Data Submission for the FY 2021 Payment Determination and Subsequent Years
1. Procedural Requirements for the FY 2021 Payment Determination and Subsequent Years
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53654 through 53655), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50898 through 50899), and the FY 2018 IPPS/LTCH PPS final rule (82 FR 38471 through 38472) for our previously finalized procedural requirements. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
2. Data Submission Requirements for the FY 2021 Payment Determination and Subsequent Years
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53655 through 53657), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50899 through 50900), and the FY 2018 IPPS/LTCH PPS final rule (82 FR 38472 through 38473) for our previously finalized data submission requirements.
Because the Medication Continuation following Discharge from an IPF (NQF #3205) measure is calculated by CMS using Medicare Fee-for-Service claims, there will be no additional data submission requirements for the FY 2021 payment determination and subsequent years. Therefore, in the FY 2020 IPF PPS proposed rule, we did not propose any changes to our previously finalized data submission policies.
3. Reporting Requirements for the FY 2021 Payment Determination and Subsequent Years
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53656 through 53657), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50900 through 50901), and the FY 2015 IPF PPS final rule (79 FR 45976 through 45977) for our previously finalized reporting requirements. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
4. Quality Measure Sampling Requirements
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53657 through 53658), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50901 through 50902), the FY 2016 IPF PPS final rule (80 FR 46717 through 46719), and the FY 2019 IPF PPS final rule (83 FR 38607 through 38608) discussions for our previously finalized sampling policies. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
5. Non-Measure Data Collection
We refer readers to the FY 2015 IPF PPS final rule (79 FR 45973), the FY 2016 IPF PPS final rule (80 FR 46717), and the FY 2019 IPF PPS final rule (83 FR 38608) for our previously finalized non-measure data collection policies. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
6. Data Accuracy and Completeness Acknowledgement (DACA) Requirements
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53658) for our previously finalized DACA requirements. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
G. Reconsideration and Appeals Procedures
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53658 through 53659) and the FY 2014 IPPS/LTCH PPS final rule (78 FR 50903) for our previously finalized reconsideration and appeals procedures. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
H. Extraordinary Circumstances Exceptions (ECE) Policy
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53659 through 53660), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50903), the FY 2015 IPF PPS final rule (79 FR 45978), and the FY 2018 IPPS/LTCH PPS final rule (82 FR 38473 through 38474) for our previously finalized ECE policies. In the FY 2020 IPF PPS proposed rule, we did not propose any changes to these policies.
VI. Collection of Information Requirements
The FY 2020 IPF PPS proposed rule did not propose any new or revised “collection of information” requirements as defined under 5 CFR 1320.3 the Paperwork Reduction Act's (PRA) implementing regulations. Nor did it contain any proposals that would have imposed any new or revised burden within the context of the PRA of 1995 (44 U.S.C. 3501 et seq.). However, we did make a number of burden adjustments based on updated Bureau of Labor Statistics (BLS) wage figures and more recent facility counts and estimated case data. These adjustments reduce our overall time estimate by 50,067 hours and increase our cost estimate by $1,820,149.
A. Collection of Information Requirements for the IPFQR Program
With regard to the IPFQR Program, we are finalizing one new measure (Medication Continuation Following Inpatient Psychiatric Discharge (NQF #3205)) that impacts the FY 2021 payment determination and subsequent years. The finalized measure is calculated by CMS using IPF submitted claims data. The claims' requirements and burden are approved by OMB under control number 0938-0050 (CMS-2552-10) for our Medicare cost report. The final measure does not impact any of the cost report's data fields or burden estimates as all worksheets and lines remain unchanged. Similarly, this final rule does not impose any new or revised collection of information requirements or burden under OMB control number 0938-1171 (CMS-10432) which contains information about our non-claims based IPFQR Program quality measure and non-quality measure information collection/reporting requirements and burden.
We refer readers to the FY 2013 IPPS/LTCH PPS final rule (77 FR 53673), the FY 2014 IPPS/LTCH PPS final rule (78 FR 50964, the FY 2015 IPF PPS final rule (79 FR 45978 through 45980), the FY 2016 IPF PPS final rule (80 FR 46720 through 46721), the FY 2017 IPPS/LTCH PPS [58] final rule (81 FR 57265 through 57266), the FY 2018 IPPS/LTCH PPS final rule (82 FR 38507 through 38508), and the FY 2019 IPF PPS final rule (83 FR 38609 through 38612) for a detailed discussion of the burden for the program requirements that we have previously adopted. Information pertaining to the requirements and burden that are currently approved by OMB can be found at reginfo.gov under control numbers 0938-0050 and 0938-1171.
B. Adjustments to IPFQR Program Burden Estimates
In the FY 2019 IPF PPS final rule (83 FR 38609), we estimated that reporting measures for the IPFQR Program could be accomplished by a Medical Records and Health Information Technician (BLS Occupation Code: 29-2071) with a median hourly wage of $18.29 per hour (as of May 2016). Since then, BLS (the Bureau of Labor Statistics) has revised their wage data with May 2017 serving as their most recent update.[59] In response, we proposed to update our cost estimates using the May 2017 figure of $18.83 per hour, an increase of $0.54 per hour or $1.08 per hour when adjusted by 100 percent to account for fringe benefits and overhead. This is necessarily a rough adjustment, both because fringe benefits and overhead costs vary significantly from employer-to-employer and because methods of estimating these costs vary widely from study-to-study. Nonetheless, we believe that doubling the hourly wage rate ($18.83 × 2 = $37.66) to estimate total cost is a reasonably accurate estimation method.
We also proposed to update our facility count and case estimates to the most recent data available. Specifically, we estimate that there are now approximately 1,679 (down from the previous estimate of 1,734) facilities and that for measures which require reporting on the entire patient population, these facilities will report on an average of 1,283 cases per facility (up from the previous estimate of 1,213). Accordingly, we proposed to adjust our currently approved cost estimate from $125,511,558 (see tables 19, 20, and 21) to $127,331,707 (see tables 22, 23, and 24).

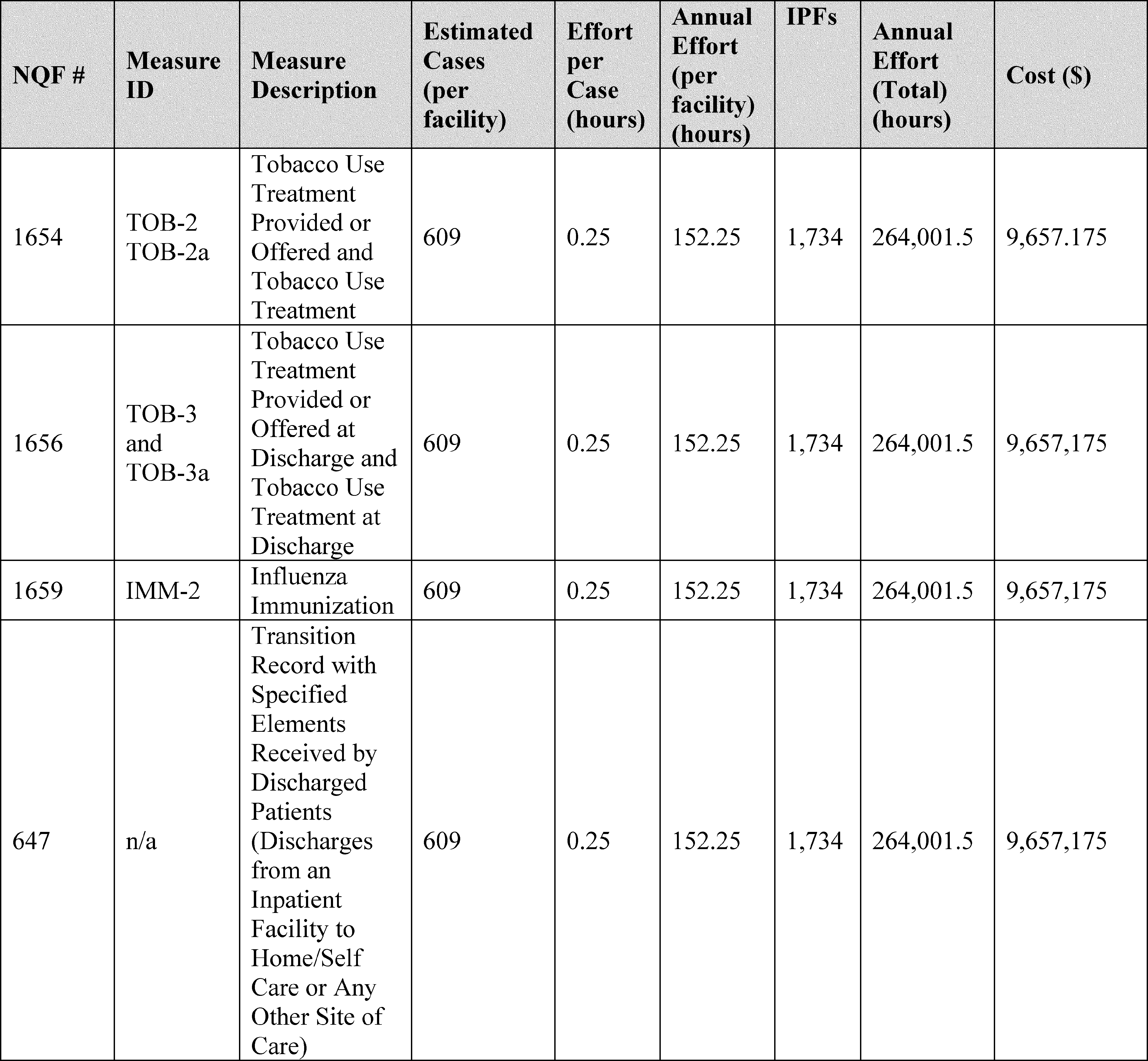


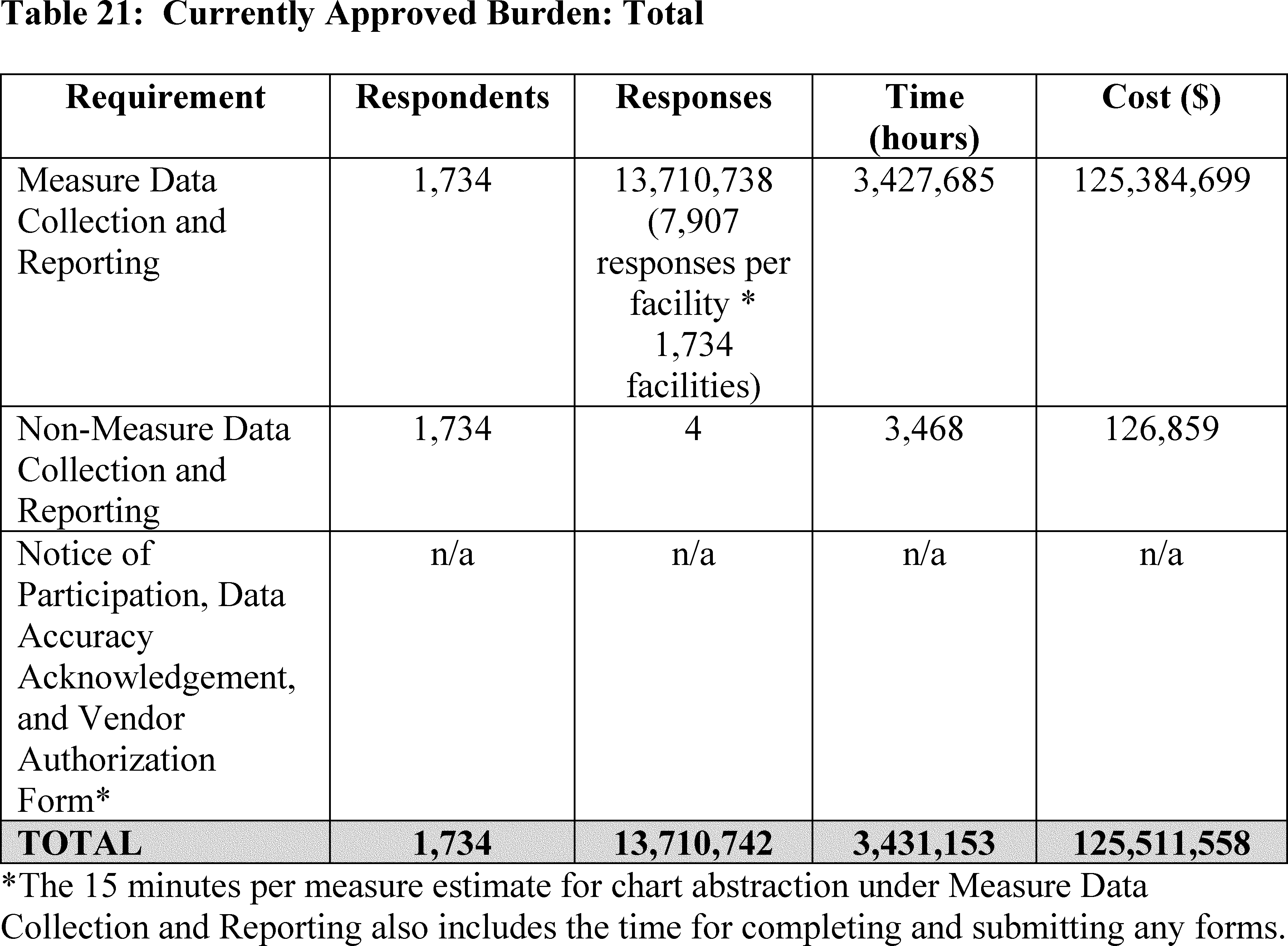



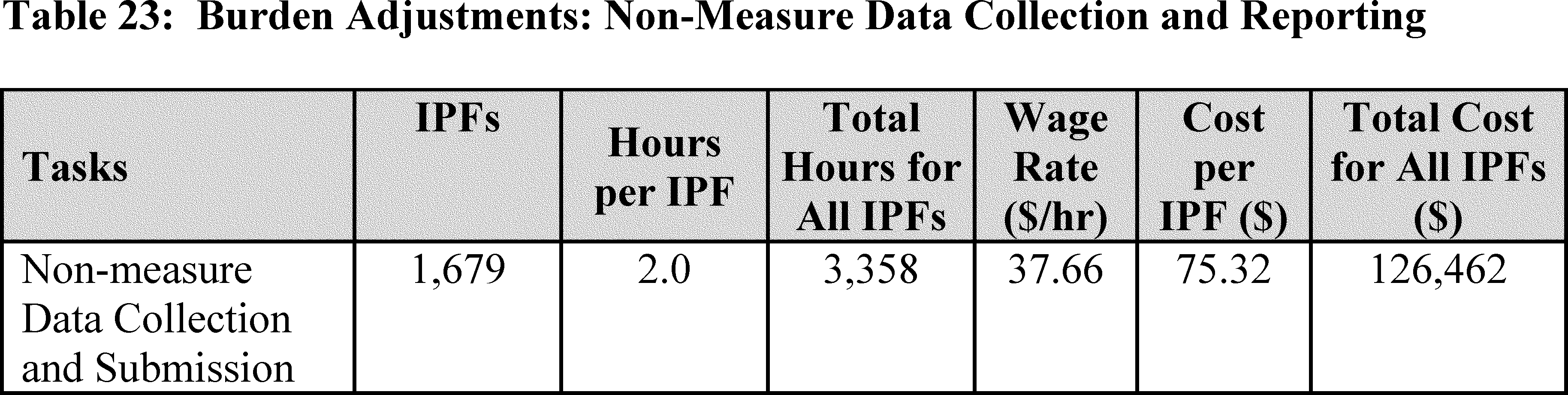

As mentioned at the beginning of this section, the adjustments are in response to updates to BLS wage figures and more recent facility counts and estimated case data. They are not a result of any of the provisions proposed in the FY 2020 IPF PPS proposed rule. The adjusted burden figures will be submitted to OMB for approval under control number 0938-1171 (CMS-10432) as a non-substantive change.
We did not receive any public comments on our proposed burden estimates.
C. Submission of PRA-Related Comments
We invited public comments on our proposed burden adjustments as well as on any of the information collection requirements/burden set out under OMB control number 0938-1171.
We did not receive any public comments on our proposed burden estimates.
VII. Regulatory Impact Statement
A. Statement of Need
This rule finalizes updates to the prospective payment rates for Medicare inpatient hospital services provided by IPFs for discharges occurring during FY 2020 (October 1, 2019 through September 30, 2020). We are finalizing our proposal to apply the 2016-based IPF market basket increase of 2.9 percent, less the productivity adjustment of 0.4 percentage point as required by 1886(s)(2)(A)(i) of the Act, and further reduced by 0.75 percentage point as required by sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act, for a final total FY 2020 payment rate update of 1.75 percent. In this final rule, we revised and rebased the IPF market basket to reflect a 2016 base year. We also aligned the IPF wage index data with the concurrent IPPS wage index data by removing the 1-year lag of the pre-floor, pre-reclassified IPPS hospital wage index upon which the IPF wage index is based. We also updated the IPF labor-related share and the IPF wage index including adoption of a new OMB designation. Finally, we updated the IPFQR Program for the FY 2021 payment determination and subsequent years.
B. Overall Impact
We have examined the impacts of this final rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96 354), section 1102(b) of the Social Security Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4), Executive Order 13132 on Federalism (August 4, 1999), the Congressional Review Act (5 U.S.C. 804(2)) and Executive Order 13771 on Reducing Regulation and Controlling Regulatory Costs (January 30, 2017).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Section 3(f) of Executive Order 12866 defines a “significant regulatory action” as an action that is likely to result in a rule: (1) Having an annual effect on the economy of $100 million or more in any 1 year, or adversely and materially affecting a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or state, local or tribal governments or communities (also referred to as “economically significant”); (2) creating a serious inconsistency or otherwise interfering with an action taken or planned by another agency; (3) materially altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raising novel legal or policy issues arising out of legal mandates, the President's priorities, or the principles set forth in the Executive Order.
A regulatory impact analysis (RIA) must be prepared for major rules with economically significant effects ($100 million or more in any 1 year). This final rule is not economically significant under Executive Order 12866, within the meaning of section 3(f)(1) of the Executive Order. However, OMB has determined that the actions are significant within the meaning of section 3(f)(4) of the Executive Order. Therefore, OMB has reviewed this final rule, and the Departments have provided the following assessment of their impact.
We estimate that the total impact of these changes for FY 2020 payments compared to FY 2019 payments will be a net increase of approximately $65 million. This reflects an $75 million increase from the update to the payment rates (+$125 million from the second quarter 2019 IGI forecast of the 2016-based IPF market basket of 2.9 percent, −$15 million for the productivity adjustment of 0.4 percentage point, and −$35 million for the “other adjustment” of 0.75 percentage point), as well as a $10 million decrease as a result of the update to the outlier threshold amount. Outlier payments are estimated to change from 2.23 percent in FY 2019 to 2.00 percent of total estimated IPF payments in FY 2020.
C. Anticipated Effects
In this section, we discuss the historical background of the IPF PPS and the impact of this final rule on the Federal Medicare budget and on IPFs.
1. Budgetary Impact
As discussed in the November 2004 and RY 2007 IPF PPS final rules, we applied a budget neutrality factor to the federal per diem base rate and ECT payment per treatment to ensure that total estimated payments under the IPF PPS in the implementation period would equal the amount that would have been paid if the IPF PPS had not been implemented. The budget neutrality factor includes the following components: Outlier adjustment, stop-loss adjustment, and the behavioral offset. As discussed in the RY 2009 IPF PPS notice (73 FR 25711), the stop-loss adjustment is no longer applicable under the IPF PPS.
As discussed in section III.D.1 of this final rule, we are updating the wage index and labor-related share in a budget neutral manner by applying a wage index budget neutrality factor to the federal per diem base rate and ECT payment per treatment. Therefore, the budgetary impact to the Medicare program of this final rule will be due to the market basket update for FY 2020 of 2.9 percent (see section III.A.4 of this final rule) less the productivity adjustment of 0.4 percentage point required by section 1886(s)(2)(A)(i) of the Act; further reduced by the “other adjustment” of 0.75 percentage point under sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act; and the update to the outlier fixed dollar loss threshold amount.
We estimate that the FY 2020 impact will be a net increase of $65 million in payments to IPF providers. This reflects an estimated $75 million increase from the update to the payment rates and a $10 million decrease due to the update to the outlier threshold amount to set total estimated outlier payments at 2.0 percent of total estimated payments in FY 2020. This estimate does not include the implementation of the required 2.0 percentage point reduction of the market basket increase factor for any IPF that fails to meet the IPF quality reporting requirements (as discussed in section V.A. of this final rule).
The RFA requires agencies to analyze options for regulatory relief of small entities if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. Most IPFs and most other providers and suppliers are small entities, either by nonprofit status or having revenues of $7.5 million to $38.5 million or less in any 1 year, depending on industry classification (for details, refer to the SBA Small Business Size Standards found at http://www.sba.gov/sites/default/files/files/Size_Standards_Table.pdf). Individuals and states are not included in the definition of a small entity.
Because we lack data on individual hospital receipts, we cannot determine the number of small proprietary IPFs or the proportion of IPFs' revenue derived from Medicare payments. Therefore, we assume that all IPFs are considered small entities.
The Department of Health and Human Services generally uses a revenue impact of 3 to 5 percent as a significance threshold under the RFA. As shown in Table 25, we estimate that the overall revenue impact of this final rule on all IPFs is to increase estimated Medicare payments by approximately 1.5 percent. As a result, since the estimated impact of this final rule is a net increase in revenue across almost all categories of IPFs, the Secretary has determined that this final rule will have a positive revenue impact on a substantial number of small entities.
In addition, section 1102(b) of the Act requires us to prepare a regulatory impact analysis if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 604 of the RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a metropolitan statistical area and has fewer than 100 beds. As discussed in section VII.C.1 of this final rule, the rates and policies set forth in this final rule will not have an adverse impact on the rural hospitals based on the data of the 255 rural excluded psychiatric units and 66 rural psychiatric hospitals in our database of 1,581 IPFs for which data were available. Therefore, the Secretary has determined that this final rule will not have a significant impact on the operations of a substantial number of small rural hospitals.
Section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA) also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2019, that threshold is approximately $154 million. This final rule does not impose spending costs on state, local, or tribal governments in the aggregate, or by the private sector of $154 million or more.
Executive Order 13132 establishes certain requirements that an agency must meet when it promulgates a final rule that imposes substantial direct requirement costs on state and local governments, preempts state law, or otherwise has Federalism implications. This final rule will not have a substantial effect on state and local governments.
2. Impact on Providers
To show the impact on providers of the changes to the IPF PPS discussed in this final rule, we compare estimated payments under the IPF PPS rates and factors for FY 2020 versus those under FY 2019. We determined the percent change in the estimated FY 2020 IPF PPS payments compared to the estimated FY 2019 IPF PPS payments for each category of IPFs. In addition, for each category of IPFs, we have included the estimated percent change in payments resulting from the update to the outlier fixed dollar loss threshold amount; the updated wage index data including the updated labor-related share; and the market basket update for FY 2020, as adjusted by the productivity adjustment according to section 1886(s)(2)(A)(i) of the Act, and the “other adjustment” according to sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act.
To illustrate the impacts of the FY 2020 changes in this final rule, our analysis begins with a FY 2019 baseline simulation model based on FY 2018 IPF payments inflated to the midpoint of FY 2019 using IHS Global Inc.'s second quarter 2019 forecast of the market basket update (see section III.A.4 of this final rule); the estimated outlier payments in FY 2019; the FY 2019 IPF wage index; the FY 2019 labor-related share; and the FY 2019 percentage amount of the rural adjustment. During the simulation, total outlier payments are maintained at 2 percent of total estimated IPF PPS payments.
Each of the following changes is added incrementally to this baseline model in order for us to isolate the effects of each change:
- The update to the outlier fixed dollar loss threshold amount.
- The FY 2020 IPF wage index and the FY 2020 labor-related share.
- The market basket update for FY 2020 of 2.9 percent less the productivity adjustment of 0.4 percentage point in accordance with section 1886(s)(2)(A)(i) of the Act and further reduced by the “other adjustment” of 0.75 percentage point in accordance with sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act, for a payment rate update of 1.75 percent.
Our final column comparison in Table 25 illustrates the percent change in payments from FY 2019 (that is, October 1, 2018, to September 30, 2019) to FY 2020 (that is, October 1, 2019, to September 30, 2020) including all the payment policy changes in this final rule.


3. Impact Results
Table 25 displays the results of our analysis. The table groups IPFs into the categories listed here based on characteristics provided in the Provider of Services (POS) file, the IPF provider specific file, and cost report data from the Healthcare Cost Report Information System:
- Facility Type.
- Location.
- Teaching Status Adjustment.
- Census Region.
- Size.
The top row of the table shows the overall impact on the 1,581 IPFs included in this analysis. In column 3, we present the effects of the update to the outlier fixed dollar loss threshold amount. We estimate that IPF outlier payments as a percentage of total IPF payments are 2.23 percent in FY 2019. Thus, we are adjusting the outlier threshold amount in this final rule to set total estimated outlier payments equal to 2.0 percent of total payments in FY 2020. The estimated change in total IPF payments for FY 2020, therefore, includes an approximate 0.23 percent decrease in payments because the outlier portion of total payments is expected to decrease from approximately 2.23 percent to 2.0 percent.
The overall impact of this outlier adjustment update (as shown in column 3 of Table 25), across all hospital groups, is to decrease total estimated payments to IPFs by 0.23 percent. The largest decrease in payments is estimated to be −0.78 percent for teaching IPFs with more than 30 percent interns and residents to beds.
In column 4, we present the effects of the budget-neutral update to the IPF wage index and the Labor-Related Share (LRS). This represents the effect of using the concurrent hospital wage data and taking into account the updated OMB delineations. That is, the impact represented in this column reflects the update from the FY 2019 IPF wage index to the final FY 2020 IPF wage index, which includes basing the FY 2020 IPF wage index on the FY 2020 pre-floor, pre-reclassified IPPS hospital wage index data, updating the OMB designations for two counties in Idaho, and updating the LRS from 74.8 percent in FY 2019 to 76.9 percent in FY 2020. We note that there is no projected change in aggregate payments to IPFs, as indicated in the first row of column 4, however, there will be distributional effects among different categories of IPFs. For example, we estimate the largest increase in payments to be 2.08 percent for Pacific IPFs, and the largest decrease in payments to be 0.83 percent for New England IPFs.
Finally, column 5 compares our estimates of the total final changes reflected in this final rule for FY 2020 to the estimates for FY 2019 (without these changes). The average estimated increase for all IPFs is approximately 1.5 percent. This estimated net increase includes the effects of the 2016-based market basket update of 2.9 percent reduced by the productivity adjustment of 0.4 percentage point, as required by section 1886(s)(2)(A)(i) of the Act and further reduced by the “other adjustment” of 0.75 percentage point, as required by sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act. It also includes the overall estimated 0.23 percent decrease in estimated IPF outlier payments as a percent of total payments from the final update to the outlier fixed dollar loss threshold amount. Column 5 also includes the distributional effects of the updates to the IPF wage index and the labor-related share.
IPF payments are estimated to increase by 1.54 percent in urban areas and 1.34 percent in rural areas. Overall, IPFs are estimated to experience a net increase in payments as a result of the updates in this final rule. The largest payment increase is estimated at 3.49 percent for IPFs in the Pacific region.
Comment: One commenter wrote that the proposed 1.7 percent estimated total IPF update was not sufficient to cover the costs of medical inflation and the growing demand for IPF services. This commenter was concerned that the update could negatively impact the financial viability of IPFs and jeopardize access.
Response: Total IPF payments were estimated to increase by 1.7 percent in the FY 2020 IPF PPS proposed rule. This 1.7 percent increase is a combination of the effects of the proposed market basket update for FY 2020 and the proposed update to the outlier threshold.
The final FY 2020 estimated increase in payments is based on a more recent estimate of the final 2016-based IPF market basket percentage increase of 2.9 percent, a more recent estimate of the MFP adjustment of 0.4 percentage point, less the 0.75 percentage point reduction (in accordance with sections 1886(s)(2)(A)(ii) and 1886(s)(3)(E) of the Act) and impact of the outlier threshold, for a total payment update of 1.75 percent.
The 2.9 percentage increase of the IPF market basket represents the FY 2020 projected increase in prices of the relative inputs used to furnish IPF services to Medicare beneficiaries. The forecasted prices of the individual inputs are based on IGI's most recent 2nd quarter 2019 forecast of the price proxies in the market basket. IGI is a nationally recognized economic and financial forecasting firm that has received multiple awards for their macroeconomic forecast accuracy of major economic indicators. CMS uses IGI's price forecasts in all of the FFS market baskets used for payment updates and has used the forecasts produced by this company for many years. In this FY 2020 final rule, we are also updating the cost weights for the IPF market basket, from 2012 to 2016, which captures changes in relative costs due to quantity and intensity. We therefore believe that the IPF market basket represents an appropriate measure of input price inflation that is expected to be realized by IPFs in FY 2020.
As stated, the Act mandates that the market basket update (which accounts for input price inflation) be adjusted for multifactor productivity and a 0.75 percentage point legislatively required adjustment. CMS does not have the authority to alter these payment adjustments, but we note that under the current law at 1886(s)(3)(E), FY 2020 is the last year that the 0.75 percentage point “other” adjustment will be made.
Estimated IPF payments are also reduced by 0.23 percent as a result of the update to the outlier threshold. Based on an updated analysis of the most recent IPF claims data for this final rule we now estimate that IPF outlier payments as a percentage of total estimated payments will be approximately 2.23 percent in FY 2019. Since this percentage exceeds our established 2 percent IPF outlier policy we are adjusting the outlier threshold amount to set total estimated outlier payments equal to 2 percent of total estimated payments in FY 2020. The estimated change in total IPF payments for FY 2020 includes an approximate 0.23 percent decrease in payments because the estimated outlier portion of total payments is estimated to decrease from 2.23 percent to 2 percent.
4. Effect on Beneficiaries
Under the IPF PPS, IPFs will receive payment based on the average resources consumed by patients for each day. We do not expect changes in the quality of care or access to services for Medicare beneficiaries under the FY 2020 IPF PPS, but we continue to expect that paying prospectively for IPF services will enhance the efficiency of the Medicare program.
5. Effects of Updates to the Inpatient Psychiatric Facilities Quality Reporting (IPFQR) Program
As discussed in section V. of this final rule and in accordance with section 1886(s)(4)(A)(i) of the Act, we will implement a 2 percentage point reduction in the market basket update when calculating the FY 2021 national per diem rate for discharges from IPFs that have failed to comply with the IPFQR Program requirements for the FY 2021 payment determination. In section III.B. of this final rule, we discuss how the 2 percentage point reduction will be applied. For the FY 2019 payment determination (that is, data submitted in CY 2018), of the 1,679 IPFs eligible for the IPFQR Program, 50 did not receive the full market basket update due to reasons specific to the IPFQR Program; 24 of these IPFs chose not to participate and 26 did not meet the requirements of the Program. Thus, we estimate similar numbers for the FY 2021 payment determination and that the IPFQR Program will have a negligible impact on overall IPF payments in FY 2021.
We are finalizing provisions that impact the FY 2021 payment determination and subsequent years. We refer readers to section VI. of this final rule for details discussing information collection requirements for the IPFQR Program. We will closely monitor the effects of this quality reporting program on IPFs and help facilitate successful reporting outcomes through ongoing stakeholder education, national trainings, and a technical help desk.
6. Regulatory Review Costs
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this final rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review this final rule, we assume that the total number of unique commenters on the most recent IPF proposed rule from FY 2020 (84 FR 16948) will be the number of reviewers of this final rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this final rule. It is possible that not all commenters reviewed the FY 2020 IPF proposed rule in detail, and it is also possible that some reviewers chose not to comment on that proposed rule. For these reasons we thought that the number of commenters would be a fair estimate of the number of reviewers of this final rule. We solicited comments on this assumption.
We also recognize that different types of entities are in many cases affected by mutually exclusive sections of this final rule; therefore, for the purposes of our estimate, we assume that each reviewer reads approximately 50 percent of this final rule.
Using the May, 2018 mean (average) wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing this final rule is $109.36 per hour, including overhead and fringe benefits (https://www.bls.gov/oes/current/oes119111.htm). Assuming an average reading speed of 250 words per minute, we estimate that it would take approximately 1.4 hours for the staff to review half of this final rule. For each IPF that reviews the final rule, the estimated cost is (1.4 hours × $109.36) or $153.10. Therefore, we estimate that the total cost of reviewing this final rule is $3,674.40 ($153.10 × 24 reviewers).
We received one comment on our assumption about the number of reviewers of the IPF PPS proposed rule.
Comment: One commenter wrote that CMS should consider the number of downloads of the IPF proposed rule in calculating regulatory review costs, since many reviewers may read the rule but not submit a comment. The commenter also noted that some organizations may download the rule once and distribute copies to others to read. This commenter suggested that CMS consider the greater of the number of downloads or of the number of unique commenters as a fair estimate of the number of reviewers. This commenter believes that this method would be a fairer assumption of the number of reviewers.
Response: We appreciate the commenter's input on our methodology. We have acknowledged that our method provides an estimate that could overstate or understate the costs of reviewing the rule. We do not believe this suggested methodology would improve the accuracy of the estimate. We do not currently have the ability to track the number of times the IPF rule is downloaded, and if we did, to know how many of those downloads are by those who are providers or similar stakeholders. We also prefer to use a methodology for estimating the number of reviewers that is consistent with the methodology that other Medicare payment systems use. As such, we will continue to use the number of commenters on the most recent proposed rule as the basis for our review cost estimate.
D. Alternatives Considered
The statute does not specify an update strategy for the IPF PPS and is broadly written to give the Secretary discretion in establishing an update methodology. Therefore, we are updating the IPF PPS using the methodology published in the November 2004 IPF PPS final rule; applying the 2016-based IPF PPS market basket update for FY 2020 of 2.9 percent, reduced by the statutorily required multifactor productivity adjustment of 0.4 percentage point and the “other adjustment” of 0.75 percentage point, along with the wage index budget neutrality adjustment to update the payment rates; finalizing a FY 2020 IPF wage index which is fully based upon the OMB CBSA designations from Bulletin 17-01 and which uses the FY 2020 pre-floor, pre-reclassified IPPS hospital wage index as its basis; and finalizing changes to the IPFQR Program.
E. Accounting Statement
As required by OMB Circular A-4 (available at www.whitehouse.gov/sites/whitehouse.gov/files/omb/circulars/A4/a-4.pdf), in Table 26, we have prepared an accounting statement showing the classification of the expenditures associated with the updates to the IPF wage index and payment rates in this final rule. Table 26 provides our best estimate of the increase in Medicare payments under the IPF PPS as a result of the changes presented in this final rule and based on the data for 1,581 IPFs in our database.
| Category | Transfers |
|---|---|
| Annualized Monetized Transfers | $65 million. |
| From Whom to Whom? | Federal Government to IPF Medicare Providers. |
F. Congressional Review Act
Pursuant to the Congressional Review Act (5 U.S.C. 801 et seq.) the Office of Information and Regulatory Affairs designated this rule as not a major rule, as defined by 5 U.S.C. 804(2).
G. Regulatory Reform Analysis Under Executive Order 13771
Executive Order 13771, titled Reducing Regulation and Controlling Regulatory Costs, was issued on January 30, 2017 and requires that the costs associated with significant new regulations “shall, to the extent permitted by law, be offset by the elimination of existing costs associated with at least two prior regulations.” This final rule is not expected to be subject to the requirements of Executive Order 13771 because it is estimated to result in no more than de minimis costs as described previously and thus is not a regulatory action for the purposes of E.O. 13771.
H. Conclusion
In accordance with the provisions of Executive Order 12866, this regulation was reviewed by the Office of Management and Budget.
Dated: July 26, 2019 .
Seema Verma,
Administrator, Centers for Medicare & Medicaid Services.
Dated: July 26, 2019.
Alex M. Azar II,
Secretary, Department of Health and Human Services.
Footnotes
1. http://www.bea.gov/papers/pdf/IOmanual_092906.pdf.
Back to Citation2. We note that the statute uses the term “rate year” (RY). However, beginning with the annual update of the inpatient psychiatric facility prospective payment system (IPF PPS) that took effect on July 1, 2011 (RY 2012), we aligned the IPF PPS update with the annual update of the ICD codes, effective on October 1 of each year. This change allowed for annual payment updates and the ICD coding update to occur on the same schedule and appear in the same Federal Register document, promoting administrative efficiency. To reflect the change to the annual payment rate update cycle, we revised the regulations at 42 CFR 412.402 to specify that, beginning October 1, 2012, the RY update period would be the 12-month period from October 1 through September 30, which we refer to as a “fiscal year” (FY) (76 FR 26435). Therefore, with respect to the IPFQR Program, the terms “rate year,” as used in the statute, and “fiscal year” as used in the regulation, both refer to the period from October 1 through September 30. For more information regarding this terminology change, we refer readers to section III. of the RY 2012 IPF PPS final rule (76 FR 26434 through 26435).
Back to Citation3. Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: A systematic review. Lancet. 2003;361(9358):653-661.
4. Glue P, Donovan MR, Kolluri S, Emir B. Metaanalysis of relapse prevention antidepressant trials in depressive disorders. The Australian and New Zealand journal of psychiatry. 2010;44(8):697-705.
Back to Citation5. Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. The American journal of psychiatry. 2004;161(4):692-699.
Back to Citation6. Gonzalez-Pinto A, Mosquera F, Alonso M, et al. Suicidal risk in bipolar I disorder patients and adherence to long-term lithium treatment. Bipolar disorders. 2006;8(5 Pt 2):618-624.
Back to Citation7. American Psychiatric Association. (2002). Practice guideline for the treatment of patients with bipolar disorder, second edition. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf.
8. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
9. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with schizophrenia: 2nd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf.
10. U.S. Department of Veterans Affairs, & U.S. Department of Defense. (2016). Management of major depressive disorder (MDD). Retrieved from: http://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf.
11. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2010) VA/DOD clinical practice guideline for management of bipolar disorder in adults. Retrieved from: http://www.healthquality.va.gov/guidelines/MH/bd/bd_305_full.pdf.
Back to Citation12. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient related outcome measures. 2014;5:43-62.
13. Hung CI. Factors predicting adherence to antidepressant treatment. Current opinion in psychiatry. 2014;27(5):344-349.
14. Lanouette NM, Folsom DP, Sciolla A, Jeste DV. Psychotropic medication nonadherence among United States Latinos: a comprehensive literature review. Psychiatric services (Washington, DC). 2009;60(2):157-174.
15. Mitchell AJ. Understanding Medication Discontinuation in Depression. BMedSci Psychiatric Times. 2007;24(4).
16. Sylvia LG, Hay A, Ostacher MJ, et al. Association between therapeutic alliance, care satisfaction, and pharmacological adherence in bipolar disorder. Journal of clinical psychopharmacology. 2013;33(3):343-350.
Back to Citation17. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip.
Back to Citation18. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip
Back to Citation19. https://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=85831.
Back to Citation20. https://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=85831.
Back to Citation21. https://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=85831
Back to Citation22. MAP Hospital Workgroup, Preliminary Analysis Worksheet. December 2016. http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=84199.
Back to Citation23. http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=84452.
Back to Citation24. http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=84452.
Back to Citation25. http://www.qualityforum.org/WorkArea/linkit.aspx?LinkIdentifier=id&ItemID=84452.
Back to Citation26. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
27. American Psychiatric Association. (2002). Practice guideline for the treatment of patients with bipolar disorder, second edition. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf.
28. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with schizophrenia: 2nd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf.
29. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2016). Management of major depressive disorder (MDD). Retrieved from: http://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf.
30. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2010) VA/DOD clinical practice guideline for management of bipolar disorder in adults. Retrieved from: http://www.healthquality.va.gov/guidelines/MH/bd/bd_305_full.pdf.
Back to Citation31. https://www.qualityforum.org/QPS/3205.
Back to Citation32. If data availability or operational issues prevent use of this performance period, we would announce the updated performance period through sub-regulatory communications including announcement on a CMS website and/or on our applicable listservs.
Back to Citation33. http://www.qualityforum.org/Projects/a-b/Behavioral_Health_2016-2017/Draft_Report_for_Comment.aspx.
Back to Citation34. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient related outcome measures. 2014;5:43-62.
35. Hung CI. Factors predicting adherence to antidepressant treatment. Current opinion in psychiatry. 2014;27(5):344-349.
36. Lanouette NM, Folsom DP, Sciolla A, Jeste DV. Psychotropic medication nonadherence among United States Latinos: a comprehensive literature review. Psychiatric services (Washington, DC). 2009;60(2):157-174.
37. Mitchell AJ. Understanding Medication Discontinuation in Depression. BMedSci Psychiatric Times. 2007;24(4).
38. Sylvia LG, Hay A, Ostacher MJ, et al. Association between therapeutic alliance, care satisfaction, and pharmacological adherence in bipolar disorder. Journal of clinical psychopharmacology. 2013;33(3):343-350.
Back to Citation39. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip.
Back to Citation40. http://www.qualityforum.org/Projects/a-b/Behavioral_Health_2016-2017/Draft_Report_for_Comment.asp.
Back to Citation41. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
42. American Psychiatric Association. (2002). Practice guideline for the treatment of patients with bipolar disorder, second edition. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf.
43. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with schizophrenia: 2nd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf.
44. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2016). Management of major depressive disorder (MDD). Retrieved from: http://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf.
45. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2010) VA/DOD clinical practice guideline for management of bipolar disorder in adults. Retrieved from: http://www.healthquality.va.gov/guidelines/MH/bd/bd_305_full.pdf.
Back to Citation46. https://www.medicare.gov/hospitalcompare/psych-measures.html.
Back to Citation47. https://www.ncbi.nlm.nih.gov/pubmed/27654151.
48. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2017.7a17.
Back to Citation49. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip.
Back to Citation50. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Version_1-0_Inpatient_Psychiatric_Facility_Medication_Continuation_Public.zip.
Back to Citation51. Ibid.
Back to Citation52. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf.
53. American Psychiatric Association. (2002). Practice guideline for the treatment of patients with bipolar disorder, second edition. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf.
54. American Psychiatric Association. (2010). Practice guideline for the treatment of patients with schizophrenia: 2nd ed. Retrieved from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf.
55. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2016). Management of major depressive disorder (MDD). Retrieved from: http://www.healthquality.va.gov/guidelines/MH/mdd/VADoDMDDCPGFINAL82916.pdf.
56. U.S. Department of Veterans Affairs & U.S. Department of Defense. (2010) VA/DOD clinical practice guideline for management of bipolar disorder in adults. Retrieved from: http://www.healthquality.va.gov/guidelines/MH/bd/bd_305_full.pdf.
Back to Citation57. For more information about the HCAHPS survey, please see https://www.ahrq.gov/cahps/surveys-guidance/hospital/about/adult_hp_survey.html.
Back to Citation58. We note that for operational reasons we sometimes publish IPFQR program requirements in the IPPS/LTCH PPS proposed and final rule as opposed to the IPF PPS proposed and final rule.
Back to Citation59. https://www.bls.gov/ooh/healthcare/medical-records-and-health-information-technicians.htm.
Back to CitationBILLING CODE 4120-01-P
BILLING CODE 4120-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
[FR Doc. 2019-16370 Filed 7-30-19; 4:15 pm]
BILLING CODE 4120-01-P
