AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Final rule with comment period.
SUMMARY:
This final rule with comment period updates the home health prospective payment system (HH PPS) payment rates and wage index for CY 2020; implements the Patient-Driven Groupings Model (PDGM), a revised case-mix adjustment methodology, for home health services beginning on or after January 1, 2020. This final rule with comment period also implements a change in the unit of payment from 60-day episodes of care to 30-day periods of care, as required by section 51001 of the Bipartisan Budget Act of 2018, hereinafter referred to the “BBA of 2018”, and finalizes a 30-day payment amount for CY 2020. Additionally, this final rule with comment period: Modifies the payment regulations pertaining to the content of the home health plan of care; allows therapist assistants to furnish maintenance therapy; and changes the split percentage payment approach under the HH PPS. For the Home Health Value-Based Purchasing (HHVBP) model, we are finalizing provisions requiring the public reporting of the Total Performance Score (TPS) and the TPS Percentile Ranking from the Performance Year 5 (CY 2020) Annual TPS and Payment Adjustment Report for each home health agency in the nine Model states that qualified for a payment adjustment for CY 2020. This final rule with comment period also finalizes the following updates to the Home Health Quality Reporting Program (HH QRP): Removal of a measure; adoption of two new measures; modification of an existing measure; and a requirement for HHA's to report standardized patient assessment data beginning with the CY 2022 HH QRP. Additionally, we are finalizing our proposal to re-designate our current HH QRP regulations in a different section of our regulations and to codify other current policies in that new regulatory section with one substantive change as well as a few technical edits. We are not finalizing our proposal to remove question 10 from all of the HH Consumer Assessment of Healthcare Providers and Systems (CAHPS) surveys. Lastly, it sets forth routine updates to the home infusion therapy payment rates for CY 2020, payment provisions for home infusion therapy services for CY 2021 and subsequent years, and solicits comments on options to enhance future efforts to improve policies related to coverage of eligible drugs for home infusion therapy.
DATES:
Effective Date: This final rule with comment period is effective January 1, 2020.
Comment Date: To be assured consideration, comments on the criteria that can be considered to allow coverage of additional drugs under the DME benefit discussed in section VI.D. of this final rule with comment period must be received at one of the addresses provided below, no later than 5 p.m. on December 30, 2019.
ADDRESSES:
In commenting, please refer to file code CMS-1711-FC. Because of staff and resource limitations, we cannot accept comments by facsimile (FAX) transmission.
Comments, including mass comment submissions, must be submitted in one of the following three ways (please choose only one of the ways listed):
1. Electronically. You may submit electronic comments on this regulation to http://www.regulations.gov. Follow the “Submit a comment” instructions.
2. By regular mail. You may mail written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1711-FC, P.O. Box 8013, Baltimore, MD 21244-8013.
Please allow sufficient time for mailed comments to be received before the close of the comment period.
3. By express or overnight mail. You may send written comments to the following address ONLY: Centers for Medicare & Medicaid Services, Department of Health and Human Services, Attention: CMS-1711-FC, Mail Stop C4-26-05, 7500 Security Boulevard, Baltimore, MD 21244-1850.
For information on viewing public comments, see the beginning of the SUPPLEMENTARY INFORMATION section.
FOR FURTHER INFORMATION CONTACT:
Hillary Loeffler, (410) 786-0456, for Home Health Prospective Payment System (HH PPS) or home infusion payment.
For general information about the Home Health Prospective Payment System (HH PPS), send your inquiry via email to: HomehealthPolicy@cms.hhs.gov.
For general information about home infusion payment, send your inquiry via email to: HomeInfusionPolicy@cms.hhs.gov.
For information about the Home Health Value-Based Purchasing (HHVBP) Model, send your inquiry via email to: HHVBPquestions@cms.hhs.gov.
For information about the Home Health Quality Reporting Program (HH QRP), send your inquiry via email to HHQRPquestions@cms.hhs.gov.
SUPPLEMENTARY INFORMATION:
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period on the following website as soon as possible after they have been received: http://www.regulations.gov. Follow the search instructions on that website to view public comments.
Table of Contents
I. Executive Summary
A. Purpose
B. Summary of the Major Provisions
C. Summary of Costs and Benefits
II. Overview of the Home Health Prospective Payment System (HH PPS)
A. Statutory Background
B. Current System for Payment of Home Health Services
C. New Home Health Prospective Payment System for CY 2020 and Subsequent Years
D. Analysis of CY 2017 HHA Cost Report Data
III. Payment Under the Home Health Prospective Payment System (HH PPS)
A. Implementation of the Patient-Driven Groupings Model (PDGM) for CY 2020
B. Implementation of a 30-Day Unit of Payment for CY 2020
C. CY 2020 HH PPS Case-Mix Weights for 60-Day Episodes of Care Spanning Implementation of the PDGM
D. CY 2020 PDGM Case-Mix Weights and Low-Utilization Payment Adjustment (LUPA) Thresholds
E. CY 2020 Home Health Payment Rate Updates
F. Payments for High-Cost Outliers under the HH PPS
G. Changes to the Split-Percentage Payment Approach for HHAs in CY 2020 and Subsequent Years
H. Change To Allow Therapist Assistants To Perform Maintenance Therapy
I. Changes to the Home Health Plan of Care Regulations at § 409.43
IV. Home Health Value-Based Purchasing (HHVBP) Model
A. Background
B. Public Reporting of Total Performance Scores and Percentile Rankings Under the HHVBP Model
C. Removal of Improvement in Pain Interfering With Activity Measure (NQF #0177)
V. Home Health Quality Reporting Program (HH QRP)
A. Background and Statutory Authority
B. General Considerations Used for the Selection of Quality Measures for the HH QRP
C. Quality Measures Currently Adopted for the CY 2021 HH QRP
D Removal of HH QRP Measures Beginning With the CY 2022 HH QRP
E. New and Modified HH QRP Quality Measures Beginning With the CY 2022 HH QRP
F. HH QRP Quality Measures, Measure Concepts, and Standardized Patient Assessment Data Elements Under Consideration for Future Years: Request for Information
G. Standardized Patient Assessment Data Reporting Beginning With the CY 2022 HH QRP
H. Standardized Patient Assessment Data by Category
I. Form, Manner, and Timing of Data Submission Under the HH QRP
J. Codification of the Home Health Quality Reporting Program Requirements
K. Home Health Care Consumer Assessment of Healthcare Providers and Systems (CAHPS®) Survey (HHCAHPS)
VI. Medicare Coverage of Home Infusion Therapy Services
A. Background and Overview
B. CY 2020 Temporary Transitional Payment Rates for Home Infusion Therapy Services
C. Home Infusion Therapy Services for CY 2021 and Subsequent Years
D. Payment Categories and Amounts for Home Infusion Therapy Services for CY 2021
E. Required Payment Adjustments for CY 2021 Home Infusion Therapy Services
F. Other Optional Payment Adjustments/Prior Authorization for CY 2021 Home Infusion Therapy Services
G. Billing Procedures for CY 2021 Home Infusion Therapy Services
VII. Waiver of Proposed Rule
VIII. Collection of Information Requirements
IX. Regulatory Impact Analysis
A. Statement of Need
B. Overall Impact
C. Anticipated Effects
D. Detailed Economic Analysis
E. Alternatives Considered
F. Accounting Statement and Tables
G. Regulatory Reform Analysis Under E.O. 13771
H. Conclusion
Regulation Text
I. Executive Summary
A. Purpose
1. Home Health Prospective Payment System (HH PPS)
This final rule with comment period updates the payment rates for home health agencies (HHAs) for calendar year (CY) 2020, as required under section 1895(b) of the Social Security Act (the Act). This rule also updates the case-mix weights under section 1895(b)(4)(A)(i) and (b)(4)(B) of the Act for 30-day periods of care beginning on or after January 1, 2020. This final rule with comment period implements the PDGM, a revised case-mix adjustment methodology that was finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56406), which also implements the removal of therapy thresholds for payment as required by section 1895(b)(4)(B)(ii) of the Act, as amended by section 51001(a)(3) of the BBA of 2018, and changes the unit of home health payment from 60-day episodes of care to 30-day periods of care, as required by section 1895(b)(2)(B) of the Act, as amended by 51001(a)(1) of the BBA of 2018. This final rule with comment period allows therapist assistants to furnish maintenance therapy; finalizes changes to the payment regulations pertaining to the content of the home health plan of care; updates technical regulations text changes which clarifies the split-percentage payment approach for newly-enrolled HHAs in CY 2020 and changes the split percentage payment approach for existing HHAs in CY 2020 and subsequent years.
2. HHVBP
This final rule with comment period finalizes public reporting of the Total Performance Score (TPS) and the TPS Percentile Ranking from the Performance Year 5 (CY 2020) Annual TPS and Payment Adjustment Report for each HHA that qualifies for a payment adjustment under the HHVBP Model for CY 2020.
3. HH QRP
This final rule with comment period finalizes changes to the Home Health Quality Reporting Program (HH QRP) requirements under the authority of section 1895(b)(3)(B)(v) of the Act.
4. Home Infusion Therapy
This final rule with comment period finalizes payment provisions for home infusion therapy services for CY 2021 and subsequent years in accordance with section 1834(u) of the Act, as added by section 5012 of the 21st Century Cures Act (Pub. L. 114-255).
B. Summary of the Major Provisions
1. Home Health Prospective Payment System (HH PPS)
Section III.A. of this final rule with comment period sets forth the implementation of the Patient-Driven Groupings Model (PDGM) as required by section 51001 of the BBA of 2018 (Pub. L. 115-123). The PDGM is an alternate case-mix adjustment methodology to adjust payments for home health periods of care beginning on and after January 1, 2020. The PDGM relies more heavily on clinical characteristics and other patient information to place patients into meaningful payment categories and eliminates the use of therapy service thresholds, as required by section 1895(b)(4)(B) of the Act, as amended by section 51001(a)(3) of the BBA of 2018. Section III.B. of this final rule with comment period implements a change in the unit of payment from a 60-day episode of care to a 30-day period of care as required by section 1895(b)(2) of the Act, as amended by section 51001(a)(1) of the BBA of 2018. Section 1895(b)(3) of the Act requires that we calculate this 30-day payment amount for CY 2020 in a budget-neutral manner such that estimated aggregate expenditures under the HH PPS during CY 2020 are equal to the estimated aggregate expenditures that otherwise would have been made under the HH PPS during CY 2020 in the absence of the change to a 30-day unit of payment. The CY 2020 30-day payment amount (for those HHAs that report the required quality data) will be $1,864.03, which reflects an adjustment of −4.36 percent to maintain overall budget neutrality under the PDGM.
Section III.C. of this final rule with comment period describes the CY 2020 case-mix weights for those 60-day episodes that span the implementation date of the PDGM and section III.D. of this rule finalizes the CY 2020 PDGM case-mix weights and LUPA thresholds for 30-day periods of care. In section III.E. of this final rule, we finalize update the home health wage index and to update the national, standardized 60-day episode of care and 30-day period of care payment amounts, the national per-visit payment amounts, and the non-routine supplies (NRS) conversion factor for 60-day episodes of care that begin in 2019 and span the 2020 implementation date of the PDGM. The home health payment update percentage for CY 2020 is 1.5 percent, as required by section 53110 of the BBA of 2018. Section III.F. of this final rule with comment period, finalizes changes change to the fixed-dollar loss ratio to 0.56 for CY 2020 under the PDGM in order to ensure that outlier payments as a percentage of total payments is closer to, but no more than, 2.5 percent, as required by section 1895(b)(5)(A) of the Act. Section III.G. of this final rule with comment period, finalized technical regulations correction at § 484.205 regarding split-percentage payments for newly-enrolled HHAs in CY 2020; and finalizes the following additional changes to the split-percentage payment approach: (1) A reduction in the up-front amount paid in response to a Request for Anticipated Payment (RAP) to 20 percent of the estimated final payment amount for both initial and subsequent 30-day periods of care for CY 2020; (2) a reduction to the up-front amount paid in response to a RAP to zero percent of the estimated final payment amount for both initial and subsequent 30-day periods of care with a late submission penalty for failure to submit the RAP within 5 calendar days of the start of care for the first 30-day period within a 60-day certification period and within 5 calendar days of day 31 for the second, subsequent 30-day period in a 60-day certification period for CY 2021; (3) the elimination of the split-percentage payment approach entirely in CY 2022, replacing the RAP with a one-time submission of a Notice of Admission (NOA) with a late submission penalty for failure to submit the NOA within 5 calendar days of the start of care. In section III.H. of this final rule with comment period, we are finalizing our proposal to allow therapist assistants to furnish maintenance therapy under the Medicare home health benefit, and section III.I. of this final rule with comment period, we finalize a change in the payment regulation text at § 409.43 related to home health plan of care requirements for payment.
2. HHVBP
In section IV. of this final rule with comment period, we are finalizing provisions requiring public reporting performance data for Performance Year (PY) 5 of the HHVBP Model. Specifically, we are finalizing the public reporting of the TPS and the TPS Percentile Ranking from the PY 5 (CY 2020) Annual TPS and Payment Adjustment Report for each HHA in the nine Model states that qualified for a payment adjustment for CY 2020.
3. HH QRP
In section V. of this final rule with comment period, we are finalizing updates to the Home Health Quality Reporting Program (HH QRP) including: The removal of one quality measure, the adoption of two new quality measures, the modification of an existing measure, and a requirement for HHAs to report standardized patient assessment data. In section V.J. of this final rule, we are finalizing our proposal to re-designate our current HH QRP regulations in a different section of our regulations and to codify other current policies in that new regulatory section with one substantive change as well as a few technical edits. Finally, in section V.K. of the rule, we are not finalizing the removal of question 10 from all HHCAHPS Surveys (both mail surveys and telephone surveys).
4. Home Infusion Therapy
In section VI.A. of this final rule with comment period, we discuss the general background of home infusion therapy services and how that relates to the implementation of the new home infusion benefit in CY 2021. Section VI.B. of this final rule with comment period discusses the updates to the CY 2020 home infusion therapy services temporary transitional payment rates, in accordance with section 1834(u)(7) of the Act. In section VI.C. of this final rule with comment period, we are finalizing our proposal to add a new subpart P under the regulations at 42 CFR part 414 to incorporate conforming regulations text regarding conditions for payment for home infusion therapy services for CY 2021 and subsequent years. Subpart P includes beneficiary qualifications and plan of care requirements in accordance with section 1861(iii) of the Act. In section VI.D. of this final rule with comment period, we finalize payment provisions for the full implementation of the home infusion therapy benefit in CY 2021 upon expiration of the home infusion therapy services temporary transitional payments in CY 2020. The home infusion therapy services payment system is to be implemented starting in CY 2021, as mandated by section 5012 of the 21st Century Cures Act. The provisions in this section include payment categories, amounts, and required and optional payment adjustments. In section VI.E. of this final rule with comment period, we finalize the use of the Geographic Adjustment Factor (GAF) to wage adjust the home infusion therapy payment as required by section 1834(u)(1)(B)(i) of the Act. In section VI.F. of this final rule with comment period, we summarize comments received on the proposed rule regarding several topics for home infusion therapy services for CY 2021 such as: Optional payment adjustments, prior authorization, and high-cost outliers. In section VI.G. of this final rule with comment period, we discuss billing procedures for CY 2021 home infusion therapy services. Lastly, given the new permanent home infusion therapy benefit to be implemented beginning January 1, 2021, which includes payment for professional services, including nursing, for parenteral drugs administered intravenously or subcutaneously for a period of 15 minutes or more through a pump that is a covered item of DME; we are soliciting comments on options to enhance future efforts to improve policies related to coverage of eligible drugs for home infusion therapy. In response to stakeholder concerns regarding the limitations of the DME LCDs for External Infusion Pumps that preclude coverage to certain infused drugs, we seek comments on the criteria CMS could consider, within the scope of the DME benefit, to allow coverage of additional home infusion drugs.
C. Summary of Costs, Transfers, and Benefits

II. Overview of the Home Health Prospective Payment System
A. Statutory Background
The Balanced Budget Act of 1997 (BBA) (Pub. L. 105-33, enacted August 5, 1997), significantly changed the way Medicare pays for Medicare home health services. Section 4603 of the BBA mandated the development of the HH PPS. Until the implementation of the HH PPS on October 1, 2000, HHAs received payment under a retrospective reimbursement system. Section 4603(a) of the BBA mandated the development of a HH PPS for all Medicare-covered home health services provided under a plan of care (POC) that were paid on a reasonable cost basis by adding section 1895 of the Act, entitled “Prospective Payment For Home Health Services.” Section 1895(b)(1) of the Act requires the Secretary to establish a HH PPS for all costs of home health services paid under Medicare. Section 1895(b)(2) of the Act required that, in defining a prospective payment amount, the Secretary will consider an appropriate unit of service and the number, type, and duration of visits provided within that unit, potential changes in the mix of services provided within that unit and their cost, and a general system design that provides for continued access to quality services.
Section 1895(b)(3)(A) of the Act required the following: (1) The computation of a standard prospective payment amount that includes all costs for HH services covered and paid for on a reasonable cost basis, and that such amounts be initially based on the most recent audited cost report data available to the Secretary (as of the effective date of the 2000 final rule), and (2) the standardized prospective payment amount be adjusted to account for the effects of case-mix and wage levels among HHAs.
Section 1895(b)(3)(B) of the Act requires the standard prospective payment amounts be annually updated by the home health applicable percentage increase. Section 1895(b)(4) of the Act governs the payment computation. Sections 1895(b)(4)(A)(i) and (b)(4)(A)(ii) of the Act require the standard prospective payment amount to be adjusted for case-mix and geographic differences in wage levels. Section 1895(b)(4)(B) of the Act requires the establishment of an appropriate case-mix change adjustment factor for significant variation in costs among different units of services.
Similarly, section 1895(b)(4)(C) of the Act requires the establishment of area wage adjustment factors that reflect the relative level of wages, and wage-related costs applicable to home health services furnished in a geographic area compared to the applicable national average level. Under section 1895(b)(4)(C) of the Act, the wage-adjustment factors used by the Secretary may be the factors used under section 1886(d)(3)(E) of the Act. Section 1895(b)(5) of the Act gives the Secretary the option to make additions or adjustments to the payment amount otherwise paid in the case of outliers due to unusual variations in the type or amount of medically necessary care. Section 3131(b)(2) of the Affordable Care Act revised section 1895(b)(5) of the Act so that total outlier payments in a given year would not exceed 2.5 percent of total payments projected or estimated. The provision also made permanent a 10 percent agency-level outlier payment cap.
In accordance with the statute, as amended by the BBA, we published a final rule in the July 3, 2000 Federal Register (65 FR 41128) to implement the HH PPS legislation. The July 2000 final rule established requirements for the new HH PPS for home health services as required by section 4603 of the BBA, as subsequently amended by section 5101 of the Omnibus Consolidated and Emergency Supplemental Appropriations Act for Fiscal Year 1999 (OCESAA), (Pub. L. 105-277, enacted October 21, 1998); and by sections 302, 305, and 306 of the Medicare, Medicaid, and SCHIP Balanced Budget Refinement Act of 1999, (BBRA) (Pub. L. 106-113, enacted November 29, 1999). The requirements include the implementation of a HH PPS for home health services, consolidated billing requirements, and a number of other related changes. The HH PPS described in that rule replaced the retrospective reasonable cost-based system that was used by Medicare for the payment of home health services under Part A and Part B. For a complete and full description of the HH PPS as required by the BBA, see the July 2000 HH PPS final rule (65 FR 41128 through 41214).
Section 5201(c) of the Deficit Reduction Act of 2005 (DRA) (Pub. L. 109-171, enacted February 8, 2006) added new section 1895(b)(3)(B)(v) to the Act, requiring HHAs to submit data for purposes of measuring health care quality, and linking the quality data submission to the annual applicable payment percentage increase. This data submission requirement is applicable for CY 2007 and each subsequent year. If an HHA does not submit quality data, the home health market basket percentage increase is reduced by 2 percentage points. In the November 9, 2006 Federal Register (71 FR 65935), we published a final rule to implement the pay-for-reporting requirement of the DRA, which was codified at § 484.225(h) and (i) in accordance with the statute. The pay-for-reporting requirement was implemented on January 1, 2007.
The Affordable Care Act made additional changes to the HH PPS. One of the changes in section 3131 of the Affordable Care Act is the amendment to section 421(a) of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) (Pub. L. 108-173, enacted on December 8, 2003) as amended by section 5201(b) of the DRA. Section 421(a) of the MMA, as amended by section 3131 of the Affordable Care Act, requires that the Secretary increase, by 3 percent, the payment amount otherwise made under section 1895 of the Act, for HH services furnished in a rural area (as defined in section 1886(d)(2)(D) of the Act) with respect to episodes and visits ending on or after April 1, 2010, and before January 1, 2016.
Section 210 of the Medicare Access and CHIP Reauthorization Act of 2015 (Pub. L. 114-10) (MACRA) amended section 421(a) of the MMA to extend the 3 percent rural add-on payment for home health services provided in a rural area (as defined in section 1886(d)(2)(D) of the Act) through January 1, 2018. In addition, section 411(d) of MACRA amended section 1895(b)(3)(B) of the Act such that CY 2018 home health payments be updated by a 1 percent market basket increase. Section 50208(a)(1) of the BBA of 2018 again extended the 3 percent rural add-on through the end of 2018. In addition, this section of the BBA of 2018 made some important changes to the rural add-on for CYs 2019 through 2022 and these changes are discussed later in this final rule with comment period.
B. Current System for Payment of Home Health Services
Generally, Medicare currently makes payment under the HH PPS on the basis of a national, standardized 60-day episode payment rate that is adjusted for the applicable case-mix and wage index. The national, standardized 60-day episode rate includes the six home health disciplines (skilled nursing, home health aide, physical therapy, speech-language pathology, occupational therapy, and medical social services). Payment for non-routine supplies (NRS) is not part of the national, standardized 60-day episode rate, but is computed by multiplying the relative weight for a particular NRS severity level by the NRS conversion factor. Payment for durable medical equipment covered under the HH benefit is made outside the HH PPS payment system. To adjust for case-mix, the HH PPS uses a 153-category case-mix classification system to assign patients to a home health resource group (HHRG). The clinical severity level, functional severity level, and service utilization are computed from responses to selected data elements in the Outcome and Assessment Information Set (OASIS) assessment instrument and are used to place the patient in a particular HHRG. Each HHRG has an associated case-mix weight which is used in calculating the payment for an episode. Therapy service use is measured by the number of therapy visits provided during the episode and can be categorized into nine visit level categories (or thresholds): 0 to 5; 6; 7 to 9; 10; 11 to 13; 14 to 15; 16 to 17; 18 to 19; and 20 or more visits.
For episodes with four or fewer visits, Medicare pays national per-visit rates based on the discipline(s) providing the services. An episode consisting of four or fewer visits within a 60-day period receives what is referred to as a low-utilization payment adjustment (LUPA). Medicare also adjusts the national standardized 60-day episode payment rate for certain intervening events that are subject to a partial episode payment adjustment (PEP adjustment). For certain cases that exceed a specific cost threshold, an outlier adjustment may also be available.
C. New Home Health Prospective Payment System for CY 2020 and Subsequent Years
In the CY 2019 HH PPS final rule with comment period (83 FR 56446), we finalized a new patient case-mix adjustment methodology, the Patient-Driven Groupings Model (PDGM), to shift the focus from volume of services to a more patient-driven model that relies on patient characteristics. For home health periods of care beginning on or after January 1, 2020, the PDGM uses timing, admission source, principal and other diagnoses, and functional impairment to case-mix adjust payments. The PDGM results in 432 unique case-mix groups. Low-utilization payment adjustments (LUPAs) will vary; instead of the current four visit threshold, each of the 432 case-mix groups has its own threshold to determine if a 30-day period of care would receive a LUPA. Additionally, non-routine supplies (NRS) are included in the base payment rate for the PDGM instead of being separately adjusted as in the current HH PPS. Also in the CY 2019 HH PPS final rule with comment period, we finalized a change in the unit of home health payment from 60-day episodes of care to 30-day periods of care, and eliminated the use of therapy thresholds used to adjust payments in accordance with section 51001 of the BBA of 2018. Thirty-day periods of care will be adjusted for outliers and partial episodes as applicable. Finally, for CYs 2020 through 2022, home health services provided to beneficiaries residing in rural counties will be increased based on rural county classification (high utilization; low population density; or all others) in accordance with section 50208 of the BBA of 2018.
D. Analysis of FY 2017 HHA Cost Report Data for 60-Day Episodes and 30-Day Periods
In the CY 2019 HH PPS proposed rule (83 FR 32348), we provided a summary of analysis on fiscal year (FY) 2016 HHA cost report data and how such data, if used, would impact our estimate of the percentage difference between Medicare payments and HHA costs. We stated in the CY 2019 HH PPS final rule with comment period (83 FR 56414) that we will continue to monitor the impacts due to policy changes and will provide the industry with periodic updates on our analysis in rulemaking and/or announcements on the HHA Center web page.
In this year's proposed rule (84 FR 34602), we examined FY 2017 HHA cost reports as this is the most recent and complete cost report data at the time of rulemaking. We include this analysis again in this final rule with comment period. We examined the estimated 60-day episode costs using FY 2017 cost reports and CY 2017 home health claims and the estimated costs for 60-day episodes by discipline and the total estimated cost for a 60-day episode for 2017 is shown in Table 2.

To estimate the costs for CY 2020, we updated the estimated 60-day episode costs with NRS by the home health market basket update, minus the multifactor productivity adjustment for CYs 2018 and 2019. In the proposed rule, we estimated the CY 2020 costs by using the home health market basket update of 1.5 percent as required by the BBA of 2018. However, for this final rule with comment period, we believe that we should be consistent with the estimation of cost calculations for purposes of analyzing the payment adequacy. This would warrant the same approach for estimating CY 2020 costs as was used for CYs 2018 and 2019. Therefore, for this final rule with comment period, we calculated the estimated CY 2020 60-day episode costs and 30-day period costs by applying each year's market basket update minus the multifactor productivity factor for that year. For CY 2020, based on IHS Global Inc. 2019 q3 forecast, the home health market basket update is forecasted to be 2.9 percent; the MFP adjustment is forecasted to be 0.3 percent resulting in a forecasted MFP-adjusted home health market basket update of 2.6 percent. The estimated costs for 60-day episodes by discipline and the total estimated cost for a 60-day episode for CY 2020 is shown in Table 3.
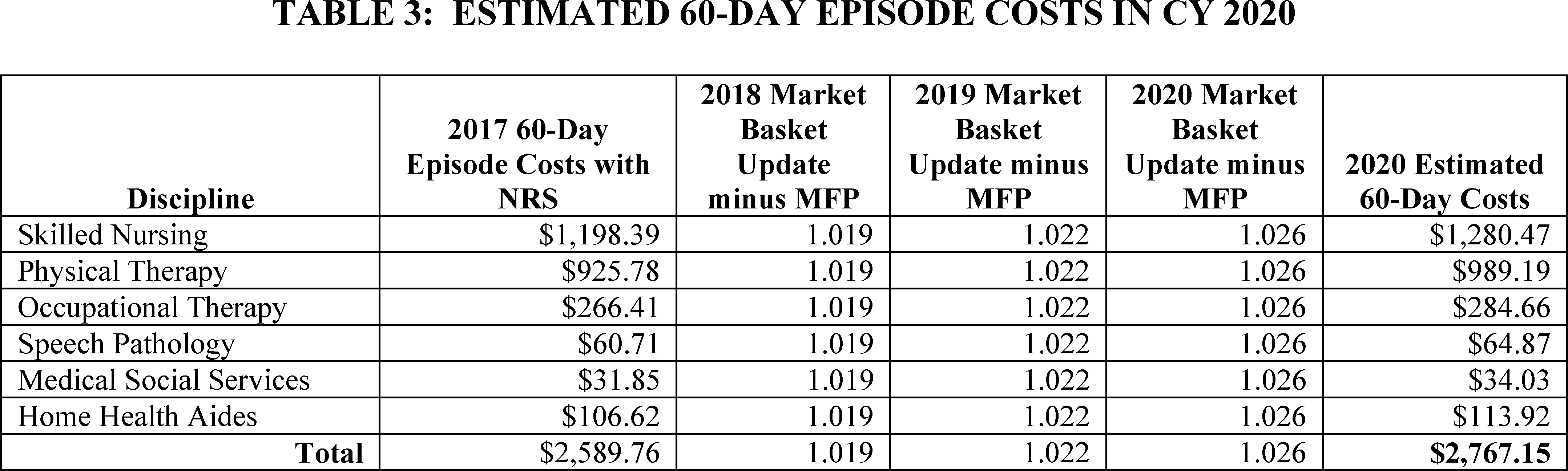
The CY 2020 60-day episode payment will be $3,220.79, approximately 16 percent more than the estimated CY 2020 60-day episode cost of $2,767.15.
Next, we also looked at the estimated costs for 30-day periods of care in 2017 using FY 2017 cost reports and CY 2017 claims. Thirty-day periods were simulated from 60-day episodes and we excluded low-utilization payment adjusted episodes and partial-episode-payment adjusted episodes. The 30-day periods were linked to OASIS assessments and covered the 60-day episodes ending in CY 2017. The estimated costs for 30-day periods by discipline and the total estimated cost for a 30-day period for 2017 is shown in Table 4.

Using the same approach as calculating the estimated CY 2020 60-day episode costs, we updated the estimated 30-day period costs with NRS by the home health market basket update, minus the multifactor productivity adjustment for CYs 2018 2019, and 2020. The estimated costs for 30-day periods by discipline and the total estimated cost for a 30-day period for CY 2020 is shown in Table 5.

The estimated, budget-neutral 30-day payment for CY 2020 is, $1,824.99 as described in section III.E. of this final rule with comment period. Updating this amount by the CY 2020 home health market basket update of 1.5 percent and the wage index budget neutrality factor results in an estimated CY 2020 30-day payment amount of $1,864.03 (as described in section III.B. of this final rule with comment period) approximately 16 percent more than the estimated CY 2020 30-day period cost of $1,608.82. After implementation of the 30-day unit of payment and the PDGM in CY 2020, we will continue to analyze the costs by discipline as well as the overall cost for a 30-day period of care to determine the effects, if any, of these changes.
III. Payment Under the Home Health Prospective Payment System (HH PPS)
A. Implementation of the Patient-Driven Groupings Model (PDGM) for CY 2020
1. Background and Legislative History
In the CY 2019 HH PPS final rule with comment period (83 FR 56406), we finalized provisions to implement changes mandated by the BBA of 2018 for CY 2020, which included a change in the unit of payment from a 60-day episode of care to a 30-day period of care, as required by section 51001(a)(1)(B), and the elimination of therapy thresholds used for adjusting home health payment, as required by section 51001(a)(3)(B). In order to eliminate the use of therapy thresholds in adjusting payment under the HH PPS, we finalized an alternative case mix-adjustment methodology, known as the Patient-Driven Groupings Model (PDGM), to be implemented for home health periods of care beginning on or after January 1, 2020.
In regard to the 30-day unit of payment, section 51001(a)(1) of the BBA of 2018 amended section 1895(b)(2) of the Act by adding a new subparagraph (B) to require the Secretary to apply a 30-day unit of service, effective January 1, 2020. Section 51001(a)(2)(A) of the BBA of 2018 added a new subclause (iv) under section 1895(b)(3)(A) of the Act, requiring the Secretary to calculate a standard prospective payment amount (or amounts) for 30-day units of service, furnished that end during the 12-month period beginning January 1, 2020, in a budget neutral manner, such that estimated aggregate expenditures under the HH PPS during CY 2020 are equal to the estimated aggregate expenditures that otherwise would have been made under the HH PPS during CY 2020 in the absence of the change to a 30-day unit of service. Section 1895(b)(3)(A)(iv) of the Act requires that the calculation of the standard prospective payment amount (or amounts) for CY 2020 be made before the application of the annual update to the standard prospective payment amount as required by section 1895(b)(3)(B) of the Act.
Section 1895(b)(3)(A)(iv) of the Act additionally requires that in calculating the standard prospective payment amount (or amounts), the Secretary must make assumptions about behavior changes that could occur as a result of the implementation of the 30-day unit of service under section 1895(b)(2)(B) of the Act and case-mix adjustment factors established under section 1895(b)(4)(B) of the Act. Section 1895(b)(3)(A)(iv) of the Act further requires the Secretary to provide a description of the behavior assumptions made in notice and comment rulemaking. CMS finalized these behavior assumptions in the CY 2019 HH PPS final rule with comment period (83 FR 56461) and these assumptions are further described in section III.B. of this final rule with comment period.
Section 51001(a)(2)(B) of the BBA of 2018 also added a new subparagraph (D) to section 1895(b)(3) of the Act. Section 1895(b)(3)(D)(i) of the Act requires the Secretary to annually determine the impact of differences between assumed behavior changes as described in section 1895(b)(3)(A)(iv) of the Act, and actual behavior changes on estimated aggregate expenditures under the HH PPS with respect to years beginning with 2020 and ending with 2026. Section 1895(b)(3)(D)(ii) of the Act requires the Secretary, at a time and in a manner determined appropriate, through notice and comment rulemaking, to provide for one or more permanent increases or decreases to the standard prospective payment amount (or amounts) for applicable years, on a prospective basis, to offset for such increases or decreases in estimated aggregate expenditures, as determined under section 1895(b)(3)(D)(i) of the Act. Additionally, 1895(b)(3)(D)(iii) of the Act requires the Secretary, at a time and in a manner determined appropriate, through notice and comment rulemaking, to provide for one or more temporary increases or decreases, based on retrospective behavior, to the payment amount for a unit of home health services for applicable years, on a prospective basis, to offset for such increases or decreases in estimated aggregate expenditures, as determined under section 1895(b)(3)(D)(i) of the Act. Such a temporary increase or decrease shall apply only with respect to the year for which such temporary increase or decrease is made, and the Secretary shall not take into account such a temporary increase or decrease in computing the payment amount for a unit of home health services for a subsequent year. And finally, section 51001(a)(3) of the BBA of 2018 amends section 1895(b)(4)(B) of the Act by adding a new clause (ii) to require the Secretary to eliminate the use of therapy thresholds in the case-mix system for CY 2020 and subsequent years.
2. Overview and CY 2020 Implementation of the PDGM
To better align payment with patient care needs and better ensure that clinically complex and ill beneficiaries have adequate access to home health care, in the CY 2019 HH PPS final rule with comment period (83 FR 56406), we finalized case-mix methodology refinements through the PDGM for home health periods of care beginning on or after January 1, 2020. We believe that the PDGM case-mix methodology better aligns payment with patient care needs and is a patient-centered model that groups periods of care in a manner consistent with how clinicians differentiate between patients and the primary reason for needing home health care. This final rule with comment period effectuates the requirements for the implementation of the PDGM, as well as finalizes updates to the PDGM case-mix weights and payment rates, which would be effective on January 1, 2020. The PDGM and a change to a 30-day unit of payment were finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56406) and, as such, there were no new policy proposals in the CY 2020 home health proposed rule on the structure of the PDGM or the change to a 30-day unit of payment. However, there were proposals related to the split-percentage payments upon implementation of the PDGM and the 30-day unit of payment as described in section III.G. of this final rule with comment period.
The PDGM uses 30-day periods of care rather than 60-day episodes of care as the unit of payment, as required by section 51001(a)(1)(B) of the BBA of 2018; eliminates the use of the number of therapy visits provided to determine payment, as required by section 51001(a)(3)(B) of the BBA of 2018; and relies more heavily on clinical characteristics and other patient information (for example, diagnosis, functional level, comorbid conditions, admission source) to place patients into clinically meaningful payment categories. A national, standardized 30-day period payment amount, as described in section III.E. of this final rule with comment period, will be adjusted by the case-mix weights as determined by the variables in the PDGM. Payment for non-routine supplies (NRS) is now included in the national, standardized 30-day payment amount. In total, there are 432 different payment groups in the PDGM. These 432 Home Health Resource Groups (HHRGs) represent the different payment groups based on five main case-mix variables under the PDGM, as shown in Figure B1, and subsequently described in more detail throughout this section.
Under this new case-mix methodology, case-mix weights are generated for each of the different PDGM payment groups by regressing resource use for each of the five categories listed in this section of this final rule with comment period (timing, admission source, clinical grouping, functional impairment level, and comorbidity adjustment) using a fixed effects model. Annually recalibrating the PDGM case-mix weights ensures that the case-mix weights reflect the most recent utilization data at the time of annual rulemaking. The final CY 2020 PDGM case-mix weights are listed in section III.D. of this final rule with comment period.

a. Timing
Under the PDGM, 30-day periods of care will be classified as “early” or “late” depending on when they occur within a sequence of 30-day periods. Under the PDGM, the first 30-day period of care will be classified as early and all subsequent 30-day periods of care in the sequence (second or later) will be classified as late. A 30-day period will not be considered early unless there is a gap of more than 60 days between the end of one period of care and the start of another. Information regarding the timing of a 30-day period of care will come from Medicare home health claims data and not the OASIS assessment to determine if a 30-day period of care is “early” or “late”. While the PDGM case-mix adjustment is applied to each 30-day period of care, other home health requirements will continue on a 60-day basis. Specifically, certifications and re-certifications continue on a 60-day basis and the comprehensive assessment will still be completed within 5 days of the start of care date and completed no less frequently than during the last 5 days of every 60 days beginning with the start of care date, as currently required by § 484.55, “Condition of participation: Comprehensive assessment of patients.”
b. Admission Source
Each 30-day period of care will also be classified into one of two admission source categories—community or institutional—depending on what healthcare setting was utilized in the 14 days prior to home health. Thirty-day periods of care for beneficiaries with any inpatient acute care hospitalizations, inpatient psychiatric facility (IPF) stays, skilled nursing facility (SNF) stays, inpatient rehabilitation facility (IRF) stays, or long-term care hospital (LTCH) stays within 14-days prior to a home health admission will be designated as institutional admissions.
The institutional admission source category will also include patients that had an acute care hospital stay during a previous 30-day period of care and within 14 days prior to the subsequent, contiguous 30-day period of care and for which the patient was not discharged from home health and readmitted (that is, the “admission date” and “from date” for the subsequent 30-day period of care do not match), as we acknowledge that HHAs have discretion as to whether they discharge the patient due to a hospitalization and then readmit the patient after hospital discharge. However, we will not categorize post-acute care stays, meaning SNF, IRF, LTCH, or IPF stays, that occur during a previous 30-day period of care and within 14 days of a subsequent, contiguous 30-day period of care as institutional (that is, the “admission date” and “from date” for the subsequent 30-day period of care do not match), as we would expect the HHA to discharge the patient if the patient required post-acute care in a different setting, or inpatient psychiatric care, and then readmit the patient, if necessary, after discharge from such setting. All other 30-day periods of care would be designated as community admissions.
Information from the Medicare claims processing system will determine the appropriate admission source for final claim payment. The OASIS assessment will not be utilized in evaluating for admission source information. We believe that obtaining this information from the Medicare claims processing system, rather than as reported on the OASIS, is a more accurate way to determine admission source information as HHAs may be unaware of an acute or post-acute care stay prior to home health admission. While HHAs can report an occurrence code on submitted claims to indicate the admission source, obtaining this information from the Medicare claims processing system allows CMS the opportunity and flexibility to verify the source of the admission and correct any improper payments as deemed appropriate. When the Medicare claims processing system receives a Medicare home health claim, the systems will check for the presence of a Medicare acute or post-acute care claim for an institutional stay. If such an institutional claim is found, and the institutional claim occurred within 14 days of the home health admission, our systems will trigger an automatic adjustment to the corresponding HH claim to the appropriate institutional category. Similarly, when the Medicare claims processing system receives a Medicare acute or post-acute care claim for an institutional stay, the systems will check for the presence of a HH claim with a community admission source payment group. If such HH claim is found, and the institutional stay occurred within 14 days prior to the home health admission, our systems will trigger an automatic adjustment of the HH claim to the appropriate institutional category. This process may occur any time within the 12-month timely filing period for the acute or post-acute claim.
However, situations in which the HHA has information about the acute or post-acute care stay, HHAs will be allowed to manually indicate on Medicare home health claims that an institutional admission source had occurred prior to the processing of an acute/post-acute Medicare claim, in order to receive higher payment associated with the institutional admission source. This will be done through the reporting of one of two admission source occurrence codes on home health claims—
- Occurrence Code 61: to indicate an acute care hospital discharge within 14 days prior to the “From Date” of any home health claim; or
- Occurrence Code 62: to indicate a SNF, IRF, LTCH, or IPF discharge with 14 days prior to the “Admission Date” of the first home health claim.
If the HHA does not include an occurrence code on the HH claim to indicate that that the home health patient had a previous acute or post-acute care stay, the period of care will be categorized as a community admission source. However, if later a Medicare acute or post-acute care claim for an institutional stay occurring within 14 days of the home health admission is submitted within the timely filing deadline and processed by the Medicare systems, the HH claim will be automatically adjusted as an institutional admission and the appropriate payment modifications will be made. For purposes of a Request for Anticipated Payment (RAP), only the final claim will be adjusted to reflect the admission source. More information regarding the admission source reporting requirements for RAP and claims submission can be found in Change Request 11081, “Home Health (HH) Patient-Drive Groupings Model (PDGM)-Split Implementation”.[1] Accordingly, the Medicare Claims Processing Manual, chapter 10,[2] has been updated to reflect all of the claims processing changes associated with implementation of the PDGM.
c. Clinical Groupings
Each 30-day period of care will be grouped into one of 12 clinical groups which describe the primary reason for which patients are receiving home health services under the Medicare home health benefit. The clinical grouping is based on the principal diagnosis reported on home health claims. The 12 clinical groups are listed and described in Table 6.

It is possible for the principal diagnosis to change between the first and second 30-day period of care and the claim for the second 30-day period of care would reflect the new principal diagnosis. HHAs would not change the claim for the first 30-day period. However, a change in the principal diagnosis does not necessarily mean that an “other follow-up” OASIS assessment (RFA 05) would need to be completed just to make the diagnoses match. However, if a patient experienced a significant change in condition before the start of a subsequent, contiguous 30-day period of care, for example due to a fall, in accordance with § 484.55(d)(1)(ii) the HHA is required to update the comprehensive assessment. The Home Health Agency Interpretive Guidelines [3] for § 484.55(d), state that a marked improvement or worsening of a patient's condition, which changes, and was not anticipated in, the patient's plan of care would be considered a “major decline or improvement in the patient's health status” that would warrant update and revision of the comprehensive assessment.[4] Additionally, in accordance with § 484.60, the total plan of care must be reviewed and revised by the physician who is responsible for the home health plan of care and the HHA as frequently as the patient's condition or needs require, but no less frequently than once every 60 days, beginning with the start of care date.
In the event of a significant change of condition warranting an updated comprehensive assessment, an “other follow-up assessment” (RFA 05) would be submitted before the start of a subsequent, contiguous 30-day period, which may reflect a change in the functional impairment level and the second 30-day claim would be grouped into its appropriate case-mix group accordingly. An “other follow-up assessment” is a comprehensive assessment conducted due to a major decline or improvement in patient's health status occurring at a time other than during the last 5 days of the episode. This assessment is done to re-evaluate the patient's condition, allowing revision to the patient's care plan as appropriate. The “Outcome and Assessment Information Set OASIS-D Guidance Manual,” effective January 1, 2019, provides more detailed guidance for the completion of an “other follow-up” assessment.[5] In this respect, two 30-day periods can have two different case-mix groups to reflect any changes in patient condition. HHAs must be sure to update the assessment completion date on the second 30-day claim if a follow-up assessment changes the case-mix group to ensure the claim can be matched to the follow-up assessment. HHAs can submit an adjustment to the original claim submitted if an assessment was completed before the start of the second 30-day period, but was received after the claim was submitted and if the assessment items would change the payment grouping.
HHAs would determine whether or not to complete a follow-up OASIS assessment for a second 30-day period of care depending on the individual's clinical circumstances. For example, if the only change from the first 30-day period and the second 30-day period is a change to the principal diagnosis and there is no change in the patient's function, the HHA may determine it is not necessary to complete a follow-up assessment. Therefore, the expectation is that HHAs would determine whether an “other follow-up” assessment is required based on the individual's overall condition, the effects of the change on the overall home health plan of care, and in accordance with the home health CoPs,[6] interpretive guidelines, and the OASIS D Guidance Manual instructions, as previously noted.
For case-mix adjustment purposes, the principal diagnosis reported on the home health claim will determine the clinical group for each 30-day period of care. Currently, billing instructions state that the principal diagnosis on the OASIS must also be the principal diagnosis on the final claim; however, we will update our billing instructions to clarify that there will be no need for the HHA to complete an “other follow-up” assessment (an RFA 05) just to make the diagnoses match. Therefore, for claim “From” dates on or after January 1, 2020, the ICD-10-CM code and principal diagnosis used for payment grouping will be from the claim rather than the OASIS. As a result, the claim and OASIS diagnosis codes will no longer be expected to match in all cases. Additional claims processing guidance, including the role of the OASIS item set is included in the Medicare Claims Processing Manual, chapter 10.
While these clinical groups represent the primary reason for home health services during a 30-day period of care, this does not mean that they represent the only reason for home health services. While there are clinical groups where the primary reason for home health services is for therapy (for example, Musculoskeletal Rehabilitation) and other clinical groups where the primary reason for home health services is for nursing (for example, Complex Nursing Interventions), home health remains a multidisciplinary benefit and payment is bundled to cover all necessary home health services identified on the individualized home health plan of care. Therefore, regardless of the clinical group assignment, HHAs are required, in accordance with the home health CoPs at § 484.60(a)(2), to ensure that the individualized home health plan of care addresses all care needs, including the disciplines to provide such care. Under the PDGM, the clinical group is just one variable in the overall case-mix adjustment for a home health period of care.
Finally, to accompany this final rule with comment period, we updated the Interactive Grouper Tool posted on both the HHA Center web page (https://www.cms.gov/center/provider-type/home-health-agency-hha-center.html) and the PDGM web page (https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/HH-PDGM.html). This Interactive Grouper Tool includes all of the ICD-10-CM diagnosis codes used in the PDGM and may be used by HHAs to generate PDGM case-mix weights for their patient census. This tool is for informational and illustrative purposes only. This Interactive Grouper Tool has been provided to assist HHAs in understanding the effects of the transition to the PDGM and will not be updated on an annual basis after CY 2020 as HHAs will have the opportunity download the HH PPS Grouper annually. The final grouper for CY 2020 will be posted with this final rule with comment period and can be found on the following website: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/CaseMixGrouperSoftware.html. Additionally, HHAs can also request a Home Health Claims-OASIS Limited Data Set (LDS) to accompany the CY 2020 HH PPS final rule with comment period to support HHAs in evaluating the effects of the PDGM. The Home Health Claims-OASIS LDS file can be requested by following the instructions on the CMS Limited Data Set (LDS) Files website: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/DUA_-_NewLDS.html.
d. Functional Impairment Level
Under the PDGM, each 30-day period of care will be placed into one of three functional impairment levels, low, medium, or high, based on responses to certain OASIS functional items as listed in Table 7.

Responses to these OASIS items are grouped together into response categories with similar resource use and each response category has associated points. A more detailed description as to how these response categories were established can be found in the technical report, “Overview of the Home Health Groupings Model” posted on the Home Health Center web page.[7] The sum of these points' results in a functional impairment level score used to group 30-day periods of care into a functional impairment level with similar resource use. The scores associated with the functional impairment levels vary by clinical group to account for differences in resource utilization. For CY 2020, we used CY 2018 claims data to update the functional points and functional impairment levels by clinical group. The updated OASIS functional points table and the table of functional impairment levels by clinical group for CY 2020 are listed in Tables 8 and 9 respectively. For ease of use, instead of listing the response categories and the associated points (as shown in Table 28 in the CY 2019 HH PPS final rule with comment period (83 FR 56478), we have reformatted the OASIS Functional Item Response Points (Table 8 to identify how the OASIS functional items used for the functional impairment level are assigned points under the PDGM. In this CY 2020 HH PPS final rule with comment period, we updated the points for the OASIS functional item response categories and the functional impairment levels by clinical group using the most recent, available claims data.


The functional impairment level will remain the same for the first and second 30-day periods of care unless there has been a significant change in condition which warranted an “other follow-up” assessment prior to the second 30-day period of care. For each 30-day period of care, the Medicare claims processing system will look for the most recent OASIS assessment based on the claims “from date.” The finalized CY 2020 functional points table and the functional impairment level thresholds table are posted on the HHA Center web page as well as on the PDGM web page.
e. Comorbidity Adjustment
Thirty-day periods will receive a comorbidity adjustment category based on the presence of certain secondary diagnoses reported on home health claims. These diagnoses are based on a home-health specific list of clinically and statistically significant secondary diagnosis subgroups with similar resource use, meaning the diagnoses have at least as high as the median resource use and are reported in more than 0.1 percent of 30-day periods of care. Home health 30-day periods of care can receive a comorbidity adjustment under the following circumstances:
Low comorbidity adjustment: There is a reported secondary diagnosis on the home health-specific comorbidity subgroup list that is associated with higher resource use.
High comorbidity adjustment: There are two or more secondary diagnoses on the home health-specific comorbidity subgroup interaction list that are associated with higher resource use when both are reported together compared to if they were reported separately. That is, the two diagnoses may interact with one another, resulting in higher resource use.
No comorbidity adjustment: A 30-day period of care will receive no comorbidity adjustment if no secondary diagnoses exist or none meet the criteria for a low or high comorbidity adjustment.
For CY 2020, there are 13 low comorbidity adjustment subgroups as identified in Table 10 and 31 high comorbidity adjustment interaction subgroups as identified in Table 11.

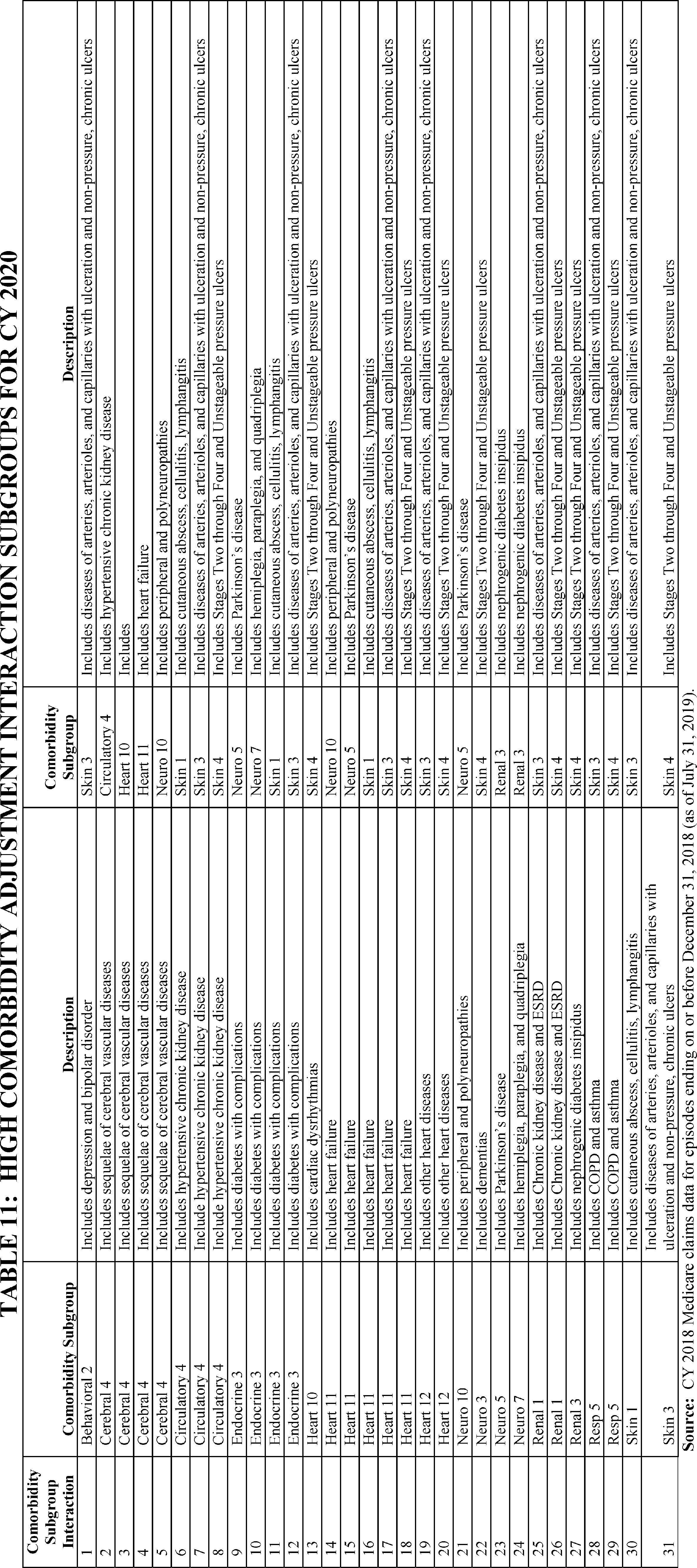
A 30-day period of care can have a low comorbidity adjustment or a high comorbidity adjustment, but not both. A 30-day period of care can receive only one low comorbidity adjustment regardless of the number of secondary diagnoses reported on the home health claim that fell into one of the individual comorbidity subgroups or one high comorbidity adjustment regardless of the number of comorbidity group interactions, as applicable. The low comorbidity adjustment amount will be the same across the subgroups and the high comorbidity adjustment will be the same across the subgroup interactions. The finalized CY 2020 low comorbidity adjustment subgroups and the high comorbidity adjustment interaction subgroups including those diagnoses within each of these comorbidity adjustments are posted on the HHA Center web page as well as on the PDGM web page.
While we did not solicit comments on the PDGM as it was finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56406), we did receive 179 comments on various components of the finalized PDGM from home health agencies, industry associations, as well as individuals. We received a few general comments on the PDGM as a whole. A few comments were received on the admission source case-mix variable, elimination of therapy thresholds, and the comorbidity adjustment; however, the majority of these comments were specific ICD 10-CM code requests to include certain previously excluded diagnosis codes as part of the clinical grouping variable or to move specific diagnosis codes from one clinical group to another. These comments and our responses are summarized in this section of this final rule with comment period.
1. General PDGM Comments
Comment: Several commenters stated they are very encouraged by CMS's efforts to develop a valid and reliable case mix adjustment model that relies on patient characteristics rather than resource use to determine the amount of payment in individual service claims. However, these commenters expressed concern that the PDGM could create financial incentives for home health agencies to under-supply needed care through inappropriate early discharge, improperly limiting the number of visits or types of services provided, or discouraging serving individuals with longer-term needs and people without a prior institutional stay. A commenter recommended that CMS monitor these issues and quality of care during initial implementation of the PDGM in ways that will allow CMS to quickly understand and address emerging problems affecting the provision of home health services. This commenter also suggested that CMS educate home health agencies as well as beneficiaries and their family caregivers about the need for beneficiaries to receive high-quality home health care that meets each Medicare beneficiary's unique needs. Other suggestions included requiring agencies to provide clear, accurate information about what Medicare covers and beneficiary appeal rights and updating CMS educational materials for beneficiaries to assist in this effort. Another commenter urged CMS to be transparent about its education budget and include information about the different mechanisms it will use for the education of providers, beneficiaries, and their family caregivers (as appropriate).
Response: We appreciate commenter support of a case-mix system based on patient-characteristics and other clinical information, rather than one based on the volume of services provided. We agree that this is a more accurate way to align payment with the cost of providing care. However, we recognize stakeholder concerns about possible perverse financial incentives that could arise as a result of transitioning to a new case-mix adjustment methodology and a change in the unit of payment. We reiterate that we expect the provision of services to be made to best meet the patient's care needs and in accordance with the home health CoPs at § 484.60 which sets forth the requirements for the content of the individualized home health plan of care which includes the types of services, supplies, and equipment required; the frequency and duration of visits to be made; as well as patient and caregiver education and training to facilitate timely discharge. Therefore, we do not expect HHAs to under-supply care or services; reduce the number of visits in response to payment; or inappropriately discharge a patient receiving Medicare home health services as these would be violations of the CoPs and could also subject HHAs to program integrity measures.
We also note that the home health CoPs at § 484.50(c) set forth patient rights, which include the patient's right to be involved in the plan of care, the right to be informed of any changes to the plan of care, as well as expected coverage, and possible beneficiary financial liability. Therefore, HHAs are already tasked with informing beneficiaries as to their rights and coverage under the Medicare home health benefit. Moreover, CMS does routinely update its public materials to ensure relevant stakeholders are informed of any policy, coverage, or payment changes. This includes updates to the Medicare Benefit Policy Manual, the “Medicare and You” Handbook, “Medicare's Home Health Benefit” booklet, and MLN Matters® articles on various aspects of the home health benefit. As with any policy, coverage, or payment change, we will update the necessary public information to ensure full transparency and to provide ample resources for beneficiaries and their families, as well as for home health agencies. The goal of the PDGM is to more accurately align home health payment with patient needs. We note that each individual policy change does not have a corresponding individual educational budget connected with its implementation; therefore this is not information we can provide. We acknowledge that the change to a new case-mix system may have unintended consequences through shifts in home health practices. However, in the CY 2020 HH PPS proposed rule, we stated that we expect the provision of services to be made to best meet the patient's care needs and in accordance with existing regulations. We also noted that we would monitor any changes in utilization patterns, beneficiary impact, and provider behavior to see if any refinements to the PDGM would be warranted, or if any concerns are identified that may signal the need for appropriate program integrity measures.
Comment: A commenter stated that under the current HH PPS, HHAs' costs are “frontloaded” and incurred regardless of whether a second 30-day period occurs within a 60-day episode. This commenter stated that CMS should account for these costs and allocate payment weights more toward the first 30-day period in each 60-day episode to ensure that payments are accurately aligned with resource use. Commenters express several concerns with the use of cost report data rather than Bureau of Labor Statistics (BLS) wage data to account for the cost of therapy services; thus, commenters recommend CMS use BLS wage-weighted minutes instead of the approach finalized in the CY 2019 final rule with comment period.
Response: We note that we provided detailed analysis on the estimated costs of 30-day periods of care using a cost-per-minute plus non-routine supply (CPM + NRS) approach in the CY 2019 HH PPS proposed rule (83 FR 32387). We also provided analysis on the average resource use by timing where early 30-day periods have higher resource use that later 30-day periods (83 FR 32392). Likewise, in the CY 2019 HH PPS final rule with comment period (83 FR 56471), we finalized the admission source case-mix variable under the PDGM where “early” 30-day periods of care receive a higher payment than “late” 30-day periods of care. Commenters supported this payment differential as it more accurately reflects HHA costs that are typically higher during the first 30-day period of care, compared to later 30-day periods of care.
When we finalized the CPM+NRS approach to calculating the costs of care in the CY 2019 HH PPS final rule with comment period, we stated that we believe that the use of HHA Medicare cost reports better reflects changes in utilization, provider payments, and supply amongst Medicare-certified HHAs that occur over time. Under the Wage-Weighted Minutes of Care (WWMC) approach, using the BLS average hourly wage rates for the entire home health care service industry does not reflect changes in Medicare home health utilization that impact costs, such as the allocation of overhead costs when Medicare home health visit patterns change. Using data from HHA Medicare cost reports better represents the total costs incurred during a 30-day period (including, but not limited to, direct patient care contract labor, overhead, and transportation costs), while the WWMC method provides an estimate of only the labor costs (wage and fringe benefit costs) related to direct patient care from patient visits that are incurred during a 30-day period.
Comment: A commenter suggested an additional alternative to consider regarding the implementation of the PDGM. Specifically, this commenter suggested a potential pilot program to test not only the PDGM but possibly the PDPM payment system for skilled nursing facilities to consider some form of a post-acute bundle with shared savings.
Response: We appreciate the commenter's suggestions for innovative ways to improve the health care system and payment models. However, we note that the change in the unit of payment and the case-mix methodology is mandated by the BBA of 2018, as such we are required to implement such changes beginning on January 1, 2020.
2. Admission Source
Comment: A commenter stated that it appears counterintuitive to have a different reimbursement for community versus institutional admission source stating that the goal of home health care is to keep the patients out of the hospital. A commenter expressed concern that even though the application of an admission source measure may seem warranted given data demonstrating different resource use, doing so may incentivize agencies to give priority to post-acute patients over those who are admitted from the community. This commenter stated that the financial impact of the PDGM admission source measure also highlights the inherent weakness of all the other PDGM measures. A few commenters supported the admission source as an indicator of predicted home health resource use.
Response: We agree that the provision of home health services may play an important role in keeping patient's out of the hospital, whether the patient is admitted to home health from an institutional source or from the community. However, the payment adjustments associated with the PDGM case-mix variables are based on the cost of providing care. As described in the CY 2018 HH PPS proposed rule (82 FR 35311), our analytic findings demonstrate that institutional admissions have significantly higher average resource use when compared with community admissions, which ultimately led to the inclusion of the admission source category within the framework of the alternative case-mix adjustment methodology refinements. Additionally, in the CY 2018 HH PPS proposed rule (82 FR 35309), we stated that in our review of related scholarly research, we found that beneficiaries admitted directly or recently from an institutional setting (acute or post-acute care (PAC)) tend to have different care needs and higher resource use than those admitted from the community, thus indicating the need for differentiated payment amounts. Furthermore, in the CY 2018 proposed rule, we provided detailed analysis and research to support the inclusion of an admission source category for case-mix adjustment. We continue to believe that having a case-mix variable accounting for admission source is clinically appropriate, will address the more intensive care needs of those admitted to home health from an institutional setting, and will more accurately align payment with the cost of providing home health care.
To address concerns that the admission source variable may create the incentive to favor institutional admission sources, we fully intend to monitor provider behavior in response to the new PDGM. As we receive and evaluate new data related to the provision of Medicare home health care under the PDGM, we will reassess the appropriateness of the payment levels for all of the case-mix variables, including admission source, to determine if HHAs are inappropriately changing their behavior to favor institutional admission sources over community. Additionally, we will share any concerning behavior or patterns with the Medicare Administrative Contractors (MACs) and other program integrity contractors, if warranted. We plan to monitor and identify any variations in the patterns of care provided to home health patients, including both increased and decreased provision of care to Medicare beneficiaries. We remind stakeholders that the purpose of case-mix adjustment is to align payment with the costs of providing care. As such, certain case-mix variables may have a more significant impact on the payment adjustment than others. However, the case-mix variables in the PDGM work in tandem to fully capture patient characteristics that translate to higher resource needs. The overall payment for a home health period of care under the PDGM is determined by the cumulative effect of all of the variables used in the case-mix adjustments. Ultimately, the goal of the PDGM is to provide more accurate payment based on the identified resource use of different patient groups.
3. Therapy Thresholds
Comment: A few commenters disagreed with the elimination of the therapy thresholds and expressed concern that the PDGM design will have a negative impact on patients who need therapy services and the HHAs that provide it. A commenter stated that therapy services are extraordinarily valuable in the care of Medicare home health beneficiaries and should be supported to the greatest degree possible. Another commenter suggested elimination of the 30-day therapy reassessment requirement stating this would duplicative and unnecessary under PDGM, given that therapy visits are no longer a payment driver, and that all visits must continue to demonstrate a skilled need, independent of a formal reassessment. Many commenters urge CMS to monitor the effects of PDGM and the implications on therapy utilization due to concerns therapy would be underutilized, which could result in beneficiaries going to inpatient settings rather than receiving care at home. Some commenters recommend further analysis to compare utilization of therapy revenue codes under the PPS and PDGM. In addition, commenters encourage CMS to use the survey process to ensure that beneficiaries continue to receive the appropriate level of therapy that were medically necessary in order to treat or manage the condition.
Response: We agree that therapy remains a valuable service for Medicare home health beneficiaries. In response to the CY 2018 and 2019 HH PPS proposed rules, the majority of commenters agreed that the elimination of therapy thresholds was appropriate because of the financial incentive to overprovide therapy services. While the functional impairment level adjustment in the PDGM is not meant to be a direct proxy for the therapy thresholds, the PDGM has other case-mix variables to adjust payment for those patients requiring multiple therapy disciplines or those chronically ill patients with significant functional impairment. We believe that also accounting for timing, source of admission, clinical group (meaning the primary reason the patient requires home health services), and the presence of comorbidities will provide the necessary adjustments to payment to ensure that care needs are met based on actual patient characteristics. Furthermore, services are to be provided in accordance with the home health plan of care established and periodically reviewed by the certifying physician. Therefore, we expect that home health agencies will continue to provide needed therapy services in accordance with the CoPs at § 484.60, which state that the individualized plan of care must specify the care and services necessary to meet the patient-specific needs as identified in the comprehensive assessment, including identification of the responsible discipline(s), and the measurable outcomes that the HHA anticipates will occur as a result of implementing and coordinating the plan of care. Upon implementation of the PDGM, we will monitor home health utilization, including the provision of therapy services. Finally, we remind commenters that section 51001(a)(3)(B) of the BBA of 2018 prohibits the use of therapy thresholds as part of the overall case-mix adjustment for CY 2020 and subsequent years. Consequently, we have no regulatory discretion in this matter.
While we appreciate commenter suggestions to further reduce burden by eliminating therapy reassessments, we did not propose to eliminate the current 30-day therapy reassessment requirement at § 409.44(c)(2)(i)(B) in the CY 2020 HH PPS proposed rule. When we finalized the 30-day therapy reassessment requirement in the CY 2015 HH PPS final rule (79 FR 66103), we stated that the qualified therapist assists the physician in evaluating level of function, helps develop the plan of care (revising it as necessary), prepares clinical and progress notes, advises and consults with the family and other agency personnel, and participates in in-service programs. Furthermore, in the CY 2015 final rule, the overwhelming majority of commenters recommended reassessing the patient at least once every 30 days as the most appropriate time frame. Commenters stated that a 30 day reassessment timeframe aligns with many state practice acts, which require that a therapist reassess the patient at least once every 30 days. As part of our response, we also referenced the American Physical Therapy Association (APTA) guidelines which state that at least once a month, the qualified therapist should conduct a supervisory visit with the therapist assistant which should include: An on-site reexamination of the patient/client; on-site review of the plan of care with appropriate revision or termination; and evaluation of need and recommendation for utilization of outside resources.[8] We also stated that we believe that requiring therapy reassessments at least once every 30 days, the CoP requirements regarding the plan of care, and the APTA guidelines together promote regular interaction between the therapist and the patient. However, we recognize the importance of decreasing unnecessary burden and we will continue to monitor home health utilization, including the provision of therapy visits, to re-evaluate any existing policies to determine if any additional changes should be proposed in future rulemaking. Likewise, we understand commenter concerns about potential underutilization of certain disciplines, especially therapy, with the elimination of therapy thresholds. The home health CoPs have requirements as to the content of the home health plan of care, as well as providing services that are ordered by the physician as indicated in the plan of care. Therefore, existing survey mechanisms are in place to help ensure patient safety and quality standards. However, as we noted in the CY 2019 HH PPS final rule with comment period, upon implementation of the PDGM, we will continue to monitor the payment system as we have done since the inception of the benefit. We will closely monitor patterns related to utilization, including changes in the composition of patients receiving the home health benefit and the types and amounts of services they are receiving, as well as any changes in the settings of care.
Comment: A few commenters support the elimination of therapy as the driver of payment and offered historical context to the potential increase in therapy utilization as it relates to the Home Health Quality Reporting Program. A commenter also identified potential opportunity for oversight and monitoring to address “problematic HHAs” that the commenter identifies as driving the therapy utilization data since the inception of the HH PPS. Another commenter stated that the elimination of therapy volumes as a determinant of reimbursement is appropriate and that they anticipate the clinical groupings based on diagnosis, along with the comorbidity adjustments will prove to be acceptable elements of payment.
MedPAC also supports the elimination of therapy as a payment factor because their March 2018 Report to Congress [9] stated concerns about the financial incentive to providing more therapy that is not necessarily tied to patient characteristics, which is a recognized vulnerability in the HH PPS. However, MedPAC believes additional monitoring is necessary regarding the 30 day payment to understand whether there is a new incentive for HHAs to provide just enough services/visits to surpass the threshold for a second 30 day payment.
Response: We appreciate commenter support regarding the elimination of the therapy thresholds for use in adjusting home health payment. We believe that elimination of the therapy thresholds is more in alignment with the intent of the home health benefit to be patient-centered and based on patient characteristics, such as functional status, and actual patient needs. Likewise, we expect that any services provided would be in accordance with all Federal and State laws, including all licensure requirements. The provision of skilled therapy services as part of a home health plan of care must also adhere to the home health CoPs, (42 CFR 484.60). We believe that the elimination of the therapy thresholds will remove the financial incentive to provide therapy solely for increased payment. Upon implementation of the PDGM and the 30-day unit of payment, we will continue to monitor home health utilization, including the provision of therapy services, as well as any shifts in disciplines to determine if any program integrity or survey efforts may be warranted.
4. Non-Routine Supplies (NRS)
Comment: A couple of commenters suggested that CMS should consider the higher costs of wound care supplies and should pay more for such supplies as part of the PDGM. Another commenter recommended that the cost of non-routine supplies (NRS) should be included in outlier payments.
Response: As finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56406), similar to the current system, NRS still would be paid prospectively under the PDGM, but the PDGM eliminates the separate case-mix adjustment model for NRS. We believe that the PDGM offers an alternative method for accounting for NRS costs and payments by grouping patients more likely to require high NRS utilization. Under the PDGM, NRS costs are reflected in the average resource use that drives the case-mix weights. If there is a high amount of NRS cost for all periods in a particular group (holding all else equal), the resource use for those periods will be higher relative to the overall average and the case-mix weight will correspondingly be higher. We appreciate the commenters' suggestion regarding the inclusion of supplies in the outlier calculation under the PDGM. In order to incorporate supply costs into the outlier calculation, significant claims payment systems modifications would be required. However, after implementation of the PDGM, we will continue to monitor the provision of NRS and we will consider whether to add supply costs to the outlier calculations and evaluate whether such a policy change is appropriate for future rulemaking.
5. Clinical Groups
Comment: Some commenters made general remarks regarding the diagnosis codes included in the clinical grouping case-mix variable. A few commenters state that elimination of certain diagnosis codes would narrow the home health benefit and may prevent access to care to which Medicare beneficiaries are legally entitled. Another commenter stated that the coding-related proposals could limit the home health benefit for eligible beneficiaries in need of skilled maintenance therapy. A commenter stated that the removal of certain diagnosis codes from the clinical grouping would essentially eliminate coverage for skilled services under the home health benefit and said that CMS should not finalize elimination of these codes and should recalculate rates with all existing codes included.
Response: The elimination of certain diagnosis codes from the HH PPS Grouper is not unique to the PDGM as we have previously removed codes from the 153-group HH PPS case-mix system that no longer have a significant impact on resource use. As stated previously, the clinical grouping is only one case-mix variable in the PDGM. These clinical groups are designed to capture the most common types of care that HHAs provide. Although the principal diagnosis code is the basis for the clinical grouping, secondary diagnosis codes and patient characteristics will be used to case-mix adjust the period further through the comorbidity adjustment and functional level. We believe that the PDGM has a robust set of clinical characteristics to ensure that payment accurately aligns with patient needs and therefore, we do not expect there to be any issues with patient access to home health services. Furthermore, eligibility for home health services remains the same as under the 153-group system. That is, individuals are eligible for home health services if the following criteria are met: The individual is confined to the home; is under the care of a physician; is receiving services under a plan of care established and periodically reviewed by a physician is in need of skilled nursing care on an intermittent basis or physical therapy or speech-language pathology therapy; has a continuing need for occupational therapy. Therefore, a patient's principal or secondary diagnoses are not sole factors in whether a patient is eligible for Medicare home health services. As such, eligible beneficiaries are entitled to their Medicare home health benefits and we do not expect there to be an access to care issue. With respect to the provision of therapy services as they relate to the home health period's clinical group, we should emphasize that although the principal diagnosis is a contributing factor in the PDGM and determines the clinical group, it is not the only consideration in determining what home health services are needed in a patient's care plan. We stated in the CY 2019 HH PPS proposed rule (83 FR 32401), that it is the responsibility of the patient's treating physician to determine if and what type of therapy (that is, maintenance or otherwise) the patient needs regardless of clinical grouping. As such, we continue to expect the ordering physician, in conjunction with the therapist, to develop and follow a plan of care for any home health patient, regardless of clinical group, as outlined in the skilled service requirements when therapy is deemed reasonable and necessary. Therefore, a home health period's clinical group should not solely determine the type and extent of therapy needed for a particular patient.
As described in the CY 2018 HH PPS proposed rule (82 FR 35313), to inform the development of the clinical groups, our home health contractor, Abt Associates and CMS conducted an extensive review of diagnosis codes to identify the primary reasons for home health services under the Medicare home health benefit. The published HHGM (predecessor to the PDGM), technical report from December 2016 [10] and the CY 2018 HH PPS proposed rule (82 FR 35314), detail several reasons why a diagnosis code was not assigned to one of the clinical groups. These included if the diagnosis code was too vague, meaning the code does not provide adequate information to support the need for skilled home health services (for example H57.9, Unspecified disorder of eye and adnexa); the code is subject to laterality for which the home health clinician could assess the appropriate side (for example, some diagnosis codes indicate laterality, specifying whether the condition occurs on the left or right, or is bilateral); the code, based on ICD 10-CM, American Hospital Association (AHA) Coding Clinic, or Medicare Code Edits (MCE) would indicate a non-home health service (for example, dental codes); the code is a manifestation code subject to a manifestation/etiology convention, meaning that the etiology code must be reported as the principal diagnosis, or the code is subject to a code first sequencing convention (for example, G99.2 myelopathy in diseases classified elsewhere); the code identifies a condition which would be unlikely to require home health services (for example, L81.2, Freckles); the code is restricted to the acute care setting per ICD 10-CM/AHA Coding Clinic, or the diagnosis indicates death as the outcome (for example S06.1X7A, Traumatic cerebral edema with loss of consciousness of any duration with death due to brain injury prior to regaining consciousness). Overall, we continue to believe that the PDGM clinical grouping includes a robust set of diagnosis codes and includes more codes than under clinical dimension of the 153-group case-mix system. Therefore, this should afford HHAs greater opportunity to more fully describe patient characteristics through principal and secondary diagnosis reporting on home health claims.
While there are certain diagnosis codes that are not assigned to a clinical group under the PDGM for the reasons described, we remind commenters that claims submitted with such codes are not denied; rather they are returned to the provider for more definitive coding. The importance of consistent, complete medical documentation cannot be overemphasized. Without such documentation, accurate diagnosis coding cannot be achieved; therefore, ICD-10-CM coding guidelines [11] state that the entire record should be reviewed to determine the specific reason for the encounter and the conditions treated. We remind stakeholders that if there is a question as to what the appropriate principal (or secondary) diagnosis should be, the HHA should query the certifying physician who is responsible for establishing the home health plan of care.
Comment: One industry association stated it had a workgroup conduct some analysis on the diagnosis codes and their assigned clinical groups and they state that it was discovered that in a significant number of instances a code assigned to one clinical grouping was also placed in a different clinical grouping. They noted that in every case they analyzed where a code was assigned to a different clinical grouping, it was assigned to the Complex Nursing group. The commenter requested clarification and CMS' rationale so they could share with other industry stakeholders.
Response: We remind commenters that in developing the case-mix weights for the PDGM, we examined the principal diagnosis codes reported by HHAs and, in order to assign periods of care into the appropriate clinical group representing the primary reason for home health services, we also looked at OASIS item, M1030, “Therapies” (identifies whether the patient is receiving intravenous, parenteral nutrition or enteral nutrition therapy at home) to see if home health patients were receiving complex therapies for which the appropriate case-mix adjustment should be made. Therefore, for those circumstances in which the workgroup's analysis of the principal diagnosis would have grouped the period of care into one of the MMTA subgroups, but the actual period was grouped into Complex Nursing Interventions, this is likely due to that period of care being assigned based on the response to OASIS item M1030, reflecting complex nursing interventions provided during the course of home health care. However, we note that for implementation of the PDGM in CY 2020 and subsequent years, we have assigned ICD-10-CM diagnosis codes to the Complex Nursing Interventions group that reflect these more complex therapies previously identified from the OASIS item M1030 (for example, Z45.2, Encounter for adjustment and management of venous access device) and we will be using the diagnosis codes reported on the home health claim and not OASIS items to assign a period of care to a clinical group for case-mix adjustment purposes.
Comment: Several commenters stated that symptom codes should be allowed to be reported as the principal diagnosis and assigned to a clinical group. A few commenters stated that disallowing symptom codes for principal diagnosis consideration will cause HHAs to report a principal diagnosis that would not truly represent the reason for the home health encounter and would force HHAs to “upcode”. A commenter remarked that there is a significant portion of the elderly population who exhibit symptomology but have declined further testing or the medical community has decided not to order expensive tests since many times the treatment remains the same. Several symptom codes were specifically mentioned for inclusion in the clinical group variable by a national industry association, as well as HHAs. Commenters suggested that the following symptom codes should be in the MS Rehab clinical group:
- R26.89, Other abnormalities of gait and mobility
- R29.6, Repeated falls
The following symptom codes were suggested to be included in the clinical group variable, but without a recommendation for a specific PDGM clinical group:
- R00.1, Bradycardia
- R41.82, Altered Mental Status
- R42, Dizziness and giddiness.
And, several commenters suggested the following symptom codes should be in the Neuro Rehab clinical group:
- R27.0, Ataxia, unspecified
- R13.10, Dysphagia
Response: As we have stated in the CY 2020 proposed rule and this final rule with comment period, we do not support or condone coding solely for purposes of higher payment (what commenters refer to as “upcoding”). In accordance with ICD-10-CM coding guidelines, the principal diagnosis reported is that “condition established after study to be chiefly responsible for occasioning the admission of the patient to the hospital for care.” For purposes of home health care admission, this would be the diagnosis chiefly responsible for home health services. Because of the home health requirements that the individual receiving home health services must be certified for such services and must have had a face-to-face encounter related to the primary reason for home health care, we believe that by the time an individual is admitted to home health, the patient has been seen by other health care providers and a diagnosis has been established. We note that we adopted a similar position as it relates hospice diagnosis reporting. In the FY 2014 hospice proposed rule (78 FR 27831), we stated that if a nonspecific, ill-defined symptom diagnosis is reported as the principal hospice diagnosis, a comprehensive, individualized patient-centered plan of care, as required, may be difficult to accurately develop and implement, and, as a result, the hospice beneficiary may not receive the full benefit of hospice services. We believe that the same principle applies to home health beneficiaries and that accurate documentation and diagnosis reporting is essential to ensure that an individualized plan of care is established to meet the patient's home health needs. Furthermore, the ICD-10-CM coding guidelines state that codes for symptoms, signs, and ill-defined conditions are not to be used as the principal diagnosis when a related definitive diagnosis has been established. Therefore, because of the inclusion of a clinical group for case-mix adjustment purposes predicated on diagnosis reporting, we believe that HHAs would improve their overall documentation and accuracy of their diagnosis code reporting to reflect patient characteristics defined by diagnosis codes, as well as other important patient information that reflects resource utilization (for example functional impairment). As such, we believe that the reporting of ill-defined symptom codes as the principal diagnosis would be less frequent.
As we stated in the CY 2019 HH PPS final rule with comment period (83 FR 56473), we believe that the majority of the R-codes (codes that describe signs and symptoms, as opposed to diagnoses) are not appropriate as principal diagnosis codes for grouping home health periods into clinical groups. We believe that the use of symptoms, signs, and abnormal clinical and laboratory findings would make it difficult to meet the requirements of an individualized plan of care as required at § 484.60. Likewise, we believe that clinically it is important for home health providers to have a clear understanding of the patients' diagnoses in order to safely and effectively furnish home health services. Interventions and treatment aimed at mitigating signs and symptoms of a condition may vary depending on the cause. For example, if a patient has been referred to home health with a diagnosis of “other abnormalities of gait and mobility” (R26.89), we believe it is important for the home health clinician to know what is precipitating the abnormality. For instance, a plan of care for a gait abnormality related to a neurological diagnosis is likely to be different from a plan of care for a gait abnormality due to a fracture or injury. Anecdotally, we have heard that the home health referral may be non-specific or that the physician may be in the process of determining a more definitive diagnosis. However, with respect to patient safety and quality of care, we believe it is important for a clinician to investigate the cause of the signs and/or symptoms for which the referral was made. This may involve calling the referring physician to gather more information regarding the gait abnormality. We note that HHAs are required under the home health CoPs at § 484.60 to participate in care coordination to assure the identification of patient needs and factors that could affect patient safety and treatment efficacy. ICD-10-CM coding guidelines are clear that R-codes are to be used when no more specific diagnosis can be made even after all the facts bearing on the case have been investigated. Therefore, these codes should not be used as a principal diagnosis for the provision of home health services while a physician may still be in the diagnostic process. By the time the patient is referred to home health and meets the qualifications of eligibility, we would expect that a more definitive code would substantiate the need for services. Furthermore, commenters have indicated a preference for greater specificity in the clinical groups, therefore, we believe this should extend to the codes within the clinical groups as well.
Regarding commenters suggesting that R29.6, Repeated falls, be included in the MS Rehab group, we note that ICD-10-CM coding guidelines state to only use R29.6 for use for encounters when a patient has recently fallen and the reason for the fall is being investigated. Given that the patient must be certified for home health services and must have had a face-to-face encounter related to the primary reason for home health services, we do not believe that this particular symptom code would be appropriate for the principal diagnosis to substantiate home health services. We believe that by the time a home health referral is made, a more clearly defined diagnosis would have been established to more accurately describe the patient's condition. However, if the patient's condition has resulted in repeated falls, the HHA would report Z91.81, History of falling, as a secondary diagnosis to describe that the patient has fallen in the past and is at future risk for falls to more accurately describe the patient's need for home health services. For the same reasons as stated throughout this response, we do not believe it appropriate to include R00.1 Bradycardia, R41.82, Altered Mental Status, or R42, Dizziness and giddiness as part of the clinical group case-mix variable because of the vague nature of symptom codes where there could be multiple reasons for such symptoms. In order to develop an appropriate, individualized home health plan of care, we believe it is clinically essential to understand the causes of such symptoms to safely and effectively provide home health services. Furthermore, it has been our longstanding policy to avoid vague diagnoses for reporting and payment purposes. Specifically, we stipulated in the 2008 HH PPS final rule (72 FR 49774) that the case-mix system avoid, to the fullest extent possible, non-specific or ambiguous ICD-9-CM codes, codes that represent general symptomatic complaints in the elderly population, and codes that lack consensus for clear diagnostic criteria within the medical community. We note that diagnosis codes R00-R99 include symptoms, signs, abnormal results of clinical or other investigative procedures, and ill-defined conditions are limited for those circumstances where there is no recorded diagnosis that is classifiable elsewhere. However, patients are referred to home health from other clinical settings (either from a facility or a community-based provider) and therefore, we believe that the medical records from such referral source should provide information as to the need for home health services, including the diagnoses established by such providers. Clinically, this information is needed to develop the individualized plan of care with patient-specific goals. In the circumstance where such information is missing or insufficient, we believe that HHAs should query these referring providers to ensure they have a clear understanding of the conditions affecting patients in need of home health services.
Regarding suggestions to include the symptom codes R27.0, Ataxia, unspecified, and R13.10, Dysphagia, in the Neuro Rehab clinical group, we reiterate our position as noted previously—that by the time a patient is admitted for home health services, there should be sufficient documentation in the patient's medical record to have an established diagnosis, and that a symptom diagnosis should not be reported as the principal diagnosis as this could be the result of other conditions besides a neurological condition and therefore, grouping the period of care into Neuro Rehab may not be appropriate. We continue to believe that the home health clinician needs appropriate, accurate clinical information, including the cause of such symptoms, in order to develop an individualized plan of care to specify the services necessary to meet the patient-specific needs.
However, we analyzed the frequency of the reporting of each of these diagnoses and we note that in 2018, there were only 3,461 30-day periods in which R27.0, Ataxia, unspecified, was reported as the principal diagnosis. However, in looking at the reported secondary diagnoses accompanying this principal diagnosis, HHAs reported established diagnoses that could explain the reason for the unspecified ataxia and would group the 30-day period of care into the Neuro Rehab group. For example, we found reported secondary diagnoses of Alzheimer's disease, Parkinson's disease, and polyneuropathy. Given that symptom diagnoses should not be reported as the principal diagnosis if there is an established diagnosis, we believe that the established diagnosis would be reported first, and the symptom code, unspecified ataxia, would be reported as a secondary diagnosis to fully reflect patient characteristics. Furthermore, in reviewing the tabular index in the CY 2020 ICD-10-CM official code set[12] for “ataxia”, there are multiple diagnosis codes available to more accurately describe the underlying condition causing the ataxia. We also note that “unspecified” codes should only be reported when the medical record is insufficient to assign a more specific code.
We also analyzed the frequency of reporting of R13.10, dysphagia, unspecified and we note that in 2018, there were approximately 28,000 30-day periods in which this particular code was reported as the principal diagnosis. In looking at the reported secondary diagnoses accompanying this principal diagnosis, we found that while there were incidences where there were other reported diagnoses which could explain the reason for the dysphagia, more often than not, there was no clear clinical picture of the possible etiology where a different reported principal diagnosis would signal the need for therapy. Furthermore, we received comments on this particular diagnosis stating that while there are diagnosis codes for dysphagia resulting from a cerebrovascular event (for example, stroke) and others resulting from somatoform disorders (for example, psychogenic dysphagia), there are very few disease-specific diagnosis codes to identify associated dysphagia (for example, dysphagia resulting from throat cancer treatment). A review of the CY 2020 ICD-10-CM official code set tabular index, showed that the majority of codes to describe dysphagia are the R13 codes. We recognize that dysphagia codes associated with a cerebrovascular event would be assigned to the Neuro Rehab clinical group and commenters stated that those patients with dysphagia due to etiologies not associated with cerebrovascular events would most often require speech-language pathology therapy if the primary reason for home health services is for the dysphagia. Given the current lack of other definitive diagnoses to describe certain forms of dysphagia, we agree that the R-codes to describe dysphagia would be acceptable for reporting the primary reason for home health services. Therefore, we will assign the following R-codes to the Neuro Rehab clinical group:
- R13.10, Dysphagia, unspecified
- R13.11, Dysphagia, oral phase
- R13.12, Dysphagia, oropharyngeal phase
- R13.13 Dysphagia, pharyngeal phase
- R13.14, Dysphagia, pharyngoesophageal phase
- R13.19, Other dysphagia
While we understand that dysphagia could be the result of non-neurological conditions, we are assigning these dysphagia groups to the Neuro Rehab group as we believe the intensity of speech-language pathology therapy would be similar to those suffering from dysphagia resulting from a neurological condition. However, we will monitor the use of these dysphagia R-codes to determine their impact on resources utilization and whether any future changes would be warranted.
Finally, we remind commenters that ICD-10-CM coding guidelines state that codes for signs and symptoms may be reported in addition to a related definitive diagnosis when the sign or symptom is not routinely associated with that diagnosis, such as signs and symptoms associated with complex syndromes. The definitive diagnosis should be sequenced before the symptom code. Signs or symptoms that are associated routinely with a disease process should not be assigned as secondary codes, unless otherwise instructed by the classification. Therefore, we expect that HHAs would report the principal and secondary diagnoses that affect the home health plan of care and justify the need for home health services.
Comment: We received specific coding comments from national industry associations as well as well as from other HHAs, with recommendations to change or add the following codes to the clinical group variable.
Response: Table 12 lists these codes and the commenters recommended clinical group, as well as our response to these recommendations:







We note that as we were examining the clinical group changes suggested by commenters, we took the opportunity to ensure consistency in the clinical group assignments and have reassigned certain diagnosis codes accordingly. Specifically, we are reassigning the following codes:

Comment: Several commenters stated that code M62.81 Muscle Weakness (generalized) should be allowed to be reported as the principal diagnosis used to assign a clinical group. Commenters stated that it is problematic to exclude this code, as there are scenarios in which patients are seen in the home for muscle weakness when the underlying etiology is unknown, or when the original condition, causing the weakness is resolved. Additionally, commenters noted that M62.81 is identified as a diagnostic code to support medical necessity for home health therapy services by the MACs within their local coverage determinations. While commenters agreed that this diagnosis lacks specificity, they stated that they disagree that this diagnosis would not be deemed medically necessary. And finally, commenters stated that when evaluating the assignation of a diagnosis code at the point of care in home health, the coding specialist must consider the available documentation.
Response: As we stated in the CY 2019 HH PPS final rule with comment period (83 FR 56474), M62.81, “Muscle weakness, generalized” is a vague code that does not clearly support a rationale for skilled services. Further, the lack of specificity for this code does not support a comprehensive plan of care. We noted that § 409.44(c)(1)(ii) states that “the patient's clinical record must include documentation describing how the course of therapy treatment for the patient's illness or injury is in accordance with accepted professional standards of clinical practice.” If there is not an identified cause of muscle weakness, then it would be questionable as to whether the course of therapy treatment would be in accordance with accepted professional standards of clinical practice.
Additionally, it is not without precedent that CMS has been disinclined to include generalized muscle weakness in the home health case-mix. In the 2008 HH PPS final rule, we identified generalized muscle weakness as a nonspecific condition that represents general symptomatic complaints in the elderly population. We stated that inclusion of this code “would threaten to move the case-mix model away from a foundation of reliable and meaningful diagnosis codes that are appropriate for home care” (72 FR 49774). The 2008 HH PPS final rule stated that the case-mix system avoid, to the fullest extent possible, non-specific or ambiguous ICD-9-CM codes, codes that represent general symptomatic complaints in the elderly population, and codes that lack consensus for clear diagnostic criteria within the medical community. Expanding upon that assertion, we stated in the CY 2019 final rule with comment period that diagnostic approaches to determining the cause of muscle weakness, polyneuropathy, and other vague conditions, combined with the expanded ICD-10 list, ensure that codes exist which more clearly describe a patient's need for home health (83 FR 56474). With respect to commenter rationale for coding generalized muscle weakness when the underlying etiology is unknown, we believe that by the time a home health referral is made, a more definitive principal diagnosis is warranted in order to justify the need for skilled services and appropriate treatment. Further, if the original condition is resolved, but the resulting muscle weakness persists as a result of the known original diagnosis, we anticipate that a more specific code exists that accounts for why the muscle weakness is on-going, such as muscle wasting or atrophy. As the commenter pointed out, the coding specialist must consider available documentation; however, as we state in the previous discussion regarding symptom codes, we believe it is important for a clinician to investigate the reason for which the referral was made. This may involve calling the referring physician if the original condition is resolved and is not included in the referral documentation.
With respect to commenter reference to the LCD for Physical Therapy in Home Health (L33942), we recognize that M62.81 is identified as a code to support medical necessity. While we are not disputing that services for this diagnosis are considered reasonable and medically necessary, we do not believe it is appropriate to list Muscle weakness, generalized as a principal diagnosis in order to group the home health period. We developed the clinical groupings in large part to clearly identify the need for the home health episode, including the skilled services involved. Allowing use of a vague code that does not clearly denote a treatment plan, would invalidate the transparency we hope to achieve in the home health payment system.
6. Comorbidities
Comment: A commenter questioned why the list of comorbidity codes stopped at the R codes and indicated there should be codes for “traumas, postoperative complications and the Z codes”. The same commenter questioned why some codes were included in the overall comorbidity list but not all were eligible for a comorbidity adjustment. A commenter requested an explanation the rationale for not including any conditions from the ICD-10-CM chapters with O, P, Q, R, S, T, or Z codes as comorbidity diagnoses as many of these seem appropriate given the significant impact these conditions have on the patient's recovery.
Another commenter questioned why blindness and other low vision codes (Neuro 11) were removed from the comorbidity grouping given their significance in patient treatment and recovery.
Response: As we described in the CY 2018 HH PPS proposed rule (82 FR 35322), we examined multiple approaches for a comorbidity adjustment in the alternate case-mix adjustment methodology and the analyses on these approaches are found in the “Overview of the Home Health Groupings Model” technical report found on the HHA Center web page. As we noted in the technical report, secondary diagnosis reporting on the OASIS and home health claims was not as robust as would be expected. As part of that analysis, we also examined claims from prior settings 90 days before the home health start of each home health episode. Again, our analysis showed that diagnosis reporting was not as robust as hypothesized, especially in Part B physician claims where diagnoses reported appeared to be specific to only the condition for which the patient sought care. Furthermore, many secondary diagnosis codes, including those associated with signs, symptoms, and other ill-defined conditions (that is, R-codes) often had an inverse relationship with resource use, meaning the presence of these symptom codes showed less resource use for home health periods of care. Based on the results of these analyses, we proposed and finalized a home health specific comorbidity list for the PDGM comorbidity adjustment, as described in the technical report and in the CY 2018 and CY 2019 HH PPS proposed and final rules. The home health-specific comorbidity list is based on the principles of patient assessment by body systems and their associated diseases, conditions, and injuries to develop larger categories of conditions that identified clinically relevant relationships associated with increased resource use. While we are aware of the prevalence of comorbidities, including those associated with symptoms, in the Medicare home health population, we note that the average number of comorbidities in the aggregate becomes the standard within that population for the purpose of payment. As such, the PDGM comorbidity adjustment includes those comorbid conditions and interaction subgroups that represent more than 0.1 percent of periods and that have at least as high as the median resource use. While there are additional comorbid diagnoses included in the home health-specific list, we note that not all diagnoses are included in a comorbidity subgroup that meets the criteria to receive an adjustment. However, it is expected that HHAs will report those secondary diagnoses that affect care planning and we will continue to evaluate reported secondary diagnoses and interactions between comorbidities to identify their impact on resource costs to determine if any future refinements to this case-mix adjustment variable are warranted.
Regarding the exclusion of diagnosis codes from the ICD-10-CM chapters starting with “O”, “P”, or “Q”, we note that these are diagnosis codes that reflect conditions of pregnancy, childbirth and the puerperium (O00-O9A),certain conditions originating in the perinatal period (P00-P96), and congenital malformations, deformations, and chromosomal abnormalities (Q00-Q99). As such, because we were examining reported diagnoses on Medicare home health claims, these were diagnoses that were not generally reported given the nature of the Medicare patient population. Secondary diagnosis codes identifying signs, symptoms and other ill-defined conditions (R-codes, R00-R99) were examined as part of our analysis for possible inclusion on the comorbidity list, however, these generally did not show any significant correlation on resource use and therefore were not included in the home health specific comorbidity diagnosis list. We note, however, that R00.1, bradycardia, unspecified, is on the comorbidity diagnosis list and is included under the comorbidity subgroup, Heart 10, which does meet the comorbidity adjustment criteria and receives additional payment. The same holds true with the codes that begin with “S” or “T”, representing injury, poisoning, and certain other consequences of external causes (S00-T88) where these codes were not frequently reported as secondary diagnoses on home health claims. Furthermore, we described in detail, in the CY 2018 proposed rule (82 FR 35322), how we developed the home health specific comorbidity diagnosis list, focusing on those chronic conditions that our literature review, and our data analysis, showed to be clinically and statistically significant on their overall impact on home health resource use. Finally, we note that there are diagnosis codes representing blindness and other low-vision conditions on the home health specific comorbidity list (the Neuro 11 subgroup). However, when analyzing CY 2018 home health claims for the CY 2020 comorbidity adjustment, these particular diagnosis codes did not represent more than 0.1 percent of periods or have at least as high as the median resource use and therefore, will not receive a comorbidity adjustment in CY 2020. We take this opportunity to remind commenters that there are diagnosis codes on the home health specific list that will not receive the adjustment in CY 2020, but that does not mean that these would never receive an adjustment. Based on our extensive literature review and previous comments received on what clinically significant secondary diagnoses to include as part of this home health specific list, we believe that if HHAs are reporting these as secondary diagnoses and they have an impact on home health resource use (that is, represent more than 0.1 percent of home health periods of care and have at least as high as the medial resource use), these diagnoses could receive a comorbidity payment adjustment in future years. As such, the comorbidity subgroups that could receive an adjustment in any given year is fluid, depending on the frequency of the reported codes and their impact on resource use. Therefore, we remind commenters of the importance of reporting secondary diagnoses on the home health claim, regardless of whether there is a comorbidity payment adjustment associated with such diagnosis. Likewise, we will continue to examine reported secondary diagnoses on home health claims and their relationship with resource use to determine whether such diagnoses should be included on the home health specific comorbidity list in future years.
Comment: A few commenters noted that there are separate instructions for reporting other/secondary diagnoses on the claim, the OASIS instructions, the CoPs and the interpretive guidelines. These commenters recommended that CMS modify all of these instructions with ICD-10-CM coding guidelines to be consistent with the expectations for reporting of diagnoses.
Response: The ICD-10-CM coding guidelines [13] define “other” (additional) diagnoses as “all conditions that coexist at the time of admission, that develop subsequently, or that affect the treatment received and/or the length of stay.” The OASIS manual instructions [14] state that “secondary diagnoses are comorbid conditions that exist at the time of the assessment, that are actively addressed in the patient's plan of care, or that have the potential to affect the patient's responsiveness to treatment and rehabilitative prognosis”. The CoPs at § 484.60 state that the home health plan of care must include all “pertinent diagnoses” and the accompanying interpretive guidelines state that this means that all “known diagnoses”. While we recognize that there could be a perceived difference between the various descriptions, we believe that these instructions essentially describe the same thing. Specifically, all of these coding instructions state to include any conditions that exist at the time of home health admission, or that develop during the course of a home health period of care, and that affect patient care planning. That is, diagnoses should be reported that affect or potentially affect patient care (and therefore would be addressed in the home health plan of care), even if such care includes observation and assessment (for actual or potential effects), teaching and training, or direct patient care interventions.
Final Decision: We note that the PDGM was finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56406), and therefore, no structural changes to this case-mix adjustment methodology have been made in this CY 2020 final rule with comment period. Therefore, we are finalizing the implementation of the PDGM for 30-day periods of care beginning on and after January 1, 2020. We are finalizing the coding changes for the clinical group as described in responses to the various diagnosis/clinical group comments. These coding changes will be reflected in the Interactive Grouper Tool posted on the HHA Center web page and also in the downloadable HH PPS grouper [15] that accompanies the publication of this final rule with comment period.
B. Implementation of a 30-Day Unit of Payment for CY 2020
Under section 1895(b)(3)(A)(iv) of the Act, we are required to calculate a 30-day payment amount for CY 2020 in a budget-neutral manner such that estimated aggregate expenditures under the HH PPS during CY 2020 are equal to the estimated aggregate expenditures that otherwise would have been made under the HH PPS during CY 2020 in the absence of the change to a 30-day unit of payment. Section 1895(b)(3)(A)(iv) of the Act also requires that in calculating a 30-day payment amount in a budget-neutral manner the Secretary must make assumptions about behavior changes that could occur as a result of the implementation of the 30-day unit of payment. In addition, in calculating a 30-day payment amount in a budget-neutral manner, we must take into account behavior changes that could occur as a result of the case-mix adjustment factors that are implemented in CY 2020. We are also required to calculate a budget-neutral 30-day payment amount before the provisions of section 1895(b)(3)(B) of the Act are applied; that is, before the home health applicable percentage increase, the adjustment if quality data are not reported, and the productivity adjustment.
In the CY 2019 HH PPS final rule with comment period (83 FR 56461), we finalized three assumptions about behavior changes that could occur in CY 2020 as a result of the implementation of the 30-day unit of payment and the implementation of the PDGM case-mix adjustment methodology:
- Clinical Group Coding: A key component of determining payment under the PDGM is the 30-day period of care's clinical group assignment, which is based on the principal diagnosis code for the patient as reported by the HHA on the home health claim. Therefore, we assume that HHAs will change their documentation and coding practices and would put the highest paying diagnosis code as the principal diagnosis code in order to have a 30-day period of care be placed into a higher-paying clinical group. While we do not support or condone coding practices or the provision of services solely to maximize payment, we often take into account in proposed rules the potential behavior effects of policy changes should they be finalized and implemented based on past evidence and as detailed in the CY 2020 proposed and this final rule with comment period.
- Comorbidity Coding: The PDGM further adjusts payments based on patients' secondary diagnoses as reported by the HHA on the home health claim. While the OASIS only allows HHAs to designate 1 primary diagnosis and 5 secondary diagnoses, the home health claim allows HHAs to designate 1 principal diagnosis and 24 secondary diagnoses. Therefore, we assume that by taking into account additional ICD-10-CM diagnosis codes listed on the home health claim (that exceed the 6 allowed on the OASIS), more 30-day periods of care will receive a comorbidity adjustment than periods otherwise would have received if we only used the OASIS diagnosis codes for payment. The comorbidity adjustment in the PDGM can increase payment by up to 20 percent.
- LUPA Threshold: Rather than being paid the per-visit amounts for a 30-day period of care subject to the low-utilization payment adjustment (LUPA) under the PDGM, we assume that for one-third of LUPAs that are 1 to 2 visits away from the LUPA threshold, HHAs will provide 1 to 2 extra visits to receive a full 30-day payment.[16] LUPAs are paid when there are a low number of visits furnished in a 30-day period of care. Under the PDGM, the LUPA threshold ranges from 2-6 visits depending on the case-mix group assignment for a particular period of care (see section III.D. of this final rule with comment period for the LUPA thresholds that correspond to the 432 case-mix groups under the PDGM).
For this final rule with comment period, in order to calculate the CY 2020 budget neutral 30-day payment amounts both with and without behavior assumptions, we first calculated the total, aggregate amount of expenditures that would occur under the current case-mix adjustment methodology (as described in section III.C. of this rule) and the 60-day episode unit of payment using the CY 2019 payment parameters (for example, CY 2019 payment rates, case-mix weights, and outlier fixed-dollar loss ratio). That resulted in a total aggregate expenditures target amount of $16.6 billion.[17] We then calculated what the 30-day payment amount would need to be set at in CY 2020, with and without behavior assumptions, while taking into account needed changes to the outlier fixed-dollar loss ratio under the PDGM in order to pay out no more than 2.5 percent of total HH PPS payments as outlier payments (refer to section III.F. of this rule) and in order for Medicare to pay out $16.6 billion in total expenditures in CY 2020 with the application of a 30-day unit of payment under the PDGM. Table 14 includes the 30-day budget-neutral payment amount for CY 2020 both with and without the behavior assumptions based on the most current data available at the time of this final rule with comment period. These amounts vary slightly from those in Table 12 of the proposed rule (84 FR 34616) due to using more up-to-date data. These payment amounts do not include the CY 2020 home health payment update of 1.5 percent.

If no behavior assumptions were made, we estimate that the CY 2020 30-day payment amount needed to achieve budget neutrality would be $1,908.18. Applying the clinical group and comorbidity coding assumptions, and the LUPA threshold assumption, as required by section 1895(b)(3)(A)(iv) of the Act, would result in the need to decrease the CY 2020 budget-neutral 30-day payment amount to $1,748.11 (an 8.389 percent decrease from $1,908.18). The CY 2020 estimated 30-day budget-neutral payment amount would be slightly less than the CY 2019 estimated 30-day budget-neutral payment amount calculated in last year's rule (that is, if the PDGM was implemented in CY 2019), which we estimated to be $1,753.68. However, the CY 2019 estimated 30-day payment amount of $1,753.68 included the CY 2019 market basket update of 2.1 percent whereas the CY 2020 estimated 30-day budget neutral payment amount of $1,748.11 does not include the 1.5 percent home health legislated payment update for CY 2020. Applying the CY 2020 Wage Index Budget Neutrality Factor and the 1.5 percent home health update as described in section III.E. of this final rule with comment period) would increase the CY 2020 national, standardized 30-day payment amount to $1,785.51. The CY 2020 estimated payment rate of $1,785.51 is approximately 11 percent more than the estimated CY 2020 30-day period cost of $1,608.82, as shown in Table 5 of this final rule with comment period.
The 30-day payment amount will be for 30-day periods of care beginning on and after January 1, 2020. Because CY 2020 is the first year of the PDGM and the change to a 30-day unit of payment, there will be a transition period to account for those home health episodes of care that span the implementation date. Therefore, for 60-day episodes (that is, not LUPA episodes) that begin on or before December 31, 2019 and end on or after January 1, 2020 (episodes that would span the January 1, 2020 implementation date), payment made under the Medicare HH PPS will be the CY 2020 national, standardized 60-day episode payment amount as described in section III.E.4.b of this final rule with comment period. For home health periods of care that begin on or after January 1, 2020, the unit of service will be a 30-day period and payment made under the Medicare HH PPS will be the CY 2020 national, standardized prospective 30-day payment amount as described in section III.E.4.d. of this final rule with comment period. For home health units of service that begin on or after December 3, 2020 through December 31, 2020 and end on or after January 1, 2021, the HHA will be paid the CY 2021 national, standardized prospective 30-day payment amount.
We note that we are also required under section 1895(b)(3)(D)(i) of the Act, as added by section 51001(a)(2)(B) of the BBA of 2018, to analyze data for CYs 2020 through 2026, after implementation of the 30-day unit of payment and new case-mix adjustment methodology, to annually determine the impact of differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures. We interpret actual behavior change to encompass both behavior changes that were previously outlined, as assumed by CMS when determining the budget-neutral 30-day payment amount for CY 2020, and other behavior changes not identified at the time the 30-day payment amount for CY 2020 is determined. We noted in the proposed rule that complete data from CYs 2020 through 2026 will be available to determine whether a prospective adjustment (increase or decrease) is needed no earlier than in years 2022 through 2028 rulemaking. However, we noted that we would analyze preliminary data after implementation of the PDGM to determine if there are any notable and consistent trends to warrant whether any changes to the national, standardized 30-day payment rate should be done earlier than CY 2022.
As noted previously, under section 1895(b)(3)(D)(ii) of the Act, we are required to provide one or more permanent adjustments to the 30-day payment amount on a prospective basis, if needed, to offset increases or decreases in estimated aggregate expenditures as calculated under section 1895(b)(3)(D)(i) of the Act. Clause (iii) of section 1895(b)(3)(D) of the Act requires the Secretary to make temporary adjustments to the 30-day payment amount, on a prospective basis, in order to offset increases or decreases in estimated aggregate expenditures, as determined under clause (i) of such section. The temporary adjustments allow us to recover excess spending or give back the difference between actual and estimated spending (if actual is less than estimated) not addressed by permanent adjustments. However, any permanent or temporary adjustments to the 30-day payment amount to offset increases or decreases in estimated aggregate expenditures as calculated under section 1895(b)(3)(D)(i) and (iii) of the Act would be subject to notice and comment rulemaking.
We reiterate that if CMS underestimates the reductions to the 30-day payment amount necessary to offset behavior changes and maintain budget neutrality, larger adjustments to the 30-day payment amount would be required in the future, by law, to ensure budget neutrality. Likewise, if CMS overestimates the reductions, we are required to make the appropriate payment adjustments accordingly as described previously.
We solicited comments on the proposed, estimated CY 2020 30-day budget neutral payment amount, as well as any potential issues that may result from taking these behavior assumptions into account when establishing the initial 30-day payment amounts for CY 2020. We did not propose any changes to the behavior assumptions finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56461). We received 186 comments on the behavior assumptions finalized in the CY 2019 HH PPS final rule with comment period and the proposed 30-day payment amount for CY 2020 from various stakeholders including home health agencies, industry associations, individual clinicians, and MedPAC. These comments and our responses are summarized in this section of this final rule with comment period.
Comment: Several commenters disagreed with the behavior assumptions finalized in the CY 2019 HH PPS final rule with comment period. Commenters added that given the current regulatory and audit environment, agencies who are coding diagnoses strictly for payment maximization must still keep their focus of care as the primary consideration in coding or their payments will be denied. Commenters went on to state that the home health agency can only code what is already in the medical record and that has been diagnosed by a physician, so there is a limit to which diagnoses may be selected. A commenter stated that CMS is creating an environment wherein agencies will have to modify their coding practices in order to survive. This commenter stated HHAs that would not normally alter their behavior without the reduction will now be forced to.
Response: We continue to believe that the behavior assumptions are reasonable given past experience with changes in provider behavior in response to payment system modifications. We refer readers to the CY 2019 HH PPS final rule with comment period (83 FR 56456), in which we provided examples of observed behavior changes resulting from payment system changes. These examples included the behavior changes resulting from the transition from diagnosis-related groups (DRGs) and the Medicare Severity (MS)-DRGs under the inpatient prospective payment system, and nominal case-mix growth observed from the 2008 changes to the HH PPS case-mix model that resulted in the current 153 home health resource groups. We also believe that there may be additional behavior changes that may result from the change to a new case-mix adjustment methodology that relies more heavily on patient characteristics. For example, given the significant number of ICD-10-CM diagnosis codes that are assigned to a clinical group, HHAs may start reporting diagnoses that were not typically reported on home health claims under the current 153-group model. As we stated in the CY 2020 HH PPS proposed rule (84 FR 34614), we do not support or condone coding practices or the provision of services solely to maximize payment. We fully expect that HHAs would report those diagnoses (both the principal diagnosis and secondary diagnoses) that reflect the primary reason for home health services and those that affect the home health plan of care. This is in accordance with ICD-10-CM coding guidelines, which state to select the principal diagnosis code that reflects the reason for the health care encounter, and to report the additional diagnoses that affect patient care in terms of clinical evaluation, therapeutic treatment, and increased nursing care or monitoring. Furthermore, the specificity and granularity of ICD-10-CM diagnosis codes provide the opportunity for HHAs to improve their diagnosis code reporting to more accurately reflect the reason for home health services and other conditions that affect the home health plan of care. If the supporting documentation from the certifying physician or the acute/post-acute care facility is lacking specificity regarding the patient's diagnoses, the HHA would be expected to query such providers in order to adequately address the patient's home health care needs.
Because one of the variables in the PDGM case-mix adjustment is the clinical grouping, we believe that HHAs would be more comprehensive in their assessment of the patient to identify all diagnoses to determine the individualized patient care needs to be addressed through the home health plan of care. More specific and accurate diagnosis reporting to identify those conditions affecting the home health plan of care and to support the need for services is appropriate. Likewise, the home health Conditions of Participation (CoPs) at § 484.60(a), require that the home health plan of care includes all pertinent diagnoses. HHAs are required to consult the physician if there are any additions or modifications to the plan of care. Therefore, any diagnoses included on the home health plan of care would have to be agreed upon by the physician responsible for the home health plan of care. More accurate and complete reporting of diagnoses is not inappropriate if in accordance with existing regulations and standards of practice. Modification of current coding practices does not mean that HHAs are engaging in inappropriate behavior nor are the coding assumptions meant to encourage any type of negative behavior change. As noted previously, ICD-10-CM diagnosis codes are granular and specific, and provide HHAs a better opportunity to report those codes that reflect the patient's conditions and support the need for home health services. We view improved diagnosis reporting as a positive change that affords HHAs the latitude to fully “paint the picture” of their patients receiving home health services.
Comment: Many commenters stated that the behavior assumptions finalized are “faulty” with no empirical evidence to support such assumptions or that the behaviors would actually occur. Most often, commenters stated that while changes in coding behavior may occur, the degree to which this may occur and the impact of the occurrence, especially in the first year of the new payment system seems to be exaggerated by CMS. Several commenters stated that their home health agencies do not “game the system” and base patients' care plans on what patients need. These commenters believe that they should not be subjected to payment cuts based on Medicare's assumptions, which they believe to be flawed. A few commenters stated that the behavior assumptions penalize those agencies who have been providing care based on patient need and not driven by therapy utilization or other behaviors solely to maximize payment. These commenters indicated that they would not change their current care practices because of this regulation and that they were essentially being punished for doing the right thing all along. They expressed concern over how they would adjust to compensate for an 8 percent reduction in the 30-day payment rate. Other commenters recommended that CMS establish monitoring programs to target providers engaging in in specific behaviors solely for payment purposes rather than “penalize all providers.” Several comments indicated that the behavioral assumptions are a punitive action against all home health agencies based on behaviors that have not happened yet and may never happen.
Response: We disagree that the finalized behavior assumptions are without empirical evidence as we have provided multiple examples of previous changes in behavior in response to payment changes, especially as they relate to coding behavior. In the CY 2020 HH PPS proposed rule (83 FR 56456), we provided examples of such evidence. For the clinical group and comorbidity assumptions when CMS implemented revisions to the home health case-mix system in 2008, subsequent analysis found that behavioral responses unrelated to patient severity caused payments to increase by 4 percent in that year—despite having increased only 1 percent per year, on average, between 2001 and 2007. CMS continued to find nominal increases in case mix unrelated to patient severity in later years and reduced payments by an average of 1.8 percent a year from 2008 through 2017 to account for this trend. We refer commenters to the impact of the coding and comorbidity assumptions in Table 14 of this rule, which is estimated to be 6.4 percent and 0.25 percent respectively, which is similar to other past coding behavior responses described previously and which were associated with the implementation of a new home health payment system.
We also provided additional examples from other Medicare payment systems where coding behaviors led to increases in payment not necessarily related to increases in patient acuity. These include the transition from DRGs to (MS) DRGs; the first year of the IRF PPS; and Maryland's transition to APR DRGs. For the LUPA assumptions, we provided the analysis of the implementation of the HH PPS where the expected rate of LUPAs (16 percent) was much higher than the actual rate of LUPAs (7 percent), indicating that HHAs were providing extra visits to receive a full 60-day episode case-mix adjusted payment amount.
Additionally, section 1895(b)(3)(A)(iv) of the Act requires us to make assumptions about behavior changes that could occur as a result of the change to a 30-day unit of payment and implementation of the PDGM when calculating a 30-day payment amount in a budget-neutral manner. These assumptions are not to account for “gaming” of the system as commenters suggest, and we stated as such in the CY 2019 HH PPS proposed rule (83 FR 56455). We clarified that CMS often takes into account anticipated behaviors when making a payment system change. By including behavior change assumptions in the proposed calculation of the 30-day payment amount, as required by statute, we did not intend to imply that HHAs would engage in unethical behavior. Furthermore in the CY 2019 HH PPS final rule with comment period (83 FR 56455), we provided detailed explanation as to why we believe that targeted actions against specific providers who may or may not be engaging in abusive coding patterns would not be effective. Explicitly, we stated that system-wide case-mix levels have risen over time throughout the country, while patient characteristics data indicate little real change in patient severity over that same time. These widespread changes make it challenging to clearly separate agencies into high and low coding change groups. While we do not believe that our overall assumptions are exaggerated, we also recognize commenter concern over the frequency of these behaviors during the first year of the payment changes.
Finally, in the CY 2019 HH PPS final rule with comment period (83 FR 56455), we stated that the behavior assumption adjustment is not meant to be punitive, rather we are required by law to make such assumptions when calculating the 30-day budget-neutral payment amount. MedPAC comments on the CY 2020 HH PPS proposed rule support the finalized behavior assumptions and it states that even with the behavior assumption adjustment, payment would still exceed estimated costs. MedPAC went on to state that most HHAs will be able to absorb the 8.01 percent adjustment.
Comment: A few commenters asserted that such behavior assumptions are not applied to other settings, should not be applied to home care, and applying behavior assumptions absent supporting data is not sound payment policy. Specifically, these commenters mention that CMS, in issuing the Skilled Nursing Facility (SNF) model, refused to make assumptions about provider behavior, stating that it would “not make any attempt to anticipate or predict provider reactions to the implementation of the proposed [payment model].”
Response: We remind commenters that CMS is required, by statute, to make assumptions about behavior changes that could occur as a result of the implementation of the 30-day unit of payment and the PDGM when calculating the 30-day payment amount in a budget neutral manner for CY 2020. Other new payment models, such as the Patient-Driven Payment Model for skilled nursing facilities did not have such a statutory requirement. In compliance with section 1895(b)(3)(A)(iv) of the Act, we believe that we have made reasonable assumptions about what behavior changes to expect with the implementation of the new home health PPS payment structure which are based on previous experience with the HH PPS, as well as other payment systems.
Comment: A commenter stated that there is no evidence to support the clinical group coding assumption. This commenter referenced the analysis of home health improper payments in the CMS 2017 Fee-for-Service Supplemental Improper Payment Data Report [18] stating improper payments due to incorrect coding was zero dollars.
Response: We note that CMS uses the Comprehensive Error Rate Testing (CERT) Program to estimate the Medicare Fee-For-Service (FFS) improper payment rate. The purpose of the CERT Program is to identify payments that should not have been made or payments made in an incorrect amount. Under the CERT Program, the definition of “incorrect coding” in the context of the home health improper payments, relates to incorrect HIPPS codes on HH claims, meaning that medical documentation supports different coding than what was billed; that the service was performed by someone other than the billing provider; that the billed service was unbundled; and that a beneficiary was discharged to a site other than the one coded on a claim.[19] For example, an improper payment is made as a result of the HIPPS code reflecting a therapy threshold not supported by entries in the medical record. Therefore, contrary to the commenter's remark, improper home health payments resulting from incorrect coding does not relate to diagnosis codes reported, rather it relates to the reported HIPPS code on home health claims. We note that the most common type of improper payment error in home health is “insufficient documentation”. This occurs when: There is missing or inadequate medical records; there is a missing certification or recertification or some element of the certification or recertification is missing; there are missing or inadequate orders; there are inconsistent records; there is a missing or inadequate plan of care; or there are multiple universal errors. For home health, “insufficient documentation” often means that the home health certification requirements, in entirety or an element, have not been submitted. Therefore, the analysis regarding the home health improper payments is not evidence to negate the clinical coding assumption. We remind commenters that our position on the coding behavior assumption is that we assume that HHAs will improve their documentation and coding behaviors to more fully account for patient characteristics that impact resource use.
Comment: A commenter supported the comorbidity assumption and stated that prior to this proposal, there was no motivation to code all of the patient's comorbidities and that under the PDGM, HHAs will have the motivation to document all conditions that affect patient care. This commenter stated that this would be a positive change in that it gives a more complete picture of acuity for the patients being cared for by the HHA and would demonstrate that HHAs are caring for very complex, chronically ill patients and perhaps keeping these patients out of more costly care settings.
Response: We agree with this commenter that the availability to report more secondary diagnoses on the home health claim would provide home health agencies with the opportunity to more comprehensively portray all of the comorbidities affecting the home health plan of care. We believe this will benefit HHAs in terms of receiving a payment adjustment to account for the services being provided to address such comorbidities.
Comment: MedPAC noted that the proposed payment reduction of 8.01 percent appears to be consistent with past trends in coding that CMS has reported and supported the behavioral assumptions. MedPAC also commented that the proposed behavior adjustment may not represent all of the behavioral changes that could occur. Specifically, MedPAC suggested that agencies could respond to the new 30-day unit of payment by providing additional visits after an initial 30-day period to trigger an additional 30-day payment, which could result in higher aggregate payments and that CMS should reduce payments to reflect this excess.
Response: We thank MedPAC for their comments. We agree that there may be other behavior changes that could result from a new case-mix system and a change in the unit of payment, including the behavior MedPAC describes. However, we are not adding a prospective adjustment to account for this additional potential behavior change for CY 2020 as we believe that the behavior changes finalized in the CY 2019 final rule with comment period are the ones best supported based on our experience with changes to payment systems for home health and other provider types. As required by the statute, we will analyze data for CYs 2020 through 2026 to annually determine the impact of differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures. This means, we would examine all behavior changes and not just those assumed to determine their impact on overall expenditures. CMS, at a time and in a manner appropriate, is required to determine whether the 30-day payment amounts needs to be increased or decreased in response to actual observed behavior change. We interpret actual observed behavior change to encompass both behavior changes that were previously outlined, as assumed by CMS when calculating the budget-neutral 30-day payment amount for CY 2020, and other behavior changes not identified at the time the 30-day payment amount for CY 2020 is determined.
Comment: Several commenters requested CMS provide expected total aggregated budget neutral HH PPS expenditures for future years and requested to further understand how the cases dropped from PDGM would be accounted for in the budget neutrality calculations. Another commenter stated that all existing work papers on the PDGM behavior adjustment by any party within CMS, including the Office of the Actuary, should be made readily available to the public through the CMS website. These comments express significant concerns that the dropped claims violate the Jimmo vs. Sebelius settlement agreement by excluding them from the analysis and not recognizing the patient needs in PDGM. Another commenter recommends that CMS should publish for public notice and comment a full description of its behavior adjustment calculation, including all the specific data used in the assessment along with the complete calculation methodology. A commenter expressed concerns that CMS is not considering the requirements of the Regulatory Flexibility Act or the Small Business Regulatory Enforcement Fairness Act, which limits the impact on small businesses. This commenter stated that many home health agencies are considered “small business” and should be afforded targeted oversight efforts rather than apply all claims to the behavioral assumption analysis. The commenter recommended that CMS consider alternatives to the behavioral adjustment that would take into account any oversight to prevent up coding or unnecessary utilization increased to offset the behavioral adjustment.
Response: We believe that it would be difficult to accurately predict total aggregate budget neutral HH PPS expenditures for future years because we cannot anticipate future year home health rate updates, which vary from year to year. Furthermore, we cannot anticipate any future legislative action that would require a set home health rate update for any given year. As such, we do not believe that providing this type of data would produce meaningful results for providers' analytic purposes. However, with the proposed and this final rule with comment period, we released the “Home Health Claims—OASIS” Limited Data Set (LDS) file, which contains information on the utilization of the Medicare Home Health benefit on the CMS website.[20] This LDS file is meant to support HHAs in evaluating the effects of the PDGM and provides detailed information for HHAs. Therefore, we believe that we have provided sufficient publically available information for HHAs to utilize so they can fully understand the effects of the PDGM.
We remind commenters that we did provide a detailed explanation as to how we calculated the behavior adjustment in the CY 2020 proposed rule (84 FR 34615). For this final rule with comment period, we used a 2018 analytic file that included 6,388,974 60-day episodes ($18 billion in total expenditures); however 9.5 percent of claims were excluded because they could not be linked to an OASIS assessment, or were RAPs without a final claim, or they were claims with zero payment amounts. After these and other exclusions, the resulting 2018 analytic file represented 5,471,454 60-day episodes and $16.6 billion in total expenditures. We do not agree that these excluded claims would be useful for inclusion of the behavior assumption adjustment, nor do we see any relationship between standard data cleaning procedures and the Jimmo v. Sebelius settlement, which addresses Medicare coverage of certain types of maintenance therapy for certain Medicare providers, and does not reflect any behavioral analyses. Furthermore, we believe the PDGM captures patient characteristics more closely associated with complex care needs of the chronically ill as we have demonstrated in our analysis of the PDGM (and previously, the HHGM). We also disagree that this rule does not consider the requirements of the Regulatory Flexibility Act or the Small Business Regulatory Enforcement Fairness Act, which limits the impact on small businesses. In fact, we are required to consider the impact of these policies as we do in the Regulatory Impact Analysis section of the proposed and final rules. Additionally, we refer commenters to Table 36 in the CY 2020 proposed rule that shows the CY 2020 estimated HHA impacts by facility type and area of the country. Even with the 8.01 percent adjustment based on assumed behavior changes, we note that smaller providers would have an estimated impact of a +2.1 percent increase in payments as a result of the PDGM and an estimated overall impact of +3.6 percent as a result of the proposed payment policies in CY 2020. Finally, as noted throughout this rule, CMS is required to reconcile the difference between assumed and observed behavior changes; that is, we are required to examine the data beginning in CY 2020 through CY 2026 to determine the impact of the differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures to determine whether any temporary adjustments for retrospective behavior or any permanent adjustments on a prospective basis are warranted to offset such increases or decreases.
Comment: A commenter recommended that CMS should factor the impact of decreased Medicare payments due to home health agency closures as part of the budget neutrality analysis. This commenter stated that evidence exists to support that a change to a new payment system will lead to agency closures and provided the example of the change from cost reimbursement payment system to the Interim Payment System and then to the Home Health Prospective Payment System, which resulted in a 30 percent reduction in the number of home health agencies. The commenter stated that the CY 2020 PDGM Agency Level Impacts file posted with the CY 2020 proposed rule is misleading because it gives an estimated PDGM revenue that does not include the adjustment due to the behavioral assumptions.
Response: We agree with commenters that there have been notable changes in the provision of home health services since the 1980s. MedPAC has provided a detailed description of the use and growth of the home health benefit and has shown how the benefit has varied substantially because of changes in coverage and payment policy in its reports.[21] We remind commenters that implementation of the inpatient hospital PPS in 1983 led to increased use of home health services as hospital lengths of stay decreased. As a result, the number of home health agencies (HHAs), users, and services expanded rapidly in the early 1990s. As the rates of use and the duration of home health episodes increased, there was concern that the benefit was serving more as a long-term care benefit.[22] The trends of the early 1990s prompted increased program integrity actions, refinements of coverage standards, temporary spending caps through an interim payment system (IPS), and the eventual replacement of the cost-based payment system with a prospective payment system in 2000. We agree that the implementation of the IPS resulted in a decrease in the number of HHAs. However, after the HH PPS was implemented, home health service use and agency supply rebounded at a rapid pace. Between 2001 and 2017, the number of home health episodes rose from 3.9 million to 6.3 million.[23] In 2017, the number of HHAs was 11,844—higher than the level of supply during the 1990s. Almost all the new agencies since implementation of the PPS have been for-profit providers. We also note that in the CY 2014 HH PPS final rule (78 FR 72282), commenters expressed similar concerns that HHAs would be forced to close in response to the rebasing adjustment to the 60-day national, standardized episode payment amount, required by section 3131(a) of the Patient Protection and Affordable Care Act (PPACA). In the CY 2014 HH PPS final rule, we finalized a 2.8 percent reduction to the national, standardized 60-day episode payment rate in each year beginning in CY 2014 through CY 2017. However, MedPAC has reported that even with these rebasing reductions, HHAs were able to adapt and there was no evidence of large-scale HHA closures or issues with access to care. In fact, MedPAC reported that changes in average payment per full episode (defined as episodes of more than four visits) underscored the limited impact of the PPACA rebasing policy that was implemented in 2014. Average payment per episode increased in the first three years of rebasing and the average payment per episode in 2016, the third year of rebasing, was 3.1 percent higher than the average payment per episode in 2013, before rebasing was implemented.[24] Therefore, we do not believe there will be large-scale HHA closures or issues with access to care as a result of the implementation of the PDGM, given past experience of HHAs adapting to payment system changes.
While we recognize that there can be a shift in provider practice patterns in response to payment changes, we believe that the PDGM puts patient characteristics and other pertinent clinical information at the forefront in adjusting home health payments to account for increases in resource use. We believe this is an improvement over other significant, past case-mix adjustment and payment changes because of the primary focus on patient characteristics that affect resource utilization. However, we are also aware that the transition to a 30-day unit of payment and implementation of a new case-mix system, the first significant payment changes to the HH PPS in almost 20 years, warrants modifications to HHA billing practices, software systems, and staff education. As we have stated since we finalized the PDGM in the CY 2019 final rule with comment period, we will continue to monitor the provision of home health services, including any changes in the composition of the disciplines providing such services, overall home health payments, and any effects on HHAs to determine if any unintended consequences result from the change in the case-mix adjustment methodology and the 30-day unit of payment that may warrant refinements in future rulemaking.
Comment: Most commenters expressed concern about the impact of the proposed 8.01 percent reduction in payment based on assumed behavior changes that HHAs may make in response to the change in the case-mix adjustment methodology and the change to a 30-day unit of payment. Commenters stated that this reduction would be one of the most significant reductions taken in any new or existing Medicare payment systems to date and would result in negative financial consequences, especially for smaller, rural HHAs that may not be able to make the changes necessary to adapt to the PDGM immediately upon implementation.
Response: We note that the overall impact on the estimated aggregate expenditures resulting from the PDGM and the 30-day unit of payment is zero given the statutory requirement that these changes are implemented in a budget-neutral manner. We appreciate commenter concerns regarding the impact of these assumptions on smaller and rural HHAs. We refer to Table 36 in the CY 2020 HH PPS proposed rule (84 FR 34706), which shows that the impact of the PDGM and the 30-day unit of payment (with behavior assumptions) on rural providers would be 3.7 percent and the impact on smaller providers (less than 100 episodes) would be 2.1 percent. Therefore, we believe that rural and smaller HHAs would recognize an increase in overall payments under the PDGM and the 30-day unit of payment.
We also remind commenters that even with the behavior assumption adjustment of 8.389 percent, the CY 2020 30-day payment rate of $1,785.51 (including the wage index standardization factor and the CY 2020 rate update) would be approximately 11 percent higher than the estimated, CY 2020 30-day period cost of $1,608.82. Additionally, in its comments on the proposed rule, MedPAC states that the analysis of payments and costs in the proposed rule suggests that payments will be more than adequate in 2020. However, we will continue to monitor the effect of the payment changes, including the impacts on smaller and rural providers to mitigate any potential unintended consequences. Moreover, we are required to examine the data beginning in CY 2020 through CY 2026 to determine the impact of the differences between assumed behavior changes and actual behavior changes on estimated aggregate expenditures to determine whether any temporary adjustments for retrospective behavior or any permanent adjustments on a prospective basis are warranted to offset such increases or decreases.
Comment: Many commenters stated that the magnitude of the 8.01 percent reduction to the home health 30-day payment rate has the potential to create negative consequences for providers transitioning to a new case-mix adjustment methodology and a change in the unit of payment. Several commenters mentioned the provider burden associated with other existing and new requirements, including HHVBP and the resumption of the Review Choice Demonstration and stated that there are too many changes occurring simultaneously and that many HHAs, especially smaller and rural providers, could not incur the costs of all of these changes all at once. Several commenters stated they recognize the statutory requirement to make such behavior assumptions when calculating the budget-neutral 30-day payment rate, but requested that CMS phase-in the behavior assumption reduction over a period of three years, rather than all at one time. Several commenters recognize the phase-out of the rural add-on is based on the Bipartisan Budget Act of 2018 with no latitude to revise the proposal, however, they suggest CMS takes this into consideration in relation to the 8.01 behavioral adjustment. Some commenters indicate the phase-out of the rural add-on payment, coupled with other payment system changes, would be difficult for rural HHAs to fiscally manage. Other commenters stated the assumption that 100 percent of providers will change coding practices and make such changes 100 percent of the time, without sufficient data, is an overestimation and suggested that reduction percentage be halved, as this is a more realistic assumption about the frequency of such behavior changes.
Response: We appreciate commenter concerns about the potential impact of the behavior assumption adjustment. We recognize that transitioning to the first significant HH PPS payment system change in almost 20 years requires a considerable amount of system changes, staff education, and modification of current billing processes. We are also cognizant that there have been recent changes to the home health CoPs, as well as a resumption of the Review Choice Demonstration, and continuation of the HHVBP for some select states. We also understand concerns by rural HHAs as to the impact of the phase-out of the rural add-on payment coupled with other changes that may challenge their fiscal management.
We continue to believe that the behavior assumptions are valid ones and supported by evidence as described in the CY 2019 final rule with comment period and the CY 2020 proposed rule. However, given the scale of the payment system changes, we agree that it might take HHAs more time before they fully implement the behavior assumed by CMS. As we noted in response to comments in the CY 2019 HH PPS final rule with comment (83 FR 56456), in the FY 2008 IPPS final rule, CMS estimated that a total adjustment of 4.8 percent would be necessary to maintain budget neutrality for the transition to the MS-DRGs (72 FR 47178). However, examining subsequent analysis of claims data for FYs 2008 and 2009, our actuaries determined that the implementation of the MS-DRG system resulted in a 2.5 percent change in documentation and coding (about half of the estimated 4.8 percent change expected) in the first year of the MS-DRGs and a 5.4 percent change in documentation and coding in the second year of the MS-DRGs. Taking into consideration the example above and the transition to the new PDGM payment system in combination with other ongoing or new home health requirements, we believe it is reasonable to apply the three previously outlined behavior change assumptions to only half of the 30-day periods in our analytic file (randomly selected). Note that since payment is made for 30-day periods, it is more accurate to apply the behavior assumptions to half the 30-day periods than to assume the magnitude of the behaviors would be halved. Therefore, taking this approach means that the resulting adjustment to the 30-day payment amount needed to maintain budget neutrality, as required by law, is an adjustment of −4.36 percent. This means that the CY 2020 30-day budget-neutral payment amount will be $1,824.99 (not including the wage index standardization factor and the 1.5 percent home health rate update for CY 2020).
We remind commenters that after implementation of the 30-day unit of payment and the PDGM, CMS is required by law to annually analyze data from CYs 2020-2026 to determine the impact of the difference between assumed behavior changes and actual behavior changes to determine if any temporary or permanent payment adjustments to the 30-day payment amount are needed to offset for such increases or decreases in estimated aggregate expenditures. Therefore, if CMS underestimates the amount of the reductions to the 30-day payment rate necessary to offset behavior changes and maintain budget neutrality for CY 2020, larger adjustments to the 30-day payment amount would be required in the future, pursuant to section 1895(b)(3)(D) of the Act, to ensure budget neutrality with respect to estimated expenditures for CY 2020. Likewise, if CMS overestimates the reductions, we are required to make the appropriate payment adjustments accordingly, as described previously. The law also requires that any permanent or temporary payment adjustment would be proposed through rulemaking. We will review data from CY 2020 to inform next year's rulemaking to determine if any change to the behavior assumption adjustment percentage should be proposed in CY 2021 (for example, if the full 8.389 percent reduction should be proposed in CY 2021 based on actual, observed data from CY 2020). While we are applying all three assumptions for establishing a 30-day payment rate, we are changing our assumption regarding the frequency with which those behaviors would occur in the first year of implementation.
Final Decision: Based on the comments received and reconsideration as to frequency of the assumed behaviors during the first year of the transition to a new unit of payment and case-mix adjustment methodology, we are finalizing a −4.36 percent behavior change assumptions adjustment in order to calculate the 30-day payment rate in a budget-neutral manner for CY 2020. This adjustment will be made using the three behavior assumptions finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56461).
The finalized 30-day budget-neutral payment amount with the −4.36 percent behavioral assumption adjustment will be $1,824.99 and the CY 2020 30-day payment rate, with the wage-index budget neutrality factor and the home health payment update of 1.5 percent, will be $1,864.03 with a fixed-dollar loss ratio of 0.56. Section III.E. of this final rule with comment period describes the CY 2020 home health payment rate update and section III.F. describes the payments for high-cost outliers and the fixed-dollar loss ratio for the CY 2020 HH PPS.
Finally, we also wish to remind stakeholders again that CMS will provide, upon request, a Home Health Claims-OASIS LDS file to accompany the CY 2020 final rule with comment period to support HHAs in evaluating the effects of the PDGM. The Home Health Claims-OASIS LDS file can be requested by following the instructions on the CMS Limited Data Set (LDS) Files website. Additionally, we have posted the CY 2020 provider-level impacts and an updated Interactive Grouper Tool on the HHA Center web page and the PDGM web page to provide HHAs with ample tools to help them understand the impact of the PDGM and the change to a 30-day unit of payment.[25]
C. CY 2020 HH PPS Case-Mix Weights for 60-Day Episodes of Care That Span the Implementation Date of the PDGM
In the CY 2015 HH PPS final rule (79 FR 66072), we finalized a policy to annually recalibrate the HH PPS case-mix weights—adjusting the weights relative to one another—using the most current, complete data available. Annual recalibration of the HH PPS case-mix weights ensures that the case-mix weights reflect, as accurately as possible, current home health resource use and changes in utilization patterns. The CY 2020 HH PPS proposed rule (84 FR 34617), outlined the implementation of the PDGM and a change in the unit of home health payment to 30-day periods of care. As such, we are recalibrating the CY 2020 case-mix weights for 30-day periods of care using the PDGM methodology. However, these recalibrated case-mix weights are not applicable for those 60-day episodes of care that begin on or before December 31, 2019 and end on or after January 1, 2020. We did not propose to separately recalibrate the case-mix weights for those 60-day episodes that span the January 1, 2020 implementation date, rather we proposed, that these 60-day episodes would be paid the national, standardized 60-day episode payment amount and would be case-mix adjusted using the CY 2019 case-mix weights as listed in Table 6 in the CY 2019 HH PPS final rule with comment period (83 FR 56422) and posted on the HHA Center web page. With the implementation of a new case-mix adjustment methodology and a move to a 30-day unit of payment, we believe this approach will be less burdensome for HHAs as they will not have to download a new, separate 153-group case-mix weight data file, in addition to the 432 case-mix weight data file for CY 2020. For those 60-day episodes that end after January 1, 2020, but where there is a continued need for home health services, we are proposed that any subsequent periods of care would be paid the 30-day national, standardized payment amount with the appropriate CY 2020 PDGM case-mix weight applied.
We solicited comments on the proposed payment for 60-day episodes of care that span the January 1, 2020 implementation date of the PDGM and the change to a 30-day unit of payment. We received a comment from an industry association and this comment and our response is summarized in this section of this final rule with comment period.
Comment: A commenter did not agree with our proposal to not recalculate the of case-mix weights for 60-day episodes that span implementation of the PDGM and the change to a 30-day unit of payment given that the national, standardized 60-day episode payment rate is being updated for CY 2020. This commenter stated that all variables that affect payment in CY 2020 should be updated for 2020.
Response: We note that we are recalibrating the case-mix weights for 30-day periods of care beginning in CY 2020 in accordance with our policy to annually recalibrate the HH PPS case-mix weights. We note that any recalibration to the case-mix weights for those 60-day episodes that span the January 1, 2020 implementation date of the new case-mix system and the change to a 30-day unit of payment would be very similar to the CY 2019 case-mix weights. We remind commenters that we did propose to update the national, standardized 60-day episode payment amount for CY 2020, which does result in an increased base rate for these episodes of care. We continue to believe that this approach to the case-mix weights for those 60-day episodes that span into CY 2020 is less burdensome for HHAs who are transitioning to a new case-mix methodology and a 30-day unit of payment.
Final Decision: We are finalizing as proposed that 60-day episodes spanning the January 1, 2020 implementation date of the PDGM and the change to a 30-day unit of payment will be paid the CY 2020 national, standardized 60-day episode payment amount of $3,220.79 (see Table 17), and will be case-mix adjusted using the CY 2019 case-mix weights as listed in the CY 2019 HH PPS final rule with comment period (83 FR 56422) and posted on the HHA Center web page.[26] Additionally, for those 60-day episodes that end after January 1, 2020, but where there is a continued need for home health services, any subsequent periods of care will be paid the CY 2020 national, standardized 30-day period payment amount (as shown in section III.E of this final rule with comment period) with the appropriate CY 2020 PDGM case-mix weight applied.
D. CY 2020 PDGM Low-Utilization Payment Adjustment (LUPA) Thresholds and PDGM Case-Mix Weights
1. CY 2020 PDGM LUPA Thresholds
Under the current 153-group payment system, a 60-day episode with four or fewer visits is paid the national per-visit amount by discipline adjusted by the appropriate wage index based on the site of service of the beneficiary, instead of the full 60-day episode payment amount. Such payment adjustments are called Low-Utilization Payment Adjustments (LUPAs). In the current payment system, approximately 7 to 8 percent of episodes are LUPAs.
LUPAs will still be paid upon implementation of the PDGM. However, the approach to calculating the LUPA thresholds has changed due to the change in the unit of payment to 30-day periods of care from 60-day episodes. As detailed in the CY 2019 HH PPS proposed rule (83 FR 32411), there are substantially more home health periods of care with four or fewer visits in a 30-day period than in 60-day episodes; therefore, we believe that the LUPA thresholds for 30-day periods of care should be correspondingly adjusted to target approximately the same percentage of LUPA episodes as under the current HH PPS case-mix system, which is approximately 7 to 8 percent of all episodes. To target approximately the same percentage of LUPAs under the PDGM, LUPA thresholds are set at the 10th percentile value of visits or 2 visits, whichever is higher, for each payment group. This means that the LUPA threshold for each 30-day period of care varies depending on the PDGM payment group to which it is assigned. In the CY 2019 HH PPS final rule with comment period (83 FR 56492), we finalized that the LUPA thresholds for each PDGM payment group will be reevaluated every year based on the most current utilization data available at the time of rulemaking. Therefore, we used CY 2018 Medicare home health claims (as of July 31, 2019) linked to OASIS assessment data for this rule. The LUPA thresholds for the CY 2020 PDGM payment groups with the corresponding Health Insurance Prospective Payment System (HIPPS) codes and the case-mix weights are listed in Table 16. Under the PDGM, if the LUPA threshold is met, the 30-day period of care will be paid the full 30-day period payment. If a 30-day period of care does not meet the PDGM LUPA visit threshold, as detailed previously, then payment will be made using the CY 2020 per-visit payment amounts. For example, if the LUPA visit threshold is four, and a 30-day period of care has four or more visits, it is paid the full 30-day period payment amount; if the period of care has three or less visits, payment is made using the per-visit payment amounts.
2. CY 2020 PDGM Case-Mix Weights
Section 1895(b)(4)(B) of the Act requires the Secretary to establish appropriate case mix adjustment factors for home health services in a manner that explains a significant amount of the variation in cost among different units of services. As finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56502), the PDGM places patients into meaningful payment categories based on patient characteristics (principal diagnosis, functional level, comorbid conditions, admission source and timing). The PDGM case-mix methodology results in 432 unique case-mix groups called Home Health Resource Groups (HHRGs).
To generate the CY 2020 PDGM case-mix weights, we utilized a data file based on home health 30-day periods of care, as reported in CY 2018 Medicare home health claims (as of July 31, 2019) linked to OASIS assessment data to obtain patient characteristics. These data are the most current and complete data available at this time. The claims data provides visit-level data and data on whether NRS was provided during the period and the total charges of NRS. We determine the case-mix weight for each of the 432 different PDGM payment groups by regressing resource use on a series of indicator variables for each of the categories using a fixed effects model as described in the steps detailed in this section of this final rule with comment period:
Step 1: Estimate a regression model to assign a functional impairment level to each 30-day period. The regression model estimates the relationship between a 30-day period's resource use and the functional status and risk of hospitalization items included in the PDGM which are obtained from certain OASIS items. We measure resource use with the cost-per-minute + NRS approach that uses information from home health cost reports. Other variables in the regression model include the 30-day period's admission source; clinical group; and 30-day period timing. We also include home health agency level fixed effects in the regression model. After estimating the regression model using 30-day periods, we divide the coefficients that correspond to the functional status and risk of hospitalization items by 10 and round to the nearest whole number. Those rounded numbers are used to compute a functional score for each 30-day period by summing together the rounded numbers for the functional status and risk of hospitalization items that are applicable to each 30-day period. Next, each 30-day period is assigned to a functional impairment level (low, medium, or high) depending on the 30-day period's total functional score. Each clinical group has a separate set of functional thresholds used to assign 30-day periods into a low, medium or high functional impairment level. We set those thresholds so that we assign roughly a third of 30-day periods within each clinical group to each functional impairment level (low, medium, or high).
Step 2: Next, a second regression model estimates the relationship between a 30-day period's resource use and indicator variables for the presence of any of the comorbidities and comorbidity interactions that were originally examined for inclusion in the PDGM. Like the first regression model, this model also includes home health agency level fixed effects and includes control variables for each 30-day period's admission source, clinical group, timing, and functional impairment level. After we estimate the model, we assign comorbidities to the low comorbidity adjustment if any comorbidities have a coefficient that is statistically significant (p-value of .05 or less) and which have a coefficient that is larger than the 50th percentile of positive and statistically significant comorbidity coefficients. If two comorbidities in the model and their interaction term have coefficients that sum together to exceed $150 and the interaction term is statistically significant (p-value of .05 or less), we assign the two comorbidities together to the high comorbidity adjustment.
Step 3: After Step 2, each 30-day period is assigned to a clinical group, admission source category, episode timing category, functional impairment level, and comorbidity adjustment category. For each combination of those variables (which represent the 432 different payment groups that comprise the PDGM), we then calculate the 10th percentile of visits across all 30-day periods within a particular payment group. If a 30-day period's number of visits is less than the 10th percentile for their payment group, the 30-day period is classified as a Low Utilization Payment Adjustment (LUPA). If a payment group has a 10th percentile of visits that is less than two, we set the LUPA threshold for that payment group to be equal to two. That means if a 30-day period has one visit, it is classified as a LUPA and if it has two or more visits, it is not classified as a LUPA.
Step 4: Finally, we take all non-LUPA 30-day periods and regress resource use on the 30-day period's clinical group, admission source category, episode timing category, functional impairment level, and comorbidity adjustment category. The regression includes fixed effects at the level of the home health agency. After we estimate the model, the model coefficients are used to predict each 30-day period's resource use. To create the case-mix weight for each 30-day period, the predicted resource use is divided by the overall resource use of the 30-day periods used to estimate the regression.
The case-mix weight is then used to adjust the base payment rate to determine each 30-day period's payment. Table 15 shows the coefficients of the payment regression used to generate the weights, and the coefficients divided by average resource use.


Table 16 presents the HIPPS code, the LUPA threshold, and the case-mix weight for each Home Health Resource Group (HHRG) in the regression model for CY 2020.










The following is a summary of the comments received and our responses to comments on the CY 2020 PDGM LUPA Thresholds and PDGM Case-Mix Weights.
Comment: A few commenters stated that the case mix weights for clinical groups that include therapy services are significantly depressed from the weights that would be assigned if CMS continued to use BLS data. These commenters expressed concern that there is a reduction in payment rates for therapy clinical groups and this would create barriers to care for patients needing therapy. These commenters urged CMS to continue to use BLS data for determining the PDGM case-mix weights.
Response: We finalized the CPM+NRS approach to calculating the costs of care in the CY 2019 HH PPS final rule with comment period and in that rule we stated that we believe that the use of HHA Medicare cost reports better reflects changes in utilization, provider payments, and supply amongst Medicare-certified HHAs that occur over time. Under a Wage-Weighted Minutes of Care (WWMC) approach, using the BLS average hourly wage rates for the entire home health care service industry does not reflect changes in Medicare home health utilization that impact costs, such as the allocation of overhead costs when Medicare home health visit patterns change. Using data from HHA Medicare cost reports better represents the total costs incurred during a 30-day period (including, but not limited to, direct patient care contract labor, overhead, and transportation costs), while the WWMC method provides an estimate of only the labor costs (wage and fringe benefit costs) related to direct patient care from patient visits that are incurred during a 30-day period. We will recalibrate the case-mix weights annually, as is currently done, to ensure that the case-mix weights accurately align with the cost of providing care.
Comment: A commenter recognized the long-term improvement of the LUPA proposal to align low acuity episodes with a lower LUPA threshold while high-acuity episodes would have higher LUPA threshold. A few commenters stated that the LUPA thresholds are confusing and recommended a more straightforward approach to pay for LUPAs. Another commenter remarked that there were some institutional admission source LUPA thresholds that had less number of visits to meet the threshold than their community admission source counterparts and questioned if this was accurate. This commenter also stated that other institutional admission source thresholds were only one visit more than their community admission source counterpart and that this seems incorrect if institutional admission sources have higher resource costs than community admission sources.
Response: Because of the change in the unit of payment from a 60-day episode to a 30-day period, the approach to calculating the LUPA thresholds needed to change in order to target approximately the same percentage of LUPAs. As we discussed in both the CYs 2018 and 2019 HH PPS proposed rules, 30-day periods of care have substantially more episodes with four or fewer visits than 60-day episodes. To create LUPA thresholds for 30-day periods of care, we finalized in the CY 2019 final rule with comment period to set the LUPA threshold at the 10th percentile value of visits or 2, whichever is higher, for each payment group, in order to target approximately the same percentage of LUPAs (approximately 7.1 percent of 30-day periods would be LUPAs (assuming no behavior change)) (83 FR 56492). We note that under the current HH PPS, LUPA episodes are billed the same as a non-LUPA episodes and this will not change under the PDGM where LUPA periods of care will be billed the same way as non-LUPA 30-day periods of care; therefore, we do not believe that this would cause any confusion related to billing.
The commenter is correct that there are some institutional admission source LUPA thresholds that are less than their community counterparts. The LUPA threshold does not necessarily relate to the case-mix weight of the 30-day period. For example, looking at the case-mix group, Behavioral Health—Low Functional Impairment, Early Timing, Low Comorbidity Adjustment:
- Community 30-day periods have an average resource use of $1,655.70 and a LUPA threshold of 4 visits.
- Institutional 30-day periods have average resource use of $1,804.17 and a LUPA threshold of 3 visits.
We remind commenters that we finalized the policy for the PDGM LUPA thresholds to target approximately the same percentage of LUPAs as under the 153 case-mix weight system using the criteria noted previously. We continue to believe that the LUPA thresholds that vary based on the case-mix assignment for the 30-day period of care in the proposed PDGM is an improvement over the current 5 visit threshold that does not vary by case-mix assignment. Likewise, in the CY 2019 HH PPS final rule with comment period (83 FR 56492), we finalized that the LUPA thresholds for each PDGM payment group will be reevaluated every year based on the most current utilization data available.
Final Decision: We are maintaining our finalized policy in the CY 2019 HH PPS final rule with comment period (83 FR 56492) to vary the LUPA thresholds for each 30-day period of care depending on the PDGM payment group to which it is assigned. Additionally, we are finalizing the CY 2020 LUPA thresholds and case-mix weights as shown in Table 16 in this final rule with comment period. We will continue to update the LUPA thresholds by payment group and will annually recalibrate the case-mix weights using the most current data available at the time of rulemaking.
E. CY 2020 Home Health Payment Rate Updates
1. CY 2020 Home Health Market Basket Update for HHAs
Section 1895(b)(3)(B) of the Act requires that the standard prospective payment amounts for CY 2020 be increased by a factor equal to the applicable home health market basket update for those HHAs that submit quality data as required by the Secretary. In the CY 2019 HH PPS final rule with comment period (83 FR 56425), we finalized a rebasing of the home health market basket to reflect 2016 Medicare cost report (MCR) data, the latest available and complete data on the actual structure of HHA costs. As such, based on the rebased 2016-based home health market basket, we finalized that the labor-related share is 76.1 percent and the non-labor-related share is 23.9 percent. A detailed description of how we rebased the HHA market basket is available in the CY 2019 HH PPS final rule with comment period (83 FR 56425 through 56436).
Section 1895(b)(3)(B) of the Act, requires that, in CY 2015 and in subsequent calendar years, except CY 2018 (under section 411(c) of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) (Pub. L. 114-10, enacted April 16, 2015)), and except in CY 2020 (under section 53110 of the Bipartisan Budget Act of 2018 (BBA) (Pub. L. 115-123, enacted February 9, 2018)), the market basket percentage under the HHA prospective payment system, as described in section 1895(b)(3)(B) of the Act, be annually adjusted by changes in economy-wide productivity. Section 1886(b)(3)(B)(xi)(II) of the Act defines the productivity adjustment to be equal to the 10-year moving average of change in annual economy-wide private nonfarm business multifactor productivity (MFP) (as projected by the Secretary for the 10-year period ending with the applicable fiscal year, calendar year, cost reporting period, or other annual period) (the “MFP adjustment”). The Bureau of Labor Statistics (BLS) is the agency that publishes the official measure of private nonfarm business MFP. Please see http://www.bls.gov/mfp, to obtain the BLS historical published MFP data.
The home health update percentage for CY 2020 would have been based on the estimated home health market basket update, specified at section 1895(b)(3)(B)(iii) of the Act, of 2.9 percent (based on IHS Global Insight Inc.'s third-quarter 2019 forecast). However, due to the requirements specified at section 1895(b)(3)(B)(vi) of the Act prior to the enactment of the BBA of 2018, the estimated CY 2020 home health market basket update of 2.9 percent would have been reduced by a MFP adjustment, as mandated by the section 3401 of the Patient Protection and Affordable Care Act (the Affordable Care Act) (Pub. L. 111-148) and currently estimated to be 0.3 percentage point for CY 2020. In effect, the home health payment update percentage for CY 2020 would have been a 2.6 percent increase. However, section 53110 of the BBA of 2018 amended section 1895(b)(3)(B) of the Act, such that for home health payments for CY 2020, the home health payment update is required to be 1.5 percent. The MFP adjustment is not applied to the BBA of 2018 mandated 1.5 percent payment update. Section 1895(b)(3)(B)(v) of the Act requires that the home health update be decreased by 2 percentage points for those HHAs that do not submit quality data as required by the Secretary. For HHAs that do not submit the required quality data for CY 2020, the home health payment update will be −0.5 percent (1.5 percent minus 2 percentage points).
2. CY 2020 Home Health Wage Index
Sections 1895(b)(4)(A)(ii) and (b)(4)(C) of the Act require the Secretary to provide appropriate adjustments to the proportion of the payment amount under the HH PPS that account for area wage differences, using adjustment factors that reflect the relative level of wages and wage-related costs applicable to the furnishing of HH services. Since the inception of the HH PPS, we have used inpatient hospital wage data in developing a wage index to be applied to HH payments. We proposed to continue this practice for CY 2020, as we continue to believe that, in the absence of HH-specific wage data that accounts for area differences, using inpatient hospital wage data is appropriate and reasonable for the HH PPS. Specifically, we proposed to use the FY 2020 pre-floor, pre-reclassified hospital wage index as the CY 2020 wage adjustment to the labor portion of the HH PPS rates. For CY 2020, the updated wage data are for hospital cost reporting periods beginning on or after October 1, 2015, and before October 1, 2016 (FY 2016 cost report data). We apply the appropriate wage index value to the labor portion of the HH PPS rates based on the site of service for the beneficiary (defined by section 1861(m) of the Act as the beneficiary's place of residence).
To address those geographic areas in which there are no inpatient hospitals, and thus, no hospital wage data on which to base the calculation of the CY 2020 HH PPS wage index, we proposed to continue to use the same methodology discussed in the CY 2007 HH PPS final rule (71 FR 65884) to address those geographic areas in which there are no inpatient hospitals. For rural areas that do not have inpatient hospitals, we proposed to use the average wage index from all contiguous Core Based Statistical Areas (CBSAs) as a reasonable proxy. Currently, the only rural area without a hospital from which hospital wage data could be derived is Puerto Rico. However, for rural Puerto Rico, we do not apply this methodology due to the distinct economic circumstances that exist there (for example, due to the close proximity to one another of almost all of Puerto Rico's various urban and non-urban areas, this methodology would produce a wage index for rural Puerto Rico that is higher than that in half of its urban areas). Instead, we proposed to continue to use the most recent wage index previously available for that area. For urban areas without inpatient hospitals, we use the average wage index of all urban areas within the state as a reasonable proxy for the wage index for that CBSA. For CY 2020, the only urban area without inpatient hospital wage data is Hinesville, GA (CBSA 25980). The CY 2020 wage index value for Hinesville, GA is 0.8322.
On February 28, 2013, OMB issued Bulletin No. 13-01, announcing revisions to the delineations of MSAs, Micropolitan Statistical Areas, and CBSAs, and guidance on uses of the delineation of these areas. In the CY 2015 HH PPS final rule (79 FR 66085 through 66087), we adopted the OMB's new area delineations using a 1-year transition.
On August 15, 2017, OMB issued Bulletin No. 17-01 in which it announced that one Micropolitan Statistical Area, Twin Falls, Idaho, now qualifies as a Metropolitan Statistical Area. The new CBSA (46300) comprises the principal city of Twin Falls, Idaho in Jerome County, Idaho and Twin Falls County, Idaho. The CY 2020 HH PPS wage index value for CBSA 46300, Twin Falls, Idaho, will be 0.8291. The August 15, 2017 Bulletin No. 17-01, Revised Delineations of Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas, and Guidance on Uses of the Delineations of These Areas, is available at https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2017/b-17-01.pdf.
The most recent OMB Bulletin (No. 18-04) was published on September 14, 2018 and is available at https://www.whitehouse.gov/wp-content/uploads/2018/09/Bulletin-18-04.pdf.
The revisions contained in OMB Bulletin No. 18-04 have no impact on the geographic area delineations that are used to wage adjust HH PPS payments.
The CY 2020 wage index is available on the CMS Home Health Prospective Payment System Regulations and Notices web page: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/Home-Health-Prospective-Payment-System-Regulations-and-Notices.html.
We received 1 comment regarding the CY 2020 Home Health wage index. The comment and our response appear in this section of this final rule with comment period:
Comment: A commenter questioned the validity of the CY 2020 wage index data in the case of the CBSA for Albany-Schenectady-Troy, noting that in the past 6 years, this CBSA has seen its wage index reduced 5.17 percent, going from 0.8647 in 2013 to a proposed CY 2020 wage index of 0.820. This commenter also suggests that the Albany-Schenectady-Troy CBSA should not be lower than any of the following other upstate New York CBSAs: Binghamton, Elmira, Glen Falls, Rochester, Syracuse, Watertown-Fort Drum and, most significantly, the “New York Rural Areas CBSA,” which is proposed to be 0.8431.
Response: As discussed in the CY 2017 HH PPS final rule (81 FR 76721), we believe that the wage index values are reflective of the labor costs in each geographic area as they reflect the costs included on the cost reports of hospitals in those specific labor market areas. The area wage index measures differences in hospital wage rates among labor market areas and compares the area wage index of the labor market area to the national average hourly wage. If a hospital or labor market area does not keep pace with the national average hourly wage in a given year, then the labor market area will see a decrease in the area wage index during that year.
We utilize efficient means to ensure and review the accuracy of the hospital cost report data and resulting wage index. Hospitals must complete the wage index survey (Worksheet S-3, Parts II and III) as part of their Medicare cost reports. Cost reports will be rejected if Worksheet S-3 is not completed. Medicare contractors perform desk reviews on all hospitals' Worksheet S- 3 wage data, and we run edits on the wage data to further ensure the accuracy and validity of the wage data. If any provider believes the underlying hospital wage data is inaccurate, the data would have to be corrected by the Medicare Administrative Contractor (MAC) within the necessary timeframe in order for the error to be corrected; otherwise the data would be deemed final for that upcoming year's wage index. The time table used for the development of the FY 2020 hospital wage index can be found at the following link: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Downloads/FY2020-Hospital-Wage-Index-Development-Time-Table.pdf. We believe that our review processes result in an accurate reflection of the applicable wages for the areas given.
3. Comment Solicitation
Historically, we have calculated the home health wage index values using unadjusted wage index values from another provider setting. Stakeholders have frequently commented on certain aspects of the home health wage index values and their impact on payments. We solicited comments on concerns stakeholders may have regarding the wage index used to adjust home health payments and suggestions for possible updates and improvements to the geographic adjustment of home health payments.
The following is a summary of the comments received on the proposed CY 2020 home health wage index comment solicitation, and our responses:
Comment: A few commenters recommended that the wage index account for areas with higher minimum wage standards. A commenter stated that the pre-floor, pre-reclassified hospital wage index is “wholly inadequate for adjusting home health costs, particularly in states like New York which has among the nation's highest labor costs now greatly exacerbated by the states implementation of a phased in $15 per hour minimum wage hike, the balance of which is unfunded by Medicare.” Another commenter suggested that CMS develop a reimbursement system adjustment providing supplemental funding to providers, such as HHAs, required to meet higher minimum wage standards, better to align reimbursement rates with cost trends impacting these providers.
Response: Regarding minimum wage standards, we note that such increases would be reflected in future data used to create the hospital wage index to the extent that these changes to state minimum wage standards are reflected in increased wages to hospital staff.
Comment: Several commenters recommended that CMS consider consulting with home health agencies to develop a home health specific wage index or explore opportunities to improve the wage index applied to home health. A commenter urges CMS to consider a home health specific wage index to support staff retention due to increased demands on meeting paperwork and regulatory requirements. The commenter notes that the current home health wage index is tied to hospital wage data, which does not reflect the true cost of hiring and retaining high quality home health staff. Another commenter suggested that CMS use home health specific data contained in home health cost reports, which contain average cost per visit. A commenter recommended that CMS use the post-reclassified wage index values for each CBSA. Another commenter indicated that “CMS should include wage data from reclassified hospitals in calculating the rural wage index for home health agencies.” The same commenter indicated that CMS should examine how population density impacts home health agency costs and then adjust the wage index by multiplying by a population density factor so that areas with a lower population density have a higher adjusted wage index. A few commenters indicated that an approach similar to that used in the FY 2020 Inpatient Hospital PPS final rule should be used, where hospitals with a wage index value that was less than the 25th percentile had their wage index increased. A commenter also suggested that a wage index floor should be established similar to the 0.8 hospice wage index floor.
Response: We thank the commenters for their comments. We will consider these recommendations for future rulemaking.
Final Decision: After considering the comments received in response to the CY 2020 HH PPS proposed rule, we are finalizing our proposal to continue to use the pre-floor, pre-reclassified hospital inpatient wage index as the wage adjustment to the labor portion of the HH PPS rates. For CY 2020, the updated wage data are for the hospital cost reporting periods beginning on or after October 1, 2015 and before October 1, 2016 (FY 2016 cost report data). The final CY 2020 wage index is available on the CMS website at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/Home-Health-Prospective-Payment-System-Regulations-and-Notices.html.
4. CY 2020 Annual Payment Update
a. Background
The Medicare HH PPS has been in effect since October 1, 2000. As set forth in the July 3, 2000 final rule (65 FR 41128), the base unit of payment under the Medicare HH PPS was a national, standardized 60-day episode payment rate. As finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56406) and as described in section III.B of this rule, the unit of home health payment will change from a 60-day episode to a 30-day period effective for those 30-day periods beginning on or after January 1, 2020. However, the standardized 60-day payment rate will apply to case-mix adjusted episodes (that is, not LUPAs) beginning on or before December 31, 2019 and ending on or after January 1, 2020. As such, the latest date such a 60-day crossover episode could end on is February 28, 2020. Those 60-day crossover episodes that begin on or before December 31, 2019, but are LUPA episodes, will be paid the national, per-visit payment rates as shown in Table 17.
As set forth in § 484.220, we adjust the national, standardized prospective payment rates by a case-mix relative weight and a wage index value based on the site of service for the beneficiary. To provide appropriate adjustments to the proportion of the payment amount under the HH PPS to account for area wage differences, we apply the appropriate wage index value to the labor portion of the HH PPS rates. In the CY 2019 HH PPS final rule with comment period (83 FR 56435), we finalized to rebase and revise the home health market basket to reflect 2016 Medicare cost report (MCR) data, the latest available and most complete data on the actual structure of HHA costs. We also finalized a revision to the labor-related share to reflect the 2016-based home health market basket Compensation (Wages and Salaries plus Benefits) cost weight. We finalized that for CY 2019 and subsequent years, the labor-related share would be 76.1 percent and the non-labor-related share would be 23.9 percent. The following are the steps we take to compute the case-mix and wage-adjusted 60-day episode (for those episodes that span the implementation date of January 1, 2020) and 30-day period rates for CY 2020:
- Multiply the national, standardized 60-day episode rate or 30-day period rate by the applicable case-mix weight.
- Divide the case-mix adjusted amount into a labor (76.1 percent) and a non-labor portion (23.9 percent).
- Multiply the labor portion by the applicable wage index based on the site of service of the beneficiary.
- Add the wage-adjusted portion to the non-labor portion, yielding the case-mix and wage adjusted 60-day episode rate or 30-day period rate, subject to any additional applicable adjustments.
We provide annual updates of the HH PPS rate in accordance with section 1895(b)(3)(B) of the Act. Section 484.225 sets forth the specific annual percentage update methodology. In accordance with section 1895(b)(3)(B)(v) of the Act and § 484.225(i), for an HHA that does not submit HH quality data, as specified by the Secretary, the unadjusted national prospective 60-day episode rate or 30-day period rate is equal to the rate for the previous calendar year increased by the applicable HH payment update, minus 2 percentage points. Any reduction of the percentage change would apply only to the calendar year involved and would not be considered in computing the prospective payment amount for a subsequent calendar year.
Medicare pays both the national, standardized 60-day and 30-day case-mix and wage-adjusted payment amounts on a split percentage payment approach for those HHAs eligible for such payments. The split percentage payment approach includes an initial percentage payment and a final percentage payment as set forth in § 484.205(b)(1) and (2). The claim that the HHA submits for the final percentage payment determines the total payment amount for the episode or period and whether we make an applicable adjustment to the 60-day or 30-day case-mix and wage-adjusted payment amount. We refer stakeholders to section III.G. of this rule regarding proposals on changes to the current split percentage policy in CY 2020 and subsequent years. The end date of the 60-day episode or 30-day period, as reported on the claim, determines which calendar year rates Medicare will use to pay the claim.
We may also adjust the 60-day or 30-day case-mix and wage-adjusted payment based on the information submitted on the claim to reflect the following:
- A low-utilization payment adjustment (LUPA) as set forth in §§ 484.205(d)(1) and 484.230.
- A partial episode payment (PEP) adjustment as set forth in §§ 484.205(d)(2) and 484.235.
- An outlier payment as set forth in §§ 484.205(d)(3) and 484.240.
b. CY 2020 National, Standardized 60-Day Episode Payment Rate
Section 1895(b)(3)(A)(i) of the Act requires that the standard, prospective payment rate and other applicable amounts be standardized in a manner that eliminates the effects of variations in relative case-mix and area wage adjustments among different home health agencies in a budget neutral manner. To determine the CY 2020 national, standardized 60-day episode payment rate for those 60-day episodes that span the implementation date of the PDGM and the change to a 30-day unit of payment, we apply a wage index budget neutrality factor and the home health payment update percentage discussed in section III.E. of this rule. We did not propose to update the case-mix weights for the 153-group case-mix methodology in CY 2020 as outlined in section III.D. of this rule. Because we will use the CY 2019 case-mix weights, we do not apply a case-mix weight budget neutrality factor to the CY 2020 60-day episode payment rate.
To calculate the wage index budget neutrality factor, we simulated total payments for non-LUPA episodes using the final CY 2020 wage index and compared it to our simulation of total payments for non-LUPA episodes using the CY 2019 wage index. By dividing the total payments for non-LUPA episodes using the CY 2020 wage index by the total payments for non-LUPA episodes using the CY 2019 wage index, we obtain a wage index budget neutrality factor of 1.0060. We apply the wage index budget neutrality factor of 1.0060 to the calculation of the CY 2020 national, standardized 60-day episode payment rate.
Next, we update the 60-day payment rate by the CY 2020 home health payment update percentage of 1.5 percent as required by section 53110 of the BBA of 2018 and as described in section III.E.1. of this rule. The CY 2020 national, standardized 60-day episode payment rate is calculated in Table 17.

The CY 2020 national, standardized 60-day episode payment rate for an HHA that does not submit the required quality data is updated by the CY 2020 home health payment update of 1.5 percent minus 2 percentage points and is shown in Table 18.

c. CY 2020 Non-Routine Medical Supply (NRS) Payment Rates for CY 2020 60-Day Episodes of Care
All medical supplies (routine and non-routine) must be provided by the HHA while the patient is under a home health plan of care. Examples of supplies that can be considered non-routine include dressings for wound care, IV supplies, ostomy supplies, catheters, and catheter supplies. Payments for NRS are computed by multiplying the relative weight for a particular severity level by the NRS conversion factor. To determine the CY 2020 NRS conversion factor, we updated the CY 2019 NRS conversion factor ($54.20) by the CY 2020 home health payment update percentage of 1.5 percent. We did not apply a standardization factor as the NRS payment amount calculated from the conversion factor is not wage or case-mix adjusted when the final claim payment amount is computed. The NRS conversion factor for CY 2020 is shown in Table 19.

Using the CY 2020 NRS conversion factor, the payment amounts for the six severity levels are shown in Table 20.

For HHAs that do not submit the required quality data, we updated the CY 2019 NRS conversion factor ($54.20) by the CY 2020 home health payment update percentage of 1.5 percent minus 2 percentage points. To determine the CY 2020 NRS conversion factor for HHAs that do not submit the required quality data we multiplied the CY 2019 NRS conversion factor ($54.20) by the CY 2020 HH Payment Update (0.995) to determine the CY 2020 NRS conversion factor ($53.93). The CY 2020 NRS conversion factor for HHAs that do not submit quality data is shown in Table 21.

The payment amounts for the various severity levels based on the updated conversion factor for HHAs that do not submit quality data are calculated in Table 22.

In CY 2020, the NRS payment amounts apply to only those 60-day episodes that begin on or before December 31, 2019, but span the implementation of the PDGM and the 30-day unit of payment on January 1, 2020 (ending in CY 2020, on or before February 28, 2020). Under the PDGM, NRS payments are included in the 30-day base payment rate.
d. CY 2020 National, Standardized 30-Day Period Payment Amount
Section 1895(b)(3)(A)(i) of the Act requires that the standard prospective payment rate and other applicable amounts be standardized in a manner that eliminates the effects of variations in relative case-mix and area wage adjustments among different home health agencies in a budget-neutral manner. To determine the CY 2020 national, standardized 30-day period payment rate, we apply a wage index budget neutrality factor; and the home health payment update percentage discussed in section III.E. of this final rule with comment period.
To calculate the wage index budget neutrality factor, we simulated total payments for non-LUPA 30-day periods using the final CY 2020 wage index and compared it to our simulation of total payments for non-LUPA 30-day periods using the CY 2019 wage index. By dividing the total payments for non-LUPA 30-day periods using the CY 2020 wage index by the total payments for non-LUPA 30-day periods using the CY 2019 wage index, we obtain a wage index budget neutrality factor of 1.0063. We would apply the wage index budget neutrality factor of 1.0063 to the calculation of the CY 2020 national, standardized 30-day period payment rate as described in section III.B. of this rule.
We note that in past years, a case-mix budget neutrality factor was annually applied to the HH PPS base rates to account for the change between the previous year's case-mix weights and the newly recalibrated case-mix weights. Since CY 2020 is the first year of PDGM, a case-mix budget neutrality factor is not applicable. However, in future years under the PDGM, we would apply a case-mix budget neutrality factor with the annual payment update in order to account for the estimated change in aggregate payments between the previous year's PDGM case-mix weights and the recalibrated PDGM case-mix weights.
Next, we update the 30-day payment rate by the CY 2020 home health payment update percentage of 1.5 percent as required by section 53110 of the BBA of 2018 and as described in section III.E. of this final rule with comment period. The CY 2020 national, standardized 30-day period payment rate is calculated in Table 23.

The CY 2020 national, standardized 30-day episode payment rate for an HHA that does not submit the required quality data is updated by the CY 2020 home health payment update of 1.5 percent minus 2 percentage points and is shown in Table 24.

e. CY 2020 National Per-Visit Rates for Both 60-Day Episodes of Care and 30-Day Periods of Care
The national per-visit rates are used to pay LUPAs and are also used to compute imputed costs in outlier calculations. The per-visit rates are paid by type of visit or HH discipline. The six HH disciplines are as follows:
- Home health aide (HH aide).
- Medical Social Services (MSS).
- Occupational therapy (OT).
- Physical therapy (PT).
- Skilled nursing (SN).
- Speech-language pathology (SLP).
To calculate the CY 2020 national per-visit rates, we started with the CY 2019 national per-visit rates. Then we applied a wage index budget neutrality factor to ensure budget neutrality for LUPA per-visit payments. We calculated the wage index budget neutrality factor by simulating total payments for LUPA episodes using the CY 2020 wage index and comparing it to simulated total payments for LUPA episodes using the CY 2019 wage index. By dividing the total payments for LUPA episodes using the CY 2020 wage index by the total payments for LUPA episodes using the CY 2019 wage index, we obtained a wage index budget neutrality factor of 1.0066. We apply the wage index budget neutrality factor of 1.0066 in order to calculate the CY 2020 national per-visit rates.
The LUPA per-visit rates are not calculated using case-mix weights. Therefore, no case-mix weight budget neutrality factor is needed to ensure budget neutrality for LUPA payments. Lastly, the per-visit rates for each discipline are updated by the CY 2020 home health payment update percentage of 1.5 percent. The national per-visit rates are adjusted by the wage index based on the site of service of the beneficiary. The per-visit payments for LUPAs are separate from the LUPA add-on payment amount, which is paid for episodes that occur as the only episode or initial episode in a sequence of adjacent episodes. The CY 2020 national per-visit rates for HHAs that submit the required quality data are updated by the CY 2020 HH payment update percentage of 1.5 percent and are shown in Table 25.

The CY 2020 per-visit payment rates for HHAs that do not submit the required quality data are updated by the CY 2020 HH payment update percentage of 1.5 percent minus 2 percentage points and are shown in Table 26.

Final Decision: We did not receive any comments on the CY 2020 home health payment rate update for CY 2020. Therefore, we are finalizing the 60-day episode payment rates for those episodes of care that span the January 1, 2020 implementation date of the change to a 30-day unit of payment; the 30-day period payment rates for periods of care beginning on and after January 1, 2020; the CY 2020 per-visit payment rates; and the home health update percentage to update the home health payment rates for CY 2020 as proposed.
f. Rural Add-On Payments for CYs 2020 Through 2022
1. Background
Section 421(a) of the Medicare Prescription Drug Improvement and Modernization Act of 2003 (MMA) (Pub. L. 108-173) required, for HH services furnished in a rural area (as defined in section 1886(d)(2)(D) of the Act), for episodes or visits ending on or after April 1, 2004, and before April 1, 2005, that the Secretary increase the payment amount that otherwise would have been made under section 1895 of the Act for the services by 5 percent. Section 5201 of the Deficit Reduction Act of 2003 (DRA) (Pub. L. 108-171) amended section 421(a) of the MMA. The amended section 421(a) of the MMA required, for HH services furnished in a rural area (as defined in section 1886(d)(2)(D) of the Act), on or after January 1, 2006, and before January 1, 2007, that the Secretary increase the payment amount otherwise made under section 1895 of the Act for those services by 5 percent.
Section 3131(c) of the Affordable Care Act amended section 421(a) of the MMA to provide an increase of 3 percent of the payment amount otherwise made under section 1895 of the Act for HH services furnished in a rural area (as defined in section 1886(d)(2)(D) of the Act), for episodes and visits ending on or after April 1, 2010, and before January 1, 2016. Section 210 of the MACRA amended section 421(a) of the MMA to extend the rural add-on by providing an increase of 3 percent of the payment amount otherwise made under section 1895 of the Act for HH services provided in a rural area (as defined in section 1886(d)(2)(D) of the Act), for episodes and visits ending before January 1, 2018.
Section 50208(a) of the BBA of 2018 amended section 421(a) of the MMA to extend the rural add-on by providing an increase of 3 percent of the payment amount otherwise made under section 1895 of the Act for HH services provided in a rural area (as defined in section 1886(d)(2)(D) of the Act), for episodes and visits ending before January 1, 2019.
2. Rural Add-on Payments for CYs 2020 Through 2022
Section 50208(a)(1)(D) of the BBA of 2018 added a new subsection (b) to section 421 of the MMA to provide rural add-on payments for episodes or visits ending during CYs 2019 through 2022. It also mandated implementation of a new methodology for applying those payments. Unlike previous rural add-ons, which were applied to all rural areas uniformly, the extension provided varying add-on amounts depending on the rural county (or equivalent area) classification by classifying each rural county (or equivalent area) into one of three distinct categories: (1) Rural counties and equivalent areas in the highest quartile of all counties and equivalent areas based on the number of Medicare home health episodes furnished per 100 individuals who are entitled to, or enrolled for, benefits under Part A of Medicare or enrolled for benefits under part B of Medicare only, but not enrolled in a Medicare Advantage plan under part C of Medicare (the “High utilization” category); (2) rural counties and equivalent areas with a population density of 6 individuals or fewer per square mile of land area and are not included in the “High utilization” category (the “Low population density” category); and (3) rural counties and equivalent areas not in either the “High utilization” or “Low population density” categories (the “All other” category).
In the CY 2019 HH PPS final rule with comment period (83 FR 56443), CMS finalized policies for the rural add-on payments for CY 2019 through CY 2022, in accordance with section 50208 of the BBA of 2018. The CY 2019 HH PPS proposed rule (83 FR 32373) described the provisions of the rural add-on payments, the methodology for applying the new payments, and outlined how we categorized rural counties (or equivalent areas) based on claims data, the Medicare Beneficiary Summary File and Census data. The data used to categorize each county or equivalent area associated with the publication of this rule is available in the “Downloads” section of the Home Health Prospective Payment System Regulations and Notices web page. In addition, an Excel file containing the rural county or equivalent area name, their Federal Information Processing Standards (FIPS) state and county codes, and their designation into one of the three rural add-on categories is available for download on the same web page.
The HH PRICER module, located within CMS' claims processing system, will increase the final CY 2020 60-day and 30-day base payment rates described in section III.E. of this rule by the appropriate rural add-on percentage prior to applying any case-mix and wage index adjustments. The CY 2020 through 2022 rural add-on percentages outlined in law are shown in Table 27.

While we did not solicit comments on the rural add-on percentages as these are mandated by the BBA of 2018, we did receive a few comments, mainly from rural HHAs. These are summarized in this section of this final rule with comment period.
Comment: MedPAC supports CMS's proposal that recognizes high-utilization counties, low-population counties, and all other counties to apply to rural add-on to remain in effect until CY 2022. MedPAC has not found systematic issues with access to home health care in rural areas nor concerns regarding rural home health margins. Furthermore, CMS's rural add-on policy supports MedPAC's recommendation to target rural payment adjustments to areas that have access challenges.
Response: We thank MedPAC for their support.
Comment: Several commenters recognized that the phase-out of the rural add-on is based on the Bipartisan Budget Act of 2018 with no latitude to revise the proposal. However, they suggested CMS take this into consideration in relation to the 8.01 percent reduction in the standardized 30-day rate to account for behavioral adjustments. Some commenters indicate the phase-out of the rural add-on payment, coupled with other payment system changes, would be difficult for rural HHAs to fiscally manage. Commenters indicated that CMS should monitor the impact of the phase-out (and determine if counties experience demographic changes year to year) and publicly report findings. A commenter recommended continued monitoring during the PDGM post-implementation period in order to determine the impact on accessibility to care and the ability of providers to fill open staffing positions.
Response: We understand commenter concerns about a phase-out of rural add-on payments and potential effects on rural HHAs. However, because the current rural add-on policy is statutory, we have no regulatory discretion to extend it. Congress would need to change the law. Additionally, section 1895(b)(3)(A)(iv) of the Act requires that in calculating a 30-day payment amount in a budget-neutral manner, the Secretary must make assumptions about behavior changes that could occur as a result of the implementation of the 30-day unit of payment and the new case-mix adjustment methodology. We remind commenters that the overall impact of the PDGM, the 30-day unit of payment, and behavioral assumptions is zero given the statutory requirement that these changes are implemented in a budget-neutral manner. CMS will continue to monitor patient access to home health services, as well as the costs associated with providing home health care in rural versus urban areas, and the impacts due to policy changes, including the changes in rural add-on payments for CYs 2019 through 2022. We will provide the industry with periodic updates on our analysis in rulemaking and/or announcements on the HHA Center web page at: https://www.cms.gov/Center/Provider-Type/Home-Health-Agency-HHA-Center.html.
Comment: Several commenters indicated that CMS should continue to ensure beneficiaries living in rural areas have adequate access to the home health benefit. Some commenters indicated that CMS should consider providing coverage for telehealth services related to therapy.
Response: We thank commenters for their suggestions as it relates to telehealth services. Section 1895(e)(1)(A) of the Act prohibits payment for services furnished via a telecommunications system if such services substitute for in person home health services ordered as part of a plan of care certified by a physician. Thus, virtual home health visits would not qualify for payment under the home health benefit. We will continue to examine the role of telehealth under the home health benefit and will consider ways to more broadly support such technology as a part of the home health benefit when used to augment the plan of care, but not replace in-person visits.
Final Decision: Policies for the provision of rural add-on payments for CY 2019 through CY 2022 were finalized in the CY 2019 HH PPS final rule with comment period (83 FR 56443), in accordance with section 50208 of the BBA of 2018. The data used to categorize each county or equivalent area are available in the Downloads section associated with the publication of this rule at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/Home-Health-Prospective-Payment-System-Regulations-and-Notices.html. In addition, an Excel file containing the rural county or equivalent area name, their Federal Information Processing Standards (FIPS) state and county codes, and their designation into one of the three rural add-on categories is available for download. The CY 2020 through 2022 rural add-on percentages outlined in law are shown in Table 27.
We are not making any changes to the policies previously finalized in last year's rulemaking in this final rule with comment period.
g. Low-Utilization Payment Adjustment (LUPA) Add-On Factors and Partial Payment Adjustments
Currently, LUPA episodes qualify for an add-on payment when the episode is the first or only episode in a sequence of adjacent episodes. As stated in the CY 2008 HH PPS final rule, LUPA add-on payments are made because the national per-visit payment rates do not adequately account for the front-loading of costs for the first LUPA episode of care as the average visit lengths in these initial LUPAs are 16 to 18 percent higher than the average visit lengths in initial non-LUPA episodes (72 FR 49848). LUPA episodes that occur as the only episode or as an initial episode in a sequence of adjacent episodes are adjusted by applying an additional amount to the LUPA payment before adjusting for area wage differences. In the CY 2014 HH PPS final rule (78 FR 72305), we changed the methodology for calculating the LUPA add-on amount by finalizing the use of three LUPA add-on factors: 1.8451 for SN; 1.6700 for PT; and 1.6266 for SLP. We multiply the per-visit payment amount for the first SN, PT, or SLP visit in LUPA episodes that occur as the only episode or an initial episode in a sequence of adjacent episodes by the appropriate factor to determine the LUPA add-on payment amount.
In the CY 2019 HH PPS final rule with comment (83 FR 56440), we finalized our policy of continuing to multiply the per-visit payment amount for the first skilled nursing, physical therapy, or speech-language pathology visit in LUPA periods that occur as the only period of care or the initial 30-day period of care in a sequence of adjacent 30-day periods of care by the appropriate add-on factor (1.8451 for SN, 1.6700 for PT, and 1.6266 for SLP) to determine the LUPA add-on payment amount for 30-day periods of care under the PDGM. For example, using the CY 2020 per-visit payment rates for those HHAs that submit the required quality data, for LUPA periods that occur as the only period or an initial period in a sequence of adjacent periods, if the first skilled visit is SN, the payment for that visit will be $276.17 (1.8451 multiplied by $149.68), subject to area wage adjustment.
Also in the CY 2019 HH PPS final rule with comment period (83 FR 56516), we finalized our policy that the process for partial payment adjustments for 30-day periods of care will remain the same as the process for 60-day episodes. The partial episode payment (PEP) adjustment is a proportion of the period payment and is based on the span of days including the start-of-care date (for example, the date of the first billable service) through and including the last billable service date under the original plan of care before the intervening event in a home health beneficiary's care defined as a—
- Beneficiary elected transfer, or
- Discharge and return to home health that would warrant, for purposes of payment, a new OASIS assessment, physician certification of eligibility, and a new plan of care.
When a new 30-day period begins due to an intervening event, the original 30-day period will be proportionally adjusted to reflect the length of time the beneficiary remained under the agency's care prior to the intervening event. The proportional payment is the partial payment adjustment. The partial payment adjustment will be calculated by using the span of days (first billable service date through and including the last billable service date) under the original plan of care as a proportion of the 30-day period. The proportion will then be multiplied by the original case-mix and wage index to produce the 30-day payment.
Final Decision: We did not receive any comments on the LUPA add-on factors or partial payment adjustments. Therefore, as finalized in the CY 2019 final rule with comment period, we will continue to multiply the per-visit payment amount for the first skilled nursing, physical therapy, or speech-language pathology visit in LUPA periods that occur as the only period of care or the initial 30-day period of care in a sequence of adjacent 30-day periods of care by the appropriate add-on factor (1.8451 for SN, 1.6700 for PT, and 1.6266 for SLP) to determine the LUPA add-on payment amount for 30-day periods of care under the PDGM. We will also retain the current PEP policy and apply such policy to 30-day periods of care under the PDGM.
F. Payments for High-Cost Outliers Under the HH PPS
1. Background
Section 1895(b)(5) of the Act allows for the provision of an addition or adjustment to the home health payment amount otherwise made in the case of outliers because of unusual variations in the type or amount of medically necessary care. Under the HH PPS, outlier payments are made for episodes whose estimated costs exceed a threshold amount for each Home Health Resource Group (HHRG). The episode's estimated cost was established as the sum of the national wage-adjusted per-visit payment amounts delivered during the episode. The outlier threshold for each case-mix group or partial episode payment (PEP) adjustment is defined as the 60-day episode payment or PEP adjustment for that group plus a fixed-dollar loss (FDL) amount. For the purposes of the HH PPS, the FDL amount is calculated by multiplying the HH FDL ratio by a case's wage-adjusted national, standardized 60-day episode payment rate, which yields an FDL dollar amount for the case. The outlier threshold amount is the sum of the wage and case-mix adjusted PPS episode amount and wage-adjusted FDL amount. The outlier payment is defined to be a proportion of the wage-adjusted estimated cost that surpasses the wage-adjusted threshold. The proportion of additional costs over the outlier threshold amount paid as outlier payments is referred to as the loss-sharing ratio.
As we noted in the CY 2011 HH PPS final rule (75 FR 70397 through 70399), section 3131(b)(1) of the Affordable Care Act amended section 1895(b)(3)(C) of the Act to require that the Secretary reduce the HH PPS payment rates such that aggregate HH PPS payments were reduced by 5 percent. In addition, section 3131(b)(2) of the Affordable Care Act amended section 1895(b)(5) of the Act by re-designating the existing language as section 1895(b)(5)(A) of the Act and revising the language to state that the total amount of the additional payments or payment adjustments for outlier episodes could not exceed 2.5 percent of the estimated total HH PPS payments for that year. Section 3131(b)(2)(C) of the Affordable Care Act also added section 1895(b)(5)(B) of the Act, which capped outlier payments as a percent of total payments for each HHA for each year at 10 percent.
As such, beginning in CY 2011, we reduced payment rates by 5 percent and targeted up to 2.5 percent of total estimated HH PPS payments to be paid as outliers. To do so, we first returned the 2.5 percent held for the target CY 2010 outlier pool to the national, standardized 60-day episode rates, the national per visit rates, the LUPA add-on payment amount, and the NRS conversion factor for CY 2010. We then reduced the rates by 5 percent as required by section 1895(b)(3)(C) of the Act, as amended by section 3131(b)(1) of the Affordable Care Act. For CY 2011 and subsequent calendar years we targeted up to 2.5 percent of estimated total payments to be paid as outlier payments, and apply a 10 percent agency-level outlier cap.
In the CY 2017 HH PPS proposed and final rules (81 FR 43737 through 43742 and 81 FR 76702), we described our concerns regarding patterns observed in home health outlier episodes. Specifically, we noted that the methodology for calculating home health outlier payments may have created a financial incentive for providers to increase the number of visits during an episode of care in order to surpass the outlier threshold; and simultaneously created a disincentive for providers to treat medically complex beneficiaries who require fewer but longer visits. Given these concerns, in the CY 2017 HH PPS final rule (81 FR 76702), we finalized changes to the methodology used to calculate outlier payments, using a cost-per-unit approach rather than a cost-per-visit approach. This change in methodology allows for more accurate payment for outlier episodes, accounting for both the number of visits during an episode of care and also the length of the visits provided. Using this approach, we now convert the national per-visit rates into per 15-minute unit rates. These per 15-minute unit rates are used to calculate the estimated cost of an episode to determine whether the claim will receive an outlier payment and the amount of payment for an episode of care. In conjunction with our finalized policy to change to a cost-per-unit approach to estimate episode costs and determine whether an outlier episode should receive outlier payments, in the CY 2017 HH PPS final rule we also finalized the implementation of a cap on the amount of time per day that would be counted toward the estimation of an episode's costs for outlier calculation purposes (81 FR 76725). Specifically, we limit the amount of time per day (summed across the six disciplines of care) to 8 hours (32 units) per day when estimating the cost of an episode for outlier calculation purposes.
Tables 25 and 26 show the CY 2020 per-visit payment rates and we will publish the cost-per-unit amounts for CY 2020 in the rate update change request, which is issued after the publication of the CY 2020 HH PPS final rule with comment period. We note that in the CY 2017 HH PPS final rule (81 FR 76724), we stated that we did not plan to re-estimate the average minutes per visit by discipline every year. Additionally, we noted that the per-unit rates used to estimate an episode's cost will be updated by the home health update percentage each year, meaning we would start with the national per-visit amounts for the same calendar year when calculating the cost-per-unit used to determine the cost of an episode of care (81 FR 76727). We note that we will continue to monitor the visit length by discipline as more recent data become available, and we may propose to update the rates as needed in the future.
In the CY 2019 HH PPS final rule with comment period (83 FR 56521), we finalized a policy to maintain the current methodology for payment of high-cost outliers upon implementation of the PDGM beginning in CY 2020 and that we will calculate payment for high-cost outliers based upon 30-day periods of care. The calculation of the proposed fixed-dollar loss ratio for CY 2020 for both the 60-day episodes that span the implementation date, and for 30-day periods of care beginning on and after January 1, 2020 is detailed in this section.
2. Fixed Dollar Loss (FDL) Ratio for CY 2020
For a given level of outlier payments, there is a trade-off between the values selected for the FDL ratio and the loss-sharing ratio. A high FDL ratio reduces the number of episodes or periods that can receive outlier payments, but makes it possible to select a higher loss-sharing ratio, and therefore, increase outlier payments for qualifying outlier episodes or periods. Alternatively, a lower FDL ratio means that more episodes or periods can qualify for outlier payments, but outlier payments per episode or per period must then be lower.
The FDL ratio and the loss-sharing ratio must be selected so that the estimated total outlier payments do not exceed the 2.5 percent aggregate level (as required by section 1895(b)(5)(A) of the Act). Historically, we have used a value of 0.80 for the loss-sharing ratio which, we believe, preserves incentives for agencies to attempt to provide care efficiently for outlier cases. With a loss-sharing ratio of 0.80, Medicare pays 80 percent of the additional estimated costs that exceed the outlier threshold amount.
In the CY 2019 HH PPS final rule with comment period (83 FR 56439), we finalized a FDL ratio of 0.51 to pay up to, but no more than, 2.5 percent of total payments as outlier payments. For CY 2020, we did not propose to update the FDL ratio for those 60-day episodes that span the implementation date of the PDGM and the change to a 30-day unit of payment. For those 30-day periods of care in CY 2020, we proposed that the FDL ratio would need to be set at 0.63 in order for outlier payments not to exceed 2.5 percent of the total payments estimated to be made under the HH PPS. In this final rule with comment period, we updated the outlier estimates for 30-day periods of care beginning on and after January 1, 2020 using updated claims data and the final CY 2020 payment rates outlined in section III.E.4 of this final rule with comment period. Given the statutory requirement that total outlier payments not exceed 2.5 percent of the total payments estimated to be made under the HH PPS, the FDL ratio for 30-day periods of care in CY 2020 would need to be set at 0.56 for 30-day periods of care based on our simulations looking at both 60-day episodes that would span into CY 2020 and 30-day periods. We note that we updated our estimate of outlier payments as a percent of total HH PPS payments using the most current and complete year of HH PPS data (CY 2018 claims data as of July 31, 2019) and therefore, the final FDL ratio has been updated accordingly.
Final Decision: We did not receive any comments on the proposed FDL ratios for 60-day episodes of care that span the January 1, 2020 implementation date of the PDGM and the change to a 30-day unit of payment or for 30-day periods of care. Therefore, we are finalizing the FDL ratio of 0.51 for 60-day episodes and 0.56 for 30-day periods of care for CY 2020.
G. Changes to the Split-Percentage Payment Approach for HHAs in CY 2020 and Subsequent Years
In the current HH PPS, there is a split-percentage payment approach to the 60-day episode of care. The first bill, a Request for Anticipated Payment (RAP), is submitted at the beginning of the initial episode for 60 percent of the anticipated final claim payment amount. The second, final bill is submitted at the end of the 60-day episode of care for the remaining 40 percent. For all subsequent episodes for beneficiaries who receive continuous home health care, the episodes are paid at a 50/50 percentage payment split. RAP submissions are operationally significant, as the RAP establishes the beneficiary's primary HHA in the common working file (CWF) so that the claims processing system can reject claims from providers or suppliers other than the primary HHA for the services and items subject to consolidated billing. As noted previously, section 1895(b)(2)(B) of the Act, as added by section 51001(a) of the BBA of 2018, requires a change in the unit of payment from a 60 days to 30 days, effective January 1, 2020. As such, in the CY 2019 HH PPS proposed rule (83 FR 32391) and in this year's CY 2020 HH PPS proposed rule (84 FR 34598), we discussed our belief that the split percentage approach to payment may no longer be needed for HHAs to maintain adequate cash flow.
In the CY 2019 HH PPS final rule with comment period (83 FR 56628), we discussed the typical RAP fraud scenario where an HHA enrolls in Medicare and proceeds to submit a large amount of RAPs in a short timeframe, the provider never submits a final claim and then shuts down the business before CMS is able to take action. In light of the potential for this type of fraud scenario, and the move to a 30-day unit of payment where HHAs can submit the final claim after 30 days, we finalized that newly-enrolled HHAs that is HHAs certified for participation in Medicare effective on or after January 1, 2019, will not receive split-percentage payments beginning in CY 2020. HHAs that are certified for participation in Medicare effective on or after January 1, 2019, will still be required to submit a “no pay” Request for Anticipated Payment (RAP) at the beginning of a period of care in order to establish the home health period of care, as well as every 30 days thereafter. Existing HHAs, meaning those HHAs that are certified for participation in Medicare with effective dates prior to January 1, 2019, would continue to receive split-percentage payments upon implementation of the PDGM and the change to a 30-day unit of payment in CY 2020. We finalized the corresponding regulations text changes at § 484.205(g)(2), which sets forth the policy for split-percentage payments for periods of care on or after January 1, 2020.
In the CY 2020 HH PPS proposed rule (84 FR 34598), we described more recent fraud schemes with existing providers where individuals or groups with the intent of perpetuating fraud enter the program by acquiring existing HHAs which allows them to circumvent Medicare's screening and enrollment process. These individuals and groups purchase existing agencies through Changes of Ownerships (CHOWs) and Changes of Information, but fail to disclose ownership changes to CMS as required by 42 CFR 424.516(e) and 489.18 (as applicable). If CMS identifies the failure to report, it can revoke the enrollment of the HHA in the Medicare program under 42 CFR 424.535(a)(1) (or under 42 CFR 424.535(a)(9) after the FY 2020 Program Integrity Enhancements to the Provider Enrollment Process final rule with comment period (84 FR 47794) is effective on November 4, 2019). However, problematic individuals or groups that engage in the above intentional reporting failures may not always be identified and, thus, CMS may not be able to remove the bad actors from the program in all relevant cases.
A situation like this, where an individual or group acquires existing HHAs and does not appropriately disclose ownership relationships to CMS, allows the individual or groups who have acquired the HHA to evade the normal enrollment screening processes enabling them to operate as if they are an existing provider. Situations like this leave CMS blind to the potentially problematic criminal history of the acquiring individual.
In order to address program integrity vulnerabilities for situations like this, as well as those where providers enroll and flood the system with RAPs solely to collect the upfront payment and never submit a final claim, we proposed in the CY 2020 HH PPS proposed rule (84 FR 34598) to lower the upfront split percentage payment from the current 60/50 percent (depending on whether period of care is the initial or subsequent period) to 20 percent in CY 2020 for both initial and subsequent 30-day periods of care and proposed to eliminate RAPs for all providers starting in CY 2021. Also, after the sunset of the RAP policy in CY 2021, we proposed to require all HHAs to submit a one-time NOA, within 5 calendar days from the start of care date, to establish that the beneficiary is under a Medicare home health period of care and also to trigger home health consolidated billing edits required under section 1842(b)(6)(F) of the Act. Moreover, we proposed that failure to submit a timely NOA, that is not submitting the NOA within 5 calendar days from the start of care date, would result in a reduction to the 30-day Medicare payment amount. We proposed that Medicare would not pay for days of home health services from the start of care date to the NOA filing date if the NOA was submitted after the 5 calendar day deadline. Likewise, we proposed that for periods of care in which an HHA fails to submit a timely NOA, no LUPA payments would be made for days that fall within the period of care prior to the submission of the NOA. We also proposed that if an exceptional circumstance is experienced by the HHA, CMS may waive the consequences of failure to submit a timely-filed NOA. Lastly, we proposed corresponding regulation text changes at § 484.205.
The following is a summary of the public comments received on the “Split Percentage Payment Approach for a 30-day Unit of Payment” and the “Notice of Admission” proposals and our responses:
Comment: Most commenters did not support the phase-out of the split percentage payment and suggested that CMS not change its current policy. Other commenters stated that CMS was implementing too many policy changes at once and requested additional time for implementation. Some commenters remarked that RAPs should continue under the PDGM to ensure there is no disruption in cash flow for providers as that would be harmful to their business. Other commenters stated that a split percentage payment phase-out should be postponed for HHAs in states that require Review Choice Demonstration (RCD) participation. There was also some commenter support to phase-out the split percentage payment over a multi-year period, starting at least one year after the implementation of the PDGM, in order to allow agencies of various sizes and geographical designations to appropriately adapt to PDGM.
Response: We continue to believe that as a result of the change in the unit of payment from a 60-day episode of care to a 30-day period of care, that a split percentage approach to payment may not be needed for HHAs to maintain an adequate cash flow. With monthly billing, HHAs have the ability to receive ongoing cash flow which we believe would mitigate concerns over having adequate funds for the provision of care, no matter the size or geographical designation of the HHA. We note that for the first year of the PDGM in CY 2020, providers will still receive a RAP payment of 20 percent which should help transition existing providers to the new payment system. We also believe that the eventual phase-out of RAPs will significantly streamline claims processing for HHAs as they would not be submitting a RAP for each 30-day period of care and instead would submit a one-time NOA. Also, HHAs have capitalization requirements which requires the agency to have available sufficient funds at the time of applying for enrollment in Medicare, at all times during the enrollment process, and during the 3-month period following the conveyance of Medicare billing privileges to the HHA. A multi-year phase-out approach, which some commenters suggest, would not help streamline claims processing for providers nor would it address the ongoing program integrity issues that we have discussed in the CY 2019 HH PPS proposed and final rules (83 FR 32391 and 83 FR 56462, respectively) and in this year's CY 2020 HH PPS proposed rule (84 FR 34638). A multi-year approach would just continue to subject the Medicare Trust Fund to additional fraud schemes in relation to the submission of RAPs. However, we will continue to monitor HHA adaptation for the split percentage phase-out with the implementation of the PDGM, and may decide whether additional adjustments are necessary in future rulemaking if an access to care issue arises.
Comment: Many commenters had concerns that CMS was modifying its RAP policy due to abuse by certain agencies. Commenters suggested that CMS should utilize their ability to restrict RAPs for agencies that abuse it instead of modifying the current RAP policy. Other commenters stated that because CMS recoups the majority of RAP overpayments, RAP policy changes were unneeded. Some commenters indicated that not all cases where a final claim is not submitted after a RAP are abusive and that CMS should address actual abuse using tools such as post payment review and audits. Commenters encouraged CMS to identify the agencies that are abusing the system and to impose more oversight through accrediting organizations and the MACs. A commenter raised their concern that removal of RAPs would increase incidents of “cherry picking.”
Response: While one of the reasons for the elimination of RAPs is to potentially stem program integrity vulnerabilities, it is not the sole reason. We remind commenters that the current median length of days for RAP submission is 12 days from the start of the 60-day episode of care. With a change in the unit of payment to a 30-day period of care, if this median length of days for RAP submissions remains constant, there is the possibility that HHAs could be simultaneously submitting a RAP and a final claim for each 30-day period of care. We believe that this defeats the purpose of the RAP to maintain adequate cash flow and only increases complexity for HHAs in their claims processing. With monthly billing, HHAs have the ability to receive an ongoing cash flow which we believe would mitigate concerns over having adequate funds for the provision of care.
CMS's use of post payment audit and review as a means to address abuse is not an appropriate intervention to prevent fraudulent or improper behavior because these are “pay and chase” solutions to a problem that demands preventive action. Post payment review and other auditing approaches are not always cost effective and as described in the proposed rule, they, by definition, are susceptible to significant program integrity abuses. We are moving beyond the pay and chase approach to program integrity structural changes wherever possible for all provider settings. To base our approach to home health program integrity on a pay and chase framework simply does not achieve the protections we need to have in place. Post payment audits and other post payment recoupment processes are not an acceptable modern technological solution for ensuring proper payment in the home health environment.
We acknowledge and appreciate the concerns commenters have raised with regards to abuse of the RAP policy by certain HHAs. We plan to continue to closely monitor RAP submissions, service utilization, payment, and quality trends which may change as a result of implementing of the PDGM and a 30-day unit of payment. If changes in practice and/or coding patterns or RAPs submissions arise, we may take further action, which may include administrative action against providers as appropriate and/or proposing changes in policy. We will also continue to work with the HHS Office of Inspector General as cases of potential provider fraud and abuse are identified.
Comment: A commenter requests CMS to clarify or identify the responsible party in a change of ownership (CHOW) when the RAP is eliminated. Another commenter stated their belief that agencies submitting RAPs would not have a limitless supply of cash and provided questions that, when answered, would pierce corporate protections and allow for civil prosecution.
Response: A change in ownership of a HHA does not change the RAP requirements. All home health agencies, including those that have undergone a change in ownership, will be subject to the elimination of RAPs when it occurs in CY 2022. Also, we believe that the new RAP policy does nothing to change any corporate protections or the rules regarding civil prosecution that exist currently.
The need for regulatory change to phase-out RAPs for existing providers is well supported by the spike in RAP fraud schemes perpetrated by existing providers. As discussed in the CY 2020 HH PPS proposed rule (84 FR 34598), the following are examples of HHAs that were identified for billing large amounts of RAPs after a CHOW, or the acquisition of an existing agency, from 2014 to the present.
Example 1: One prior investigation illustrates an individual intent on perpetrating the HH RAP fraud who took advantage of the acquisition of an existing agency. The investigation was initiated based on a lead generated by the Fraud Prevention System (FPS). Per the Provider Enrollment, Chain and Ownership System (PECOS), the provider had an effective date that was followed by a CHOW. The investigation was aided by a whistleblower coming forward who stated that the new owners of the agency completed the transaction with the intent to submit large quantities of fraudulent claims with the expressed purpose of receiving inappropriate payment from Medicare. Notwithstanding the quick actions taken to prevent further inappropriate payments, the fraud scheme resulted in improper payments of RAPs and final claims in the amount of $1.3 million.
Example 2: One investigation involved a HHA located in Michigan that submitted home health claims for beneficiaries located in California and Florida. Further analysis found that, after a CHOW, the HHA submitted RAPs with no final claims. CMS discovered that the address of record for the HHA was vacant for an extended period of time. In addition, we determined that although the HHA had continued billing and receiving payments for RAP claims, it had not submitted a final claim in 10 months. Ultimately, the HHA submitted a total of $50,234,430 in RAP claims and received $37,204,558 in RAP payments.
Example 3: A HHA submitted a significant spike in the number of RAPs following an ownership change. The investigation identified that in the period following the CHOW there were RAP payments totaling $12 million and thousands of RAPs that were submitted for which apparently no services were rendered.
Example 4: An Illinois HHA was identified through analysis of CHOW information. Three months after, the HHA had a CHOW, and the provider subsequently submitted a spike in RAP suppressions. All payments to the provider were suspended. Notwithstanding, the provider was paid $3.6 million in RAPs.
Although CMS has attempted to address these vulnerabilities through extensive monitoring, audits and investigations, there continue to be cases of individual HHAs causing large RAP fraud losses. Recently, a September 27, 2019 DOJ press release highlighted a number of charges brought against individuals involved in certain health care fraud schemes: https://www.justice.gov/opa/pr/midwest-health-care-fraud-law-enforcement-action-results-charges-against-53-individuals. We consider these fraudulent improper payments a significant vulnerability to the Medicare Trust Funds. We continue to believe that we need proactive interventions and approaches to prevent these kinds of events from happening, and that the financial impact to HHAs will be minimal under the change from a 60-day to 30-day episode of care. Likewise, we believe that the RAP phase-out and eventual elimination of split-percentage payments would serve to mitigate potential fraud schemes while minimally impacting HHAs due to the switch to a 30-day unit of payment.
Comment: A few commenters expressed support for the NOA and recognized that the NOA would be necessary to alert the claims processing system of a home health period of care due to the required consolidated billing requirements. Other commenters stated that the use of a NOA would place burden on HHAs in the form of additional paperwork/coordination, and that the NOA requirements were excessive and CMS should consider not requiring HHAs to complete the OASIS or acquiring a signed plan of care before accepting the NOA. Some commenters indicated that the only information that should be required to submit the NOA are items like the “beneficiary's name and a start of care date” and/or a verbal order to begin care. A commenter suggested that the NOA be optional in CY 2021 and mandatory in CY 2022.
Response: We thank those commenters for their support and recognition of the need for a NOA. Specifically, we agree that having a one-time submission of a NOA within 5 calendar days of the start of care, establishing that the beneficiary is under a Medicare home health period of care, will cut down on claims denials, help trigger consolidated billing edits sooner and may streamline claims processing for HHAs. The NOA also provides other HHAs the capability to determine if a beneficiary is already under a Medicare home health period of care; thereby, reduces the administrative burden associated with determining a beneficiary's period of care, reimbursement cancelations, and general beneficiary coordination issues. After reviewing all of the comments received regarding the information needed to submit the NOA, we agree with commenters that since the NOA does not have a payment tied to its submission, the requirements to fulfill the NOA should not mirror the requirements associated with the submission of a RAP. As such, we agree with commenters that the NOA submission criteria should require only the necessary information needed to begin Medicare home health services for the beneficiary. Therefore, the only information we will require for the NOA, starting in CY 2022, will be: (1) A written or verbal order from the physician (containing the services required for the initial visit) signed and dated by the physician, and if verbal, signed and dated by the registered nurse or qualified therapist (as defined in § 484.115) responsible for furnishing or supervising the ordered service in the plan of care signed by the physician; and (2) for the HHA to conduct the initial start of care visit. We believe these requirements represent the minimum amount of information that is sufficient for establishing a home health period of care and is information that the home health agency would already have as part of the medical record for beneficiaries admitted to home health.
Comment: Some commenters requested that CMS consider adopting a simple mechanism for timely notification, such as requiring HHAs to make notations in the CWF or through the EDI. Other commenters stated that submitting a NOA within 5 calendar days from the start of care is problematic and that many HHAs would be unable to meet that short timeframe. Instead of the 5 calendar day timely filing requirement, some commenters suggested lengthening the timeframe to 10-14 calendar days to submit a NOA. Other commenters recommended that CMS postpone the NOA requirements until CY 2022 or later, to allow HHAs time to adjust to the new PDGM 30-day unit of payment.
Response: There is currently no mechanism that would allow providers the ability to make any kind of notation in the CWF. Even if the creation of such a mechanism was feasible, the program integrity concerns of allowing providers to make their own notations in CWF would be exchanging one program integrity vulnerability (the upfront RAP payments) for another (allowing providers to make their own notations in the CWF). A NOA is needed to identify the initial home health period of care for each beneficiary after the elimination of RAPs. Failure to provide such notification, (which triggers the home health consolidated billing edits and establishes the home health period of care in the CWF), could lead to an increase in claims denials. Moreover, not having an NOA potentially could result in an increase in appeals and an increase in situations where other providers, including other HHAs, would not have easily accessible information on whether a patient was already being treated by another provider. As we envision it, the home health NOA process would be operationalized through an EDI submission, similar to that used for submission of the hospice Notice of Election (NOE). The purpose of an EDI submission, for NOEs for hospice or NOAs for home health, is to minimize data entry errors. Because there is already a Medicare claims processing notification, for benefit admission, in place, we believe that this should make the home health NOA process more consistent and timely for HHAs. Additionally, the use of a one-time NOA would streamline HHAs claims processing as the need for submitting a RAP for every period of care would be eliminated. The HHA would only be submitting the NOA once at the start of care which would minimize provider administrative burden for each beneficiary whom the HHA provides home health services.
Concerning the 5 calendar day timely-filing requirement, CMS considered different time frames for the submission of the one-time NOA, including a 7 calendar day timeframe in which to submit a timely-filed NOA. However, to be consistent with similar requirements in other settings (for example, in hospice where the NOE must be submitted within 5 calendar days), we believe the 5 calendar day timely-filing requirement would ensure that the Medicare claims processing system is alerted as soon as possible to mitigate any potential claims denials of other providers for services that should be covered under the home health benefit. Furthermore, the longer the NOA submission timeframe, the higher the uncertainty for providers to determine home health periods of care for a beneficiary. Having a policy for submitting a NOA within 5 calendar days, when compared to the commenter suggested 10-14 calendar days, will create an environment where there is less confusion and administrative burden for HHAs, when determining home health periods of care. After reviewing comments, we have decided to limit the requirements to submit the NOA to only require a verbal order from the physician (containing the services required for the initial visit) signed and dated by the registered nurse or qualified therapist (as defined in § 484.115) responsible for furnishing or supervising the ordered service in the plan of care signed by the physician, and that the HHA conduct the start of care visit. Also, in response to comments received, as well as CMS operational issues, we will delay the implementation of the NOA requirement until CY 2022, and instead will require that HHAs submit a “no-pay” RAP for CY 2021. However, for CY 2021, HHAs would be required to submit the “no-pay” RAP within five calendar days after the start of each 30-day period of care as this would have been the requirement for the NOA, if the NOA requirement would have been finalized for 2021. Furthermore, in alignment with the proposed NOA process, we will also apply a reduction to home health payment if the “no-pay” RAP is not submitted timely. That is, there will be a non-timely submission reduction in payment amount tied to late submission of any “no-pay” RAPs when the HHA does not submit the RAP within 5 calendar days from the start of care date for the first 30-day period of care in a 60-day certification period and within 5 calendar days of day 31 for the second 30-day period of care in the 60-day certification period. This reduction in payment amount would be calculated the same way as the NOA non-timely filing policy where the reduction in payment amount would be equal to a 1/30th reduction to the wage-adjusted 30-day period payment amount for each day from the home health start of care date until the date the HHA submits the “no-pay” RAP. We are adopting such changes under a “good cause” waiver of proposed rulemaking (see section VII. of this final rule with comment period).
Comment: A number of commenters opposed CMS' proposal to impose a financial penalty on HHAs for failing to submit a timely NOA and instead recommended that CMS consider making the notice of admission a survey requirement in the future. A commenter strongly urged that the NOA submission component be thoroughly vetted with input from providers, EHR vendors, MACs; and another recommended that CMS provide education to assist home health providers with appropriately adapting to all changes.
Response: Currently the RAP establishes an HHA as the primary HHA for the beneficiary during that timeframe and also alerts the claims processing system that a beneficiary is under a home health episode and triggers the consolidated billing edits required by law under section 1842(b)(6)(F) of the Act. Also, under the current structure of the RAP, providers receive an upfront split-percentage payment upon submission of the RAP, providing an incentive for submitting the RAP as early as possible, which also ensures the triggering of the consolidated billing edits. Without a potential payment impact associated with the submission of a NOA, the HHA could submit the NOA when they submit their final claim, which would delay turning on the consolidated billing edits, thus having an adverse effect on other providers providing services to a beneficiary that were likely unaware that the beneficiary was already under a home health episode of care. Therefore, we believe that having a penalty or a reduction in the payment amount for NOAs submitted after the 5 calendar day timely filing requirement is appropriate to aid in expediting the submission of the NOA, triggering consolidated billing edits as soon as possible and reducing claim rejections for other providers who are providing care for a beneficiary who is already under a home health episode. Additionally, our proposal to assess a financial reduction in payment amount for late NOA submission is in alignment with current hospice policy for timely submission of the hospice Notice of Election (NOE). Hospices are paid a bundled per diem payment amount for each day a beneficiary is under a hospice election. If the hospice NOE is not submitted timely (that is, within five calendar dates of the date of election), Medicare will not cover and pay for the days of hospice care from the hospice admission date to the date the NOE is submitted to the Medicare contractor. We have found the reduction in payment amount for failure to submit an NOE to be an effective tool in ensuring timely NOE submission and believe it would be appropriate to apply a similar policy to home health. As proposed in the CY 2020 HH PPS proposed rule (84 FR 34640), if an HHA failed to submit a timely NOA, the reduction in payment amount would be equal to a 1/30th reduction to the wage-adjusted 30-day period payment amount for each day from the home health start of care date until the date the HHA submitted the NOA. For example, if an HHA submits their NOA one day late (with an NOA submission 6 days after the start of care), the result would be a 20 percent reduction to the 30-day payment amount. Also, if an HHA submits their NOA 25 days late (with an NOA submission 30 days after the start of care), there would be a 100 percent reduction to the payment The reduction in payment amount (R) to the full 30-day period payment amount would be calculated as follows:
- Step 1: The number of calendar days (d) from the start of care until the NOA is submitted divided by 30 days;
- Step 2: The fraction from step 1 is multiplied by the case-mix and wage adjusted 30-day period payment amount (P).
The formula for the reduction in payment amount would be R = (d/30) × P.
We proposed that there would be no NOA reduction in payment amount if the NOA is submitted timely (that is, within the first 5 calendar days starting with the start of care date). Likewise, for periods of care in which an HHA fails to submit a timely NOA, no LUPA payments would be made for days that fall within the period of care prior to the submission of the NOA. We stated that these days would be a provider liability, the payment reduction could not exceed the total payment of the claim, and that the provider may not bill the beneficiary for these days. Once the NOA is received, all claims for both initial and subsequent episodes of care would compare the receipt date of the NOA to the HH period of care start date to determine whether a late NOA reduction applies. This will be an automated process performed by the claims processing system.
We disagree with the commenters' suggestion to make the NOA a survey requirement as the NOA, like the current RAP, serves to identify that the beneficiary is under a home health period of care and trigger consolidated billing edits and to establish the home health period of care in the Medicare claims processing system. Survey requirements are to ensure health and safety standards in accordance with the home health CoPs; whereas, the NOA serves a claims processing function for payment. Therefore, we believe tying the NOA timely submission requirement to payment is appropriate to mitigate any potential denial/recoupment issues that might occur if other providers file claims for providing services to a beneficiary under a home health period of care before a NOA is submitted.
In the CY 2019 HH PPS proposed rule (83 FR 32390), as well as in this year's CY 2020 HH PPS proposed rule (84 FR 34639), we solicited for comments on the need for HHAs to submit an NOA within 5 calendar days from the start of care to capture that HHA as the primary agency for the beneficiary during their home health episode of care. The comments we received from both the CY 2019 and 2020 HH PPS proposed rules aided in the development of our final NOA policy. We appreciate the careful review of the NOA policy and the feedback we received. Given that the NOA process will be new for HHAs, we will provide education and develop materials for guidance on the NOA policy, including MLN Matters® articles and manual guidance.
Comment: A commenter stated their concerns regarding the how the NOA policy would apply in situations where beneficiaries have a Medicare Advantage Plan but changes coverage to traditional Medicare during open enrollment or when the patient qualifies for a special enrollment while receiving home health services under an existing plan of care.
Response: In this scenario, the HHA would likely fall into one of the established timely filing exceptions for NOAs. To pursue this potential exception, the HHA would file for an exception with their MAC to request a waiver of the timely filing requirement associated with submitting the NOA. If the MAC determines that the circumstance meets the criteria for an exception, the HHA would receive the full 30-day payment amount despite filing the NOA more than 5 calendar days after the start of care.
Comment: Commenters expressed concern regarding all of the changes occurring in CY 2020 with implementation of the PDGM and transitioning to a 30-day unit of payment and these commenters stated HHAs will not have sufficient time to make additional changes to their software systems and business processes to accommodate a NOA process in CY 2021. Commenters questioned whether the Medicare claims processing system would be ready for a NOA process in CY 2021 and cited past issues with the hospice NOE process.
Response: We appreciate commenter concerns about instituting a NOA process in CY 2021 after having to make other system changes to accommodate the PDGM and a 30-day unit of payment in CY 2020. Likewise, we recognize operational issues with the Medicare claims processing system that may make a CY 2021 implementation date overly ambitious. Specifically, because of the way the current claims processing system is developed, any final claim submitted for payment must reconcile to a RAP or the claim will be denied. Because of the changes that would be required to perform this function, we are not able to do a redesign of the claims processing system so that a final claim is processed without matching it to a RAP in time for CY 2021 implementation. Therefore, we will delay implementation of a NOA process until CY 2022 in order to redesign the claims processing system to ensure accurate final claim/RAP matching.
We also agree that we want the home health NOA process to implement in a way where submission errors are minimized. The intent of a NOA process is not to be punitive to providers and we believe that delaying implementation of a NOA process until CY 2022 will allow sufficient time for both HHA and Medicare systems to be modified to accommodate submission of the NOA while mitigating any unintended consequences.
Final Decision: We are finalizing the following policies as they relate to split-percentage payments, Requests for Anticipated Payment (RAPs), and submission of a Notice of Admission (NOA):
For CY 2020:
We are finalizing the proposal to decrease the upfront split-percentage payment for 30-day periods of care beginning on and after January 1, 2020 from 60/50 percent (depending on whether the period of care is the initial or subsequent period) to 20 percent for each 30-day period, for existing HHAs, meaning HHAs certified for participation in Medicare effective on or before December 31, 2018. We remind commenters that in the CY 2019 HH PPS final rule with comment period (83 FR 56463), we finalized a policy that newly-enrolled HHAs (that is, those HHAs certified for participation in Medicare on or after January 1, 2019) will not receive split-percentage payments for periods of care beginning on or after January 1, 2020 and are required to submit a “no-pay” RAP for each 30-day period of care.
For CY 2021:
We are finalizing to lower the split-percentage payment to zero for all HHAs (that is, existing HHAs as well as newly-enrolled HHAs who receive no split-percentage payments in CY 2020) and for all 30-day periods of care beginning on or after January 1, 2021. For CY 2021, all HHAs will submit a “no-pay” RAP at the beginning of each 30-day period to allow the beneficiary to be claimed in the CWF and also to trigger the consolidated billing edits. This means that existing HHAs (those certified for participation in Medicare on or before December 31, 2018) will have their initial split-percentage payment reduced from 20 percent in CY 2020 to zero percent in CY 2021 for all 30-day periods of care and will submit a “no-pay” RAP for all 30-day periods of care in CY 2021. Newly enrolled HHAs (those certified for participation in Medicare on or after January 1, 2019) will continue to submit “no-pay” RAPs at the beginning of a 30-day period of care in order to establish the home health period of care, as well as every 30 days thereafter in CY 2021. Therefore, in CY 2021 all HHAs (both existing and newly-enrolled HHAs) will submit a “no pay” RAP until RAP elimination and the implementation of the one-time NOA policy in CY 2022.
However, the “no-pay” RAP for all HHAs in CY 2021 will require less information before the RAP can be submitted. Since we are removing the upfront payment associated with the RAP, we are relaxing the required information needed to submit the “no-pay” RAP. Starting in CY 2021, we are finalizing a policy that the information needed to submit a “no-pay” RAP will mirror the NOA policy we are finalizing in this rule. Specifically, we are finalizing a policy that submission of “no-pay” RAPs can be made when the following criteria have been met:
(1) The appropriate physician's written or verbal order that sets out the services required for the initial visit has been received and documented as required at §§ 484.60(b) and 409.43(d);
(2) The initial visit within the 60-day certification period must have been made and the individual admitted to home health care.
We are also finalizing a provision which will allow the advance submission of certain RAPs in CY 2021 such that in instances where the plan of care dictates that multiple 30-day periods of care will be required to effectively treat the beneficiary, we will allow the HHA to submit both the RAP for the first 30-day period of care and the RAP for the second 30-day period of care (for a 60-day certification) at the same time to help further reduce provider administrative burden. Additionally, for CY 2021, we are finalizing a policy where there will be a non-timely submission reduction in payment amount tied to late submission of any “no-pay” RAPs when the HHA does not submit the RAP within 5 calendar days from the start of care date for the first 30-day period of care in a 60-day certification period and within 5 calendar days of day 31 for the second 30-day period of care in the 60-day certification period. This reduction in payment amount would be calculated the same way as the NOA non-timely filing policy where the reduction in payment amount would be equal to a 1/30th reduction to the wage-adjusted 30-day period payment amount for each day from the home health start of care date until the date the HHA submits the “no-pay” RAP. We are also finalizing exceptions to the timely filing consequences of the RAP requirements. The RAP timely-filing policies are in alignment with the substance of the timely-filing NOA provisions proposed in the CY 2020 proposed rule (84 FR 34639).
For CY 2022:
Starting in CY 2022, we are finalizing that submission of RAPs will be eliminated and instead we are finalizing the implementation of a one-time NOA submission policy for all HHAs. We are finalizing a policy that all HHAs must submit a NOA to their Medicare contractor within 5 calendar days from the start of care date. The NOA is a one-time submission to establish the home health period of care and covers contiguous 30-day periods of care until the individual is discharged from Medicare home health services. We are also finalizing that NOA submission criteria will require HHAs having a verbal or written order from the physician that contains the services required for the initial visit, and that the HHA has conducted an initial visit at the start of care. We are finalizing that there will be a non-timely submission reduction in payment amount tied to any late submission of NOAs when the HHA does not submit the NOA within 5 calendar days from the start of care. That is, if an HHA failed to submit a timely NOA, the reduction in payment amount would be equal to a 1/30th reduction to the wage-adjusted 30-day period payment amount for each day from the home health start of care date until the date the HHA submitted the NOA. We are also finalizing exceptions to the timely filing consequences of the NOA requirements. Moreover, we are finalizing the corresponding regulation text changes at § 484.205 to effectuate these split-percentage payment, RAP and NOA policies.
Finally, as we noted in the CY 2020 HH PPS proposed rule, after publication of the CY 2019 HH PPS final rule with comment period, we note that there was an error in titling of the regulations text changes associated with § 484.205(g)(2) when the CY 2019 HH PPS final rule with comment period went to the Federal Register. Specifically, paragraph (g)(2)(iii) was incorrectly titled “Split percentage payments on or after January 1, 2019”. The title of this paragraph implies that split percentage payments are not made to newly-enrolled HHAs beginning on or after January 1, 2019, which is contradictory to the finalized policy on split percentage-payments for newly enrolled HHAs. We finalized a policy in the CY 2019 final rule with comment period that newly-enrolled HHAs will not receive split-percentage payments beginning in CY 2020. As such, in the CY 2020 proposed rule, we proposed to make a correction to the regulations text title to accurately reflect the finalized policy that newly-enrolled HHAs will not receive split-percentage payments beginning in CY 2020. We did not receive any comments on this proposed change. However, because of proposed revisions to split-percentage payments in the CY 2020 proposed rule, the finalized revised title correction, previously at paragraph (g)(2)(iii), has been redesignated to § 484.205(g)(2)(ii). The full revisions to the text at § 484.205 are found in the regulations text section of this final rule with comment period. We are adopting both the revised title change from the CY 2019 HH PPS final rule with comment period and the finalized changes in this final rule with comment period under a “good cause” waiver of proposed rulemaking as the final policy mirrors that of the proposed NOA policy.
We note that the regulation at § 484.205(g)(2)(ii), as it relates to split percentage payments for newly-enrolled HHAs under the HH PPS beginning in CY 2020, is separate from the placement of new HHAs into a provisional period of enhanced oversight under the authority of section 6401(a)(3) of the Affordable Care Act, which amended section 1866(j)(3) of the Act. The provisional period of enhanced oversight became effective in February 2019. More information regarding the provisional period of enhanced oversight can be found in the February 15, 2019 MLN Matters article: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/SE19005.pdf.
H. Regulatory Change To Allow Therapist Assistants To Perform Maintenance Therapy
In the CY 2020 HH PPS proposed rule (84 FR 34640) we recognized that, while a therapist assistant is able to perform restorative therapy under the Medicare home health benefit, the regulations at § 409.44(c)(2)(iii)(C) state that only a qualified therapist, and not an assistant, can perform maintenance therapy. We explained that although Medicare allows for skilled maintenance therapy in a SNF and other outpatient settings, the type of clinician that can provide the therapy services varies by setting. In some settings both the therapist and the therapist assistant can deliver the skilled maintenance therapy services, and in other settings, only the therapist can deliver the skilled maintenance therapy services. For example, Medicare regulations allow therapist assistants to provide maintenance therapy in a SNF, but not in the home health setting. We noted that commenters on the CY 2019 Physician Fee Schedule final rule (83 FR 59654) expressed concerns about shortages of therapists. That rule also finalized payment for outpatient therapy services for which payment is made for services that are furnished by a therapist assistant.
Therefore, we stated that we believe it would be appropriate to allow therapist assistants to perform maintenance therapy services under a maintenance program established by a qualified therapist under the home health benefit, if acting within the therapy scope of practice defined by state licensure laws. We clarified that the qualified therapist would still be responsible for the initial assessment; plan of care; maintenance program development and modifications; and reassessment every 30 days, in addition to supervising the services provided by the therapist assistant. We stated that this would allow home health agencies more latitude in resource utilization, and potentially address the concern regarding therapist shortages in home health. We also noted that allowing assistants to perform maintenance therapy would be consistent with other post-acute care settings, including SNFs. As such, we proposed to modify the regulations at § 409.44(c)(2)(iii)(C) to allow therapist assistants (rather than only therapists) to perform maintenance therapy under the Medicare home health benefit.
We solicited comments regarding this proposal and welcomed feedback on whether this proposal would require therapists to provide more frequent patient reassessment or maintenance program review when the services are being performed by a therapist assistant. We also solicited comments on whether we should revise the description of the therapy codes to indicate maintenance services performed by a physical or occupational therapist assistant (G0151 and G0157) versus a qualified therapist, or simply remove the therapy code indicating the establishment or delivery of a safe and effective physical therapy maintenance program, by a physical therapist (G0159). And finally, we welcomed comments on the importance of tracking whether a visit is for maintenance or restorative therapy or whether it would be appropriate to only identify whether the service is furnished by a qualified therapist or an assistant in addition to any possible effects on the quality of care that could result by allowing therapist assistants to perform maintenance therapy.
The following is a summary of the comments received and our responses to comments on the proposed regulatory change to allow therapist assistants to perform maintenance therapy:
Comment: All commenters were supportive of the proposal to change the regulations at § 409.44(c)(2)(iii)(C) to allow therapist assistants to perform maintenance therapy under the home health benefit. Commenters stated that, as therapist assistants provide skilled professional services in the home, are licensed in practice, and are bound by the same ethical standards as therapists, assistants are qualified to provide maintenance therapy. Additionally, commenters stated that allowing HHAs to utilize therapist assistants within their scope of practice to provide maintenance therapy as well as restorative therapy, will support continued access to therapy services and improve overall quality of care.
Response: We thank commenters for their support of this proposal to allow therapist assistants to practice at the top of their licensure as well as allowing HHAs the flexibility to ensure beneficiary access to all available levels of therapy and resources.
Comment: Several commenters noted that the proposed rule and regulations text referenced “physical therapist assistants” and requested clarification regarding whether proposed § 409.44(c)(2)(iii)(C) allows all therapist assistants (physical, occupational, and speech-language pathology) to perform maintenance therapy.
Response: The proposed changes at § 409.44(c)(2)(iii)(C) would allow therapist assistants from all therapy disciplines to perform maintenance therapy within their scope of practice. The reference to physical therapist assistants in the preamble language was an example used to highlight, in general, licensure requirements for therapist assistants. However, the example was in regard to the regulations at § 484.115(g) and (i), which is in reference to the personnel qualifications of both occupational and physical therapist assistants. We thank the commenters for pointing out that the regulations text however, only referenced physical therapist assistants, and note that § 409.44(c)(2)(iii)(C)(1) and (2) has been changed to “therapist assistants,” and not “physical therapist assistants.” We thank commenters for their careful review of this proposal and for pointing out this important clarification.
Comment: Commenters provided mixed recommendations regarding the importance of tracking whether a visit is for maintenance or restorative therapy and whether the service is furnished by a qualified therapist or a therapist assistant. A few commenters stated that this data would be relevant to future discussions on changes in intensity/duration of therapy services delivered under the Patient-Driven Groupings Model. Other commenters noted that, as both therapists and therapist assistants are considered “qualified” and provide skilled care, it would not be necessary to collect this information. And finally we received a few comments stating that allowing therapist assistants to perform maintenance therapy would not require the supervising therapist to provide more frequent assessments, as this provision would align the requirement with the existing standard in other settings and for restorative therapy under home health.
Response: We thank all commenters for their recommendations and will take all comments under consideration for future rule-making and analysis.
Final Decision: We are finalizing our proposal to allow therapist assistants to perform maintenance therapy under the home health benefit. We are finalizing the proposed regulations text at § 409.44(c)(2)(iii)(C)(1) and (2) with a modification to reflect that all therapist assistants, rather than only physical therapist assistants, can perform maintenance therapy.
I. Changes to the Home Health Plan of Care Regulations at § 409.43
As a condition for payment of Medicare home health services, the regulations at § 409.43(a), home health plan of care content requirements, state that the plan of care must contain those items listed in § 484.60(a) that specify the standards relating to a plan of care that an HHA must meet in order to participate in the Medicare program. The home health CoPs at § 484.60(a) set forth the content requirements of the individualized home health plan of care. In the January 13, 2017 final rule, “Medicare and Medicaid Program: Conditions of Participation for Home Health Agencies” (82 FR 4504), we finalized changes to the plan of care requirements under the home health CoPs by reorganizing the existing plan of care content requirements at § 484.18(a), adding two additional plan of care content requirements, and moving the plan of care content requirements to § 484.60(a). Specifically, in addition to the longstanding plan of care content requirements previously listed at § 484.18(a), a home health plan of care must also include the following:
- A description of the patient's risk for emergency department visits and hospital readmission, and all necessary interventions to address the underlying risk factors; and
- Information related to any advance directives.
The new content requirements for the plan of care at § 484.60(a) became effective January 13, 2018 (82 FR 31729) and the Interpretive Guidelines to accompany the new CoPs were released on August 31, 2018. Since implementation of the new home health CoP plan of care requirements, we stated in subregulatory guidance in the Medicare Benefit Policy Manual, chapter 7,[27] that the plan of care must include the identification of the responsible discipline(s) providing home health services, and the frequency and duration of all visits, as well as those items required by the CoPs that establish the need for such services (§ 484.60(a)(2)(iii) and (iv)). Although not legally binding, the revised guidance in the Medicare Benefit Policy Manual is our preferred policy; therefore, in the CY 2020 HH PPS proposed rule, we stated that the current requirements at § 409.43(a) may be overly prescriptive and may interfere with timely payment for otherwise eligible episodes of care. To mitigate these potential issues, we proposed to change the regulations text at § 409.43(a). Specifically, we proposed to change the regulations text to state that for HHA services to be covered, the individualized plan of care must specify the services necessary to meet the patient-specific needs identified in the comprehensive assessment. In addition, the plan of care must include the identification of the responsible discipline(s) and the frequency and duration of all visits as well as those items listed in § 484.60(a) that establish the need for such services. All care provided must be in accordance with the plan of care. While these newly-added plan of care items at § 484.60(a) remain a CoP requirement, we believe that violations for an HHA inadvertently omitting required items are best addressed through the survey process, rather than through claims denials for otherwise eligible periods of care.
We solicited comments on the proposal to change to the regulations text at § 409.43 to state that the home health plan of care must include those items listed in § 484.60(a) that establish the need for such services.
The following is a summary of the comments received, primarily from HHAs, on the proposed changes to the home health plan of care regulations.
Comment: Commenters overwhelmingly supported the proposal without modifications. In addition, commenters agreed that the individualized plan of care must specify services necessary to meet patient-specific needs, which would be documented in the comprehensive assessment. Commenters also agreed and supported CMS using the survey process to address violations of required missing information or items.
Response: We thank commenters for their support of this proposal. We agree that this may help mitigate any claims denials resulting from these two items missing from the plan of care and we believe that violations for missing required items are best addressed through the survey process, rather than through claims denials for otherwise eligible periods of care.
Final Decision: We are finalizing to change the regulations text at § 409.43(a) to state that for HHA services to be covered, the individualized plan of care must specify the services necessary to meet the patient-specific needs identified in the comprehensive assessment. In addition, the plan of care must include the identification of the responsible discipline(s) and the frequency and duration of all visits as well as those items listed in § 484.60(a) that establish the need for such services. All care provided must be in accordance with the plan of care.
IV. Home Health Value-Based Purchasing (HHVBP) Model
A. Background
As authorized by section 1115A of the Act and finalized in the CY 2016 HH PPS final rule (80 FR 68624) and in the regulations at 42 CFR part 484, subpart F, we began testing the HHVBP Model on January 1, 2016. The HHVBP Model has an overall purpose of improving the quality and delivery of home health care services to Medicare beneficiaries. The specific goals of the Model are to: (1) Provide incentives for better quality care with greater efficiency; (2) study new potential quality and efficiency measures for appropriateness in the home health setting; and (3) enhance the current public reporting process.
Using the randomized selection methodology finalized in the CY 2016 HH PPS final rule, we selected nine states for inclusion in the HHVBP Model, representing each geographic area across the nation. All Medicare-certified Home Health Agencies (HHAs) providing services in Arizona, Florida, Iowa, Maryland, Massachusetts, Nebraska, North Carolina, Tennessee, and Washington are required to compete in the Model. The HHVBP Model uses the waiver authority under section 1115A(d)(1) of the Act to adjust Medicare payment rates under section 1895(b) of the Act based on the competing HHAs' performance on applicable measures. The maximum payment adjustment percentage increases incrementally, upward or downward, over the course of the HHVBP Model in the following manner: (1) 3 percent in CY 2018; (2) 5 percent in CY 2019; (3) 6 percent in CY 2020; (4) 7 percent in CY 2021; and (5) 8 percent in CY 2022. Payment adjustments are based on each HHA's Total Performance Score (TPS) in a given performance year (PY), which is comprised of performance on: (1) A set of measures already reported via the Outcome and Assessment Information Set (OASIS), completed Home Health Consumer Assessment of Healthcare Providers and Systems (HHCAHPS) surveys, and select claims data elements; and (2) three New Measures for which points are achieved for reporting data.
In the CY 2017 HH PPS final rule (81 FR 76741 through 76752), CY 2018 HH PPS final rule (83 FR 51701 through 51706), and CY 2019 HH PPS final rule with comment (83 FR 56527 through 56547), we finalized changes to the HHVBP Model. Some of those changes included adding and removing measures from the applicable measure set, revising our methodology for calculating benchmarks and achievement thresholds at the state level, creating an appeals process for recalculation requests, and revising our methodologies for weighting measures and assigning improvement points.
B. Public Reporting of Total Performance Scores and Percentile Rankings Under the HHVBP Model
As stated previously and discussed in prior rulemaking, one of the goals of the HHVBP Model is to enhance the current public reporting processes for home health. In the CY 2016 HH PPS final rule, we finalized our proposed reporting framework for the HHVBP Model, including both the annual and quarterly reports that are made available to competing HHAs and a separate, publicly available quality report (80 FR 68663 through 68665). We stated that such publicly available performance reports would inform home health industry stakeholders (consumers, physicians, hospitals) as well as all competing HHAs delivering care to Medicare beneficiaries within selected state boundaries on their level of quality relative to both their peers and their own past performance, and would also provide an opportunity to confirm that the beneficiaries referred for home health services are being provided the best quality of care available. We further stated that we intended to make public competing HHAs' TPSs with the intention of encouraging providers and other stakeholders to utilize quality ranking when selecting an HHA. As summarized in the CY 2016 final rule (80 FR 68665), overall, commenters generally encouraged the transparency of data pertaining to the HHVBP Model. Commenters offered that to the extent possible, accurate comparable data would provide HHAs the ability to improve care delivery and patient outcomes, while better predicting and managing quality performance and payment updates.
We have continued to discuss and solicit comments on the scope of public reporting under the HHVBP Model in subsequent rulemaking. In the CY 2017 final rule (81 FR 76751 through 76752), we discussed the public display of total performance scores, stating that annual publicly available performance reports would be a means of developing greater transparency of Medicare data on quality and aligning the competitive forces within the market to deliver care based on value over volume. We stated our belief that the public reporting of competing HHAs' performance scores under the HHVBP Model would support our continued efforts to empower consumers by providing more information to help them make health care decisions, while also encouraging providers to strive for higher levels of quality. We explained that we have employed a variety of means (CMS Open Door Forums, webinars, a dedicated help desk, and a web-based forum where training and learning resources are regularly posted) to facilitate direct communication, sharing of information and collaboration to ensure that we maintain transparency while developing and implementing the HHVBP Model. This same care was taken with our plans to publicly report performance data, through collaboration with other CMS components that use many of the same quality measures. We also noted that section 1895(b)(3)(B)(v) of the Act requires HHAs to submit patient-level quality of care data using the OASIS and the HHCAHPS, and that section 1895(b)(3)(B)(v)(III) of the Act states that this quality data is to be made available to the public. Thus, HHAs have been required to collect OASIS data since 1999 and report HHCAHPS data since 2012.
We solicited further public comment in the CY 2019 HH PPS proposed rule (83 FR 32438) on which information from the Annual Total Performance Score and Payment Adjustment Report (Annual Report) should be made publicly available. We noted that HHAs have the opportunity to review and appeal their Annual Report as outlined in the appeals process finalized in the CY 2017 HH PPS final rule (81 FR 76747 through 76750). Examples of the information included in the Annual Report are the agency name, address, TPS, payment adjustment percentage, performance information for each measure used in the Model (for example, quality measure scores, achievement, and improvement points), state and cohort information, and percentile ranking. We stated that based on the public comments received, we would consider what information, specifically from the Annual Report, we may consider proposing for public reporting in future rulemaking.
As we summarized in the CY 2019 HH PPS final rule with comment (83 FR 56546 through 56547), several commenters expressed support for publicly reporting information from the Annual Total Performance Score and Payment Adjustment Report, as they believed it would better inform consumers and allow for more meaningful and objective comparisons among HHAs. Other commenters suggested that CMS consider providing the percentile ranking for HHAs along with their TPS and expressed interest in publicly reporting all information relevant to the HHVBP Model. Several commenters expressed concern with publicly displaying HHAs' TPSs, citing that the methodology is still evolving and pointing out that consumers already have access to data on the quality measures in the Model on Home Health Compare. Another commenter believed that publicly reporting data just for states included in the HHVBP Model could be confusing for consumers.
As we stated in the CY 2020 HH PPS proposed rule, our belief remains that publicly reporting HHVBP data would enhance the current home health public reporting processes as it would better inform beneficiaries when choosing an HHA, while incentivizing HHAs to improve quality. Although the data made public would only pertain to the final performance year of the Model, we believe that publicly reporting HHVBP data for Performance Year 5 would nonetheless incentivize HHAs to improve performance. Consistent with our discussion in prior rulemaking of the information that we are considering for public reporting under the HHVBP Model, we proposed to publicly report on the CMS website the following two points of data from the final CY 2020 (PY) 5 Annual Report for each participating HHA in the Model that qualified for a payment adjustment for CY 2020: (1) The HHA's TPS from PY 5; and (2) the HHA's corresponding PY 5 TPS Percentile Ranking. We stated that we were considering making these data available on the HHVBP Model page of the CMS Innovation website (https://innovation.cms.gov/initiatives/home-health-value-based-purchasing-model). We further stated that these data would be reported for each such competing HHA by agency name, city, state, and by the agency's CMS Certification Number (CCN). We expect that these data would be made public after December 1, 2021, the date by which we intend to complete the CY 2020 Annual Report appeals process and issuance of the final Annual Report to each HHA.
As discussed in prior rulemaking, we believe the public reporting of such data would further enhance quality reporting under the Model by encouraging participating HHAs to provide better quality of care through focusing on quality improvement efforts that could potentially improve their TPS. In addition, we believe that publicly reporting performance data that indicates overall performance may assist beneficiaries, physicians, discharge planners, and other referral sources in choosing higher-performing HHAs within the nine Model states and allow for more meaningful and objective comparisons among HHAs on their level of quality relative to their peers.
As discussed in the proposed rule, we believe that the TPS would be more meaningful if the corresponding TPS Percentile Ranking were provided so consumers can more easily assess an HHA's relative performance. We stated that we would also provide definitions for the HHVBP TPS and the TPS Percentile Ranking methodology to ensure the public understands the relevance of these data points and how they were calculated.
We further stated that under our proposal, the data reported would be limited to one year of the Model. We believe this strikes a balance between allowing for public reporting under the Model for the reasons discussed while heeding commenters' concerns about reporting performance data for earlier performance years of the HHVBP Model. We believe publicly reporting the TPS and TPS Percentile Ranking for CY 2020 would enhance quality reporting under the Model by encouraging participating HHAs to provide better quality of care and would promote transparency, and could enable beneficiaries to make better informed decisions about where to receive care.
We solicited comment on our proposal to publicly report the TPS and TPS Percentile Ranking from the final CY 2020 PY 5 Annual Report for each HHA in the nine Model states that qualified for a payment adjustment for CY 2020. We also solicited comment on our proposed amendment to § 484.315 to reflect this policy. Specifically, we proposed to add new paragraph (d) to specify that CMS will report, for Performance Year 5, the TPS and the percentile ranking of the TPS for each competing HHA on the CMS website.
The following is a summary of public comments received and our responses:
Comment: The majority of commenters supported our proposal to publicly report these performance data under the HHVBP Model, citing that the data are appropriate for public reporting and, although limited to performance during the final year of the Model, such information would be beneficial for members of the public in the nine states and potentially be valuable to beneficiaries. A commenter encouraged CMS to make additional performance data available beyond our proposal and to provide a link on the Home Health Compare (HHC) website alerting consumers that this supplemental information is available. One commenter advised CMS to provide greater clarity on the TPS and TPS Percentile Ranking, regarding how the data is measured and how it compares to the star rating data on HHC, by providing guidance to the general public that there will likely be instances where an HHA is a 4 or 5 star agency but not as high of a performer under the HHVBP Model. The commenter expressed concern that the different information available through HHC and the HHVBP Model publicly reported information may confuse the public.
Response: As discussed in the proposed rule, we anticipate making the HHVBP Model performance data available on the HHVBP Model page website at https://innovation.cms.gov/initiatives/home-health-value-based-purchasing-model. We will take under consideration the commenter's suggestion for also alerting the public of the availability of the Model performance data on the HHC website. In addition, as discussed in the proposed rule, to accompany the data, we will also provide definitions for the HHVBP TPS and the TPS Percentile Ranking methodology, as well as descriptions of the scoring methodology, on the CMS website to ensure the public understands the relevance of these data points and how they were calculated. We will report data by state, CCN, and agency name. As the HHVBP Model performance data is supplemental to the star ratings, we intend to also include a reference to the star ratings available on the CMS website.
Comment: One commenter stated that this information is already available on the HHC website and questioned the utility of reporting this information for only the fifth and final year of the model. Another commenter stated that the information is not easily understood by Medicare beneficiaries or caregivers and is not sufficiently impactful. Furthermore, the commenter stated that the impact of HHVBP, from a fiscal and quality perspective, is not yet fully understood, recent changes in quality metrics for the Model are not yet fully integrated, and more changes are likely needed before HHA-specific results should be publicly displayed.
Response: We continue to believe that publicly reporting HHVBP performance data would incentivize HHAs to improve quality performance under the Model and enhance the current home health public reporting processes to assist consumers, patients, providers, stakeholders and referral sources in making informed choices on their home health care services. We note that the specific information we proposed to publicly report is not currently provided on HHC, and that the HHVBP performance data would supplement the information provided on HHC by together providing a more comprehensive assessment of an HHA's performance across a range of quality measures, including the two new composite measures included in the HHVBP Model's measure set effective performance year 4 (CY 2019). While the publicly reported data would be limited to the final performance year of the model, we believe providing this data would benefit beneficiaries by encouraging participating HHAs to further improve the quality of care they provide.
We agree that it is important to ensure the public can understand the data we publicly report on the HHVBP Model, and as previously discussed, will provide accompanying information with the publicly reported data to promote public understanding. With regard to the recent changes to the Model, in the CY 2019 HH PPS Final Rule, we finalized changes to the quality measures and scoring methodology for the HHVBP Model. We would only be publicly reporting data from the CY 2020 performance year, which will be the second performance year to which these changes in the quality measures and scoring methodology have applied. Prior to publicly reporting the CY 2020 performance data, we will have provided participating HHAs with multiple reports on their performance under the modified methodology. Moreover, as discussed in the proposed rule, we expect that these data would be made public after December 1, 2021, the date by which we intend to complete the CY 2020 Annual Report appeals process and issuance of the final Annual Report to each HHA. Finally, we currently have a publicly available report for PY1 on the evaluation of the HHVBP Model on the CMS Innovation Center website and will have more information forthcoming about the impact of the Model.
Comment: One commenter encouraged CMS to continue to develop and share quality data. However, they also expressed concerns with public reporting, particularly for providers who are not participating in the HHVBP Model, but are located in markets that overlap with HHVBP states. The commenter requested that CMS ensure that the variation of participation by geography does not give advantages or disadvantages to providers based purely on state line because HHAs located in a HHVBP Model state may have more publicly available quality information than HHAs outside of those Model states. The commenter expressed concern that HHAs in non-participating states would not have the same quality information publicly available as the participating HHAs, which could be confusing to consumers and referral sources when selecting an agency.
Response: As stated in our response to the previous commenter's concern, the TPS and TPS Percentile Ranking would supplement the information publicly reported through the HHC star ratings and other public resources, which include information about both HHVBP Model participating and non-participating HHAs and therefore can be used by patients or providers to review quality information on HHAs in non-HHVBP Model states. The HHVBP Model performance data would be publicly reported only for participating HHAs in the nine states that qualified for a payment adjustment percentage based on their Total Performance Score in the fifth and final performance year (CY 2020) of the Model. We believe that making these HHVBP Model performance data available on the CMS Innovation Center's HHVBP Model web page, along with information about what this data represents and how it was calculated, will minimize any potential confusion.
Final Decision: For the reasons stated and after consideration of the comments received, we are finalizing the public reporting of the Total Performance Score and Total Performance Score Percentile Ranking from the final CY 2020 PY 5 Annual Report for each HHA in the nine HHVBP Model states that qualified for a payment adjustment for CY 2020. We are also finalizing our proposed amendment to § 484.315 to reflect this policy. As discussed in the proposed rule and in this final rule with comment period, we expect that these data will be made available on the HHVBP Model page of the CMS Innovation Center website after December 1, 2021, the date by which we intend to complete the CY 2020 Annual Report appeals process and issuance of the final Annual Report to each HHA.
We received several out-of-scope comments, including requests to expand the HHVBP Model and for more information about when we may consider expansion. We thank the commenters for their interest and will address any future changes through rulemaking. We also note that HHVBP Model evaluation reports are currently publicly available on the CMS website (https://innovation.cms.gov/initiatives/home-health-value-based-purchasing-model), which will be updated with forthcoming reports.
C. Removal of Improvement in Pain Interfering With Activity Measure (NQF #0177)
As discussed in section V.C of this final rule with comment period, after careful consideration of the concerns raised by commenters, the responses provided to those concerns and the discussion of alignment across the QRPs, CMS is finalizing the removal of the Improvement in Pain Interfering with Activity Measure (NQF #0177) from the HH QRP beginning with the CY 2022 HH QRP under measure removal Factor 7: Collection or public reporting of a measure leads to negative unintended consequences other than patient harm. HHAs will no longer be required to submit OASIS Item M1242, Frequency of Pain Interfering with Patient's Activity or Movement for the purposes of this measure beginning January 1, 2021. Data for this measure will be publicly reported on HH Compare until April 2020. As we discussed in the CY 2020 HH PPS proposed rule (84 FR 34643), as HHAs would continue to be required to submit their data for this measure through CY 2020, we do not anticipate any impact on the collection of this data and the inclusion of the measure in the HHVBP Model's applicable measure set for the final performance year (CY 2020) of the Model.
V. Home Health Care Quality Reporting Program (HH QRP)
A. Background and Statutory Authority
The HH QRP is authorized by section 1895(b)(3)(B)(v) of the Act. Section 1895(b)(3)(B)(v)(II) of the Act requires that for 2007 and subsequent years, each HHA submit to the Secretary in a form and manner, and at a time, specified by the Secretary, such data that the Secretary determines are appropriate for the measurement of health care quality. To the extent that an HHA does not submit data in accordance with this clause, the Secretary shall reduce the home health market basket percentage increase applicable to the HHA for such year by 2 percentage points. As provided at section 1895(b)(3)(B)(vi) of the Act, depending on the market basket percentage increase applicable for a particular year, the reduction of that increase by 2 percentage points for failure to comply with the requirements of the HH QRP and further reduction of the increase by the productivity adjustment (except in 2018 and 2020) described in section 1886(b)(3)(B)(xi)(II) of the Act may result in the home health market basket percentage increase being less than 0.0 percent for a year, and may result in payment rates under the Home Health PPS for a year being less than payment rates for the preceding year.
For more information on the policies we have adopted for the HH QRP, we refer readers to the following rules:
- CY 2007 HH PPS final rule (71 FR 65888 through 65891).
- CY 2008 HH PPS final rule (72 FR 49861 through 49864).
- CY 2009 HH PPS update notice (73 FR 65356).
- CY 2010 HH PPS final rule (74 FR 58096 through 58098).
- CY 2011 HH PPS final rule (75 FR 70400 through 70407).
- CY 2012 HH PPS final rule (76 FR 68574).
- CY 2013 HH PPS final rule (77 FR 67092).
- CY 2014 HH PPS final rule (78 FR 72297).
- CY 2015 HH PPS final rule (79 FR 66073 through 66074).
- CY 2016 HH PPS final rule (80 FR 68690 through 68695).
- CY 2017 HH PPS final rule (81 FR 76752).
- CY 2018 HH PPS final rule (82 FR 51711 through 51712).
- CY 2019 HH PPS final rule with comment period (83 FR 56547).
B. General Considerations Used for the Selection of Quality Measures for the HH QRP
For a detailed discussion of the considerations we historically use for measure selection for the HH QRP quality, resource use, and others measures, we refer readers to the CY 2016 HH PPS final rule (80 FR 68695 through 68696). In the CY 2019 HH PPS final rule with comment (83 FR 56548 through 56550) we also finalized the factors we consider for removing previously adopted HH QRP measures.
C. Quality Measures Currently Adopted for the CY 2021 HH QRP
The HH QRP currently includes 19 [28] measures for the CY 2021 program year, as outlined in Table 28.

D. Removal of HH QRP Measures Beginning With the CY 2022 HH QRP
In line with our Meaningful Measures Initiative, in the CY 2020 HH PPS proposed rule (84 FR 34644 through 34645), we proposed to remove one measure from the HH QRP beginning with the CY 2022 HH QRP.
1. Removal of the Improvement in Pain Interfering With Activity Measure (NQF #0177)
We are removing pain-associated quality measures from our quality reporting programs in an effort to mitigate any potential unintended, over-prescription of opioid medications inadvertently driven by these measures. In the CY 2020 HH PPS proposed rule (84 FR 34644 and 34645), we proposed to remove the Improvement in Pain Interfering with Activity Measure (NQF #0177) from the HH QRP beginning with the CY 2022 HH QRP under our measure removal Factor 7: Collection or public reporting of a measure leads to negative unintended consequences other than patient harm.
In the CY 2007 HH PPS final rule (71 FR 65888 through 65891), we adopted the Improvement in Pain Interfering with Activity Measure beginning with the CY 2007 HH QRP. The measure was NQF-endorsed (NQF #0177) in March 2009. This risk-adjusted outcome measure reports the percentage of HH episodes during which the patient's frequency of pain with activity or movement improved. The measure is calculated using OASIS Item M1242, Frequency of Pain Interfering with Patient's Activity or Movement.[29]
We evaluated the Improvement in Pain Interfering with Activity Measure (NQF #0177) and determined that the measure could have unintended consequences with respect to responsible use of opioids for the management of pain. In 2018, CMS published a comprehensive roadmap, available at https://www.cms.gov/About-CMS/Agency-Information/Emergency/Downloads/Opioid-epidemic-roadmap.pdf, which outlined the agency's efforts to address national issues around prescription opioid misuse and overuse. Because the Medicare program pays for a significant amount of prescription opioids, the roadmap was designed to promote appropriate stewardship of these medications that can provide a medical benefit but also carry a risk for patients, including those receiving home health. One key component of this strategy is to prevent new cases of opioid use disorder, through education, guidance and monitoring of opioid prescriptions. When used correctly, prescription opioids are helpful for treating pain. However, effective non-opioid pain treatments are available to providers and CMS is working to promote their use.
Although we are not aware of any scientific studies that support an association between the prior or current iterations of the Improvement in Pain Interfering with Activity Measure (NQF #0177) and opioid prescribing practices, out of an abundance of caution and to avoid any potential unintended consequences, we proposed to remove the Improvement in Pain Interfering with Activity Measure (NQF #0177) from the HH QRP beginning with the CY 2022 HH QRP under measure removal Factor 7: Collection or public reporting of a measure leads to negative unintended consequences other than patient harm.
We stated in the proposed rule that if we finalized this proposal, HHAs would no longer be required to submit OASIS Item M1242, Frequency of Pain Interfering with Patient's Activity or Movement for the purposes of this measure beginning January 1, 2021. We stated we are unable to remove M1242 earlier due to the timelines associated with implementing changes to OASIS. We also stated that if we finalized this proposal, data for this measure would be publicly reported on HH Compare until April 2020.
We invited public comment on this proposal and received several comments. A discussion of these comments, along with our responses follows.
Comment: Several commenters supported our proposal to remove the Improvement in Pain Interfering with Activity Measure (NQF #0177) from the HH QRP as well as the associated OASIS item M1242 used to calculate the measure. One commenter supported removing the measure but recommended that CMS retain M1242 for purposes of risk-adjustment. A few commenters expressed support for CMS' proposal to add new, standardized pain assessment items to the OASIS that would enable the agency to continue collecting data on pain.
Response: We appreciate commenters' support for our proposal to remove the Improvement in Pain Interfering with Activity Measure (NQF #0177) as part of the overall HHS strategy to address opioid misuse. We note that we do not have the authority under the HH QRP to retain the OASIS item M1242 for risk-adjustment purposes once removed from the HH QRP. We will evaluate the SPADE Items in section V.H.3. of this final rule with comment period for risk adjustment use in the future.
Comment: Several commenters requested that CMS develop or share its plans to address pain management in its quality reporting programs (QRPs) in the future after the related measures and data elements are removed, noting that the agency should be consistent in its approach to addressing patient pain. One commenter recommended that CMS track the HHA's approach to appropriate teaching of non-pharmacological pain management options as a part of the individualized care plan.
Response: In the CY 2020 HH PPS proposed rule (84 FR 34672 through 34675) we proposed to add new, standardized patient assessment data elements on pain to the OASIS such that agencies would continue to collect information on patient pain that could support care planning, quality improvement, and potential quality measurement, including risk adjustment. In section V.H.3. of this rule, we have finalized the adoption of the three new pain data elements. We believe their inclusion on the next version of the OASIS will underscore the priority of managing pain. In addition, the CMS Roadmap to Address the Opioid Epidemic includes emphasis on non-pharmacological options for managing pain as critical in the efforts to reduce over-reliance on and misuse of opioids. We are committed to continuing to communicate our strategy for both promoting pain management and appropriate use of opioids.
Comment: The majority of commenters did not support the proposal to remove the Improvement in Pain Interfering with Activity Measure (NQF #0177). Several commenters stated that pain is an important concern for home health patients and that information on pain was valuable to the care team and for quality improvement. These commenters noted that pain can be a root cause of declining health and well-being and is linked to patient quality of life. Some commenters said that measuring pain improvement helps assess treatment efficacy.
Other commenters noted the lack of evidence that measuring pain level in home health is linked to increased opioid use. One commenter additionally noted that generally home health agencies do not prescribe opioids.
While some commenters appreciated CMS' efforts to address the opioid epidemic, they opposed removal of this measure, expressing concern that this removal could decrease the priority of efforts to manage pain, including chronic pain. A few commenters noted that greater emphasis on pain management and impact, as well as promoting and educating providers on non-pharmacological pain management strategies and care plans, were important to addressing opioid misuse.
Response: We appreciate the feedback given by the commenters and acknowledge the concerns raised. We agree that pain is an important concern for home health patients. In response to recommendations from the President's Commission on Combatting Drug Addiction and the Opioid Crisis, to comply with the requirements of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act (Pub. L. 115-271), and to avoid any potential unintended consequences, in the CY 2019 OPPS/ASC final rule (83 FR 59149) we finalized to update the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) patient experience of care survey measure by removing three recently revised pain communication questions. We proposed the removal of the Improvement in Pain Interfering with Activity Measure (NQF #0177) measure in the spirit of alignment with these efforts.
Additionally, we proposed the removal of this measure to minimize any potential overprescribing of opioids associated with incentives to improve scoring on the measure. We have particular concern with quality measures that assess directly or indirectly whether or not a patient's pain has improved, as we believe such measures may more directly incentivize over-prescribing of opioids. We have addressed this specific issue in previous rule-making. In the FY 2017 IPPS/LTCH PPS final rule (82 FR 38342), we similarly finalized refinements to the HCAHPS Survey measure pain management questions, removing questions such as “During this hospital stay, how often was your pain well controlled?” and “During this hospital stay, how often did the hospital staff do everything they could to help you with your pain?”, to minimize such incentives. We plan to further evaluate this issue across all programs.
Comment: Several commenters expressed concern that removal of M1242 would leave the OASIS without any items to assess pain, noting that pain interference not only captures pain intensity, but also the impact of pain on function.
Response: Given the adoption of the new pain items, in section V.H.3. of this rule the OASIS would continue to contain items that assess pain and the impact on function. CMS will require HHAs to report OASIS M1242 through December 31, 2020. CMS will begin requiring reporting of the new pain items finalized in section V.H.3. of this rule January 1, 2021. This timeline will ensure that there is no gap in the assessment and reporting of pain for this population.
Final Decision: After careful consideration of the concerns raised by commenters, the responses provided to those concerns and the discussion of alignment across the QRPs, we are finalizing our proposal to remove the Improvement in Pain Interfering with Activity Measure (NQF #0177) from the HH QRP beginning with the CY 2022 HH QRP under measure removal Factor 7: Collection or public reporting of a measure leads to negative unintended consequences other than patient harm. HHAs will no longer be required to submit OASIS Item M1242, Frequency of Pain Interfering with Patient's Activity or Movement for the purposes of this measure beginning January 1, 2021. Data for this measure will be publicly reported on HH Compare until April 2020.
E. New and Modified HH QRP Quality Measures Beginning With the CY 2022 HH QRP
In the CY 2020 HH PPS proposed rule (84 FR 34645 through 34650), we proposed to adopt two process measures for the HH QRP under section 1895(b)(3)(B)(v)(IV)(aa) of the Act, both of which would satisfy section 1899B(c)(1)(E)(ii) of the Act, which requires that the quality measures specified by the Secretary include measures with respect to the quality measure domain titled “Accurately communicating the existence of and providing for the transfer of health information and care preferences of an individual to the individual, family caregiver of the individual, and providers of services furnishing items and services to the individual, when the individual transitions from a [post-acute care] PAC provider to another applicable setting, including a different PAC provider, a hospital, a critical access hospital, or the home of the individual.” Given the length of this domain title, hereafter, we will refer to this quality measure domain as “Transfer of Health Information.”
The two measures we proposed to adopt are: (1) Transfer of Health Information to Provider-Post-Acute Care; and (2) Transfer of Health Information to Patient-Post-Acute Care. Both of these proposed measures support our Meaningful Measures priority of promoting effective communication and coordination of care, specifically the Meaningful Measure area of the transfer of health information and interoperability.
In addition to the two measure proposals, we proposed to update the specifications for the Discharge to Community-Post Acute Care (PAC) HH QRP measure to exclude baseline nursing facility (NF) residents from the measure.
1. Transfer of Health Information to the Provider-Post-Acute Care (PAC) Measure
The Transfer of Health Information to the Provider-Post-Acute Care (PAC) Measure is a process-based measure that assesses whether or not a current reconciled medication list is given to the admitting provider when a patient is discharged/transferred from his or her current PAC setting.
(a) Background
In 2013, 22.3 percent of all acute hospital discharges were discharged to PAC settings, including 11 percent who were discharged to home under the care of a home health agency, and 9 percent who were discharged to SNFs.[30] The proportion of patients being discharged from an acute care hospital to a PAC setting was greater among beneficiaries enrolled in Medicare fee-for-service (FFS), underscoring the importance of the measure. Among Medicare FFS patients discharged from an acute hospital, 42 percent went directly to PAC settings. Of that 42 percent, 20 percent were discharged to a SNF, 18 percent were discharged to an HHA, three percent were discharged to an IRF, and one percent were discharged to an LTCH.[31]
The transfer and/or exchange of health information from one provider to another can be done verbally (for example, clinician-to-clinician communication in-person or by telephone), paper-based (for example, faxed or printed copies of records), and via electronic communication (for example, through a health information exchange network using an electronic health/medical record, and/or secure
messaging). Health information, such as medication information, that is incomplete or missing increases the likelihood of a patient or resident safety risk, and is often life-threatening.[32 33 34 35 36 37] Poor communication and coordination across health care settings contributes to patient complications, hospital readmissions, emergency department visits, and medication errors.38 39 40 41 42 43 44 45 46 47 48 49 Communication has been cited as the third most frequent root cause in sentinel events, which The Joint Commission defines [50] as a patient safety event that results in death, permanent harm, or severe temporary harm. Failed or ineffective patient handoffs are estimated to play a role in 20 percent of serious preventable adverse events.[51] When care transitions are enhanced through care coordination activities, such as expedited patient information flow, these activities can reduce duplication of care services and costs of care, resolve conflicting care plans, and prevent medical errors.[52 53 54 55 56 57]
Care transitions across health care settings have been characterized as complex, costly, and potentially hazardous, and may increase the risk for multiple adverse outcomes.[58 59] The rising incidence of preventable adverse events, complications, and hospital readmissions have drawn attention to the importance of the timely transfer of health information and care preferences at the time of transition. Failures of care coordination, including poor communication of information, were estimated to cost the U.S. health care system between $25 billion and $45 billion in wasteful spending in 2011.[60] The communication of health information and patient care preferences is critical to ensuring safe and effective transitions from one health care setting to another.[61 62]
Patients in PAC settings often have complicated medication regimens and require efficient and effective communication and coordination of care between settings, including detailed transfer of medication information.[63 64 65] Patients in PAC settings may be vulnerable to adverse health outcomes due to insufficient medication information on the part of their health care providers, and the higher likelihood for multiple comorbid chronic conditions, polypharmacy, and complicated transitions between care settings.[66 67] Preventable adverse drug events (ADEs) may occur after hospital discharge in a variety of settings including PAC.[68] For older patients discharged from the hospital, 80 percent of the medication errors occurring during patient handoffs relate to miscommunication between providers [69] and for those transferring to an HHA, medication errors typically relate to transmission of inaccurate discharge medication lists.[70] Medication errors and one-fifth of ADEs occur during transitions between settings, including admission to or discharge from a hospital to home or a PAC setting, or transfer between hospitals.[71 72]
Patients in PAC settings often take multiple medications. Consequently, PAC providers regularly are in the position of starting complex new medication regimens with little knowledge of the patients or their medication history upon admission. Medication discrepancies in PAC are common, such as those identified in transition from hospital to SNF [73] and hospital to home.[74] In one small intervention study, approximately 90 percent of the sample of 101 patients experienced at least one medication discrepancy in the transition from hospital to home care.[75]
We would define a reconciled medication list as a list of the current prescribed and over the counter (OTC) medications, nutritional supplements, vitamins, and homeopathic and herbal products administered by any route to the patient/resident at the time of discharge or transfer. Medications may also include but are not limited to total parenteral nutrition (TPN) and oxygen. The current medications should include those that are: (1) Active, including those that will be discontinued after discharge; and (2) those held during the stay and planned to be continued/resumed after discharge. If deemed relevant to the patient's/resident's care by the subsequent provider, medications discontinued during the stay may be included.
A reconciled medication list often includes important information about: (1) The patient/resident—including their name, date of birth, information, active diagnoses, known medication and other allergies, and known drug sensitivities and reactions; and (2) each medication, including the name, strength, dose, route of medication administration, frequency or timing, purpose/indication, any special instructions (for example, crush medications), and, for any held medications, the reason for holding the medication and when medication should resume. This information can improve medication safety. Additional information may be applicable and important to include in the medication list such as the patient's/resident's weight and date taken, height and date taken, patient's preferred language, patient's ability to self-administer medication, when the last dose of the medication was administered by the discharging provider, and when the final dose should be administered (for example, end of treatment). This is not an exhaustive list of the information that could be included in the medication list. The suggested elements detailed in the previous definition are for guidance purposes only and are not a requirement for the types of information to be included in a reconciled medication list in order to meet the measure criteria.
(b) Stakeholder and TEP Input
The Transfer of Health Information to the Provider-Post-Acute Care (PAC) measure was developed after consideration of feedback we received from stakeholders and four TEPs convened by our contractors. Further, the measure was developed after evaluation of data collected during two pilot tests we conducted in accordance with the CMS Measures Management System Blueprint.
Our measure development contractors convened a TEP, which met on September 27, 2016,[76] January 27, 2017, and August 3, 2017 [77] to provide input on a prior version of this measure. Based on this input, we updated the measure concept in late 2017 to include the transfer of a specific component of health information—medication information. Our measure development contractors reconvened a TEP on April 20, 2018 for the purpose of obtaining expert input on the proposed measure, including the measure's reliability, components of face validity, and the feasibility of implementing the measure across PAC settings. Overall, the TEP was supportive of the measure, affirming that the measure provides an opportunity to improve the transfer of medication information. A summary of the April 20, 2018 TEP proceedings titled “Transfer of Health Information TEP Meeting 4-June 2018” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Our measure development contractors solicited stakeholder feedback on the proposed measure by requesting comment on the CMS Measures Management System Blueprint website, and accepted comments that were submitted from March 19, 2018 to May 3, 2018. The comments received expressed overall support for the measure. Several commenters suggested ways to improve the measure, primarily related to what types of information should be included at transfer. We incorporated this input into development of the proposed measure. The summary report for the March 19 to May 3, 2018 public comment period titled “IMPACT—Medication -Profile- Transferred -Public- Comment- Summary- Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
(c) Pilot Testing
The measure was tested between June and August 2018 in a pilot test that involved 24 PAC facilities/agencies, including five IRFs, six SNFs, six LTCHs, and seven HHAs. The 24 pilot sites submitted a total of 801 records. Analysis of agreement between coders within each participating facility (266 qualifying pairs) indicated a 93-percent agreement for this measure. Overall, pilot testing enabled us to verify its reliability, components of face validity, and feasibility of being implemented across PAC settings. Further, more than half of the sites that participated in the pilot test stated during the debriefing interviews that the measure could distinguish facilities or agencies with higher quality medication information transfer from those with lower quality medication information transfer at discharge. The pilot test summary report is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
(d) Measure Applications Partnership (MAP) Review and Related Measures
We included the measure on the 2018 Measures Under Consideration (MUC) list for HH QRP. The NQF-convened MAP Post-Acute Care-Long Term Care (PAC LTC) Workgroup met on December 10, 2018 and provided input on this proposed Transfer of Health Information to the Provider-Post-Acute Care measure. The MAP conditionally supported this measure pending NQF endorsement, noting that the measure can promote the transfer of important medication information. The MAP also suggested that CMS consider a measure that can be adapted to capture bi-directional information exchange and recommended that the medication information transferred include important information about supplements and opioids. More information about the MAP's recommendations for this measure is available at: http://www.qualityforum.org/Projects/i-m/MAP/PAC-LTC_Workgroup/2019_Considerations_for_Implementing_Measures_Draft_Report.aspx .
As part of the measure development and selection process, we identified one NQF-endorsed quality measure related to the measure, titled Documentation of Current Medications in the Medical Record (NQF #0419e, CMS eCQM ID: CMS68v8). This measure was adopted as one of the recommended adult core clinical quality measures for eligible professionals for the EHR Incentive Program beginning in 2014, and was adopted under the Merit-based Incentive Payment System (MIPS) quality performance category beginning in 2017. The measure is calculated based on the percentage of visits for patients aged 18 years and older for which the eligible professional or eligible clinician attests to documenting a list of current medications using all resources immediately available on the date of the encounter.
The Transfer of Health Information to the Provider-Post-Acute Care measure addresses the transfer of medication information whereas the NQF-endorsed measure #0419e assesses the documentation of medications, but not the transfer of such information. Further, the measure utilizes standardized patient assessment data elements (SPADEs), which is a requirement for measures specified under the Transfer of Health Information measure domain under section 1899B(c)(1)(E) of the Act, whereas NQF #0419e does not. After review of the NQF-endorsed measure, we determined that the Transfer of Health Information to Provider-Post-Acute Care measure better addresses the Transfer of Health Information measure domain, which requires that at least some of the data used to calculate the measure be collected as standardized patient assessment data through post-acute care assessment instruments.
Section 1899B(e)(2)(A) of the Act requires that measures specified by the Secretary under section 1899B of the Act be endorsed by the consensus-based entity with a contract under section 1890(a) of the Act, which is currently the NQF. However, when a feasible and practical measure has not been NQF endorsed for a specified area or medical topic determined appropriate by the Secretary, section 1899B(e)(2)(B) of the Act allows the Secretary to specify a measure that is not NQF endorsed as long as due consideration is given to the measures that have been endorsed or adopted by the consensus-based entity under a contract with the Secretary. For these reasons, we believe that there is currently no feasible NQF-endorsed measure that we could adopt under section 1899B(c)(1)(E) of the Act. However, we note that we intend to submit the measure to the NQF for consideration of endorsement when feasible.
(e) Quality Measure Calculation
The Transfer of Health Information to the Provider-Post-Acute Care (PAC) quality measure is calculated as the proportion of quality episodes with a discharge/transfer assessment indicating that a current reconciled medication list was provided to the admitting provider at the time of discharge/transfer.
The measure denominator is the total number of quality episodes ending in discharge/transfer to an “admitting provider,” which is defined as: a short-term general hospital, intermediate care, home under care of another organized home health service organization or a hospice, a hospice in an institutional facility, a SNF, an LTCH, an IRF, an inpatient psychiatric facility, or a critical access hospital (CAH). These providers were selected for inclusion in the denominator because they represent admitting providers captured by the current discharge location items on the OASIS. The measure numerator is the number of HH quality episodes (Start of Care or Resumption of Care OASIS assessment and a Transfer or Discharge OASIS Assessment) indicating a current reconciled medication list was provided to the admitting provider at the time of discharge/transfer. The measure also collects data on how information is exchanged in PAC facilities, informing consumers and providers on how information was transferred at discharge/transfer. Data pertaining to how information is transferred by PAC providers to other providers and/or to patients/family/caregivers will provide important information to consumers, improving shared-decision making while selecting PAC providers. For additional technical information about this measure, including information about the measure calculation and the standardized items used to calculate this measure, we referred readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and Standardized Patient Assessment Data Elements,” available on the website at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. The data source for the quality measure is the OASIS assessment instrument for HH patients.
For more information about the data submission requirements we proposed for this measure, we refer readers to section V.L.2. of this final rule with comment period.
We invited public comment on this proposal and received one comment specific to this measure. A discussion of this comment, along with our responses, appears below. The remaining comments we received on this measure also addressed the second transfer of health information that we proposed to adopt. Those comments, along with our responses and our final decision concerning both measures, can be found in section V.E.2 of this final rule with comment period.
Comment: One commenter expressed concerns that the proposed Transfer of Health Information to the Provider-Post-Acute Care quality measure denominator does not recognize the importance of transmitting the medication list to providers, such as therapists, that are not included in the proposed definition of “admitting provider.
Response: We appreciate the suggestion to expand the Transfer of Health Information to The Provider-Post-Acute Care measure to assess the transfer of health information to include other providers such as physical therapists. We recognize the importance of all provider disciplines. Our proposed definition of “admitting provider” for purposes of the proposed measure was informed through our measure development and pilot testing process, and it focuses upon providers that can be readily identified through the discharge location item on the OASIS. This would not preclude the sharing of information that will help inform providers such as therapist who may be involved in the patients care once transferred or discharged. At this time, we believe that the current means of provider identification will improve the reliability and validity of the measure.
2. Transfer of Health Information to the Patient-Post-Acute Care (PAC) Measure
The Transfer of Health Information to the Patient-Post-Acute Care (PAC) measure is a process-based measure that assesses whether or not a current reconciled medication list was provided to the patient, family, and/or caregiver when the patient was discharged from a PAC setting to a private home/apartment, a board and care home, assisted living, a group home or transitional living.
(a) Background
In 2013, 22.3 percent of all acute hospital discharges were discharged to PAC settings, including 11 percent who were discharged to home under the care of a home health agency.[78] The communication of health information, such as a reconciled medication list, is critical to ensuring safe and effective patient transitions from health care settings to home and/or other community settings. Incomplete or missing health information, such as medication information, increases the likelihood of a risk to patient safety, often life-threatening.79 80 81 82 [83] Individuals who use PAC care services are particularly vulnerable to adverse health outcomes due to their higher likelihood of having multiple comorbid chronic conditions, polypharmacy, and complicated transitions between care settings.84 [85] Upon discharge to home, individuals in PAC settings may be faced with numerous medication changes, new medication regimes, and follow-up details.86 87 [88] The efficient and effective communication and coordination of medication information may be critical to prevent potentially deadly adverse events. When care coordination activities enhance care transitions, these activities can reduce duplication of care services and costs of care, resolve conflicting care plans, and prevent medical errors.89 [90]
Finally, the transfer of a patient's discharge medication information to the patient, family, and/or caregiver is a common practice and supported by discharge planning requirements for participation in Medicare and Medicaid programs.[91 92] Most PAC EHR systems generate a discharge medication list to promote patient participation in medication management, which has been shown to be potentially useful for improving patient outcomes and transitional care.[93]
(b) Stakeholder and TEP Input
The measure was developed after consideration of feedback we received from stakeholders, and four TEPs convened by our contractors. Further, the measure was developed after evaluation of data collected during two pilot tests, we conducted in accordance with the CMS MMS Blueprint.
Our measure development contractors convened a TEP which met on September 27, 2016,[94] January 27, 2017, and August 3, 2017 [95] to provide input on a prior version of this measure. Based on this input, we updated the measure concept in late 2017 to include the transfer of a specific component of health information—medication information. Our measure development contractors reconvened this TEP on April 20, 2018 to seek expert input on the measure. Overall, the TEP members supported the measure, affirming that the measure provides an opportunity to improve the transfer of medication information. Most of the TEP members believed that the measure could improve the transfer of medication information to patients, families, and caregivers. Several TEP members emphasized the importance of transferring information to patients and their caregivers in a clear manner using plain language. A summary of the April 20, 2018 TEP proceedings titled “Transfer of Health Information TEP Meeting 4—June 2018” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html .
Our measure development contractors solicited stakeholder feedback on the measure by requesting comment on the CMS MMS Blueprint website, and accepted comments that were submitted from March 19, 2018 to May 3, 2018. Several commenters noted the importance of ensuring that the instruction provided to patients and caregivers is clear and understandable to promote transparent access to medical record information and meet the goals of the IMPACT Act. The summary report for the March 19 to May 3, 2018 public comment period titled “IMPACT- Medication Profile Transferred Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html
(c) Pilot Testing
Between June and August 2018, we held a pilot test involving 24 PAC facilities/agencies, including five IRFs, six SNFs, six LTCHs, and seven HHAs. The 24 pilot sites submitted a total of 801 assessments. Analysis of agreement between coders within each participating facility (241 qualifying pairs) indicated 87 percent agreement for this measure. Overall, pilot testing enabled us to verify its reliability, components of face validity, and feasibility of being implemented the proposed measure across PAC settings. Further, more than half of the sites that participated in the pilot test stated, during debriefing interviews, that the measure could distinguish facilities or agencies with higher quality medication information transfer from those with lower quality medication information transfer at discharge. The pilot test summary report is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. The summary report for pilot testing conducted in 2017 of a previous version of the data element, at that time intended for benchmarking purposes only, is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
(d) Measure Applications Partnership (MAP) Review and Related Measures
This measure was submitted to the 2018 MUC list for HH QRP. The NQF-convened MAP PAC-LTC Workgroup met on December 10, 2018 and provided input on the use of the proposed Transfer of Health Information to the Patient-Post Acute-Care measure. The MAP conditionally supported this measure pending NQF endorsement, noting that the measure can promote the transfer of important medication information to the patient. The MAP recommended that providers transmit medication information to patients that is easy to understand because health literacy can impact a person's ability to take medication as directed. More information about the MAP's recommendations for this measure is available at: http://www.qualityforum.org/Projects/i-m/MAP-PAC-LTC_Workgroup/2019_Considerations_for_Implementing_Measures_Draft_Report.aspx.
Section 1899B(e)(2)(A) of the Act requires that measures specified by the Secretary under section 1899B of the Act be endorsed by the entity with a contract under section 1890(a) of the Act, which is currently the NQF. However, when a feasible and practical measure has not been NQF-endorsed for a specified area or medical topic determined appropriate by the Secretary, section 1899B(e)(2)(B) of the Act allows the Secretary to specify a measure that is not NQF-endorsed as long as due consideration is given to the measures that have been endorsed or adopted by the consensus organization identified by the Secretary. Therefore, in the absence of any NQF-endorsed measures that address the Transfer of Health Information to the Patient-Post-Acute Care (PAC), which requires that at least some of the data used to calculate the measure be collected as standardized patient assessment data through the post-acute care assessment instruments, we believe that there is currently no feasible NQF-endorsed measure that we could adopt under section 1899B(c)(1)(E) of the Act. However, we note that we intend to submit the measure to the NQF for consideration of endorsement when feasible.
(e) Quality Measure Calculation
The calculation of the Transfer of Health Information to Patient-Post-Acute Care measure would be based on the proportion of quality episodes with a discharge assessment indicating that a current reconciled medication list was provided to the patient, family, and/or caregiver at the time of discharge.
The measure denominator is the total number of HH quality episodes ending in discharge to a private home/apartment without any further services, a board and care home, assisted living, a group home or transitional living. These health care providers and settings were selected for inclusion in the denominator because they represent discharge locations captured by items on the OASIS. The measure numerator is the number of HH quality episodes with an OASIS discharge assessment indicating a current reconciled medication list was provided to the patient, family, and/or caregiver at the time of discharge. We believe that data pertaining to how information is transferred by PAC providers to other providers and/or to patients/family/caregivers will provide important information to consumers, improving shared-decision making while selecting PAC providers. For technical information about this measure including information about the measure calculation, we refer readers to the document titled “Proposed Specifications for HH QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
For more information about the data submission requirements we proposed for this measure, we refer readers to section V.L.2. of this final rule with comment period.
Commenters submitted the following comments on the two proposed transfer of health information measures that we proposed to adopt, beginning with the CY 2022 HH QRP. A discussion of these comments, along with our responses, appears in this section of this final rule with comment period.
Comment: The majority of commenters supported CMS's proposal to adopt the Transfer of Health Information to the Provider-Post-Acute Care quality measure and Transfer of Health to the Patient-Post-Acute Care quality measure beginning with the CY 2022 HH QRP. Many cited the importance of timely and accurate discharge documentation to ensure patient safety.
Response: We appreciate commenters' support for adoption of the Transfer of Health Information quality measures beginning with the CY 2022 QRP. We concur that timely information sharing during the care transfer process is critical to a safe patient transfer.
Comment: Multiple commenters stated that all measures used in the HH QRP should be endorsed by the National Quality Forum.
Response: While section 1899B(e)(2)(A) of the Act requires that any measure specified by the Secretary be endorsed by the entity with a contract under section 1890(a) of the Act, which is currently the National Quality Form (NQF), when a feasible and practical measure has not been NQF endorsed for a specified area or medical topic determined appropriate by the Secretary, section 1899B(e)(2)(B) of the Act allows the Secretary to specify a measure that is not NQF endorsed as long as due consideration is given to the measures that have been endorsed or adopted by a consensus organization identified by the Secretary. While these two measures are not currently NQF-endorsed, we recognize that the NQF endorsement process is an important part of measure development. As discussed in the CY 2020 HH PPS proposed rule (84 FR 34647 through 34648), there is currently no feasible NQF-endorsed measure that we could adopt under section 1899B(c)(1)(E) of the Act that better addresses the Transfer of Health Information measure domain. We plan to submit the measures for NQF endorsement consideration as soon as feasible.
Comment: A few commenters recommended that we expedite the timeline for beginning the collection of data on these measures. These commenters also recommended that we refrain from making any new revisions to the OASIS, such as adding new items for at least five years if we finalize the proposed changes.
Response:
In the case of the Transfer of Health Information-Provider and Transfer of Health-Patient Post-Acute Care quality measures, the timeline outlined is intended to give providers sufficient time to become familiar with the new measures and participate in trainings and other stakeholder engagement initiatives prior to submitting data on the measures. In response to the request for not making any new revisions, we will take this recommendation under consideration.
Comment: Several commenters expressed concern about anticipated additional burden of collecting the additional assessment data needed to calculate these measures.
Response: We are mindful of burden that may occur from the collection and reporting of data and measures we adopt for our quality reporting programs. The timely and complete transfer of information focuses on the medication list, as recommended by our TEP and through public comment. The transfer of health information measures are each calculated using a single OASIS item and based upon the TEP feedback and pilot test findings, we do not believe that it will be overly burdensome for HHAs to report these items. We also believe that these measures will likely drive improvements in the transfer of medication information between providers and with patients, families, and caregivers and thus justify the additional burden being imposed.
Comment: A few commenters recommended CMS adopt fewer process measures and more outcome measures for the HH QRP.
Response: While we agree that outcome measures are important, and have worked to consistently adopt outcome and claims-based measures, we also believe that process measures, are important and necessary to promote the quality of care furnished by HHAs. The proposed transfer of health measures in particular will ensure care is coordinated at the time of discharge.
Comment: One commenter recommended that the data element for the Transfer of Health Information to the Patient-Post-Acute-Care should be clear that if a Medicare beneficiary has a family caregiver, then that caregiver should receive the list if the beneficiary and family caregiver consent, even if it is also provided to the patient and that the patient, family, or caregiver should be given a chance to ask questions about the medication list to ensure they understand it.
Response: The Transfer of Health Information to the Patient-Post-Acute Care data element asks about the transfer of a reconciled medication list to the patient, family and/or caregiver. We acknowledge the importance of family and/or caregivers and encourage collaboration between the HHA and the family or caregiver when authorized by the patient. HHA staff routinely provide opportunities for family and/or caregivers to identify questions.
Comment: A few commenters requested CMS to clarify what is meant by “reconciled [medication] list” and that the contents of a reconciled medication list are left up to the discretion of the provider.
Response: Suggested elements detailed in the definition are for guidance purposes only and are not a requirement in order to meet the measure criteria. Defining the completeness of the medication list is left to the discretion of the providers and patients who are coordinating this care.
Comment: One commenter questioned the alignment of these proposed measures with the rule “Revisions to Requirements for Discharge Planning for Hospitals, Critical Access Hospitals, and Home Health Agencies” (CMS-3317-F) and requested CMS ensure alignment of an electronic option to transmit this information that aligns with the requirements in the Discharge Planning final rule.
Response: The final rule, “Revisions to Requirements for Discharge Planning for Hospitals, Critical Access Hospitals, and Home Health Agencies” (CMS-3317-F) was finalized on September 30, 2019 (84 FR 51836). In the Discharge Planning final rule, we established that effective November 29, 2019 an HHA must establish an effective discharge planning process for each patient when discharged to another PAC setting and establish a standard for the contents of the discharge summary. In addition, we established that an HHA must comply with additional requests from the receiving facility or agency when necessary for the treatment of the patient. We have worked closely with our counterparts in the agency to ensure proper alignment of this policy proposal and the requirements in our Discharge Planning final rule. We would like to note that neither policy contains a requirement for electronic options to transmit the medication list or Discharge planning information electronically. CMS is committed to furthering interoperability in post-acute care and we encourage HHAs that are electronically capturing discharge information to exchange that information electronically with providers who have the capacity to accept it.
Comment: A commenter noted that an HHA may not find out information about a transfer to an inpatient facility until after the fact and may not know to which facility the patient has been transferred.
Response: We acknowledge that there are times when a home health agency may not be notified timely about a transfer to an inpatient facility. This situation would prevent the HHA from being able to transfer the medication information to the new facility. To address this particular concern we have approved a Not Applicable (NA) response at the Transfer to Inpatient Facility time point.
Final Decision: After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Transfer of Health Information to the Provider-Post-Acute Care (PAC) and Transfer of Health Information to the Patient-Post-Acute Care (PAC) Measures under section 1899B(c)(1)(E) of the Act beginning with the CY 2022 HH QRP as proposed.
3. Update to the Discharge to Community (DTC)-Post Acute Care (PAC) Home Health (HH) Quality Reporting Program (QRP) Measure
In the CY 2020 HH PPS proposed rule (84 FR 34650 through 34651), we proposed to update the specifications for the DTC--PAC HH QRP measure (NQF #3477) to exclude baseline nursing facility (NF) residents from the measure. This measure exclusion aligns with the updates to measure exclusions for the DTC-PAC measures that we finalized in the FY 2020 SNF QRP, IRF QRP, and LTHC QRP final rules. The DTC--PAC HH QRP measure (NQF #3477) assesses successful discharge to the community from an HHA, with successful discharge to the community including no unplanned re-hospitalizations and no death in the 31 days following discharge. We adopted this measure in the CY 2017 HH PPS final rule (81 FR 76765 through 76770).
The DTC-PAC HH QRP measure (NQF #3477) does not currently exclude baseline NF residents. We have now developed a methodology to identify and exclude baseline NF residents using the Minimum Data Set (MDS) and have conducted additional measure testing work. To identify baseline NF residents, we examine any historical MDS data in the 180 days preceding the qualifying prior acute care admission and index HH episode of care start date. Presence of only an Omnibus Budget Reconciliation Act (OBRA) assessment (not a SNF PPS assessment) with no intervening community discharge between the OBRA assessment and acute care admission date flags the index HH episode of care as baseline NF resident. We assessed the impact of the baseline NF resident exclusion on HH patient- and agency-level discharge to community rates using CY 2016 and CY 2017 Medicare FFS claims data. Baseline NF residents represented 0.13 percent of the measure population after all measure exclusions were applied. The national observed patient-level discharge to community rate was 78.05 percent when baseline NF residents were included in the measure, increasing to 78.08 percent when they were excluded from the measure. After excluding baseline NF residents to align with current or proposed exclusions in other PAC settings, the agency-level risk-standardized discharge to community rate ranged from 3.21 percent to 100 percent, with a mean of 77.39 percent and standard deviation of 17.27 percentage points, demonstrating a performance gap in this domain. That is, the results show that there is a wide range in measure results, emphasizing the opportunity for providers to improve their measure performance.
Accordingly, in the CY 2020 HH PPS proposed rule (84 FR 34650 through 34651), we proposed to exclude baseline NF residents from the DTC-PAC HH QRP measure beginning with the CY 2021 HH QRP. We proposed to define “baseline NF residents” for purposes of this measure as HH patients who had a long-term NF stay in the 180 days preceding their hospitalization and HH episode, with no intervening community discharge between the NF stay and qualifying hospitalization. We are currently using MDS assessments, which are required quarterly for NF residents, to identify baseline NF residents. A 180-day lookback period ensures that we will capture both quarterly OBRA assessments identifying NF residency and any discharge assessments to determine if there was a discharge to community from NF.
For additional technical information regarding the DTC-PAC HH QRP measure (NQF #3477), including technical information about the proposed exclusion, we referred readers to the document titled “Proposed Specifications for HH QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We invited public comment on this proposal and received several comments. A discussion of these comments, along with our responses, appears in this section of this final rule with comment period.
Comment: The majority of commenters supported CMS' proposal to exclude baseline nursing home residents from the DTC-PAC HH QRP measure (NQF #3477), and expressed appreciation for CMS' responsiveness to stakeholder feedback.
Response: CMS appreciates commenters' support for excluding NF residents from the DTC-PAC HH QRP measure (NQF #3477).
Comment: MedPAC did not support the proposed exclusion of baseline nursing facility residents from the DTC—PAC HH QRP measure (NQF #3477). They suggested that CMS instead expand their definition of “return to the community” to include baseline nursing home residents returning to the nursing home where they live, as this represents their home or community. MedPAC also stated that providers should be held accountable for the quality of care they provide for as much of their Medicare patient population as feasible.
Response: We agree with MedPAC that providers should be held accountable for the quality of care for as much of their Medicare population as feasible. However, we believe this exclusion is necessary to enhance the validity of this measure. For baseline nursing facility residents, the goal of care is successful discharge back to their residence at the nursing facility, which is considered an unsuccessful outcome in this measure, rather than a discharge to the community (defined as home/self-care without HH services). The use of risk adjustment is inappropriate when the measurable outcome of success is not the goal of care for this population.
Community is traditionally understood as representing non-institutional settings by policy makers, providers, and other stakeholders. Including long-term care NF in the definition of community would confuse this long-standing concept of community and would misalign with CMS' definition of community in patient assessment instruments. We conceptualized this measure using the traditional definition of “community” and specified the measure as a discharge to community measure, rather than a discharge to baseline residence measure.
Baseline NF residents represent an inherently different patient population with not only a significantly lower likelihood of discharge to community settings, but also a higher likelihood of post-discharge readmissions and death compared with PAC patients who did not live in a NF at baseline. The inherent differences in patient characteristics and PAC processes and goals of care for baseline NF residents and non-NF residents are significant enough that we do not believe risk adjustment using a NF flag would provide adequate control. While we acknowledge that a return to nursing home for baseline NF residents represents a return to their home, this outcome does not align with our measure concept. Thus, we have chosen to exclude baseline NF residents from the measure.
Comment: One commenter noted that the Discharge to Community measure may incentivize inappropriate discharges, adding that the community is not always the best option for some patients. This commenter further noted that this measure could result in agencies not accepting certain types of patients.
Response: We appreciate the importance of incentivizing holistic, patient-specific health decisions and to that end The Discharge to Community measure is risk adjusted based on multiple initial patient characteristics, including diagnoses and previous hospitalizations. This risk adjustment accounts for potentially higher risk of readmission or death and addresses any incentives to not admit or inappropriately discharge high-risk patients.
Final Decision: After consideration of the public comments, we are finalizing our proposal to exclude baseline NF residents from the DTC-PAC HH QRP measure (NQF #3477) beginning with the CY 2021 HH QRP. We are also finalizing our proposal to define “baseline NF residents” for purposes of this measure as HH patients who had a long-term NF stay in the 180 days preceding their hospitalization and HH episode, with no intervening community discharge between the NF stay and qualifying hospitalization.
F. HH QRP Quality Measures, Measure Concepts, and Standardized Patient Assessment Data Elements Under Consideration for Future Years: Request for Information
In the CY 2020 HH PPS proposed rule (84 FR 34651), we sought input on the importance, relevance, appropriateness, and applicability of each of the measures, standardized patient assessment data elements (SPADEs), and measure concepts under consideration listed in the Table 29 for future years in the HH QRP.
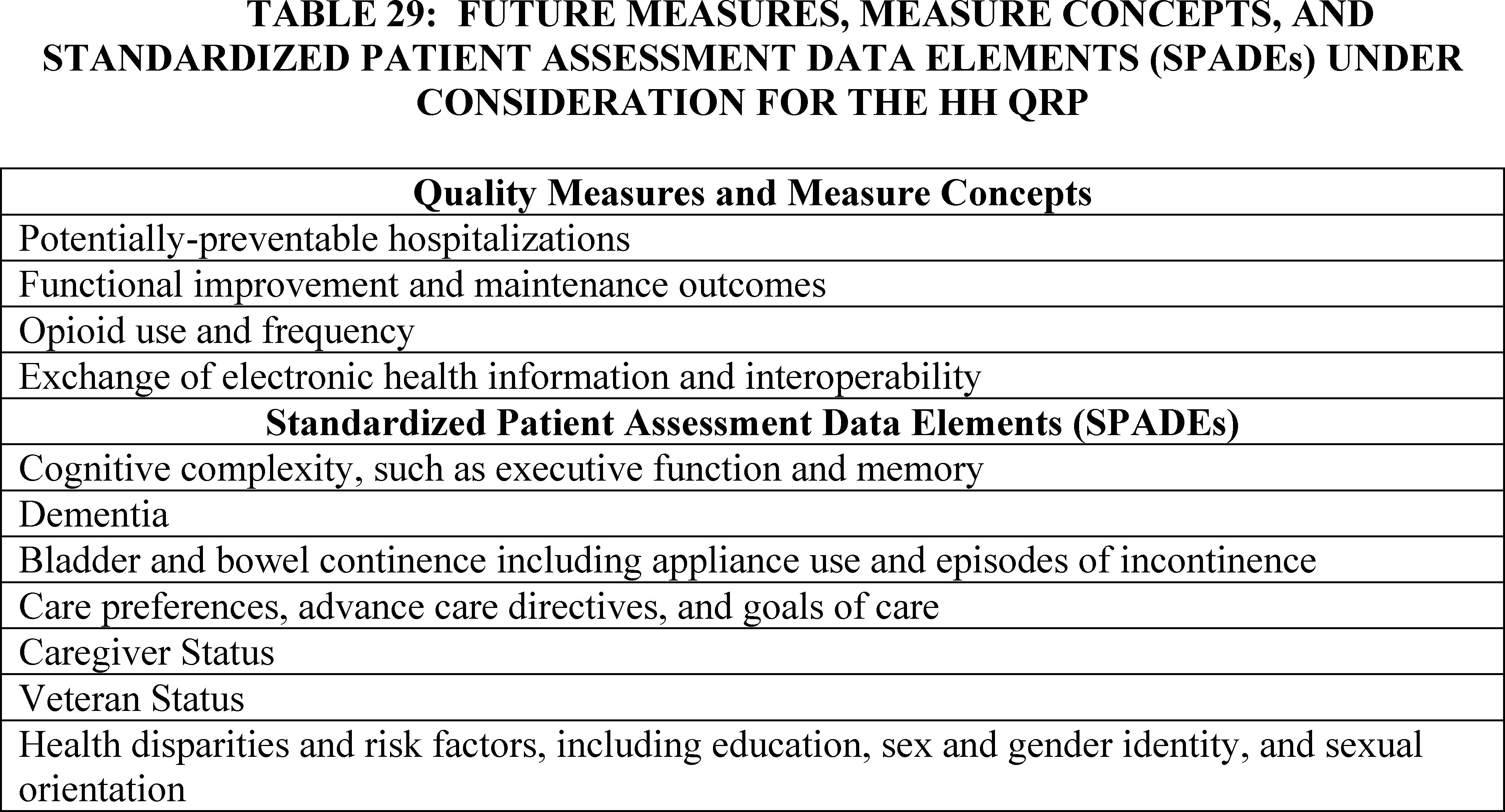
While we are not responding to comment submissions in response to this Request for Information in the CY 2020 HH PPS final rule with comment period, nor are we finalizing any of these measures, measure concepts, and SPADEs under consideration for the HH QRP in this CY 2020 HH PPS final rule with comment period, we appreciate all commenter suggestions and intend to use this input to inform our future measure and SPADE development efforts.
Comment: A number of commenters supported the broad range of measures and data elements suggested as future additions to the OASIS and the HH QRP. One provider stated strong support for CMS's plans to adopt an exchange of health information measure, stressing the need for adoption of interoperable health information technology in PAC settings and in this case in home health. A number of providers supported future adoption of functional improvement outcome measures while a few commenters stressed the value of having maintenance measures focused on patients who are not likely to improve. Another commenter stressed the need for avoiding unintended consequences in punishing HHAs with patients who are expected to decline. A commenter supported the opioid use and frequency quality measure, but stressed the need to ensure that providers aren't penalized for appropriately prescribing medications. Another commenter expressed concern that the adoption of an opioid use and frequency measure may adversely affect the appropriate use of opioids. A few providers suggested a criterion of CMS only including measures in the HH QRP program that have already received NQF endorsement. A few others suggested that CMS strongly pursue removing less useful measures and data elements from the HH QRP at the time in which new measures or data elements are considered for supplementing the HH QRP.
With respect to future SPADE proposals, one commenter strongly supported introduction of a caregiver status data element. A few other commenters suggested the need to add data elements that address housing and food security to any social determinants of health SPADEs under consideration. One commenter stressed the need for current and future SPADEs to more adequately account for patients with a broader range of speech, hearing, and swallowing abilities. Finally, one commenter suggested that CMS should not consider introducing any data element that has not already undergone data testing since this limits the ability of providers and the general public to provide input into potential implementation implications of the data elements.
We appreciate the feedback submitted on these issues.
G. Standardized Patient Assessment Data Reporting Beginning With the CY 2022 HH QRP
Section 1895(b)(3)(B)(v)(IV)(bb) of the Act requires that, for CY 2019 (beginning January 1, 2019) and each subsequent year, HHAs report standardized patient assessment data required under section 1899B(b)(1) of the Act. Section 1899B(a)(1)(C) of the Act requires, in part, the Secretary to modify the PAC assessment instruments in order for PAC providers, including HHAs, to submit SPADEs under the Medicare program. Section 1899B(b)(1)(A) of the Act requires that PAC providers must submit SPADEs under applicable reporting provisions, (which for HHAs is the HH QRP) with respect to the admissions and discharges of an individual (and more frequently as the Secretary deems appropriate), and section 1899B(b)(1)(B) defines standardized patient assessment data as data required for at least the quality measures described in section 1899B(c)(1) of the Act and that is with respect to the following categories: (1) Functional status, such as mobility and self-care at admission to a PAC provider and before discharge from a PAC provider; (2) cognitive function, such as ability to express ideas and to understand, and mental status, such as depression and dementia; (3) special services, treatments, and interventions, such as need for ventilator use, dialysis, chemotherapy, central line placement, and total parenteral nutrition; (4) medical conditions and comorbidities, such as diabetes, congestive heart failure, and pressure ulcers; (5) impairments, such as incontinence and an impaired ability to hear, see, or swallow; and (6) other categories deemed necessary and appropriate by the Secretary.
In the CY 2018 HH PPS proposed rule (82 FR 35355 through 35371), we proposed to adopt SPADEs that would satisfy the first five categories. While many commenters expressed support for our adoption of SPADEs, including support for our broader standardization goal and support for the clinical usefulness of specific proposed SPADEs in general, we did not finalize the majority of our SPADE proposals in recognition of the concern raised by many commenters that we were moving too fast to adopt the SPADEs and modify our assessment instruments in light of all of the other requirements we were also adopting under the IMPACT Act at that time (82 FR 51737 through 51740). In addition, we noted our intention to conduct extensive testing to ensure that the standardized patient assessment data elements we select are reliable, valid, and appropriate for their intended use (82 FR 51732 through 51733).
However, we did, finalize the adoption of SPADEs for two of the categories described in section 1899B(b)(1)(B) of the Act: (1) Functional status: Data elements currently reported by HHAs to calculate the measure Application of Percent of Long-Term Care Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That Addresses Function (NQF #2631) along with the additional data elements in Section GG: Functional Abilities and Goals; and (2) Medical conditions and comorbidities: The data elements used to calculate the pressure ulcer measures, Percent of Residents or Patients with Pressure Ulcers That Are New or Worsened (Short Stay) (NQF #0678) and the replacement measure, Changes in Skin Integrity Post-Acute Care: Pressure Ulcer/Injury. We stated that these data elements were important for care planning, known to be valid and reliable, and already being reported by HHAs for the calculation of quality measures (82 FR 51733 through 51735).
Since we issued the CY 2018 HH PPS final rule, HHAs have had an opportunity to familiarize themselves with other new reporting requirements that we have adopted under the IMPACT Act. We have also conducted further testing of the proposed SPADEs, as described more fully elsewhere in this final rule with comment period, and believe that this testing supports their use in our PAC assessment instruments. Therefore, we proposed to adopt many of the same SPADEs that we previously proposed to adopt, along with other SPADEs.
In the CY 2020 HH PPS proposed rule (84 FR 34652), we proposed that HHAs would be required to report these SPADEs beginning with the CY 2022 HH QRP. If finalized as proposed, HHAs would be required to report this data with respect to admissions and discharges that occur between January 1, 2021 and June 30, 2021 for the CY 2022 HH QRP. Beginning with the CY 2023 HH QRP, we proposed that HHAs must report data with respect to admissions and discharges that occur the successive calendar year (for example, data from FY 2021 for the CY 2023 HH QRP and data from FY 2022 for the CY 2024 HH QRP). For the purposes of the HH QRP, we proposed that HHAs must submit SPADEs with respect to start of care (SOC), resumption of care (ROC), and discharge with the exception of Hearing, Vision, Race, and Ethnicity SPADEs, which will only be collected with respect to SOC. We proposed to use SOC for purposes of admissions because, in the HH setting, the start of care is functionally the same as an admission.
We proposed that HHAs that submit the Hearing, Vision, Race, and Ethnicity SPADEs with respect to SOC only will be deemed to have submitted those SPADEs with respect to both admission and discharge, because it is unlikely that the assessment of those SPADEs at admission will differ from the assessment of the same SPADEs at discharge.
We considered the burden of assessment-based data collection and aimed to minimize additional burden by evaluating whether any data that is currently collected through one or more PAC assessment instruments could be collected as SPADE. In selecting the proposed SPADEs, we also took into consideration the following factors with respect to each data element:
- Overall clinical relevance.
- Interoperable exchange to facilitate care coordination during transitions in care.
- Ability to capture medical complexity and risk factors that can inform both payment and quality.
- Scientific reliability and validity, general consensus agreement for its usability.
In identifying the SPADEs proposed, we additionally drew on input from several sources, including TEPs, public input, and the results of a recent National Beta Test of candidate data elements conducted by our data element (hereafter “National Beta Test”), contractor.
The National Beta Test collected data from 3,121 patients and residents across 143 LTCHs, SNFs, IRFs, and HHAs from November 2017 to August 2018 to evaluate the feasibility, reliability, and validity of candidate data elements across PAC settings. The National Beta Test also gathered feedback on the candidate data elements from staff who administered the test protocol in order to understand usability and workflow of the candidate data elements. More information on the methods, analysis plan, and results for the National Beta Test can be found in the document titled, “Development and Evaluation of Candidate Standardized Patient Assessment Data Elements: Findings from the National Beta Test (Volume 2),” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Further, to inform the proposed SPADEs, we took into account feedback from stakeholders, as well as from technical and clinical experts, including feedback on whether the candidate data elements would support the factors described previously. Where relevant, we also took into account the results of the Post-Acute Care Payment Reform Demonstration (PAC PRD) that took place from 2006 to 2012.
We invited public comment on these proposals and received several comments. A discussion of these comments, along with our responses, appears in this section of this final rule with comment period.
Comment: A majority of commenters expressed support for the adoption of the SPADEs within the categories of: Cognitive function and mental status; special services, treatments, and interventions; medical condition and comorbidity data; and impairments. Supporters of the SPADE proposals highlighted the benefit of assessing the areas of SPADEs across post-acute care settings.
Response: CMS thanks the commenters for their support of the goals of standardization and of the proposed SPADEs. We selected the proposed SPADEs in part because of the attributes that the commenters noted.
Comment: Some commenters suggested the need to remove duplicative items in the OASIS and to continually assess the value of the proposed data elements. A number of commenters expressed overall concern with the adoption of the SPADEs due to an anticipated increase in administrative burden for providers. Commenters recommended mitigating this burden through introducing SPADEs over a number of years instead of all at one time. Numerous commenters supported the following recommendations:
1. CMS should issue a draft of the assessment tool no later than 6 months prior to the implementation date, to allow for staff training and other necessary preparations required for agency implementation;
2. CMS should use the authority permitted by the IMPACT Act to waive the Paperwork Reduction Act (PRA) requirements related to modification of the assessment tools for providers subject to the IMPACT Act and expedite CMS's ability to issue a final version of the revised OASIS instrument in a timely manner;
3. CMS should refrain from issuing any revisions to the OASIS instrument for at least 5 years after the 2021 implementation of the proposed changes.
Response: Our development and selection process for the SPADEs prioritized data elements essential to comprehensive patient care. While the introduction of SPADEs will require some additional burden, we maintain that there will be significant benefit associated with each of the SPADEs to providers and patients, in that they are clinically useful (for example, for care planning), they support patient-centered care, and they will promote interoperability and data exchange between providers.
We appreciate the importance of avoiding undue burden and will continue to evaluate and consider any burden the IMPACT Act and the HH QRP places on home health providers. In implementing the IMPACT Act thus far, we have taken into consideration any new burden that our requirements might place on PAC providers. We were also cognizant of the changes that providers will need to make to implement these additions to the OASIS. In CY 2018 HH PPS final rule (82 FR 51732), we provided information about goals, scope, and timeline for implementing SPADEs, as well as updated HHAs about ongoing development and testing of data elements through other public forums. In terms of the timing of the release of the OASIS, we plan to publish a draft of the revised OASIS instrument in early 2020.
Comment: Some commenters suggested that CMS implement the SPADES more slowly than proposed.
Response: We believe the current schedule is appropriate because it aligns with the requirements of the IMPACT Act and because of our efforts to date to prepare for the implementation of new cross-setting SPADES. Our development and selection process for the SPADEs we are adopting in this final rule with comment period reflect prioritized data elements that are essential to comprehensive patient care. We maintain that there will be significant benefit associated with each of the SPADEs to providers and patients, in that they are clinically useful (for example, for care planning), they support patient-centered care, and they will promote interoperability and data exchange between providers. We therefore believe that the proposed implementation timeline for the SPADEs is appropriate.
Comment: One commenter expressed concerns about the methodology of the National Beta Test, noting their belief that the sample was not nationally representative.
Response: The National Beta Test was designed to generate valid and robust national SPADE performance estimates for each of the four PAC provider types. This required acceptable geographic diversity, sufficient sample size, and reasonable coverage of the range of clinical characteristics. To meet these requirements, the National Beta Test was carefully designed so that data could be collected from a wide range of environments (such as geographic regions, and PAC providers of different types, sizes, and ownership), allowing for thorough evaluation of candidate SPADE performance in all PAC settings. The approach included a stratified random sample, to maximize generalizability, and subsequent analyses included extensive checks on the sampling design.
In a document that we issued in conjunction with the proposed rule (entitled “Proposed Specifications for HH QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html), we described key findings from the National Beta Test related to the proposed SPADEs. We refer readers to an initial volume of the National Beta Test report that details the methodology of the field test (“Development and Evaluation Candidate Standardized Patient Assessment Data Elements: Findings from the National Beta Test (Volume 2),” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html).
Comment: Some commenters recommended that CMS leverage electronic health record initiatives to better utilize SPADEs in home health agencies.
Response: It is our intention to use the SPADE data to inform the common standards and definitions to facilitate interoperable exchange of data. We believe that a core, standardized set of data elements that could be shared across PAC and other provider types is an important first step to foster this interoperability between providers. We are hopeful that by requiring the collection of standardized data, the SPADEs may spur providers, such as home health agencies, to adopt health information technology that eases the burden associated with data collection and data exchange. Further, we believe that the collection of these SPADEs reflect common clinical practice and will improve discharge planning, as well as address errors that can occur during transition from one setting to the next. We note the collection of the SPADEs is one of many tasks to supporting interoperability. We will take into consideration how best to decrease burden from data collection including our manual processes. Additionally, we will take into consideration ways to help incentivize providers to adopt health information technology.
Comment: Some commenters stated support for the proposed SPADEs, but noted reservations that the SPADEs aren't sufficient to address all areas of assessment. One commenter described the SPADEs as an appropriate start, but noted that the SPADEs cannot stand alone, and must be built upon in order to be useful for risk adjustment and quality measurement.
Response: We believe that the SPADEs as proposed represent an important core set of information about clinical status and patient characteristics that may be used for risk adjustment. Additionally, we will continue to assess the use of the SPADES across our PAC settings, including the feasibility, reliability, validity and usability of the data elements in future risk adjustment models and quality measures. We also welcome continued input, recommendations, and feedback from stakeholders about ways to improve assessment and quality measurement for PAC providers, including ways that the SPADEs could be used in the HH QRP. Input can be shared with CMS through our PAC Quality Initiatives email address PACQualityInitiative@cms.hhs.gov .
H. Standardized Patient Assessment Data by Category
1. Cognitive Function and Mental Status Data
A number of underlying conditions, including dementia, stroke, traumatic brain injury, side effects of medication, metabolic and/or endocrine imbalances, delirium, and depression, can affect cognitive function and mental status in PAC patient and resident populations.[96] The assessment of cognitive function and mental status by PAC providers is important because of the high percentage of patients and residents with these conditions,[97] and because these assessments provide opportunity for improving quality of care.
Symptoms of dementia may improve with pharmacotherapy, occupational therapy, or physical activity,98 99 [100] and promising treatments for severe traumatic brain injury are currently being tested.[101] For older patients and residents diagnosed with depression, treatment options to reduce symptoms and improve quality of life include antidepressant medication and psychotherapy,102 103 104 [105] and targeted services, such as therapeutic recreation, exercise, and restorative nursing, to increase opportunities for psychosocial interaction.[106]
In alignment with our Meaningful Measures Initiative, accurate assessment of cognitive function and mental status of patients and residents in PAC is expected to make care safer by reducing harm caused in the delivery of care; promoting effective prevention and treatment of chronic disease; strengthening person and family engagement as partners in their care; and promoting effective communication and coordination of care. For example, standardized assessment of cognitive function and mental status of patients and residents in PAC will support establishing a baseline for identifying changes in cognitive function and mental status (for example, delirium), anticipating the patient's or resident's ability to understand and participate in treatments during a PAC stay, ensuring patient and resident safety (for example, risk of falls), and identifying appropriate support needs at the time of discharge or transfer. SPADEs will enable or support clinical decision-making and early clinical intervention; person-centered, high quality care through facilitating better care continuity and coordination; better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable SPADEs assessing cognitive function and mental status are needed in order to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events. We describe each of the proposed cognitive function and mental status data SPADEs elsewhere in the final rule.
We invited comment on our proposals to collect as standardized patient assessment data the following data with respect to cognitive function and mental status. Commenters submitted the following comments related to the proposed rule's discussion of the cognitive function and mental status data elements.
Comment: A number of commenters supported the proposed use of the BIMS and CAM, but also raised concerns with the lack of sensitivity of these assessments for identifying mild to moderate cognitive impairment that can impact performance of activities of daily living (ADLs).
Response: We acknowledge the limitations of the proposed SPADEs to fully assess all areas of cognition and mental status. We strived to balance the scope and level of detail of the data elements against the potential burden placed on patients and providers. In our past work, we evaluated the potential of several different cognition assessments for use as standardized data elements in PAC settings. We ultimately decided on the data elements in our proposal as a starting point, and we welcome continued input, recommendations, and feedback from stakeholders about additional data elements for standardization, which can be shared with CMS through our PAC Quality Initiatives email address: PACQualityInitiative@cms.hhs.gov.
Comment: Another provider recommended supplementing the BIMS and CAM specifically with the Development of Outpatient Therapy Payment Alternatives (DOTPA) items for post-acute assessments. They suggest that DOTPA items, coupled with a functional screen to detect practical problems, need to be administered during PAC assessments.
Response: We evaluated the suitability of the DOTPA, as well as other screening tools that targeted functional cognition, by engaging our TEP, through “alpha” feasibility testing, and through soliciting input from stakeholders. At the second TEP meeting in March 2017, members questioned the use of data elements that rely on assessor observation and judgment, such as DOTPA CARE tool items, and favored other assessments of cognition that required patient interview or patient actions. The TEP also discussed performance-based assessment of functional cognition. These are assessments that require patients to respond by completing a simulated task, such as ordering from a menu, or reading medication instructions and simulating the taking of medications, as required by the Performance Assessment of Self-Care Skills (PASS) items. In Alpha 2 feasibility testing, which was conducted between April and July 2017, we included a subset of items from the DOTPA as well as the PASS. Findings of that test identified several limitations of the DOTPA items for use as SPADEs, such as the length of time to administer (5 to 7 minutes). In addition, interrater reliability was highly variable among the DOTPA items, both overall and across settings, with some items showing very low agreement (as low as 0.34) and others showing excellent agreement (as high as 0.81). Similarly, findings of the Alpha 2 feasibility test identified several limitations of the PASS for use as SPADEs. The PASS was relatively time-intensive to administer (also 5 to 7 minutes), many patients in HHAs needed assistance completing the PASS tasks, and missing data were prevalent. Unlike the DOTPA items, interrater reliability was consistently high overall for PASS (ranging from 0.78 to 0.92), but the high reliability was not deemed to outweigh fundamental feasibility concerns related to administration challenges. A summary report for the Alpha 2 feasibility testing titled “Development and Maintenance of Standardized Cross Setting Patient Assessment Data for Post-Acute Care: Summary Report of Findings from Alpha 2 Pilot Testing” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Alpha-2-SPADE-Pilot-Summary-Document.pdf. While we received support for the DOTPA, PASS, and other assessments of functional cognition, commenters also raised concerns about the reliability of the DOTPA, given that it is based on staff evaluation, and the feasibility of the PASS, given that the simulated medication task requires props, such as a medication bottle with printed label and pill box, which may not be accessible in all settings.
Based on the input from our TEP, results of alpha feasibility testing, and input from stakeholders, we decided to propose the BIMS for standardization at this time due to the body of research literature supporting its feasibility and validity, its relative brevity, and its existing use in the MDS and IRF-PAI.
a. Brief Interview for Mental Status (BIMS)
In the CY 2020 HH PPS proposed rule (84 FR 34653 through 34654), we proposed that the data elements that comprise the BIMS meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35356 through 35357), dementia and cognitive impairment are associated with long-term functional dependence and, consequently, poor quality of life and increased health care costs and mortality.[107] This makes assessment of mental status and early detection of cognitive decline or impairment critical in the PAC setting. The intensity of routine nursing care is higher for patients and residents with cognitive impairment than those without, and dementia is a significant variable in predicting readmission after discharge to the community from PAC providers.[108]
The BIMS is a performance-based cognitive assessment screening tool that assesses repetition, recall with and without prompting, and temporal orientation. The data elements that make up the BIMS are seven questions on the repetition of three words, temporal orientation, and recall that result in a cognitive function score. The BIMS was developed to be a brief objective screening tool with a focus on learning and memory. As a brief screener, the BIMS was not designed to diagnose dementia or cognitive impairment, but rather to be a relatively quick and easy to score assessment that could identify cognitively impaired patients as well as those who may be at risk for cognitive decline and require further assessment. It is currently in use in two of the PAC assessments: The MDS in SNFs and the IRF-PAI used by IRFs. For more information on the BIMS, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The data elements that comprise the BIMS were first proposed as SPADEs in the CY 2018 HH PPS proposed rule (82 FR 35356 through 35357). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 expressed support for use of the BIMS, noting that it is reliable, feasible to use across settings, and will provide useful information about patients and residents. We also stated that those commenters had noted that the data collected through the BIMS will provide a clearer picture of patient or resident complexity, help with the care planning process, and be useful during care transitions and when coordinating across providers. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, we received public comments in support of the use of the BIMS in the HH setting. However, a commenter suggested the BIMS should be administered with respect to both admission and discharge, and another commenter encouraged its use at follow-up assessments. Another commenter expressed support for the BIMS to assess significant cognitive impairment, but a few commenters suggested alternative cognitive assessments as more appropriate for the HH settings, such as assessments that would capture mild cognitive impairment and “functional cognition.”
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the BIMS was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the BIMS to be feasible and reliable for use with PAC patients and residents. We stated in the proposed rule that more information about the performance of the BIMS in the National Beta Test could be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018 for the purpose of soliciting input on the BIMS, and the TEP supported the assessment of patient or resident cognitive status with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Some commenters expressed concern that the BIMS, if used alone, may not be sensitive enough to capture the range of cognitive impairments, including mild cognitive impairment (MCI). A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We understand the concerns raised by stakeholders that BIMS, if used alone, may not be sensitive enough to capture the range of cognitive impairments, including functional cognition and MCI, but note that the purpose of the BIMS data elements as SPADEs is to screen for cognitive impairment in a broad population. We also acknowledge that further cognitive tests may be required based on a patient's condition and will take this feedback into consideration in the development of future standardized assessment data elements. However, taking together the importance of assessing cognitive status, stakeholder input, and strong test results, we proposed that the BIMS data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act and to adopt the BIMS as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect the BIMS as standardized patient assessment data. We did not receive additional comments specific to the BIMS. General comments on the category of Cognitive Function and Mental Status are discussed in section V.H.1 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the BIMS as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
b. Confusion Assessment Method (CAM)
In the CY 2020 HH PPS proposed rule (84 FR 34654 through 34655), we proposed that the data elements that comprise the Confusion Assessment Method (CAM) meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35357), the CAM was developed to identify the signs and symptoms of delirium. It results in a score that suggests whether a patient or resident should be assigned a diagnosis of delirium. Because patients and residents with multiple comorbidities receive services from PAC providers, it is important to assess delirium, which is associated with a high mortality rate and prolonged duration of stay in hospitalized older adults.[109] Assessing these signs and symptoms of delirium is clinically relevant for care planning by PAC providers.
The CAM is a patient assessment instrument that screens for overall cognitive impairment, as well as distinguishes delirium or reversible confusion from other types of cognitive impairment. The CAM is currently in use in two of the PAC assessments: A four-item version of the CAM is used in the MDS in SNFs, and a six-item version of the CAM is used in the LTCH CARE Data Set (LCDS) in LTCHs. We proposed the four-item version of the CAM that assesses acute change in mental status, inattention, disorganized thinking, and altered level of consciousness. For more information on the CAM, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The data elements that comprise the CAM were first proposed as SPADEs in the CY 2018 HH PPS proposed rule (82 FR 35357). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on the CAM from August 12 to September 12, 2016 expressed support for use of the CAM, noting that it would provide important information for care planning and care coordination and, therefore, contribute to quality improvement. We also stated that those commenters had noted the CAM is particularly helpful in distinguishing delirium and reversible confusion from other types of cognitive impairment. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, a commenter expressed support for the CAM to assess significant cognitive impairment but noted that functional cognition should also be assessed. Another commenter suggested the CAM was not suitable for the HH setting and noted that the additional cognition items would be redundant with existing assessment items in the OASIS data set.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the CAM was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the CAM to be feasible and reliable for use with PAC patients and residents. More information about the performance of the CAM in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, although they did not specifically discuss the CAM data elements, the TEP supported the assessment of patient or resident cognitive status with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing delirium, stakeholder input, and strong test results, we proposed that the CAM data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act and to adopt CAM as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposals to collect as standardized patient assessment data the following data with respect to the CAM. We did not receive any comments specific to the CAM. General comments on the category of Cognitive Function and Mental Status are discussed in section V.H.1 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the CAM data elements as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
c. Patient Health Questionnaire—2 to 9 (PHQ-2 to 9)
In CY 2020 HH PPS proposed rule (84 FR 34655 through 34656), we proposed that the Patient Health Questionnaire—2 to 9 (PHQ-2 to 9) data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act. The proposed data elements are based on the PHQ-2 mood interview, which focuses on only the two cardinal symptoms of depression, and the longer PHQ-9 mood interview, which assesses presence and frequency of nine signs and symptoms of depression. The name of the data element, the PHQ-2 to 9, refers to an embedded skip pattern that transitions patients with a threshold level of symptoms in the PHQ-2 to the longer assessment of the PHQ-9. The skip pattern is described in detail in this section of this final rule with comment period.
As described in the CY 2018 HH PPS proposed rule (82 FR 35358 through 35359), depression is a common and under-recognized mental health condition. Assessments of depression help PAC providers better understand the needs of their patients and residents by: Prompting further evaluation after establishing a diagnosis of depression; elucidating the patient's or resident's ability to participate in therapies for conditions other than depression during their stay; and identifying appropriate ongoing treatment and support needs at the time of discharge.
The proposed PHQ-2 to 9 is based on the PHQ-9 mood interview. The PHQ-2 consists of questions about only the first two symptoms addressed in the PHQ-9: Depressed mood and anhedonia (inability to feel pleasure), which are the cardinal symptoms of depression. The PHQ-2 has performed well as both a screening tool for identifying depression, to assess depression severity, and to monitor patient mood over time.[110 111] If a patient demonstrates signs of depressed mood and anhedonia under the PHQ-2, then the patient is administered the lengthier PHQ-9. This skip pattern (also referred to as a gateway) is designed to reduce the length of the interview assessment for patients who fail to report the cardinal symptoms of depression. The design of the PHQ-2 to 9 reduces the burden that would be associated with the full PHQ-9, while ensuring that patients with indications of depressive symptoms based on the PHQ-2 receive the longer assessment.
Components of the proposed data elements are currently used in the OASIS for HHAs (PHQ-2) and the MDS for SNFs (PHQ-9). We proposed to add the additional data elements of the PHQ-9 to the OASIS to replace M1730, Depression Screening. We are proposed to alter the administration instructions for the existing and new data elements to adopt the PHQ-2 to 9 gateway logic, meaning that administration of the full PHQ-9 is contingent on patient responses to questions about the cardinal symptoms of depression. For more information on the PHQ-2 to 9, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The PHQ-2 data elements were first proposed as SPADEs in the CY 2018 HH proposed rule (82 FR 35358 through 35359). In that proposed rule, we stated that the proposal was informed by input we received from the TEP convened by our data element contractor on April 6 and 7, 2016. The TEP members particularly noted that the brevity of the PHQ-2 made it feasible to administer with low burden for both assessors and PAC patients or residents. A summary of the April 6 and 7, 2016 TEP meeting titled “SPADE Technical Expert Panel Summary (First Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
That rule proposal was also informed by public input that we received through a call for input published on the CMS Measures Management System Blueprint website. Input was submitted from August 12 to September 12, 2016 on three versions of the PHQ depression screener: The PHQ-2; the PHQ-9; and the PHQ-2 to 9 with the skip pattern design. Many commenters were supportive of the standardized assessment of mood in PAC settings, given the role that depression plays in well-being. Several commenters expressed support for an approach that would use PHQ-2 as a gateway to the longer PHQ-9 while still potentially reducing burden on most patients and residents, as well as test administrators, and ensuring the administration of the PHQ-9, which exhibits higher specificity,[112] for patients and residents who showed signs and symptoms of depression on the PHQ-2. A summary report for to the September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, we received public comments in support of the PHQ-2, with a few commenters noting the limitation that the PHQ-2 is not appropriate for patients who are physically or cognitively impaired.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the PHQ-2 to 9 data elements were included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the PHQ-2 to 9 to be feasible and reliable for use with PAC patients and residents. More information about the performance of the PHQ-2 to 9 in the National Beta Test can be found in the document titled, “Final Specifications for CY 2020 HH QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the PHQ-2 to 9. The TEP was supportive of the PHQ-2 to 9 data element set as a screener for signs and symptoms of depression. The TEP's discussion noted that symptoms evaluated by the full PHQ-9 (for example, concentration, sleep, appetite) had relevance to care planning and the overall well-being of the patient or resident, but that the gateway approach of the PHQ-2 to 9 would be appropriate as a depression screening assessment, as it depends on the well-validated PHQ-2 and focuses on the cardinal symptoms of depression. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing depression, stakeholder input, and strong test results, we proposed that the PHQ-2 to 9 data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act and to adopt the PHQ-2 to 9 data elements as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposals to collect as standardized patient assessment data the PHQ-2 to 9 data elements. We did not receive comments specific to the PHQ-2 to 9 data elements. General comments on this category of Cognitive Function and Mental Status are discussed in section V.H.1 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the PHQ-2 to 9 data elements as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
2. Special Services, Treatments, and Interventions Data
Special services, treatments, and interventions performed in PAC can have a major effect on an individual's health status, self-image, and quality of life. The assessment of these special services, treatments, and interventions in PAC is important to ensure the continuing appropriateness of care for the patients and residents receiving them, and to support care transitions from one PAC provider to another, an acute care hospital, or discharge. In alignment with our Meaningful Measures Initiative, accurate assessment of special services, treatments, and interventions of patients and residents served by PAC providers is expected to make care safer by reducing harm caused in the delivery of care; promoting effective prevention and treatment of chronic disease; strengthening person and family engagement as partners in their care; and promoting effective communication and coordination of care.
For example, standardized assessment of special services, treatments, and interventions used in PAC can promote patient and resident safety through appropriate care planning (for example, mitigating risks such as infection or pulmonary embolism associated with central intravenous access), and identifying life-sustaining treatments that must be continued, such as mechanical ventilation, dialysis, suctioning, and chemotherapy, at the time of discharge or transfer. Standardized assessment of these data elements will enable or support: Clinical decision-making and early clinical intervention; person-centered, high quality care through, for example, facilitating better care continuity and coordination; better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable data elements assessing special services, treatments, and interventions are needed to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events. We provide rationale and further support for each of the proposed data elements and in the document titled, “Proposed Specifications for CY 2020 HH QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
A TEP convened by our data element contractor provided input on the data elements for special services, treatments, and interventions. In a meeting held on January 5 and 6, 2017, the TEP found that these data elements are appropriate for standardization because they would provide useful clinical information to inform care planning and care coordination. The TEP affirmed that assessment of these services and interventions is standard clinical practice, and that the collection of these data by means of a list and checkbox format would conform to common workflow for PAC providers. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Comments on the category of special services, treatments, and interventions were also submitted by stakeholders during the CY 2018 HH PPS proposed rule (82 FR 35359 through 35369) public comment period. A few commenters expressed support for the special services, treatments, and interventions data elements but requested that a vendor be contracted to support OASIS questions and answers. A commenter noted that many of these data elements were redundant with current assessment items and encouraged CMS to eliminate the redundancy by removing items similar to the proposed data elements. Another commenter noted that collecting these data elements on patients that come to the HH setting from non-affiliated entities can be challenging. The Medicare Payment Advisory Commission supported the addition of data elements related to specific services, treatments, and interventions, but cautioned that such data elements, when used for risk adjustment, may be susceptible to inappropriate manipulation by providers and expressed that CMS may want to consider requiring a physician signature to attest that the reported service was reasonable and necessary. We did not propose to require a physician signature because the existing Conditions of Participation for HHAs already require accurate reporting of patient assessment data, and a physician signature would be redundant. We reported this comment in order to accurately represent the public comments received on these proposals in the CY 2017 HH PPS proposed rule.
We invited comment on our proposals to collect as standardized patient assessment data the following data with respect to special services, treatments, and interventions.
Comment: A number of commenters questioned whether data elements in the SPADE category of Special Services, Treatments, and Interventions were applicable to home health, due to their low prevalence and that these data elements would place an undue burden on providers.
Response: We appreciate the commenters' concern that clinical treatments or response categories documented by some SPADEs are uncommon overall and/or unlikely in the HH setting. We understand that not all SPADEs will be equally relevant to all patients and/or PAC providers. However, we assert that even relatively rare treatments or clinical situations, such as a patient undergoing chemotherapy while receiving PAC services, or having a feeding tube, are important to document, both for care planning within the setting and for transfer of information to the next setting of care. We note that the assessment of many of the less frequently occurring treatments and conditions is formatted as a “check all that apply” list, which minimizes burden. When treatments do not apply the assessor need only check one row for “None of the Above.”
a. Cancer Treatment: Chemotherapy (IV, Oral, Other)
In CY 2020 HH PPS proposed rule (84 FR 34657 through 34658), we proposed that the Chemotherapy (IV, Oral, Other) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35359 through 35360), chemotherapy is a type of cancer treatment that uses drugs to destroy cancer cells. It is sometimes used when a patient has a malignancy (cancer), which is a serious, often life-threatening or life-limiting condition. Both intravenous (IV) and oral chemotherapy have serious side effects, including nausea/vomiting, extreme fatigue, risk of infection due to a suppressed immune system, anemia, and an increased risk of bleeding due to low platelet counts. Oral chemotherapy can be as potent as chemotherapy given by IV but can be significantly more convenient and less resource-intensive to administer. Because of the toxicity of these agents, special care must be exercised in handling and transporting chemotherapy drugs. IV chemotherapy is administered either peripherally or more commonly given via an indwelling central line, which raises the risk of bloodstream infections. Given the significant burden of malignancy, the resource intensity of administering chemotherapy, and the side effects and potential complications of these highly-toxic medications, assessing the receipt of chemotherapy is important in the PAC setting for care planning and determining resource use. The need for chemotherapy predicts resource intensity, both because of the complexity of administering these potent, toxic drug combinations under specific protocols, and because of what the need for chemotherapy signals about the patient's underlying medical condition. Furthermore, the resource intensity of IV chemotherapy is higher than for oral chemotherapy, as the protocols for administration and the care of the central line (if present) for IV chemotherapy require significant resources.
The Chemotherapy (IV, Oral, Other) data element consists of a principal data element (Chemotherapy) and three response option sub-elements: IV chemotherapy, which is generally resource-intensive; Oral chemotherapy, which is less invasive and generally requires less intensive administration protocols; and a third category, Other, provided to enable the capture of other less common chemotherapeutic approaches. This third category is potentially associated with higher risks and is more resource intensive due to chemotherapy delivery by other routes (for example, intraventricular or intrathecal). If the assessor indicates that the patient is receiving chemotherapy on the principal Chemotherapy data element, the assessor would then indicate by which route or routes (IV, Oral, Other) the chemotherapy is administered.
A single Chemotherapy data element that does not include the proposed three sub-elements is currently in use in the MDS in SNFs. For more information on the Chemotherapy (IV, Oral, Other) data element, we refer readers to the document titled “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Chemotherapy data element was first proposed as a SPADE in the CY 2018 HH PPS proposed rule (82 FR 35359 through 35360). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 expressed support for the IV Chemotherapy data element and suggested it be included as standardized patient assessment data. We also stated that those commenters had noted that assessing the use of chemotherapy services is relevant to share across the care continuum to facilitate care coordination and care transitions and noted the validity of the data element. Commenters also noted the importance of capturing all types of chemotherapy, regardless of route, and stated that collecting data only on patients and residents who received chemotherapy by IV would limit the usefulness of this standardized data element. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Chemotherapy data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Chemotherapy data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Chemotherapy data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Chemotherapy data element in the National Beta Test can be found in the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018 for the purpose of soliciting input on the special services, treatments, and interventions. Although the TEP members did not specifically discuss the Chemotherapy data element, the TEP members supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing chemotherapy, stakeholder input, and strong test results, we proposed that the Chemotherapy (IV, Oral, Other) data element with a principal data element and three sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Chemotherapy (IV, Oral, Other) data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Chemotherapy (IV, Oral, Other) data element.
Comment: One commenter agreed that it is important to know if a patient is receiving chemotherapy for cancer and the method of administration, but also expressed concern about the lack of an association with a patient outcome. This commenter noted that implications of chemotherapy for patients needing speech-language pathology services include chemotherapy-related cognitive impairment, dysphagia, and speech- and voice-related deficits.
Response: We appreciate the commenter's concern. We agree with the commenter that chemotherapy can create related treatment needs for patients, such as the examples noted by the commenter. However, we believe that it is not feasible for SPADEs to capture all of a patient's needs related to any given treatment, and we maintain that the Special Services, Treatments, and Interventions SPADEs provide a common foundation of clinical assessment, which can be built on by the individual provider or a patient's care team.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Chemotherapy (IV, Oral, Other) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
b. Cancer Treatment: Radiation
In CY 2020 HH PPS proposed rule (84 FR 34658), we proposed that the Radiation data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35360), radiation is a type of cancer treatment that uses high-energy radioactivity to stop cancer by damaging cancer cell DNA, but it can also damage normal cells. Radiation is an important therapy for particular types of cancer, and the resource utilization is high, with frequent radiation sessions required, often daily for a period of several weeks. Assessing whether a patient or resident is receiving radiation therapy is important to determine resource utilization because PAC patients and residents will need to be transported to and from radiation treatments, and monitored and treated for side effects after receiving this intervention. Therefore, assessing the receipt of radiation therapy, which would compete with other care processes given the time burden, would be important for care planning and care coordination by PAC providers.
The proposed data element consists of the single Radiation data element. The Radiation data element is currently in use in the MDS for SNFs. For more information on the Radiation data element, we refer readers to the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Radiation data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35360). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 expressed support for the Radiation data element, noting its importance and clinical usefulness for patients and residents in PAC settings, due to the side effects and consequences of radiation treatment on patients and residents that need to be considered in care planning and care transitions, the feasibility of the item, and the potential for it to improve quality. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Radiation data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Radiation data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Radiation data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Radiation data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP members did not specifically discuss the Radiation data element, the TEP members supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing radiation, stakeholder input, and strong test results, we proposed that the Radiation data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Radiation data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Radiation data element.
Comment: One commenter expressed concern that the radiation data element assesses whether a patient is receiving radiation for cancer treatment, but does not identify the rationale for and outcomes associated with radiation. The commenter noted that implications of radiation for patients needing speech-language pathology services include reduced head and neck range of motion due to radiation or severe fibrosis, scar bands, and reconstructive surgery complications and that these can impact both communication and swallowing abilities.
Response: We appreciate the commenter's concern. We agree with the commenter that radiation can create related treatment needs for patients, such as the examples noted by the commenter. However, we believe that it is not feasible for SPADEs to capture all of a patient's needs related to any given treatment, and we maintain that the Special Services, Treatments, and Interventions SPADEs provide a common foundation of clinical assessment, which can be built on by the individual provider or a patient's care team.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Radiation data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
c. Respiratory Treatment: Oxygen Therapy (Intermittent, Continuous, High-Concentration Oxygen Delivery System)
In CY 2020 HH PPS proposed rule (84 FR 34658 through 34659), we proposed that the Oxygen Therapy (Intermittent, Continuous, High-Concentration Oxygen Delivery System) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35360 through 35361), we proposed a data element related to oxygen therapy. Oxygen therapy provides a patient or resident with extra oxygen when medical conditions such as chronic obstructive pulmonary disease, pneumonia, or severe asthma prevent the patient or resident from getting enough oxygen from breathing. Oxygen administration is a resource-intensive intervention, as it requires specialized equipment such as a source of oxygen, delivery systems (for example, oxygen concentrator, liquid oxygen containers, and high-pressure systems), the patient interface (for example, nasal cannula or mask), and other accessories (for example, regulators, filters, tubing). The data element proposed here captures patient or resident use of three types of oxygen therapy (intermittent, continuous, and high-concentration oxygen delivery system), which reflects the intensity of care needed, including the level of monitoring and bedside care required. Assessing the receipt of this service is important for care planning and resource use for PAC providers.
The proposed data element, Oxygen Therapy, consists of the principal Oxygen Therapy data element and three sub-elements: Continuous (whether the oxygen was delivered continuously, typically defined as > =14 hours per day); Intermittent; or High-concentration oxygen delivery system. Based on public comments and input from expert advisors about the importance and clinical usefulness of documenting the extent of oxygen use, we added a third sub-element, high-concentration oxygen delivery system, to the sub-elements, which previously included only intermittent and continuous. If the assessor indicates that the patient is receiving oxygen therapy on the principal oxygen therapy data element, the assessor would then indicate the type of oxygen the patient receives (for example, Continuous, Intermittent, High-concentration oxygen delivery system).
These three proposed sub-elements were developed based on similar data elements that assess oxygen therapy, currently in use in the MDS for SNFs (“Oxygen Therapy”), previously used in the OASIS-C2 for HHAs (“Oxygen (intermittent or continuous)”), and a data element tested in the PAC PRD that focused on intensive oxygen therapy (“High O2 Concentration Delivery System with FiO2 >40 percent”). For more information on the proposed Oxygen Therapy (Continuous, Intermittent, High-concentration oxygen delivery system) data element, we refer readers to the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Oxygen Therapy (Continuous, Intermittent) data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35360 through 35361). In that proposed rule, we stated that the proposal was informed by input we received on the single data element, Oxygen (inclusive of intermittent and continuous oxygen use), through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 expressed the importance of the Oxygen data element, noting feasibility of this item in PAC, and the relevance of it to facilitating care coordination and supporting care transitions, but suggesting that the extent of oxygen use be documented. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Oxygen Therapy (Continuous, Intermittent) data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Oxygen Therapy data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Oxygen Therapy data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Oxygen Therapy data element in the National Beta Test can be found in the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, although the TEP did not specifically discuss the Oxygen Therapy data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing oxygen therapy, stakeholder input, and strong test results, we proposed that the Oxygen Therapy (Continuous, Intermittent, High-Concentration Oxygen Delivery System) data element with a principal data element and three sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Oxygen (Continuous, Intermittent, High-Concentration Oxygen Delivery System) data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Oxygen Therapy data element. We did not receive any comments specific to the Oxygen Therapy data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the Oxygen Therapy (Intermittent, Continuous, High-Concentration Oxygen Delivery System) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
d. Respiratory Treatment: Suctioning (Scheduled, As Needed)
In CY 2020 HH PPS proposed rule (84 FR 34659 through 34661), we proposed that the Suctioning (Scheduled, As needed) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35361 through 35362), suctioning is a process used to clear secretions from the airway when a person cannot clear those secretions on his or her own. It is done by aspirating secretions through a catheter connected to a suction source. Types of suctioning include oropharyngeal and nasopharyngeal suctioning, nasotracheal suctioning, and suctioning through an artificial airway such as a tracheostomy tube. Oropharyngeal and nasopharyngeal suctioning are a key part of many patients' or residents' care plans, both to prevent the accumulation of secretions than can lead to aspiration pneumonias (a common condition in patients and residents with inadequate gag reflexes), and to relieve obstructions from mucus plugging during an acute or chronic respiratory infection, which often lead to desaturations and increased respiratory effort. Suctioning can be done on a scheduled basis if the patient is judged to clinically benefit from regular interventions, or can be done as needed when secretions become so prominent that gurgling or choking is noted, or a sudden desaturation occurs from a mucus plug. As suctioning is generally performed by a care provider rather than independently, this intervention can be quite resource intensive. It also signifies an underlying medical condition that prevents the patient from clearing his/her secretions effectively (such as after a stroke, or during an acute respiratory infection). Generally, suctioning is necessary to ensure that the airway is clear of secretions which can inhibit successful oxygenation of the individual. The intent of suctioning is to maintain a patent airway, the loss of which can lead to death, or complications associated with hypoxia.
The Suctioning (Scheduled, As Needed) data element consists of the principal data element, and two sub-elements: Scheduled and As Needed. These sub-elements capture two types of suctioning. Scheduled indicates suctioning based on a specific frequency, such as every hour; as needed means suctioning only when indicated. If the assessor indicates that the patient is receiving suctioning on the principal Suctioning data element, the assessor would then indicate the frequency (Scheduled, As needed). The proposed data element is based on an item currently in use in the MDS in SNFs which does not include our proposed two sub-elements, as well as data elements tested in the PAC PRD that focused on the frequency of suctioning required for patients and residents with tracheostomies (“Trach Tube with Suctioning: Specify most intensive frequency of suctioning during stay [Every _ hours]”). For more information on the Suctioning data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Suctioning data element was first proposed as standardized patient assessment data elements in the CY 2018 HH PPS proposed rule (82 FR 35361 through 35362). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 expressed support for the Suctioning data element currently used in the MDS in SNFs. The input noted the feasibility of this item in PAC, and the relevance of this data element to facilitating care coordination and supporting care transitions. We also stated that those commenters had suggested that we examine the frequency of suctioning to better understand the use of staff time, the impact on a patient or resident's capacity to speak and swallow, and intensity of care required. Based on these comments, we decided to add two sub-elements (Scheduled and As needed) to the suctioning element. The proposed Suctioning data element includes both the principal Suctioning data element that is included on the MDS in SNFs and two sub-elements, Scheduled and As needed. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Suctioning data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Suctioning data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Suctioning data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Suctioning data element in the National Beta Test can be found in the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Suctioning data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicited additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing suctioning, stakeholder input, and strong test results, we proposed that the Suctioning (Scheduled, As needed) data element with a principal data element and two sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Suctioning (Scheduled, As Needed) data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Suctioning data element.
Comment: One commenter requested that respiratory treatment—suctioning data element also assess the frequency of suctioning, as it can impact resource utilization and potential medication changes in the plan of care.
Response: We appreciate the commenter's feedback that the response options for this data element may not fully capture impacts to resource utilization and care plans. The Suctioning data element includes sub-elements to identify if suctioning is performed on a “Scheduled” or “As Needed” basis, but it does not directly assess the frequency of suctioning by, for example, asking an assessor to specify how often suctioning is scheduled. This data element differentiates between patients who only occasionally need suctioning and patients for whom assessment of suctioning needs is a frequent and routine part of the care (that is, where suctioning is performed on a schedule according to physician instructions). In our work to identify standardized patient assessment data elements, we have strived to balance the scope and level of detail of the data elements against the potential burden placed on patients and providers. We further clarify that any SPADE is intended as a minimum assessment and does not limit the ability of providers to conduct a more comprehensive evaluation of a patient's situation to identify the potential impacts on outcomes that the commenter describes.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Suctioning (Scheduled, As Needed) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
e. Respiratory Treatment: Tracheostomy Care
In CY 2020 HH PPS proposed rule (84 FR 34661), we proposed that the Tracheostomy Care data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35362), a tracheostomy provides an air passage to help a patient or resident breathe when the usual route for breathing is obstructed or impaired. Generally, in all of these cases, suctioning is necessary to ensure that the tracheostomy is clear of secretions, which can inhibit successful oxygenation of the individual. Often, individuals with tracheostomies are also receiving supplemental oxygenation. The presence of a tracheostomy, albeit permanent or temporary, warrants careful monitoring and immediate intervention if the tracheostomy becomes occluded or if the device used becomes dislodged. While in rare cases the presence of a tracheostomy is not associated with increased care demands (and in some of those instances, the care of the ostomy is performed by the patient) in general the presence of such as device is associated with increased patient risk, and clinical care services will necessarily include close monitoring to ensure that no life-threatening events occur as a result of the tracheostomy. In addition, tracheostomy care, which primarily consists of cleansing, dressing changes, and replacement of the tracheostomy cannula is also a critical part of the care plan. Regular cleansing is important to prevent infection such as pneumonia and to prevent any occlusions with which there are risks for inadequate oxygenation.
The proposed data element consists of the single Tracheostomy Care data element. The proposed data element is currently in use in the MDS for SNFs (“Tracheostomy care”). For more information on the Tracheostomy Care data element, we refer readers to the document titled “Proposed Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Tracheostomy Care data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35362). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on the Tracheostomy Care data element from August 12 to September 12, 2016 supported this data element, noting the feasibility of this item in PAC, and the relevance of this data element to facilitating care coordination and supporting care transitions. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Tracheostomy Care data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Tracheostomy Care data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Tracheostomy Care data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Tracheostomy Care data element in the National Beta Test can be found in the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Tracheostomy Care data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing tracheostomy care, stakeholder input, and strong test results, we proposed that the Tracheostomy Care data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Tracheostomy Care data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Tracheostomy Care data element.
Comment: A commenter, noted the importance of tracheostomy care and determining whether a patient is receiving tracheostomy care, as it helps with risk adjustment and identifying increased resource utilization. The commenter recommended that the SPADE be expanded to ask about the size of the tracheostomy and whether the tracheostomy has a cuff or is fenestrated.
Response: Risk adjustment determinations is an issue that we continue to evaluate in all of our QRPs, including the HH QRP. We will note this issue for further analysis in our future work to determine how the SPADEs will be used. With regard to the commenter's request to expand the Tracheostomy Care SPADE to include more detail about the type of tracheostomy, we do not believe that this level of clinical detail is necessary to fulfill the purposes of the SPADEs, which are to support care coordination, care planning, and future quality measures. We believe the broad indication that a patient is receiving Tracheostomy Care will be sufficient for the purposes of standardization and quality measurement.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Tracheostomy Care data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
f. Respiratory Treatment: Non-Invasive Mechanical Ventilator (BiPAP, CPAP)
In CY 2020 HH PPS proposed rule (84 FR 34661 through 34662), we proposed that the Non-invasive Mechanical Ventilator (Bilevel Positive Airway Pressure [BiPAP], Continuous Positive Airway Pressure [CPAP]) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35362 through 35363), BiPAP and CPAP are respiratory support devices that prevent the airways from closing by delivering slightly pressurized air via electronic cycling throughout the breathing cycle (BiPAP) or through a mask continuously (CPAP). Assessment of non-invasive mechanical ventilation is important in care planning, as both CPAP and BiPAP are resource-intensive (although less so than invasive mechanical ventilation) and signify underlying medical conditions about the patient or resident who requires the use of this intervention. Particularly when used in settings of acute illness or progressive respiratory decline, additional staff (for example, respiratory therapists) are required to monitor and adjust the CPAP and BiPAP settings and the patient or resident may require more nursing resources.
The proposed data element, Non-invasive Mechanical Ventilator (BIPAP, CPAP), consists of the principal Non-invasive Mechanical Ventilator data element and two response option sub-elements: BiPAP and CPAP. If the assessor indicates that the patient is receiving non-invasive mechanical ventilation on the principal Non-invasive Mechanical Ventilator data element, the assessor would then indicate which type (BIPAP, CPAP). Data elements that assess non-invasive mechanical ventilation are currently included on LCDS for the LTCH setting (“Non-invasive Ventilator (BIPAP, CPAP)”), and the MDS for the SNF setting (“Non-invasive Mechanical Ventilator (BiPAP/CPAP)”). For more information on the Non-invasive Mechanical Ventilator data element, we refer readers to the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs”, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Non-invasive Mechanical Ventilator data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35362 through 35363). In that proposed rule, we stated that the proposal was informed by input we received from August 12 to September 12, 2016 on a single data element, BiPAP/CPAP, that captures equivalent clinical information but uses a different label than the data element currently used in the MDS in SNFs and LCDS in LTCHs, expressing support for this data element, noting the feasibility of these items in PAC, and the relevance of this data element for facilitating care coordination and supporting care transitions. In addition, we also stated that some commenters supported separating out BiPAP and CPAP as distinct sub-elements, as they are therapies used for different types of patients and residents. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Non-invasive Mechanical Ventilator data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Non-invasive Mechanical Ventilator data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Non-invasive Mechanical Ventilator data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Non-invasive Mechanical Ventilator data element in the National Beta Test can be found in the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Non-invasive Mechanical Ventilator data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing non-invasive mechanical ventilation, stakeholder input, and strong test results, we proposed that the Non-Invasive Mechanical Ventilator (BiPAP, CPAP) data element with a principal data element and two sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element as standardized patient assessment data for use in the HH QRP.
We sought comment on our proposal to collect as standardized patient assessment data the Non-Invasive Mechanical Ventilator data element. We did not receive any comments specific to the Non-Invasive Mechanical Ventilator data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
g. Respiratory Treatment: Invasive Mechanical Ventilator
In CY 2020 HH PPS proposed rule (84 FR 34662 through 34663),we proposed that the Invasive Mechanical Ventilator data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35363 through 35364), invasive mechanical ventilation includes ventilators and respirators that ventilate the patient through a tube that extends via the oral airway into the pulmonary region or through a surgical opening directly into the trachea. Thus, assessment of invasive mechanical ventilation is important in care planning and risk mitigation. Ventilation in this manner is a resource-intensive therapy associated with life-threatening conditions without which the patient or resident would not survive. However, ventilator use has inherent risks requiring close monitoring. Failure to adequately care for the patient or resident who is ventilator dependent can lead to iatrogenic events such as death, pneumonia and sepsis. Mechanical ventilation further signifies the complexity of the patient's underlying medical or surgical condition. Of note, invasive mechanical ventilation is associated with high daily and aggregate costs.[113]
The proposed data element, Invasive Mechanical Ventilator, consists of a single data element. Data elements that capture invasive mechanical ventilation are currently in use in the MDS in SNFs and LCDS in LTCHs. For more information on the Invasive Mechanical Ventilator data element, we refer readers to the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Invasive Mechanical Ventilator data element was first proposed as a SPADE in the CY 2018 HH PPS proposed rule (82 FR 35363 through 35364). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on data elements that assess invasive ventilator use and weaning status that were tested in the PAC PRD (“Ventilator—Weaning” and “Ventilator—Non-Weaning”) from August 12 to September 12, 2016 expressed support for this data element, highlighting the importance of this information in supporting care coordination and care transitions. We also stated that some commenters had expressed concern about the appropriateness for standardization given: The prevalence of ventilator weaning across PAC providers; the timing of administration; how weaning is defined; and how weaning status in particular relates to quality of care. These public comments guided our decision to propose a single data element focused on current use of invasive mechanical ventilation only, which does not attempt to capture weaning status. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Invasive Mechanical Ventilator data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Invasive Mechanical Ventilator data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Invasive Mechanical Ventilator data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Invasive Mechanical Ventilator data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Invasive Mechanical Ventilator data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing invasive mechanical ventilation, stakeholder input, and strong test results, we proposed that the Invasive Mechanical Ventilator data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Invasive Mechanical Ventilator data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposals to collect as standardized patient assessment data the Invasive Mechanical Ventilator data element.
Comment: One commenter expressed concern that the invasive mechanical ventilator element only assesses whether or not a patient is on a mechanicalventilator. The commenter suggested CMS consider collecting data to track functional outcomes related to progress towards independence in communication and swallowing.
Response: In our evaluation of the suitability of data elements for SPADEs, we examined the clinical usefulness of candidate SPADEs across the full range of PAC providers, including HHAs. We intend to use the SPADEs to inform care planning and comparing of assessment data for standardized measures. We believe that assessing the use of an invasive mechanical ventilator is a useful point of information to inform care planning and further assessment, such as related to functional outcomes. We wish to clarify that the proposed SPADEs are not intended to replace comprehensive clinical evaluation and in no way preclude providers from conducting further patient evaluation or assessments in their settings as they believe are necessary and useful. However, we will take into consideration functional outcomes, overall, that are related to progress towards independence in communication and swallowing in future modifications.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Invasive Mechanical Ventilator data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
h. Intravenous (IV) Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other)
In CY 2020 HH PPS proposed rule (84 FR 34663 through 34664), we proposed that the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35364 through 35365), when we proposed a similar set of data elements related to IV medications, IV medications are solutions of a specific medication (for example, antibiotics, anticoagulants) administered directly into the venous circulation via a syringe or intravenous catheter. IV medications are administered via intravenous push, single, intermittent, or continuous infusion through a tube placed into the vein. Further, IV medications are more resource intensive to administer than oral medications, and signify a higher patient complexity (and often higher severity of illness). The clinical indications for each of the sub-elements of the IV Medications data elements (Antibiotics, Anticoagulants, Vasoactive Medications, and Other) are very different. IV antibiotics are used for severe infections when: The bioavailability of the oral form of the medication would be inadequate to kill the pathogen; an oral form of the medication does not exist; or the patient is unable to take the medication by mouth. IV anticoagulants refer to anti-clotting medications (that is, “blood thinners”). IV anticoagulants are commonly used for hospitalized patients who have deep venous thrombosis, pulmonary embolism, or myocardial infarction, as well as those undergoing interventional cardiac procedures. Vasoactive medications refer to the IV administration of vasoactive drugs, including vasopressors, vasodilators, and continuous medication for pulmonary edema, which increase or decrease blood pressure or heart rate. The indications, risks, and benefits of each of these classes of IV medications are distinct, making it important to assess each separately in PAC. Knowing whether or not patients and residents are receiving IV medication and the type of medication provided by each PAC provider will improve quality of care.
The IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, and Other) data element we proposed consists of a principal data element (IV Medications) and four response option sub-elements: Antibiotics, Anticoagulants, Vasoactive Medications, and Other. The Vasoactive Medications sub-element was not proposed in the CY 2018 HH PPS proposed rule (82 FR 35364 through 35365). We added the Vasoactive Medications sub-element to our proposal in order to harmonize the proposed IV Mediciations element with the data currently collected in the LCDS.
If the assessor indicates that the patient is receiving IV medications on the principal IV Medications data element, the assessor would then indicate which types of medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other). An IV Medications data element is currently in use on the MDS in SNFs and there is a related data element in OASIS that collects information on Intravenous and Infusion Therapies. For more information on the IV Medications data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs, available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
An IV Medications data element was first proposed as standardized patient assessment data elements in the CY 2018 HH PPS proposed rule (82 FR 35364 through 35365). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on Vasoactive Medications from August 12 to September 12, 2016 supported this data element with one commenter noting the importance of this data element in supporting care transitions. We also stated that those commenters had criticized the need for collecting specifically Vasoactive Medications, giving feedback that the data element was too narrowly focused. In addition, public comment received indicated that the clinical significance of vasoactive medications administration alone was not high enough in PAC to merit mandated assessment, noting that related and more useful information could be captured in an item that assessed all IV medication use. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for IV Medications data elements.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the IV Medications data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the IV Medications data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the IV Medications data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the IV Medications data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing IV medications, stakeholder input, and strong test results, we proposed that the IV Medications (Antibiotics, Anticoagulation, Vasoactive Medications, Other) data element with a principal data element and four sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element as standardized patient assessment data for use in the HH QRP.
We sought public comment on our proposal to collect as standardized patient assessment data the IV Medications data element. We did not receive any comments specific to the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
i. Transfusions
In CY 2020 HH PPS proposed rule (84 FR 34664 through 34665), we proposed that the Transfusions data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35365), transfusion refers to introducing blood, blood products, or other fluid into the circulatory system of a person. Blood transfusions are based on specific protocols, with multiple safety checks and monitoring required during and after the infusion in case of adverse events. Coordination with the provider's blood bank is necessary, as well as documentation by clinical staff to ensure compliance with regulatory requirements. In addition, the need for transfusions signifies underlying patient complexity that is likely to require care coordination and patient monitoring, and impacts planning for transitions of care, as transfusions are not performed by all PAC providers.
The proposed data element consists of a single Transfusions data element. A data element on transfusion is currently in use in the MDS in SNFs (“Transfusions”) and a data element tested in the PAC PRD (“Blood Transfusions”) was found feasible for use in each of the four PAC settings. For more information on the Transfusions data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Transfusions data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35365).
In response to our proposal in the CY 2018 HH PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Transfusions data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Transfusions data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Transfusions data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Transfusions data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Transfusions data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing transfusions, stakeholder input, and strong test results, we proposed that the Transfusions data element that is currently in use in the MDS meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Transfusions data element as standardized patient assessment data for use in the HH QRP.
We invited public comment on our proposal to collect as standardized patient assessment data the Transfusions data element. We did not receive any comments specific to the Transfusions data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the Transfusions data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
j. Dialysis (Hemodialysis, Peritoneal Dialysis)
In CY 2020 HH PPS proposed rule (84 FR 34655 through 34656), we proposed that the Dialysis (Hemodialysis, Peritoneal Dialysis) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35365 through 35366), dialysis is a treatment primarily used to provide replacement for lost kidney function. Both forms of dialysis (hemodialysis and peritoneal dialysis) are resource intensive, not only during the actual dialysis process but before, during and following. Patients and residents who need and undergo dialysis procedures are at high risk for physiologic and hemodynamic instability from fluid shifts and electrolyte disturbances as well as infections that can lead to sepsis. Further, patients or residents receiving hemodialysis are often transported to a different facility, or at a minimum, to a different location in the same facility. Close monitoring for fluid shifts, blood pressure abnormalities, and other adverse effects is required prior to, during and following each dialysis session. Nursing staff typically perform peritoneal dialysis at the bedside, and as with hemodialysis, close monitoring is required.
The proposed data element, Dialysis (Hemodialysis, Peritoneal Dialysis) consists of the principal Dialysis data element and two response option sub-elements: Hemodialysis and Peritoneal Dialysis. If the assessor indicates that the patient is receiving dialysis on the principal Dialysis data element, the assessor would then indicate which type (Hemodialysis, Peritoneal Dialysis). The principal Dialysis data element is currently included on the MDS in SNFs and the LCDS for LTCHs and assesses the overall use of dialysis. As the result of public feedback described, in this final rule with comment period, we proposed data elements that include the principal Dialysis data element and two sub-elements (Hemodialysis and Peritoneal Dialysis). For more information on the Dialysis data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Dialysis data element was first proposed as standardized patient assessment data elements in the CY 2018 HH PPS proposed rule (82 FR 35365 through 35366). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on a singular Hemodialysis data element from August 12 to September 12, 2016 supported the assessment of hemodialysis and recommended that the data element be expanded to include peritoneal dialysis. We also stated that those commenters had supported the singular Hemodialysis data element, noting the relevance of this information for sharing across the care continuum to facilitate care coordination and care transitions, the potential for this data element to be used to improve quality, and the feasibility for use in PAC. In addition, we received comment that the item would be useful in improving patient and resident transitions of care. We also noted that several commenters had stated that peritoneal dialysis should be included in a standardized data element on dialysis and recommended collecting information on peritoneal dialysis in addition to hemodialysis. The rationale for including peritoneal dialysis from commenters included the fact that patients and residents receiving peritoneal dialysis will have different needs at post-acute discharge compared to those receiving hemodialysis or not having any dialysis. Based on these comments, the Hemodialysis data element was expanded to include a principal Dialysis data element and two sub-elements, Hemodialysis and Peritoneal Dialysis. We proposed the expanded version of the Dialysis data element that includes two types of dialysis. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Dialysis data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Dialysis data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Dialysis data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Dialysis data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although they did not specifically discuss the Dialysis data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing dialysis, stakeholder input, and strong test results, we proposed that the Dialysis (Hemodialysis, Peritoneal Dialysis) data element with a principal data element and two sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Dialysis (Hemodialysis, Peritoneal Dialysis) data element as standardized patient assessment data for use in the HH QRP.
We invited public comment on our proposal to collect as standardized patient assessment data the Dialysis data element. We did not receive any comments specific to the Dialysis (Hemodialysis, Peritoneal Dialysis) data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the Dialysis (Hemodialysis, Peritoneal dialysis) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
k. Intravenous (IV) Access (Peripheral IV, Midline, Central Line)
In CY 2020 HH PPS proposed rule (84 FR 34666 through 34667), we proposed that the IV Access (Peripheral IV, Midline, Central Line) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35366), patients or residents with central lines, including those peripherally inserted or who have subcutaneous central line “port” access, always require vigilant nursing care to keep patency of the lines and ensure that such invasive lines remain free from any potentially life-threatening events such as infection, air embolism, or bleeding from an open lumen. Clinically complex patients and residents are likely to be receiving medications or nutrition intravenously. The sub-elements included in the IV Access data element distinguish between peripheral access and different types of central access. The rationale for distinguishing between a peripheral IV and central IV access is that central lines confer higher risks associated with life-threatening events such as pulmonary embolism, infection, and bleeding.
The proposed data element, IV Access (Peripheral IV, Midline, Central Line), consists of the principal IV Access data element and three response option sub-elements: Peripheral IV, Midline, and Central Line. The proposed IV Access data element is not currently included on any of the PAC assessment instruments, although there is a related response option in the M1030 data element in the OASIS. We proposed to replace the existing “Intravenous or Infusion Therapy” response option of the M1030 data element in the OASIS with the IV Access (Peripheral IV, Midline, Central Line) data element. For more information on the IV Access data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The IV Access data element was first proposed as standardized patient assessment data elements in the CY 2018 HH PPS proposed rule (82 FR 35366). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input was submitted on one of the PAC PRD data elements, Central Line Management, from August 12 to September 12, 2016. A central line is one type of IV access. We stated that those commenters had supported the assessment of central line management and recommended that the data element be broadened to also include other types of IV access. Several commenters noted feasibility and importance of facilitating care coordination and care transitions. However, a few commenters recommended that the definition of this data element be broadened to include peripherally inserted central catheters (“PICC lines”) and midline IVs. Based on public comment feedback and in consultation with expert input, described elsewhere in this final rule with comment period, we created an overarching IV Access data element with sub-elements for other types of IV access in addition to central lines (that is, peripheral IV and midline). This expanded version of IV Access is the data element being proposed. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the IV Access data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the IV Access data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the IV Access data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the IV Access data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the IV Access data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing IV access, stakeholder input, and strong test results, we proposed that the IV access (Peripheral IV, Midline, Central Line) data element with a principal data element and three sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the IV Access (Peripheral IV, Midline, Central Line) data element as standardized patient assessment data for use in the HH QRP.
We invited public comment on our proposal to collect as standardized patient assessment data the IV Access data element. We did not receive any comments specific to the IV Access (Peripheral IV, Midline, Central Line) data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the Intravenous (IV) Access (Peripheral IV, Midline, Central line) data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
l. Nutritional Approach: Parenteral/IV Feeding
In CY 2020 HH PPS proposed rule (84 FR 345667 through 34668), we proposed that the Parenteral/IV Feeding data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35366 through 35367), parenteral nutrition/IV feeding refers to a patient or resident being fed intravenously using an infusion pump, bypassing the usual process of eating and digestion. The need for parenteral nutrition/IV feeding indicates a clinical complexity that prevents the patient or resident from meeting his or her nutritional needs internally, and is more resource intensive than other forms of nutrition, as it often requires monitoring of blood chemistries and maintenance of a central line. Therefore, assessing a patient's or resident's need for parenteral feeding is important for care planning and resource use. In addition to the risks associated with central and peripheral intravenous access, total parenteral nutrition is associated with significant risks such as embolism and sepsis.
The proposed data element consists of the single Parenteral/IV Feeding data element. The proposed Parenteral/IV Feeding data element is currently in use in the MDS for SNFs, and equivalent or related data elements are in use in the LCDS, IRF-PAI, and OASIS. We proposed to replace the existing “Parenteral nutrition (TPN or lipids)” response option of the M1030 data element in the OASIS with the proposed Parenteral/IV Feeding data element. For more information on the Parenteral/IV Feeding data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Parenteral/IV Feeding data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35366 through 35367). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on Total Parenteral Nutrition (an item with nearly the same meaning as the proposed data element, but with the label used in the PAC PRD), which was included in a call for public input from August 12 to September 12, 2016. We stated that commenters had supported this data element, noting its relevance to facilitating care coordination and supporting care transitions. After the public comment period, the Total Parenteral Nutrition data element was renamed Parenteral/IV Feeding, to be consistent with how this data element is referred to in the MDS in SNFs. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. In response to our proposal in the CY 2018 HH PPS proposed rule, two commenters expressed support for the Parenteral/IV Feeding data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Parenteral/IV Feeding data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Parenteral/IV Feeding data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Parenteral/IV Feeding data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Parenteral/IV Feeding data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing parenteral/IV feeding, stakeholder input, and strong test results, we proposed that the Parenteral/IV Feeding data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Parenteral/IV Feeding data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Parenteral/IV Feeding data element.
Comment: One commenter was supportive of collecting the Parenteral/IV Feeding data element, but noted that it should not be a substitute for capturing information related to swallowing which reflects additional patient complexity and resource use.
Response: We agree that the Parenteral/IV Feeding SPADE should not be used as a substitute for an assessment of a patient's swallowing function. The proposed SPADEs are not intended to replace comprehensive clinical evaluation and in no way preclude providers from conducting further patient evaluation or assessments in their settings as they believe are necessary and useful. We agree that information related to swallowing can capture patient complexity. However, we also note that Parenteral/IV Feeding data element captures a different construct than an evaluation of swallowing. That is, the Parenteral/IV Feeding data element captures a patient's need to receive calories and nutrients intravenously, while an assessment of swallowing would capture a patient's functional ability to safely consume food/liquids orally for digestion in their gastrointestinal tract.
After careful consideration of the public comment we received on the Parenteral/IV Feeding data element, we are finalizing our proposal to adopt the Parenteral/IV Feeding data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
m. Nutritional Approach: Feeding Tube
In CY 2020 HH PPS proposed rule (84 FR 34668 through 34669), we proposed that the Feeding Tube data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35367 through 35368), the majority of patients admitted to acute care hospitals experience deterioration of their nutritional status during their hospital stay, making assessment of nutritional status and method of feeding if unable to eat orally very important in PAC. A feeding tube can be inserted through the nose or the skin on the abdomen to deliver liquid nutrition into the stomach or small intestine. Feeding tubes are resource intensive and, therefore, are important to assess for care planning and resource use. Patients with severe malnutrition are at higher risk for a variety of complications.[114] In PAC settings, there are a variety of reasons that patients and residents may not be able to eat orally (including clinical or cognitive status).
The proposed data element consists of the single Feeding Tube data element. The Feeding Tube data element is currently included in the MDS for SNFs, and in the OASIS for HHAs, where it is labeled “Enteral Nutrition (nasogastric, gastrostomy, jejunostomy, or any other artificial entry into the alimentary canal)”. A related data element, collected in the IRF-PAI for IRFs (Tube/Parenteral Feeding), assesses use of both feeding tubes and parenteral nutrition. We proposed to rename “Enteral nutrition (nasogastric, gastrostomy, jejunostomy, or any other artificial entry into the alimentary canal)” data element to “Feeding Tube,” and adopt it as a SPADE for the HH QRP. For more information on the Feeding Tube data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Feeding Tube data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35367 through 35368). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on an Enteral Nutrition data element (which is the same as the data element we proposed in the CY 2020 HH PPS proposed rule (84 FR 34668), but is used in the OASIS under a different name) from August 12 to September 12, 2016 supported the data element, noting the importance of assessing enteral nutrition status for facilitating care coordination and care transitions. After the public comment period, the Enteral Nutrition data element used in public comment was renamed Feeding Tube, indicating the presence of an assistive device. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, a few commenters expressed support for the Feeding Tube data element. A commenter also recommended that the term “enteral feeding” be used instead of “feeding tube.”
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Feeding Tube data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Feeding Tube data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Feeding Tube data element in the National Beta Test can be found in the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Feeding Tube data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing feeding tubes, stakeholder input, and strong test results, we proposed that the Feeding Tube data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Feeding Tube data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Feeding Tube data element.
Comment: In regard to the nutritional approach—feeding tube data element, one commenter noted that in addition to identifying if the patient is on a feeding tube or not, it would be important to assess the patient's progression towards oral feeding within this data element, as this impacts the tube feeding regimen.
Response: We agree that progression to oral feeding is important for care planning and transfer. We wish to clarify that the proposed SPADEs are not intended to replace comprehensive clinical evaluation and in no way preclude providers from conducting further patient evaluation or assessments in their settings as they believe are necessary and useful. However, we will take this recommendation into consideration in future work on standardized data elements.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Feeding Tube data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
n. Nutritional Approach: Mechanically Altered Diet
In CY 2020 HH PPS proposed rule (84 FR 34669), we proposed that the Mechanically Altered Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35368), the Mechanically Altered Diet data element refers to food that has been altered to make it easier for the patient or resident to chew and swallow, and this type of diet is used for patients and residents who have difficulty performing these functions. Patients with severe malnutrition are at higher risk for a variety of complications.[115]
In PAC settings, there are a variety of reasons that patients and residents may have impairments related to oral feedings, including clinical or cognitive status. The provision of a mechanically altered diet may be resource intensive, and can signal difficulties associated with swallowing/eating safety, including dysphagia. In other cases, it signifies the type of altered food source, such as ground or puree that will enable the safe and thorough ingestion of nutritional substances and ensure safe and adequate delivery of nourishment to the patient. Often, patients and residents on mechanically altered diets also require additional nursing supports such as individual feeding, or direct observation, to ensure the safe consumption of the food product. Assessing whether a patient or resident requires a mechanically altered diet is therefore important for care planning and resource identification.
The proposed data element consists of the single Mechanically Altered Diet data element. The proposed data element for a mechanically altered diet is currently included on the MDS for SNFs. A related data element for modified food consistency/supervision is currently included on the IRF-PAI for IRFs. Another related data element is included in the OASIS for HHAs that collects information about independent eating that requires “a liquid, pureed or ground meat diet.” For more information on the Mechanically Altered Diet data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Mechanically Altered Diet data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35368).
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Mechanically Altered Diet data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Mechanically Altered Diet data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Mechanically Altered Diet data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Mechanically Altered Diet data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Mechanically Altered Diet data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing mechanically altered diet, stakeholder input, and strong test results, we proposed that the Mechanically Altered Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Mechanically Altered Diet data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Mechanically Altered Diet data element.
Comment: One commenter was concerned that the Mechanically Altered Diet data element does not capture clinical complexity and does not provide any insight into resource allocation because it only measures whether the patient needs a mechanically altered diet and not, for example, the extent of help a patient needs in consuming his or her meal.
Response: We believe that assessing patients' needs for mechanically altered diets captures one piece of information about resource intensity. That is, patients with this special nutritional requirement may require additional nutritional planning services, special meals, and staff to ensure that meals are prepared and served in the way the patient needs. Additional factors that would affect resource allocation, such as those noted by the commenter, are not captured by this data element. We have attempted to balance the scope and level of detail of the data elements against the potential burden placed on providers who must complete the assessment. We will take this suggestion into consideration in future refinement of the clinical SPADEs.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Mechanically Altered Diet data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
o. Nutritional Approach: Therapeutic Diet
In CY 2020 HH PPS proposed rule (84 FR 34670), we proposed that the Therapeutic Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35368 through 35369), a therapeutic diet refers to meals planned to increase, decrease, or eliminate specific foods or nutrients in a patient's or resident's diet, such as a low-salt diet, for the purpose of treating a medical condition. The use of therapeutic diets among patients and residents in PAC provides insight on the clinical complexity of these patients and residents and their multiple comorbidities. Therapeutic diets are less resource intensive from the bedside nursing perspective, but do signify one or more underlying clinical conditions that preclude the patient from eating a regular diet. The communication among PAC providers about whether a patient is receiving a particular therapeutic diet is critical to ensure safe transitions of care.
The proposed data element consists of the single Therapeutic Diet data element. The Therapeutic Diet data element is currently in use in the MDS for SNFs. For more information on the Therapeutic Diet data element, we refer readers to the document titled, “Final Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Therapeutic Diet data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35368 through 35369).
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter expressed support for the Therapeutic Diet data element and encouraged CMS to align with the Academy of Nutrition and Dietetics definition of “therapeutic diet.”
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Therapeutic Diet data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Therapeutic Diet data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Therapeutic Diet data element in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. Although the TEP did not specifically discuss the Therapeutic Diet data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing therapeutic diet, stakeholder input, and strong test results, we proposed that the Therapeutic Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Therapeutic data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardize patient assessment data the Therapeutic Diet data element. We did not receive any additional comments specific to the Therapeutic Diet data element. General comments on the category of Special Services, Treatments, and Interventions Data are discussed in section V.H.2 of this final rule with comment period.
Accordingly, we are finalizing our proposal to adopt the Therapeutic Diet data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
p. High-Risk Drug Classes: Use and Indication
In CY 2020 HH PPS proposed rule (84 FR 34670 through 34672), we proposed that the High-Risk Drug Classes: Use and Indication data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
Most patients and residents receiving PAC services depend on short- and long-term medications to manage their medical conditions. However, as a treatment, medications are not without risk; medications are in fact a leading cause of adverse events. A study by the U.S. Department of Health and Human Services found that 31 percent of adverse events that occurred in 2008 among hospitalized Medicare beneficiaries were related to medication.[116] Moreover, changes in a patient's condition, medications, and transitions between care settings put patients and residents at risk of medication errors and adverse drug events (ADEs). ADEs may be caused by medication errors such as drug omissions, errors in dosage, and errors in dosing frequency.[117]
ADEs are known to occur across different types of healthcare. For example, the incidence of ADEs in the outpatient setting has been estimated at 1.15 ADEs per 100 person-months,[118] while the rate of ADEs in the long-term care setting is approximately 9.80 ADEs per 100 resident-months.[119] In the hospital setting, the incidence has been estimated at 15 ADEs per 100 admissions.[120] In addition, approximately half of all hospital-related medication errors and 20 percent of ADEs occur during transitions within, admission to, transfer to, or discharge from a hospital.[121 122 123] ADEs are more common among older adults, who make up most patients and residents receiving PAC services. The rate of emergency department visits for ADEs is three times higher among adults 65 years of age and older compared to that among those younger than age 65.[124]
Understanding the types of medication a patient is taking and the reason for its use are key facets of a patient's treatment with respect to medication. Some classes of drugs are associated with more risk than others.[125] We proposed one High-Risk Drug Class data element with six sub-elements. The six medication classes response options are: Anticoagulants; antiplatelets; hypoglycemics (including insulin); opioids; antipsychotics; and antibiotics. These drug classes are high-risk due to the adverse effects that may result from use. In particular, bleeding risk is associated with anticoagulants and antiplatelets; [126 127] fluid retention, heart failure, and lactic acidosis are associated with hypoglycemics; [128] misuse is associated with opioids;[129] fractures and strokes are associated with antipsychotics; [130 131] and various adverse events such as central nervous systems effects and gastrointestinal intolerance are associated with antimicrobials,[132] the larger category of medications that include antibiotics. Moreover, some medications in five of the six drug classes included as response options in this data element are included in the 2019 Updated Beers Criteria® list as potentially inappropriate medications for use in older adults.[133] Finally, although a complete medication list should record several important attributes of each medication (for example, dosage, route, stop date), recording an indication for the drug is of crucial importance.[134]
The High-Risk Drug Classes: Use and Indication data element requires an assessor to record whether or not a patient is taking any medications within six drug classes. The six response options for this data element are high-risk drug classes with particular relevance to PAC patients and residents, as identified by our data element contractor. The six data response options are Anticoagulants, Antiplatelets, Hypoglycemics, Opioids, Antipsychotics, and Antibiotics. For each drug class, the assessor is asked to indicate if the patient is taking any medications within the class, and, for drug classes in which medications were being taken, whether indications for all drugs in the class are noted in the medical record. For example, for the response option Anticoagulants, if the assessor indicates that the patient is taking anticoagulant medication, the assessor would then indicate if an indication is recorded in the medication record for the anticoagulant(s).
The High-Risk Drug Classes: Use and Indication data element that is being proposed as a SPADE was developed as part of a larger set of data elements to assess medication reconciliation, the process of obtaining a patient's multiple medication lists and reconciling any discrepancies. For more information on the High-Risk Drug Classes: Use and Indication data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We sought public input on the relevance of conducting assessments on medication reconciliation and specifically on the proposed High-Risk Drug Classes: Use and Indication data element. Our data element contractor presented data elements related to medication reconciliation to the TEP convened on April 6 and 7, 2016. The TEP supported a focus on high-risk drugs, because of higher potential for harm to patients and residents, and were in favor of a data element to capture whether or not indications for medications were recorded in the medical record. A summary of the April 6 and 7, 2016 TEP meeting titled “SPADE Technical Expert Panel Summary (First Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. Medication reconciliation data elements were also discussed at a second TEP meeting on January 5 and 6, 2017, convened by our data element contractor.
At this meeting, the TEP agreed about the importance of evaluating the medication reconciliation process, but disagreed about how this could be accomplished through standardized assessment. The TEP also disagreed about the usability and appropriateness of using the Beers Criteria to identify high-risk medications,[135] although they were supportive of the other six drug classes named in the draft version of the data element, which are the six drug classes being proposed as response options in the proposed High-Risk Drug Classes: Use and Indications SPADE. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We received public input on data elements related to medication reconciliation through a call for input published on the CMS Measures Management System Blueprint website. In input received from April 26 to June 26, 2017, several commenters expressed support for the medication reconciliation data elements that were put on display, noting the importance of medication reconciliation in preventing medication errors and stating that the items seemed feasible and clinically useful. A few commenters were critical of the choice of ten drug classes posted during that comment period—the six drug classes in the proposed SPADE, along with antidepressants, diuretics, antianxiety, and hypnotics—arguing that ADEs are not limited to high-risk drugs, and raised issues related to training assessors to correctly complete a valid assessment of medication reconciliation. A summary report for the April 26 to June 26, 2017 public comment period titled “SPADE May-June 2017 Public Comment Summary Report” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The High-Risk Drug Classes: Use and Indication data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the High-Risk Drug Classes: Use and Indication data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the High-Risk Drug Classes: Use and Indication data element in the National Beta Test can be found in the document titled, ”Final Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018. The TEP acknowledged the challenges of assessing medication safety, and were supportive of some of the data elements focused on medication reconciliation that were tested in the National Beta Test. The TEP was especially supportive of the focus on the six high-risk drug classes—which they identified from among other options during the second convening of the TEP, described previously—and of using these classes to assess whether the indication for a drug is recorded. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. These activities provided updates on the field-testing work and solicited feedback on data elements considered for standardization, including the High-Risk Drug Classes: Use and Indication data element. One stakeholder group was critical of the six drug classes included as response options in the High-Risk Drug Classes: Use and Indication data element, noting that potentially risky medications (for example, muscle relaxants) are not included in this list; that there may be important differences between drugs within classes (for example, more recent versus older style antidepressants); and that drug allergy information is not captured. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, one commenter questioned whether the time to complete the High-Risk Drug Classes: Use and Indication data element would differ across settings. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing high-risk drugs and for whether or not indications are noted for high-risk drugs, stakeholder input, and strong test results, we proposed that the High-Risk Drug Classes: Use and Indication data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the High-Risk Drug Classes: Use and Indication data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the High-Risk Drug Classes: Use and Indication data element.
Comment: One commenter raised the concern of assessing some high risk drug classes, noting that assessing each patient for use of opioids and antipsychotics could discourage appropriate use of these medications in those with advanced illness or receiving palliative care.
Response: We acknowledge commenters' concerns about potential unintended consequences of limiting use of medications for patients with a clinical need. We remain confident that HHAs will continue to focus on appropriate management of pain and mental health issues for all patients as part of their commitment to quality of care and ongoing quality improvement efforts. CMS is also committed to monitor incoming assessment data related to pain for unintended consequences and will be prepared to take necessary steps based on monitoring findings.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the High-Risk Drug Classes: Use and Indication data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
3. Medical Condition and Comorbidity Data
Assessing medical conditions and comorbidities is critically important for care planning and safety for patients and residents receiving PAC services, and the standardized assessment of selected medical conditions and comorbidities across PAC providers is important for managing care transitions and understanding medical complexity.
We discuss our proposals for data elements related to the medical condition of pain as standardized patient assessment data. Appropriate pain management begins with a standardized assessment, and thereafter establishing and implementing an overall plan of care that is person-centered, multi-modal, and includes the treatment team and the patient. Assessing and documenting the effect of pain on sleep, participation in therapy, and other activities may provide information on undiagnosed conditions and comorbidities and the level of care required, and do so more objectively than subjective numerical scores. With that, we assess that taken separately and together, these proposed data elements are essential for care planning, consistency across transitions of care, and identifying medical complexities, including undiagnosed conditions. We also conclude that it is the standard of care to always consider the risks and benefits associated with a personalized care plan, including the risks of any pharmacological therapy, especially opioids.[136] We also conclude that in addition to assessing and appropriately treating pain through the optimum mix of pharmacologic, non-pharmacologic, and alternative therapies, while being cognizant of current prescribing guidelines, clinicians in partnership with patients are best able to mitigate factors that contribute to the current opioid crisis.[137] [138] [139]
In alignment with our Meaningful Measures Initiative, accurate assessment of medical conditions and comorbidities of patients and residents in PAC is expected to make care safer by reducing harm caused in the delivery of care; promoting effective prevention and treatment of chronic disease; strengthening person and family engagement as partners in their care; and promoting effective communication and coordination of care. The proposed SPADEs will enable or support clinical decision-making and early clinical intervention; person-centered, high quality care through: Facilitating better care continuity and coordination; better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable data elements assessing medical conditions and comorbidities are needed in order to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events.
We invited comment on our proposals to collect as standardized patient assessment data the following data with respect to medical conditions and comorbidities.
a. Pain Interference (Pain Effect on Sleep, Pain Interference With Therapy Activities, and Pain Interference With Day-to-Day Activities).
In acknowledgement of the opioid crisis, we specifically sought comment on whether or not we should add these pain items in light of those concerns. Commenters were asked to address to what extent collection of the data through patient queries might encourage providers to prescribe opioids.
In CY 2020 HH PPS proposed rule (84 FR 34673 through 34675), we proposed that a set of three data elements on the topic of Pain Interference (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) meet the definition of standardized patient assessment data with respect to medical conditions and comorbidities under section 1899B(b)(1)(B)(iv) of the Act.
The practice of pain management began to undergo significant changes in the 1990s because the inadequate, non-standardized, non-evidence-based assessment and treatment of pain became a public health issue.[140] In pain management, a critical part of providing comprehensive care is performance of a thorough initial evaluation, including assessment of both the medical and any biopsychosocial factors causing or contributing to the pain, with a treatment plan to address the causes of pain and to manage pain that persists over time.[141] Quality pain management, based on current guidelines and evidence-based practices, can minimize unnecessary opioid prescribing both by offering alternatives or supplemental treatment to opioids and by clearly stating when they may be appropriate, and how to utilize risk-benefit analysis for opioid and non-opioid treatment modalities.[142]
Pain is not a surprising symptom in PAC patients and residents, where healing, recovery, and rehabilitation often require regaining mobility and other functions after an acute event. Standardized assessment of pain that interferes with function is an important first step toward appropriate pain management in PAC settings. The National Pain Strategy called for refined assessment items on the topic of pain, and describes the need for these improved measures to be implemented in PAC assessments.[143] Further, the focus on pain interference, as opposed to pain intensity or pain frequency, was supported by the TEP convened by our data element contractor as an appropriate and actionable metric for assessing pain. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We appreciate the important concerns related to the misuse and overuse of opioids in the treatment of pain and to that end we note that in this final rule with comment period we have also proposed a SPADE in section V.H.2.p. of this rule that assess for the use of, as well as importantly the indication for the use of high risk drugs, including opioids. Further, in the CY 2017 HH PPS final rule (81 FR 76780) we adopted the Drug Regimen Review Conducted With Follow-Up for Identified Issues—Post Acute Care (PAC) HH QRP measure, which assesses whether PAC providers were responsive to potential or actual clinically significant medication issue(s) including issues associated with use and misuse of opioids for pain management, when such issues were identified.
We also note that the proposed SPADEs related to pain assessment are not associated with any particular approach to management. Since the use of opioids is associated with serious complications, particularly in the elderly, an array of successful non-pharmacologic and non-opioid approaches to pain management may be considered.[144] [145] [146] PAC providers have historically used a range of pain management strategies, including non-steroidal anti-inflammatory drugs, ice, transcutaneous electrical nerve stimulation (TENS) therapy, supportive devices, acupuncture, and the like. In addition, non-pharmacological interventions implemented for pain management include, but are not limited to, biofeedback, application of heat/cold, massage, physical therapy, nerve block, stretching and strengthening exercises, chiropractic, electrical stimulation, radiotherapy, and ultrasound.[147] [148] [149]
We believe that standardized assessment of pain interference will support PAC clinicians in applying best-practices in pain management for chronic and acute pain, consistent with current clinical guidelines. For example, the standardized assessment of both opioids and pain interference would support providers in successfully tapering patients/residents who arrive in the PAC setting with long-term use of opioids onto non-pharmacologic treatments and non-opioid medications, as recommended by the Society for Post-Acute and Long-Term Care Medicine,[150] and consistent with HHS's 5-Point Strategy To Combat the Opioid Crisis [151] which includes “Better Pain Management.”
The Pain Interference data element set consists of three data elements: Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities. Pain Effect on Sleep assesses the frequency with which pain affects a patient's sleep. Pain Interference with Therapy Activities assesses the frequency with which pain interferes with a patient's ability to participate in therapies. The Pain Interference with Day-to-Day Activities assesses the extent to which pain interferes with a patient's ability to participate in day-to-day activities excluding therapy.
A similar data element on the effect of pain on activities is currently included in the OASIS. A similar data element on the effect on sleep is currently included in the MDS instrument in SNFs. We proposed to add the Pain Interference data element set (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) to the OASIS and to remove M1242, Frequency of Pain Interfering with Patient's Activity or Movement. For more information on the Pain Interference data elements, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We sought public input on the relevance of conducting assessments on pain and specifically on the larger set of Pain Interview data elements included in the National Beta Test. The proposed data elements were supported by comments from the TEP meeting held by our data element contractor on April 7 to 8, 2016. The TEP affirmed the feasibility and clinical utility of pain as a concept in a standardized assessment. The TEP agreed that data elements on pain interference with ability to participate in therapies versus other activities should be addressed. Further, during a more recent convening of the same TEP on September 17, 2018, the TEP supported the interview-based pain data elements included in the National Beta Test. The TEP members were particularly supportive of the items that focused on how pain interferes with activities (that is, Pain Interference data elements) because understanding the extent to which pain interferes with function would enable clinicians to determine the need for appropriate pain treatment. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We held a public comment period in 2016 to solicit feedback on the standardization of pain and several other items that were under development in prior efforts, through a call for input published on the CMS Measures Management System Blueprint website. From the prior public comment period, we included several pain data elements (Pain Effect on Sleep; Pain Interference—Therapy Activities; Pain Interference—Other Activities) in a second call for public comment, also published on the CMS Measures Management System Blueprint website, open from April 26 to June 26, 2017. The items we sought comment on were modified from all stakeholder and test efforts. Commenters provided general comments about pain assessment in general in addition to feedback on the specific pain items. A few commenters shared their support for assessing pain, the potential for pain assessment to improve the quality of care, and for the validity and reliability of the data elements. Commenters affirmed that the item of pain and the effect on sleep would be suitable for PAC settings. Commenters' main concerns included redundancy with existing data elements, feasibility and utility for cross-setting use, and the applicability of interview-based items to patients and residents with cognitive or communication impairments, and deficits. A summary report for the April 26 to June 26, 2017 public comment period titled “SPADE May-June 2017 Public Comment Summary Report” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Pain Interference data elements were included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Pain Interference data elements to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Pain Interference data elements in the National Beta Test can be found in the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018 for the purpose of soliciting input on the proposed standardized patient assessment data elements. The TEP supported the interview-based pain data elements included in the National Beta Test. The TEP members were particularly supportive of the items that focused on how pain interferes with activities (that is, Pain Interference data elements), because understanding the extent to which pain interferes with function would enable clinicians to determine the need for pain treatment. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, one commenter expressed strong support for the proposed pain SPADEs and was encouraged by the fact that this portion of the assessment surpasses pain presence. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing the effect of pain on function, stakeholder input, and strong test results, we proposed that the set of Pain Interference data elements (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day—to-Day Activities) meet the definition of standardized patient assessment data with respect to medical conditions and comorbidities under section 1899B(b)(1)(B)(iv) of the Act and to adopt the Pain Interference data elements (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal and received the following comments related to our proposal to adopt the Pain Interference (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) data elements.
Comment: Some commenters noted specific support for the introduction of the new pain data elements that can assist providers in care planning.
Response: CMS thanks commenters for their support of the pain interference data elements. We believe that standardized assessment of pain interference will support PAC clinicians in applying best-practices in pain management for chronic and acute pain, consistent with current clinical guidelines.
Comment: A commenter expressed concerns about the suitability of the Pain Interference data elements for use in patients with cognitive and communication deficits and recommended CMS consider the use of non-verbal means to allow patients to respond to SPADEs related to pain.
Response: We appreciate the commenter's concern surrounding pain assessment with patients with cognitive and communication deficits. The Pain Interference interview SPADEs require that a patient be able to communicate, whether verbally, in writing, or using another method. Assessors may use non-verbal means to administer the questions (for example, providing the questions and response in writing for a patient with severe hearing impairment). Patients who are unable to communicate by any means would not be required to complete the Pain Interference interview SPADEs. In addition, we note that evidence suggests that pain presence can be reliably assessed in non-communicative patients through structural observational protocols. To that end, we tested observational pain presence elements in the National Beta Test, but chose not to propose those data elements as SPADEs at this time. We will take the commenter's concern into consideration as the SPADEs are monitored and refined in the future.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Pain Interference (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) data elements as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
4. Impairment Data
Hearing and vision impairments are conditions that, if unaddressed, affect activities of daily living, communication, physical functioning, rehabilitation outcomes, and overall quality of life. Sensory limitations can lead to confusion in new settings, increase isolation, contribute to mood disorders, and impede accurate assessment of other medical conditions. Failure to appropriately assess, accommodate, and treat these conditions increases the likelihood that patients and residents will require more intensive and prolonged treatment. Onset of these conditions can be gradual, so individualized assessment with accurate screening tools and follow-up evaluations are essential to determining which patients and residents need hearing- or vision-specific medical attention or assistive devices and accommodations, including auxiliary aids and/or services, and to ensure that person-directed care plans are developed to accommodate a patient's or resident's needs. Accurate diagnosis and management of hearing or vision impairment would likely improve rehabilitation outcomes and care transitions, including transition from institutional-based care to the community. Accurate assessment of hearing and vision impairment would be expected to lead to appropriate treatment, accommodations, including the provision of auxiliary aids and services during the stay, and ensure that patients and residents continue to have their vision and hearing needs met when they leave the facility. In addition, entities that receive Federal financial assistance, such as through Medicare Parts A, C, and D, must take appropriate steps to ensure effective communication for individuals with disabilities, including provision of appropriate auxiliary aids and services.[152]
In alignment with our Meaningful Measures Initiative, we expect accurate individualized assessment, treatment, and accommodation of hearing and vision impairments of patients and residents in PAC to make care safer by reducing harm caused in the delivery of care; promoting effective prevention and treatment of chronic disease; strengthening person and family engagement as partners in their care; and promoting effective communication and coordination of care. For example, standardized assessment of hearing and vision impairments used in PAC will support ensuring patient safety (for example, risk of falls), identifying accommodations needed during the stay, and appropriate support needs at the time of discharge or transfer. Standardized assessment of these data elements will enable or support clinical decision-making and early clinical intervention; person-centered, high quality care (for example, facilitating better care continuity and coordination); better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable data elements assessing hearing and vision impairments are needed to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events.
Comments on the category of impairments were also submitted by stakeholders during the CY 2018 HH PPS proposed rule (82 FR 35369 through 35371) public comment period. We received public comments regarding the Hearing and Vision data elements; no additional comments were received about impairments in general.
We invited comment on our proposals to collect as standardized patient assessment data the Hearing and Vision data elements with respect to impairments.
a. Hearing
In CY 2020 HH PPS proposed rule (84 FR 34675 through 34676), we proposed that the Hearing data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35369 through 35370), accurate assessment of hearing impairment is important in the PAC setting for care planning and resource use. Hearing impairment has been associated with lower quality of life, including poorer physical, mental, and social functioning, and emotional health.[153 154] Treatment and accommodation of hearing impairment led to improved health outcomes, including but not limited to quality of life.[155] For example, hearing loss in elderly individuals has been associated with depression and cognitive impairment,[156 157 158] higher rates of incident cognitive impairment and cognitive decline,[159] and less time in occupational therapy.[160] Accurate assessment of hearing impairment is important in the PAC setting for care planning and defining resource use.
The proposed data element consists of the single Hearing data element. This data consists of one question that assesses level of hearing impairment. This data element is currently in use in the MDS in SNFs. For more information on the Hearing data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Hearing data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35369 through 35370). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted on the PAC PRD form of the data element (“Ability to Hear”) from August 12 to September 12, 2016, recommended that hearing, vision, and communication assessments be administered at the beginning of patient assessment process. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter noted that resources would be needed for a change in the OASIS to account for the Hearing data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Hearing data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Hearing data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Hearing data element in the National Beta Test can be found in the document titled, ”Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on January 5 and 6, 2017 for the purpose of soliciting input on all the SPADEs, including the Hearing data element. The TEP affirmed the importance of standardized assessment of hearing impairment in PAC patients and residents. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, a commenter expressed support for the Hearing data element and suggested administration at the beginning of the patient assessment to maximize utility. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Due to the relatively stable nature of hearing impairment, we proposed that HHAs that submit the Hearing data element with respect to SOC will be deemed to have submitted with respect to discharge. Taking together the importance of assessing hearing, stakeholder input, and strong test results, we proposed that the Hearing data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act and to adopt the Hearing data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Hearing data element.
Comment: With regard to the hearing data element, one commenter suggested that CMS consider how hearing impairment impacts a patient's ability to respond to the assessment tool in general.
Response: We intend to reinforce assessment tips and item rationale through training, open door forums, and future rulemaking efforts. In the existing guidance manual for the OASIS, we offer tips for administration that direct assessors to take appropriate steps to accommodate sensory and communication impairments when conducting the assessment.
After careful consideration of the public comment we received, we are finalizing our proposal to adopt the Hearing data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
b. Vision
In CY 2020 HH PPS proposed rule (84 FR 34676 through 35677), we proposed that the Vision data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act.
As described in the CY 2018 HH PPS proposed rule (82 FR 35370 through 35371), evaluation of an individual's ability to see is important for assessing risks such as falls and provides opportunities for improvement through treatment and the provision of accommodations, including auxiliary aids and services, which can safeguard patients and residents and improve their overall quality of life. Further, vision impairment is often a treatable risk factor associated with adverse events and poor quality of life. For example, individuals with visual impairment are more likely to experience falls and hip fracture, have less mobility, and report depressive symptoms.161 162 163 164 165 166 [167] Individualized initial screening can lead to life-improving interventions such as accommodations, including the provision of auxiliary aids and services, during the stay and/or treatments that can improve vision and prevent or slow further vision loss. In addition, vision impairment is often a treatable risk factor associated with adverse events which can be prevented and accommodated during the stay. Accurate assessment of vision impairment is important in the HH setting for care planning and defining resource use.
The proposed data element consists of the single Vision (Ability To See in Adequate Light) data element that consists of one question with five response categories. The Vision data element that we proposed for standardization was tested as part of the development of the MDS for SNFs and is currently in use in that assessment. A similar data element, but with different wording and fewer response option categories, is in use in the OASIS. We are proposed to add the Vision (Ability to See in Adequate Light) data element to the OASIS to replace M1200, Vision. For more information on the Vision data element, we refer readers to the document titled, “Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Vision data element was first proposed as a standardized patient assessment data element in the CY 2018 HH PPS proposed rule (82 FR 35370 through 35371). In that proposed rule, we stated that the proposal was informed by input we received from August 12 to September 12, 2016, on the Ability to See in Adequate Light data element (version tested in the PAC PRD with three response categories) through a call for input published on the CMS Measures Management System Blueprint website. The data element on which we solicited input differed from the proposed data element, but input submitted from August 12 to September 12, 2016 supported the assessment of vision in PAC settings and the useful information a vision data element would provide. We also stated that commenters had noted that the Ability to See item would provide important information that would facilitate care coordination and care planning, and consequently improve the quality of care. Other commenters suggested it would be helpful as an indicator of resource use and noted that the item would provide useful information about the abilities of patients and residents to care for themselves. Additional commenters noted that the item could feasibly be implemented across PAC providers and that its kappa scores from the PAC PRD support its validity. Some commenters noted a preference for MDS version of the Vision data element over the form put forward in public comment, citing the widespread use of this data element. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the CY 2018 HH PPS proposed rule, one commenter noted that resources would be needed for a change in the OASIS to account for the Vision data element.
Subsequent to receiving comments on the CY 2018 HH PPS proposed rule, the Vision data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Vision data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Vision data element in the National Beta Test can be found in the document titled, Proposed Specifications for HH QRP Quality Measures and SPADEs,” available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on January 5 and 6, 2017 for the purpose of soliciting input on all the SPADEs including the Vision data element. The TEP affirmed the importance of standardized assessment of vision impairment in PAC patients and residents. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, a commenter expressed support for the Vision data element and suggested administration at the beginning of the patient assessment to maximize utility. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on SPADEs Received After November 27, 2018 Stakeholder Meeting” is available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-ownloads-and-Videos.html.
Due to the relatively stable nature of vision impairment, we proposed that HHAs that submit the Vision data element with respect to SOC will be deemed to have submitted with respect to discharge. Taking together the importance of assessing vision, stakeholder input, and strong test results, we proposed that the Vision data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act and to adopt the Vision data element as standardized patient assessment data for use in the HH QRP.
We invited comment on our proposal to collect as standardized patient assessment data the Vision data element. We did not receive any comments on this category of impairment data or on the Vision data element.
Accordingly, we are finalizing our proposal to adopt the Vision data element as standardized patient assessment data beginning with the CY 2022 HH QRP as proposed.
5. New Category: Social Determinants of Health
a. Social Determinants of Health Data Collection To Inform Measures and Other Purposes
Subparagraph (A) of section 2(d)(2) of the IMPACT Act requires CMS to assess appropriate adjustments to quality measures, resource measures, and other measures, and to assess and implement appropriate adjustments to payment under Medicare based on those measures, after taking into account studies conducted by ASPE on social risk factors (described elsewhere in this final rule with comment period) and other information, and based on an individual's health status and other factors. Subparagraph (C) of section 2(d)(2) of the IMPACT Act further requires the Secretary to carry out periodic analyses, at least every three years, based on the factors referred to subparagraph (A) so as to monitor changes in possible relationships. Subparagraph (B) of section 2(d)(2) of the IMPACT Act requires CMS to collect or otherwise obtain access to data necessary to carry out the requirement of the paragraph (both assessing adjustments described previously in such subparagraph (A) and for periodic analyses in such subparagraph (C)). Accordingly we proposed to use our authority under subparagraph (B) of section 2(d)(2) of the IMPACT Act to establish a new data source for information to meet the requirements of subparagraphs (A) and (C) of section 2(d)(2). In the CY 2020 HH PPS proposed rule (84 FR 34677 through 34684), we proposed to collect and access data about social determinants of health (SDOH) in order to perform CMS' responsibilities under subparagraphs (A) and (C) of section 2(d)(2) of the IMPACT Act, as explained in more detail elsewhere in this final rule with comment period. Social determinants of health, also known as social risk factors, or health-related social needs, are the socioeconomic, cultural and environmental circumstances in which individuals live that impact their health. We proposed to collect information on seven proposed SDOH SPADE data elements relating to race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation; a detailed discussion of each of the proposed SDOH data elements is found in section IV.A.7.f.(ii). of this final rule with comment period.
We also proposed to use the OASIS, the current version being OASIS-D, described as the PAC assessment instrument for home health agencies under section 1899B(a)(2)(B)(i) of the Act, to collect these data via an existing data collection mechanism. We believe this approach will provide CMS with access to data with respect to the requirements of section 2(d)(2) of the IMPACT Act, while minimizing the reporting burden on PAC health care providers by relying on a data reporting mechanism already used and an existing system to which PAC providers are already accustomed.
The IMPACT Act includes several requirements applicable to the Secretary, in addition to those imposing new data reporting obligations on certain PAC providers as discussed in section IV.A.7.f.(2). of this final rule with comment period. Subparagraphs (A) and (B) of section 2(d)(1) of the IMPACT Act require the Secretary, acting through the Office of the Assistant Secretary for Planning and Evaluation (ASPE), to conduct two studies that examine the effect of risk factors, including individuals' socioeconomic status, on quality, resource use and other measures under the Medicare program. The first ASPE study was completed in December 2016 and is discussed in this final rule with comment period, and the second study is to be completed in the fall of 2019. We recognize that ASPE, in its studies, is considering a broader range of social risk factors than the SDOH data elements in this final, and address both PAC and non-PAC settings. We acknowledge that other data elements may be useful to understand, and that some of those elements may be of particular interest in non-PAC settings. For example, for beneficiaries receiving care in the community, as opposed to an in-patient facility, housing stability and food insecurity may be more relevant. We will continue to take into account the findings from both of ASPE's reports in future policy making.
One of the ASPE's first actions under the IMPACT Act was to commission the National Academies of Sciences, Engineering and Medicine (NASEM) to define and conceptualize socioeconomic status for the purposes of ASPE's two studies under section 2(d)(1) of the IMPACT Act. The NASEM convened a panel of experts in the field and conducted an extensive literature review. Based on the information collected, the 2016 NASEM panel report titled, “Accounting for Social Risk Factors in Medicare Payment: Identifying Social Risk Factors,” concluded that the best way to assess how social processes and social relationships influence key health-related outcomes in Medicare beneficiaries is through a framework of social risk factors instead of socioeconomic status. Social risk factors discussed in the NASEM report include socioeconomic position, race, ethnicity, gender, social context, and community context. These factors are discussed at length in chapter 2 of the NASEM report, entitled “Social Risk Factors.” [168] Consequently NASEM framed the results of its report in terms of “social risk factors” rather than “socioeconomic status” or “sociodemographic status.” The full text of the “Social Risk Factors” NASEM report is available for reading on the website at https://www.nap.edu/read/21858/chapter/1.
Each of the data elements we proposed to collect and access pursuant to our authority under section 2(d)(2)(B) of the IMPACT Act is identified in the 2016 NASEM report as a social risk factor that has been shown to impact care use, cost and outcomes for Medicare beneficiaries. CMS uses the term social determinants of health (SDOH) to denote social risk factors, which is consistent with the objectives of Healthy People 2020.[169]
ASPE issued its first Report to Congress, entitled “Social Risk Factors and Performance Under Medicare's Value-Based Purchasing Programs,” under section 2(d)(1)(A) of the IMPACT Act on December 21, 2016.[170] Using NASEM's social risk factors framework, ASPE focused on the following social risk factors, in addition to disability: (1) Dual enrollment in Medicare and Medicaid as a marker for low income; (2) residence in a low-income area; (3) Black race; (4) Hispanic ethnicity; and (5) residence in a rural area. ASPE acknowledged that the social risk factors examined in its report were limited due to data availability. The report also noted that the data necessary to meaningfully attempt to reduce disparities and identify and reward improved outcomes for beneficiaries with social risk factors have not been collected consistently on a national level in post-acute care settings. Where these data have been collected, the collection frequently involves lengthy questionnaires. More information on the Report to Congress on Social Risk Factors and Performance under Medicare's Value-Based Purchasing Programs, including the full report, is available on the website at https://aspe.hhs.gov/social-risk-factors-and-medicares-value-based-purchasing-programs-reports.
Section 2(d)(2) of the IMPACT Act relates to CMS activities and imposes several responsibilities on the Secretary relating to quality, resource use, and other measures under Medicare. As mentioned previously, under of subparagraph (A) of section 2(d)(2) of the IMPACT Act, the Secretary is required, on an ongoing basis, taking into account the ASPE studies and other information, and based on an individual's health status and other factors, to assess appropriate adjustments to quality, resource use, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. Section 2(d)(2)(A)(i) of the IMPACT Act applies to measures adopted under subsections (c) and (d) of section 1899B of the Act and to other measures under Medicare. However, our ability to perform these analyses, and assess and make appropriate adjustments is hindered by limits of existing data collections on SDOH data elements for Medicare beneficiaries. In its first study in 2016, in discussing the second study, ASPE noted that information related to many of the specific factors listed in the IMPACT Act, such as health literacy, limited English proficiency, and Medicare beneficiary activation, are not available in Medicare data.
Subparagraph 2(d)(2)(A) of the IMPACT Act specifically requires the Secretary to take the studies and considerations from ASPE's reports to Congress, as well as other information as appropriate, into account in assessing and implementing adjustments to measures and related payments based on measures in Medicare. The results of the ASPE's first study demonstrated that Medicare beneficiaries with social risk factors tended to have worse outcomes on many quality measures, and providers who treated a disproportionate share of beneficiaries with social risk factors tended to have worse performance on quality measures. As a result of these findings, ASPE suggested a three-pronged strategy to guide the development of value-based payment programs under which all Medicare beneficiaries receive the highest quality healthcare services possible. The three components of this strategy are to: (1) Measure and report quality of care for beneficiaries with social risk factors; (2) set high, fair quality standards for care provided to all beneficiaries; and (3) reward and support better outcomes for beneficiaries with social risk factors. In discussing how measuring and reporting quality for beneficiaries with social risk factors can be applied to Medicare quality payment programs, the report offered nine considerations across the three-pronged strategy, including enhancing data collection and developing statistical techniques to allow measurement and reporting of performance for beneficiaries with social risk factors on key quality and resource use measures.
Congress, in section 2(d)(2)(B) of the IMPACT Act, required the Secretary to collect or otherwise obtain access to the data necessary to carry out the provisions of paragraph (2) of section 2(d)(2) of the IMPACT Act through both new and existing data sources. Taking into consideration NASEM's conceptual framework for social risk factors discussed previously, ASPE's study, and considerations under section 2(d)(1)(A) of the IMPACT Act, as well as the current data constraints of ASPE's first study and its suggested considerations, we proposed to collect and access data about SDOH under section 2(d)(2) of the IMPACT Act. Our collection and use of the SDOH data described in section IV.A.7.f.(i). of this final rule with comment period, under section 2(d)(2) of the IMPACT Act, would be independent of our proposal discussed in this final rule with comment period in section IV.A.7.f.(2). of the preamble of this final rule with comment period and our authority to require submission of that data for use as SPADE under section 1899B(a)(1)(B) of the Act.
Accessing standardized data relating to the SDOH data elements on a national level is necessary to permit CMS to conduct periodic analyses, to assess appropriate adjustments to quality measures, resource use measures, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. We agree with ASPE's observations, in the value-based purchasing context, that the ability to measure and track quality, outcomes, and costs for beneficiaries with social risk factors over time is critical as policymakers and providers seek to reduce disparities and improve care for these groups. Collecting the data as proposed will provide the basis for our periodic analyses of the relationship between an individual's health status and other factors and quality, resource, and other measures, as required by section 2(d)(2) of the IMPACT Act, and to assess appropriate adjustments. These data would also permit us to develop the statistical tools necessary to maximize the value of Medicare data, reduce costs and improve the quality of care for all beneficiaries. Collecting and accessing SDOH data in this way also supports the three-part strategy put forth in the first ASPE report, specifically ASPE's consideration to enhance data collection and develop statistical techniques to allow measurement and reporting of performance for beneficiaries with social risk factors on key quality and resource use measures.
For the reasons discussed previously, we proposed under section 2(d)(2) of the IMPACT Act, to collect the data on the following SDOH: (1) Race, as described in section V.G.5.b.(1). of this final rule with comment period; (2) Ethnicity, described in section V.G.5.b.(1). of this final rule with comment period; (3) Preferred Language, as described in section V.G.5.(ii).(2). of this final rule with comment period; (4) Interpreter Services, as described in section V.G.5.b.(2). of this final rule with comment period; (5) Health Literacy, as described in section V.G.5.b.(3). of this final rule with comment period; (6) Transportation, as described in section V.G.5.(ii).(4). of this final rule with comment period; and (7) Social Isolation, as described in section V.G.5.b.(5). of this final rule with comment period. 84 FR 34677 through 34684. These data elements are discussed in more detail in section V.G.5. of this final rule with comment period.
Comment: One commenter noted that CMS did not state explicitly in the rule whether it anticipates the SDOH SPADEs will be used in adjusting measures and whether it believes that the IMPACT Act's requirements make it likely the SPADEs will be considered for use in future adjustments. The commenters recommended that CMS be circumspect and transparent in its approaches to incorporating the data elements proposed in payment and quality adjustments, such as by collecting stakeholder feedback before implementing any adjustments.
Response: We thank the commenter for their comment. We intend to use this data to assess the impact that the social determinants of health have on health outcomes. We will continue to work with stakeholders to promote transparency and support providers who serve vulnerable populations, promote high quality care, and refine and further implement SDOH SPADEs. We appreciate the comment on collecting stakeholder feedback before implementing any adjustments to measures based on the SDOH SPADEs. Collection of this data will help us identify potential disparities, conduct analyses, and assess whether any risk adjustments or other type of adjustments are needed. Any future policy development based on this data would be done transparently, and involve solicitation of stakeholder feedback through the notice and comment rulemaking process as appropriate.
Comment: Some commenters stated that the inclusion of the new proposed SPADEs, including SDOH data elements, will be burdensome for providers and agencies to implement. Commenters stated that CMS should explore obtaining this data through Medicare claims. They suggested that the agency should explain why certain data elements can only be obtained through OASIS and other patient assessment tools, rather than through other means, and asked that CMS lay out a multi-year plan for implementation because the current proposal for implementation is not feasible. The commenters suggested that CMS consider reducing the number of SDOH SPADE metrics to ensure questions and overall categories do not create an undue burden and that the new SPADE measures be transitioned by category in a stepwise fashion, allowing achievement of the IMPACT Act requirements while interoperability continues to be strengthened. They also urged CMS to consider a two-year voluntary submission period when additional SPADEs are adopted into the HH QRP to allow for vendor development, facility integration, and staff training, and recommended that CMS provides funding and administrative support for standardizing electronic medical records to ensure effective operability across all post-acute sites.
Response: We thank the commenters for their comments, and we agree that it is important to to minimize burden on providers. Under subsections (A) and (C) of section 2(d)(2, the IMPACT Act requires that CMS periodically assess appropriate adjustments to quality, resource use, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. Section 2(d)(2)(A)(i) of the IMPACT Act applies to measures adopted under subsections (c) and (d) of section 1899B of the Act and to other measures under Medicare. However, as stated above in this section, our ability to perform these analyses, and assess and make appropriate adjustments is hindered by limits of existing data collections on SDOH data elements for Medicare beneficiaries. In its first study in 2016, ASPE noted that information related to many of the specific factors listed in the IMPACT Act, such as health literacy, limited English proficiency, and Medicare beneficiary activation, are not available in Medicare data. We will collect this SDOH data under the authority of subsection (B) of section 2(d)(2) to obtain this level of detail. We will provide technical assistance to organizations as they implement these requirements and believe that the implementation timeline we proposed and are finalizing in this rule is sufficient because some of the data elements required may have already been collected by HHAs.
Comment: A few commenters noted concerns that the expanded comprehensive assessment added documentation and that the length of time it will take their clinicians to collect this data would be burdensome. The commenters stated that CMS should not add additional documentation burden to clinicians that add little value to patients or agencies who provide skilled home health services. They stated that CMS should not require agencies to collect SDOH data, which agencies have no ability to address or impact because it only increases time, cost, and frustration for patients and clinicians during the start of care while CMS intends to decrease cash flow during the same period.
Response: We thank the commenters for their comments. We are mindful of the increased obligation that is required though this additional data collection. However, this data collection is highly valuable. Accessing standardized data relating to the SDOH data elements on a national level is necessary to permit CMS to conduct periodic analyses, to assess appropriate adjustments to quality measures, resource use measures, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. Collecting the data as proposed will provide the basis for our periodic analyses of the relationship between an individual's health status and other factors and quality, resource use, and other measures, as required by section 2(d)(2) of the IMPACT Act, and to assess appropriate adjustments.
b. Standardized Patient Assessment Data
Section 1899B(b)(1)(B)(vi) of the Act authorizes the Secretary to collect SPADEs with respect to other categories deemed necessary and appropriate. In the CY 2020 HH PPS proposed rule (84 FR 34679) we proposed to create a Social Determinants of Health SPADE category under section 1899B(b)(1)(B)(vi) of the Act. In addition to collecting SDOH data for the purposes outlined previously, under section 2(d)(2)(B), we also proposed to collect as SPADE these same data elements (race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation) under section 1899B(b)(1)(B)(vi) of the Act. We believe that this proposed new category of Social Determinants of Health will inform provider understanding of individual patient risk factors and treatment preferences, facilitate coordinated care and care planning, and improve patient outcomes. We proposed to deem this category necessary and appropriate, for the purposes of SPADE, because using common standards and definitions for PAC data elements is important in ensuring interoperable exchange of longitudinal information between PAC providers and other providers to facilitate coordinated care, continuity in care planning, and the discharge planning process from post-acute care settings.
All of the Social Determinants of Health data elements we proposed under section 1899B(b)(1)(B)(vi) of the Act have the capacity to take into account treatment preferences and care goals of patients and to inform our understanding of patient complexity and risk factors that may affect care outcomes. While acknowledging the existence and importance of additional SDOH, we proposed to assess some of the factors relevant for patients receiving post-acute care that PAC settings are in a position to impact through the provision of services and supports, such as connecting patients with identified needs with transportation programs, certified interpreters, or social support programs.
As previously mentioned, and described in more detail elsewhere in this final rule with comment period, we proposed to adopt the following seven data elements as SPADE under the proposed Social Determinants of Health category: Race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation. To select these data elements, we reviewed the research literature, a number of validated assessment tools and frameworks for addressing SDOH currently in use (for example, Health Leads, NASEM, Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE), and ICD-10), and we engaged in discussions with stakeholders. We also prioritized balancing the reporting burden for PAC providers with our policy objective to collect SPADEs that will inform care planning and coordination and quality improvement across care settings. Furthermore, incorporating SDOH data elements into care planning has the potential to reduce readmissions and help beneficiaries achieve and maintain their health goals.
We also considered feedback received during a listening session that we held on December 13, 2018. The purpose of the listening session was to solicit feedback from health systems, research organizations, advocacy organizations, state agencies, and other members of the public on collecting patient-level data on SDOH across care settings, including consideration of race, ethnicity, spoken language, health literacy, social isolation, transportation, sex, gender identity, and sexual orientation. We also gave participants an option to submit written comments. A full summary of the listening session, titled “Listening Session on Social Determinants of Health Data Elements: Summary of Findings,” includes a list of participating stakeholders and their affiliations, and is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We solicited comment on these proposals and received the following comments. A discussion of these comments, along with our responses, appears in this section of this final rule with comment period.
Comment: Several commenters supported the inclusion of the seven proposed SDOH data elements, “race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation” as data elements collected by HHAs. A commenter noted that this supports the increasing attention on the critical role that social factors place in individual and population health and the growing body of evidence that shows addressing health-related social needs through enhanced clinical-community linkages can improve health outcomes and reduce costs. Another commenter stated that there are gaps in assessing SDOH and they appreciate the considerable time and energy that CMS has invested to develop these SPADEs.
Response: We thank the commenters for their support, and we agree that collecting SDOH data elements can be useful in identifying and addressing health disparities.
Comment: A few commenters expressed support for moving toward population health and outcomes through the SDOH SPADEs, requested clarification as to what the data will be used for, and inquired whether the data is already collected in other manners.
Response: We thank the commenters for the feedback. We proposed the collection of SDOH SPADEs as part of the requirements outlined in section 1899B(b)(1) of the Act, and more specifically under the category of standardized patient assessment data that we specified under section 1899B(b)(1)(B)(vi) of the Act. SDOH data for home health beneficiaries is not systematically available for home health providers at this time. Collection of this data will enhance patient care, interoperability, and coordinated care. The availability of standardized data through this collection allows for common standards and definitions to be used among the providers, thus ensuring interoperable exchange in longitudinal information between post-acute care providers and other providers. Additionally, standardizing the collection of SDOH SPADES will allow providers to have a better understanding of individual patient's risk factors and treatment preferences, to facilitate better coordinated care and care planning for their patients, and to monitor for improvements in patient outcomes. Further, we are collecting these new SDOH SPADE data elements under the authority of section 2(d)(2) of the IMPACT ACT in order to assess appropriate adjustments to quality, resource use, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures.
Comment: Several commenters supported the inclusion of the seven proposed SDOH data elements in the OASIS assessment instrument, as HHAs serve populations affected by social determinants, but recommend including additional factors within the SDOH SPADE category to ensure that the full spectrum of social needs is examined. One commenter suggested evaluating the abilities of the caregiver to support the patient's care needs since any deficit could pose a risk to the health and safety of the patient with advanced illness. A few other commenters suggested that CMS consider adding level of education, food insecurity, and the ability to secure medications to the SDOH assessment. Several commenters stated that collecting sexual orientation and gender identity data alongside the SDOH data elements is important in post-acute care because sexual and gender minorities experience unique cultural and environmental factors, including discrimination and stigma, which can negatively affect access to elder services, health services and health outcomes, and these identities also intersect with the proposed SDOH data elements in unique ways that can create additional barriers to care.
Response: We thank the commenters for the comments and agree that SDOH should include a wide and ever-changing array of elements. In considering which SDOH we proposed to collect, we balanced our policy objective to collect SPADES that will inform care planning and coordination and quality improvement across care settings with the reporting burden for PAC providers. To select these data elements, we reviewed the research literature, a number of validated assessment tools and frameworks for addressing SDOH currently in use (for example, Health Leads, National Academics of Sciences, Engineering, and Medicine (NASEM), Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE), and ICD-10). We also engaged in discussions with stakeholders. Ultimately, we decided to propose SDOH SPADE data elements, some of which were identified in the 2016 NASEM report, which was commissioned by Office of the Assistant Secretary for Planning and Evaluation (ASPE). We will take the commenters' suggestion to include additional or different SDOH under advisement as we continue to improve and refine the SPADEs.
Comment: One commenter noted that it is unknown what the most useful social risk data to collect is, and that collecting a comprehensive record comes with significant administrative burden. They support transforming general data collection categories into more discrete data points that can be analyzed and aggregated for programmatic strategies. They encouraged CMS to be mindful of meaningful collection and the potential for data overload as well as the ability to leverage existing data sources from across care settings. Since SDOH have impacts far beyond the post-acute care (PAC) setting, they cautioned CMS not to require data collection that cannot be readily gathered, shared or replicated beyond the PAC setting. For healthcare settings that have more established EHRs, the collection of SDOH should be aligned and associated costs for gathering, sharing or replicating considered. They also encouraged CMS to consider leveraging data points from primary care visits and urged CMS to take a holistic view of SDOH across the care continuum so that all care settings may gather, collect or leverage this data efficiently and so that the collection will yield the utmost impact.
Response: We thank the commenter for the comment, and we agree that collecting SDOH data elements can be useful in identifying and addressing health disparities. We also agree with the feedback that we should be mindful of meaningful collection of SDOH data collection efforts so that data elements that are selected are useful. This is one of the reasons why we proposed SDOH SPADE data elements that were identified in the 2016 National Academies of Sciences, Engineering, and Medicine (NASEM) report, which was commissioned by the Office of the Assistant Secretary for Planning and Evaluation (ASPE). Regarding the commenter's suggestion that we consider how it can align existing and future SDOH data elements to minimize burden on providers, we agree that it is important to minimize duplication efforts and align data collection as appropriate and to the extent possible, and will take this under advisement for future consideration. We also intend to solicit on the issue of whether we should collect SDOH data in other health care settings.
(1) Race and Ethnicity
The persistence of racial and ethnic disparities in health and health care is widely documented, including in PAC settings.171 172 173 174 [175] Despite the trend toward overall improvements in quality of care and health outcomes, the Agency for Healthcare Research and Quality, in its National Healthcare Quality and Disparities Reports, consistently indicates that racial and ethnic disparities persist, even after controlling for factors such as income, geography, and insurance.[176] For example, racial and ethnic minorities tend to have higher rates of infant mortality, diabetes and other chronic conditions, and visits to the emergency department, and lower rates of having a usual source of care and receiving immunizations such as the flu vaccine.[177] Studies have also shown that African Americans are significantly more likely than white Americans to die prematurely from heart disease and stroke.[178] However, our ability to identify and address racial and ethnic health disparities has historically been constrained by data limitations, particularly for smaller populations groups such as Asians, American Indians and Alaska Natives, and Native Hawaiians and other Pacific Islanders.[179]
The ability to improve understanding of and address racial and ethnic disparities in PAC outcomes requires the availability of better data. There is currently a Race and Ethnicity data element, collected in the MDS, LCDS, IRF-PAI, and OASIS, that consists of a single question, which aligns with the 1997 Office of Management and Budget (OMB) minimum data standards for federal data collection efforts.[180] The 1997 OMB Standard lists five minimum categories of race: (1) American Indian or Alaska Native; (2) Asian; (3) Black or African American; (4) Native Hawaiian or Other Pacific Islander; (5) and White. The 1997 OMB Standard also lists two minimum categories of ethnicity: (1) Hispanic or Latino; and (2) Not Hispanic or Latino. The 2011 HHS Data Standards requires a two-question format when self-identification is used to collect data on race and ethnicity. Large federal surveys such as the National Health Interview Survey, Behavioral Risk Factor Surveillance System, and the National Survey on Drug Use and Health, have implemented the 2011 HHS race and ethnicity data standards. CMS has similarly updated the Medicare Current Beneficiary Survey, Medicare Health Outcomes Survey, and the Health Insurance Marketplace Application for Health Coverage with the 2011 HHS data standards. More information about the HHS Race and Ethnicity Data Standards are available on the website at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=54 .
In the CY 2020 HH PPS proposed rule (84 FR 34680 through 34681), we proposed to revise the current Race and Ethnicity data element for purposes of this proposal to conform to the 2011 HHS Data Standards for person-level data collection, while also meeting the 1997 OMB minimum data standards for race and ethnicity. Rather than one data element that assesses both race and ethnicity, we proposed two separate data elements: One for Race and one for Ethnicity, that would conform with the 2011 HHS Data Standards and the 1997 OMB Standard. In accordance with the 2011 HHS Data Standards, a two-question format would be used for the proposed race and ethnicity data elements.
The proposed Race data element asks, “What is your race?” We proposed to include 14 response options under the race data element: (1) White; (2) Black or African American; (3) American Indian or Alaska Native; (4) Asian Indian; (5) Chinese; (6) Filipino; (7) Japanese; (8) Korean; (9) Vietnamese; (10) Other Asian; (11) Native Hawaiian; (12) Guamanian or Chamorro; (13) Samoan; and, (14) Other Pacific Islander.
The proposed Ethnicity data element asks, “Are you Hispanic, Latino/a, or Spanish origin?” We proposed to include five response options under the ethnicity data element: (1) Not of Hispanic, Latino/a, or Spanish origin; (2) Mexican, Mexican American, Chicano; (3) Puerto Rican; (4) Cuban; and (5) Another Hispanic, Latino, or Spanish Origin.
We believe that the two proposed data elements for race and ethnicity conform to the 2011 HHS Data Standards for person-level data collection, while also meeting the 1997 OMB minimum data standards for race and ethnicity, because under those standards, more detailed information on population groups can be collected if those additional categories can be aggregated into the OMB minimum standard set of categories.
In addition, we received stakeholder feedback during the December 13, 2018 SDOH listening session on the importance of improving response options for race and ethnicity as a component of health care assessments and for monitoring disparities. Some stakeholders emphasized the importance of allowing for self-identification of race and ethnicity for more categories than are included in the 2011 HHS Standard to better reflect state and local diversity, while acknowledging the burden of coding an open-ended health care assessment question across different settings.
We believe that the proposed modified race and ethnicity data elements more accurately reflect the diversity of the U.S. population than the current race/ethnicity data element included in MDS, LCDS, IRF-PAI, and OASIS.[181 182 183 184] We believe, and research consistently shows, that improving how race and ethnicity data are collected is an important first step in improving quality of care and health outcomes. Addressing disparities in access to care, quality of care, and health outcomes for Medicare beneficiaries begins with identifying and analyzing how SDOH, such as race and ethnicity, align with disparities in these areas.[185] Standardizing self-reported data collection for race and ethnicity allows for the equal comparison of data across multiple healthcare entities.[186] By collecting and analyzing these data, CMS and other healthcare entities will be able to identify challenges and monitor progress. The growing diversity of the U.S. population and knowledge of racial and ethnic disparities within and across population groups supports the collection of more granular data beyond the 1997 OMB minimum standard for reporting categories. The 2011 HHS race and ethnicity data standard includes additional detail that may be used by PAC providers to target quality improvement efforts for racial and ethnic groups experiencing disparate outcomes. For more information on the Race and Ethnicity data elements, we refer readers to the document titled “Proposed Specifications for HH QRP Measures and SPADEs,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of race and ethnicity data among IRFs, HHAs, SNFs, and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Race and Ethnicity data elements described previously as SPADEs with respect to the proposed Social Determinants of Health category.
Specifically, we proposed to replace the current Race/Ethnicity data element, M0140, with the proposed Race and Ethnicity data elements. Due to the stable nature of Race/Ethnicity, we proposed that HHAs that submit the Race and Ethnicity SPADEs with respect to SOC only will be deemed to have submitted those SPADEs with respect to SOC, ROC, and discharge, because it is unlikely that the assessment of those SPADEs with respect to SOC will differ from the assessment of the same SPADES with respect to ROC and discharge.
We solicited comment on these proposals.
Commenters submitted the following comments related to the proposed rule's discussion of the Race and Ethnicity SPADEs. A discussion of these comments, along with our responses, appears in this section of this final rule with comment period.
Comment: A few commenters questioned the response options for race. One commenter noted that the response options for race do not align with those used in other government data, such as the U.S. Census or the Office of Management and Budget (OMB). Some of the commenters also stated these responses are not consistent with the recommendations made in the 2009 NAESM (formerly Institute of Medicine) report. One commenter pointed out that the report recommended using broader OMB race categories and granular ethnicities chosen from a national standard set that can be “rolled up” into the broader categories. The commenters stated that it is unclear how CMS chose the 14 response options under the race data element and the five options under the ethnicity element and worried that these response options would add to the confusion that already may exist for patients about what terms like “race” and “ethnicity” mean for the purposes of health care data collection. The commenter also noted that CMS should confer directly with experts in the issue to ensure patient assessments are collecting the right data in the right way before these SDOH SPADEs are finalized. Another commenter noted that the response options for race may not include all races that should be reflected, such as Native African and Middle Eastern. The commenter stated that the item should include “check all that apply.” They encouraged CMS to provide rationale for the finalized list of response options. A commenter also urged CMS to review the Race/Ethnicity options to ensure they align with the www.wh.gov definitions as they are requirements for the Consolidated-Clinical Document Architecture (C-CDA) and referenced in the US Core Data for Interoperability (USCDI). They pointed out that the SDOH elements will need to align options with the current Consumer Assessment of Healthcare Providers and Systems (CAHPS) requirements and other data reporting requirements, reducing burden for providers to gather this information in multiple locations. The commenter stated that this alignment is imperative to ensure data elements are referenced from a single source of data entry for use across multiple data reporting requirements and that this careful review will help avoid administrative burdens.
Response: We agree that data elements used by CMS should, to the extent possible, cross-reference with those used by other agencies. The proposed race and ethnicity categories align with and are rolled up into the 1997 OMB minimum data standards and conforming with the 2011 HHS Data Standards at https://aspe.hhs.gov/basic-report/hhs-implementation-guidance-data-collection-standards-race-ethnicity-sex-primary-language-and-disability-status . The race and ethnicity data element that we proposed also includes “Check all that apply” language. As provided in the rationale of the proposed rule (84 FR 34680 through 34681), the 14 race categories and the 5 ethnicity categories conform with the 2011 HHS Data Standards for person-level data collection, which were developed in fulfillment of section 4302 of the Affordable Care Act that required the Secretary of HHS to establish data collection standards for race, ethnicity, sex, primary language, and disability status.
The Section 4302 Standards Workgroup was formed through the HHS Data Council, which is the principal, senior internal Departmental forum and advisory body to the Secretary on health and human services data policy and which coordinates HHS data collection and analysis activities. The Workgroup included representatives from HHS, the OMB, and the Census Bureau. The Workgroup examined current federal data collection standards, adequacy of prior testing, and quality of the data produced in prior surveys; consulted with statistical agencies and programs; reviewed OMB data collection standards and the 2009 Institute of Medicine report Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement; sought input from national experts; and built on its members' experience with collecting and analyzing demographic data. As a result of this Workgroup, a set of data collection standards were developed, and then published for public comment. This set of data collection standards is referred to as the 2011 HHS Data Standards (https://aspe.hhs.gov/basic-report/hhs-implementation-guidance-data-collection-standards-race-ethnicity-sex-primary-language-and-disability-status). The categories of race and ethnicity under the 2011 HHS Data Standards allow for more detailed information to be collected and the additional categories under the 2011 HHS Data Standards can be aggregated into the OMB minimum standards set of categories. As noted in the proposed rule, we conducted a listening session regarding the proposed SDOH data elements regarding the importance of improving response options for race and ethnicity as a component of health care assessments and for monitoring disparities. Some stakeholders emphasized the importance of allowing for self-identification of race and ethnicity for more categories than are included in the 2011 HHS Data Standards to better reflect state and local diversity.
Regarding the commenter who urged CMS to review the proposed race and ethnicity elements to ensure they align with the www.wh.gov definitions, we believe the commenter may be referring to the 1997 OMB minimum data standards as the White House's definitions. If so, then as provided earlier in this response, the race and ethnicity categories that were proposed do align with and are rolled up into the 1997 OMB minimum data standards, which also align with CAHPS reporting requirements.
Comment: One commenter stated that the degree of detail required for the social determinants of health sections A1005 ethnicity (focus on Hispanic, Latino/and Spanish origin) and A1010 race may be regarded as intrusive and offensive to patients. This could potentially cause refusal of home care or affect the provider-patient relationship and patient satisfaction.
Response: We thank the commenter for their comment. Accessing standardized data relating to the SDOH data elements on a national level is necessary to permit CMS to conduct periodic analyses, to assess appropriate adjustments to quality measures, resource use measures, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. Collecting the data as proposed will provide the basis for our periodic analyses of the relationship between an individual's health status and other factors and quality, resource use, and other measures, as required by section 2(d)(2) of the IMPACT Act, and to assess appropriate adjustments. Moreover, collection of race and ethnicity data, along with the other SDOH data elements, contributes to higher quality patient outcomes due to the ability to use the standardized, interoperable data to facilitate coordinated care and improved patient outcomes. Collection of data for these purposes is authorized under 1899B(a)(1)(B). With the high value of collecting this data in mind, we do acknowledge the commenter's concerns about the potential for patients to view the collection of this data as intrusive and offensive, leading to service refusal or damaging the provide-patient relationship and patient satisfaction. We will monitor the implementation of these new data elements and modify the rule as appropriate.
Providers are required to ask patients for responses to every SPADE data element question required in this rule for the HH QRP, including every SDOH SPADE question. However, patients are not required to respond to any of the SDOH SPADE questions. If the patient declines to or is unable to answer an SDOH SPADE question, the provider must indicate this non-response in the documentation. Therefore, we believe that the patient's wishes and concerns about privacy and whether the question is intrusive are respected and adequately protected under this policy.
(2) Preferred Language and Interpreter Services
More than 64 million Americans speak a language other than English at home, and nearly 40 million of those individuals have limited English proficiency (LEP).[187] Individuals with LEP have been shown to receive worse care and have poorer health outcomes, including higher readmission rates.[188 189 190] Communication with individuals with LEP is an important component of high quality health care, which starts by understanding the population in need of language services. Unaddressed language barriers between a patient and provider care team negatively affects the ability to identify and address individual medical and non-medical care needs, to convey and understand clinical information, as well as discharge and follow up instructions, all of which are necessary for providing high quality care. Understanding the communication assistance needs of patients with LEP, including individuals who are Deaf or hard of hearing, is critical for ensuring good outcomes.
Presently, the preferred language of patients and need for interpreter services are assessed in two PAC assessment tools. The LCDS and the MDS use the same two data elements to assess preferred language and whether a patient or resident needs or wants an interpreter to communicate with health care staff. The MDS initially implemented preferred language and interpreter services data elements to assess the needs of SNF residents and patients and inform care planning. For alignment purposes, the LCDS later adopted the same data elements for LTCHs. The 2009 NASEM (formerly Institute of Medicine) report on standardizing data for health care quality improvement emphasizes that language and communication needs should be assessed as a standard part of health care delivery and quality improvement strategies.[191]
In developing our proposal for a standardized language data element across PAC settings, we considered the current preferred language and interpreter services data elements that are in LCDS and MDS. We also considered the 2011 HHS Primary Language Data Standard and peer-reviewed research. The current preferred language data element in LCDS and MDS asks, “What is your preferred language?” Because the preferred language data element is open-ended, the patient is able to identify their preferred language, including American Sign Language (ASL). Finally, we considered the recommendations from the 2009 NASEM (formerly Institute of Medicine) report, “Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement.” In it, the committee recommended that organizations evaluating a patient's language and communication needs for health care purposes, should collect data on the preferred spoken language and on an individual's assessment of his/her level of English proficiency.
A second language data element in LCDS and MDS asks, “Do you want or need an interpreter to communicate with a doctor or health care staff?” and includes yes or no response options. In contrast, the 2011 HHS Primary Language Data Standard recommends either a single question to assess how well someone speaks English or, if more granular information is needed, a two-part question to assess whether a language other than English is spoken at home and if so, identify that language. However, neither option allows for a direct assessment of a patient's preferred spoken or written language nor whether they want or need interpreter services for communication with a doctor or care team, both of which are an important part of assessing patient needs and the care planning process. More information about the HHS Data Standard for Primary Language is available on the website at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=54.
Research consistently recommends collecting information about an individual's preferred spoken language and evaluating those responses for purposes of determining language access needs in health care.[192] However, using “preferred spoken language” as the metric does not adequately account for people whose preferred language is ASL, which would necessitate adopting an additional data element to identify visual language. The need to improve the assessment of language preferences and communication needs across PAC settings should be balanced with the burden associated with data collection on the provider and patient. Therefore we proposed to use the Preferred Language and Interpreter Services data elements currently in use on the MDS and LCDS, on the OASIS.
In addition, we received feedback during the December 13, 2018 listening session on the importance of evaluating and acting on language preferences early to facilitate communication and allowing for patient self-identification of preferred language. Although the discussion about language was focused on preferred spoken language, there was general consensus among participants that stated language preferences may or may not accurately indicate the need for interpreter services, which supports collecting and evaluating data to determine language preference, as well as the need for interpreter services. An alternate suggestion was made to inquire about preferred language specifically for discussing health or health care needs. While this suggestion does allow for ASL as a response option, we do not have data indicating how useful this question might be for assessing the desired information and thus we are not including this question in our proposal.
Improving how preferred language and need for interpreter services data are collected is an important component of improving quality by helping PAC providers and other providers understand patient needs and develop plans to address them. For more information on the Preferred Language and Interpreter Services data elements, we refer readers to the document titled “Final Specifications for HH QRP Measures and SPADEs,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of language data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Preferred Language and Interpreter Services data elements currently used on the LCDS and MDS, and described previously, as SPADES with respect to the Social Determinants of Health category.
Comment: Some commenters noted that preferred language, need for an interpreter, access to transportation, and social isolation are unlikely to change between admission and discharge. One commenter disagrees with CMS's statement in the SNF, IRF and LTCH PPS FY 2020 final rules that “[patient] circumstances may have changed over the duration of their admission,” and might change the answers to the health literacy, access to transportation and social isolation items. They acknowledge that for the SNF, IRF, and LTCH QRPs, CMS will allow providers to collect the Language Preference and Interpreter Services at just admission and they felt that CMS should do the same for other SDOH SPADES and just require that they be collected at admission. For example, they noted that Health Literacy is the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions, and it is difficult to see how these elemental skills would change over the course of a month-long HH episode. Thus, they encouraged CMS to only require collection of all SDOH SPADEs with respect to admission only.
Response: We thank the commenters for their comments. We agree that Preferred Language and Interpreter Services should just be collected at admission given that a patient's response is unlikely to change. We disagree with the commenters that Health Literacy, Transportation and Social Isolation are unlikely to change from admission to discharge. Unlike the Vision, Hearing, Race, Ethnicity, Preferred Language, and Interpreter Services SPADEs, we believe that the response to this data element is likely to change from admission to discharge for some patients. For example, some patients may develop health issues, such as cognitive decline, during their stay that could impact their response to health literacy thus changing their status at discharge. Cognitive decline can impact a patient's ability to process and understand health information. Similarly, losing a loved one or caregiver, which can happen at any time, could impact someone's response on social isolation and access to transportation. It is common for caregivers to provide emotional support and access to transportation for those for those that they provide caregiving. Therefore, we are finalizing that the Preferred Language and Interpreter Services data elements would just be collected at admission, which will align with the collection of those elements in the IRF, SNF, and LTCH QRPs. We refer the reader to section V.L of this final rule with comment period, where we discuss the collection points for other SDOH SPADEs. For Health Literacy, Transportation, and Social Isolation, we are finalizing that these elements be collected upon admission and discharge, as described in these sections of this final rule with comment period.
(3) Health Literacy
The Department of Health and Human Services defines health literacy as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.” [193] Similar to language barriers, low health literacy can interfere with communication between the provider and patient and the ability for patients or their caregivers to understand and follow treatment plans, including medication management. Poor health literacy is linked to lower levels of knowledge about health, worse health outcomes, and the receipt of fewer preventive services, but higher medical costs and rates of emergency department use.[194]
Health literacy is prioritized by Healthy People 2020 as an SDOH.[195] Healthy People 2020 is a long-term, evidence-based effort led by the Department of Health and Human Services that aims to identify nationwide health improvement priorities and improve the health of all Americans. Although not designated as a social risk factor in NASEM's 2016 report on accounting for social risk factors in Medicare payment, the NASEM report noted that Health literacy is impacted by other social risk factors and can affect access to care as well as quality of care and health outcomes.[196] Assessing for health literacy across PAC settings would facilitate better care coordination and discharge planning. A significant challenge in assessing the health literacy of individuals is avoiding excessive burden on patients and health care providers. The majority of existing, validated health literacy assessment tools use multiple screening items, generally with no fewer than four, which would make them burdensome if adopted in MDS, LCDS, IRF-PAI, and OASIS.
The Single Item Literacy Screener (SILS) question asks, “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” Possible response options are: (1) Never; (2) Rarely; (3) Sometimes; (4) Often; and (5) Always. The SILS question, which assesses reading ability (a primary component of health literacy), tested reasonably well against the 36 item Short Test of Functional Health Literacy in Adults (S-TOFHLA), a thoroughly vetted and widely adopted health literacy test, in assessing the likelihood of low health literacy in an adult sample from primary care practices participating in the Vermont Diabetes Information System.[197 198] The S-TOFHLA is a more complex assessment instrument developed using actual hospital related materials such as prescription bottle labels and appointment slips, and often considered the instrument of choice for a detailed evaluation of health literacy.[199] Furthermore, the S-TOFHLA instrument is proprietary and subject to purchase for individual entities or users.[200] Given that SILS is publicly available, shorter and easier to administer than the full health literacy screen, and research found that a positive result on the SILS demonstrates an increased likelihood that an individual has low health literacy, we proposed to use the single-item reading question for health literacy in the standardized data collection across PAC settings. We believe that use of this data element will provide sufficient information about the health literacy of HH patients to facilitate appropriate care planning, care coordination, and interoperable data exchange across PAC settings.
In addition, we received feedback during the December 13, 2018 SDOH listening session on the importance of recognizing health literacy as more than understanding written materials and filling out forms, as it is also important to evaluate whether patients understand their conditions. However, the NASEM recently recommended that health care providers implement health literacy universal precautions instead of taking steps to ensure care is provided at an appropriate literacy level based on individualized assessment of health literacy.[201] Given the dearth of Medicare data on health literacy and gaps in addressing health literacy in practice, we recommend the addition of a health literacy data element.
The proposed Health Literacy data element is consistent with considerations raised by NASEM and other stakeholders and research on health literacy, which demonstrates an impact on health care use, cost, and outcomes.[202] For more information on the proposed Health Literacy data element, we refer readers to the document titled “Proposed Specifications for HH QRP Measures and SPADEs,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of health literacy data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the SILS question, described previously for the Health Literacy data element, as SPADE under the Social Determinants of Health category. We proposed to add the Health Literacy data element to the OASIS. We solicited comment on this proposal. A discussion of the comment, along with our response, appears in this of this final rule with comment period.
Comment: One commenter stated that the health literacy question could be improved to capture whether the patient can read, understand, and implement/respond to the information. In addition, the commenter stated that the question does not take into account whether a patient's need for help is due to limited vision, which is different from the purpose of the separate Vision Impairment data element. Another possible question the commenter suggested was “How often do you have difficulty?” The commenter suggested that a single construct may not be sufficient for this area, depending on the aspect of health literacy that CMS intends to identify.
Response: We appreciate this commenter's suggestions. We proposed the Single Item Literacy Screener (SILS) to minimize burden and based on stakeholder feedback. We also conducted a listening session regarding the proposed SDOH data elements regarding the importance of collecting health literacy as a component of health care assessments and the listening session stakeholders generally supported the SILS option. Regarding the potential impacts of impaired vision, we do want to note that this rule adopts a vision data element that will be included on the OASIS instrument. The data on a patient's vision will be helpful with the health literacy question to gain a comprehensive picture of the patient's functioning.
(4) Transportation
Transportation barriers commonly affect access to necessary health care, causing missed appointments, delayed care, and unfilled prescriptions, all of which can have a negative impact on health outcomes.[203] Access to transportation for ongoing health care and medication access needs, particularly for those with chronic diseases, is essential to successful chronic disease management. Adopting a data element to collect and analyze information regarding transportation needs across PAC settings would facilitate the connection to programs that can address identified needs. We therefore proposed to adopt as SPADE a single transportation data element that is from the Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE) assessment tool and currently part of the Accountable Health Communities (AHC) Screening Tool.
The proposed Transportation data element from the PRAPARE tool asks, “Has a lack of transportation kept you from medical appointments, meetings, work, or from getting things needed for daily living?” The three response options are: (1) Yes, it has kept me from medical appointments or from getting my medications; (2) Yes, it has kept me from non-medical meetings, appointments, work, or from getting things that I need; and (3) No. The patient would be given the option to select all responses that apply. We proposed to use the transportation data element from the PRAPARE Tool, with permission from National Association of Community Health Centers (NACHC), after considering research on the importance of addressing transportation needs as a critical SDOH.[204]
The proposed data element is responsive to research on the importance of addressing transportation needs as a critical SDOH and would adopt the Transportation item from the PRAPARE tool.[205] This data element comes from the national PRAPARE social determinants of health assessment protocol, developed and owned by NACHC, in partnership with the Association of Asian Pacific Community Health Organization, the Oregon Primary Care Association, and the Institute for Alternative Futures. Similarly the Transportation data element used in the AHC Screening Tool was adapted from the PRAPARE tool. The AHC screening tool was implemented by the Center for Medicare and Medicaid Innovation's AHC Model and developed by a panel of interdisciplinary experts that looked at evidence-based ways to measure SDOH, including transportation. While the transportation access data element in the AHC screening tool serves the same purposes as our proposed SPADE collection about transportation barriers, the AHC tool has binary yes or no response options that do not differentiate between challenges for medical versus non-medical appointments and activities. We believe that this is an important nuance for informing PAC discharge planning to a community setting, as transportation needs for non-medical activities may differ than for medical activities and should be taken into account.[206] We believe that use of this data element will provide sufficient information about transportation barriers to medical and non-medical care for HH patients to facilitate appropriate discharge planning and care coordination across PAC settings. As such, we proposed to adopt the Transportation data element from PRAPARE. More information about development of the PRAPARE tool is available on the website at https://protect2.fireeye.com/url?k=7cb6eb44-20e2f238-7cb6da7b-0cc47adc5fa2-1751cb986c8c2f8c&u=http://www.nachc.org/prapare.
In addition, we received stakeholder feedback during the December 13, 2018 SDOH listening session on the impact of transportation barriers on unmet care needs. While recognizing that there is no consensus in the field about whether providers should have responsibility for resolving patient transportation needs, discussion focused on the importance of assessing transportation barriers to facilitate connections with available community resources.
Adding a Transportation data element to the collection of SPADE would be an important step to identifying and addressing SDOH that impact health outcomes and patient experience for Medicare beneficiaries. For more information on the Transportation data element, we refer readers to the document titled “Final Specifications for HH QRP Measures and SPADEs,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of transportation data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Transportation data element described previously as SPADE with respect to the proposed Social Determinants of Health category. If finalized as proposed, we would add the Transportation data element to the OASIS.
We solicited comment on this proposal. A discussion of the comment received, along with our responses appears in this section of this final rule with comment period.
Comment: One commenter supported the collection of data to capture the reason(s) transportation affects a patient's access to health care. The commenter appreciated the inclusion of these items on the HHA and encouraged exploration of quality measures in this area as transportation is an extremely important instrumental activity of daily living to effectively transition to the community.
Response: We thank the commenter for the comment and we will consider this feedback as we continue to improve and refine our quality measures.
(5) Social Isolation
Distinct from loneliness, social isolation refers to an actual or perceived lack of contact with other people, such as living alone or residing in a remote area.[207 208] Social isolation tends to increase with age, is a risk factor for physical and mental illness, and a predictor of mortality.[209 210 211] Post-acute care providers are well-suited to design and implement programs to increase social engagement of patients, while also taking into account individual needs and preferences. Adopting a data element to collect and analyze information about social isolation for patients receiving HH services and across PAC settings would facilitate the identification of patients who are socially isolated and who may benefit from engagement efforts.
We proposed to adopt as SPADE a single social isolation data element that is currently part of the AHC Screening Tool. The AHC item was selected from the Patient-Reported Outcomes Measurement Information System (PROMIS®) Item Bank on Emotional Distress, and asks, “How often do you feel lonely or isolated from those around you?” The five response options are: (1) Never; (2) Rarely; (3) Sometimes; (4) Often; and (5) Always.[212] The AHC Screening Tool was developed by a panel of interdisciplinary experts that looked at evidence-based ways to measure SDOH, including social isolation. More information about the AHC Screening Tool is available on the website at https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf.
In addition, we received stakeholder feedback during the December 13, 2018 SDOH listening session on the value of receiving information on social isolation for purposes of care planning. Some stakeholders also recommended assessing social isolation as an SDOH as opposed to social support.
The proposed Social Isolation data element is consistent with NASEM considerations about social isolation as a function of social relationships that impacts health outcomes and increases mortality risk, as well as the current work of a NASEM committee examining how social isolation and loneliness impact health outcomes in adults 50 years and older. We believe that adding a Social Isolation data element would be an important component of better understanding patient complexity and the care goals of patients, thereby facilitating care coordination and continuity in care planning across PAC settings. For more information on the Social Isolation data element, we refer readers to the document titled “Proposed Specifications for HH QRP Measures and SPADEs,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of data about social isolation among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Social Isolation data element described previously as SPADE with respect to the proposed Social Determinants of Health category. We proposed to add the Social Isolation data element to the OASIS.
We solicited comment on this proposal. A discussion of the comment, along with our response, appears in this section of this final rule with comment period.
Comment: One commenter stated that the proposed question on social isolation may solicit different answers based on the time horizon considered by the beneficiary as beneficiaries who are newly admitted to an HHA may have experienced differing levels of social isolation throughout their time in acute and post-acute care due to interactions with health care providers, emergency providers, and friends or family visiting due to hospitalization. The commenter believes this question could be improved by adding timeframe to the question. For example, “How often have you felt lonely or isolated from those around you in the past six months?”.
Response: We thank the commenter for this comment and we will take it under advisement for future consideration. The social isolation question proposed is currently part of the Accountable Health Communities (AHC) Screening Tool. The AHC item was selected from the Patient-Reported Outcomes Measurement Information System (PROMIS®) Item Bank on Emotional Distress. At this time, we do not believe that we should add a time horizon to the social isolation question. During cognitive testing of the proposed social isolation question, there was no evidence of confusion related to the time covered.[213] We will continue to monitor if this is an area that needs further clarification to satisfy the social isolation data element.
After consideration of the public comments, we are finalizing our proposals to collect SDOH data for the purposes of section 2(d)(2) of the IMPACT Act and section 1899B(b)(1)(B)(vi) of the Act as follows. With regard to Race, Ethnicity, Health Literacy, Transportation, and Social Isolation, we are finalizing our proposals as proposed. In response to stakeholder comments, we are finalizing that HHAs that submit the Preferred Language and Interpreter Services SPADEs with respect to admission will be deemed to have submitted with respect to both admission and discharge.
J. Codification of the Home Health Quality Reporting Program Requirements
To promote alignment of the HH QRP and the SNF QRP, IRF QRP, and LTCH QRP regulatory text, we believe that with the exception of the provision governing the 2 percentage point reduction to the update of the unadjusted national standardized prospective payment rate, it is appropriate to codify the requirements that apply to the HH QRP in a single section of our regulations. Accordingly, in the CY 2020 HH PPS proposed rule (84 FR 34684 through 34685), we proposed to amend 42 CFR chapter IV, subchapter G, by creating a new § 484.245, titled “Home Health Quality Reporting Program”.
The provisions we proposed to codify were as follows:
- The HH QRP participation requirements at § 484.245(a) (72 FR 49863).
- The HH QRP data submission requirements at § 484.245(b)(1), including—
++ Data on measures specified under section 1899B(c)(1) and 1899B(d)(1) of the Act;
++ Standardized patient assessment data required under section 1899B(b)(1) of the Act (82 FR 51735 through 51736); and
++ Quality data specified under section 1895(b)(3)(B)(v)(II) of the Act including the HHCAHPS survey data submission requirements at § 484.245(b)(1)(iii)(A) through (E) (redesignated from § 484.250(b) through (c)(3) and striking § 484.250(a)(2)).
- The HH QRP data submission form, manner, and timing requirements at § 484.245(b)(2).
- The HH QRP exceptions and extension requirements at § 484.245(c) (redesignated from § 484.250(d)(1) through (d)(4)(ii)).
- The HH QRP's reconsideration policy at § 484.245(d) (redesignated from § 484.250(e)(1) through (4)).
- The HH QRP appeals policy at § 484.245(e) (redesignated from § 484.250(f)).
We also note the following codification proposals:
- The addition of the HHCAHPS and HH QRP acronyms to the definitions at § 484.205.
- The removal of the regulatory provision in § 484.225(b) regarding the unadjusted national prospective 60-day episode rate for HHAs that submit their quality data as specified by the Secretary.
- The redesignation of the regulatory provision in § 484.225(c) to § 484.225(b) regarding the unadjusted national prospective 60-day episode rate for HHAs that do not submit their quality data as specified by the Secretary.
- The redesignation of the regulatory provision in § 484.225(d) to § 484.225(c) regarding the national, standardized prospective 30-day payment amount. The cross-reference in newly redesignated paragraph (c) would also be revised.
Comment: One commenter supported the proposed codification of the HH QRP requirements.
Response: CMS appreciates the support from the commenter for the codification of the HH QRP requirements.
Comment: One commenter did not support the codification of the HH QRP requirements because of a concern that the current program favors patients whose health status will improve, and does not adequately consider patients whose status will just be maintained by home health services. The commenter believes that codification of the current requirements will reinforce the lack of attention given to appropriate delivery of maintenance nursing and therapy services.
Response: We believe it is important to codify policies that apply to the HHAs as it reflects the policies that apply to HHA's relative to the HH QRP. We do not agree with the recommendation to not codify our policies.
Final Decision: After careful consideration of the public comments received, we are finalizing our proposal to codify requirements for the HH QRP and note that we have made both a substantive change and technical edits.
K. Home Health Care Consumer Assessment of Healthcare Providers and Systems (CAHPS®) Survey (HHCAHPS)
In the CY 2020 HH PPS proposed rule (84 FR 34685), we proposed to remove Question 10 from all HHCAHPS Surveys (both mail surveys and telephone surveys) which says, “In the last 2 months of care, did you and a home health provider from this agency talk about pain?” which is one of seven questions (they are questions 3, 4, 5, 10, 12, 13 and 14) in the “Special Care Issues” composite measure, beginning July 1, 2020. The “Special Care Issues” composite measure also focuses on home health agency staff discussing home safety, the purpose of the medications that are being taken, side effects of medications, and when to take medications. In the initial development of the HHCAHPS Survey, this question was included in the survey since home health agency staff talk about pain to identify any emerging issues (for example, wounds that are getting worse) every time they see their home health patients.
We proposed to remove the pain question from the HHCAHPS Survey and pain items from the OASIS data sets to avoid potential unintended consequences that may arise from their inclusion in CMS surveys and datasets. The reason that CMS proposed removing this particular pain question is consistent with the proposed removal of pain items from OASIS in section IV.D.1. of this final rule with comment period and is also consistent with the removal of pain items from the Hospital CAHPS Survey. The removal of the pain question from CMS surveys and removal of pain items from CMS data sets is to avoid potential unintended consequences that arise from their inclusion in CMS surveys and datasets. We welcomed comments about the proposed removal of Q10 from the HHCAHPS Survey. In the initial development of the HHCAHPS Survey, this question was included in the survey, and, consequently, from the “Special Care Issues” measure. The HHCAHPS Survey is available on the official website for HHCAHPS, at https://homehealthcahps.org.
We solicited comment on this proposal. A discussion of the comments, along with our responses, appears in this section of this final rule with comment period.
Comment: We received a few comments supporting the removal of Question 10. Commenters supporting the proposal to remove the pain question either did not give a reason, or stated it would reduce burden. Two commenters supported the question's removal due to the unintended consequences of using pain killers.
Response: We thank the commenters for their support.
Comment: The majority of commenters opposed the removal of Question 10. There were a number of reasons that commenters opposed the proposal to remove Q10 from the HHCAHPS survey and, consequently, from the HHCAHPS Specific Care Issues measure. Some commenters stated that pain assessment is a critical component of the home health care patient assessment protocol and should be measured as part of a patient experience of care survey. Several commenters contended that there is no evidence that the discussion of pain is linked to opioid misuse. Commenters wrote that home health providers are unable to prescribe opioids and other medications so there would be no direct impact on opioid prescribing. Some commenters said that because the presence of pain is related to the ability to function, it is important to determine if pain is causing a patient to have limited activity. Other commenters noted that talking about pain is part of the physical therapist's assessment of patients in home health care.
Some commenters thought that Question 10 provides an opportunity to assess if home health agency staff are asking their patients about pain to presumably follow-up with steps to address the patients' pain and discomfort. An example is that a patient with diabetic complications may not feel pain in their feet and by the time they feel pain in a wound in their foot, it is likely that the wound's infection will be in a critical state causing significant discomfort.
Response: We appreciate these comments and agree that monitoring pain is critical in the home health setting to monitor how patients are recovering and to identify emergent issues. Whether the question is on the survey or not, we expect home health agencies to continue to monitor pain in the home health setting.
Final Decision: Based upon the comments received, we have evaluated our proposal to take into consideration points raised by commenters and also concerns raised within HHS. Commenters noted that monitoring of pain is critical and we agree that it is imperative to continue to monitor the management of pain. HHS reviewers also noted that removal of this question would potentially affect the validity of the survey and we also agree with their concern. Therefore, we are not finalizing our proposal to remove Question 10 from all HHCAHPS Surveys.
L. Form, Manner, and Timing of Data Submission Under the HH QRP
1. Background
Section 484.250 requires HHAs to submit OASIS data and Home Health Care Consumer Assessment of Healthcare Providers and Systems Survey (HHCAHPS) data to meet the quality reporting requirements of section 1895(b)(3)(B)(v) of the Act. Not all OASIS data described in § 484.55(b) and (d) are necessary for purposes of complying with the quality reporting requirements of section 1895(b)(3)(B)(v) of the Act. OASIS data items may be used for other purposes unrelated to the HH QRP, including payment, survey and certification, the HH VBP Model, or care planning. Any OASIS data that are not submitted for the purposes of the HH QRP are not used for purposes of determining HH QRP compliance.
2. Schedule for Reporting the Transfer of Health Information Quality Measures Beginning With the CY 2022 HH QRP
As discussed in section V.E. of this final rule with comment period, we are finalizing our proposal to adopt the Transfer of Health Information to Provider-Post-Acute Care (PAC) and Transfer of Health Information to Patient-Post-Acute Care (PAC) quality measures beginning with the CY 2022 HH QRP. We are also finalizing our proposal that HHAs would report the data on those measures using the OASIS. In addition, we are also finalizing that HHAs would be required to collect data on both measures for patients beginning with patients discharged or transferred on or after January 1, 2021. HHAs would be required to report these data for the CY 2022 HH QRP at discharge and transfer between January 1, 2021 and June 30, 2021. Following the initial reporting period for the CY 2022 HH QRP, subsequent years for the HH QRP would be based on 12 months of such data reporting beginning with July 1, 2021 through June 30, 2022 for the CY 2023 HH QRP.
3. Schedule for Reporting Standardized Patient Assessment Data Elements Beginning With the CY 2022 HH QRP
As discussed in section V.G. of this final rule with comment period, we finalized to adopt additional SPADEs beginning with the CY 2022 HH QRP. We finalized that HHAs would report the data using the OASIS. HHAs would be required to collect the SPADEs for episodes beginning or ending on or after January 1, 2021. We also finalized that HHAs that submit the Hearing, Vision, Race, Ethnicity, Preferred Language and Interpreter Services SPADEs with respect to SOC will be deemed to have submitted those SPADEs with respect to SOC, ROC, and discharge, because it is unlikely that the assessment of those SPADEs with respect to SOC will differ from the assessment of the same SPADES with respect to ROC or discharge. HHAs would be required to report the remaining SPADES for the CY 2022 HH QRP at SOC, ROC, and discharge time points between January 1, 2021 and June 30, 2021. Following the initial reporting period for the CY 2022 HH QRP, subsequent years for the HH QRP would be based on 12 months of such data reporting beginning with July 1, 2021 through June 30, 2022 for the CY 2023 HH QRP.
4. Input Sought To Expand the Reporting of OASIS Data Used for the HH QRP To Include Data on All Patients Regardless of Their Payer
We continue to believe that the reporting of all-payer data under the HH QRP would add value to the program and provide a more accurate representation of the quality provided by HHA's. In the CY 2018 HH PPS final rule (82 FR 51736 through 51737), we received and responded to comments sought for data reporting related to assessment based measures, specifically on whether we should require quality data reporting on all HH patients, regardless of payer, where feasible. Several commenters supported data collection of all patients regardless of payer but other commenters did express concerns about the burden imposed on the HHAs as a result of OASIS reporting for all patients, including healthcare professionals spending more time with documentation and less time providing patient care, and the need to increase staff hours or hire additional staff. A commenter requested CMS provide additional explanation of what the benefit would be to collecting OASIS data on all patients regardless of payer.
We are sensitive to the issue of burden associated with data collection and acknowledge concerns about the additional burden required to collect quality data on all patients. We are aware that while some providers use a separate assessment for private payers, many HHA's currently collect OASIS data on all patients regardless of payer to assist with clinical and work flow implications associated with maintaining two distinct assessments. We believe collecting OASIS data on all patients regardless of payer will allow us to ensure data that is representative of quality provided to all patients in the HHA setting and therefore, allow us to better determine whether HH Medicare beneficiaries receive the same quality of care that other patients receive. We also believe it is the overall goal of the IMPACT Act to standardize data and measures in the four PAC programs to permit longitudinal analysis of the data. The absence of all payer data limits CMS's ability to compare all patients receiving services in each PAC setting, as was intended by the Act.
We plan to consider expanding the reporting of OASIS data used for the HH QRP to include data on all patients, regardless of their payer, in future rulemaking. Collecting data on all HHA patients, regardless of their payer would align our data collection requirements under the HH QRP with the data collection requirements currently adopted for the Long-Term Care Hospital (LTCH) QRP and the Hospice QRP. Additionally, collection of data on all patients, regardless of their payer was proposed but not finalized in the FY 2020 rules for the Skilled Nursing Facility (SNF) QRP (84 FR 17678 through 17679) and the Inpatient Rehabilitation Facilities (IRF) QRP (84 FR 17326 through 17327). To assist us regarding a future proposal, in the CY 2020 HH PPS proposed rule (84 FR 34598), we sought input on the following questions related to requiring quality data reporting on all HH patients, regardless of payer:
- Do you agree there is a need to collect OASIS data for the HH QRP on all patients regardless of payer?
- What percentage of your HHA's patients are you not currently reporting OASIS data for the HH QRP?
- Are there burden issues that need to be considered specific to the reporting of OASIS data on all HH patients, regardless of their payer?
- What differences, if any, do you notice in patient mix or in outcomes between those patients that you currently report OASIS data, and those patients that you do not report data for the HH QRP?
- Are there other factors that should be considered prior to proposing to expand the reporting of OASIS data used for the HH QRP to include data on all patients, regardless of their payer?
We did not propose to expand the reporting of OASIS data used for the HH QRP to include data on all HHA patients regardless of payer. We stated, however, that we welcomed comments on this topic, including comments related to the questions noted previously, and that we would take all recommendations received into consideration.
Comment: Several commenters supported expanding the reporting of OASIS data used for the HH QRP to include data on all patients regardless of their payer in the future. Commenters supporting all-payer collection cited alignment with data collection requirements for other PAC providers, as well as other quality programs, such as the Merit-based Incentive Payment System. Other reasons cited by commenters included more accurate representation of the quality of care furnished by HHAs to the entire HH population, the ability of such data to better guide quality improvement activities, and the reduction of current administrative efforts made by HHAs to ensure that only OASIS data for Medicare and Medicaid patients are reported to CMS. For example, one large HHA noted that OASIS data are already completed for approximately 80 percent of their patients. A state association commented that a survey of its members found that 52 percent of respondents currently use the OASIS assessment tool for all of their patients, regardless of payer, while 48 percent indicated that they do not.
Several commenters raised the need for explicit authorization to submit data for other payers, and noted this could create additional administrative burden if patient-level affirmation was required. Commenters asked if agencies would need to develop a waiver or consent for information release to be signed by patients covered by payers other than Medicare in order to report their OASIS data to CMS. One commenter recommended that CMS conduct a nationally-representative survey to inform this decision.
The majority of commenters opposed expanding OASIS data reporting to all-payers, most frequently noting the additional administrative burden this would entail. A few commenters noted that the additional data collection was not aligned with the Patients over Paperwork initiative. One commenter specifically raised as an issue the burden of training private-duty nurses on completing the OASIS. Even when data are collected for all patients, some commenters noted that there would be additional costs of submitting those data to CMS.
Several commenters also had concerns that the data collection could implicate HIPAA and questioned how CMS would plan to use these data, which is protected personal health information requested by a government entity that is not the patient's payer. One commenter requested that CMS provide the evidence-basis for expanding OASIS data collection to all payers.
Several commenters noted there was no difference in care provided to patients by payer type. Commenters stated that payer mix varies considerably between agencies, with anywhere from 10 to 50 percent patients being commercially-insured. One commenter noted over fifty percent of their patients are Medicare patients, which they believed is a sufficiently representative sample for quality reporting programs.
Several commenters described differences between commercially-insured patients and Medicare patients, with commenters reporting that commercially-insured patients are usually younger and healthier, and recover more quickly. In addition to the differences in patient demographics, commenters noted that coverage of services tends to differ between Medicare and commercial insurance, and that some commercial insurance providers restrict the number of home health visits in ways that might alter the effectiveness of services for patient outcomes. They also noted that commercial insurers do not have a “homebound” requirement for patients and would not likely reimburse the cost of OASIS data collection. Some commenters had concerns on how these differences might adversely affect the quality results and administrative burden.
Response: We appreciate all of the feedback that we received on this issue and we will take it into consideration in our future policy and propose it in future rulemaking whereby HHAs would be required to collect and submit data on HH patients regardless of their payer.
VI. Medicare Coverage of Home Infusion Therapy Services
A. Background and Overview
1. Background
Section 5012 of the 21st Century Cures Act (“the Cures Act”) (Pub. L. 114-255), which amended sections 1861(s)(2) and 1861(iii) of the Act, established a new Medicare home infusion therapy benefit. The Medicare home infusion therapy benefit covers the professional services, including nursing services, furnished in accordance with the plan of care, patient training and education (not otherwise covered under the durable medical equipment benefit), remote monitoring, and monitoring services for the provision of home infusion drugs, furnished by a qualified home infusion therapy supplier.
Section 50401 of the BBA of 2018 amended section 1834(u) of the Act by adding a new paragraph (7) that establishes a home infusion therapy services temporary transitional payment for eligible home infusion suppliers for certain items and services furnished in coordination with the furnishing of transitional home infusion drugs beginning January 1, 2019. This temporary payment covers the same items and previously listed services, as defined in section 1861(iii)(2)(A) and (B) of the Act, related to the administration of home infusion drugs. The temporary transitional payment began on January 1, 2019 and will end the day before the full implementation of the home infusion therapy benefit on January 1, 2021, as required by section 5012 of the 21st Century Cures Act.
In the CY 2019 HH PPS final rule with comment period (83 FR 56046), we finalized the implementation of temporary transitional payments for home infusion therapy services to begin on January 1, 2019. In addition, we implemented the establishment of regulatory authority for the oversight of national accrediting organizations (AOs) that accredit home infusion therapy suppliers, and their CMS-approved home infusion therapy accreditation programs.
2. Overview of Infusion Therapy
Infusion drugs can be administered in multiple health care settings, including inpatient hospitals, skilled nursing facilities (SNFs), hospital outpatient departments (HOPDs), physicians' offices, and in the home. Traditional fee-for-service (FFS) Medicare provides coverage for infusion drugs, equipment, supplies, and administration services. However, Medicare coverage requirements and payment vary for each of these settings. Infusion drugs, equipment, supplies, and administration are all covered by Medicare in the inpatient hospital, SNFs, HOPDs, and physicians' offices.
Generally, Medicare payment under Part A for the drugs, equipment, supplies, and services are bundled, meaning a single payment is made on the basis of expected costs for clinically-defined episodes of care. For example, if a beneficiary is receiving an infusion drug during an inpatient hospital stay, the Part A payment for the drug, supplies, equipment, and drug administration is included in the diagnosis-related group (DRG) payment to the hospital under the Medicare inpatient prospective payment system. Beneficiaries are liable for the Medicare inpatient hospital deductible and no coinsurance for the first 60 days. Similarly, if a beneficiary is receiving an infusion drug while in a SNF under a Part A stay, the payment for the drug, supplies, equipment, and drug administration are included in the SNF prospective payment system payment. After 20 days of SNF care, there is a daily beneficiary cost-sharing amount through day 100 when the beneficiary becomes responsible for all costs for each day after day 100 of the benefit period.
Under Medicare Part B, certain items and services are paid separately while other items and services may be packaged into a single payment together. For example, in an HOPD and in a physician's office, the drug is paid separately, generally at the average sales price (ASP) plus 6 percent (77 FR 68210).[214] Medicare also makes a separate payment to the physician or HOPD for administering the drug. The separate payment for infusion drug administration in an HOPD and in a physician's office generally includes a base payment amount for the first hour and a payment add-on that is a different amount for each additional hour of administration. The beneficiary is responsible for the 20 percent coinsurance under Medicare Part B.
Medicare FFS covers outpatient infusion drugs under Part B, “incident to” a physician's service, provided the drugs are not usually self-administered by the patient. Drugs that are “not usually self-administered,” are defined in our manual according to how the Medicare population as a whole uses the drug, not how an individual patient or physician may choose to use a particular drug. For the purpose of this exclusion, the term “usually” means more than 50 percent of the time for all Medicare beneficiaries who use the drug. The term “by the patient” means Medicare beneficiaries as a collective whole. Therefore, if a drug is self-administered by more than 50 percent of Medicare beneficiaries, the drug is generally excluded from Part B coverage. This determination is made on a drug-by-drug basis, not on a beneficiary-by-beneficiary basis.[215] The MACs review the Self-Administered Drug (SAD) exclusion lists on a regular basis.[216]
Home infusion therapy involves the intravenous or subcutaneous administration of drugs or biologicals to an individual at home. Certain drugs can be infused in the home, but the nature of the home setting presents different challenges than the settings previously described. Generally, the components needed to perform home infusion include the drug (for example, antivirals, immune globulin), equipment (for example, a pump), and supplies (for example, tubing and catheters). Likewise, nursing services are usually necessary to train and educate the patient and caregivers on the safe administration of infusion drugs in the home. Visiting nurses often play a large role in home infusion. These nurses typically train the patient or caregiver to self-administer the drug, educate on side effects and goals of therapy, and visit periodically to assess the infusion site and provide dressing changes. Depending on patient acuity or the complexity of the drug administration, certain infusions may require more training and education, especially those that require special handling or pre-or post-infusion protocols. The home infusion process typically requires coordination among multiple entities, including patients, physicians, hospital discharge planners, health plans, home infusion pharmacies, and, if applicable, home health agencies.
With regard to payment for home infusion therapy under traditional Medicare, drugs are generally covered under Part B or Part D. Certain infusion pumps, supplies (including home infusion drugs) and the services required to furnish the drug, (that is, preparation and dispensing), and nursing are covered in some circumstances through the Part B durable medical equipment (DME) benefit, the Medicare home health benefit, or some combination of these benefits. In accordance with section 50401 of the Bipartisan Budget Act (BBA) of 2018, beginning on January 1, 2019, for CYs 2019 and 2020, Medicare implemented temporary transitional payments for home infusion therapy services furnished in coordination with the furnishing of transitional home infusion drugs. This payment, for home infusion therapy services, is only made if a beneficiary is furnished certain drugs and biologicals administered through an item of covered DME, and payable only to suppliers enrolled in Medicare as pharmacies that provide external infusion pumps and external infusion pump supplies (including the home infusion drug). With regard to the coverage of the home infusion drugs, Medicare Part B covers a limited number of home infusion drugs through the DME benefit if: (1) The drug is necessary for the effective use of an external infusion pump classified as DME and determined to be reasonable and necessary for administration of the drug; and (2) the drug being used with the pump is itself reasonable and necessary for the treatment of an illness or injury. Additionally, in order for the infusion pump to be covered under the DME benefit, it must be appropriate for use in the home (§ 414.202).
Only certain types of infusion pumps are covered under the DME benefit. The Medicare National Coverage Determinations Manual, chapter 1, part 4, section 280.14 describes the types of infusion pumps that are covered under the DME benefit.[217] For DME external infusion pumps, Medicare Part B covers the infusion drugs and other supplies and services necessary for the effective use of the pump. Through the Local Coverage Determination (LCD) for External Infusion Pumps (L33794), the DME Medicare administrative contractors (MACs) specify the details of which infusion drugs are covered with these pumps. Examples of covered Part B DME infusion drugs include, among others, certain IV drugs for heart failure and pulmonary arterial hypertension, immune globulin for primary immune deficiency (PID), insulin, antifungals, antivirals, and chemotherapy, in limited circumstances.
3. Home Infusion Therapy Legislation
a. 21st Century Cures Act
Effective January 1, 2021, section 5012 of the 21st Century Cures Act (Pub. L. 114-255) (Cures Act) created a separate Medicare Part B benefit category under section 1861(s)(2)(GG) of the Act for coverage of home infusion therapy services needed for the safe and effective administration of certain drugs and biologicals administered intravenously, or subcutaneously for an administration period of 15 minutes or more, in the home of an individual, through a pump that is an item of DME. The infusion pump and supplies (including home infusion drugs) will continue to be covered under the Part B DME benefit. Section 1861(iii)(2) of the Act defines home infusion therapy to include the following items and services: The professional services, including nursing services, furnished in accordance with the plan, training and education (not otherwise paid for as DME), remote monitoring, and other monitoring services for the provision of home infusion therapy and home infusion drugs furnished by a qualified home infusion therapy supplier, which are furnished in the individual's home. Section 1861(iii)(3)(B) of the Act defines the patient's home to mean a place of residence used as the home of an individual as defined for purposes of section 1861(n) of the Act. As outlined in section 1861(iii)(1) of the Act, to be eligible to receive home infusion therapy services under the home infusion therapy benefit, the patient must be under the care of an applicable provider (defined in section 1861(iii)(3)(A) of the Act as a physician, nurse practitioner, or physician's assistant), and the patient must be under a physician-established plan of care that prescribes the type, amount, and duration of infusion therapy services that are to be furnished. The plan of care must be periodically reviewed by the physician in coordination with the furnishing of home infusion drugs (as defined in section 1861(iii)(3)(C) of the Act). Section 1861(iii)(3)(C) of the Act defines a “home infusion drug” under the home infusion therapy benefit as a drug or biological administered intravenously, or subcutaneously for an administration period of 15 minutes or more, in the patient's home, through a pump that is an item of DME as defined under section 1861(n) of the Act. This definition does not include insulin pump systems or any self-administered drug or biological on a self-administered drug exclusion list.
Section 1861(iii)(3)(D)(i) of the Act defines a “qualified home infusion therapy supplier” as a pharmacy, physician, or other provider of services or supplier licensed by the state in which supplies or services are furnished. The provision specifies qualified home infusion therapy suppliers must furnish infusion therapy to individuals with acute or chronic conditions requiring administration of home infusion drugs; ensure the safe and effective provision and administration of home infusion therapy on a 7-day-a-week, 24-hour-a-day basis; be accredited by an organization designated by the Secretary; and meet other such requirements as the Secretary deems appropriate, taking into account the standards of care for home infusion therapy established by Medicare Advantage (MA) plans under Part C and in the private sector. The supplier may subcontract with a pharmacy, physician, other qualified supplier or provider of medical services, in order to meet these requirements.
Section 1834(u)(1) of the Act requires the Secretary to implement a payment system under which, beginning January 1, 2021, a single payment is made to a qualified home infusion therapy supplier for the items and services (professional services, including nursing services; training and education; remote monitoring, and other monitoring services). The single payment must take into account, as appropriate, types of infusion therapy, including variations in utilization of services by therapy type. In addition, the single payment amount is required to be adjusted to reflect other factors such as geographic wage index and other costs that may vary by region, patient acuity, and complexity of drug administration. The single payment may be adjusted to reflect outlier situations, and other factors as deemed appropriate by the Secretary, which are required to be done in a budget-neutral manner. Section 1834(u)(2) of the Act specifies certain items that “the Secretary may consider” in developing the HIT payment system: “the costs of furnishing infusion therapy in the home, consult[ation] with home infusion therapy suppliers, . . . payment amounts for similar items and services under this part and part A, and . . . payment amounts established by Medicare Advantage plans under part C and in the private insurance market for home infusion therapy (including average per treatment day payment amounts by type of home infusion therapy)”. Section 1834(u)(3) of the Act specifies that annual updates to the single payment are required to be made, beginning January 1, 2022, by increasing the single payment amount by the percent increase in the Consumer Price Index for all urban consumers (CPI-U) for the 12-month period ending with June of the preceding year, reduced by the 10-year moving average of changes in annual economy-wide private nonfarm business multifactor productivity (MFP). Under section 1834(u)(1)(A)(iii) of the Act, the single payment amount for each infusion drug administration calendar day, including the required adjustments and the annual update, cannot exceed the amount determined under the fee schedule under section 1848 of the Act for infusion therapy services if furnished in a physician's office. This statutory provision limits the single payment amount so that it cannot reflect more than 5 hours of infusion for a particular therapy per calendar day. Section 1834(u)(4) of the Act also allows the Secretary discretion, as appropriate, to consider prior authorization requirements for home infusion therapy services. Finally, section 5012(c)(3) of the 21st Century Cures Act amended section 1861(m) of the Act to exclude home infusion therapy from the HH PPS beginning on January 1, 2021.
b. Bipartisan Budget Act of 2018
Section 50401 of the Bipartisan Budget Act of 2018 (Pub. L. 115-123) amended section 1834(u) of the Act by adding a new paragraph (7) that established a home infusion therapy services temporary transitional payment for eligible home infusion suppliers for certain items and services furnished in coordination with the furnishing of transitional home infusion drugs, beginning January 1, 2019. This payment covers the same items and services as defined in section 1861(iii)(2)(A) and (B) of the Act, furnished in coordination with the furnishing of transitional home infusion drugs. Section 1834(u)(7)(A)(iii) of the Act defines the term “transitional home infusion drug” using the same definition as “home infusion drug” under section 1861(iii)(3)(C) of the Act, which is a parenteral drug or biological administered intravenously, or subcutaneously for an administration period of 15 minutes or more, in the home of an individual through a pump that is an item of DME as defined under section 1861(n) of the Act. The definition of “home infusion drug” excludes “a self-administered drug or biological on a self-administered drug exclusion list” but the definition of “transitional home infusion drug” notes that this exclusion shall not apply if a drug described in such clause is identified in clauses (i), (ii), (iii) or (iv) of 1834(u)(7)(C) of the Act. Section 1834(u)(7)(C) of the Act sets out the Healthcare Common Procedure Coding System (HCPCS) codes for the drugs and biologicals covered under the DME LCD for External Infusion Pumps (L33794), as the drugs covered during the temporary transitional period. In addition, section 1834(u)(7)(C) of the Act states that the Secretary shall assign to an appropriate payment category drugs which are covered under the DME LCD for External Infusion Pumps (L33794) and billed under HCPCS codes J7799 (Not otherwise classified drugs, other than inhalation drugs, administered through DME) and J7999 (Compounded drug, not otherwise classified), or billed under any code that is implemented after the date of the enactment of this paragraph and included in such local coverage determination or included in sub-regulatory guidance as a home infusion drug.
Section 1834(u)(7)(E)(i) of the Act states that payment to an eligible home infusion supplier or qualified home infusion therapy supplier for an infusion drug administration calendar day in the individual's home refers to payment only for the date on which professional services, as described in section 1861(iii)(2)(A) of the Act, were furnished to administer such drugs to such individual. This includes all such drugs administered to such individual on such day. Section 1842(u)(7)(F) of the Act defines “eligible home infusion supplier” as a supplier who is enrolled in Medicare as a pharmacy that provides external infusion pumps and external infusion pump supplies, and that maintains all pharmacy licensure requirements in the State in which the applicable infusion drugs are administered.
As set out at section 1834(u)(7)(C) of the Act, identified HCPCS codes for transitional home infusion drugs are assigned to three payment categories, as identified by their corresponding HCPCS codes, for which a single amount will be paid for home infusion therapy services furnished on each infusion drug administration calendar day. Payment category 1 includes certain intravenous infusion drugs for therapy, prophylaxis, or diagnosis, including antifungals and antivirals; inotropic and pulmonary hypertension drugs; pain management drugs; and chelation drugs. Payment category 2 includes subcutaneous infusions for therapy or prophylaxis, including certain subcutaneous immunotherapy infusions. Payment category 3 includes intravenous chemotherapy infusions, including certain chemotherapy drugs and biologicals. The payment category for subsequent transitional home infusion drug additions to the LCD and compounded infusion drugs not otherwise classified, as identified by HCPCS codes J7799 and J7999, will be determined by the DME MACs.
In accordance with section 1834(u)(7)(D) of the Act, each payment category is paid at amounts in accordance with the Physician Fee Schedule (PFS) for each infusion drug administration calendar day in the individual's home for drugs assigned to such category, without geographic adjustment. Section 1834(u)(7)(E)(ii) of the Act requires that in the case that two (or more) home infusion drugs or biologicals from two different payment categories are administered to an individual concurrently on a single infusion drug administration calendar day, one payment for the highest payment category will be made.
4. Summary of CY 2019 Home Infusion Therapy Provisions
In the CY 2019 Home Health Prospective Payment System (HH PPS) final rule with comment period, (83 FR 56579) we finalized the implementation of the home infusion therapy services temporary transitional payments under paragraph (7) of section 1834(u) of the Act. These services are furnished in the individual's home to an individual who is under the care of an applicable provider (defined in section 1861(iii)(3)(A) of the Act as a physician, nurse practitioner, or physician's assistant) and where there is a plan of care established and periodically reviewed by a physician prescribing the type, amount, and duration of infusion therapy services. Only eligible home infusion suppliers can bill for the temporary transitional payments. Therefore, in accordance with section 1834(u)(7)(F) of the Act, we clarified that this means that existing DME suppliers that are enrolled in Medicare as pharmacies that provide external infusion pumps and external infusion pump supplies, who comply with Medicare's DME Supplier and Quality Standards, and maintain all pharmacy licensure requirements in the State in which the applicable infusion drugs are administered, are considered eligible home infusion suppliers.
Section 1834(u)(7)(C) of the Act assigns transitional home infusion drugs, identified by the HCPCS codes for the drugs and biologicals covered under the DME LCD for External Infusion Pumps (L33794),[218] into three payment categories, for which we established a single payment amount in accordance with section 1834(u)(7)(D) of the Act. This section states that each single payment amount per category will be paid at amounts equal to the amounts determined under the PFS established under section 1848 of the Act for services furnished during the year for codes and units of such codes, without geographic adjustment. Therefore, we created a new HCPCS G-code for each of the three payment categories and finalized the billing procedure for the temporary transitional payment for eligible home infusion suppliers. We stated that the eligible home infusion supplier would submit, in line-item detail on the claim, a G-code for each infusion drug administration calendar day. The claim should include the length of time, in 15-minute increments, for which professional services were furnished. The G-codes can be billed separately from, or on the same claim as, the DME, supplies, or infusion drug, and are processed through the DME MACs. On August 10, 2018, we issued Change Request: R4112CP: Temporary Transitional Payment for Home Infusion Therapy Services for CYs 2019 and 2020 [219] outlining the requirements for the claims processing changes needed to implement this payment.
And last, we finalized the definition of “infusion drug administration calendar day” in regulation as the day on which home infusion therapy services are furnished by skilled professional(s) in the individual's home on the day of infusion drug administration. The skilled services provided on such day must be so inherently complex that they can only be safely and effectively performed by, or under the supervision of, professional or technical personnel (42 CFR 486.505). Section 1834(u)(7)(E)(i) of the Act clarifies that this definition is with respect to the furnishing of “transitional home infusion drugs” and “home infusion drugs” to an individual by an “eligible home infusion supplier” and a “qualified home infusion therapy supplier.” The definition of “infusion drug administration calendar day” applies to both the temporary transitional payment in CYs 2019 and 2020 and the permanent home infusion therapy benefit to be implemented beginning in CY 2021. Although we finalized this definition in regulation in the CY 2019 HH PPS final rule with comment period (83 FR 56583), we stated that we would carefully monitor the effects of this definition on access to care and that, if warranted and if within the limits of our statutory authority, we would engage in additional rulemaking or guidance regarding this definition. In that same rule, we solicited additional comments on this interpretation and on its effects on access to care.
B. CY 2020 Temporary Transitional Payment Rates for Home Infusion Therapy Services
In the CY 2020 HH PPS proposed rule (84 FR 34689) we discussed section 1834(u)(7) of the Act that established a home infusion therapy services temporary transitional payment for eligible home infusion suppliers for certain items and services furnished to administer home infusion drugs. This temporary payment covers the cost of the professional services, training and education, monitoring, and remote monitoring services, as defined in section 1861(iii)(2)(A) and (B) of the Act, related to the administration of home infusion drugs. The temporary transitional payment began on January 1, 2019 and will end the day before the full implementation of the home infusion therapy benefit on January 1, 2021, as required by section 5012 of the 21st Century Cures Act. The list of transitional home infusion drugs and the payment categories for the temporary transitional payment for home infusion therapy services can be found in Tables 55 and 56 in the CY 2019 HH PPS proposed rule (83 FR 32465 and 32466).[220]
Section 1834(u)(7)(D)(i) of the Act sets the payment amounts for each category equal to the amounts determined under the PFS established under section 1848 of the Act for services furnished during the year for codes and units for such codes specified without application of geographic adjustment under section 1848(e) of the Act. That is, the payment amounts are based on the PFS rates for the Current Procedural Terminology (CPT) codes corresponding to each payment category. For eligible home infusion suppliers to bill for the temporary transitional payments for home infusion therapy services for an infusion drug administration calendar day, we created a G-code associated with each of the three payment categories. The J-codes for eligible home infusion drugs, the G-codes associated with each of the three payment categories, and instructions for billing for the temporary transitional home infusion therapy payments are found in the August 10, 2018 Change Request 10836, “Temporary Transitional Payment for Home Infusion Therapy Services for CYs 2019 and 2020.” [221] Therefore, as proposed, CMS will update the temporary transitional payment amounts based on the CPT code payment amounts in the CY 2020 PFS final rule. At the time of publication of this final rule with comment period, we do not yet have the CY 2020 PFS final rates; however, in accordance with the CY 2020 HH PPS proposed rule, the temporary transitional payments starting on January 1, 2020 will be based on the PFS amounts as specified in section 1834(u)(7)(D) of the Act. We will publish these updated rates in the CY 2020 PFS final rule,[222] and will publish the updated CY 2020 temporary transitional payment rates in the January 2020 DMEPOS fee schedule file.[223] We received a few comments on the proposed rule regarding the CY 2020 temporary transitional payment rates for home infusion therapy. The following are our responses:
Comment: A commenter stated that the lack of defined PFS rates presents a hardship to suppliers when creating budgets for CY 2020. This commenter also suggested that CMS include provisions for geographic adjustments to the temporary transitional payment. The commenter stated that geographic adjustment is necessary in light of nursing shortages noted in several areas of our country, and stated that the shortage of qualified professionals results in costs in recruitment, retention, and wages, and requested that CMS consider these challenges when reviewing the lack of geographic adjustment for the temporary transitional payments.
Response: The proposed CY 2020 PFS rates for the infusion CPT codes can be found at the following link: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1715-P.html. The final rates will be posted in the CY 2020 PFS final rule, which we expect will be on display by November 1, 2019. The temporary transitional rates for home infusion therapy services will continue to be posted on the DMEPOS fee schedule file, which can be found at the following link: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/DMEPOSFeeSched/DMEPOS-Fee-Schedule.html. The CY 2020 rates as previously discussed, will be posted by January 1, 2020.
Regarding geographic adjustment, the temporary transitional payment is statutorily limited to the payment methodology as set forth in section 1834(u)(7)(D) of the Act, which states that each payment category is paid at amounts in accordance with the PFS for drugs assigned to such category without geographic adjustment.
Comment: A commenter requested that CMS clarify that nurse practitioners are authorized to establish the home infusion plan of care during the temporary transitional period. The commenter expressed understanding that, as the full payment provisions for the home infusion benefit proposed in this year's rule do not go into effect until CY 2021, there is no statutory requirement that only a physician can establish the plan of care during the transitional payment period.
Response: In the Home Infusion Therapy Services Temporary Transitional Payment Frequently Asked Questions (FAQs), we stated that the eligibility criteria for home infusion therapy services includes the patient being under a plan of care established and periodically reviewed by a physician prescribing the type, amount, and duration of infusion therapy services. The FAQs can be found at the following link: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/Home-Infusion-Therapy/Downloads/Home-Infusion-Therapy-Services-Temp-Transitional-Payment-FAQs.pdf. The BBA of 2018 gives CMS the authority to implement requirements during the transitional payment period outside of rulemaking. Therefore, we are maintaining our previously-stated requirement that only the physician can establish and review the plan during the transitional payment period.
C. Home Infusion Therapy Services for CY 2021 and Subsequent Years
Upon completion of the temporary transitional payments for home infusion therapy services at the end of CY 2020, we will be implementing the permanent payment system for home infusion therapy services under Section 5012 of the 21st Century Cures Act (Pub. L. 114-255) beginning January 1, 2021. In the CY 2020 HH PPS proposed rule (84 FR 34690), we proposed provisions regarding payment for home infusion therapy services for CY 2021 and beyond in order to allow adequate time for eligible home infusion therapy suppliers to make any necessary software and business process changes for implementation on January 1, 2021.
We explained that section 1861(iii) of the Act establishes certain provisions related to home infusion therapy with respect to the requirements that must be met for Medicare payment to be made to qualified home infusion therapy suppliers, and that these provisions serve as the basis for determining the scope of the home infusion drugs eligible for coverage of home infusion therapy services; outline beneficiary qualifications and plan of care requirements; and establish who can bill for payment under the benefit.
Additionally, as previously discussed, in the CY 2019 HH PPS final rule with comment period (83 FR 56583), we solicited additional comments on our interpretation of the definition of “infusion drug administration calendar day” and on its potential effects on access to care. Although we did not propose a change to the definition, we received comments on both the CY 2019 HH PPS final rule with comment period and the CY 2020 HH PPS proposed rule with respect to our interpretation.
Of the timely correspondence received in response to the CY 2020 HH PPS proposed rule, approximately 52 comments pertained to the home infusion therapy benefit. The following is a summary of the proposed rule provisions, comments received, and our responses.
1. Infusion Drug Administration Calendar Day
In general, the comments received on the CY 2019 HH PPS final rule with comment period and the CY 2020 HH PPS proposed rule regarding “infusion drug administration calendar day” were similar to those received on the CY 2019 HH PPS proposed rule, and focused primarily on the proposed definition as it pertains to the “professional services” covered under the benefit.
Comment: Commenters continued to disagree with the final definition of “infusion drug administration calendar day,” and stated that payment for home infusion therapy services should include any day that a home infusion drug is infused, and not just a day on which a professional is in the home furnishing services. Specifically, commenters on the CY 2019 HH PPS final rule with comment period recommended that CMS immediately amend the definition at 42 CFR 486.505 to eliminate the requirement that a skilled professional be in the home in order for reimbursement to occur. The majority of the comments pertaining to the home infusion benefit on the CY 2020 HH PPS proposed rule reiterated this recommendation and called on CMS to revise the existing definition of infusion drug administration calendar day to allow for reimbursement of home infusion services “each day that an infusion drug physically enters the patient's body, irrespective of whether a skilled professional is in the individual's home.” Conversely, MedPAC continued to support CMS' definition of infusion drug administration calendar day.
Response: As we stated in the CY 2019 HH PPS final rule with comment period, the definition at 42 CFR 486.505 is consistent with section 1861(iii)(1) of the Act, which defines the term “home infusion therapy” as the items and services furnished by a qualified home infusion supplier, which are furnished in the individual's home. Additionally, section 1834(u)(7)(E)(i) of the Act states that payment to an eligible home infusion supplier or qualified home infusion therapy supplier for an infusion drug administration calendar day in the individual's home, refers to payment only for the date on which professional services (as described in section 1861(iii)(2)(A) of the Act) were furnished to administer such drugs to such individual. In other words, section 1834(u)(7) makes clear that while the single payment covers both professional services under section 1861(iii)(2)(A) and training and education, remote monitoring, and other monitoring services under section 1861(iii)(2)(B), payment is only issued on certain days—days on which professional services are provided in the patient's home.
Comment: Commenters stated that by not defining “professional services” and limiting payment to a day on which a skilled professional is in the home, CMS fails to capture a broader cross-section of professional services that do not occur in the patient's home, but that are critical to ensure the safe and effective provision of home infusion therapy services. Several commenters specified that these services include compounding and dispensing of the drug; however, some commenters also identified “remote pharmacy services” that they believe should be included in the payment. Commenters on the CY 2020 HH PPS proposed rule elaborated on the notion of “remote pharmacy services,” stating that these services include initial and ongoing pharmacist assessments; clinical care planning; drug preparation and compounding; care coordination; medication reconciliation; monitoring, (including remote monitoring) for adverse events and response to therapy; drug therapy evaluation and design; pharmacist interventions and subsequent therapeutic recommendations to prescribers; patient education; and all other associated professional work.
Response: The drugs identified for coverage of home infusion therapy services are paid under the Part B DME benefit. Therefore, the services related to the furnishing of the drug, remote or otherwise, are paid under the DME benefit. Furthermore, a “qualified home infusion therapy supplier” as defined in section 1861(iii)(3)(D)(i) of the Act, is not required to furnish services related to the furnishing of the drug. In the CY 2019 HH PPS final rule with comment period CMS stated that we acknowledge that pharmacy services are closely related to the home infusion therapy benefit; however, at this time pharmacy services, furnished by a Medicare-enrolled DMEPOS supplier, associated with the preparation and dispensing of home infusion drugs are covered under the Part B DME benefit and are not part of the specific home infusion therapy benefit (83 FR 56563).
In the CY 2019 HH PPS proposed rule (83 FR 32467) we stated that the DME supplier standards require the DME supplier to document that it or another qualified party has at an appropriate time provided beneficiaries with the necessary information and instructions on how to use Medicare-covered items safely and effectively.[224] Therefore, the professional services covered under the home infusion benefit would include a limited amount of training and education on the provision of home infusion drugs that is not already covered under the DME benefit regarding the appropriate and safe use of the equipment.
In accordance with section 1861(iii)(1)(B), an individual must be under a plan of care established by a physician, prescribing the type, amount, and duration of infusion therapy services, in coordination with the furnishing of home infusion drugs. In order to avoid being overly prescriptive, we did not define “professional services” or enumerate a list of services that are covered under the benefit. We did not want to inadvertently omit services which may be necessary for an individual patient or particular therapy or course of treatment, as determined by the physician responsible for the plan of care. As previously discussed and in the CY 2019 proposed rule, the services provided under the home infusion therapy benefit are distinct from those required and paid under the DME benefit (that is, instruction on how to safely and effectively use the DME equipment) and :
- Training and education on care and maintenance of vascular access devices:
++ Hygiene education
++ Instruction on what to do in the event of a dislodgement or occlusion
++ Education on signs and symptoms of infection
++ Teaching and training on flushing and locking the catheter
++ Dressing changes and site care
- Patient assessment and evaluation:
++ Review of patient's history and assessment of current physical and mental status, including obtaining vital signs
++ Assessment of any adverse effects or infusion complications
++ Evaluation of family and caregiver support
++ Review of prescribed treatment and any concurrent oral and/or over-the-counter Treatments
++ Obtaining blood for lab-work
- Medication and disease management education:
++ Instruction on self-monitoring
++ Education on lifestyle and nutritional modifications
++ Education regarding drug mechanism of action, side effects, interactions with other medications, adverse and infusion-related reactions
++ Education regarding therapy goals and progress
++ Instruction on administering pre-medications and inspection of medication prior to use
++ Education regarding household and contact precautions and/or spills
- Remote monitoring services
- Monitoring services:
++ Communicating with patient regarding changes in condition and treatment plan
++ Monitoring patient response to therapy
++ Assessing compliance
Comment: A few commenters stated that Medicare's interpretation of “infusion drug administration calendar day” under the home infusion therapy benefit is inadequate to cover the cost of care, and that consequently, home infusion suppliers would be forced to discontinue home infusion therapy services to Medicare beneficiaries. Some commenters specifically identified subcutaneous immunoglobulin, stating that administration of this biological requires virtually no professional services in the home, and therefore the home infusion supplier would never be reimbursed for the “pharmacy-based” services furnished outside of the home. Commenters stated that this would impede access to these services and force patients to receive their infusions in the physician's office, outpatient department, hospital, or nursing home, which are more costly and clinically less appropriate.
Response: The single payment for the home infusion therapy services is only made when a skilled professional is in the patient's home on a day of drug administration. This single payment does not include the DME external infusion pump, supplies (including the home infusion drug), and related services paid under the DME benefit. Medicare payment for an infusion drug administration calendar day is separate from the payment for DME items and services, therefore, a supplier could still be paid for DME items and services under the DME benefit, even if it does not receive payment for home infusion therapy services. Additionally, the home infusion therapy services payment is a single bundled payment amount, set equal to the administration services furnished in a physician's office for each infusion drug administration calendar day, regardless of the actual length of the visit. Therefore, it is unclear why suppliers would limit access to patients requiring “virtually no services in the home,” when suppliers are still being paid for the DME, supplies (including the home infusion drug), and services covered under the DME benefit, as well as an additional payment for professional services equal to a set amount of hours, regardless of the actual visit length, when a home visit is furnished.
Comment: A commenter noted anecdotally that since the implementation of the transitional benefit DME suppliers have begun to consolidate or no longer accept new patients under the Part B benefit, and anticipate that more beneficiaries will face access barriers. Commenters requested that CMS make utilization data from 2019 available for public review to allow for a full assessment of how the current policy has impacted access and/or contributed to provider consolidation.
Response: As we stated in the CY 2019 HH PPS final rule with comment period, CMS will monitor home infusion therapy utilization to determine what, if any, effects on access to care occur after implementation of the temporary transitional payments for home infusion therapy. Since the implementation of these payments on January 1, 2019 we have been collecting quarterly data on the number of home infusion therapy users; volume of infusion therapy prescription fills, including by category and individual drugs; and number of DME suppliers furnishing home infusion therapy. We have been monitoring changes in trends between quarters, nationwide trends, and trends across the payment categories and among individual drugs, beneficiary characteristics, and by geographic variation. We have also been monitoring trend data from the past before the implementation of the temporary transitional home infusion therapy payments. Based on the claims data from Q1 2016 to Q4 2018, we found that overall, the utilization of infusion services in Q4 2018 shows a steadily increasing trend across all three care settings (home, outpatient, and physician's office). Specifically, both the numbers of prescription fills and claims for the transitional infusion drugs in the home setting increased steadily in Q4 2018, compared to the previous quarter. Additionally, although there has been fluctuation in the number of DME suppliers supplying transitional home infusion drugs, from Q1 2016 through Q3 2018, the number has increased between Q3 and Q4, indicating that access to services has not been negatively impacted since the drug pricing change from average wholesale price (AWP) to average sales price (ASP) plus 6 percent took effect on January 1, 2017. We will continue to monitor and analyze claims data in order to determine whether, and how access to home infusion therapy services has been impacted since the implementation of the home infusion benefit in CY 2019. We are currently still receiving and analyzing claims data during this time period; however, we note that home infusion utilization for Q1 2019 has been stable and shown slight increases since Q1 2017. We also note that this monitoring and analysis is unrelated to CMS's legal interpretation of the term “infusion drug administration calendar day.” We anticipate releasing our analysis of claims data from Q1 2016 through CY 2019 once we have more complete data for CY 2019.
2. Home Infusion Drugs
In the CYs 2019 and 2020 Home Health Prospective Payment System (HH PPS) proposed rules (83 FR 32466 and 84 FR 34690) we discussed the relationship between the home infusion therapy benefit and the DME benefit. We stated that, as there is no separate Medicare Part B DME payment for the professional services associated with the administration of certain home infusion drugs covered as supplies necessary for the effective use of external infusion pumps, we consider the home infusion therapy benefit to be a separate payment in addition to the existing payment for the DME external infusion pump, supplies (including the home infusion drug), and services covered under the DME benefit. We stated that, consistent with the definition of “home infusion therapy,” the home infusion therapy payment explicitly and separately pays for the professional services related to the administration of the drugs identified on the DME LCD for External Infusion Pumps (L33794),[225] when such services are furnished in the individual's home. For purposes of the temporary transitional payments for home infusion therapy services in CYs 2019 and 2020, the term “transitional home infusion drug” includes the HCPCS codes for the drugs and biologicals covered under this LCD for External Infusion Pumps. We also noted that although section 1834(u)(7)(A)(iii) of the Act defines the term “transitional home infusion drug,” section 1834(u)(7)(A)(iii) of the Act does not specify the HCPCS codes for home infusion drugs for which home infusion therapy services will be covered beginning in CY 2021.
Section 1861(iii)(3)(C) of the Act defines “home infusion drug” as a parenteral drug or biological administered intravenously, or subcutaneously for an administration period of 15 minutes or more, in the home of an individual through a pump that is an item of durable medical equipment (as defined in section 1861(n) of the Act). Such term does not include insulin pump systems or self-administered drugs or biologicals on a self-administered drug exclusion list. As noted in the proposed rule, this definition not only specifies that the drug or biological must be administered through a pump that is an item of DME, but references the statutory definition of DME at 1861(n) of the Act. Therefore, we stated that this means that “home infusion drugs” are defined as parenteral drugs and biologicals administered intravenously, or subcutaneously for an administration period of 15 minutes or more, in the home of an individual through a pump that is an item of DME covered under the Medicare Part B DME benefit, pursuant to the statutory definition set out at section 1861(iii)(3)(C) of the Act, and incorporated by cross reference at section 1834(u)(7)(A)(iii) of the Act.
Comment: A commenter requested clarification regarding the applicability of payment for services under the home infusion benefit specifically with regard to the administration of intravenous immunoglobulin (IVIG). The commenter noted that we stated in the proposed rule that payment category 1 would include any subsequent intravenous infusion drug additions, and stated that a plain reading of the statutory language indicates that IVIG products would meet the definition of a home infusion drug administered intravenously and thus, would be covered under the home infusion therapy payment beginning in CY 2021. This commenter stated that the proposed codes for home infusion therapy services payment categories, however, do not reflect how IVIG services will be addressed. Similarly, another commenter recommended including IV antibacterial drugs to the list of home infusion drugs eligible for services beginning in CY 2021.
Response: As discussed in the CY 2020 HH PPS proposed rule (84 FR 34690), we stated that Medicare payment for home infusion therapy services is for services furnished in coordination with the furnishing of the intravenous and subcutaneous infusion drugs and biologicals specified on the DME LCD for External Infusion Pumps (L33794), with the exception of insulin pump systems and drugs and biologicals on a self-administered drug exclusion list. In order for the drugs and biologicals to be covered under the Part B DME benefit they must require infusion through an external infusion pump. If the drug or biological can be infused through a disposable pump or by a gravity drip, it does not meet this criterion. IVIG does not require an external infusion pump for administration purposes and therefore, would not be covered under the DME LCD for External Infusion Pumps. We note that a DME external infusion pump is also not covered under the Medicare Intravenous Immune Globulin Demonstration. The Frequently Asked Questions (FAQs) regarding this demonstration state that it is up the supplier to determine the services and supplies appropriate and necessary to administer the IVIG in any given situation, and that this may or may not include the use of a pump.[226] Furthermore, the LCD specifically states that intravenous immune globulin products are not covered under this LCD and specifies that DME coverage of subcutaneous immune globulin (SCIG) applies only to those products that are specifically labeled as subcutaneous administration products. This means that immune globulin labeled for both intravenous and subcutaneous use would not be covered under the LCD.
The reference to payment category 1 including any subsequent intravenous drug or biological additions is in reference to the DME LCD for External Infusion Pumps (L33794). In the CY 2020 HH PPS proposed rule (84 FR 34687) we stated that the DME Medicare Administrative Contractors (MACs) specify the details of which infusion drugs are covered with these pumps through local coverage policies. We also gave examples of covered Part B DME infusion drugs, which we stated currently include, among others, certain IV drugs for heart failure and pulmonary arterial hypertension; immune globulin for primary immune deficiency (PID); insulin; antifungals and antivirals; and chemotherapy, in limited circumstances. As previously discussed, the immune globulin for PID currently covered under the DME LCD for External Infusion Pumps (L33794) is only immune globulin which is administered subcutaneously, not intravenously, and is paid under payment category 2 of the temporary transitional home infusion therapy services payment. If the MACs determine that additional intravenous infusion drugs or biologicals (excluding chemotherapy drugs or other highly complex drugs and biologicals, as those would be paid under payment category 3) meet the criteria to be added to the DME LCD for External Infusion Pumps (L33794), then home infusion therapy services for these newly added intravenous drugs would be covered under payment category 1. Likewise, although there are a few antifungal and antiviral drugs covered under the DME LCD for External Infusion Pumps (L33794), there are currently no antibacterial drugs included and therefore, services for these drugs would not be covered under the home infusion therapy benefit at this time. In general, antibiotics do not require the use of a DME external infusion pump and can be given through an elastomeric pump or by gravity infusion.
Comment: Commenters requested coverage of home infusion therapy services for other drugs and biologicals currently covered under the DME LCD for External Infusion Pumps (L33794). A commenter recommended we cover services for Carbidopa 5 Mg/Levodopa 20 Mg enteral suspension and Hizentra, a subcutaneous immunoglobulin. The commenter noted that the pump and supplies for Carbidopa/Levodopa are billed to DME, similar to immune globulin, and recommended services be covered under payment category 2. Regarding Hizentra, the commenter urged CMS to either extend coverage for services under the home infusion benefit in CY 2021 or remove Hizentra from the self-administered drug exclusion list. Also with regard to the self-administered drug exclusion lists, another commenter encouraged CMS to consider giving additional guidance to the MACs regarding the process and time involved in administering SCIG therapies. Lastly, a commenter recommended identifying all such drugs administered via external infusion pumps covered under the DME benefit as “home infusion drugs.”
Response: As noted previously, section 1861(iii)(3)(C) of the Act defines a “home infusion drug” as a parenteral drug or biological administered intravenously or subcutaneously. Although we clarified that a “home infusion drug” is a drug or biological included on the DME LCD for External Infusion Pumps (L33794), there are drugs and biologicals on this LCD that do not meet the definition of “home infusion drug” required by statute. While Carbidopa/Levodopa is on the DME LCD, because it is an enteral infusion and not administered intravenously or subcutaneously, it does not meet the statutory definition of home infusion drug. Additionally, in the CY 2020 HH PPS proposed rule, we identified additional drugs covered under the temporary transitional payment that would be excluded from the permanent benefit because they, similarly, do not meet the statutory definition of home infusion drug. We stated that Ziconotide and Floxuridine are not considered “home infusion drugs” because they are not administered either subcutaneously or intravenously (84 FR 34695). Section 1861(iii)(3)(C) of the Act also excludes insulin pump systems and any drugs or biologicals on self-administered drug exclusion lists from the definition of home infusion drug. Therefore, this provision excludes Hizentra, which is on a self-administered drug exclusion list, from the benefit beginning in CY 2021. Because this is a statutory exclusion, CMS does not have the authority to extend coverage under the home infusion benefit for services related to drugs and biologicals on these lists. In the CY 2020 HH PPS proposed rule we discuss that the determination for which drugs and biologicals belong on a self-administered drug exclusion list is made on a drug by drug basis, taking into account whether a drug is self-administered by more than 50 percent of Medicare beneficiaries (84 FR 34687). Chapter 15, section 50.2 of the Medicare Benefit Policy Manual [227] addresses the specific policy for making this determination in general, therefore, further guidance to the MACs regarding specific therapies is unnecessary.
Comment: Many commenters expressed concern that relying on the DME LCD for External Infusion Pumps limits the ability for new and/or innovative drugs to be added under the home infusion therapy benefit. Commenters indicated that the LCD process and the DME criteria is such that the DME MACs continue to evaluate drugs based on the notion that only drugs that patients can self-administer, or that a caregiver can administer for the patient, can be added. Commenters recommended that CMS require the DME MACs to increase transparency of their coverage policy by further detailing the criteria used to make coverage determinations and ensuring that coverage determinations follow current clinical practice guidelines and patient need. Another commenter urged CMS to clarify that Medicare covers the cost of pump maintenance for the duration of the drug's use in treating the beneficiary and further clarify that pumps supplied per the benefit remain the property of the pharmacy and are returnable when the beneficiary ceases service.
Response: As detailed in section VI.C.1.a. of the CY 2020 HH PPS proposed rule, home infusion drugs are those drugs and biologicals identified on the DME LCD for External Infusion Pumps (L33794). This does not however, limit the scope of drugs to only those drugs and biologicals which are currently on this LCD at this time. Table 30 lists the drugs and biologicals which are currently on the DME LCD for External Infusion Pumps (L33794), and which also meet the definition of a home infusion drug; however, it is important to note that this list is not static. The DME criteria used to determine which items are included on the LCD for External Infusion Pumps, as well as the cost of pump maintenance, is out of the scope of this final rule with comment period, which focuses on the home infusion therapy benefit. However, in response to stakeholder concerns regarding the limitations of the DME LCDs for External Infusion Pumps that preclude coverage to certain infused drugs, we are soliciting comments on the criteria CMS could consider to allow coverage of additional drugs under the DME benefit.
With regard to transparency in the LCD Development Process, the 21st Century Cures Act required a summary of the evidence and a publication of a written explanation of the rationale to be included in the LCD. The new LCD development process that includes these procedures is outlined in Chapter 13 of the Medicare Program Integrity Manual (PIM); pub. 100-08 (found at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html) and went into effect on January 1, 2019. Therefore, the new LCD development requirements do not apply to local coverage policies prior to the effective date of January 1, 2019.
In addition, the mechanism that allows the Medicare Administrative Contractors (MACs) to change coverage continues to be the LCD reconsideration process. The LCD reconsideration process allows any stakeholder to submit new evidence to ask for a reconsideration of the policy. The full LCD reconsideration process and requirements are also located at Chapter 13 of the PIM. We encourage stakeholders with additional evidence to engage their MAC in consultation regarding the available evidence that was not considered in the initial review, or to sensitize the MAC of emerging evidence that could be useful in an upcoming reconsideration once published.
3. Patient Eligibility and Plan of Care Requirements
Subparagraphs (A) and (B) of section 1861(iii)(1) of the Act set forth beneficiary eligibility and plan of care requirements for “home infusion therapy.” In accordance with section 1861(iii)(1)(A) of the Act, the beneficiary must be under the care of an applicable provider, defined in section 1861(iii)(3)(A) of the Act as a physician, nurse practitioner, or physician assistant. In accordance with section 1861(iii)(1)(B) of the Act, the beneficiary must also be under a plan of care, established by a physician (defined at section 1861(r)(1) of the Act), prescribing the type, amount, and duration of infusion therapy services that are to be furnished, and periodically reviewed, in coordination with the furnishing of home infusion drugs under Part B.
Based on these statutory requirements, we proposed to make a number of revisions to the regulations to implement the home infusion therapy services payment system beginning on January 1, 2021. We proposed to add a new 42 CFR part 414, subpart P, to implement the home infusion therapy services conditions for payment. In accordance with the standards at § 486.520, we proposed conforming regulations text, at § 414.1505, requiring that home infusion therapy services be furnished to an eligible beneficiary by, or under arrangement with, a qualified home infusion therapy supplier that meets the health and safety standards for qualified home infusion therapy suppliers at § 486.520(a) through (c). We also proposed at § 414.1510 that, as a condition for payment, qualified home infusion therapy suppliers must ensure that a beneficiary meets certain eligibility criteria for coverage of services, as well as ensure that certain plan of care requirements are met. We proposed at § 414.1510 to require that a beneficiary must be under the care of an applicable provider, defined in section 1861(iii)(3)(A) of the Act as a physician, nurse practitioner, or physician assistant. Additionally, we proposed at § 414.1510, to require that a beneficiary must be under a plan of care, established by a physician. In accordance with section 1861(iii)(1)(B) of the Act, a physician is defined at section 1861(r)(1) of the Act, as a doctor of medicine or osteopathy legally authorized to practice medicine and surgery by the State in which he performs such function or action. We proposed to require at § 414.1515, that the plan of care must contain those items listed in § 486.520(b). We also stated that in addition to the type of home infusion therapy services to be furnished, the physician's orders for services in the plan of care must also specify at what frequency the services will be furnished, as well as the healthcare professional that will furnish each of the ordered services. The following is a summary of the comments received on the proposed conditions for payment, which include patient eligibility and plan of care requirements, and our responses.
Comment: A commenter stated that proposed § 414.1515(c) does not provide applicable providers the authority to properly manage home infusion patients under their care. The commenter noted that while the statute says that a physician is required to establish and periodically review the plan of care, the patient can be under the care of an applicable provider, which does not have to be a physician. Commenters disagreed with the portion of proposed § 414.1515(c) which states that a physician must sign and date the plan of care upon any changes to the plan of care, and stated that this is not required by statute and prevents an applicable provider from managing a patient under his/her care when the applicable provider is not the ordering physician. This commenter requested that CMS remove this language from proposed § 414.1515 or amend the language to state that the “ordering physician or applicable provider must sign and date the plan of care upon any changes to the plan of care.”
Response: We appreciate the commenter's review of the regulatory language and recognition that in accordance with section 1861(iii)(1)(A) of the Act, the patient must be under the care of an applicable provider, which as defined in 1861(iii)(3)(A) of the Act, is a physician, nurse practitioner, or physician assistant. Additionally, section 1861(iii)(1)(B) of the Act, states that the beneficiary must be under a plan of care, established by a physician (defined at section 1861(r)(1) of the Act). Therefore, for payment purposes, the plan of care must be established and reviewed by a physician. This means that all services billed to Medicare have to be reflected in the plan of care, which is required to be established and reviewed by the physician, which includes any changes or updates to the plan, as stated in the regulatory language. We will consider whether an applicable provider can update the plan of care for future rulemaking.
Comment: Several commenters recommended that CMS adopt a timeframe for the physician review of the plan of care. Some commenters specifically recommended that CMS require the physician to review the plan of care at least every 90 days.
Response: As section 1861(iii)(1)(B) of the Act states that the plan of care must be periodically reviewed by a physician in coordination with the furnishing of home infusion drugs, we believe this to mean that the home infusion plan of care must be established and reviewed by the physician, in consultation with the DME supplier responsible for furnishing the home infusion drugs. Additionally, the DME Quality Standards require suppliers to work collaboratively with the physician prescribing the drug, who is ultimately responsible for any changes in type, dosage, and frequency of medication. Therefore, as coordination is required between the entity responsible for furnishing the drug, and both the entities (if they are not the same entity) responsible for ordering the home infusion therapy services and the home infusion drug, we would expect all entities to be involved in the care coordination process.
However, we do recognize the integral part the plan of care plays in care coordination between providers, particularly when the physician ordering the home infusion drug is not the same physician establishing the home infusion therapy plan of care. Coordination between the physician ordering the home infusion drug, the physician ordering the home infusion services, and the DME supplier furnishing the home infusion drug is imperative in providing safe and effective home infusion therapy. Coordination would likely include review of the patient assessment and evaluation, including interpretation of lab results as they pertain to changes in medication type, dose, or frequency. And, as many of the home infusion drugs and biologicals likely require weekly bloodwork and close monitoring, a current home infusion therapy plan of care is essential in order to ensure that the qualified home infusion therapy supplier is providing the appropriate professional services, including patient monitoring, to ensure that administration is safe and effective. Additionally, these drugs and biologicals treat a variety of both acute and chronic conditions. Treatment regimens and schedules will likely vary in length and intensity depending on the drug, individual response to therapy, and disease progression. As such, patient needs, including interventions and monitoring, will likely fluctuate based on short-term and long-term goals of the varying treatment regimens. For this reason, in order to ensure that therapy is safe and effective throughout the course of treatment, the physician responsible for the home infusion therapy plan of care should review the plan on a regular basis, in coordination with the DME supplier.
We received comments on the proposed health and safety standards in the CY 2019 HH PPS proposed rule stating that establishing timeframe requirements could conflict with State laws, creating duplicative requirements, which may add burden to home infusion therapy suppliers. Therefore, we stated in the CY 2019 HH PPS final rule with comment period that we would not include specific timeframes for the review of the plan of care, and will defer to existing State laws and regulations (83 FR 56563). However, we will take the recommendations received on the CY 2020 HH PPS proposed rule regarding establishing a timeframe for physician review under consideration for future rulemaking.
Comment: Several commenters recommended that CMS require that home infusion suppliers document the following in the plan of care: Drug name, strength, and dosage; frequency of administration; route of administration; method of administration; and a care plan for the following professional services: Patient assessments; drug therapy evaluation and design; drug preparation and compounding; care coordination; monitoring and remote monitoring; and nursing services.
Response: The CY 2019 HH PPS final rule with comment period finalized the plan of care requirements for home infusion therapy suppliers. Section 486.520(b) requires that the home infusion therapy supplier ensure that all patients have a plan of care established by a physician that prescribes the type, amount, and duration of home infusion therapy services that are to be furnished. The plan of care would also include the specific medication, including the prescribed dosage and frequency, as well as the professional services to be utilized for treatment. In addition, the plan of care would specify the care and services necessary to meet the patient-specific needs (83 FR 56562). Additionally, proposed § 414.1515 requires, as a condition for payment, that in addition to the elements indicated in § 486.520(b), the physician's orders for services in the plan of care must also specify at what frequency the services will be furnished, as well as the healthcare professional that will furnish each of the ordered services. These required elements capture the majority of the commenters' recommendations; however, any additional regulatory plan of care elements would be required to go through notice and comment rulemaking.
Comment: Several commenters recommended that CMS add a requirement that the same physician be responsible for signing the DME detailed written order (DWO) and the home infusion therapy plan of care. Commenters stated that because CMS is proposing to allow the DME supplier and the home infusion therapy supplier to be different entities, there is a risk for medication errors resulting from conflicting orders being obtained by the individual providers involved in the patient's care.
Response: We recognize the commenter's concern; however, the statute does not specify that the home infusion plan of care must be established by the same physician who orders the DME and signs the DWO. While we would expect that in most cases the physician ordering the home infusion therapy services is the same physician ordering the DME and the infusion drug, we recognize that this may not always be the case. However, § 486.520(a) requires that in addition to the professional services utilized for treatment, the home infusion plan of care must include the specific home infusion drug or biological, along with the prescribed dosage and frequency of the medication. Therefore, regardless of whether the physician ordering the home infusion drug is the same physician ordering the home infusion therapy services, there must be care coordination between both entities in order to meet the plan of care requirements under § 486.520(a).
Comment: A commenter noted that in the CY 2019 HH PPS final rule with comment period, CMS finalized the definition of “applicable provider” at § 486.505 as “a physician, a nurse practitioner, and a physician assistant;” however, the regulatory language under 42 CFR 486.505 uses the term “nurse provider” rather than “nurse practitioner.” The commenter therefore, requested a technical edit of 42 CFR 486.505 to change the language to read “nurse practitioner” in accordance with the statutory definition at 1861(iii)(3)(A) of the Act.
Response: We thank the commenter for his/her review of the regulatory language and agree that the language at § 486.505 should be changed from “nurse provider” to “nurse practitioner” and will be modified accordingly.
Final Decision: We are finalizing, as proposed, the home infusion therapy services conditions for payment at 42 CFR part 414, subpart P.
In addition, in response to the comment made regarding terminology, we will amend the regulations at § 486.505 to change the term “nurse provider” to “nurse practitioner.” We are also amending § 414.1550(a)(1) and (2) to include “or service.” Although these changes were not proposed in the proposed rule, we are adopting the changes here under a “good cause” waiver of proposed rulemaking. The specific changes we are making in the regulations are simply technical corrections in the language and do not reflect any additional substantive changes. Therefore, we find that undertaking further notice and comment procedures to incorporate these corrections into this final rule with comment period is unnecessary and contrary to the public interest.
4. Qualified Home Infusion Therapy Suppliers and Professional Services
Section 1861(iii)(3)(D)(i) of the Act defines a “qualified home infusion therapy supplier” as a pharmacy, physician, or other provider of services or supplier licensed by the State in which the pharmacy, physician, or provider of services or supplier furnishes items or services. The qualified home infusion therapy supplier must: Furnish infusion therapy to individuals with acute or chronic conditions requiring administration of home infusion drugs; ensure the safe and effective provision and administration of home infusion therapy on a 7-day-a-week, 24-hour a-day basis; be accredited by an organization designated by the Secretary; and meet such other requirements as the Secretary determines appropriate. Importantly, neither the statute, nor the health and safety standards and accreditation requirements, outlined in 42 CFR part 486, require the qualified home infusion therapy supplier to furnish the pump, home infusion drug, or related pharmacy services. Therefore, in the CY 2020 HH PPS proposed rule, we noted that the infusion pump, drug, and other supplies, and the services required to furnish these items (that is, the compounding and dispensing of the drug) remain covered under the DME benefit.
We stated in the CY 2020 HH PPS proposed rule that we did not specifically enumerate a list of “professional services” for which the qualified home infusion therapy supplier is responsible in order to avoid limiting services or the involvement of providers of services or suppliers that may be necessary in the care of an individual patient (84 FR 34692). However, we noted that, under section 1862(a)(1)(A) of the Act, no payment can be made for Medicare services under Part B that are not reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member, unless explicitly authorized by statute. We stated that this means that the qualified home infusion therapy supplier is responsible for the reasonable and necessary services related to the administration of the home infusion drug in the individual's home. These services may require some degree of care coordination or monitoring outside of an infusion drug administration calendar day; however, payment for these services is built into the bundled payment for an infusion drug administration calendar day.
Comment: A commenter supported CMS' efforts to promote supplier participation in Medicare home infusion therapy services and improve access for beneficiaries by giving them more choices of providers under the benefit.
Response: We thank the commenter for this recognition and also anticipate that the breadth of providers able to become accredited as qualified home infusion therapy suppliers will help ensure continued access to home infusion services.
Comment: A commenter referenced the discussion of billing for chronic care management and remote patient monitoring codes associated with the home infusion benefit. The commenter indicated that CMS only references ordering physicians and does not mention applicable providers, and stated that CMS should clarify that these codes, and other care coordination services, are billable by the applicable provider managing the patient's care. Another commenter suggested adding teaching and training users to self-administer using a pump, troubleshooting pump issues (for example, telephonically or via video monitoring); and providing clinical/quality assessments such as monitoring the efficacy of drugs (for example, number of infections for a user of immune globulin diagnosed with primary immunodeficiency (PID)) to the proposed list of remote monitoring services.
Response: The discussion referencing the PFS chronic care management and remote monitoring codes was regarding the services for which a provider can bill separately under the PFS and was referenced in order to separate these services from the care coordination included in the bundled services under the single unit of payment for home infusion therapy suppliers. These are not codes for which home infusion therapy suppliers can bill separately under the home infusion therapy benefit, therefore, which providers can bill for these codes is out of the scope of the CY 2020 HH PPS final rule with comment period.
Additionally, as we did not propose a list of remote monitoring services considered professional services under the home infusion therapy benefit, it is unclear if the comment regarding teaching and training on the pump pertains specifically to the CY 2020 HH PPS proposed rule. However, we will note that the commenter's suggestion that the infusion therapy supplier engage in training and education on the item of DME, address services already covered under the DME benefit, and would not be covered under the home infusion therapy benefit. Additionally, in the CY 2019 HH PPS proposed rule, although we did not define home infusion therapy professional services, we did give examples of services we believe fall under the home infusion therapy benefit. Clinical assessments, including monitoring efficacy of drug therapy, was included in these examples (83 FR 32468).
Comment: Several commenters expressed concern about care coordination between different entities providing services under various benefits. These commenters stated that the proposed rule tasked the home infusion therapy supplier with furnishing the necessary services to administer the drug in the home, but does not require the qualified home infusion therapy supplier to furnish the pump, home infusion drug, or related pharmacy services. Commenters stated that because “CMS' interpretation” allows the DME supplier and the home infusion therapy supplier to be separate entities, this could potentially create confusion about roles and responsibilities. Further, commenters indicated that CMS makes no requirement for the provider of HIT services to coordinate directly with the DME supplier. A commenter stated that typically, commercial payers structure the home infusion benefit as a pharmacy-coordinated service, where the pharmacy assumes responsibility for case managing the therapy and provides oversight of all the professional services. The commenter noted that under the commercial payer structure, the pharmacy is the entity contracted to supply the drugs, equipment, and supplies, and because of the dependency between these two components of care, commercial payers and accreditation organizations never separate the case management from the supplier of the drug, equipment, and supplies. Commenters recommended that the Secretary add a new requirement that the home infusion therapy supplier be enrolled in the DME program as a pharmacy that provides external infusion pumps and supplies, and that maintains all pharmacy licensure and accreditation requirements, and that all components of the home infusion benefit should be billed by the same provider, including professional services, drugs, pumps, and supplies.
Response: We recognize that there may be various providers and suppliers involved in a patient's care in the provision of home infusion therapy and the importance of care coordination. While the supplier furnishing the DME, home infusion drug, and related services may be the supplier furnishing the home infusion services, the statute does not require that the DME supplier also furnish home infusion therapy services. Section 1861(iii)(3)(D)(i) of the Act defines a “qualified home infusion therapy supplier” as a pharmacy, physician, or other provider of services or supplier licensed by the State in which the pharmacy, physician, or provider of services or supplier furnishes items or services. There is no provision requiring the home infusion therapy supplier to furnish the infusion pump, drug, or other supplies. Further, section 1861(iii)(3)(D)(ii) of the Act allows a qualified home infusion therapy supplier to sub-contract with a pharmacy, physician, provider of services, or supplier to provide these services. Additionally, section 1861(u) of the Act defines “provider of services” to mean a hospital, critical access hospital, skilled nursing facility, comprehensive outpatient rehabilitation facility, home health agency, hospice program, or, for purposes of sections 1814(g) and 1835(e) of the Act, a fund. Therefore, any of the previously noted entities who meet the Medicare accreditation requirements for home infusion therapy suppliers is eligible to enroll as a qualified home infusion therapy supplier.
We also do not anticipate a lapse in care coordination in the case that the home infusion therapy supplier is not the same entity furnishing the DME, drug, and related services. Section 1861(iii)(1)(B) of the Act states that the home infusion therapy plan of care must be established and periodically reviewed by a physician in coordination with the furnishing of home infusion drugs. As previously stated, this means that the home infusion plan of care must be established and reviewed by a physician in consultation with the DME supplier responsible for furnishing the home infusion drug and related services. Likewise, as discussed in the CY 2020 HH PPS proposed rule, the DME Quality Standards require the supplier (furnishing the infusion drug) to consult with the physician prescribing the infusion drug as needed to confirm the order and to recommend any necessary changes, refinements, or additional evaluation to the prescribed equipment item(s), and/or service(s) (84 FR 34692). Therefore, as the DME supplier is required to consult with the physician prescribing the infusion drug, initially and upon any changes in medication or orders, and the physician responsible for drafting the home infusion plan of care is required to consult with the DME supplier and the home infusion therapy supplier, we would expect the home infusion therapy plan of care to be current. Furthermore, proposed § 414.1515 requires that the home infusion plan of care contain the items indicated in § 486.520(b), which includes the specific medication, the prescribed dosage and frequency, as well as the professional services to be utilized for treatment, including the care and services necessary to meet patient-specific needs. Additionally, proposed § 414.1515 requires the plan of care to include the healthcare professional that will furnish each of the ordered services. Therefore, while the home infusion therapy supplier may not be the DME supplier, the home infusion plan of care must contain the required contents, as previously discussed, and established in coordination with the furnishing of the infusion drug. For this reason, in order to ensure that therapy is safe and effective throughout the course of treatment, as required by section 1861(iii)(1)(B) of the Act, the physician who orders the home infusion therapy services must review the plan of care on a regular basis, in coordination with the DME supplier, who is also required to consult with the physician prescribing the infusion drug.
Comment: A commenter requested that CMS clarify whether there will be a grace period for accreditation, and whether or not more accrediting bodies be added.
Response: Home Infusion Therapy (HIT) Accreditation Organizations will be held to the same expectations as our remaining accreditation organizations. The home infusion therapy application procedures and ongoing responsibilities are provided at 42 CFR part 488, subpart L. Any accreditation organization will be allowed to apply to be a CMS Approved Deeming Accreditation Organization for Home Infusion Therapy, if the organization meets all of the requirements provided at 42 CFR 488.1010. Applications will be considered for the January 1, 2021 designation deadline, if the application is received by April 1, 2020.
Comment: Several commenters indicated that reimbursement under the DME benefit is inadequate to cover the home infusion therapy professional services and stated that Congress understood that the breadth and frequency of these services exceeds the scope of the DME benefit. Other commenters stated that the home infusion therapy payment was intended to make up for the drug pricing change from AWP to ASP plus 6 percent. Commenters stated that it is for these reasons that Congress created the home infusion therapy benefit and intended for these services, most notably those provided remotely by a pharmacist, to be reimbursed without regard to overlap with the DME benefit or contingent on the patient's nursing needs. Additionally, commenters stated that it is notable that Congress exempted training and education that is not otherwise paid for as DME from the professional services reimbursement, but made no such exemption for professional services, remote monitoring and monitoring services, or the other professional services referenced in the proposed rule.
Response: We are unsure of whether Congressional intent for the home infusion benefit was to reimburse providers for the change in drug pricing. However, in general, Medicare does not implement new benefits in order to subsidize other existing benefits. Additionally, because the home infusion therapy services payment does not include payment for the DME or the home infusion drug, the adequacy of the drug pricing is out of the scope for this final rule with comment period. Although the commenter stated that the home infusion therapy payment is for services “without regard to overlap with DME,” it is important to note that Medicare does not make duplicative payment for services, therefore we would not require two benefits to furnish the same services.
Additionally, CMS did not define or enumerate the professional services under the home infusion therapy benefit in order to avoid inadvertently excluding certain services. However, we agree that it is notable that training and education not otherwise paid for as DME is exempted from the professional services covered under the home infusion therapy benefit. The training and education provided under the DME benefit are services that would likely be furnished in the patient's home. Therefore, in order to avoid making duplicative payment, the training and education furnished under the DME benefit is explicitly excluded from the home infusion therapy services payment. Furthermore, as we noted in the CY 2019 HH PPS proposed rule, we consider the home infusion benefit principally to be a separate payment in addition to the existing payment made under the DME benefit, thus explicitly and separately paying for the home infusion therapy services (83 FR 32466). Therefore, the professional services covered under the DME benefit are not covered under the home infusion benefit. While the two benefits exist in tandem, the services are unique to each benefit and billed and paid for under separate payment systems.
5. Home Infusion Therapy and the Interaction With Home Health
In the proposed rule, we discussed the potential for overlap between the new home infusion therapy benefit and the home health benefit. We stated that a beneficiary is not required to be considered homebound in order to be eligible for the home infusion therapy benefit; however, there may be instances where a beneficiary under a home health plan of care also requires home infusion therapy services. Additionally, because section 5012 of the 21st Century Cures Act amends section 1861(m) of the Act to exclude home infusion therapy from home health services effective on January 1, 2021, we stated that a beneficiary may utilize both benefits concurrently.
Furthermore, because both the home health agency and the qualified home infusion therapy supplier furnish services in the individual's home, and may potentially be the same entity, we stated that the best process for payment for furnishing home infusion therapy services to beneficiaries who qualify for both benefits is as outlined in the CY 2019 HH PPS proposed rule (83 FR 32469). If a patient receiving home infusion therapy is also under a home health plan of care, and receives a visit that is unrelated to home infusion therapy, then payment for the home health visit would be covered by the HH PPS and billed on the home health claim. When the home health agency furnishing home health services is also the qualified home infusion therapy supplier furnishing home infusion services, and a home visit is exclusively for the purpose of furnishing items and services related to the administration of the home infusion drug, the home health agency would submit a home infusion therapy services claim under the home infusion therapy benefit. If the home visit includes the provision of other home health services in addition to, and separate from, home infusion therapy services, the home health agency would submit both a home health claim under the HH PPS and a home infusion therapy claim under the home infusion therapy benefit. However, the agency must separate the time spent furnishing services covered under the HH PPS from the time spent furnishing services covered under the home infusion therapy benefit. DME is excluded from the consolidated billing requirements governing the HH PPS (42 CFR 484.205) and therefore, the DME items and services (including the home infusion drug and related services) will continue to be paid for outside of the HH PPS. If the qualified home infusion therapy supplier is not the same entity as the home health agency furnishing the home health services, the home health agency would continue to bill under the HH PPS on the home health claim, and the qualified home infusion therapy supplier would bill for the services related to the administration of the home infusion drugs on the home infusion therapy services claim.
Comment: Several commenters expressed concern that home health agencies will not be able to bill for the home infusion therapy services for beneficiaries under a home health plan of care, unless they are also accredited as a home infusion therapy supplier. Commenters expressed concern that this is in contrast to the full coverage currently available for beneficiaries under the home health benefit, and that beneficiaries will now be responsible for a 20 percent coinsurance. Additionally, commenters stated that the home health agency would be responsible for providing the pump, medication, and infusion supplies if they did obtain the designation, and expressed concern that many HHAs believe that this is outside of their scope of practice. Commenters stated that HHAs will restrict the availability of infusion services and limit those patients needing infusion services, forcing many of these patients to receive their infusions at another setting rather than receiving them at home. A commenter recommended that the home infusion benefit should only be available for beneficiaries who are not homebound, and infusion services for otherwise eligible home health beneficiaries should remain under the home health benefit.
Response: We understand commenter concern regarding home infusion therapy services under the home health benefit; however, section 5012 of the 21st Century Cures Act amends section 1861(m) of the Act to exclude home infusion therapy from home health services effective January 1, 2021. Therefore, home infusion therapy will no longer be provided to homebound patients under the home health benefit. Home infusion therapy services will now be provided under the home infusion benefit for both homebound and non-homebound beneficiaries. It is also important to note, that the HHA is not responsible for furnishing the pump, related supplies, or the infusion medication. Further, the HHA is already required to arrange for the DME and related infusion services for patients under a home health plan of care. In the case that an HHA also becomes accredited as a home infusion therapy supplier, the HHA would continue to meet the requirements under the Home Health Conditions of Participation (CoPs) as well as the home infusion therapy supplier requirements as set out in Part 486, Subpart I, of which DME services, including pharmacy services associated with the preparation and dispensing of home infusion drugs are not included. We acknowledged in the CY 2019 HH PPS final rule with comment period that while these services are closely related to the home infusion therapy benefit, they remain covered under the Part B DME benefit and are not part of the Medicare home infusion therapy benefit (83 FR 56563).
6. Public Comments Regarding Notification of Infusion Therapy Options Available Prior To Furnishing Home Infusion Therapy Services
Section 1834(u)(6) of the Act requires that prior to the furnishing of home infusion therapy to an individual, the physician who establishes the plan described in section 1861(iii)(1) of the Act for the individual shall provide notification (in a form, manner, and frequency determined appropriate by the Secretary) of the options available (such as home, physician's office, hospital outpatient department) for the furnishing of infusion therapy under this part.
We recognize there are several possible forms, manners, and frequencies that physicians may use to notify patients of their infusion therapy treatment options. For example, a physician may verbally discuss the treatment options with the patient during the visit and annotate the treatment decision in the medical record before establishing the infusion plan. Some physicians may also provide options in writing to the patient in the hospital discharge papers or office visit summaries, as well as retain a written patient attestation that all options were provided and considered. The frequency of discussing these options could vary based on a routine scheduled visit or according to the individual's clinical needs.
We solicited comments in the CY 2020 PFS proposed rule (84 FR 40716), as well as the CY 2020 HH PPS proposed rule (84 FR 34694), regarding the appropriate form, manner, and frequency that any physician must use to provide notification of the treatment options available to his/her patient for the furnishing of infusion therapy (home or otherwise) under Medicare Part B. We also solicited comments on any additional interpretations of this notification requirement and whether this requirement is already being met under the temporary transitional payment for home infusion therapy services.
The following is a summary of the related comments received on both solicitations.
Comment: Several commenters supported the proposed examples of the physician verbally discussing the infusion therapy options and annotating the resulting decision in the medical record and initial plan of care. Many commenters stated that written materials may be a helpful supplement to a verbal conversation, but written materials should not be the sole means of beneficiary notification. They emphasized that infusion therapy options should be verbally discussed so the patient, and any family caregiver, may have an opportunity to get immediate answers to questions that may not be addressed in written materials. Many commenters encouraged CMS to consider minimizing the paperwork burden and confusion that written documents or patient attestations could impose on physicians and patients.
Commenters recommended that the conversation should include how the infusion therapy options differ in terms of effectiveness, safety, time, comfort, convenience, location, frequency, and out-of-pocket costs. Some commenters specifically noted that beneficiaries are subject to the standard 20 percent coinsurance with this new Part B benefit; and the ordering physician should be aware of the patient's insurance status and therefore assist them in making informed decisions about their care.
Some commenters recommended the policy should allow for other professionals, such as social workers, home health nurses, and other staff to assist the treating physician with this notification in order to remove unnecessary administrative burden for clinicians. Commenters also requested that the notification policy include requirements would be simple and easy for physicians to implement, and that would retain the current flexibility for physicians to use multiple notification mechanisms as directly suggested by beneficiaries, advocates and stakeholders.
One commenter requested that CMS follow similar procedures for other electronically prompted beneficiary notifications. Another commenter recommended that CMS develop a single standardized format for this notice to avoid benefit denials and delays in therapy. Another commenter suggested that CMS establish a training program for physicians, hospitals and contractors prior to implementation.
A commenter requested that CMS permit sufficient time for physicians to research the available home infusion therapy options. Another commenter requested that CMS create a web page where a beneficiary or referring clinician can research if there is a home infusion therapy supplier in the beneficiary's geographic location that is capable of delivering these services, and that the supplier is enrolled and approved by Medicare.
A few commenters asked that this notification be required only when the drug regimen is available and appropriate for home infusion therapy. They suggested that notification should not be required if there are certain safety risks associated with infusion therapy in that patient's home or if the home infusion therapy option is not available in the patient's geographic area.
Regarding the frequency of notification, one commenter suggested that only one streamlined notice be required at the start of therapy because many therapies have a duration for the life of the beneficiary. Two commenters specified that notification of options should be discussed and documented in the patient record whenever a new infusion therapy treatment is deemed necessary by the physician and anytime thereafter if there are changes in patient condition or circumstances that would affect the patient's choices.
Response: We appreciate the commenters' support and recommendations and will take the comments into consideration as we continue developing future policy through notice-and-comment rulemaking effective for home infusion therapy services beginning CY 2021 and for subsequent years.
D. Payment Categories and Amounts for Home Infusion Therapy Services for CY 2021
In the CY 2020 HH PPS proposed rule we discussed section 1834(u)(1)(A)(i) of the Act, which requires the Secretary to implement a payment system under which a single payment is made to a qualified home infusion therapy supplier for items and services furnished by a qualified home infusion therapy supplier in coordination with the furnishing of home infusion drugs. Section 1834(u)(1)(A)(ii) of the Act states that a unit of single payment under this payment system is for each infusion drug administration calendar day in the individual's home, and requires the Secretary, as appropriate, to establish single payment amounts for different types of infusion therapy, taking into account variation in utilization of nursing services by therapy type. Section 1834(u)(1)(A)(iii) of the Act provides a limitation to the single payment amount, requiring that it shall not exceed the amount determined under the PFS (under section 1848 of the Act) for infusion therapy services furnished in a calendar day if furnished in a physician office setting. Furthermore, such single payment shall not reflect more than 5 hours of infusion for a particular therapy in a calendar day. Section 1834(u)(1)(B)(ii) of the Act requires the payment amount to reflect patient acuity and complexity of drug administration.
We stated that the best way to establish a single payment amount that varies by utilization of nursing services and reflects patient acuity and complexity of drug administration, is to group home infusion drugs by J-code into payment categories reflecting similar therapy types. Therefore, each payment category would reflect variations in infusion drug administration services. We proposed to maintain the three payment categories, with the associated J-codes, utilized currently under the temporary transitional payment. We stated that this utilizes an already established framework for assigning a unit of single payment (per category), accounting for different therapy types, which in turn, reflects variations in nursing utilization, complexity of drug administration, and patient acuity. We stated that retaining the three current payment categories would maintain consistency with the already established payment methodology and ensure a smooth transition between the temporary transitional payments and the permanent payment system to be implemented beginning with CY 2021. Table 30 provides the list of J-codes associated with the infusion drugs that fall within each of the payment categories. We also noted that there are a few drugs for which services are included under the transitional benefit that would not be defined as home infusion drugs under the permanent benefit beginning with CY 2021.

We stated in the proposed rule that the language at section 1834(u)(1)(A)(ii) of the Act is consistent with section 1834(u)(7)(B)(iv) of the Act, which establishes “single payment amounts” for the temporary transitional payments for home infusion therapy services. We also reiterated that a “single payment amount” for an infusion drug administration calendar day means that all home infusion therapy services, which include professional services, including nursing; training and education; remote monitoring; and monitoring, are built into the day on which the services are furnished in the home and the drug is being administered. In other words, payment for an infusion drug administration calendar day is a bundled payment amount per visit. As such, because payment for an infusion drug administration calendar day under the permanent benefit is also a “unit of single payment,” we proposed to carry forward the payment methodology as outlined in section 1834(u)(7)(A) of the Act for the temporary transitional payments. We proposed to pay a single payment amount for each infusion drug administration calendar day in the individual's home for drugs assigned under each proposed payment category. Each proposed payment category amount would be in accordance with the six infusion CPT codes identified in section 1834(u)(7)(D) of the Act. However, because section 1834(u)(1)(A)(iii) of the Act states that the single payment shall not exceed more than 5 hours of infusion for a particular therapy in a calendar day, we proposed that the single payment amount be set at an amount equal to 5 hours of infusion therapy administration services in a physician's office for each infusion drug administration calendar day, rather than retaining the current rate under the temporary transitional payment, equal to 4 hours. We stated that a single unit of payment equal to 5 hours of infusion therapy services in a physician's office is a reasonable approach to account for the bundled services included under the home infusion therapy benefit. We stated that setting the payment amount at the maximum amount allowed by statute would reflect the varying degrees of care among individual patients within each category and from visit to visit for the same patient. It would also ensure that payment for home infusion therapy services adequately covers the different patient care needs and level of complexity of services provided, while remaining a unit of single payment. While the single unit of payment for the temporary transitional payments was set at 4 hours by law, the law for the permanent benefit provides more latitude for home infusion therapy services payments beginning in CY 2021. We stated that furnishing care in the patient's home is fundamentally different from furnishing care in the physician's office due to healthcare professionals being unable to achieve the economies of scale in the home that can be achieved in an office setting. Therefore, the single unit of payment is a bundle that is made on the basis of expected costs for clinically-defined episodes of care, where some episodes of care for similar patients with similar care needs cost more than others. While the payment rates for each of the three payment categories are higher than the home health per-visit nursing rate of $149.68, the rate for medical social services is $239.92. As we did not limit this benefit to only nursing visits, the home infusion therapy rates for subsequent visits are comparable to the home health per visit amounts. The home infusion therapy rates reflect the increased complexity of the professional services provided per category, and as required by law. We continue to believe that increasing the payment amount to 5 hours will better account for all of the home infusion therapy services covered under the benefit, including nursing; training and education; remote monitoring; and monitoring provided on an infusion drug administration calendar day.
We also stated that setting the payment amounts for each proposed payment category in accordance with the CPT infusion code amounts under the PFS accounts for variation in utilization of nursing services, patient acuity, and complexity of drug administration. Medicare PFS valuation of CPT codes uses a combination of the time and complexity used to furnish the service, as well as the amount and value of resources used. We explained that one component used to value the CPT code, the non-facility practice expense relative value unit (RVU), is based, in part, on the amount and complexity of services furnished by nursing and ancillary clinical staff involved in the procedure or service, and that therefore, the values of the CPT infusion code amounts, in accordance with the different payment categories, reflect variations in nursing utilization, patient acuity, and complexity of drug administration, as they are directly proportionate to the clinical labor involved in furnishing the infusion services in the patient's home.
We also recognized that often the first visit furnished by a home infusion therapy supplier to furnish services in the patient's home may be longer or more resource intensive than subsequent visits. In accordance with section 1834(u)(1)(C) of the Act, which allows the Secretary discretion to adjust the single payment amount to reflect outlier situations and other factors as the Secretary determines appropriate, in a budget neutral manner, we proposed increasing the payment amounts for each of the three payment categories for the first visit by the relative payment for a new patient rate over an existing patient rate using the physician evaluation and management (E/M) payment amounts for a given year. Overall this adjustment would be budget-neutral, in accordance with the requirement at section 1834(u)(1)(C)(ii) of the Act, resulting in a small decrease to the payment amounts for any subsequent visits. We stated that the first visit payment amount is only issued on the first home visit to initiate home infusion therapy services furnished by the qualified home infusion therapy supplier, and that any changes in the plan of care or drug regimen, including the addition of drugs or biologicals that may change the payment category, would not trigger a first visit payment amount. We stated that if a patient receiving home infusion therapy services is discharged, the home infusion therapy services claim must show a patient status code to indicate a discharge with a gap of more than 60 days in order to bill a first visit again if the patient is readmitted. This means that upon re-admission, there cannot be a G-code billed for this patient in the past 60 days, and the last G-code billed for this patient must show that the patient had been discharged. A qualified home infusion therapy supplier could bill the first visit payment amount on day 61 for a patient who had previously been discharged from service. We also recognized that many beneficiaries have been receiving services during the temporary transitional payment period, and as a result, many of these patients already have a working knowledge of their pump and may need less start-up time with the nurse during their initial week of visits during the permanent benefit. Therefore, we stated that suppliers would not be able to bill for the initial visit amount for those patients who have been receiving services under the temporary transitional payment, and have billed a G-code within the past 60 days.
And finally, we stated that we plan on monitoring home infusion therapy service lengths of visits, both initial and subsequent, in order to evaluate whether the data substantiates this increase or whether we should re-evaluate whether, or how much, to increase the initial visit payment amount.
The following is a summary of the comments received on the proposed CY 2021 home infusion therapy categories and payment amounts, and our responses:
Comment: A few commenters' stated that the proposed categories do not necessarily reflect the acuity or complexity of drug administration. These commenters did not suggest other methods for grouping drugs but recommended that CMS reimburse all home infusion professional services at the proposed rate for payment category 3 (1 hour at CPT 96413 and 4 hours at CPT 96415). MedPAC recommended that CMS use 2019 home infusion therapy claims data to evaluate the three categories and consider whether modifications to the three categories are appropriate in next year's proposed rule.
Response: While commenters' did not provide a rationale as to why they believe all infusion drug administration calendar days should be paid at the payment category 3 rate, it is important to reiterate that CMS is required to account for varying therapy types under the payment system. Section 1834(u) of the Act requires the Secretary to implement a payment system under which a single payment is made to a home infusion therapy supplier for the items and services (professional services, including nursing services; training and education; remote monitoring, and other monitoring services), beginning January 1, 2021. The single payment must take into account, as appropriate, types of infusion therapy, including variations in utilization of services by therapy type. In addition, the single payment amount is required to be adjusted to reflect geographic wage index and other costs that may vary by region, patient acuity, and complexity of drug administration. Paying a single payment amount at the category 3 rate for the professional services for all home infusion drugs would not take into account types of infusion therapy, including the variation in utilization of nursing services, patient acuity, and complexity of drug administration.
We appreciate MedPAC's suggestion to evaluate the three categories and consider whether modifications are appropriate for next year's rule. We will continue to monitor home infusion utilization using the temporary transitional payment claims data, including visit length. If adjustments to any of the home infusion therapy provisions are warranted based on this data analysis, we will address such changes in future rulemaking.
Comment: A commenter stated that the CPT description for the category three CPT codes are more expansive than only chemotherapy drugs, and noted that it can be used for “injection and intravenous infusion chemotherapy and other highly complex drug or highly complex biologic agent administration.”
Response: We recognize that the CPT code associated with payment category 3 home infusion drugs also includes other highly complex drugs and biologicals; however, currently the only drugs on the LCD for External Infusion Pumps (L33794) that are appropriate for this category are the cancer chemotherapy drugs. In the event that additional drugs or biologicals are added to the DME LCD, then potentially more drugs and biologicals (other than cancer chemotherapy drugs) would be included in payment category 3.
Comment: The majority of commenters supported the 5 hour payment rate; however, these commenters continued to disagree with the definition of “infusion drug administration calendar day.” Several commenters also stated they would support retaining the three payment categories and the rates that were established in the Bipartisan Budget Act of 2018 if CMS were to pay on each day the patient receives an infusion drug, regardless of whether a professional is in the home. MedPAC disagreed with the increase from a 4 hour payment rate to a 5 hour payment rate without sufficient evidence that this increase is warranted, or that increasing the aggregate level of payment to the maximum level permitted by statute is an appropriate approach for addressing variation in costs across patients. MedPAC also suggested considering other approaches to address variation in costs such as developing a payment adjuster for patient acuity or complexity of drug administration.
Response: We thank the commenters for their support for setting the payment rate to 5 hours of infusion in a physician's office. We believe that a single unit of payment equal to 5 hours of infusion therapy services in a physician's office is a reasonable approach to account for the bundled services included under the home infusion therapy benefit. We understand MedPAC's concern regarding the lack of evidence that such an increase in the number of hours is warranted. However, because the home infusion therapy payment must take into account, as appropriate, types of infusion therapy, including variations in utilization of services by therapy type, yet remain a single payment amount, we do believe that setting the payment rate to the maximum amount set in statute recognizes the variety and amount of services included in the payment. Also, because we are implementing a payment system for a new Medicare benefit, we do not have sufficient data in order to examine situations for which payment adjustment (for example, a case-mix adjustment system) may be appropriate. As previously discussed, we plan to continue to monitor visit length in order to determine if adjustments in the payment methodology are needed. However, as we do not collect cost report data for suppliers, it is unclear how we would be able to evaluate data regarding variations in cost across patients.
We remind commenters that we finalized the definition of “infusion drug administration calendar day” in the CY 2019 HH PPS final rule with comment period (83 FR 56583) and we did not propose changes to this definition in the CY 2020 HH PPS proposed rule. Our responses to additional comments received on the CY 2019 HH PPS final rule with comment period with regard to this definition are addressed in section VI.C.1. of this final rule with comment period. Therefore, payment for home infusion therapy services beginning in CY 2021 will be for those days on which a skilled professional is in the patient's home furnishing home infusion therapy services during a day of drug administration.
Comment: Commenters were overwhelmingly in support of the proposed payment adjustment for the first visit. Commenters appreciated the recognition that new patients require more time and education. A commenter agreed that it is reasonable to expect that the first home infusion therapy visit will have higher associated costs, but encouraged CMS to examine claims data as it becomes available in order to determine an appropriate payment rate for the first versus subsequent visits.
Response: We thank commenters for their support of this proposal, and as previously stated, do plan on monitoring visit lengths in order to determine if the data substantiates this adjustment.
Comment: A few commenters recommended collecting the data necessary to construct a permanent rate that reflects the complexity and duration of services necessary to deliver home infusion therapy, will incentivize the delivery of safe, effective, high-quality care, and will inform future policy discussions as new and emerging medications become available.
Response: We appreciate commenters' recommendations and will consider them for the future as well as continue to monitor home infusion therapy utilization through the collection and analysis of claims data as previously discussed.
Final Decision: We are finalizing our proposal to maintain the three payment categories currently being utilized under the temporary transitional payments for home infusion therapy services. We are finalizing that each category payment amount will be in accordance with the six CPT infusion codes under the PFS and equal to 5 hours of infusion services in a physician's office. And finally, we are finalizing our proposal to increase the payment amounts for each of the three payment categories for the first visit by the relative payment for a new patient rate over an existing patient rate using the physician evaluation and management (E/M) payment amounts for a given year, in a budget neutral manner, resulting in a small decrease to the payment amounts for any subsequent visits. Payment will be made for each infusion drug administration calendar day in accordance with the definition finalized in the CY 2019 final rule with comment period (83 FR 56583). We will continue to evaluate the home infusion therapy benefit and if appropriate and within the scope of our statutory authority, make adjustments to the payment methodology to maximize utilization of the home infusion therapy benefit, while protecting the integrity of the Medicare program.
In response to stakeholder concerns regarding the limitations of the DME LCDs for external infusion pumps that preclude coverage to certain infused drugs, we seek comments on the criteria CMS could consider to allow coverage of additional drugs under the DME benefit. In order for a drug to be covered as a supply under the Medicare DME benefit, the drug itself must require administration through an external infusion pump. Under this benefit, the DME Supplier Standards require that the supplier train the patient and/or caregiver to operate the equipment safely and effectively in the home. As such, the patient and/or caregiver must be able to use the equipment on his/her own. For this reason, the DME LCDs for External Infusion Pumps do not currently include drugs that the patient and/or caregiver would not be able to infuse in the home without a healthcare professional present. However, given the new permanent home infusion therapy benefit to be implemented beginning January 1, 2021, which includes payment for professional services, including nursing; we are soliciting comments on options to enhance future efforts to improve policies related to coverage of eligible drugs for home infusion therapy (for example, whether coverage could include instances where diseases or conditions prevent a patient from being able to self-infuse, such as due to a neurodegenerative disease). We believe that any changes to the DME and home infusion therapy benefits must first ensure that the DME and supplies covered fall within the scope of the DME benefit, and also balance concerns of promoting access to innovative treatments with patient safety and cost-efficient delivery and monitoring of drug infusions relative to the facility setting (for example, physician office or hospital outpatient department).
Table 31 shows the payment categories with the CPT codes and units for such codes for home infusion therapy services in CY 2021 and subsequent calendar years. Table 32 illustrates the 5-hour payment rates (using the proposed CY 2020 PFS amounts) reflecting the increased payment for the first visit and the decreased payment for all subsequent visits. The actual home infusion payment rates will be updated in next year's rule using the CY 2021 PFS amounts.


E. Required Payment Adjustments for CY 2021 Home Infusion Therapy Services
1. Home Infusion Therapy Geographic Wage Index Adjustment
Section 1834(u)(1)(B)(i) of the Act requires that the single payment amount be adjusted to reflect a geographic wage index and other costs that may vary by region. In the 2019 HH PPS proposed rule (83 FR 32467) we stated that we were considering using the Geographic Practice Cost Indices (GPCIs) to account for regional variations in wages and adjust the payment for home infusion therapy professional services; however, after further analysis and consideration we stated that we determined that the geographic adjustment factor (GAF) is a more appropriate option to adjust home infusion therapy payments based on differences in geographic area wages.
The GAF is a weighted composite of each PFS locality's work, practice expense (PE), and malpractice (MP) GPCIs, and represents the combined impact of the three GPCI components. The GAF is calculated by multiplying the work, PE and MP GPCIs by the corresponding national cost share weight: work (50.886 percent), PE (44.839 percent), and MP (4.295 percent).[228] The work GPCI reflects the relative costs of physician labor by region. The PE GPCI measures the relative cost difference in the mix of goods and services comprising practice expenses among the PFS localities as compared to the national average of these costs. The MP GPCI measures the relative regional cost differences in the purchase of professional liability insurance (PLI). The GAF is updated at least every 3 years per statute and reflects a 1.5 work GPCI floor for services furnished in Alaska as well as a 1.0 PE GPCI floor for services furnished in frontier states (Montana, Nevada, North Dakota, South Dakota and Wyoming).
The GAF is not specific to any of the home infusion drug categories, so the GAF payment rate would equal the unadjusted rate multiplied by the GAF for each locality level, without a labor share adjustment. As such, based on locality, the GAF adjusted payment rate would be calculated using the following formula:

We would apply the appropriate GAF value to the home infusion therapy single payment amount based on the site of service of the beneficiary. There are currently 112 total PFS localities, 34 of which are statewide areas (that is, only one locality for the entire state). There are 10 states with 2 localities, 2 states having 3 localities, 1 state having 4 localities, and 3 states having 5 or more localities. The combined District of Columbia, Maryland, and Virginia suburbs; Puerto Rico; and the Virgin Islands are the remaining three localities. Beginning in 2017, California's locality structure was modified to increase its number of localities from 9, under the previous locality structure, to 27 under the new Metropolitan Statistical Area based locality structure defined by the Office of Management and Budget (OMB).
The list of GAFs by locality for this final rule with comment period is available as a downloadable file at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/Home-Infusion-Therapy/Overview.html.
We considered other alternatives to using the GAF such as the hospital wage index (HWI), the GPCI, and using just the practice expense component of the GPCI. However, we proposed use of the GAF to geographically wage adjust home infusion therapy for CY 2021 and subsequent years. We stated that the GAF is the best option for geographic wage adjustment, as it is the most operationally feasible. Utilizing the GAF would allow adjustments to be made while leveraging systems that are already in place. There are already mechanisms in place to geographically adjust using the GAF and applying this option would require less system changes. The adjustment would happen on the PFS and be based on the beneficiary zip code submitted on the 837P/CMS-1500 professional and supplier claims form. The GAF is further discussed in the CY 2017 PFS final rule (81 FR 80170). The final CY 2020 and CY 2021 GAF values for each payment locality, when available, will be posted along with the final rule with comment period at: https://www.cms.gov/Center/Provider-Type/Home-Health-Agency-HHA-Center.html.
We proposed that the application of the geographic wage adjustment be budget neutral so there is no overall cost impact. However, this results in some adjusted payments being higher than the average and others being lower. In order to make the application of the GAF budget neutral we will apply a budget-neutrality factor. If the rates were set for 2020 the budget neutrality factor would be 0.9985. The budget neutrality factor will be recalculated for 2021 in next year's rule using 2019 utilization data from the first year of the temporary transitional payment period.
We received a comment that supported the use of geographic adjustment for the home infusion therapy benefit in CY 2021; however, we did not receive any comments specifically regarding the use of the GAF, or any other wage adjustment, to geographically adjust the home infusion therapy payment amounts.
Comment: A commenter stated support for the use of geographic payment indexing to ensure that in higher cost markets, reimbursement is in line with expenses.
Response: We appreciate the commenter's support, and will note that geographic adjustment is a statutory requirement for the home infusion therapy benefit beginning in CY 2021.
Final Decision: We are finalizing our proposal to use the GAF to geographically adjust the home infusion therapy payment amounts in CY 2021 and subsequent calendar years.
2. Consumer Price Index
Subparagraphs (A) and (B) of section 1834(u)(3) of the Act specify annual adjustments to the single payment amount that are required to be made beginning January 1, 2022. In accordance with these sections we stated that we would increase the single payment amount by the percent increase in the Consumer Price Index for all urban consumers (CPI-U) for the 12-month period ending with June of the preceding year, reduced by the 10-year moving average of changes in annual economy-wide private nonfarm business multifactor productivity (MFP). Accordingly, this may result in a percentage being less than 0.0 for a year, and may result in payment being less than such payment rates for the preceding year.
F. Other Optional Payment Adjustments/Prior Authorization for CY 2021 Home Infusion Therapy Services
1. Prior Authorization
Section 1834(u)(4) of the Act allows the Secretary discretion, as appropriate, to apply prior authorization for home infusion therapy services. Generally, prior authorization requires that a decision by a health insurer or plan be rendered to confirm health care service, treatment plan, prescription drug, or durable medical equipment is medically necessary.[229] Prior authorization helps to ensure that a service, such as home infusion therapy, is being provided appropriately.
In the CY 2020 HH PPS proposed rule (84 FR 34701), we discussed comments received on the CY 2019 HH PPS proposed rule solicitation of comments regarding whether and how prior authorization could potentially be applied under the home infusion benefit. We noted that the majority of commenters were concerned that applying prior authorization would risk denying or delaying timely access to needed services, as an expeditious transition of care is clinically and economically important in home infusion therapy.
Ultimately, we agreed with commenters and stated that we do not consider prior authorization to be appropriate for the home infusion therapy benefit at this time, as the benefit is contingent on the requirement that a home infusion drug or biological be administered through a Medicare Part B covered pump that is an item of DME. We stated that we will monitor the provision of home infusion therapy services and revisit the need for prior authorization if issues arise.
We received a few comments on the CY 2020 HH PPS proposed rule regarding the use of prior authorization for the home infusion therapy benefit in CY 2021:
Comment: A commenter stated that requiring prior authorization from the prescriber for home infusion therapy services will not improve the safety or efficacy of care, as site of care choices in this context are only initiated by the prescribing physician. The commenter stated that the home infusion therapy supplier cannot unilaterally switch the care setting, and stated that further mandating prior authorization only delays initiation of home infusion therapy for the patient and adds administrative burden and costs to the process. Another commenter stated that implementing prior authorization for home infusion therapy, or any other home health service would be a duplication of physician effort (who have already determined reasonable and necessary), may result in delay of care, and potentially lead to a prior denial for legitimate care.
Response: We thank the commenters for their comments. As stated previously, we agree that prior authorization is not necessary for home infusion therapy at this time, but will continue to monitor the provision of home infusion therapy services and revisit the need for prior authorization if issues arise.
2. Payments for High-Cost Outliers for Home Infusion Therapy Services
Section 1834(u)(1)(C) of the Act allows for discretionary adjustments which may include outlier situations and other factors as the Secretary determines appropriate. In the 2020 HH PPS proposed rule (84 FR 34701) we discussed comments received on the CY 2019 HH PPS proposed rule, regarding situations that may incur an outlier payment and potential designs for an outlier payment calculation. We stated that we planned to monitor the need for such payment and if necessary address outlier situations in future rule making. We received a comment regarding outliers for home infusion therapy services.
Comment: MedPAC suggested that although it may be premature to develop a system of outliers, developing such a system would be preferable to increasing aggregate payments for the purpose of addressing cost variation.
Response: We thank MedPAC for this recommendation and will pay close attention to any situations that would potentially be appropriate for an outlier payment, and if necessary address these situations in future rulemaking.
G. Billing Procedures for CY 2021 Home Infusion Therapy Services
Finally, in the CY 2020 HH PPS proposed rule we discussed billing procedures for home infusion therapy services for CY 2021 and subsequent years. We stated that because a qualified home infusion therapy supplier is only required to enroll in Medicare as a Part B supplier, and is not required to enroll as a DME supplier, it is more practicable to process home infusion therapy service claims through the A/B MACs and the Multi-Carrier System (MCS) for Medicare Part B claims. DME suppliers, also enrolled as qualified home infusion therapy suppliers, would continue to submit DME claims through the DME MACs; however, they would also be required to submit home infusion therapy service claims to the A/B MACs for processing. Therefore, the qualified home infusion therapy supplier will submit all home infusion therapy service claims on the 837P/CMS-1500 professional and supplier claims form to the A/B MACs. DME suppliers, concurrently enrolled as qualified home infusion therapy suppliers, would need to submit one claim for the DME, supplies, and drug on the 837P/CMS-1500 professional and supplier claims form to the DME MAC and a separate 837P/CMS-1500 professional and supplier claims form for the home infusion therapy professional services to the A/B MAC. We stated that because the home infusion therapy services are contingent upon a home infusion drug J-code being billed, home infusion therapy suppliers must ensure that the appropriate drug associated with the visit is billed with the visit or no more than 30 days prior to the visit. We also plan to add the home infusion G-codes to the PFS, incorporating the required annual and geographic wage adjustments. Home infusion therapy suppliers will include a modifier on the appropriate G-code to differentiate the first visit from all subsequent visits, as well as a modifier to indicate when a patient has been discharged from service. We will issue a Change Request (CR) providing more detailed instruction regarding billing and policy information for home infusion therapy services, prior to implementation of the CY 2021 home infusion benefit.
Comment: Several commenters had concerns about the home infusion therapy supplier enrollment process with the A/B MACs, as the majority of suppliers are only enrolled as DME suppliers and only bill the DME MACs. They stated that the 855B A/B enrollment form does not include a category for “home infusion therapy supplier” and urged CMS to offer enrollment guidance. Commenters also pointed out that the DME supplier is not required to be in the same state as the patient, which allows the supplier to distribute drugs and supplies across a broad geographical region, thereby allowing continued service for Medicare beneficiaries who spend parts of the year in different states. They encouraged CMS to ensure that home infusion therapy suppliers are able to enroll in such a way that they can identify their pharmacy as a practice location and base-operation from which they schedule and dispatch nursing related home infusion services; allow for jurisdictional enrollment and billing of HIT services without the requirement to have a physical location within the jurisdiction; and allow for DME suppliers, also accredited as qualified home infusion therapy suppliers, to complete a single A/B MAC application identifying all areas that they schedule and dispatch the nursing component of home infusion therapy.
Response: We thank commenters for their review of the billing procedures outlined in the proposed rule. We recognize that the enrollment process will be new for the DME suppliers enrolling concurrently as home infusion therapy suppliers; however, we encourage commenters not to conflate DME suppliers with home infusion therapy suppliers. The DME taxonomy code, which, as the commenter pointed out, allows for pharmacy-based, decentralized patient care that does not require a physical brick-and mortar location, will not be affected by the requirement for home infusion therapy suppliers enrollment through the A/B MACs. DME suppliers are not required to enroll with the A/B MACs but instead they will continue to enroll with the National Supplier Clearinghouse, and their billing processes for equipment and supplies, including infusion drugs, will not change. Only if they become accredited as a home infusion therapy supplier, would they complete an additional enrollment with the A/B MACs in order to submit home infusion therapy service claims. We do understand that some current DME suppliers enrolling as home infusion therapy suppliers may not have brick-and-mortar locations per the A/B MAC requirements; however, and plan to issue more complete guidance for these providers.
We also recognize there is currently not a “home infusion therapy supplier” type on the 855B enrollment form, and are considering creating one for home infusion supplier enrollment. In the meantime, providers can enroll using the “other” option. We are currently examining and working on all other aspects of the enrollment process and appreciate and will take all commenter suggestions under consideration as we continue developing guidance for suppliers.
VII. Waiver of Proposed Rulemaking
We ordinarily publish a notice of proposed rulemaking in the Federal Register and invite public comment before the provisions of a rule take effect in accordance with section 553(b) of the Administrative Procedure Act (APA) (5 U.S.C. 553(b)). However, we can waive this notice and comment procedure if the Secretary finds, for good cause, that the notice and comment process is impracticable, unnecessary, or contrary to the public interest, and incorporates a statement of the finding and the reasons therefore in the rule. This home health proposed rule has previously been subjected to notice and comment procedures. These corrections do not make substantive changes to this policy. Specifically, we amended the definition of “applicable provider” at § 486.505 to read “nurse practitioner” rather than “nurse provider.” Additionally, we amended § 414.1550(a)(1) and (2) to include “or service”. The specific changes we are making in the regulations are simply technical corrections in the language and do not reflect any additional substantive changes. Therefore, we find that undertaking further notice and comment procedures to incorporate these corrections into the CY 2020 final rule with comment period is unnecessary and contrary to the public interest.
Additionally, we are finalizing the submission of a “no-pay” RAP within five calendar days after the start of each 30-day period of care for CY 2021. We are also finalizing to apply a payment reduction if the “no-pay” RAP is not submitted timely. These changes were not proposed in the proposed rule, however, we are adopting the change here under a “good cause” waiver of proposed rulemaking. The specific changes we are making are in accordance with the proposed NOA policy for CY 2021. However, we are delaying the submission of a NOA until CY 2022 to allow sufficient time to make system changes to accommodate the NOA process. We note that if the NOA policy would have been finalized for CY 2021, the payment reduction for an untimely filed NOA would also be applied. Therefore, finalizing a “no-pay” RAP policy, as opposed to a NOA policy, with an untimely submission payment reduction in CY 2021 does not reflect any additional substantive changes to what was proposed. Therefore, we find that undertaking further notice and comment procedures to incorporate this correction into the final rule with comment period is unnecessary and contrary to the public interest.
VIII. Collection of Information Requirements
Under the Paperwork Reduction Act of 1995, we are required to provide 30-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the Office of Management and Budget (OMB) for review and approval. In order to fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the Paperwork Reduction Act of 1995 requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
In section V. of this final rule with comment period, we are finalizing our proposed updates to the HH QRP with the exception of the removal of Question 10 from all HHCAHPS survey as discussed in Section V.K. We believe that the burden associated with the HH QRP provisions is the time and effort associated with data collection and reporting. As of February 1, 2019, there are approximately 11,385 HHAs reporting quality data to CMS under the HH QRP. For the purposes of calculating the costs associated with the collection of information requirements, we obtained mean hourly wages for these staff from the U.S. Bureau of Labor Statistics' May 2018 National Occupational Employment and Wage Estimates (https://www.bls.gov/oes/current/oes_nat.htm). To account for overhead and fringe benefits (100 percent), we have doubled the hourly wage. These amounts are detailed in Table 33.

As discussed in section V.D. of this final rule with comment period, we are finalizing the removal of the Improvement in Pain Interfering with Activity Measure (NQF #0177) from the HH QRP beginning with the CY 2022 HH QRP under our measure removal Factor 7: Collection or public reporting of a measure leads to negative unintended consequences other than patient harm. Additionally, we finalized the removal of OASIS item M1242. Removing M1242 will result in a decrease in burden of 0.3 minutes of clinical staff time to report data at start of care (SOC), 0.3 minutes of clinical staff time to report data at resumption of care (ROC) and 0.3 minutes of clinical staff time to report data at Discharge.
As discussed in section V.E. of this final rule with comment period, we are finalizing the adoption of two new measures: (1) Transfer of Health Information to Provider-Post-Acute Care (PAC); and (2) Transfer of Health Information to Patient-Post-Acute Care (PAC), beginning with the CY 2022 HH QRP. We estimate the data elements for the Transfer of Health Information quality measures will take 0.6 minutes of clinical staff time to report data at Discharge and 0.3 minutes of clinical staff time to report data at Transfer of Care (TOC).
In section V.G. of this final rule with comment period, we are finalizing the collection of standardized patient assessment data beginning with the CY 2022 HH QRP. We estimate the SPADEs will take 10.05 minutes of clinical staff time to report data at SOC, 9.15 minutes of clinical staff time to report at ROC, and 10.95 minutes of clinical staff time to report data at Discharge.
We estimate that there would be a net increase in clinician burden per OASIS assessment of 9.75 minutes at SOC, 8.85 minutes at ROC, 0.3 minutes at TOC, and 11.25 minutes at Discharge as a result of the HH QRP proposals finalized in this rule.
The OASIS is completed by RNs or PTs, or very occasionally by occupational therapists (OT) or speech language pathologists (SLP/ST). Data from 2018 show that the SOC/ROC OASIS is completed by RNs (approximately 84.5 percent of the time), PTs (approximately 15.2 percent of the time), and other therapists, including OTs and SLP/STs (approximately 0.3 percent of the time). Based on this analysis, we estimated a weighted clinician average hourly wage of $74.58, inclusive of fringe benefits, using the hourly wage data in Table 33. Individual providers determine the staffing resources necessary.
Table 34 shows the total number of OASIS assessments submitted by HHAs in CY 2018 and estimated burden at each time point.

Based on the data in Table 34, for the 11,385 active Medicare-certified HHAs in February 2019, we estimate the total average increase in cost associated with changes to the HH QRP at approximately $15,081.76 per HHA annually, or $171,705,794.10 for all HHAs annually. This corresponds to an estimated increase in clinician burden associated with changes to the HH QRP of approximately 202.2 hours per HHA annually, or 2,302,303.5 hours for all HHAs annually. This estimated increase in burden will be accounted for in the information collection under OMB control number 0938-1279.
IX. Regulatory Impact Analysis
A. Statement of Need
1. Home Health Prospective Payment System (HH PPS)
Section 1895(b)(1) of the Act requires the Secretary to establish a HH PPS for all costs of home health services paid under Medicare. In addition, section 1895(b) of the Act requires: (1) The computation of a standard prospective payment amount include all costs for home health services covered and paid for on a reasonable cost basis and that such amounts be initially based on the most recent audited cost report data available to the Secretary; (2) the prospective payment amount under the HH PPS to be an appropriate unit of service based on the number, type, and duration of visits provided within that unit; and (3) the standardized prospective payment amount be adjusted to account for the effects of case-mix and wage levels among HHAs. Section 1895(b)(3)(B) of the Act addresses the annual update to the standard prospective payment amounts by the HH applicable percentage increase. Section 1895(b)(4) of the Act governs the payment computation. Sections 1895(b)(4)(A)(i) and (b)(4)(A)(ii) of the Act requires the standard prospective payment amount to be adjusted for case-mix and geographic differences in wage levels. Section 1895(b)(4)(B) of the Act requires the establishment of appropriate case-mix adjustment factors for significant variation in costs among different units of services. Lastly, section 1895(b)(4)(C) of the Act requires the establishment of wage adjustment factors that reflect the relative level of wages, and wage-related costs applicable to home health services furnished in a geographic area compared to the applicable national average level.
Section 1895(b)(3)(B)(iv) of the Act provides the Secretary with the authority to implement adjustments to the standard prospective payment amount (or amounts) for subsequent years to eliminate the effect of changes in aggregate payments during a previous year or years that were the result of changes in the coding or classification of different units of services that do not reflect real changes in case-mix. Section 1895(b)(5) of the Act provides the Secretary with the option to make changes to the payment amount otherwise paid in the case of outliers because of unusual variations in the type or amount of medically necessary care. Section 1895(b)(3)(B)(v) of the Act requires HHAs to submit data for purposes of measuring health care quality, and links the quality data submission to the annual applicable percentage increase. Section 50208 of the BBA of 2018 (Pub. L. 115-123) requires the Secretary to implement a new methodology used to determine rural add-on payments for CYs 2019 through 2022.
Sections 1895(b)(2) and 1895(b)(3)(A) of the Act, as amended by section 51001(a)(1) and 51001(a)(2) of the BBA of 2018 respectively, require the Secretary to implement a 30-day unit of service, effective for CY 2020, and calculate a 30-day payment amount for CY 2020 in a budget neutral manner, respectively. In addition, section 1895(b)(4)(B) of the Act, as amended by section 51001(a)(3) of the BBA of 2018 requires the Secretary to eliminate the use of the number of therapy visits provided to determine payment, also effective for CY 2020.
2. HHVBP
The HHVBP Model applies a payment adjustment based on an HHA's performance on quality measures to test the effects on quality and expenditures.
3. HH QRP
Section 1895(b)(3)(B)(v) of the Act requires HHAs to submit data for purposes of measuring heath care quality, and links the quality data submission to the annual applicable percentage increase.
4. Home Infusion Therapy
Section 1834(u)(1) of the Act, as added by section 5012 of the 21st Century Cures Act, requires the Secretary to establish a home infusion therapy services payment system under Medicare. Under this payment system a single payment would be made to a qualified home infusion therapy supplier for items and services furnished by a qualified home infusion therapy supplier in coordination with the furnishing of home infusion drugs. Section 1834(u)(1)(A)(ii) of the Act states that a unit of single payment is for each infusion drug administration calendar day in the individual's home. The Secretary shall, as appropriate, establish single payment amounts for types of infusion therapy, including to take into account variation in utilization of nursing services by therapy type. Section 1834(u)(1)(A)(iii) of the Act provides a limitation to the single payment amount, requiring that it shall not exceed the amount determined under the Physician Fee Schedule (under section 1848 of the Act) for infusion therapy services furnished in a calendar day if furnished in a physician office setting, except such single payment shall not reflect more than 5 hours of infusion for a particular therapy in a calendar day. Section 1834(u)(1)(B)(i) of the Act requires that the single payment amount be adjusted by a geographic wage index. Finally, section 1834(u)(1)(C) of the Act allows for discretionary adjustments which may include outlier payments and other factors as deemed appropriate by the Secretary, and are required to be made in a budget neutral manner. This payment system would become effective for home infusion therapy items and services furnished on or after January 1, 2021, and is not reflective of cost estimates for CY 2020.
Section 50401 of the BBA of 2018 amended section 1834(u) of the Act, by adding a new paragraph (7). The paragraph establishes a home infusion therapy temporary transitional payment for eligible home infusion therapy suppliers for items and services associated with the furnishing of transitional home infusion drugs for CYs 2019 and 2020. Under this payment methodology (as described in section VI.B. of this final rule with comment period), the Secretary established three payment categories at amounts equal to the amounts determined under the Physician Fee Schedule established under section 1848 of the Act. This rule continues this categorization for services furnished during CY 2020 for codes and units of such codes, determined without application of the geographic adjustment.
B. Overall Impact
We have examined the impacts of this rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96-354), section 1102(b) of the Social Security Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4), Executive Order 13132 on Federalism (August 4, 1999), the Congressional Review Act (5 U.S.C. 804(2)), and Executive Order 13771 on Reducing Regulation and Controlling Regulatory Costs (January 30, 2017).
A regulatory impact analysis (RIA) must be prepared for major rules with economically significant effects ($100 million or more in any 1 year). We estimate that this rulemaking is “economically significant” as measured by the $100 million threshold, and hence also a major rule under the Congressional Review Act. Accordingly, we have prepared a Regulatory Impact Analysis that to the best of our ability presents the costs and benefits of the rulemaking.
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Section 3(f) of Executive Order 12866 defines a “significant regulatory action” as an action that is likely to result in a rule: (1) Having an annual effect on the economy of $100 million or more in any 1 year, or adversely and materially affecting a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or state, local or tribal governments or communities (also referred to as “economically significant”); (2) creating a serious inconsistency or otherwise interfering with an action taken or planned by another agency; (3) materially altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raising novel legal or policy issues arising out of legal mandates, the President's priorities, or the principles set forth in the Executive Order. Given that we note the follow costs associated with the provisions of this final rule with comment period:
- HH PPS—The net transfer impact related to the changes in payments under the HH PPS for CY 2020 is estimated to be $250 million (1.3 percent). This reflects the effects of the CY 2020 home health payment update percentage of 1.5 percent ($290 million increase), and a 0.2 percent decrease in payments due to the rural add-on percentages mandated by the Bipartisan Budget Act of 2018 for CY 2020 ($40 million decrease). The home health wage index update for CY 2020 and the updated FDL ratio that will be used for outlier payments in CY 2020 are both budget-neutral.
- HHVBP—The savings impacts related to the HHVBP Model as a whole are estimated at $378 million for CYs 2018 through 2022. We do not believe the policy finalized in this final rule with comment period would affect the prior estimate.
- HH QRP—The cost impact for HHA's related to proposed changes to the HH QRP are estimated at $167.8 million.
- Home Infusion Therapy—The CY 2020 cost impact related to the routine updates to the temporary transitional payments for home infusion therapy in CY 2020 is an estimated 1.9 percent, or $1.2 million, decrease in payments to home infusion therapy suppliers in CY 2020 based on the proposed CY 2020 Physician Fee Schedule (PFS) payment amounts for such services (the final CY 2020 PFS payment amounts were not available in time for this final rule with comment period). The cost impact in CY 2021 related to the implementation of the permanent home infusion therapy benefit is estimated to be a $2 million reduction in payments to home infusion therapy suppliers.
C. Anticipated Effects
1. HH PPS and Home Infusion Therapy
The RFA requires agencies to analyze options for regulatory relief of small entities, if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. Most hospitals and most other providers and suppliers are small entities, either by nonprofit status or by having revenues of less than $7.5 million to $38.5 million in any one year. For the purposes of the RFA, we estimate that almost all HHAs and home infusion therapy suppliers are small entities as that term is used in the RFA. Individuals and states are not included in the definition of a small entity. The economic impact assessment is based on estimated Medicare payments (revenues) and HHS's practice in interpreting the RFA is to consider effects economically “significant” only if greater than 5 percent of providers reach a threshold of 3 to 5 percent or more of total revenue or total costs. The majority of HHAs' visits are Medicare paid visits and therefore the majority of HHAs' revenue consists of Medicare payments. Based on our analysis, we conclude that the policies in this final rule with comment period will result in an estimated total impact of 3 to 5 percent or more on Medicare revenue for greater than 5 percent of HHAs and home infusions therapy suppliers. Therefore, the Secretary has determined that this HH PPS final rule with comment period will have a significant economic impact on a substantial number of small entities. We refer stakeholders to Tables 35 and 36 which contain some information on the numbers of small entities impacted by the rule.
In addition, section 1102(b) of the Act requires us to prepare a final RIA if a rule has a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 604 of RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a metropolitan statistical area and has fewer than 100 beds. This rule is not applicable to hospitals. Therefore, the Secretary has determined this final rule with comment period will not have a significant economic impact on the operations of small rural hospitals.
Section 202 of the Unfunded Mandates Reform Act of 1995 (UMRA) also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2019, that threshold is approximately $150 million. This rule is not anticipated to have an effect on State, local, or tribal governments, in the aggregate, or on the private sector of $150 million or more.
Executive Order 13132 establishes certain requirements that an agency must meet when it promulgates a proposed rule (and subsequent final rule) that imposes substantial direct requirement costs on state and local governments, preempts State law, or otherwise has Federalism implications. We have reviewed this final rule with comment period under these criteria of Executive Order 13132, and have determined that it will not impose substantial direct costs on state or local governments.
One commenter expressed concerns that CMS is not considering the requirements of the Regulatory Flexibility Act or the Small Business Regulatory Enforcement Fairness Act, which limits the impact on small businesses. We refer commenters to section III.B. of this final rule with comment period for our response to this comment.
2. HHVBP
Under the HHVBP Model, the first payment adjustment was applied in CY 2018 based on PY 1 (2016) data and the final payment adjustment will apply in CY 2022 based on PY 5 (2020) data. In the CY 2016 HH PPS final rule, we estimated that the overall impact of the HHVBP Model from CY 2018 through CY 2022 was a reduction of approximately $380 million (80 FR 68716). In the CYs 2017, 2018, and 2019 HH PPS final rules, we estimated that the overall impact of the HHVBP Model from CY 2018 through CY 2022 was a reduction of approximately $378 million (81 FR 76795, 82 FR 51751, and 83 FR 56593, respectively). We do not believe the policy that we are finalizing will affect the prior estimate.
3. HH QRP
Section VIII. of this final rule with comment period provides a detailed description of the net increase in burden associated with changes to the HH QRP. We have estimated this associated burden beginning with CY 2021 because HHAs will be required to submit data beginning with that calendar year. The cost impact related to OASIS item collection as a result of the changes to the HH QRP is estimated to be a net increase of approximately $171.7 million in annualized cost to HHAs, discounted at 7 percent relative to year 2016, over a perpetual time horizon beginning in CY 2021.
4. Regulatory Review Cost Estimation
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this final rule with comment period, we must estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that would review the rule, we assume that the total number of unique reviewers of this year's final rule with comment period would be the similar to the number of reviewers on last year's final rule with comment period. We acknowledge that this assumption may understate or overstate the costs of reviewing this rule. It is possible that not all commenters reviewed this year's rule with comment period in detail, and it is also possible that some reviewers chose not to comment on the proposed rule. For these reasons we believe that the number of past commenters would be a fair estimate of the number of reviewers of this rule. We also recognize that different types of entities are in many cases affected by mutually exclusive sections of this final rule with comment period, and therefore for the purposes of our estimate we assume that each reviewer reads approximately 50 percent of the rule. While we solicited comments on the approach in estimating the number of entities which would review the proposed rule and the assumption of how much of the rule reviewers would read, we did not receive any comments. Therefore, using the wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing this rule with comment period is $109.36 per hour, including overhead and fringe benefits (https://www.bls.gov/oes/current/oes_nat.htm). Assuming an average reading speed of 250 words per minute, we estimate that it would take approximately 5 hours for the staff to review half of this final rule with comment period, which consists of approximately 152,000 words. For each HHA that reviews the final rule with comment period, the estimated cost is $546.80 (5 hours × $109.36). Therefore, we estimate that the total cost of reviewing this final rule with comment period is $292,632 ($546.80 × 537 reviewers).
D. Detailed Economic Analysis
1. HH PPS
This final rule with comment period finalizes updates to Medicare payments under the HH PPS for the CY 2020. This rule with comment period also implements changes in the case-mix adjustment methodology for home health periods of care beginning on and after January 1, 2020 and implements the change in the unit of payment from 60-day episodes to 30-day periods. The impact analysis of this final rule with comment period presents the estimated expenditure effects of policy changes finalized in this rule. We use the latest data and best analysis available, but we do not make adjustments for future changes in such variables as number of visits or case-mix.
This analysis incorporates the latest estimates of growth in service use and payments under the Medicare HH benefit, based primarily on Medicare claims data from 2018. We note that certain events may combine to limit the scope or accuracy of our impact analysis, because such an analysis is future-oriented and, thus, susceptible to errors resulting from other changes in the impact time period assessed. Some examples of such possible events are newly-legislated general Medicare program funding changes made by the Congress, or changes specifically related to HHAs. In addition, changes to the Medicare program may continue to be made as a result of the Affordable Care Act, or new statutory provisions. Although these changes may not be specific to the HH PPS, the nature of the Medicare program is such that the changes may interact, and the complexity of the interaction of these changes could make it difficult to predict accurately the full scope of the impact upon HHAs.
Table 35 represents how HHA revenues are likely to be affected by the policy changes in this rule for CY 2020. For this analysis, we used an analytic file with linked CY 2018 OASIS assessments and HH claims data for dates of service that ended on or before December 31, 2018 (as of July 31, 2019). The first column of Table 35 classifies HHAs according to a number of characteristics including provider type, geographic region, and urban and rural locations. The second column shows the number of facilities in the impact analysis. The third column shows the payment effects of the CY 2020 wage index. The fourth column shows the payment effects of the CY 2020 rural add-on payment provision in statute. The fifth column shows the effects of the implementation of the PDGM case-mix methodology for CY 2020. The sixth column shows the payment effects of the CY 2020 home health payment update percentage as required by section 53110 of the BBA of 2018. And the last column shows the combined effects of all the policies finalized in this rule with comment period.
Overall, it is projected that aggregate payments in CY 2020 would increase by 1.3 percent. As illustrated in Table 35, the combined effects of all of the changes vary by specific types of providers and by location. We note that some individual HHAs within the same group may experience different impacts on payments than others due to the distributional impact of the CY 2020 wage index, the extent to which HHAs are affected by changes in case-mix weights between the current 153-group case-mix model and the case-mix weights under the 432-group PDGM, the percentage of total HH PPS payments that were subject to the low-utilization payment adjustment (LUPA) or paid as outlier payments, and the degree of Medicare utilization.


2. HHVBP
As discussed in section IV. of this final rule with comment period, for the HHVBP Model, we proposed and are finalizing the public reporting of certain performance data for PY 5 (CY 2020) of the Model. This finalized policy does not affect our analysis of the distribution of payment adjustments for PY 5 as presented in the CY 2019 HH PPS final rule with comment period. Therefore, we are not providing a detailed analysis.
3. HH QRP
Failure to submit data required under section 1895(b)(3)(B)(v) of the Act with respect to a calendar year will result in the reduction of the annual home health market basket percentage increase otherwise applicable to a HHA for that calendar year by 2 percentage points. For the CY 2019 payment determination, 1,286 of the 11,444 active Medicare-certified HHAs, or approximately 11.2 percent, did not receive the full annual percentage increase. Information is not available to determine the precise number of HHAs that would not meet the requirements to receive the full annual percentage increase for the CY 2020 payment determination.
As discussed in section V.D. of this final rule with comment period, we proposed to remove one measure beginning with the CY 2022 HH QRP. The measure we proposed to remove is Improvement in Pain Interfering with Activity Measure (NQF #0177). As discussed in section V.E. of this final rule with comment period, we proposed to add two measures beginning with the CY 2022 HH QRP. The two measures we proposed to adopt are: (1) Transfer of Health Information to Provider-Post-Acute Care; and (2) Transfer of Health Information to Patient-Post-Acute Care. As discussed in section V.G. of this final rule with comment period, we are also proposed to collect standardized patient assessment data beginning with the CY 2022 HH QRP. Section VII. of this final rule with comment period provides a detailed description of the net increase in burden associated with these proposed changes. We have estimated this associated burden beginning with CY 2021 because HHAs will be required to submit data beginning with that calendar year. The cost impact related to OASIS item collection as a result of the changes to the HH QRP is estimated to be a net increase of approximately $167.8 million in annualized cost to HHAs, discounted at 7 percent relative to year 2016, over a perpetual time horizon beginning in CY 2021.
4. Home Infusion Therapy Services Payment
a. Home Infusion Therapy Services Temporary Transitional Payment
The impact due to the updated payment amounts for furnishing home infusion therapy services is determined based on the rates published in the physician fee schedule established under section 1848 of the Act. At the time of publication of this final rule with comment period, the CY 2020 PFS final payment rates were not available. However, we estimate the impact in CY 2020, based on the CY 2020 PFS proposed rates, would result in a 1.9 percent decrease in overall payments for home infusion therapy suppliers receiving temporary transitional payments.
b. Home Infusion Therapy Services Payment for CY 2021 and Subsequent Years
The following analysis applies to payment for home infusion therapy as set forth in section 1834(u)(1) of the Act, as added by section 5012 of the 21st Century Cures Act (Pub. L. 114-255), and accordingly, describes the preliminary impact for CY 2021 only. We should also note that as payment amounts are contingent on the Physician Fee Schedule (PFS) rates, this impact analysis will be affected by whether rates increase or decrease in CY 2021. We used CY 2018 claims data to identify beneficiaries with DME claims containing 1 of the codes identified on the DME LCD for External Infusion Pumps (L33794), excluding drugs that are statutorily excluded from coverage under the permanent home infusion therapy benefit. These include insulin, drugs and biologicals listed on self-administered drug exclusion lists, and drugs administered by routes other than intravenous or subcutaneous infusion. Because we do not have complete data for CY 2019 (the first year of the temporary transitional payments), we used the visit assumptions identified in the CY 2019 HH PPS final rule with comment period. We calculated the total weeks of care, which is the sum of weeks of care across all beneficiaries found in each category (as determined from the CY 2018 claims). Weeks of care for categories 1 and 3 are defined as the week of the last infusion drug or pump claim minus the week of the first infusion drug or pump claim plus one. Additionally for these categories, we assumed 2 visits for the initial week of care, with 1 visit per week for all subsequent weeks in order to estimate the total visits of care per category. For category 2, we assumed 1 visit per month, or 12 visits per year. For this analysis, we did not factor in an increase in beneficiaries receiving home infusion therapy services due to switching from physician's offices or outpatient centers. Because home infusion therapy services under Medicare are contingent on utilization of the DME benefit, we anticipate utilization will remain fairly stable and that there will be no significant changes in the settings of care where current infusion therapy is provided. We will continue to monitor utilization to determine if referral patterns change significantly during the temporary transitional payment period, and once the permanent benefit is implemented in CY 2021.
Table 36 reflects the estimated wage-adjusted beneficiary impact, representative of a 4-hour payment rate, compared to a 5-hour payment rate, excluding statutorily excluded drugs and biologicals. Column 3 represents the percent change from the estimated CY 2020 transitional payment to the estimated CY 2021 payment after applying the geographic adjustment factor (GAF). Column 4 represents the percent change from the estimated CY 2021 payment after applying the GAF to the estimated CY 2021 payment after removing the statutorily excluded drugs and biologicals. Column 5 represents the percent change from the estimated CY 2021 payment after applying the GAF and removing the statutorily excluded drugs and biologicals to the estimated CY 2021 payment, and after applying the higher reimbursement rate. Overall, we estimate a 3.6 percent decrease ($2 million) in payments to home infusion therapy suppliers in CY 2021.

E. Alternatives Considered
1. HH PPS
This final rule with comment period contains a range of policies, including some provisions related to specific statutory provisions. The preceding preamble provides descriptions of the statutory provisions that are addressed, identifies those policies when discretion has been exercised, presents rationale for our final policies and, where relevant, alternatives that were considered.
2. HHVBP
With regard to our proposal to publicly report on the CMS website the CY 2020 (PY 5) Total Performance Score (TPS) and the percentile ranking of the TPS for each competing HHA that qualifies for a payment adjustment in CY 2020, we also considered not making this Model performance data public, and whether there was any potential cost to stakeholders and beneficiaries if the data were to be misinterpreted. However, for the reasons discussed in section IV. of this final rule with comment period, we are finalizing the public reporting of the HHVBP Model performance data for PY 5 as proposed. We believe that providing definitions for the HHVBP TPS and the TPS Percentile Ranking methodology would address any such concerns by ensuring the public understands the relevance of these data points and how they were calculated. We also considered the financial costs associated with our proposal to publicly report HHVBP data, but do not anticipate such costs to CMS, stakeholders or beneficiaries, as CMS already calculates and reports the TPS and TPS Percentile Ranking in the Annual Reports to HHAs. As discussed in section IV of this final rule with comment period, we believe the public reporting of such data would further enhance quality reporting under the Model by encouraging participating HHAs to provide better quality of care through focusing on quality improvement efforts that could potentially improve their TPS. In addition, we believe that publicly reporting performance data that indicates overall performance may assist beneficiaries, physicians, discharge planners, and other referral sources in choosing higher-performing HHAs within the nine Model states and allow for more meaningful and objective comparisons among HHAs on their level of quality relative to their peers.
3. HH QRP
We believe that removing the Pain Interfering with Activity Measure (NQF #0177) from the HH QRP beginning with the CY 2022 HH QRP would reduce negative unintended consequences. We proposed the removal of the measure under Meaningful Measures Initiative measure removal Factor 7: Collection or public reporting of a measure leads to negative unintended consequences other than patient harm. We considered alternatives to this measure and no appropriate alternative measure is ready at this time. Out of an abundance of caution to potential harm from over-prescription of opioid medications inadvertently driven by this measure, we have determined that removing the current pain measure is the most appropriate provision.
The finalization of the proposed adoption of two transfer of health information process measures is vital to satisfying section 1899B(c)(1)(E)(ii) of the Act, which requires that the quality measures specified by the Secretary include measures with respect to the quality measure domain of accurately communicating the existence of and providing for the transfer of health information and care preferences of an individual when the individual transitions from a PAC provider to another applicable setting. We believe adopting these measures best addresses the requirements of the IMPACT Act for this domain. We considered not adopting these proposals and doing additional analyses for a future implementation. This approach was not viewed as a viable alternative because of the extensive effort invested in creating the best measures possible and failure to adopt measures in the domain of transfer of health information puts CMS at risk of not meeting the legislative mandate of the IMPACT Act.
Collecting and reporting standardized patient assessment data under the HH QRP is required under section 1899B(b)(1) of the Act. We have carefully considered assessment items for each of the categories of assessment data and believe these proposals best addressed the requirements of the Act for the HH QRP. The proposed SPADEs are items that received additional national testing after they were proposed in the CY 2018 HH PPS proposed rule (82 FR 35354 through 35371) and more extensively vetted. These items have been carefully considered and the alternative of not proposing to adopt standardized patient assessment data will result in CMS not meeting our legislative mandate under the IMPACT Act.
4. Home Infusion Therapy
This final rule with comment period contains a range of policies, including some provisions related to specific statutory provisions. The preceding preamble provides descriptions of the statutory provisions that are addressed, identifies those policies when discretion has been exercised, presents rationale for our final policies and, where relevant, alternatives that were considered.
F. Accounting Statement and Tables
As required by OMB Circular A-4 (available at https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/circulars/A4/a-4.pdf), in Table 37, we have prepared an accounting statement showing the classification of the transfers and costs associated with the CY 2020 HH PPS provisions of this rule. Table 38 shows the burden to HHA's for submission of OASIS. Table 39 provides our best estimate of the increase in Medicare payments to home infusion therapy suppliers for home infusion therapy beginning in CY 2021.



G. Regulatory Reform Analysis Under E.O. 13771
Executive Order 13771, entitled “Reducing Regulation and Controlling Regulatory Costs,” was issued on January 30, 2017 and requires that the costs associated with significant new regulations “shall, to the extent permitted by law, be offset by the elimination of existing costs associated with at least two prior regulations.” This final rule with comment period is considered an E.O. 13771 regulatory action. We estimate the rule generates $169.9 million in annualized costs in 2016 dollars, discounted at 7 percent relative to year 2016 over a perpetual time horizon. Details on the estimated costs of this rule can be found in the preceding and subsequent analyses.
H. Conclusion
1. HH PPS for CY 2020
In conclusion, we estimate that the net impact of the HH PPS policies in this rule is an increase of 1.3 percent, or $250 million, in Medicare payments to HHAs for CY 2020. This reflects the effects of the CY 2020 home health payment update percentage of 1.5 percent ($290 million increase), and a 0.2 percent decrease in payments due to the declining rural add-on percentages mandated by the Bipartisan Budget Act of 2018 for CY 2020 ($40 million decrease). The home health wage index update for CY 2020 and the updated FDL ratio that will be used for outlier payments in CY 2020 are both budget-neutral. Effects of the implementation of the PDGM and the change to a 30-day unit of payment are also budget-neutral.
2. HHVBP
In conclusion, as noted previously for the HHVBP Model, we are finalizing our proposal to publicly report performance data for PY 5 (CY 2020) of the Model. This finalized policy does not affect our analysis of the distribution of payment adjustments for PY 5 as presented in the CY 2019 HH PPS final rule with comment period.
We estimate there would be no net impact (to include either a net increase or reduction in payments) for this final rule with comment period in Medicare payments to HHAs competing in the HHVBP Model. However, the overall economic impact of the HHVBP Model is an estimated $378 million in total savings from a reduction in unnecessary hospitalizations and SNF usage as a result of greater quality improvements in the home health industry over the life of the HHVBP Model.
3. HH QRP
In conclusion, we estimate that the changes to OASIS item collection as a result of the changes to the HH QRP effective on January 1, 2021 result in a net additional annualized cost of $167.8 million, discounted at 7 percent relative to year 2016, over a perpetual time horizon beginning in CY 2021.
4. Home Infusion Therapy
a. Home Infusion Therapy Services Temporary Transitional Payment for CY 2020
In conclusion, we estimate a 1.9 percent, or $1.2 million, decrease in payments to home infusion therapy suppliers in CY 2020 based on the proposed CY 2020 Physician Fee Schedule (PFS) payment amounts for such services established under section 1848 of the Act (the final CY 2020 PFS payment amounts were not available in time for this final rule with comment period).
b. Home Infusion Therapy Services Payment for CY 2021
In conclusion, we estimate that the net impact of the payment for home infusion therapy services for CY 2021 is approximately $2 million in reduced payments to home infusion therapy suppliers.
This analysis, together with the remainder of this preamble, provides an initial Regulatory Flexibility Analysis.
In accordance with the provisions of Executive Order 12866, this final rule with comment period was reviewed by the OMB.
List of Subjects
42 CFR Part 409
- Health facilities
- Medicare
42 CFR Part 414
- Administrative practice and procedure
- Health facilities
- Health professions
- Kidney diseases
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 484
- Health facilities
- Health professions
- Medicare
- Reporting and recordkeeping requirements
42 CFR Part 486
- Grant programs—health
- Health facilities
- Medicare
- Reporting and recordkeeping requirements
- X-rays
For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services amends 42 CFR chapter IV as follows:
PART 409—HOSPITAL INSURANCE BENEFITS
1. The authority citation for part 409 continues to read as follows:
2. Section 409.43 is amended by revising paragraph (a) to read as follows:
(a) Contents. An individualized plan of care must be established and periodically reviewed by the certifying physician.
(1) The HHA must be acting upon a physician plan of care that meets the requirements of this section for HHA services to be covered.
(2) For HHA services to be covered, the individualized plan of care must specify the services necessary to meet the patient-specific needs identified in the comprehensive assessment.
(3) The plan of care must include the identification of the responsible discipline(s) and the frequency and duration of all visits as well as those items listed in § 484.60(a) of this chapter that establish the need for such services. All care provided must be in accordance with the plan of care.
3. Section 409.44 is amended by revising paragraph (c)(2)(iii)(C) to read as follows:
(c) * * *
(2) * * *
(iii) * * *
(C) The unique clinical condition of a patient may require the specialized skills of a qualified therapist or therapist assistant to perform a safe and effective maintenance program required in connection with the patient's specific illness or injury. Where the clinical condition of the patient is such that the complexity of the therapy services required—
(1) Involve the use of complex and sophisticated therapy procedures to be delivered by the therapist or the therapist assistant in order to maintain function or to prevent or slow further deterioration of function; or
(2) To maintain function or to prevent or slow further deterioration of function must be delivered by the therapist or the therapist assistant in order to ensure the patient's safety and to provide an effective maintenance program, then those reasonable and necessary services must be covered.
PART 414—PAYMENT FOR PART B MEDICAL AND OTHER HEALTH SERVICES
4. The authority citation for part 414 continues to read as follows:
5. Add subpart P to read as follows:
- 414.1500
- Basis, purpose, and scope.
- 414.1505
- Requirement for payment.
- 414.1510
- Beneficiary qualifications for coverage of services.
- 414.1515
- Plan of care requirements.
- 414.1550
- Basis of payment.
Subpart P—Home Infusion Therapy Services Payment
Conditions for Payment
This subpart implements section 1861(iii) of the Act with respect to the requirements that must be met for Medicare payment to be made for home infusion services furnished to eligible beneficiaries.
In order for home infusion therapy services to qualify for payment under the Medicare program the services must be furnished to an eligible beneficiary by, or under arrangements with, a qualified home infusion therapy supplier that meets the following requirements:
(a) The health and safety standards for qualified home infusion therapy suppliers at § 486.520(a) through (c) of this chapter.
(b) All requirements set forth in §§ 414.1510 through 414.1550.
To qualify for Medicare coverage of home infusion therapy services, a beneficiary must meet each of the following requirements:
(a) Under the care of an applicable provider. The beneficiary must be under the care of an applicable provider, as defined in section 1861(iii)(3)(A) of the Act as a physician, nurse practitioner, or physician assistant.
(b) Under a physician plan of care. The beneficiary must be under a plan of care that meets the requirements for plans of care specified in § 414.1515.
(a) Contents. The plan of care must contain those items listed in § 486.520(b) of this chapter that specify the standards relating to a plan of care that a qualified home infusion therapy supplier must meet in order to participate in the Medicare program.
(b) Physician's orders. The physician's orders for services in the plan of care must specify at what frequency the services will be furnished, as well as the discipline that will furnish the ordered professional services. Orders for care may indicate a specific range in frequency of visits to ensure that the most appropriate level of services is furnished.
(c) Plan of care signature requirements. The plan of care must be signed and dated by the ordering physician prior to submitting a claim for payment. The ordering physician must sign and date the plan of care upon any changes to the plan of care.
Payment System
(a) General rule. For home infusion therapy services furnished on or after January 1, 2021, Medicare payment is made on the basis of 80 percent of the lesser of the following:
(1) The actual charge for the item or service.
(2) The fee schedule amount for the item or service, as determined in accordance with the provisions of this section.
(b) Unit of single payment. A unit of single payment is made for items and services furnished by a qualified home infusion therapy supplier per payment category for each infusion drug administration calendar day, as defined at § 486.505 of this chapter.
(c) Initial establishment of the payment amounts. In calculating the initial single payment amounts for CY 2021, CMS determined such amounts using the equivalent to 5 hours of infusion services in a physician's office as determined by codes and units of such codes under the annual fee schedule issued under section 1848 of the Act as follows:
(1) Category 1. (i) Includes certain intravenous infusion drugs for therapy, prophylaxis, or diagnosis, including antifungals and antivirals; inotropic and pulmonary hypertension drugs; pain management drugs; chelation drugs; and other intravenous drugs as added to the durable medicare equipment local coverage determination (DME LCD) for external infusion pumps.
(ii) Payment equals 1 unit of 96365 plus 4 units of 96366.
(2) Category 2. (i) Includes certain subcutaneous infusion drugs for therapy or prophylaxis, including certain subcutaneous immunotherapy infusions.
(ii) Payment equals 1 unit of 96369 plus 4 units of 96370.
(3) Category 3. (i) Includes intravenous chemotherapy infusions, including certain chemotherapy drugs and biologicals.
(ii) Payment equals 1 unit of 96413 plus 4 units of 96415.
(4) Initial visit. (i) For each of the three categories listed in paragraphs (c)(1) through (3) of this section, the payment amounts are set higher for the first visit by the qualified home infusion therapy supplier to initiate the furnishing of home infusion therapy services in the patient's home and lower for subsequent visits in the patient's home. The difference in payment amounts is a percentage based on the relative payment for a new patient rate over an existing patient rate using the annual physician fee schedule evaluation and management payment amounts for a given year and calculated in a budget neutral manner.
(ii) The first visit payment amount is subject to the following requirements if a patient has previously received home infusion therapy services:
(A) The previous home infusion therapy services claim must include a patient status code to indicate a discharge.
(B) If a patient has a previous claim for HIT services, the first visit home infusion therapy services claim subsequent to the previous claim must show a gap of more than 60 days between the last home infusion therapy services claim and must indicate a discharge in the previous period before a HIT supplier may submit a home infusion therapy services claim for the first visit payment amount.
(d) Required payment adjustments. The single payment amount represents payment in full for all costs associated with the furnishing of home infusion therapy services and is subject to the following adjustments:
(1) An adjustment for a geographic wage index and other costs that may vary by region, using an appropriate wage index based on the site of service of the beneficiary.
(2) Beginning in 2022, an annual increase in the single payment amounts from the prior year by the percentage increase in the Consumer Price Index (CPI) for all urban consumers (United States city average) for the 12-month period ending with June of the preceding year.
(3)(i) An annual reduction in the percentage increase described in paragraph (d)(2) of this section by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act.
(ii) The application of the paragraph (c)(3)(i) of this section may result in the both of the following:
(A) A percentage being less than zero for a year.
(B) Payment being less than the payment rates for the preceding year.
(e) Medical review. All payments under this system may be subject to a medical review adjustment reflecting the following:
(1) Beneficiary eligibility.
(2) Plan of care requirements.
(3) Medical necessity determinations.
PART 484—HOME HEALTH SERVICES
6. The authority citation for part 484 continues to read as follows:
7. Section 484.202 is amended by adding the definitions of “HHCAHPS” and “HH QRP” in alphabetical order to read as follows:
HHCAHPS stands for Home Health Care Consumer Assessment of Healthcare Providers and Systems.
HH QRP stands for Home Health Quality Reporting Program.
8. Section 484.205 is amended by—
a. Revising paragraph (g)(2);
b. Adding paragraphs (g)(3) and (4);
c. Revising the heading for paragraph (h); and
d. Adding paragraphs (i) and (j).
The revisions and additions read as follows:
(g) * * *
(2) Split percentage payments for periods beginning on or after January 1, 2020 through December 31, 2020—(i) HHAs certified for participation on or before December 31, 2018. (A) The initial payment for all 30-day periods is paid to an HHA at 20 percent of the case-mix and wage-adjusted 30-day payment rate.
(B) The residual final payment for all 30-day periods is paid at 80 percent of the case-mix and wage-adjusted 30-day payment rate.
(ii) HHAs certified for participation in Medicare on or after January 1, 2019. Split percentage payments are not made to HHAs that are certified for participation in Medicare effective on or after January 1, 2019. Newly enrolled HHAs must submit a request for anticipated payment, which is set at 0 percent, at the beginning of every 30-day period. An HHA that is certified for participation in Medicare effective on or after January 1, 2019 receives a single payment for a 30-day period of care after the final claim is submitted.
(3) Split percentage payments for periods beginning on or after January 1, 2021 through December 31, 2021. All HHAs must submit a request for anticipated payment within 5 calendar days after the start of care date for initial 30-day periods and within 5 calendar days after the “from date” for each subsequent 30-day period of care, which is set at 0 percent at the beginning of every 30-day period. HHAs receive a single payment for a 30-day period of care after the final claim is submitted.
(4) Payments for periods beginning on or after January 1, 2022. All HHAs must submit a Notice of Admission (NOA) at the beginning of the initial 30-day period of care as described in paragraph (j) of this section. HHAs receive a single payment for a 30-day period of care after the final claim is submitted.
(h) Requests for anticipated payment (RAP) for 30-day periods of care starting on January 1, 2020 through December 31, 2020. * * *
(i) Submission of RAPs for CY 2021—(1) General. All HHAs must submit a RAP, which is to be paid at 0 percent, within 5 calendar days after the start of care and within 5 calendar days after the “from date” for each subsequent 30-day period of care.
(2) Criteria for RAP submission for CY 2021. The HHA shall submit RAPs only when all of the following conditions are met:
(i) Once physician's written or verbal orders that contain the services required for the initial visit have been received and documented as required at §§ 484.60(b) and 409.43(d) of this chapter.
(ii) The initial visit within the 60-day certification period must have been made and the individual admitted to home health care.
(3) Consequences of failure to submit a timely RAP. When a home health agency does not file the required RAP for its Medicare patients within 5 calendar days after the start of each 30-day period of care—
(i) Medicare does not pay for those days of home health services based on the “from date” on the claim to the date of filing of the RAP;
(ii) The wage and case-mix adjusted 30-day period payment amount is reduced by 1/30th for each day from the home health based on the “from date” on the claim until the date of filing of the RAP;
(iii) No LUPA payments are made that fall within the late period;
(iv) The payment reduction cannot exceed the total payment of the claim; and
(v)(A) The non-covered days are a provider liability; and
(B) The provider must not bill the beneficiary for the non-covered days.
(4) Exception to the consequences for filing the RAP late. (i) CMS may waive the consequences of failure to submit a timely-filed RAP specified in paragraph (i)(3) of this section.
(ii) CMS determines if a circumstance encountered by a home health agency is exceptional and qualifies for waiver of the consequence specified in paragraph (i)(3) of this section.
(iii) A home health agency must fully document and furnish any requested documentation to CMS for a determination of exception. An exceptional circumstance may be due to, but is not limited to the following:
(A) Fires, floods, earthquakes, or similar unusual events that inflict extensive damage to the home health agency's ability to operate.
(B) A CMS or Medicare contractor systems issue that is beyond the control of the home health agency.
(C) A newly Medicare-certified home health agency that is notified of that certification after the Medicare certification date, or which is awaiting its user ID from its Medicare contractor.
(D) Other situations determined by CMS to be beyond the control of the home health agency.
(j) Submission of Notice of Admission (NOA)—(1) For periods of care that begin on and after January 1, 2022. For all 30-day periods of care after January 1, 2022, all HHAs must submit a Notice of Admission (NOA) to their Medicare contractor within 5 calendar days after the start of care date. The NOA is a one-time submission to establish the home health period of care and covers contiguous 30-day periods of care until the individual is discharged from Medicare home health services.
(2) Criteria for NOA submission. In order to submit the NOA, the following criteria must be met:
(i) Once a physician's written or verbal orders that contains the services required for the initial visit have been received and documented as required at §§ 484.60(b) and 409.43(d) of this chapter.
(ii) The initial visit must have been made and the individual admitted to home health care.
(3) Consequences of failure to submit a timely Notice of Admission. When a home health agency does not file the required NOA for its Medicare patients within 5 calendar days after the start of care—
(i) Medicare does not pay for those days of home health services from the start date to the date of filing of the notice of admission;
(ii) The wage and case-mix adjusted 30-day period payment amount is reduced by 1/30th for each day from the home health start of care date until the date of filing of the NOA;
(iii) No LUPA payments are made that fall within the late NOA period;
(iv) The payment reduction cannot exceed the total payment of the claim; and
(v)(A) The non-covered days are a provider liability; and
(B) The provider must not bill the beneficiary for the non-covered days.
(4) Exception to the consequences for filing the NOA late. (i) CMS may waive the consequences of failure to submit a timely-filed NOA specified in paragraph (j)(3) of this section.
(ii) CMS determines if a circumstance encountered by a home health agency is exceptional and qualifies for waiver of the consequence specified in paragraph (j)(3) of this section.
(iii) A home health agency must fully document and furnish any requested documentation to CMS for a determination of exception. An exceptional circumstance may be due to, but is not limited to the following:
(A) Fires, floods, earthquakes, or similar unusual events that inflict extensive damage to the home health agency's ability to operate.
(B) A CMS or Medicare contractor systems issue that is beyond the control of the home health agency.
(C) A newly Medicare-certified home health agency that is notified of that certification after the Medicare certification date, or which is awaiting its user ID from its Medicare contractor.
(D) Other situations determined by CMS to be beyond the control of the home health agency.
9. Section 484.225 is amended by—
a. Removing paragraph (b);
b. Redesignating paragraphs (c) and (d) as paragraphs (b) and (c); and
c. In newly redesignated paragraph (c), removing the phrase “paragraphs (a) through (c) of this section” and adding in its place the phrase “paragraphs (a) and (b) of this section”.
10. Add § 484.245 to read as follows:
(a) Participation. Beginning January 1, 2007, an HHA must report Home Health Quality Reporting Program (HH QRP) data in accordance with the requirements of this section.
(b) Data submission. (1) Except as provided in paragraph (d) of this section, and for a program year, an HHA must submit all of the following to CMS:
(i) Data on measures specified under sections 1899B(c)(1) and 1899B(d)(1) of the Act.
(ii) Standardized patient assessment data required under section 1899B(b)(1) of the Act.
(iii) Quality data required under section 1895(b)(3)(B)(v)(II) of the Act, including HHCAHPS survey data. For purposes of HHCAHPS survey data submission, the following additional requirements apply:
(A) Patient count. An HHA that has less than 60 eligible unique HHCAHPS patients must annually submit to CMS their total HHCAHPS patient count to CMS to be exempt from the HHCAHPS reporting requirements for a calendar year.
(B) Survey requirements. An HHA must contract with an approved, independent HHCAHPS survey vendor to administer the HHCAHPS on its behalf.
(C) CMS approval. CMS approves an HHCAHPS survey vendor if the applicant has been in business for a minimum of 3 years and has conducted surveys of individuals and samples for at least 2 years.
(1) For HHCAHPS, a “survey of individuals” is defined as the collection of data from at least 600 individuals selected by statistical sampling methods and the data collected are used for statistical purposes.
(2) All applicants that meet the requirements in this paragraph (b)(1)(iii)(C) are approved by CMS.
(D) Disapproval by CMS. No organization, firm, or business that owns, operates, or provides staffing for an HHA is permitted to administer its own HHCAHPS Survey or administer the survey on behalf of any other HHA in the capacity as an HHCAHPS survey vendor. Such organizations are not be approved by CMS as HHCAHPS survey vendors.
(E) Compliance with oversight activities. Approved HHCAHPS survey vendors must fully comply with all HHCAHPS oversight activities, including allowing CMS and its HHCAHPS program team to perform site visits at the vendors' company locations.
(2) The data submitted under paragraph (b) of this section must be submitted in the form and manner, and at a time, specified by CMS.
(c) Exceptions and extension requirements. (1) An HHA may request and CMS may grant exceptions or extensions to the reporting requirements under paragraph (b) of this section for one or more quarters, when there are certain extraordinary circumstances beyond the control of the HHA.
(2) An HHA may request an exception or extension within 90 days of the date that the extraordinary circumstances occurred by sending an email to CMS HHAPU reconsiderations at HHAPUReconsiderations@cms.hhs.gov that contains all of the following information:
(i) HHA CMS Certification Number (CCN).
(ii) HHA Business Name.
(iii) HHA Business Address.
(iv) CEO or CEO-designated personnel contact information including name, title, telephone number, email address, and mailing address (the address must be a physical address, not a post office box).
(v) HHA's reason for requesting the exception or extension.
(vi) Evidence of the impact of extraordinary circumstances, including, but not limited to, photographs, newspaper, and other media articles.
(vii) Date when the HHA believes it will be able to again submit data under paragraph (b) of this section and a justification for the proposed date.
(3) Except as provided in paragraph (c)(4) of this section, CMS does not consider an exception or extension request unless the HHA requesting such exception or extension has complied fully with the requirements in this paragraph (c).
(4) CMS may grant exceptions or extensions to HHAs without a request if it determines that one or more of the following has occurred:
(i) An extraordinary circumstance, such as an act of nature, affects an entire region or locale.
(ii) A systemic problem with one of CMS's data collection systems directly affects the ability of an HHA to submit data under paragraph (b) of this section.
(d) Reconsiderations. (1)(i) HHAs that do not meet the quality reporting requirements under this section for a program year will receive a letter of noncompliance via the United States Postal Service and the CMS-designated data submission system.
(ii) An HHA may request reconsideration no later than 30 calendar days after the date identified on the letter of non-compliance.
(2) Reconsideration requests may be submitted to CMS by sending an email to CMS HHAPU reconsiderations at HHAPureConsiderations@cms.hhs.gov containing all of the following information:
(i) HHA CCN.
(ii) HHA Business Name.
(iii) HHA Business Address.
(iv) CEO or CEO-designated personnel contact information including name, title, telephone number, email address, and mailing address (the address must be a physical address, not a post office box).
(v) CMS identified reason(s) for non-compliance as stated in the non-compliance letter.
(vi) Reason(s) for requesting reconsideration, including all supporting documentation.
(3) CMS does not consider a reconsideration request unless the HHA has complied fully with the submission requirements in paragraphs (d)(1) and (2) of this section.
(4) CMS makes a decision on the request for reconsideration and provide notice of the decision to the HHA via letter sent via the United States Postal Service.
(e) Appeals. An HHA that is dissatisfied with CMS' decision on a request for reconsideration submitted under paragraph (d) of this section may file an appeal with the Provider Reimbursement Review Board (PRRB) under 42 CFR part 405, subpart R.
11. Section 484.250 is revised to read as follows:
An HHA must submit to CMS the OASIS data described at § 484.55(b) and (d) as is necessary for CMS to administer the payment rate methodologies described in §§ 484.215, 484.220, 484.230, 484.235, and 484.240.
12. Section 484.315 is amended by revising the section heading and adding paragraph (d) to read as follows:
(d) For performance year 5, CMS publicly reports the following for each competing home health agency on the CMS website:
(1) The Total Performance Score.
(2) The percentile ranking of the Total Performance Score.
PART 486—CONDITIONS FOR COVERAGE OF SPECIALIZED SERVICES FURNISHED BY SUPPLIERS
13. The authority citation for part 486 continues to read as follows:
14. Section 486.505 is amended by revising the definition of “Applicable provider” to read as follows:
Applicable provider means a physician, a nurse practitioner, and a physician assistant.
Dated: October 24, 2019.
Seema Verma,
Administrator, Centers for Medicare and Medicaid Services.
Dated: October 28, 2019.
Alex M. Azar II,
Secretary, Department of Health and Human Services.
Footnotes
1. Home Health (HH) Patient-Driven Groupings Model (PDGM)—Split Implementation Change Request. February 15, 2019. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2019Downloads/R4244CP.pdf.
Back to Citation2. Medicare Claims Processing Manual Chapter 10—Home Health Agency Billing. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c10.pdf.
Back to Citation3. Home Health Agency (HHA) Interpretive Guidelines. August 31, 2018. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/QSO18-25-HHA.pdf.
Back to Citation4. State Operations Manual (SOM), Appendix B. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/QSO18-25-HHA.pdf.
Back to Citation5. Outcome and Assessment Information Set OASIS-D Guidance Manual. January 1, 2019. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HomeHealthQualityInits/Downloads/OASIS-D-Guidance-Manual-final.pdf.
Back to Citation6. Home Health Conditions of Participation. https://www.ecfr.gov/cgi-bin/text-idx?SID=fb4353988ab209999ca866efc142a601&mc=true&node=pt42.5.484&rgn=div5.
Back to Citation7. Overview of the Home Health Groupings Model. November 18, 2016. https://downloads.cms.gov/files/hhgm%20technical%20report%20120516%20sxf.pdf.
Back to Citation8. Direction and Supervision of the Physical Therapist Assistant. August 30, 2018. http://www.apta.org/uploadedFiles/APTAorg/About_Us/Policies/Practice/DirectionSupervisionPTA.pdf.
Back to Citation9. MedPAC Report to Congress, Home health care services, March 2018. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_ch9_sec.pdf?sfvrsn=0.
Back to Citation10. “Overview of the Home Health Groupings Model” Technical Report. November 18, 2016. https://downloads.cms.gov/files/hhgm%20technical%20report%20120516%20sxf.pdf.
Back to Citation11. “ICD-10-CM Official Guidelines for Coding and Reporting FY 2020 (October 1, 2019-September 30, 2020). https://www.cms.gov/Medicare/Coding/0ICD10/Downloads/2020-Coding-Guidelines.pdf.
Back to Citation12. 2020 ICD-10-CM web page. https://www.cms.gov/Medicare/Coding/ICD10/2020-ICD-10-CM.html.
Back to Citation13. ICD-10-CM Official Guidelines for Coding and Reporting FY 2020. https://www.cdc.gov/nchs/data/icd/10cmguidelines-FY2020_final.pdf.
Back to Citation14. “Outcome and Assessment Information Set OASIS-D Guidance Manual”, Effective January 1, 2019 https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HomeHealthQualityInits/Downloads/draft-OASIS-D-Guidance-Manual-7-2-2018.pdf.
Back to Citation15. Home Health PPS Software web page. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HomeHealthPPS/CaseMixGrouperSoftware.html.
Back to Citation16. Current data suggest that what would be about 1/3 of the LUPA episodes with visits near the LUPA threshold move up to become non-LUPA episodes. We assume this experience will continue under the PDGM, with about 1/3 of those episodes 1 or 2 visits below the thresholds moving up to become non-LUPA episodes.
Back to Citation17. The final 2018 analytic file included 6,3388,974 60-day episodes ($18.0 billion in total expenditures as shown on the claim). Of these, 609,947 (9.5 percent) were excluded because they could not be linked to OASIS assessments or because of the claims data cleaning process reasons listed in section III.F.1 of this rule. We note that of the 609,947 excluded claims, 142,206 were excluded because they were RAPs without a final claim or they were claims with zero payment amounts, resulting in $17.9 billion in total expenditures (as shown on the claim). After removing all 609,947 excluded claims, the 2018 analytic file consisted of 5,779,027 60-day episodes ($16.6 billion in total expenditures ass shown on the claim). 60-day episodes of duration longer than 30 days were divided into two 30-day periods in order to calculate the 30-day payment amounts. As noted in section III.F.1 of this rule, there were instances where 30-day periods were excluded from the 2018 analytic file (for example, we could not match the period to a start of care or resumption of care OASIS to determine the functional level under the PDGM, the 30-day period did not have any skilled visits, or because information necessary to calculate payment was missing from claim record). The final 2018 analytic file used to calculate budget neutrality consisted of 9,336,898 30-day periods ($16.6 billion in total expenditures that are simulated under the PDGM) drawn from 5,471,454 60-day episodes.
Back to Citation18. 2017 Medicare Fee-for-Service Supplemental Improper Payment Data. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/CERT/Downloads/2017-Medicare-FFS-Improper-Payment.pdf.
Back to Citation19. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/CERT/Downloads/IntroductiontoComprehensiveErrorRateTesting.pdf.
Back to Citation20. Home Health Prospective Payment System (HH PPS) Limited Data Set (LDS) web page. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/Home_Health_PPS_LDS.html.
Back to Citation21. MedPAC report, “Home Care Services”, March 2019. http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch9_sec.pdf?sfvrsn=0.
Back to Citation22. Government Accountability Office. 1996. Medicare: Home health utilization expands while program controls deteriorate. GAO/HEHS-96-16. Washington, DC: GAO.
Back to Citation23. MedPAC report, “Home Care Services”, March 2019. http://www.medpac.gov/docs/default-source/reports/mar19_medpac_ch9_sec.pdf?sfvrsn=0.
Back to Citation24. MedPAC Report, Home Care Services”, chapter 9, March 2018. http://www.medpac.gov/docs/default-source/reports/mar18_medpac_ch9_sec.pdf?sfvrsn=0.
Back to Citation25. Home Health Agency (HHA) Center web page. https://www.cms.gov/center/provider-type/home-health-agency-hha-center.html.
Back to Citation26. Home Health Agency web page. https://www.cms.gov/center/provider-Type/home-Health-Agency-HHA-Center.html.
Back to Citation27. Medicare Benefit Policy Manual, Chapter 7—Home Health Services https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c07.pdf.
Back to Citation28. The HHCAHPS has five component questions that together are used to represent one NQF-endorsed measure.
Back to Citation29. Measure specifications can be found in the Home Health Process Measures Table on the Home Health Quality Measures website https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HomeHealthQualityInits/Downloads/Home-Health-Outcome-Measures-Table-OASIS-D-11-2018c.pdf.
Back to Citation30. Tian, W. “An all-payer view of hospital discharge to post-acute care,” May 2016. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp.
Back to Citation31. Ibid.
Back to Citation32. Kwan, J.L., Lo, L., Sampson, M., & Shojania, K.G., “Medication reconciliation during transitions of care as a patient safety strategy: a systematic review,” Annals of Internal Medicine, 2013, Vol. 158(5), pp. 397-403.
33. Boockvar, K.S., Blum, S., Kugler, A., Livote, E., Mergenhagen, K.A., Nebeker, J.R., & Yeh, J., “Effect of admission medication reconciliation on adverse drug events from admission medication changes,” Archives of Internal Medicine, 2011, Vol. 171(9), pp. 860-861.
34. Bell, C.M., Brener, S.S., Gunraj, N., Huo, C., Bierman, A.S., Scales, D C., & Urbach, D.R., “Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases,” JAMA, 2011, Vol. 306(8), pp. 840-847.
35. Basey, A.J., Krska, J., Kennedy, T.D., & Mackridge, A.J., “Prescribing errors on admission to hospital and their potential impact: a mixed-methods study,” BMJ Quality & Safety, 2014, Vol. 23(1), pp. 17-25.
36. Desai, R., Williams, C.E., Greene, S.B., Pierson, S., & Hansen, R.A., “Medication errors during patient transitions into nursing homes: characteristics and association with patient harm,” The American Journal of Geriatric Pharmacotherapy, 2011, Vol. 9(6), pp. 413-422.
37. Boling, P.A., “Care transitions and home health care,” Clinical Geriatric Medicine, 2009, Vol.25(1), pp. 135-48.
Back to Citation38. Barnsteiner, J.H., “Medication Reconciliation: Transfer of medication information across settings—keeping it free from error,” The American Journal of Nursing, 2005, Vol. 105(3), pp. 31-36.
39. Arbaje, A.I., Kansagara, D.L., Salanitro, A.H., Englander, H.L., Kripalani, S., Jencks, S.F., & Lindquist, L.A., “Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs,” Journal of General Internal Medicine, 2014, Vol. 29(6), pp. 932-939.
40. Jencks, S.F., Williams, M.V., & Coleman, E.A., “Rehospitalizations among patients in the Medicare fee-for-service program,” New England Journal of Medicine, 2009, Vol. 360(14), pp. 1418-1428.
41. Institute of Medicine. “Preventing medication errors: quality chasm series,” Washington, DC: The National Academies Press 2007. Available at: https://www.nap.edu/read/11623/chapter/1
42. Kitson, N.A., Price, M., Lau, F.Y., & Showler, G., “Developing a medication communication framework across continuums of care using the Circle of Care Modeling approach,” BMC Health Services Research, 2013, Vol. 13(1), pp. 1-10.
43. Mor, V., Intrator, O., Feng, Z., & Grabowski, D.C., “ The revolving door of rehospitalization from skilled nusing facilities” Health Affairs, 2010, Vol. 29(1), pp. 57-64.
44. Institute of Medicine. “Preventing medication errors: quality chasm series,” Washington, DC: The National Academies Press 2007. Available at: https://www.nap.edu/read/11623/chapter/1.
45. Kitson, N.A., Price, M., Lau, F.Y., & Showler, G., “Developing a medication communication framework across continuums of care using the Circle of Care Modeling approach,” BMC Health Services Research, 2013, Vol. 13(1), pp. 1-10.
46. Forster, A.J., Murff, H.J., Peterson, J. F., Gandhi, T.K., & Bates, D.W., “The incidence and severity of adverse events affecting patients after discharge from the hospital.” Annals of Internal Medicine, 2003,138(3), pp. 161-167.
47. King, B.J., Gilmore‐Bykovskyi, A.L., Roiland, R.A., Polnaszek, B.E., Bowers, B.J., & Kind, A.J. “The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study,” Journal of the American Geriatrics Society, 2013, Vol. 61(7), 1095-1102.
48. Lattimer, C. (2011). When it comes to transitions in patient care, effective communication can make all the difference. Generations, 35(1), 69-72.
49. Vognar, L., & Mujahid, N. (2015). Healthcare transitions of older adults: an overview for the general practitioner. Rhode Island Medical Journal (2013), 98(4), 15-18.
Back to Citation50. The Joint Commission, “Sentinel Event Policy” available at https://www.jointcommission.org/sentinel_event_policy_and_procedures/
Back to Citation51. The Joint Commission. “Sentinel Event Data Root Causes by Event Type 2004 -2015.” 2016. Available at: https://www.jointcommission.org/assets/1/23/jconline_Mar_2_2016.pdf.
Back to Citation52. Mor, V., Intrator, O., Feng, Z., & Grabowski, D. C., “The revolving door of rehospitalization from skilled nursing facilities,” Health Affairs, 2010, Vol. 29(1), pp. 57-64.
53. Institute of Medicine, “Preventing medication errors: quality chasm series,” Washington, DC: The National Academies Press, 2007. Available at: https://www.nap.edu/read/11623/chapter/1.
54. Starmer, A.J., Sectish, T.C., Simon, D.W., Keohane, C., McSweeney, M.E., Chung, E.Y., Yoon, C.S., Lipsitz, S.R., Wassner, A.J., Harper, M.B., & Landrigan, C.P., “Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle,” JAMA, 2013, Vol. 310(21), pp. 2262-2270.
55. Pronovost, P., M.M.E. Johns, S. Palmer, R.C. Bono, D.B. Fridsma, A. Gettinger, J. Goldman, W. Johnson, M. Karney, C. Samitt, R.D. Sriram, A. Zenooz, and Y.C. Wang, Editors. Procuring Interoperability: Achieving High-Quality, Connected, and Person-Centered Care. Washington, DC, 2018 National Academy of Medicine. Available at: https://nam.edu/wp-content/uploads/2018/10/Procuring-Interoperability_web.pdf.
56. Balaban RB, Weissman JS, Samuel PA, & Woolhandler, S., “Redefining and redesigning hospital discharge to enhance patient care: a randomized controlled study,” J Gen Intern Med, 2008, Vol. 23(8), pp. 1228-33.
57. Siefferman, J.W., Lin, E., & Fine, J.S. (2012). Patient safety at handoff in rehabilitation medicine. Physical Medicine and Rehabilitation Clinics of North America, 23(2), 241-257.
Back to Citation58. Arbaje, A.I., Kansagara, D.L., Salanitro, A.H., Englander, H.L., Kripalani, S., Jencks, S.F., & Lindquist, L.A., “Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs,” Journal of General Internal Medicine, 2014, Vol 29(6), pp. 932-939.
59. Simmons, S., Schnelle, J., Slagle, J., Sathe, N.A., Stevenson, D., Carlo, M., & McPheeters, M.L., “Resident safety practices in nursing home settings.” Technical Brief No. 24 (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2015-00003-I.) AHRQ Publication No. 16-EHC022-EF. Rockville, MD: Agency for Healthcare Research and Quality. May 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK384624/.
Back to Citation60. Berwick, D.M. & Hackbarth, A.D. “Eliminating Waste in US Health Care,” JAMA, 2012, Vol. 307(14), pp.1513-1516.
Back to Citation61. McDonald, K.M., Sundaram, V., Bravata, D.M., Lewis, R., Lin, N., Kraft, S.A. & Owens, D.K. Care Coordination. Vol. 7 of: Shojania K.G., McDonald K.M., Wachter R.M., Owens D.K., editors. “Closing the quality gap: A critical analysis of quality improvement strategies.” Technical Review 9 (Prepared by the Stanford University-UCSF Evidence-based Practice Center under contract 290-02-0017). AHRQ Publication No. 04(07)-0051-7. Rockville, MD: Agency for Healthcare Research and Quality. June 2006. Available at: https://www.ncbi.nlm.nih.gov/books/NBK44015/.
62. Lattimer, C., “When it comes to transitions in patient care, effective communication can make all the difference,” Generations, 2011, Vol. 35(1), pp. 69-72.
Back to Citation63. Starmer A.J, Spector N.D., Srivastava R., West, D.C., Rosenbluth, G., Allen, A.D., Noble, E.L., & Landrigen, C.P., “Changes in medical errors after implementation of a handoff program,” N Engl J Med, 2014, Vol. 37(1), pp. 1803-1812.
64. Kruse, C.S. Marquez, G., Nelson, D., & Polomares, O., “The use of health information exchange to augment patient handoff in long-term care: a systematic review,” Applied Clinical Informatics, 2018, Vol. 9(4), pp. 752-771
65. Brody, A.A., Gibson, B., Tresner-Kirsch, D., Kramer, H., Thraen, I., Coarr, M.E., & Rupper, R., “High prevalence of medication discrepancies between home health referrals and Centers for Medicare and Medicaid Services home health certification and plan of care and their potential to affect safety of vulnerable elderly adults,” Journal of the American Geriatrics Society, 2016, Vol. 64(11), pp. e166-e170.
Back to Citation66. Chhabra, P.T., Rattinger, G.B., Dutcher, S.K., Hare, M.E., Parsons, K.,L., & Zuckerman, I.H., “Medication reconciliation during the transition to and from long-term care settings: a systematic review,” Res Social Adm Pharm, 2012, Vol. 8(1), pp. 60-75.
67. Levinson, D.R., & General, I., “Adverse events in skilled nursing facilities: national incidence among Medicare beneficiaries.” Washington, DC: U.S. Department of Health and Human Services, Office of the Inspector General, February 2014. Available at: https://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf.
Back to Citation68. Battles J., Azam I., Grady M., & Reback K., “Advances in patient safety and medical liability,” AHRQ Publication No. 17-0017-EF. Rockville, MD: Agency for Healthcare Research and Quality, August 2017. Available at: https://www.ahrq.gov/sites/default/files/publications/files/advances-complete_3.pdf.
Back to Citation69. Siefferman, J.W., Lin, E., & Fine, J.S. (2012). Patient safety at handoff in rehabilitation medicine. Physical Medicine and Rehabilitation Clinics of North America, 23(2), 241-257.
Back to Citation70. Hale, J., Neal, E.B., Myers, A., Wright, K.H.S., Triplett, J., Brown, L.B., & Mixon, A.S. (2015). Medication Discrepancies and Associated Risk Factors Identified in Home Health patients. Home Healthcare Now, 33(9), 493-499 https://doi.org/10.1097/NHH.0000000000000290.
Back to Citation71. Barnsteiner, J.H., “Medication Reconciliation: Transfer of medication information across settings—keeping it free from error,” The American Journal of Nursing, 2005, Vol. 105(3), pp. 31-36.
72. Gleason, K.M., Groszek, J.M., Sullivan, C., Rooney, D., Barnard, C., Noskin, G.A., “Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients,” American Journal of Health System Pharmacy, 2004, Vol. 61(16), pp. 1689-1694.
Back to Citation73. Tjia, J., Bonner, A., Briesacher, B.A., McGee, S., Terrill, E., Miller, K., “Medication discrepancies upon hospital to skilled nursing facility transitions,” J Gen Intern Med, 2009, Vol. 24(5), pp. 630-635.
Back to Citation74. Corbett C.L., Setter S.M., Neumiller J.J., & Wood, I.D., “Nurse identified hospital to home medication discrepancies: implications for improving transitional care”, Geriatr Nurs, 2011 Vol. 31(3), pp.188-96.
Back to Citation75. Corbett C.L., Setter S.M., Neumiller J.J., & Wood, I.D., “Nurse identified hospital to home medication discrepancies: implications for improving transitional care”, Geriatr Nurs, 2011 Vol. 31(3), pp.188-96.
Back to Citation76. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-Information-TEP_Summary_Report_Final-June-2017.pdf.
Back to Citation77. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-Information-TEP-Meetings-2-3/Summary-Report_Final_Feb2018.pdf.
Back to Citation78. Tian, W. “An all-payer view of hospital discharge to postacute care,” May 2016. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp.
Back to Citation79. Kwan, J.L., Lo, L., Sampson, M., & Shojania, K.G., “Medication reconciliation during transitions of care as a patient safety strategy: a systematic review,” Annals of Internal Medicine, 2013, Vol. 158(5), pp. 397-403.
80. Boockvar, K.S., Blum, S., Kugler, A., Livote, E., Mergenhagen, K.A., Nebeker, J.R., & Yeh, J., “Effect of admission medication reconciliation on adverse drug events from admission medication changes,” Archives of Internal Medicine, 2011, Vol. 171(9), pp. 860-861.
81. Bell, C.M., Brener, S.S., Gunraj, N., Huo, C., Bierman, A.S., Scales, D.C., & Urbach, D.R., “Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases,” JAMA, 2011, Vol. 306(8), pp. 840-847.
82. Basey, A.J., Krska, J., Kennedy, T.D., & Mackridge, A.J., “Prescribing errors on admission to hospital and their potential impact: a mixed-methods study,” BMJ Quality & Safety, 2014, Vol. 23(1), pp. 17-25.
83. Desai, R., Williams, C.E., Greene, S.B., Pierson, S., & Hansen, R.A., “Medication errors during patient transitions into nursing homes: characteristics and association with patient harm,” The American Journal of Geriatric Pharmacotherapy, 2011, Vol. 9(6), pp. 413-422.
Back to Citation84. Brody, A.A., Gibson, B., Tresner-Kirsch, D., Kramer, H., Thraen, I., Coarr, M.E., & Rupper, R. “High prevalence of medication discrepancies between home health referrals and Centers for Medicare and Medicaid Services home health certification and plan of care and their potential to affect safety of vulnerable elderly adults,”
Journal of the American Geriatrics Society, 2016, Vol. 64(11), pp. e166-e170.
85. Chhabra, P.T., Rattinger, G.B., Dutcher, S.K., Hare, M.E., Parsons, K.L., & Zuckerman, I.H., “Medication reconciliation during the transition to and from long-term care settings: a systematic review,” Res Social Adm Pharm, 2012, Vol. 8(1), pp. 60-75.
Back to Citation86. Brody, A.A., Gibson, B., Tresner-Kirsch, D., Kramer, H., Thraen, I., Coarr, M.E., & Rupper, R. “High prevalence of medication discrepancies between home health referrals and Centers for Medicare and Medicaid Services home health certification and plan of care and their potential to affect safety of vulnerable elderly adults,” Journal of the American Geriatrics Society, 2016, Vol. 64(11), pp. e166-e170.
87. Bell, C.M., Brener, S.S., Gunraj, N., Huo, C., Bierman, A.S., Scales, D.C., & Urbach, D.R., “Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases,” JAMA, 2011, Vol. 306(8), pp. 840-847.
88. Sheehan, O.C., Kharrazi, H., Carl, K.J., Leff, B., Wolff, J.L., Roth, D.L., Gabbard, J., & Boyd, C. M., “Helping older adults improve their medication experience (HOME) by addressing medication regimen complexity in home healthcare,” Home Healthcare Now. 2018, Vol. 36(1) pp. 10-19.
Back to Citation89. Mor, V., Intrator, O., Feng, Z., & Grabowski, D.C., “The revolving door of rehospitalization from skilled nursing facilities,” Health Affairs, 2010, Vol. 29(1), pp. 57-64.
90. Starmer, A.J., Sectish, T.C., Simon, D.W., Keohane, C., McSweeney, M.E., Chung, E.Y., Yoon, C.S., Lipsitz, S.R., Wassner, A.J., Harper, M.B., & Landrigan, C.P., “Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle,” JAMA, 2013, Vol. 310(21), pp. 2262-2270.
Back to Citation91. CMS, “Revision to state operations manual (SOM), Hospital Appendix A—Interpretive Guidelines for 42 CFR 482.43, Discharge Planning” May 17, 2013. Available at: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-32.pdf.
92. The State Operations Manual Guidance to Surveyors for Long Term Care Facilities (Guidance § 483.21(c)(1) Rev. 11-22-17) for discharge planning process. Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf.
Back to Citation93. Toles, M., Colon-Emeric, C., Naylor, M.D., Asafu-Adjei, J., Hanson, L.C., “Connect-home: transitional care of skilled nursing facility patients and their caregivers,” Am Geriatr Soc., 2017, Vol. 65(10), pp. 2322-2328.
Back to Citation94. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-ssessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-nformation-TEP_Summary_Report_Final-June-2017.pdf.
Back to Citation95. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/;Transfer-of-Health-Information-TEP-Meetings-2-3-Summary-Report_Final_Feb2018.pdf.
Back to Citation96. National Institute on Aging. (2014). Assessing Cognitive Impairment in Older Patients. A Quick Guide for Primary Care Physicians. Retrieved from: https://www.nia.nih.gov/alzheimers/publication/assessing-cognitive-impairment-older-patients.
Back to Citation97. Gage B., Morley M., Smith L., et al. (2012). Post-Acute Care Payment Reform Demonstration (Final report, Volume 4 of 4). Research Triangle Park, NC: RTI International.
Back to Citation98. Casey D.A., Antimisiaris D., O'Brien J. (2010). Drugs for Alzheimer's Disease: Are They Effective? Pharmacology & Therapeutics, 35, 208-11.
99. Graff M.J., Vernooij-Dassen M.J., Thijssen M., Dekker J., Hoefnagels W.H., Rikkert M.G.O. (2006). Community Based Occupational Therapy for Patients with Dementia and their Care Givers: Randomised Controlled Trial. BMJ, 333(7580): 1196.
100. Bherer L., Erickson K.I., Liu-Ambrose T. (2013). A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. Journal of Aging Research, 657508.
Back to Citation101. Giacino J.T., Whyte J., Bagiella E., et al. (2012). Placebo-controlled trial of amantadine for severe traumatic brain injury. New England Journal of Medicine, 366(9), 819-826.
Back to Citation102. Alexopoulos G.S., Katz I.R., Reynolds C.F. 3rd, Carpenter D., Docherty J.P., Ross R.W. (2001). Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. Journal of Psychiatric Practice, 7(6), 361-376.
103. Arean P.A., Cook B.L. (2002). Psychotherapy and combined psychotherapy/pharmacotherapy for late life depression. Biological Psychiatry, 52(3), 293-303.
104. Hollon S.D., Jarrett R.B., Nierenberg A.A., Thase M.E., Trivedi M., Rush A.J. (2005). Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? Journal of Clinical Psychiatry, 66(4), 455-468.
105. Wagenaar D, Colenda CC, Kreft M, Sawade J, Gardiner J, Poverejan E. (2003). Treating depression in nursing homes: practice guidelines in the real world. J Am Osteopath Assoc. 103(10), 465-469.
Back to Citation106. Crespy SD, Van Haitsma K, Kleban M, Hann CJ. Reducing Depressive Symptoms in Nursing Home Residents: Evaluation of the Pennsylvania Depression Collaborative Quality Improvement Program. J Healthc Qual. 2016. Vol. 38, No. 6, pp. e76-e88.
Back to Citation107. Agüero-Torres, H., Fratiglioni, L., Guo, Z., Viitanen, M., von Strauss, E., & Winblad, B. (1998). “Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study.” Am J of Public Health 88(10): 1452-1456.
Back to Citation108. RTI International. Proposed Measure Specifications for Measures Proposed in the FY 2017 IRF QRP NPRM. Research Triangle Park, NC. 2016.
Back to Citation109. Fick, D.M., Steis, M.R., Waller, J.L., & Inouye, S.K. (2013). “Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults.” J of Hospital Med 8(9): 500-505.
Back to Citation110. Li, C., Friedman, B., Conwell, Y., & Fiscella, K. (2007). “Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people.” J of the A Geriatrics Society, 55(4): 596-602.
111. Löwe, B., Kroenke, K., & Gräfe, K. (2005). “Detecting and monitoring depression with a two-item questionnaire (PHQ-2).” J of Psychosomatic Research, 58(2): 163-171.
Back to Citation112. Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Annals of family medicine. 2010; 8(4):348-53. doi: 10.1370/afm.1139 pmid:20644190; PubMed Central PMCID: PMC2906530.
Back to Citation113. Wunsch, H., Linde-Zwirble, W. T., Angus, D. C., Hartman, M. E., Milbrandt, E. B., & Kahn, J. M. (2010). “The epidemiology of mechanical ventilation use in the United States.” Critical Care Med 38(10): 1947-1953.
Back to Citation114. Dempsey, D.T., Mullen, J.L., & Buzby, G.P. (1988). “The link between nutritional status and clinical outcome: Can nutritional intervention modify it?” Am J of Clinical Nutrition, 47(2): 352-356.
Back to Citation115. Dempsey, D.T., Mullen, J.L., & Buzby, G.P. (1988). “The link between nutritional status and clinical outcome: Can nutritional intervention modify it?” Am J of Clinical Nutrition, 47(2): 352-356.
Back to Citation116. U.S. Department of Health and Human Services. Office of Inspector General. Daniel R. Levinson. Adverse Events in Hospitals: National Incidence Among Medicare Beneficiaries. OEI-06-09-00090. November 2010.
Back to Citation117. Boockvar KS, Liu S, Goldstein N, Nebeker J, Siu A, Fried T. Prescribing discrepancies likely to cause adverse drug events after patient transfer. Qual Saf Health Care. 2009;18(1):32-6.
Back to Citation118. Gandhi TK, Seger AC, Overhage JM, et al. Outpatient adverse drug events identified by screening electronic health records. J Patient Saf 2010;6:91-6.doi:10.1097/PTS.0b013e3181dcae06.
Back to Citation119. Gurwitz JH, Field TS, Judge J, Rochon P, Harrold LR, Cadoret C, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med. 2005; 118(3):251±8. Epub 2005/03/05. https://doi.org/10.1016/j.amjmed.2004.09.018 PMID: 15745723.
Back to Citation120. Hug BL, Witkowski DJ, Sox CM, Keohane CA, Seger DL, Yoon C, Matheny ME, Bates DW. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009; 76:1192-1198. [PubMed: 19759525]
Back to Citation121. Barnsteiner JH. Medication reconciliation: transfer of medication information across settings-keeping it free from error. J Infus Nurs. 2005;28(2 Suppl):31-36.
122. Rozich J, Roger, R. Medication safety: one organization's approach to the challenge. Journal of Clinical Outcomes Management. 2001(8):27-34.
123. Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm. 2004;61(16):1689-1695.
Back to Citation124. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. doi: 10.1001/jama.2016.16201.
Back to Citation125. Ibid.
Back to Citation126. Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis. 2013;35(3):312-319. doi: 10.1007/s11239-013-0899-7.
127. Melkonian M, Jarzebowski W, Pautas E. Bleeding risk of antiplatelet drugs compared with oral anticoagulants in older patients with atrial fibrillation: A systematic review and meta‐analysis. J Thromb Haemost. 2017;15:1500-1510. DOI: 10.1111/jth.13697.
Back to Citation128. Hamnvik OP, McMahon GT. Balancing Risk and Benefit with Oral Hypoglycemic Drugs. The Mount Sinai journal of medicine, New York. 2009; 76:234-243.
Back to Citation129. Naples JG, Gellad WF, Hanlon JT. The Role of Opioid Analgesics in Geriatric Pain Management. Clin Geriatr Med. 2016;32(4):725-735.
Back to Citation130. Rigler SK, Shireman TI, Cook-Wiens GJ, Ellerbeck EF, Whittle JC, Mehr DR, Mahnken JD. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc. 2013; 61(5):715-722. [PubMed: 23590366].
131. Wang S, Linkletter C, Dore D et al. Age, antipsychotics, and the risk of ischemic stroke in the Veterans Health Administration. Stroke 2012;43:28-31. doi:10.1161/STROKEAHA.111.617191.
Back to Citation132. Faulkner CM, Cox HL, Williamson JC. Unique aspects of antimicrobial use in older adults. Clin Infect Dis. 2005;40(7):997-1004.
Back to Citation133. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019; 00:1-21. DOI: 10.1111/jgs.15767.
Back to Citation134. Li Y, Salmasian H, Harpaz R, Chase H, Friedman C. Determining the reasons for medication prescriptions in the EHR using knowledge and natural language processing. AMIA Annu Symp Proc. 2011;2011:768-76.
Back to Citation135. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society. Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015; 63:2227-2246.
Back to Citation136. Department of Health and Human Services: Pain Management Best Practices Inter-Agency Task Force. Draft Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. Accessed April 1, 2019. https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf
Back to Citation137. Department of Health and Human Services: Pain Management Best Practices Inter-Agency Task Force. Draft Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. Accessed April 1, 2019. https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf
Back to Citation138. Fishman SM, Carr DB, Hogans B, et al. Scope and Nature of Pain- and Analgesia-Related Content of the United States Medical Licensing Examination (USMLE). Pain Med Malden Mass. 2018;19(3):449-459. doi:10.1093/pm/pnx336.
Back to Citation139. Fishman SM, Young HM, Lucas Arwood E, et al. Core competencies for pain management: results of an interprofessional consensus summit. Pain Med Malden Mass. 2013;14(7):971-981. doi:10.1111/pme.12107.
Back to Citation140. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press (US); 2011. http://www.ncbi.nlm.nih.gov/books/NBK91497/.
Back to Citation141. Department of Health and Human Services: Pain Management Best Practices Inter-Agency Task Force. Draft Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. Accessed April 1, 2019. https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf.
Back to Citation142. National Academies. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington DC: National Academies of Sciences, Engineering, and Medicine.; 2017.
Back to Citation143. National Pain Strategy: A Comprehensive Population-Health Level Strategy for Pain. https://iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf.
Back to Citation144. Chau, D.L., Walker, V., Pai, L., & Cho, L.M. (2008). Opiates and elderly: use and side effects. Clinical interventions in aging, 3 (2), 273-8.
Back to Citation145. Fine, P.G. (2009). Chronic Pain Management in Older Adults: Special Considerations. Journal of Pain and Symptom Management, 38(2): S4-S14.
Back to Citation146. Solomon, D.H., Rassen, J.A., Glynn, R.J., Garneau, K., Levin, R., Lee, J., & Schneeweiss, S.. (2010). Archives Internal Medicine, 170(22):1979-1986.
Back to Citation147. Byrd L. Managing chronic pain in older adults: a long-term care perspective. Annals of Long-Term Care: Clinical Care and Aging. 2013;21(12):34-40.
Back to Citation148. Kligler, B., Bair, M.J., Banerjea, R. et al. (2018). Clinical Policy Recommendations from the VHA State-of-the-Art Conference on Non-Pharmacological Approaches to Chronic Musculoskeletal Pain. Journal of General Internal Medicine, 33 (Suppl 1): 16. https://doi.org/10.1007/s11606-018-4323-z.
Back to Citation149. Chou, R., Deyo, R., Friedly, J., et al. (2017). Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. An nals of Internal Medicine, 166 (7):493-505.
Back to Citation150. Society for Post-Acute and Long-Term Care Medicine (AMDA). (2018). Opioids in Nursing Homes: Position Statement. https://paltc.org/opioids%20in%20nursing%20homes.
Back to Citation151. https://www.hhs.gov/opioids/about-the-epidemic/hhs-response/index.html.
Back to Citation152. Section 504 of the Rehabilitation Act of 1973, section1557 of the Affordable Care Act, and their respective implementing regulations. More information is available at: https://www.hhs.gov/civil-rights/for-individuals/disability/index.html, and https://www.hhs.gov/civil-rights/for-individuals/section-1557/index.html.
Back to Citation153. Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults . Gerontologist. 2003;43(5):661-668.
154. Hawkins K, Bottone FG, Jr., Ozminkowski RJ, et al. The prevalence of hearing impairment and its burden on the quality of life among adults with Medicare Supplement Insurance. Qual Life Res. 2012; 21(7):1135-1147.
Back to Citation155. Horn KL, McMahon NB, McMahon DC, Lewis JS, Barker M, Gherini S. Functional use of the Nucleus 22-channel cochlear implant in the elderly. The Laryngoscope. 1991; 101(3):284-288.
Back to Citation156. Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options—a mini-review. Gerontology. 2010; 56(3):351-358.
157. Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing Loss Prevalence and Risk Factors Among Older Adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011; 66A(5):582-590.
158. Hawkins K, Bottone FG, Jr., Ozminkowski RJ, et al. The prevalence of hearing impairment and its burden on the quality of life among adults with Medicare Supplement Insurance. Qual Life Res. 2012; 21(7):1135-1147.
Back to Citation159. Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing Loss and Incident Dementia. Arch Neurol. 2011; 68(2):214-220.
Back to Citation160. Cimarolli VR, Jung S. Intensity of Occupational Therapy Utilization in Nursing Home Residents: The Role of Sensory Impairments. J Am Med Dir Assoc. 2016;17(10):939-942.
Back to Citation161. Colon-Emeric CS, Biggs DP, Schenck AP, Lyles KW. Risk factors for hip fracture in skilled nursing facilities: who should be evaluated? Osteoporos Int. 2003;14(6):484-489.
162. Freeman EE, Munoz B, Rubin G, West SK. Visual field loss increases the risk of falls in older adults: the Salisbury eye evaluation. Invest Ophthalmol Vis Sci. 2007;48(10):4445-4450.
163. Keepnews D, Capitman JA, Rosati RJ. Measuring patient-level clinical outcomes of home health care. J Nurs Scholarsh. 2004;36(1):79-85.
164. Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002;57(3):M181-185.
165. Prager AJ, Liebmann JM, Cioffi GA, Blumberg DM. Self-reported Function, Health Resource Use, and Total Health Care Costs Among Medicare Beneficiaries With Glaucoma. JAMA ophthalmology. 2016;134(4):357-365.
166. Rovner BW, Ganguli M. Depression and disability associated with impaired vision: The MoVies Project. J Am Geriatr Soc. 1998;46(5):617-619.
167. Tinetti ME, Ginter SF. The nursing home life-space diameter. A measure of extent and frequency of mobility among nursing home residents. J Am Geriatr Soc. 1990;38(12):1311-1315.
Back to Citation168. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Chapter 2. Washington, DC: The National Academies Press.
Back to Citation169. Social Determinants of Health. Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. (February 2019).
Back to Citation170. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. 2016. Report to Congress: Social Risk Factors and Performance Under Medicare's Value-Based Payment Programs. Washington, DC.
Back to Citation171. 2017 National Healthcare Quality and Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; September 2018. AHRQ Pub. No. 18-0033-EF.
172. Fiscella, K. and Sanders, M.R. Racial and Ethnic Disparities in the Quality of Health Care. (2016). Annual Review of Public Health. 37:375-394.
173. 2018 National Impact Assessment of the Centers for Medicare & Medicaid Services (CMS) Quality Measures Reports. Baltimore, MD: U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services; February 28, 2018.
174. Smedley, B.D., Stith, A.Y., & Nelson, A.R. (2003). Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC, National Academy Press.
175. Chase, J., Huang, L. and Russell, D. (2017). Racial/ethnic disparities in disability outcomes among post-acute home care patients. J of Aging and Health. 30(9):1406-1426.
Back to Citation176. National Healthcare Quality and Disparities Reports. (December 2018). Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/research/findings/nhqrdr/index.html.
Back to Citation177. National Center for Health Statistics. Health, United States, 2017: With special feature on mortality. Hyattsville, Maryland. 2018.
Back to Citation178. HHS. Heart disease and African Americans. 2016b. (October 24, 2016). http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=19.
Back to Citation179. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States; Baciu A, Negussie Y, Geller A, et al., editors. Communities in Action: Pathways to Health Equity. Washington (DC): National Academies Press (US); 2017 Jan 11. 2, The State of Health Disparities in the United States. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425844/.
Back to Citation180. “Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity (Notice of Decision)”. Federal Register 62:210 (October 30, 1997) pp. 58782-58790. Available from: https://www.govinfo.gov/content/pkg/FR/1997/10/30/pdf/97/28653.pdf.
Back to Citation181. Penman-Aguilar, A., Talih, M., Huang, D., Moonesinghe, R., Bouye, K., Beckles, G. (2016). Measurement of Health Disparities, Health Inequities, and Social Determinants of Health to Support the Advancement of Health Equity. J Public Health Manag Pract. 22 Suppl 1: S33-42.
182. Ramos, R., Davis, J.L., Ross, T., Grant, C.G., Green, B.L. (2012). Measuring health disparities and health inequities: do you have REGAL data? Qual Manag Health Care. 21(3):176-87.
183. IOM (Institute of Medicine). 2009. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: The National Academies Press.
184. “Revision of Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity: Proposals From Federal Interagency Working Group (Notice and Request for Comments).” Federal Register 82: 39 (March 1, 2017) p. 12242.
Back to Citation185. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States; Baciu A, Negussie Y, Geller A, et al., editors. Communities in Action: Pathways to Health Equity. Washington (DC): National Academies Press (US); 2017 Jan 11. 2, The State of Health Disparities in the United States. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425844/.
Back to Citation186. IOM (Institute of Medicine). 2009. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: The National Academies Press.
Back to Citation187. U.S. Census Bureau, 2013-2017 American Community Survey 5-Year Estimates.
Back to Citation188. Karliner LS, Kim SE, Meltzer DO, Auerbach AD. Influence of language barriers on outcomes of hospital care for general medicine inpatients. J Hosp Med. 2010 May-Jun;5(5):276-82. doi: 10.1002/jhm.658.
189. Kim EJ, Kim T, Paasche-Orlow MK, et al. Disparities in Hypertension Associated with Limited English Proficiency. J Gen Intern Med. 2017 Jun;32(6):632-639. doi: 10.1007/s11606-017-3999-9.
190. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Washington, DC: The National Academies Press.
Back to Citation191. IOM (Institute of Medicine). 2009. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: The National Academies Press.
Back to Citation192. Guerino, P. and James, C. Race, Ethnicity, and Language Preference in the Health Insurance Marketplaces 2017 Open Enrollment Period. Centers for Medicare & Medicaid Services, Office of Minority Health. Data Highlight: Volume 7—April 2017. Available at https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Data-Highlight-Race-Ethnicity-and-Language-Preference-Marketplace.pdf.
Back to Citation193. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National action plan to improve health literacy. Washington (DC): Author; 2010.
Back to Citation194. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Washington, DC: The National Academies Press.
Back to Citation195. Social Determinants of Health. Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. (February 2019).
Back to Citation196. U.S. Department of Health & Human Services, Office of the Assistant Secretary for Planning and Evaluation. Report to Congress: Social Risk Factors and Performance Under Medicare's Value-Based Purchasing Programs. Available at https://aspe.hhs.gov/pdf-report/report-congress-social-risk-factors-and-performance-under-medicares-value-based-purchasing-programs. Washington, DC: 2016.
Back to Citation197. Morris, N.S., MacLean, C.D., Chew, L.D., & Littenberg, B. (2006). The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC family practice, 7, 21. doi:10.1186/1471-2296-7-21.
198. Brice, J.H., Foster, M.B., Principe, S., Moss, C., Shofer, F.S., Falk, R.J., Ferris, M.E., DeWalt, D.A. (2013). Single-item or two-item literacy screener to predict the S-TOFHLA among adult hemodialysis patients. Patient Educ Couns. 94(1):71-5.
Back to Citation199. University of Miami, School of Nursing & Health Studies, Center of Excellence for Health Disparities Research. Test of Functional Health Literacy in Adults (TOFHLA). (March 2019). Available from: https://elcentro.sonhs.miami.edu/research/measures-library/tofhla/index.html.
Back to Citation200. Nurss, J.R., Parker, R.M., Williams, M.V., &Baker, D.W. David W. (2001). TOFHLA. Peppercorn Books & Press. Available from: http://www.peppercornbooks.com/catalog/information.php?info_id=5.
Back to Citation201. Hudson, S., Rikard, R.V., Staiculescu, I. & Edison, K. (2017). Improving health and the bottom line: The case for health literacy. In Building the case for health literacy: Proceedings of a workshop. Washington, DC: The National Academies Press.
Back to Citation202. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for Social Risk Factors in Medicare Payment: Identifying Social Risk Factors. Washington, DC: The National Academies Press.
Back to Citation203. Syed, S.T., Gerber, B.S., and Sharp, L.K. (2013). Traveling Towards Disease: Transportation Barriers to Health Care Access. J Community Health. 38(5): 976-993.
Back to Citation204. Health Research & Educational Trust. (2017, November). Social determinants of health series: Transportation and the role of hospitals. Chicago, IL. Available at www.aha.org/transportation.www.aha.org/transportation.
Back to Citation205. Health Research & Educational Trust. (2017, November). Social determinants of health series: Transportation and the role of hospitals. Chicago, IL. Available at www.aha.org/transportation.
Back to Citation206. Northwestern University. (2017). PROMIS Item Bank v. 1.0—Emotional Distress—Anger—Short Form 1.
Back to Citation207. Tomaka, J., Thompson, S., and Palacios, R. (2006). The Relation of Social Isolation, Loneliness, and Social Support to Disease Outcomes Among the Elderly. J of Aging and Health. 18(3): 359-384.
208. Social Connectedness and Engagement Technology for Long-Term and Post-Acute Care: A Primer and Provider Selection Guide. (2019). Leading Age. Available at https://www.leadingage.org/white-papers/social-connectedness-and-engagement-technology-long-term-and-post-acute-care-primer-and#1.1.
Back to Citation209. Landeiro, F., Barrows, P., Nuttall Musson, E., Gray, A.M., and Leal, J. (2017). Reducing Social Loneliness in Older People: A Systematic Review Protocol. BMJ Open. 7(5): e013778.
210. Ong, A.D., Uchino, B.N., and Wethington, E. (2016). Loneliness and Health in Older Adults: A Mini-Review and Synthesis. Gerontology. 62:443-449.
211. Leigh-Hunt, N., Bagguley, D., Bash, K., Turner, V., Turnbull, S., Valtorta, N., and Caan, W. (2017). An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 152:157-171.
Back to Citation212. Northwestern University. (2017). PROMIS Item Bank v. 1.0—Emotional Distress—Anger—Short Form 1.
Back to Citation213. National Association of Community Health Centers, “PRAPARE” available at http://www.nachc.org/research-and-data/prapare/.
Back to Citation214. https://www.govinfo.gov/content/pkg/FR-2012-11-15/pdf/2012-26902.pdf.
Back to Citation215. Medicare Benefit Policy Manual, Chapter 15, “Covered Medical and Other Health Services”, section 50.2—Determining Self-Administration of Drug or Biological. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c15.pdf.
Back to Citation216. Self-Administered Drug (SAD) Exclusion List Report. www.cms.gov/medicare-coverage-database/reports/sad-exclusion-list-report.aspx.
Back to Citation217. Medicare National Coverage Determinations (NCD) Manual. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/internet-Only-Manuals-IOMs-Items/CMS014961.html.
Back to Citation218. Local Coverage Determination (LCD): External Infusion Pumps (L33794). https://www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=33794&ver=83&Date=05%2f15%2f2019&DocID=L33794&bc=iAAAABAAAAAA&.
Back to Citation219. Temporary Transitional Payment for Home Infusion Therapy Services for CYs 2019 and 2020. August 10, 2018. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2018Downloads/R4112CP.pdf.
Back to Citation220. CY 2019 HH PPS proposed rule (83 FR 32465 and 32466). https://www.govinfo.gov/content/pkg/FR-2018-07-12/pdf/2018-14443.pdf.
Back to Citation221. CR 10836. Temporary Transitional Payment for Home Infusion Therapy Services for CYs 2019 and 2020. August 10, 2018. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2018Downloads/R4112CP.pdf.
Back to Citation222. Medicare Physician Fee Schedule. https://www.cms.gov/apps/physician-fee-schedule/.
Back to Citation223. January 2019 DMEPOS Fee Schedules. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/DMEPOSFeeSched/DMEPOS-Fee-Schedule-Items/DME19-A.html?DLPage=1&DLEntries=10&DLSort=2&DLSortDir=descending.
Back to Citation224. https://www.cms.gov/Medicare/wwwProvider-Enrollment-and-Certification/MedicareProviderSupEnroll/wwwdownloads/DMEPOSSupplierStandards.pdf.
Back to Citation225. Local Coverage Determination (LCD): External Infusion Pumps (L33794). https://med.noridianmedicare.com/wwwdocuments/2230703/7218263/External+wwwInfusion+Pumps+LCD+and+PA.
Back to Citation226. https://innovation.cms.gov/wwwinitiatives/IVIG/supplierfaq.html.
Back to Citation227. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c15.pdf.
Back to Citation228. GAF = (.50886 × Work GPCI) + (.44839 × PE GPCI) + (.04295 × MP GPCI).
Back to Citation229. Preauthorization. https://www.healthcare.gov/glossary/preauthorization/.
Back to CitationBILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
[FR Doc. 2019-24026 Filed 10-31-19; 4:15 pm]
BILLING CODE 4120-01-P
