AGENCY:
Centers for Medicare & Medicaid Services (CMS), HHS.
ACTION:
Final rule.
SUMMARY:
This final rule updates the prospective payment rates for inpatient rehabilitation facilities (IRFs) for federal fiscal year (FY) 2020. As required by the statute, this final rule includes the classification and weighting factors for the IRF prospective payment system's (PPS) case-mix groups (CMGs) and a description of the methodologies and data used in computing the prospective payment rates for FY 2020. This final rule rebases and revises the IRF market basket to reflect a 2016 base year rather than the current 2012 base year. Additionally, this final rule revises the CMGs and updates the CMG relative weights and average length of stay (LOS) values beginning with FY 2020, based on analysis of 2 years of data (FYs 2017 and 2018). Although we proposed to use a weighted motor score to assign patients to CMGs, we are finalizing based on public comments the use of an unweighted motor score to assign patients to CMGs beginning with FY 2020. Additionally, we are finalizing the removal of one item from the motor score. We are updating the IRF wage index to use the concurrent fiscal year inpatient prospective payment system (IPPS) wage index beginning with FY 2020. We are amending the regulations to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. For the IRF Quality Reporting Program (QRP), we are adopting two new measures, modifying an existing measure, and adopting new standardized patient assessment data elements. We are also making updates to reflect our migration to a new data submission system.
DATES:
Effective date: These regulations are effective on October 1, 2019.
Applicability dates: The updated IRF prospective payment rates are applicable for IRF discharges occurring on or after October 1, 2019, and on or before September 30, 2020 (FY 2020). The new and updated quality measures and reporting requirements under the IRF QRP are applicable for IRF discharges occurring on or after October 1, 2020.
FOR FURTHER INFORMATION CONTACT:
Gwendolyn Johnson, (410) 786-6954, for general information.
Catie Kraemer, (410) 786-0179, for information about the IRF payment policies and payment rates.
Kadie Derby, (410) 786-0468, for information about the IRF coverage policies.
Kate Brooks, (410) 786-7877, for information about the IRF quality reporting program.
SUPPLEMENTARY INFORMATION:
Inspection of Public Comments: All comments received before the close of the comment period are available for viewing by the public, including any personally identifiable or confidential business information that is included in a comment. We post all comments received before the close of the comment period as soon as possible after they have been received at http://www.regulations.gov. Follow the search instructions on that website to view public comments.
The IRF PPS Addenda along with other supporting documents and tables referenced in this final rule are available through the internet on the CMS website at http://www.cms.hhs.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/.
Executive Summary
A. Purpose
This final rule updates the prospective payment rates for IRFs for FY 2020 (that is, for discharges occurring on or after October 1, 2019, and on or before September 30, 2020) as required under section 1886(j)(3)(C) of the Social Security Act (the Act). As required by section 1886(j)(5) of the Act, this final rule includes the classification and weighting factors for the IRF PPS's case-mix groups (CMGs) and a description of the methodologies and data used in computing the prospective payment rates for FY 2020. This final rule also rebases and revises the IRF market basket to reflect a 2016 base year, rather than the current 2012 base year. Additionally, this final rule revises the CMGs and updates the CMG relative weights and average LOS values beginning with FY 2020, based on analysis of 2 years of data (FYs 2017 and 2018). Although we proposed to use a weighted motor score to assign patients to CMGs, we are finalizing based on public comments the use of an unweighted motor score to assign patients to CMGs beginning with FY 2020. Additionally, we are finalizing the removal of one item from the motor score. We are also updating the IRF wage index to use the concurrent FY IPPS wage index for the IRF PPS beginning with FY 2020. We are also amending the regulations at 42 CFR 412.622 to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. For the IRF QRP, we are adopting two new measures, modifying an existing measure, and adopting new standardized patient assessment data elements. We also include updates related to the system used for the submission of data and related regulation text. We are not finalizing our proposal requiring that IRFs submit data on measures and standardized patient assessment data for which the source of the data is the IRF-PAI to all patients, regardless of payer, but plan to propose this policy in future rulemaking.
B. Summary of Major Provisions
In this final rule, we use the methods described in the FY 2019 IRF PPS final rule (83 FR 38514) to update the prospective payment rates for FY 2020 using updated FY 2018 IRF claims and the most recent available IRF cost report data, which is FY 2017 IRF cost report data. This final rule also rebases and revises the IRF market basket to reflect a 2016 base year rather than the current 2012 base year. Additionally, this final rule revises the CMGs and updates the CMG relative weights and average LOS values beginning with FY 2020, based on analysis of 2 years of data (FYs 2017 and 2018). Although we proposed to use a weighted motor score to assign patients to CMGs, we are finalizing based on public comments the use of an unweighted motor score to assign patients to CMGs beginning with FY 2020. Additionally, we are finalizing the removal of one item from the motor score. We are also updating the IRF wage index to use the concurrent FY IPPS wage index for the IRF PPS beginning in FY 2020. We are also amending the regulations at § 412.622 to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. We also update requirements for the IRF QRP.
C. Summary of Impacts

I. Background
A. Historical Overview of the IRF PPS
Section 1886(j) of the Act provides for the implementation of a per-discharge PPS for inpatient rehabilitation hospitals and inpatient rehabilitation units of a hospital (collectively, hereinafter referred to as IRFs). Payments under the IRF PPS encompass inpatient operating and capital costs of furnishing covered rehabilitation services (that is, routine, ancillary, and capital costs), but not direct graduate medical education costs, costs of approved nursing and allied health education activities, bad debts, and other services or items outside the scope of the IRF PPS. Although a complete discussion of the IRF PPS provisions appears in the original FY 2002 IRF PPS final rule (66 FR 41316) and the FY 2006 IRF PPS final rule (70 FR 47880), we are providing a general description of the IRF PPS for FYs 2002 through 2019.
Under the IRF PPS from FY 2002 through FY 2005, the prospective payment rates were computed across 100 distinct CMGs, as described in the FY 2002 IRF PPS final rule (66 FR 41316). We constructed 95 CMGs using rehabilitation impairment categories (RICs), functional status (both motor and cognitive), and age (in some cases, cognitive status and age may not be a factor in defining a CMG). In addition, we constructed five special CMGs to account for very short stays and for patients who expire in the IRF.
For each of the CMGs, we developed relative weighting factors to account for a patient's clinical characteristics and expected resource needs. Thus, the weighting factors accounted for the relative difference in resource use across all CMGs. Within each CMG, we created tiers based on the estimated effects that certain comorbidities would have on resource use.
We established the federal PPS rates using a standardized payment conversion factor (formerly referred to as the budget-neutral conversion factor). For a detailed discussion of the budget-neutral conversion factor, please refer to our FY 2004 IRF PPS final rule (68 FR 45684 through 45685). In the FY 2006 IRF PPS final rule (70 FR 47880), we discussed in detail the methodology for determining the standard payment conversion factor.
We applied the relative weighting factors to the standard payment conversion factor to compute the unadjusted prospective payment rates under the IRF PPS from FYs 2002 through 2005. Within the structure of the payment system, we then made adjustments to account for interrupted stays, transfers, short stays, and deaths. Finally, we applied the applicable adjustments to account for geographic variations in wages (wage index), the percentage of low-income patients, location in a rural area (if applicable), and outlier payments (if applicable) to the IRFs' unadjusted prospective payment rates.
For cost reporting periods that began on or after January 1, 2002, and before October 1, 2002, we determined the final prospective payment amounts using the transition methodology prescribed in section 1886(j)(1) of the Act. Under this provision, IRFs transitioning into the PPS were paid a blend of the federal IRF PPS rate and the payment that the IRFs would have received had the IRF PPS not been implemented. This provision also allowed IRFs to elect to bypass this blended payment and immediately be paid 100 percent of the federal IRF PPS rate. The transition methodology expired as of cost reporting periods beginning on or after October 1, 2002 (FY 2003), and payments for all IRFs now consist of 100 percent of the federal IRF PPS rate.
Section 1886(j) of the Act confers broad statutory authority upon the Secretary to propose refinements to the IRF PPS. In the FY 2006 IRF PPS final rule (70 FR 47880) and in correcting amendments to the FY 2006 IRF PPS final rule (70 FR 57166), we finalized a number of refinements to the IRF PPS case-mix classification system (the CMGs and the corresponding relative weights) and the case-level and facility-level adjustments. These refinements included the adoption of the Office of Management and Budget's (OMB) Core-Based Statistical Area (CBSA) market definitions; modifications to the CMGs, tier comorbidities; and CMG relative weights, implementation of a new teaching status adjustment for IRFs; rebasing and revising the market basket index used to update IRF payments, and updates to the rural, low-income percentage (LIP), and high-cost outlier adjustments. Beginning with the FY 2006 IRF PPS final rule (70 FR 47908 through 47917), the market basket index used to update IRF payments was a market basket reflecting the operating and capital cost structures for freestanding IRFs, freestanding inpatient psychiatric facilities (IPFs), and long-term care hospitals (LTCHs) (hereinafter referred to as the rehabilitation, psychiatric, and long-term care (RPL) market basket). Any reference to the FY 2006 IRF PPS final rule in this final rule also includes the provisions effective in the correcting amendments. For a detailed discussion of the final key policy changes for FY 2006, please refer to the FY 2006 IRF PPS final rule.
In the FY 2007 IRF PPS final rule (71 FR 48354), we further refined the IRF PPS case-mix classification system (the CMG relative weights) and the case-level adjustments, to ensure that IRF PPS payments would continue to reflect as accurately as possible the costs of care. For a detailed discussion of the FY 2007 policy revisions, please refer to the FY 2007 IRF PPS final rule.
In the FY 2008 IRF PPS final rule (72 FR 44284), we updated the prospective payment rates and the outlier threshold, revised the IRF wage index policy, and clarified how we determine high-cost outlier payments for transfer cases. For more information on the policy changes implemented for FY 2008, please refer to the FY 2008 IRF PPS final rule.
After publication of the FY 2008 IRF PPS final rule (72 FR 44284), section 115 of the Medicare, Medicaid, and SCHIP Extension Act of 2007 (Pub. L. 110-173, enacted December 29, 2007) (MMSEA) amended section 1886(j)(3)(C) of the Act to apply a zero percent increase factor for FYs 2008 and 2009, effective for IRF discharges occurring on or after April 1, 2008. Section 1886(j)(3)(C) of the Act required the Secretary to develop an increase factor to update the IRF prospective payment rates for each FY. Based on the legislative change to the increase factor, we revised the FY 2008 prospective payment rates for IRF discharges occurring on or after April 1, 2008. Thus, the final FY 2008 IRF prospective payment rates that were published in the FY 2008 IRF PPS final rule (72 FR 44284) were effective for discharges occurring on or after October 1, 2007, and on or before March 31, 2008, and the revised FY 2008 IRF prospective payment rates were effective for discharges occurring on or after April 1, 2008, and on or before September 30, 2008. The revised FY 2008 prospective payment rates are available on the CMS website at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/Data-Files.html.
In the FY 2009 IRF PPS final rule (73 FR 46370), we updated the CMG relative weights, the average LOS values, and the outlier threshold; clarified IRF wage index policies regarding the treatment of “New England deemed” counties and multi-campus hospitals; and revised the regulation text in response to section 115 of the MMSEA to set the IRF compliance percentage at 60 percent (the “60 percent rule”) and continue the practice of including comorbidities in the calculation of compliance percentages. We also applied a zero percent market basket increase factor for FY 2009 in accordance with section 115 of the MMSEA. For more information on the policy changes implemented for FY 2009, please refer to the FY 2009 IRF PPS final rule.
In the FY 2010 IRF PPS final rule (74 FR 39762) and in correcting amendments to the FY 2010 IRF PPS final rule (74 FR 50712), we updated the prospective payment rates, the CMG relative weights, the average LOS values, the rural, LIP, teaching status adjustment factors, and the outlier threshold; implemented new IRF coverage requirements for determining whether an IRF claim is reasonable and necessary; and revised the regulation text to require IRFs to submit patient assessments on Medicare Advantage (MA) (formerly called Medicare Part C) patients for use in the 60 percent rule calculations. Any reference to the FY 2010 IRF PPS final rule in this final rule also includes the provisions effective in the correcting amendments. For more information on the policy changes implemented for FY 2010, please refer to the FY 2010 IRF PPS final rule.
After publication of the FY 2010 IRF PPS final rule (74 FR 39762), section 3401(d) of the Patient Protection and Affordable Care Act (Pub. L. 111-148, enacted March 23, 2010), as amended by section 10319 of the same Act and by section 1105 of the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152, enacted March 30, 2010) (collectively, hereinafter referred to as “PPACA”), amended section 1886(j)(3)(C) of the Act and added section 1886(j)(3)(D) of the Act. Section 1886(j)(3)(C) of the Act requires the Secretary to estimate a multifactor productivity (MFP) adjustment to the market basket increase factor, and to apply other adjustments as defined by the Act. The productivity adjustment applies to FYs from 2012 forward. The other adjustments apply to FYs 2010 to 2019.
Sections 1886(j)(3)(C)(ii)(II) and 1886(j)(3)(D)(i) of the Act defined the adjustments that were to be applied to the market basket increase factors in FYs 2010 and 2011. Under these provisions, the Secretary was required to reduce the market basket increase factor in FY 2010 by a 0.25 percentage point adjustment. Notwithstanding this provision, in accordance with section 3401(p) of the PPACA, the adjusted FY 2010 rate was only to be applied to discharges occurring on or after April 1, 2010. Based on the self-implementing legislative changes to section 1886(j)(3) of the Act, we adjusted the FY 2010 prospective payment rates as required, and applied these rates to IRF discharges occurring on or after April 1, 2010, and on or before September 30, 2010. Thus, the final FY 2010 IRF prospective payment rates that were published in the FY 2010 IRF PPS final rule (74 FR 39762) were used for discharges occurring on or after October 1, 2009, and on or before March 31, 2010, and the adjusted FY 2010 IRF prospective payment rates applied to discharges occurring on or after April 1, 2010, and on or before September 30, 2010. The adjusted FY 2010 prospective payment rates are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRF-Rules-and-Related-Files.html.
In addition, sections 1886(j)(3)(C) and (D) of the Act also affected the FY 2010 IRF outlier threshold amount because they required an adjustment to the FY 2010 RPL market basket increase factor, which changed the standard payment conversion factor for FY 2010. Specifically, the original FY 2010 IRF outlier threshold amount was determined based on the original estimated FY 2010 RPL market basket increase factor of 2.5 percent and the standard payment conversion factor of $13,661. However, as adjusted, the IRF prospective payments were based on the adjusted RPL market basket increase factor of 2.25 percent and the revised standard payment conversion factor of $13,627. To maintain estimated outlier payments for FY 2010 equal to the established standard of 3 percent of total estimated IRF PPS payments for FY 2010, we revised the IRF outlier threshold amount for FY 2010 for discharges occurring on or after April 1, 2010, and on or before September 30, 2010. The revised IRF outlier threshold amount for FY 2010 was $10,721.
Sections 1886(j)(3)(C)(ii)(II) and 1886(j)(3)(D)(i) of the Act also required the Secretary to reduce the market basket increase factor in FY 2011 by a 0.25 percentage point adjustment. The FY 2011 IRF PPS notice (75 FR 42836) and the correcting amendments to the FY 2011 IRF PPS notice (75 FR 70013) described the required adjustments to the FY 2010 and FY 2011 IRF PPS prospective payment rates and outlier threshold amount for IRF discharges occurring on or after April 1, 2010, and on or before September 30, 2011. It also updated the FY 2011 prospective payment rates, the CMG relative weights, and the average LOS values. Any reference to the FY 2011 IRF PPS notice in this final rule also includes the provisions effective in the correcting amendments. For more information on the FY 2010 and FY 2011 adjustments or the updates for FY 2011, please refer to the FY 2011 IRF PPS notice.
In the FY 2012 IRF PPS final rule (76 FR 47836), we updated the IRF prospective payment rates, rebased and revised the RPL market basket, and established a new QRP for IRFs in accordance with section 1886(j)(7) of the Act. We also consolidated, clarified, and revised existing policies regarding IRF hospitals and IRF units of hospitals to eliminate unnecessary confusion and enhance consistency. For more information on the policy changes implemented for FY 2012, please refer to the FY 2012 IRF PPS final rule.
The FY 2013 IRF PPS notice (77 FR 44618) described the required adjustments to the FY 2013 prospective payment rates and outlier threshold amount for IRF discharges occurring on or after October 1, 2012, and on or before September 30, 2013. It also updated the FY 2013 prospective payment rates, the CMG relative weights, and the average LOS values. For more information on the updates for FY 2013, please refer to the FY 2013 IRF PPS notice.
In the FY 2014 IRF PPS final rule (78 FR 47860), we updated the prospective payment rates, the CMG relative weights, and the outlier threshold amount. We also updated the facility-level adjustment factors using an enhanced estimation methodology, revised the list of diagnosis codes that count toward an IRF's 60 percent rule compliance calculation to determine “presumptive compliance,” revised sections of the IRF patient assessment instrument (IRF-PAI), revised requirements for acute care hospitals that have IRF units, clarified the IRF regulation text regarding limitation of review, updated references to previously changed sections in the regulations text, and updated requirements for the IRF QRP. For more information on the policy changes implemented for FY 2014, please refer to the FY 2014 IRF PPS final rule.
In the FY 2015 IRF PPS final rule (79 FR 45872) and the correcting amendments to the FY 2015 IRF PPS final rule (79 FR 59121), we updated the prospective payment rates, the CMG relative weights, and the outlier threshold amount. We also revised the list of diagnosis codes that count toward an IRF's 60 percent rule compliance calculation to determine “presumptive compliance,” revised sections of the IRF-PAI, and updated requirements for the IRF QRP. Any reference to the FY 2015 IRF PPS final rule in this final rule also includes the provisions effective in the correcting amendments. For more information on the policy changes implemented for FY 2015, please refer to the FY 2015 IRF PPS final rule.
In the FY 2016 IRF PPS final rule (80 FR 47036), we updated the prospective payment rates, the CMG relative weights, and the outlier threshold amount. We also adopted an IRF-specific market basket that reflects the cost structures of only IRF providers, a blended 1-year transition wage index based on the adoption of new OMB area delineations, a 3-year phase-out of the rural adjustment for certain IRFs due to the new OMB area delineations, and updates for the IRF QRP. For more information on the policy changes implemented for FY 2016, please refer to the FY 2016 IRF PPS final rule.
In the FY 2017 IRF PPS final rule (81 FR 52056) and the correcting amendments to the FY 2017 IRF PPS final rule (81 FR 59901), we updated the prospective payment rates, the CMG relative weights, and the outlier threshold amount. We also updated requirements for the IRF QRP. Any reference to the FY 2017 IRF PPS final rule in this final rule also includes the provisions effective in the correcting amendments. For more information on the policy changes implemented for FY 2017, please refer to the FY 2017 IRF PPS final rule.
In the FY 2018 IRF PPS final rule (82 FR 36238), we updated the prospective payment rates, the CMG relative weights, and the outlier threshold amount. We also revised the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnosis codes that are used to determine presumptive compliance under the “60 percent rule,” removed the 25 percent payment penalty for IRF-PAI late transmissions, removed the voluntary swallowing status item (Item 27) from the IRF-PAI, summarized comments regarding the criteria used to classify facilities for payment under the IRF PPS, provided for a subregulatory process for certain annual updates to the presumptive methodology diagnosis code lists, adopted the use of height/weight items on the IRF-PAI to determine patient body mass index (BMI) greater than 50 for cases of single-joint replacement under the presumptive methodology, and updated requirements for the IRF QRP. For more information on the policy changes implemented for FY 2018, please refer to the FY 2018 IRF PPS final rule.
In the FY 2019 IRF PPS final rule (83 FR 38514), we updated the prospective payment rates, the CMG relative weights, and the outlier threshold amount. We also alleviated administrative burden for IRFs by removing the FIMTM instrument and associated Function Modifiers from the IRF-PAI beginning in FY 2020 and revised certain IRF coverage requirements to reduce the amount of required paperwork in the IRF setting beginning in FY 2019. Additionally, we incorporated certain data items located in the Quality Indicators section of the IRF-PAI into the IRF case-mix classification system using analysis of 2 years of data (FYs 2017 and 2018) beginning in FY 2020. For the IRF QRP, we adopted a new measure removal factor, removed two measures from the IRF QRP measure set, and codified a number of program requirements in our regulations. For more information on the policy changes implemented for FY 2019, please refer to the FY 2019 IRF PPS final rule.
B. Provisions of the PPACA Affecting the IRF PPS in FY 2012 and Beyond
The PPACA included several provisions that affect the IRF PPS in FYs 2012 and beyond. In addition to what was previously discussed, section 3401(d) of the PPACA also added section 1886(j)(3)(C)(ii)(I) of the Act (providing for a “productivity adjustment” for FY 2012 and each subsequent fiscal year). The productivity adjustment for FY 2020 is discussed in section VI.D. of this final rule. Section 1886(j)(3)(C)(ii)(II) of the Act provides that the application of the productivity adjustment to the market basket update may result in an update that is less than 0.0 for a fiscal year and in payment rates for a fiscal year being less than such payment rates for the preceding fiscal year.
Sections 3004(b) of the PPACA and section 411(b) of the Medicare Access and CHIP Reauthorization Act of 2015 (Pub. L. 114-10, enacted April 16, 2015) (MACRA) also addressed the IRF PPS. Section 3004(b) of PPACA reassigned the previously designated section 1886(j)(7) of the Act to section 1886(j)(8) of the Act and inserted a new section 1886(j)(7) of the Act, which contains requirements for the Secretary to establish a QRP for IRFs. Under that program, data must be submitted in a form and manner and at a time specified by the Secretary. Beginning in FY 2014, section 1886(j)(7)(A)(i) of the Act requires the application of a 2 percentage point reduction to the market basket increase factor otherwise applicable to an IRF (after application of paragraphs (C)(iii) and (D) of section 1886(j)(3) of the Act) for a fiscal year if the IRF does not comply with the requirements of the IRF QRP for that fiscal year. Application of the 2 percentage point reduction may result in an update that is less than 0.0 for a fiscal year and in payment rates for a fiscal year being less than such payment rates for the preceding fiscal year. Reporting-based reductions to the market basket increase factor are not cumulative; they only apply for the FY involved. Section 411(b) of MACRA amended section 1886(j)(3)(C) of the Act by adding paragraph (iii), which required us to apply for FY 2018, after the application of section 1886(j)(3)(C)(ii) of the Act, an increase factor of 1.0 percent to update the IRF prospective payment rates.
C. Operational Overview of the Current IRF PPS
As described in the FY 2002 IRF PPS final rule (66 FR 41316), upon the admission and discharge of a Medicare Part A Fee-for-Service (FFS) patient, the IRF is required to complete the appropriate sections of a PAI, designated as the IRF-PAI. In addition, beginning with IRF discharges occurring on or after October 1, 2009, the IRF is also required to complete the appropriate sections of the IRF-PAI upon the admission and discharge of each Medicare Advantage (MA) patient, as described in the FY 2010 IRF PPS final rule (74 FR 39762 and 74 FR 50712). All required data must be electronically encoded into the IRF-PAI software product. Generally, the software product includes patient classification programming called the Grouper software. The Grouper software uses specific IRF-PAI data elements to classify (or group) patients into distinct CMGs and account for the existence of any relevant comorbidities.
The Grouper software produces a five-character CMG number. The first character is an alphabetic character that indicates the comorbidity tier. The last four characters are numeric characters that represent the distinct CMG number. Free downloads of the Inpatient Rehabilitation Validation and Entry (IRVEN) software product, including the Grouper software, are available on the CMS website at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/Software.html.
Once a Medicare Part A FFS patient is discharged, the IRF submits a Medicare claim as a Health Insurance Portability and Accountability Act of 1996 (Pub. L. 104-191, enacted August 21, 1996) (HIPAA) compliant electronic claim or, if the Administrative Simplification Compliance Act of 2002 (Pub. L. 107-105, enacted December 27, 2002) (ASCA) permits, a paper claim (a UB-04 or a CMS-1450 as appropriate) using the five-character CMG number and sends it to the appropriate Medicare Administrative Contractor (MAC). In addition, once a MA patient is discharged, in accordance with the Medicare Claims Processing Manual, chapter 3, section 20.3 (Pub. 100-04), hospitals (including IRFs) must submit an informational-only bill (Type of Bill (TOB) 111), which includes Condition Code 04 to their MAC. This will ensure that the MA days are included in the hospital's Supplemental Security Income (SSI) ratio (used in calculating the IRF LIP adjustment) for fiscal year 2007 and beyond. Claims submitted to Medicare must comply with both ASCA and HIPAA.
Section 3 of the ASCA amended section 1862(a) of the Act by adding paragraph (22), which requires the Medicare program, subject to section 1862(h) of the Act, to deny payment under Part A or Part B for any expenses for items or services for which a claim is submitted other than in an electronic form specified by the Secretary. Section 1862(h) of the Act, in turn, provides that the Secretary shall waive such denial in situations in which there is no method available for the submission of claims in an electronic form or the entity submitting the claim is a small provider. In addition, the Secretary also has the authority to waive such denial in such unusual cases as the Secretary finds appropriate. For more information, see the “Medicare Program; Electronic Submission of Medicare Claims” final rule (70 FR 71008). Our instructions for the limited number of Medicare claims submitted on paper are available at http://www.cms.gov/manuals/downloads/clm104c25.pdf.
Section 3 of the ASCA operates in the context of the administrative simplification provisions of HIPAA, which include, among others, the requirements for transaction standards and code sets codified in 45 CFR part 160 and part 162, subparts A and I through R (generally known as the Transactions Rule). The Transactions Rule requires covered entities, including covered health care providers, to conduct covered electronic transactions according to the applicable transaction standards. (See the CMS program claim memoranda at http://www.cms.gov/ElectronicBillingEDITrans/ and listed in the addenda to the Medicare Intermediary Manual, Part 3, section 3600).
The MAC processes the claim through its software system. This software system includes pricing programming called the “Pricer” software. The Pricer software uses the CMG number, along with other specific claim data elements and provider-specific data, to adjust the IRF's prospective payment for interrupted stays, transfers, short stays, and deaths, and then applies the applicable adjustments to account for the IRF's wage index, percentage of low-income patients, rural location, and outlier payments. For discharges occurring on or after October 1, 2005, the IRF PPS payment also reflects the teaching status adjustment that became effective as of FY 2006, as discussed in the FY 2006 IRF PPS final rule (70 FR 47880).
D. Advancing Health Information Exchange
The Department of Health and Human Services (HHS) has a number of initiatives designed to encourage and support the adoption of interoperable health information technology and to promote nationwide health information exchange to improve health care. The Office of the National Coordinator for Health Information Technology (ONC) and CMS work collaboratively to advance interoperability across settings of care, including post-acute care.
To further interoperability in post-acute care, we developed a Data Element Library (DEL) to serve as a publicly-available centralized, authoritative resource for standardized data elements and their associated mappings to health IT standards. The DEL furthers CMS' goal of data standardization and interoperability. These interoperable data elements can reduce provider burden by allowing the use and exchange of healthcare data, support provider exchange of electronic health information for care coordination, person-centered care, and support real-time, data driven, clinical decision making. Standards in the Data Element Library (https://del.cms.gov/) can be referenced on the CMS website and in the ONC Interoperability Standards Advisory (ISA). The 2019 ISA is available at https://www.healthit.gov/isa.
The 21st Century Cures Act (Pub. L. 114-255, enacted December 13, 2016) (Cures Act), requires HHS to take new steps to enable the electronic sharing of health information ensuring interoperability for providers and settings across the care continuum. In another important provision, Congress defined “information blocking” as practices likely to interfere with, prevent, or materially discourage access, exchange, or use of electronic health information, and established new authority for HHS to discourage these practices. In March 2019, ONC and CMS published the proposed rules, “21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program,” (84 FR 7424) and “Interoperability and Patient Access” (84 FR 7610) to promote secure and more immediate access to health information for patients and healthcare providers through the implementation of information blocking provisions of the Cures Act and the use of standardized application programming interfaces (APIs) that enable easier access to electronic health information. We solicited comment on the two proposed rules. We invited providers to learn more about these important developments and how they are likely to affect IRFs.
II. Summary of Provisions of the Proposed Rule
In the FY 2020 IRF PPS proposed rule, we proposed to update the IRF prospective payment rates for FY 2020 and to rebase and revise the IRF market basket to reflect a 2016 base year rather than the current 2012 base year. We also proposed to replace the previously finalized unweighted motor score with a weighted motor score to assign patients to CMGs and remove one item from the score beginning with FY 2020 and to revise the CMGs and update the CMG relative weights and average LOS values beginning with FY 2020, based on analysis of 2 years of data (FYs 2017 and 2018). We also proposed to use the concurrent FY IPPS wage index for the IRF PPS beginning with FY 2020. We also solicited comments on stakeholder concerns regarding the appropriateness of the wage index used to adjust IRF payments. We proposed to amend the regulations at § 412.622 to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF.
The proposed policy changes and updates to the IRF prospective payment rates for FY 2020 are as follows:
- Describe a proposed weighted motor score to replace the previously finalized unweighted motor score to assign a patient to a CMG, the removal of one item from the score, and revisions to the CMGs beginning on October 1, 2019, based on analysis of 2 years of data (FYs 2017 and 2018) using the Quality Indicator items in the IRF-PAI. This includes proposed revisions to the CMG relative weights and average LOS values for FY 2020, in a budget neutral manner, as discussed in section III. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17249 through 17260).
- Describe the proposed rebased and revised IRF market basket to reflect a 2016 base year rather than the current 2012 base year as discussed in section V. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17261 through 17273).
- Update the IRF PPS payment rates for FY 2020 by the proposed market basket increase factor, based upon the most current data available, with a proposed productivity adjustment required by section 1886(j)(3)(C)(ii)(I) of the Act, as described in section V. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17274 through 17275).
- Describe the proposed update to the IRF wage index to use the concurrent FY IPPS wage index and the FY 2020 proposed labor-related share in a budget-neutral manner, as described in section V. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17276 through 17279).
- Describe the continued use of FY 2014 facility-level adjustment factors, as discussed in section IV. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17260 through 17261).
- Describe the calculation of the IRF standard payment conversion factor for FY 2020, as discussed in section V. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17280 through 17282).
- Update the outlier threshold amount for FY 2020, as discussed in section VI. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17283 through 17284).
- Update the cost-to-charge ratio (CCR) ceiling and urban/rural average CCRs for FY 2020, as discussed in section VI. of the FY 2020 IRF PPS proposed rule (84 FR 17244 at 17284).
- Describe the proposed amendments to the regulations at § 412.622 to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF, as discussed in section VII. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17284 through 17285).
- Updates to the requirements for the IRF QRP, as discussed in section VIII. of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17285 through 17330).
III. Analysis and Response to Public Comments
We received 1,257 timely responses from the public, many of which contained multiple comments on the FY 2020 IRF PPS proposed rule (84 FR 17244). The majority consisted of form letters, in which we received multiple copies of two types of identically-worded letters that had been signed and submitted by different individuals. We received comments from various trade associations, IRFs, individual physicians, therapists, clinicians, health care industry organizations, and health care consulting firms. The following sections, arranged by subject area, include a summary of the public comments that we received, and our responses.
IV. Refinements to the Case-Mix Classification System Beginning With FY 2020
A. Background
Section 1886(j)(2)(A) of the Act requires the Secretary to establish CMGs for payment under the IRF PPS and a method of classifying specific IRF patients within these groups. Under section 1886(j)(2)(B) of the Act, the Secretary must assign each CMG an appropriate weighting factor that reflects the relative facility resources used for patients classified within the group as compared to patients classified within other groups. Additionally, section 1886(j)(2)(C)(i) of the Act requires the Secretary from time to time to adjust the established classifications and weighting factors as appropriate to reflect changes in treatment patterns, technology, case-mix, number of payment units for which payment is made under title XVIII of the Act, and other factors which may affect the relative use of resources. Such adjustments must be made in a manner so that changes in aggregate payments under the classification system are a result of real changes and are not a result of changes in coding that are unrelated to real changes in case mix.
In the FY 2019 IRF PPS final rule (83 FR 38533 through 38549), we finalized the removal of the Functional Independence Measure (FIMTM) instrument and associated Function Modifiers from the IRF-PAI and the incorporation of an unweighted additive motor score derived from 19 data items located in the Quality Indicators section of the IRF-PAI beginning with FY 2020 (83 FR 38535 through 38536, 38549). As discussed in section IV.B of this final rule, based on further analysis to examine the potential impact of weighting the motor score, we proposed to replace the previously finalized unweighted motor score with a weighted motor score and remove one item from the score beginning with FY 2020.
Additionally, as noted in the FY 2019 IRF PPS final rule (83 FR 38534), the incorporation of the data items from the Quality Indicator section of the IRF-PAI into the IRF case-mix classification system necessitates revisions to the CMGs to ensure that IRF payments are calculated accurately. We finalized the use of data items from the Quality Indicators section of the IRF-PAI to construct the functional status scores used to classify IRF patients in the IRF case-mix classification system for purposes of establishing payment under the IRF PPS beginning with FY 2020, but modified our proposal based on public comments to incorporate 2 years of data (FYs 2017 and 2018) into our analyses used to revise the CMG definitions (83 FR 38549). We stated that any changes to the proposed CMG definitions resulting from the incorporation of an additional year of data (FY 2018) into the analysis would be addressed in future rulemaking prior to their implementation beginning in FY 2020. As discussed in section III.C of the FY 2020 IRF PPS proposed rule (84 FR 17244, 17250 through 17260), we proposed to revise the CMGs based on analysis of 2 years of data (FYs 2017 and 2018) beginning with FY 2020. We also proposed to update the relative weights and average LOS values associated with the revised CMGs beginning with FY 2020.
B. Proposed Use of a Weighted Motor Score Beginning With FY 2020
As noted in the FY 2019 IRF PPS final rule (83 FR 38535), the IRF case-mix classification system currently uses a weighted motor score based on FIMTM data items to assign patients to CMGs under the IRF PPS through FY 2019. More information on the development and implementation of this motor score can be found in the FY 2006 IRF PPS final rule (70 FR 47896 through 47900). In the FY 2019 IRF PPS final rule (83 FR 38535 through 38536, 38549), we finalized the incorporation of an unweighted additive motor score derived from 19 data items located in the Quality Indicators section of the IRF-PAI beginning with FY 2020. We did not propose a weighted motor score at the time, because we believed that the unweighted motor score would facilitate greater understanding among the provider community, as it is less complex. However, we also noted that we would take comments in favor of a weighted motor score into consideration in future analysis. In response to feedback we received from various stakeholders and professional organizations regarding the use of an unweighted motor score and requesting that we consider weighting the motor score, we extended our contract with Research Triangle Institute, International (RTI) to examine the potential impact of weighting the motor score. Based on this analysis, discussed further below, we believed that a weighted motor score would improve the accuracy of payments to IRFs and proposed to replace the previously finalized unweighted motor score with a weighted motor score to assign patients to CMGs beginning with FY 2020.
The previously finalized motor score is calculated by summing the scores of the 19 data items, with equal weight applied to each item. The 19 data items are (83 FR 38535):
- GG0130A1 Eating.
- GG0130B1 Oral hygiene.
- GG0130C1 Toileting hygiene.
- GG0130E1 Shower/bathe self.
- GG0130F1 Upper-body dressing.
- GG0130G1 Lower-body dressing.
- GG0130H1 Putting on/taking off footwear.
- GG0170A1 Roll left and right.
- GG0170B1 Sit to lying.
- GG0170C1 Lying to sitting on side of bed.
- GG0170D1 Sit to stand.
- GG0170E1 Chair/bed-to-chair transfer.
- GG0170F1 Toilet transfer.
- GG0170I1 Walk 10 feet.
- GG0170J1 Walk 50 feet with two turns.
- GG0170K1 Walk 150 feet.
- GG0170M1 One step curb.
- H0350 Bladder continence.
- H0400 Bowel continence.
In response to feedback we received from various stakeholders and professional organizations requesting that we consider applying weights to the motor score, we extended our contract with RTI to explore the potential of applying unique weights to each of the 19 items in the motor score.
As part of their analysis, RTI examined the degree to which the items used to construct the motor score were related to one another and adjusted their weighting methodology to account for their findings. RTI considered a number of different weighting methodologies to develop a weighted index that would increase the predictive power of the IRF case-mix classification system while at the same time maintaining simplicity. RTI used regression analysis to explore the relationship of the motor score items to costs. This analysis was undertaken to determine the impact of each of the items on cost and then to weight each item in the index according to its relative impact on cost. Based on findings from this analysis, we proposed to remove the item GG0170A1 Roll left and right from the motor score as this item was found to have a high degree of multicollinearity with other items in the motor score and would have resulted in either a negative or non-significant coefficient. As such, we did not believe it would be appropriate to include this item in the motor score calculation. Using the revised motor score composed of the remaining 18 items identified above, RTI designed a weighting methodology for the motor score that could be applied uniformly across all RICs. For a more detailed discussion of the analysis used to construct the weighted motor score, we refer readers to the March 2019 technical report entitled “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System”, available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/Research.html. Findings from this analysis suggested that the use of a weighted motor score index slightly improves the ability of the IRF PPS to predict patient costs. Based on this analysis, we proposed to use a weighted motor score for the purpose of determining IRF payments.
Table 1 shows the proposed weights for each component of the motor score, averaged to 1, obtained through the regression analysis.

We proposed to determine the motor score by applying each of the weights indicated in Table 1 to the score of each corresponding item, as finalized in the FY 2019 IRF PPS final rule (83 FR 38535 through 38537), and then summing the weighted scores for each of the 18 items that compose the motor score.
We received several comments on the proposal to replace the previously finalized unweighted motor score with a weighted motor score to assign patients to CMGs under the IRF PPS and our proposal to remove the item GG0170A1 Roll left and right from the calculation of the motor score beginning with FY 2020, that is, for all discharges beginning on or after October 1, 2019. As summarized in more detail below, with the exception of one comment from MedPAC, the commenters overwhelmingly requested that CMS delay implementation of a weighted motor score and use an unweighted motor score to assign patients to CMGs until we can more fully analyze and work with stakeholders on developing a weighted motor score methodology.
In response to public comments, we carefully considered whether to finalize the proposed weighted motor score or go back to using an unweighted motor score to assign patients to CMGs. Although the proposed weighted motor score results in a slight improvement in the ability of the IRF PPS to predict patient costs and thus the accuracy of IRF PPS payments (less than 0.18 difference in accuracy between the weighted and the unweighted motor scores), we acknowledge the unweighted motor score is conceptually simpler and, as such, believe it will ease providers' transition to the use of the data items located in the Quality Indicators section of the IRF-PAI (also referred to as section GG items). Thus, we are finalizing based on public comments the use of an unweighted motor score to assign patients to CMGs beginning with FY 2020. We appreciate the commenters' suggestions on the weighting methodology and will take them into consideration as we explore possible refinements to the case-mix classification system in the future.
Comment: Although several commenters noted appreciation for the fact that we analyzed a weighted motor score in response to their comments on the FY 2019 IRF PPS proposed rule (83 FR 38546), these same commenters expressed concerns with the actual weight values that CMS proposed for FY 2020, as indicated in Table 1, and stated that we should go back to an unweighted motor score so that we can do further analysis and collaborate with stakeholders to further refine the weighting methodology. Some commenters expressed concern that CMS might be proposing higher weights for the self-care items than for the mobility items, in contrast to the current weighted motor score, which weights mobility items higher than self-care items. Some commenters specifically requested that CMS explain why the weight for the eating item increased from 0.6 under the current weighting methodology to 2.7 under the proposed methodology, and requested we explain what we believe this change will mean for patients with eating deficits. Commenters were also generally concerned by what they suggested were large differences in the weight value assignments between the current and proposed motor score.
Response: We used simple ordinary least squares regression analysis of the data that IRFs submitted to us in FYs 2017 and 2018 to calculate the proposed weight values for the motor score, in response to stakeholder feedback on the FY 2019 IRF PPS proposed rule (83 FR 38546). Commenters are correct that the proposed weights for the motor score items, in comparison with the current weights, shift some of the weight from the mobility to the self-care items. We also note that the proposed weights assigned to the bowel and bladder function items increased compared with the current weights. These changes are all reflective of the data the IRFs submitted to us in FYs 2017 and 2018.
Regarding the proposed increase in the weight for the eating item, it is important to note key differences in the coding guidelines between the FIMTM eating item and the section GG eating item that may have contributed to the change in the relative importance of this item for predicting IRF costs. For item GG0130A, Eating, assistance with tube feedings is not considered when coding this item. If a patient does not eat or drink by mouth but is instead tube fed, item GG0130A must be coded as 88—“Not attempted due to medical condition or safety concerns” or 09—“Not applicable”. Both of these responses would be recoded to a 01—“Dependent” for the purposes of assigning the patient to a CMG. This differs from the coding instructions for the FIMTM eating item used in the current motor score, which takes into consideration assistance with tube feedings in scoring the item. For example, according to the FIMTM instructions, a patient who could administer the tube feeding completely independently could receive a score of 7-Complete independence on the eating item.
In regards to the suggested differences in the weight value assignments between the current and proposed methodologies, we note that in certain cases the proposed weights were divided among multiple items in the motor score that were found to be highly correlated to avoid overweighting any particular measure of function. For instance, the three items (GG0170I1, GG0170J1, and GG0170K1) that assess walking function were each assigned a proposed weight of 0.8. When summed together, the weight value for walking under the proposed methodology is 2.4, which is slightly higher than the weight value of 1.6 for the single walking item used in the current motor score.
Comment: One commenter disagreed with the removal of item GG0170A1 roll left and right from the motor score and noted it is an important functional task in the IRF setting. Some commenters questioned the use of averaging values across pairs of items that were correlated and inquired why the roll left and right item was removed from the motor score while other correlated items were not removed. Commenters also inquired about the use of the item “walk 10 feet” to derive the weights for the “walk 50 feet” and “walk 150 feet” items.
Response: We appreciate the commenter's concerns regarding the removal of item GG0170A1 from the motor score. As described in detail in the technical report, “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System,” the roll left and right item was found to have a high degree of multicollinearity with other standardized patient assessment elements and to be inversely correlated with costs after controlling for each of the other self-care and mobility items. This relationship persisted when this item was paired with the other correlated items. The continued inclusion of this item in the motor score would have resulted in either a negative or non-significant coefficient. As such, we do not believe it is appropriate to include this item in the construction of the motor score. The other item pairs that were found to be correlated did not generate negative or non-significant coefficients, and were therefore maintained in the calculation of the motor score.
Unlike the FIMTM instrument, the items from the quality indicator section of the IRF-PAI sometimes use more than one item to measure functional areas. As discussed in more detail in the technical report, we noted that a few items were found to be highly correlated. Because of the correlation, we proposed to use an average score for some items so as to avoid introducing bias or inappropriately overweighting any particular functional area. We note this methodology is consistent with the methodology used under the Patient Driven Payment Model (PDPM), as described in more detail in the FY 2019 SNF final rule (83 FR 39204) and the accompanying technical report entitled “Skilled Nursing Facilities Patient-Driven Payment Model Technical Report” available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/SNFPPS/therapyresearch.html.
Regarding the “walk 10 feet” item, that item was used to derive the weights for the “walk 50 feet” and “walk 150 feet” items as these three items were found to be highly correlated and the “walk 150 feet” item had a high proportion of observations coded on admission with “activity not attempted” codes.
Comment: Some commenters requested that CMS apply the current motor score weights associated with the FIMTM items to the revised motor score while other commenters requested that CMS postpone weighting the motor score until additional data can be collected and analyzed. While a few commenters were supportive of using a weighted motor score, other commenters suggested that CMS use a 1-year payment model or phase in the use of a weighted motor score.
Response: We do not believe it would be appropriate to apply the weight values associated with the FIMTM items to the components of the revised motor score, as these weights would not accurately reflect how the various components of the revised motor score contribute to predicting patient costs. We used simple ordinary least squares regression analysis of the data that IRFs submitted to us in FYs 2017 and 2018 to calculate the proposed weight values for the revised motor score. Changes in patient demographics, treatment practices, technology, and other factors that may affect the relative use of resources in an IRF since the motor score weights were originally calculated have likely contributed to changes in the weight values applied across the self-care and mobility items. We proposed to apply weights to the motor score items because RTI's analysis indicated that a weighted motor score would improve the classification of patients into CMGs, which in turn would improve the accuracy of payments to IRFs. However, as discussed above, in response to public comments, we carefully considered whether to finalize the proposed weighted motor score or go back to using an unweighted motor score to assign patients to CMGs. Although the proposed weighted motor score results in a slight improvement in the ability of the IRF PPS to predict patient costs and thus the accuracy of IRF PPS payments (less than 0.18 difference in accuracy between the weighted and the unweighted motor scores), we acknowledge the unweighted motor score is conceptually simpler and, as such, believe it will ease providers' transition to the use of the data items located in the Quality Indicators section of the IRF-PAI (also referred to as section GG items). Thus, we are finalizing based on public comments the use of an unweighted motor score, in which each of the 18 items have a weight of 1, to assign patients to CMGs beginning with FY 2020.
Comment: Commenters expressed concern that the analysis performed by RTI did not explicitly follow the analysis conducted by RAND when the motor score weights were developed for FY 2006 (70 FR 47896 through 47900) and that RTI based their analyses on 2 years of data instead of several years of data. Additionally, commenters requested more information on the other weighting methodologies that RTI considered.
Response: We disagree with the commenters that the RAND analysis for FY 2006 used more years of data than RTI's analysis for the FY 2020 proposed rule. As discussed in the FY 2006 IRF PPS final rule (70 FR 47897), RAND performed regression analysis on less than 2 full years of data (calendar year (CY) 2002 and FY 2003) to derive the current motor score weights. In contrast, RTI used 2 full years of data (FYs 2017 and 2018) to perform the analysis for the weighted motor score proposed in the FY 2020 IRF PPS proposed rule. As the FYs 2017 and 2018 data portrays the most recent and complete picture of patients under the IRF PPS, we believe it was sufficient and appropriate to utilize for the analysis for the proposed rule.
While RTI utilized a different weighting methodology than was used by RAND in 2006, the overall model prediction using the weighted motor score developed by RAND and the weighted motor score developed by RTI is extremely similar. The model using the CMGs based on the standardized patient assessment data elements and comorbidity tiers to predict wage-adjusted costs of care has an r-squared value is 0.3358, while the r-squared value is 0.3169 for the CMGs in the current IRF PPS. This is indicative of similar model performance regardless of model specification. The item weights that the RAND work notes as “optimally weighted” are weights that were constructed separately for each RIC. These were not the weights that were used in the final weights developed by RAND.
RTI also examined weighing methodologies utilizing a general linear model (GLM) and log transformed ordinary least squares (OLS) regression models, as well as the OLS model described in more detail in the technical report. All three models had comparable model fit and generated similar item weights. Based on the greater simplicity achieved through the use of the OLS regression model we believe using the OLS regression was appropriate to maintain simplicity and transparency in the payment system.
Comment: Commenters disagreed with the omission of the wheelchair mobility items from the items used to construct the motor score.
Response: We appreciate the commenters' concerns about wheelchair-dependent patients. As most recently discussed in the FY 2019 IRF PPS final rule (83 FR 38546) in response to similar stakeholder comments, we explained our rationale for not including the wheelchair mobility items in the construction of the finalized motor score. We continue to believe that the higher resource needs of wheelchair dependent patients in IRFs will be better accounted for by not including a wheelchair item in the motor score at this time. Patients that are considered wheelchair dependent or unable to walk will be accounted for through the “not attempted” response codes captured through other items, especially some of the walking items, that are included in the motor score. In this way, we ensure that IRFs will be appropriately compensated for the higher costs they incur in treating wheelchair-dependent patients. We refer readers to the FY 2019 IRF PPS final rule (83 FR 38546) and the technical report entitled “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System” for more information on the rationale as to why this item was not included in the calculation of the motor score.
Comment: Commenters expressed concern with the weighted motor score and questioned the reliability and validity of the weighted motor score. Some commenters stated that they believe the weighted and unweighted motor scores have shown little to no correlation with the weighted motor score currently in use, and therefore, questioned if the weighted motor score could accurately measure patient severity.
Response: We disagree with the commenters' suggestion that unweighted and weighted motor scores have shown little to no correlation with the weighted motor score currently in use as our analysis shows a strong correlation between the scores. In addition, each of the proposed Quality Indicators data items that were included in the motor score were found to have statistically significant correlation with IRF costs. As discussed in the technical report “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System” the use of a weighted motor score was found to increase the predictive ability of the payment model.
Comment: Commenters requested that CMS make available the data utilized in the analyses including patient assessment data, matching claims data, and additional facility and cost report data to enable stakeholders to replicate the analyses.
Response: We appreciate the commenters' feedback regarding the types of information that would be most useful to them in replicating our analyses. We are unable to make patient assessment and claims data publicly available on the CMS website because these data contain personally identifiable information. However, we believe that we released sufficient information in the proposed rule, the accompanying data files, and the technical report entitled “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System,” to enable stakeholders to submit meaningful comments on the underlying analyses and methodologies used to revise the IRF case-mix classification system, to pose alternative approaches, and to assess the impacts of the proposed revisions.
Comment: A few commenters noted that they did not believe that CMS has performed the thorough data analyses and engagement with the provider community that are necessary prior to making significant changes to the existing IRF PPS. These commenters requested that we solicit additional feedback from the stakeholder community, including convening technical advisory panels (TEPs), to provide additional transparency into the underlying analyses and to delay implementation of a weighted motor score until we conduct additional engagements with stakeholders.
Response: We value transparency in our processes and will continue to engage stakeholders in future development of payment policies. We appreciate the offers from stakeholders to assist in the development of future revisions to payment policies and we recognize the value from these partnerships. However, for something as analytically simple as running a regression analysis to determine the weights for the motor score items that best reflect patients' resource needs in the IRF, we do not believe that a TEP is necessary.
As noted above, although the proposed weighted motor score results in a slight improvement in the ability of the IRF PPS to predict patient costs and thus the accuracy of IRF PPS payments (less than 0.18 difference in accuracy between the weighted and the unweighted motor scores), we acknowledge the unweighted motor score is conceptually simpler and, as such, believe it will ease providers' transition to the use of the data items located in the Quality Indicators section of the IRF-PAI (also referred to as section GG items). Thus, we are finalizing based on public comments the use of an unweighted motor score to assign patients to CMGs beginning with FY 2020. We appreciate the stakeholders' comments on this topic and will take them into consideration for future analysis.
Comment: A few commenters requested that CMS provide additional information regarding the provider specific impact analysis file that accompanied the rule, such as a data dictionary describing the data used to calculate the impacts.
Response: In conjunction with the release of the FY 2020 IRF PPS proposed rule, we posted a provider-specific impact analysis file that compared estimated payments to providers for FY 2020 without the proposed revisions to the CMGs with estimated payments to providers for FY 2020 with the proposed revisions to the CMGs. We believe that this file gives IRFs added information to enable them to see how their individual payments would be affected by the proposed changes to the CMGs. We updated this provider specific impact analysis file shortly after it was initially posted to include additional information regarding the underlying data used to calculate the provider specific impacts, and we believe that this additional information is responsive to commenters' requests. The file can be downloaded from the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRF-Rules-and-Related-Files.html. We appreciate the commenters' suggestions regarding the additional types of information that would be most useful to them to further facilitate understanding of our analyses.
As previously discussed, we proposed a weighted motor score as it was found to slightly improve the predicative ability of the case-mix system and thus the accuracy of IRF PPS payments. However, nearly all of the comments we received requested that we revert to an unweighted motor score for the various reasons discussed above. While we continue to believe that a weighted motor score is slightly more accurate, the difference is small, and in light of the conceptual simplicity achieved through the use of an unweighted motor score, which we believe will ease providers' transition to the use of the data items located in the Quality Indicators section of the IRF-PAI, we are finalizing the use of an unweighted motor score, in which each of the 18 items used in the score have an equal weight of 1, to assign patients to CMGs beginning with FY 2020. Additionally, we are finalizing the proposed removal of one item (GG0170A1 Roll left to right) from the motor score beginning with FY 2020. Effective for all discharges beginning on or after October 1, 2019, we will use an unweighted motor score as indicated in Table 2 to determine a beneficiary's CMG placement.

C. Revisions to the CMGs and Updates to the CMG Relative Weights and Average Length of Stay Values Beginning With FY 2020
In the FY 2019 IRF PPS final rule (83 FR 38549), we finalized the use of data items from the Quality Indicators section of the IRF-PAI to construct the functional status scores used to classify IRF patients in the IRF case-mix classification system for purposes of establishing payment under the IRF PPS beginning with FY 2020, but modified our proposal based on public comments to incorporate 2 years of data (FYs 2017 and 2018) into our analyses used to revise the CMG definitions. We stated that any changes to the proposed CMG definitions resulting from the incorporation of an additional year of data (FY 2018) into the analysis would be addressed in future rulemaking prior to their implementation beginning in FY 2020. Additionally, we stated that we would also update the relative weights and average LOS values associated with any revised CMG definitions in future rulemaking.
As noted in the FY 2020 IRF PPS proposed rule (84 FR 17251), we continued our contract with RTI to support us in developing proposed revisions to the CMGs used under the IRF PPS based on analysis of 2 years of data (FYs 2017 and 2018). The process RTI uses for its analysis, which is based on a Classification and Regression Tree (CART) algorithm, is described in detail in the FY 2019 IRF PPS final rule (83 FR 38536 through 38540). RTI used this analysis to revise the CMGs utilizing FYs 2017 and 2018 claim and assessment data and to develop revised CMGs that reflect the use of the data items collected in the Quality Indicators section of the IRF-PAI, incorporating the proposed weighted motor score described in the FY 2020 IRF PPS proposed rule. However, as discussed in section IV.B of this final rule, we are finalizing based on public comments the use of an unweighted motor score to assign patients to a CMGs beginning in with FY 2020.
To develop the proposed revised CMGs, RTI used CART analysis to divide patients into payment groups based on similarities in their clinical characteristics and relative costs. As part of this analysis, RTI imposed some typically-used constraints on the payment group divisions (for example, on the minimum number of cases that could be in the resulting payment groups and the minimum dollar payment amount differences between groups) to identify the optimal set of payment groups. For a more detailed discussion of the analysis used to revise the CMGs for FY 2020, we refer readers to the March 2019 technical report entitled, “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System” available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/Research.html. Additionally, we refer readers to the FY 2020 IRF PPS proposed rule (84 FR 17250 through 17260) for more information on the proposed revisions to the CMGs.
As noted above, we are finalizing the use of an unweighted motor score beginning with FY 2020. As the motor score is a key input in the CART analysis used to revise the CMGs, the use of the unweighted motor score required that the CART analysis be rerun utilizing the unweighted motor score. RTI utilized the same methodology described in the FY 2020 IRF PPS proposed rule (84 FR 17250 through 17260) to support us in developing revisions to the CMGs, incorporating the unweighted motor score, as described in section IV.B of this final rule. The revised CMGs can be found in Table 3.
After developing the revised CMGs, RTI then calculated the relative weights and average LOS values for each revised CMG using the same methodologies that we have used to update the CMG relative weights and average LOS values each fiscal year since 2009 (when we implemented an update to this methodology). More information about the methodology used to update the CMG relative weights can be found in the FY 2009 IRF PPS final rule (73 FR 46372 through 46374). For FY 2020, we proposed to use the FYs 2017 and 2018 IRF claims and FY 2017 IRF cost report data to update the CMG relative weights and average LOS values. In calculating the CMG relative weights, we use a hospital-specific relative value method to estimate operating (routine and ancillary services) and capital costs of IRFs. As noted in the FY 2019 IRF PPS final rule (83 FR 38521), this is the same methodology that we have used to update the CMG relative weights and average LOS values each fiscal year since we implemented an update to the methodology in the FY 2009 IRF PPS final rule (73 FR 46372 through 46374). More information on the methodology used to update calculate the CMG relative weights and average LOS values can found in the March 2019 technical report entitled “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System” available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/Research.html. Consistent with the methodology that we have used to update the IRF classification system in each instance in the past, we proposed to update the relative weights associated with the revised CMGs for FY 2020 in a budget neutral manner by applying a budget neutrality factor to the standard payment amount. To calculate the appropriate budget neutrality factor for use in updating the FY 2020 CMG relative weights, we used the following steps:
Step 1. Calculate the estimated total amount of IRF PPS payments for FY 2020 (with no changes to the CMG relative weights).
Step 2. Calculate the estimated total amount of IRF PPS payments for FY 2020 by applying the changes to the CMGs and the associated CMG relative weights (as described in this final rule).
Step 3. Divide the amount calculated in step 1 by the amount calculated in step 2 to determine the budget neutrality factor (1.0016) that would maintain the same total estimated aggregate payments in FY 2020 with and without the changes to the CMGs and the associated CMG relative weights.
Step 4. Apply the budget neutrality factor (1.0016) to the FY 2019 IRF PPS standard payment amount after the application of the budget-neutral wage adjustment factor.
We note that, as we typically do, we updated our data between the FY 2020 IRF PPS proposed and final rules to ensure that we use the most recent available data in calculating IRF PPS payments. Additionally, we are finalizing the use of unweighted motor score beginning in with FY 2020 which generated revisions to the CMGs and relative weights. Based on our analysis using this updated data and an unweighted motor score, we now estimate a budget neutrality factor of (1.0010) to maintain the same total estimated aggregate payments in FY 2020 with and without the changes to the CMGs and the associated CMG relative weights. For FY 2020 we will apply the budget neutrality factor (1.0010) to the FY 2019 IRF PPS standard payment amount after the application of the budget-neutral wage adjustment factor.
The relative weights and average LOS values for those revised CMGs (found in Table 3) were calculated using the same methodology described in the FY 2020 IRF PPS proposed rule, which is the same methodology that we have used to update the CMG relative weights and average LOS values each fiscal year since we implemented an update to the methodology in FY 2009. The revised CMGs (reflecting the unweighted motor score) and their respective descriptions, as well as the comorbidity tiers, corresponding relative weights and the average LOS values for each CMG and tier for FY 2020 are shown in Table 3. The average LOS for each CMG is used to determine when an IRF discharge meets the definition of a short-stay transfer, which results in a per diem case level adjustment. In section V.H. of this final rule, we discuss the proposed use of the existing methodology to calculate the standard payment conversion factor for FY 2020.
We received a number of comments on the proposed revisions to the CMGs based on analysis of 2 years of data (FYs 2017 and 2018) and the proposed updates to the relative weights and average LOS values associated with the revised CMGs beginning with FY 2020, that is, for all discharges beginning on or after October 1, 2019, which are summarized below.
Comment: A number of commenters were appreciative of the use of 2 years of data to revise the CMGs; however, commenters expressed concern with the proposed CMG revisions and suggested that these changes could result in payment rate compression or a misalignment between payments and the costs of caring for patients. Commenters suggested payment compression would result in reduced payments for higher acuity patients and increased payments for lower acuity patients which could compromise access to care for patients with certain impairments. Additionally, some commenters questioned why there would be fewer CMGs within some RICs and suggested having fewer CMGs would also contribute to payment rate compression.
Response: We disagree with the commenters that revisions to CMGs will lead to payment rate compression or could compromise access to care for any particular group of patients. As the revised CMGs are reflective of the data that IRFs submitted to us in FYs 2017 and 2018, we believe the revised CMGs reflect the distinct resource needs of the current Medicare IRF population. We believe the revised CMGs more accurately predict resource use in IRFs and better align payments with the expected costs of treating patients in the IRF setting. As such, we believe that the revised CMGs may in fact improve access to and quality of care for IRF patients by increasing the accuracy of IRF payments to providers.
Regarding why some RICs would have fewer CMGs, we refer the commenters to the Technical Report entitled “Analyses to Inform the Use of Standardized Patient Assessment Data Elements in the Inpatient Rehabilitation Facility Prospective Payment System” that describes in detail the analysis used to derive the CMGs and the criteria required to generate additional payment groups. As noted in the FY 2020 IRF PPS proposed rule (84 FR 17250 through 17252), RTI imposed some typically-used constraints in their analysis to identify the proposed set of payment groups. These constraints consisted of a minimum number of stays within a node, a 0.5 percentage point increase of explanatory power, and monotonicity across the CMGs within each RIC. We do not believe it would be appropriate to generate additional CMGs that did not improve the predicative ability of the model beyond what was produced through the CART analysis utilizing the constraints above. We note that while the CART analysis generated fewer CMGs within some RICs, it generated a greater number of CMGs within other RICs and that the overall number of CMGs increases through these revisions to the case-mix classification system. We do not believe having fewer CMGs within any RIC will contribute to payment rate compression as we believe these revisions better align payments with the expected costs of treating patients in IRFs.
Additionally, we disagree with the commenters' statements that the CMG revisions will result in higher payments for lower acuity patients and reduced payments for higher acuity patients. Our analysis has found that higher function is associated with a slight reduction in payment under the revised CMGs and that lower function is associated with a slight increase in payments. The purpose of the proposed revisions to the CMGs is to align payments more appropriately with the costs of caring for all types of patients in IRFs. As such, we do not believe that the revisions will result in higher payments for lower acuity patients. We appreciate the commenters' concerns and will continue to monitor the IRF data closely to ensure that IRF payments are appropriately aligned with costs of care and that Medicare patients continue to have appropriate access to IRF services.
Comment: Several commenters expressed concerns that the proposed CMG revisions could cause a significant redistribution of payments among IRF provides. These commenters indicated that they believe the section GG items make patients appear to be less severe and requested additional information on how patients would be redistributed among the revised CMGs. Additionally, commenters encouraged CMS to monitor the data based on these changes and to update the model if necessary in the future.
Response: We agree with the commenters that the revisions to the CMGs may result in some redistribution of payments among providers. As noted in the FY 2019 IRF PPS final rule (83 FR 38547), the scales and coding instructions are slightly different between the item sets used to derive the existing CMGs and those used to derive the revised CMGs. As such, these differences may result in some patients grouping into different CMGs that more accurately account for the expected resource needs of the patient. While we cannot make individual Medicare beneficiary data publically available, we believe we released adequate information for stakeholders to determine how beneficiaries could be distributed across the revised CMGs. We appreciate the commenters' suggestions to conduct monitoring activities and make future updates to the case-mix classification system and will take this into consideration in the future.
Comment: Commenters expressed concern with the use of section GG items to assign a patient to a CMG and suggested that these items are not sensitive enough and do not capture patients' true burden of care. Commenters also expressed concern with the reliability of the data collected through these items and suggested that the data is not accurate or valid.
Response: As discussed in detail in the FY 2019 IRF PPS final rule (83 FR 38541), we believe that the data items located in the Quality Indicators section of the IRF-PAI are sensitive and accurately capture the functional and cognitive status of patients and can also be used to accurately assess changes in patients' functional status. As noted above, RTI found that the model predicting costs using the CMGs derived from the items located in the Quality Indicators section of the IRF-PAI had a slightly higher R-squared value than models using the current CMGs which are derived from items in the FIMTM instrument, indicating that the revised CMGs more accurately predict resource use in IRFs than the CMGs that are currently utilized. As the data collected in the Quality Indicators section of the IRF-PAI have been collected nationally for all IRFs since October 1, 2016, we believe the data to be accurate and valid at this time. We also believe it is the responsibility of the IRF to submit accurate and valid data that adheres to the coding guidelines detailed in the IRF-PAI training manual.
Comment: Commenters expressed concern with the cognition items collected on the IRF-PAI and their omission from the revised CMGs. A few commenters noted the importance of cognitive impairment in the IRF setting and encouraged CMS to conduct further analysis of the relationship between cognitive function and resource use in the IRF setting and to improve the items that are used to measure cognitive function.
Response: We appreciate the commenters' concerns with the cognitive items that are collected on the IRF-PAI. As we discussed in the FY 2019 IRF PPS final rule (83 FR 38546), the cognitive items that we used for this analysis are the best ones that we have for use at the present time. Unfortunately, we found that including these cognitive items in generating the CMGs would have resulted in lower payments for patients with higher cognitive deficits. This result does not make sense from a clinical perspective, and could have the unintended consequence of reducing access to IRF care for more cognitively impaired beneficiaries. Thus, we determined that it would be better at this time to remove the CMG splits that were generated by the cognitive items. We appreciate the commenters' suggestion to incorporate improved cognition measures into the IRF-PAI and will take this into consideration in the future.
Comment: Commenters suggested that CMS has not provided sufficient education, training materials, or supporting documentation regarding the functional items to support their use in developing a payment model. Some commenters suggested revisions to the existing training materials while other commenters requested that CMS provide additional training, monitor the data, and modify the case mix groupings as needed.
Response: We disagree with the commenters that we have provided insufficient training or guidance on proper coding of this data. We believe we have provided adequate training opportunities for IRFs on coding the Quality Indicator data items, including multiple in-person training opportunities, webinars, on-line training and on-going help desk guidance. We are committed to providing information and support that will allow providers to accurately interpret and complete quality reporting items and we will continue to provide these types of opportunities to the IRF community. We thank the commenters for their suggestions to improve the training materials and we appreciate the commenters' suggestions to continue to monitor the data and make updates to the case-mix classification system when necessary.
After careful consideration of the comments received, we are finalizing revisions to the CMGs based on analysis of 2 years of data (FYs 2017 and 2018) and the incorporation of the unweighted motor score described in section IV.B of this final rule. The revised CMGs that will be effective October 1, 2019 are presented below in Table 3. We refer readers to Table 20 in section XIII.C of this final rule for more information on the distributional effects of revisions to the CMGs. For a provider specific impact analysis for this change, we refer readers to the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRF-Rules-and-Related-Files.html. We are also updating the relative weights and average LOS values associated with the revised CMGs (reflecting an unweighted motor score) beginning with FY 2020.



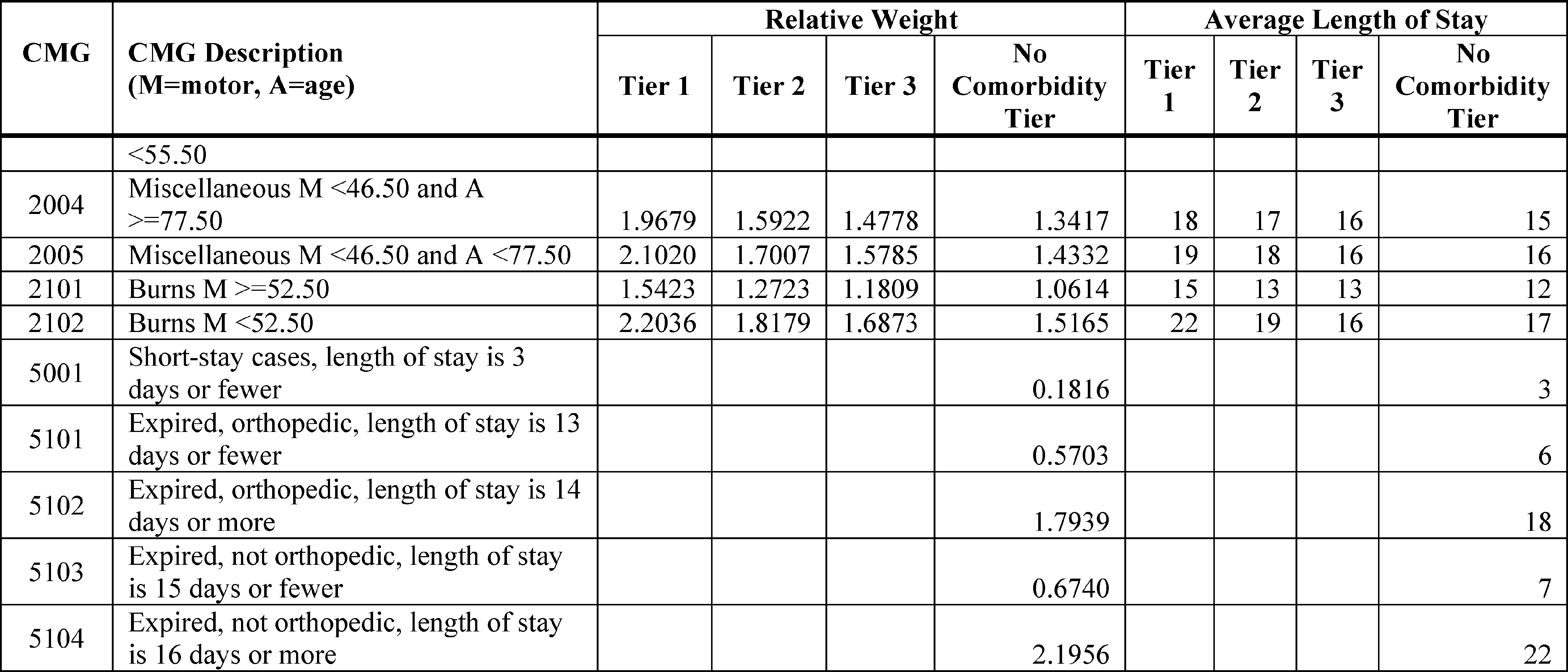
V. Facility-Level Adjustment Factors
Section 1886(j)(3)(A)(v) of the Act confers broad authority upon the Secretary to adjust the per unit payment rate by such factors as the Secretary determines are necessary to properly reflect variations in necessary costs of treatment among rehabilitation facilities. Under this authority, we currently adjust the prospective payment amount associated with a CMG to account for facility-level characteristics such as an IRF's LIP, teaching status, and location in a rural area, if applicable, as described in § 412.624(e).
Based on the substantive changes to the facility-level adjustment factors that were adopted in the FY 2014 IRF PPS final rule (78 FR 47860, 47868 through 47872), in the FY 2015 IRF PPS final rule (79 FR 45872, 45882 through 45883), we froze the facility-level adjustment factors at the FY 2014 levels for FY 2015 and all subsequent years (unless and until we propose to update them again through future notice-and-comment rulemaking). For FY 2020, we will continue to hold the adjustment factors at the FY 2014 levels as we continue to monitor the most current IRF claims data available and continue to evaluate and monitor the effects of the FY 2014 changes.
VI. FY 2020 IRF PPS Payment Update
A. Background
Section 1886(j)(3)(C) of the Act requires the Secretary to establish an increase factor that reflects changes over time in the prices of an appropriate mix of goods and services included in the covered IRF services. According to section 1886(j)(3)(A)(i) of the Act, the increase factor shall be used to update the IRF prospective payment rates for each FY. Section 1886(j)(3)(C)(ii)(I) of the Act requires the application of a productivity adjustment. Thus, in the FY 2020 IRF proposed rule, we proposed to update the IRF PPS payments for FY 2020 by a market basket increase factor as required by section 1886(j)(3)(C) of the Act based upon the most current data available, with a productivity adjustment as required by section 1886(j)(3)(C)(ii)(I) of the Act (84 FR 17261).
We have utilized various market baskets through the years in the IRF PPS. For a discussion of these market baskets, we refer readers to the FY 2016 IRF PPS final rule (80 FR 47046).
Beginning with FY 2016, we finalized the use of a 2012-based IRF market basket, using Medicare cost report (MCR) data for both freestanding and hospital-based IRFs (80 FR 47049 through 47068). Beginning with FY 2020, we proposed to rebase and revise the IRF market basket to reflect a 2016 base year. In the following discussion, we provide an overview of the proposed market basket and describe the methodologies used to determine the operating and capital portions of the proposed 2016-based IRF market basket.
B. Overview of the 2016-Based IRF Market Basket
The 2016-based IRF market basket is a fixed-weight, Laspeyres-type price index. A Laspeyres price index measures the change in price, over time, of the same mix of goods and services purchased in the base period. Any changes in the quantity or mix of goods and services (that is, intensity) purchased over time relative to a base period are not measured.
The index itself is constructed in three steps. First, a base period is selected (for the proposed IRF market basket, the base period is 2016), total base period costs are estimated for a set of mutually exclusive and exhaustive cost categories, and each category is calculated as a proportion of total costs. These proportions are called cost weights. Second, each cost category is matched to an appropriate price or wage variable, referred to as a price proxy. In nearly every instance where we have selected price proxies for the various market baskets, these price proxies are derived from publicly available statistical series that are published on a consistent schedule (preferably at least on a quarterly basis). In cases where a publicly available price series is not available (for example, a price index for malpractice insurance), we have collected price data from other sources and subsequently developed our own index to capture changes in prices for these types of costs. Finally, the cost weight for each cost category is multiplied by the established price proxy. The sum of these products (that is, the cost weights multiplied by their price levels) for all cost categories yields the composite index level of the market basket for the given time period. Repeating this step for other periods produces a series of market basket levels over time. Dividing the composite index level of one period by the composite index level for an earlier period produces a rate of growth in the input price index over that timeframe.
As previously noted, the market basket is described as a fixed-weight index because it represents the change in price over time of a constant mix (quantity and intensity) of goods and services needed to furnish IRF services. The effects on total costs resulting from changes in the mix of goods and services purchased after the base period are not measured. For example, an IRF hiring more nurses after the base period to accommodate the needs of patients would increase the volume of goods and services purchased by the IRF, but would not be factored into the price change measured by a fixed-weight IRF market basket. Only when the index is rebased would changes in the quantity and intensity be captured, with those changes being reflected in the cost weights. Therefore, we rebase the market basket periodically so that the cost weights reflect recent changes in the mix of goods and services that IRFs purchase to furnish inpatient care between base periods.
C. Rebasing and Revising of the IRF PPS Market Basket
As discussed in the FY 2016 IRF PPS final rule (80 FR 47050), the 2012-based IRF market basket reflects the Medicare cost reports for both freestanding and hospital-based facilities.
Beginning with FY 2020, we proposed to rebase and revise the 2012-based IRF market basket to a 2016 base year reflecting both freestanding and hospital-based IRFs. Below we provide a detailed description of our methodology used to develop the proposed 2016-based IRF market basket. This proposed methodology is generally similar to the methodology used to develop the 2012-based IRF market basket with the exception of the proposed derivation of the Home Office Contract Labor cost weight using the MCR data as described in section VI.C.a.(6) of this final rule.
1. Development of Cost Categories and Weights for the 2016-Based IRF Market Basket
a. Use of Medicare Cost Report Data
We proposed a 2016-based IRF market basket that consists of seven major cost categories and a residual derived from the 2016 Medicare cost reports (CMS Form 2552-10) for freestanding and hospital-based IRFs. The seven cost categories are Wages and Salaries, Employee Benefits, Contract Labor, Pharmaceuticals, Professional Liability Insurance (PLI), Home Office Contract Labor, and Capital. The residual category reflects all remaining costs not captured in the seven cost categories. The 2016 cost reports include providers whose cost reporting period began on or after October 1, 2015, and prior to September 30, 2016. We selected 2016 as the base year because we believe that the Medicare cost reports for this year represent the most recent, complete set of MCR data available for developing the IRF market basket at the time of the proposed rule.
Since our goal is to establish cost weights that were reflective of case mix and practice patterns associated with the services IRFs provide to Medicare beneficiaries, as we did for the 2012-based IRF market basket, we proposed to limit the cost reports used to establish the 2016-based IRF market basket to those from facilities that had a Medicare average LOS that was relatively similar to their facility average LOS. We believe that this requirement eliminates statistical outliers and ensures a more accurate market basket that reflects the costs generally incurred during a Medicare-covered stay. The Medicare average LOS for freestanding IRFs is calculated from data reported on line 14 of Worksheet S-3, part I. The Medicare average LOS for hospital-based IRFs is calculated from data reported on line 17 of Worksheet S-3, part I. We proposed to include the cost report data from IRFs with a Medicare average LOS within 15 percent (that is, 15 percent higher or lower) of the facility average LOS to establish the sample of providers used to estimate the 2016-based IRF market basket cost weights. We proposed to apply this LOS edit to the data for IRFs to exclude providers that serve a population whose LOS would indicate that the patients served are not consistent with a LOS of a typical Medicare patient. We note that this is the same LOS edit that we applied to develop the 2012-based IRF market basket. This process resulted in the exclusion of about eight percent of the freestanding and hospital-based IRF Medicare cost reports. Of those excluded, about 18 percent were freestanding IRFs and 82 percent were hospital-based IRFs. This ratio is relatively consistent with the ratio of the universe of freestanding to hospital-based IRF providers.
We then used the cost reports for IRFs that met this requirement to calculate the costs for the seven major cost categories (Wages and Salaries, Employee Benefits, Contract Labor, Professional Liability Insurance, Pharmaceuticals, Home Office Contract Labor, and Capital) for the market basket. For comparison, the 2012-based IRF market basket utilized the Bureau of Economic Analysis Benchmark Input-Output data rather than MCR data to derive the Home Office Contract Labor cost weight. A more detailed discussion of this methodological change is provided in section VI.C.1.a.(6). of this final rule.
Similar to the 2012-based IRF market basket major cost weights, the proposed 2016-based IRF market basket cost weights reflect Medicare allowable costs (routine, ancillary and capital)—costs that are eligible for reimbursement through the IRF PPS.
For freestanding IRFs, total Medicare allowable costs would be equal to the total costs as reported on Worksheet B, part I, column 26, lines 30 through 35, 50 through 76 (excluding 52 and 75), 90 through 91, and 93. For hospital-based IRFs, total Medicare allowable costs would be equal to the total costs for the IRF inpatient unit after the allocation of overhead costs (Worksheet B, part I, column 26, line 41) and a proportion of total ancillary costs reported on Worksheet B, part I, column 26, lines 50 through 76 (excluding 52 and 75), 90 through 91, and 93. We proposed to calculate the portion of ancillary costs attributable to the hospital-based IRF for a given ancillary cost center by multiplying total facility ancillary costs for the specific cost center (as reported on Worksheet B, part I, column 26) by the ratio of IRF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3 for hospital-based IRFs) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3 for all relevant PPS [that is, IPPS, IRF, IPF and skilled nursing facility (SNF)]). We proposed to use these methods to derive levels of total costs for IRF providers. This is the same methodology used for the 2012-based IRF market basket. With this work complete, we then set about deriving cost levels for the seven major cost categories and then derive a residual cost weight reflecting all other costs not classified.
(1) Wages and Salaries Costs
For freestanding IRFs, we proposed to derive Wages and Salaries costs as the sum of routine inpatient salaries, ancillary salaries, and a proportion of overhead (or general service cost centers in the Medicare cost reports) salaries as reported on Worksheet A, column 1. Since overhead salary costs are attributable to the entire IRF, we only include the proportion attributable to the Medicare allowable cost centers. We proposed to estimate the proportion of overhead salaries that are attributed to Medicare allowable costs centers by multiplying the ratio of Medicare allowable area salaries (Worksheet A, column 1, lines 50 through 76 (excluding 52 and 75), 90 through 91, and 93) to total salaries (Worksheet A, column 1, line 200) times total overhead salaries (Worksheet A, column 1, lines 4 through 18). This is the same methodology used in the 2012-based IRF market basket.
For hospital-based IRFs, we proposed to derive Wages and Salaries costs as the sum of inpatient routine salary costs (Worksheet A, column 1, line 41) for the hospital-based IRF and the overhead salary costs attributable to this IRF inpatient unit; and ancillary salaries plus a portion of overhead salary costs attributable to the ancillary departments utilized by the hospital-based IRF.
We proposed to calculate hospital-based ancillary salary costs for a specific cost center (Worksheet A, column 1, lines 50 through 76 (excluding 52 and 75), 90 through 91, and 93) using salary costs from Worksheet A, column 1, multiplied by the ratio of IRF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3, for IRF subproviders) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3, for all relevant PPS units [that is, IPPS, IRF, IPF and a SNF]). For example, if hospital-based IRF Medicare physical therapy costs represent 30 percent of the total Medicare physical therapy costs for the entire facility, then 30 percent of total facility physical therapy salaries (as reported in Worksheet A, column 1, line 66) would be attributable to the hospital-based IRF. We believe it is appropriate to use only a portion of the ancillary costs in the market basket cost weight calculations since the hospital-based IRF only utilizes a portion of the facility's ancillary services. We believe the ratio of reported IRF Medicare costs to reported total Medicare costs provides a reasonable estimate of the ancillary services utilized, and costs incurred, by the hospital-based IRF.
We proposed to calculate the portion of overhead salary costs attributable to hospital-based IRFs by first calculating total noncapital overhead costs (Worksheet B, part I, columns 4-18, line 41, less Worksheet B, part II, columns 4-18, line 41). We then multiply total noncapital overhead costs by an overhead ratio equal to the ratio of total facility overhead salaries (as reported on Worksheet A, column 1, lines 4-18) to total facility noncapital overhead costs (as reported on Worksheet A, column 1 and 2, lines 4-18). This methodology assumes the proportion of total costs related to salaries for the overhead cost center is similar for all inpatient units (that is, acute inpatient or inpatient rehabilitation).
We proposed to calculate the portion of overhead salaries attributable to each ancillary department by first calculating total noncapital overhead costs attributable to each specific ancillary department (Worksheet B, part I, columns 4-18 less, Worksheet B, part II, columns 4-18). We then identify the portion of these noncapital overhead costs attributable to Wages and Salaries by multiplying these costs by the overhead ratio defined as the ratio of total facility overhead salaries (as reported on Worksheet A, column 1, lines 4-18) to total overhead costs (as reported on Worksheet A, column 1 & 2, lines 4-18). Finally, we identified the portion of these overhead salaries for each ancillary department that is attributable to the hospital-based IRF by multiplying by the ratio of IRF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3, for hospital-based IRFs) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3, for all relevant PPS units [that is, IPPS, IRF, IPF and SNF]). This is the same methodology used to derive the 2012-based IRF market basket.
(2) Employee Benefits Costs
Effective with the implementation of CMS Form 2552-10, we began collecting Employee Benefits and Contract Labor data on Worksheet S-3, part V.
For 2016 MCR data, the majority of providers did not report data on Worksheet S-3, part V; particularly, approximately 48 percent of freestanding IRFs and 40 percent of hospital-based IRFs reported data on Worksheet S-3, part V. However, we believe we have a large enough sample to enable us to produce a reasonable Employee Benefits cost weight. Again, we continue to encourage all providers to report these data on the Medicare cost report.
For freestanding IRFs, we proposed Employee Benefits costs would be equal to the data reported on Worksheet S-3, part V, column 2, line 2. We note that while not required to do so, freestanding IRFs also may report Employee Benefits data on Worksheet S-3, part II, which is applicable to only IPPS providers. For those freestanding IRFs that report Worksheet S-3, part II, data, but not Worksheet S-3, part V, we proposed to use the sum of Worksheet S-3, part II, lines 17, 18, 20, and 22, to derive Employee Benefits costs. This proposed method allows us to obtain data from about 30 more freestanding IRFs than if we were to only use the Worksheet S-3, part V, data as was done for the 2012-based IRF market basket.
For hospital-based IRFs, we proposed to calculate total benefit costs as the sum of inpatient unit benefit costs, a portion of ancillary benefits, and a portion of overhead benefits attributable to the routine inpatient unit and a portion of overhead benefits attributable to the ancillary departments. We proposed inpatient unit benefit costs be equal to Worksheet S-3, part V, column 2, line 4. We proposed that the portion of overhead benefits attributable to the routine inpatient unit and ancillary departments be calculated by multiplying ancillary salaries for the hospital-based IRF and overhead salaries attributable to the hospital-based IRF (determined in the derivation of hospital-based IRF Wages and Salaries costs as described above) by the ratio of total facility benefits to total facility salaries. Total facility benefits is equal to the sum of Worksheet S-3, part II, column 4, lines 17-25, and total facility salaries is equal to Worksheet S-3, part II, column 4, line 1.
(3) Contract Labor Costs
Contract Labor costs are primarily associated with direct patient care services. Contract labor costs for other services such as accounting, billing, and legal are calculated separately using other government data sources as described in section VI.C.3. of this final rule. To derive contract labor costs using Worksheet S-3, part V, data, for freestanding IRFs, we proposed Contract Labor costs be equal to Worksheet S-3, part V, column 1, line 2. As we noted for Employee Benefits, freestanding IRFs also may report Contract Labor data on Worksheet S-3, part II, which is applicable to only IPPS providers. For those freestanding IRFs that report Worksheet S-3, part II data, but not Worksheet S-3, part V, we proposed to use the sum of Worksheet S-3, part II, lines 11 and 13, to derive Contract Labor costs.
For hospital-based IRFs, we proposed that Contract Labor costs would be equal to Worksheet S-3, part V, column 1, line 4. As previously noted, for 2016 MCR data, while there were providers that did report data on Worksheet S-3, part V, many providers did not complete this worksheet. However, we believe we have a large enough sample to enable us to produce a reasonable Contract Labor cost weight. We continue to encourage all providers to report these data on the Medicare cost report.
(4) Pharmaceuticals Costs
For freestanding IRFs, we proposed to calculate pharmaceuticals costs using non-salary costs reported on Worksheet A, column 7, less Worksheet A, column 1, for the pharmacy cost center (line 15) and drugs charged to patients cost center (line 73).
For hospital-based IRFs, we proposed to calculate pharmaceuticals costs as the sum of a portion of the non-salary pharmacy costs and a portion of the non-salary drugs charged to patient costs reported for the total facility. We proposed that non-salary pharmacy costs attributable to the hospital-based IRF would be calculated by multiplying total pharmacy costs attributable to the hospital-based IRF (as reported on Worksheet B, part I, column 15, line 41) by the ratio of total non-salary pharmacy costs (Worksheet A, column 2, line 15) to total pharmacy costs (sum of Worksheet A, columns 1 and 2 for line 15) for the total facility. We proposed that non-salary drugs charged to patient costs attributable to the hospital-based IRF would be calculated by multiplying total non-salary drugs charged to patient costs (Worksheet B, part I, column 0, line 73 plus Worksheet B, part I, column 15, line 73, less Worksheet A, column 1, line 73) for the total facility by the ratio of Medicare drugs charged to patient ancillary costs for the IRF unit (as reported on Worksheet D-3 for hospital-based IRFs, column 3, line 73) to total Medicare drugs charged to patient ancillary costs for the total facility (equal to the sum of Worksheet D-3, column 3, line 73 for all relevant PPS [that is, IPPS, IRF, IPF and SNF]).
(5) Professional Liability Insurance Costs
For freestanding IRFs, we proposed that Professional Liability Insurance (PLI) costs (often referred to as malpractice costs) would be equal to premiums, paid losses and self-insurance costs reported on Worksheet S-2, part I, columns 1 through 3, line 118. For hospital-based IRFs, we proposed to assume that the PLI weight for the total facility is similar to the hospital-based IRF unit since the only data reported on this worksheet is for the entire facility, as we currently have no means to identify the proportion of total PLI costs that are only attributable to the hospital-based IRF. Therefore, hospital-based IRF PLI costs are equal to total facility PLI (as reported on Worksheet S-2, part I, columns 1 through 3, line 118) divided by total facility costs (as reported on Worksheet A, columns 1 and 2, line 200) times hospital-based IRF Medicare allowable total costs. Our assumption is that the same proportion of expenses are used among each unit of the hospital.
(6) Home Office/Related Organization Contract Labor Costs
For the 2016-based IRF market basket, we proposed to determine the home office/related organization contract labor costs using MCR data. The 2012-based IRF market basket used the 2007 Benchmark Input-Output (I-O) expense data published by the Bureau of Economic Analysis (BEA) to derive these costs (80 FR 47057). A more detailed explanation of the general methodology using the BEA I-O data is provided in section VI.C.3. of this final rule. For freestanding and hospital-based IRFs, we proposed to calculate the home office contract labor cost weight (using data reported on Worksheet S-3, part II, column 4, lines 14, 1401, 1402, 2550, and 2551) and total facility costs (Worksheet B, part I, column 26, line 202). We proposed to use total facility costs as the denominator for calculating the home office contract labor cost weight as these expenses reported on Worksheet S-3, part II reflect the entire hospital facility. Our assumption is that the same proportion of expenses are used among each unit of the hospital. For the 2012-based IRF market basket, we calculated the home office cost weight using expense data for North American Industry Classification System (NAICS) code 55, Management of Companies and Enterprises (80 FR 47067).
(7) Capital Costs
For freestanding IRFs, we proposed that capital costs would be equal to Medicare allowable capital costs as reported on Worksheet B, part II, column 26, lines 30 through 35, 50 through 76 (excluding 52 and 75), 90 through 91, and 93.
For hospital-based IRFs, we proposed that capital costs would be equal to IRF inpatient capital costs (as reported on Worksheet B, part II, column 26, line 41) and a portion of IRF ancillary capital costs. We calculate the portion of ancillary capital costs attributable to the hospital-based IRF for a given cost center by multiplying total facility ancillary capital costs for the specific ancillary cost center (as reported on Worksheet B, part II, column 26) by the ratio of IRF Medicare ancillary costs for the cost center (as reported on Worksheet D-3, column 3 for hospital-based IRFs) to total Medicare ancillary costs for the cost center (equal to the sum of Worksheet D-3, column 3 for all relevant PPS [that is, IPPS, IRF, IPF and SNF]). For example, if hospital-based IRF Medicare physical therapy costs represent 30 percent of the total Medicare physical therapy costs for the entire facility, then 30 percent of total facility physical therapy capital costs (as reported in Worksheet B, part II, column 26, line 66) would be attributable to the hospital-based IRF.
b. Final Major Cost Category Computation
After we derive costs for the major cost categories for each provider using the MCR data as previously described, we proposed to trim the data for outliers. For the Wages and Salaries, Employee Benefits, Contract Labor, Pharmaceuticals, Professional Liability Insurance, and Capital cost weights, we first divide the costs for each of these six categories by total Medicare allowable costs calculated for the provider to obtain cost weights for the universe of IRF providers. We then remove those providers whose derived cost weights fall in the top and bottom 5 percent of provider specific derived cost weights to ensure the exclusion of outliers. After the outliers have been excluded, we sum the costs for each category across all remaining providers. We then divide this by the sum of total Medicare allowable costs across all remaining providers to obtain a cost weight for the 2016-based IRF market basket for the given category.
The proposed trimming methodology for the Home Office Contract Labor cost weight is slightly different than the proposed trimming methodology for the other six cost categories as described above. For the Home Office Contract Labor cost weight, since we are using total facility data rather than Medicare-allowable costs associated with IRF services, we proposed to trim the freestanding and hospital-based IRF cost weights separately. For each of the providers, we first divide the home office contract labor costs by total facility costs to obtain a Home Office Contract Labor cost weight for the universe of IRF providers. We then proposed to trim only the top 1 percent of providers to exclude outliers while also allowing providers who have reported zero home office costs to remain in the Home Office Contract Labor cost weight calculations as not all providers will incur home office costs. After removing these outliers, we are left with a trimmed data set for both freestanding and hospital-based providers. We then proposed to sum the costs for each category (freestanding and hospital-based) across all remaining providers. We next divide this by the sum of total facility costs across all remaining providers to obtain a freestanding and hospital-based cost weight. Lastly, we proposed to weight these two cost weights together using the Medicare-allowable costs to derive a Home Office Contract Labor cost weight for the 2016-based IRF market basket.
Finally, we proposed to calculate the residual “All Other” cost weight that reflects all remaining costs that are not captured in the seven cost categories listed.
We received a few comments on our proposed derivation of the Home Office Contract Labor cost weight from the Medicare cost reports, which are summarized below.
Comment: Commenters expressed concern with the proposed methodology change to the Home Office Contract Labor cost weight. These commenters stated that CMS had not provided sufficient rationale for this change in methodology nor has CMS provided a discussion of how these data points were reasonably validated and tested. One commenter requested that CMS provide stakeholders with more information on the rationale and the data validation methodologies employed in the final rule.
The commenters expressed concern with the sample of IRFs reporting the home office cost data and found based on their analysis that reporting was between 50 to 65 percent. These commenters suggested that this was due to these cost report line items being an optional category for IRFs under Medicare cost reporting requirements. One of the commenters further expressed concern with the methodology and approach that CMS applied in determining IRF unit Home Office Contract Labor amounts, specifically the assumption that hospital-based IRFs utilize the same proportion of home office expenses as the rest of the acute care hospital in which it is located. The commenter stated that typically IRF units are a very small part of the larger parent acute care hospital and that the larger systems do not spend the same proportional time and resources on these units compared to hospital system as a whole. They stated that this assumption likely overstates the Home Office Contract Labor cost weight.
Based on these concerns, the commenters requested that CMS not finalize its proposed changes to the Home Office Contract Labor cost category and instead finalize use of the previous methodology relating to this category that was used for the 2012-based market basket. One commenter also requested that CMS revisit this potential change with adequate explanation and data in future rulemaking.
Response: We appreciate the commenters' concerns on the proposed methodological change for the Home Office Contract Labor cost weight. We proposed to revise our methodology and use the 2016 IRF MCR data to calculate the Home Office Contract Labor costs rather than the 2012 Benchmark I-O data because it reflected more up-to-date data and we believe it to be an improvement over the use of the BEA Benchmark I-O data that is not specific to IRFs. The MCR data allows us to calculate Home Office Contract Labor Costs for freestanding and IRF hospital-based facilities.
We disagree with the commenters' concern that the MCR data completion rates for the Home Office Contract Labor costs are inadequate to obtain a cost weight. When developing the proposed 2016-based IRF market basket, we conducted a thorough analysis of the MCR data and our proposed Home Office Contract Labor cost weight methodology. We found that approximately 90 percent of freestanding IRFs reported having a home office, of which over 50 percent reported home office compensation data on Worksheet S-3, part II. The composition of the providers (by ownership-type and region) that reported both wage index data (including those who do not have a home office) and home office contract labor cost data were similarly representative to all freestanding IRFs. A sensitivity analysis of calculating a reweighted Home Office Contract Labor cost weight based on ownership-type and region produced a Home Office Contract Labor cost weight similar to the proposed 3.7 percent weight.
For additional sensitivity testing, recognizing that some of the freestanding IRFs with home offices may not have completed the applicable fields on the MCR, we calculated a weight using only freestanding IRFs that reported having a home office (Worksheet S-2, part I, line 140). This produced a Home Office Contract Labor cost weight nearly identical to the freestanding IRF 2016 cost weight using our proposed methodology. Based on this analysis, we believe that the sample of providers included in the Home Office Contract Labor cost weight are a technically representative sample of all IRF providers.
Regarding IRF units, we recognize the commenter's concern that they represent a small proportion of the total facility. We believe that the assumption that IRFs utilize the same proportion of home office expenses as the rest of the acute care hospital is reasonable. The use of total facility data assumes the facility Home Office Contract Labor cost weight is equal to the Home Office Contract Labor cost weight for the IRF unit. Further analysis of the MCR data shows IRF unit direct patient care costs (as reported on Worksheet B, part I, column 0, line 41) account for about one percent of total facility costs (excluding capital, Administrative and General (A&G), and Employee Benefit department costs). Similarly, A&G costs (Worksheet B, part I, column 0, line 5), where Home Office Contract Labor costs are likely captured, allocated to the IRF unit account for a similar proportion of direct patient care costs with about one percent of total A&G costs. We also found the proportion of allocated A&G costs for other larger, more medically-complex hospital units (such as the intensive care, surgical care, and operating room) were consistent with direct patient care cost proportions and the proportions for these units were higher than the proportion of the A&G expenses allocated to the IRF unit. This supports the commenter's claim that hospitals allocate less A&G costs to less medically-complex services (as measured by costs). Our proposed calculation would adhere to this assumption as well since the facility level cost weight is applied to the IRF Medicare allowable total costs representing these relatively less medically-complex services. Furthermore, the Benchmark I-O methodology used in the 2012-based IRF market basket also assumes that the IRF relative costs are the same as those of the hospital total facility. We invite the commenters to submit additional data that would help in this area for consideration in future rulemaking.
We disagree with the commenters' request to use the Benchmark I-O data to calculate the Home Office Contract Labor cost weight rather than the proposed 2016 MCR data. We believe the proposed methodology is a technical improvement over the prior methodology because it represents more recent data that is representative compositionally and geographically of IRFs. It is also is the same data used to determine the other major cost weights in the 2016-based market basket and the proportion of the Home Office Contract Labor cost weight that is allocated to the Professional Fees: Labor-related and Professional Fees: Nonlabor-related cost weights. We believe the assumptions made by using the total facility data for the hospital-based IRFs are reasonable and supported by the MCR data on A&G cost allocation. Finally, we note that the methodological change accounts for only 0.2 percentage point of the 2.0 percentage points change in the labor-related share.
After careful consideration of comments, we are finalizing our methodology for deriving the major cost weights as proposed.
Table 4 presents the cost weights for these major cost categories calculated from the Medicare cost reports for the 2016-based IRF market basket, as well as for the 2012-based IRF market basket.

As we did for the 2012-based IRF market basket, we proposed to allocate the Contract Labor cost weight to the Wages and Salaries and Employee Benefits cost weights based on their relative proportions under the assumption that contract labor costs are comprised of both wages and salaries and employee benefits. The Contract Labor allocation proportion for Wages and Salaries is equal to the Wages and Salaries cost weight as a percent of the sum of the Wages and Salaries cost weight and the Employee Benefits cost weight. For the proposed rule, this rounded percentage is 81 percent; therefore, we proposed to allocate 81 percent of the Contract Labor cost weight to the Wages and Salaries cost weight and 19 percent to the Employee Benefits cost weight. The 2012-based IRF market basket percentage was also 81 percent (80 FR 47056). We did not receive any specific public comments on our proposed allocation of Contract Labor. Therefore, we are finalizing our method of allocating Contract Labor as proposed.
Table 5 shows the Wages and Salaries and Employee Benefit cost weights after Contract Labor cost weight allocation for both the 2016-based IRF market basket and 2012-based IRF market basket.

c. Derivation of the Detailed Operating Cost Weights
To further divide the “All Other” residual cost weight estimated from the 2016 MCR data into more detailed cost categories, we proposed to use the 2012 Benchmark I-O “Use Tables/Before Redefinitions/Purchaser Value” for NAICS 622000, Hospitals, published by the BEA. This data is publicly available at http://www.bea.gov/industry/io_annual.htm. For the 2012-based IRF market basket, we used the 2007 Benchmark I-O data, the most recent data available at the time (80 FR 47057).
The BEA Benchmark I-O data are scheduled for publication every 5 years with the most recent data available for 2012. The 2007 Benchmark I-O data are derived from the 2012 Economic Census and are the building blocks for BEA's economic accounts. Thus, they represent the most comprehensive and complete set of data on the economic processes or mechanisms by which output is produced and distributed.[1] BEA also produces Annual I-O estimates; however, while based on a similar methodology, these estimates reflect less comprehensive and less detailed data sources and are subject to revision when benchmark data becomes available. Instead of using the less detailed Annual I-O data, we proposed to inflate the 2012 Benchmark I-O data forward to 2016 by applying the annual price changes from the respective price proxies to the appropriate market basket cost categories that are obtained from the 2012 Benchmark I-O data. We repeat this practice for each year. We then proposed to calculate the cost shares that each cost category represents of the inflated 2012 data. These resulting 2016 cost shares are applied to the All Other residual cost weight to obtain the detailed cost weights for the 2016-based IRF market basket. For example, the cost for Food: Direct Purchases represents 5.0 percent of the sum of the “All Other” 2012 Benchmark I-O Hospital Expenditures inflated to 2016; therefore, the Food: Direct Purchases cost weight represents 5.0 percent of the 2016-based IRF market basket's “All Other” cost category (22.2 percent), yielding a “final” Food: Direct Purchases cost weight of 1.1 percent in the 2016-based IRF market basket (0.05 * 22.2 percent = 1.1 percent).
Using this methodology, we proposed to derive seventeen detailed IRF market basket cost category weights from the 2016-based IRF market basket residual cost weight (22.2 percent). These categories are: (1) Electricity; (2) Fuel, Oil, and Gasoline; (3) Food: Direct Purchases; (4) Food: Contract Services; (5) Chemicals; (6) Medical Instruments; (7) Rubber & Plastics; (8) Paper and Printing Products; (9) Miscellaneous Products; (10) Professional Fees: Labor-related; (11) Administrative and Facilities Support Services; (12) Installation, Maintenance, and Repair; (13) All Other Labor-related Services; (14) Professional Fees: Nonlabor-related; (15) Financial Services; (16) Telephone Services; and (17) All Other Nonlabor-related Services. We note that for the 2012-based IRF market basket, we had a Water and Sewerage cost weight. For the 2016-based IRF market basket, we proposed to include Water and Sewerage costs in the Electricity cost weight due to the small amount of costs in this category.
For the 2012-based IRF market basket, we used the I-O data for NAICS 55 Management of Companies to derive the Home Office Contract Labor cost weight, which were classified in the Professional Fees: Labor-related and Professional Fees: Nonlabor-related cost weights. As previously discussed, we proposed to use the MCR data to derive the Home Office Contract Labor cost weight, which we would further classify into the Professional Fees: Labor-related or Professional Fees: Nonlabor-related categories.
We did not receive any specific comments on the derivation of the detailed operating cost weights. In this final rule, we are finalizing our methodology for deriving the detailed operating cost weights as proposed.
d. Derivation of the Detailed Capital Cost Weights
As described in section VI.C.1.a.(6) of this final rule, we proposed a Capital-Related cost weight of 9.0 percent as obtained from the 2016 Medicare cost reports for freestanding and hospital-based IRF providers. We proposed to then separate this total Capital-Related cost weight into more detailed cost categories.
Using 2016 Medicare cost reports, we were able to group Capital-Related costs into the following categories: Depreciation, Interest, Lease, and Other Capital-Related costs. For each of these categories, we proposed to determine separately for hospital-based IRFs and freestanding IRFs what proportion of total capital-related costs the category represents.
For freestanding IRFs, we proposed to derive the proportions for Depreciation, Interest, Lease, and Other Capital-related costs using the data reported by the IRF on Worksheet A-7, which is similar to the methodology used for the 2012-based IRF market basket.
For hospital-based IRFs, data for these four categories were not reported separately for the hospital-based IRF; therefore, we proposed to derive these proportions using data reported on Worksheet A-7 for the total facility. We assumed the cost shares for the overall hospital are representative for the hospital-based IRF unit. For example, if depreciation costs make up 60 percent of total capital costs for the entire facility, we believe it is reasonable to assume that the hospital-based IRF would also have a 60 percent proportion because it is a unit contained within the total facility. This is the same methodology used for the 2012-based IRF market basket (80 FR 47057).
To combine each detailed capital cost weight for freestanding and hospital-based IRFs into a single capital cost weight for the 2016-based IRF market basket, we proposed to weight together the shares for each of the categories (Depreciation, Interest, Lease, and Other Capital-related costs) based on the share of total capital costs each provider type represents of the total capital costs for all IRFs for 2016. Applying this methodology results in proportions of total capital-related costs for Depreciation, Interest, Lease and Other Capital-related costs that are representative of the universe of IRF providers. This is the same methodology used for the 2012-based IRF market basket (80 FR 47057 through 47058).
Lease costs are unique in that they are not broken out as a separate cost category in the 2016-based IRF market basket. Rather, we proposed to proportionally distribute these costs among the cost categories of Depreciation, Interest, and Other Capital-Related, reflecting the assumption that the underlying cost structure of leases is similar to that of capital-related costs in general. As was done under the 2012-based IRF market basket, we proposed to assume that 10 percent of the lease costs as a proportion of total capital-related costs represents overhead and assign those costs to the Other Capital-Related cost category accordingly. We proposed to distribute the remaining lease costs proportionally across the three cost categories (Depreciation, Interest, and Other Capital-Related) based on the proportion that these categories comprise of the sum of the Depreciation, Interest, and Other Capital-related cost categories (excluding lease expenses). This resulted in three primary capital-related cost categories in the 2016-based IRF market basket: Depreciation, Interest, and Other Capital-Related costs. This is the same methodology used for the 2012-based IRF market basket (80 FR 47058). The allocation of these lease expenses are shown in Table 6.
Finally, we proposed to further divide the Depreciation and Interest cost categories. We proposed to separate Depreciation into the following two categories: (1) Building and Fixed Equipment; and (2) Movable Equipment. We proposed to separate Interest into the following two categories: (1) Government/Nonprofit; and (2) For-profit.
To disaggregate the Depreciation cost weight, we need to determine the percent of total Depreciation costs for IRFs that are attributable to Building and Fixed Equipment, which we hereafter refer to as the “fixed percentage.” For the 2016-based IRF market basket, we proposed to use slightly different methods to obtain the fixed percentages for hospital-based IRFs compared to freestanding IRFs.
For freestanding IRFs, we proposed to use depreciation data from Worksheet A-7 of the 2016 Medicare cost reports. However, for hospital-based IRFs, we determined that the fixed percentage for the entire facility may not be representative of the hospital-based IRF unit due to the entire facility likely employing more sophisticated movable assets that are not utilized by the hospital-based IRF. Therefore, for hospital-based IRFs, we proposed to calculate a fixed percentage using: (1) Building and fixture capital costs allocated to the hospital-based IRF unit as reported on Worksheet B, part I, line 41; and (2) building and fixture capital costs for the top five ancillary cost centers utilized by hospital-based IRFs. We proposed to weight these two fixed percentages (inpatient and ancillary) using the proportion that each capital cost type represents of total capital costs in the 2016-based IRF market basket. We proposed to then weight the fixed percentages for hospital-based and freestanding IRFs together using the proportion of total capital costs each provider type represents. For both freestanding and hospital-based IRFs, this is the same methodology used for the 2012-based IRF market basket (80 FR 47058).
To disaggregate the Interest cost weight, we determined the percent of total interest costs for IRFs that are attributable to government and nonprofit facilities, which is hereafter referred to as the “nonprofit percentage,” as price pressures associated with these types of interest costs tend to differ from those for for-profit facilities. For the 2016-based IRF market basket, we proposed to use interest costs data from Worksheet A-7 of the 2016 Medicare cost reports for both freestanding and hospital-based IRFs. We proposed to determine the percent of total interest costs that are attributed to government and nonprofit IRFs separately for hospital-based and freestanding IRFs. We then proposed to weight the nonprofit percentages for hospital-based and freestanding IRFs together using the proportion of total capital costs that each provider type represents.
We did not receive any specific public comments on the derivation of the detailed capital cost weights. In this final rule, we are finalizing our methodology for deriving the detailed capital cost weights as proposed. Table 6 provides the detailed capital cost share composition estimated from the 2016 IRF Medicare cost reports. These detailed capital cost share composition percentages are applied to the total Capital-Related cost weight of 9.0 percent explained in detail in section VI.C.1.a.(6) of this final rule.

e. 2016-Based IRF Market Basket Cost Categories and Weights
Table 7 compares the cost categories and weights for the final 2016-based IRF market basket compared to the 2012-based IRF market basket.
2. Selection of Price Proxies
After developing the cost weights for the 2016-based IRF market basket, we selected the most appropriate wage and price proxies currently available to represent the rate of price change for each expenditure category. For the majority of the cost weights, we base the price proxies on U.S. Bureau of Labor Statistics (BLS) data and group them into one of the following BLS categories:
- Employment Cost Indexes. Employment Cost Indexes (ECIs) measure the rate of change in employment wage rates and employer costs for employee benefits per hour worked. These indexes are fixed-weight indexes and strictly measure the change in wage rates and employee benefits per hour. ECIs are superior to Average Hourly Earnings (AHE) as price proxies for input price indexes because they are not affected by shifts in occupation or industry mix, and because they measure pure price change and are available by both occupational group and by industry. The industry ECIs are based on the NAICS and the occupational ECIs are based on the Standard Occupational Classification System (SOC).
- Producer Price Indexes. Producer Price Indexes (PPIs) measure the average change over time in the selling prices received by domestic producers for their output. The prices included in the PPI are from the first commercial transaction for many products and some services (https://www.bls.gov/ppi/).
- Consumer Price Indexes. Consumer Price Indexes (CPIs) measure the average change over time in the prices paid by urban consumers for a market basket of consumer goods and services (https://www.bls.gov/cpi/). CPIs are only used when the purchases are similar to those of retail consumers rather than purchases at the producer level, or if no appropriate PPIs are available.
We evaluate the price proxies using the criteria of reliability, timeliness, availability, and relevance:
- Reliability. Reliability indicates that the index is based on valid statistical methods and has low sampling variability. Widely accepted statistical methods ensure that the data were collected and aggregated in a way that can be replicated. Low sampling variability is desirable because it indicates that the sample reflects the typical members of the population. (Sampling variability is variation that occurs by chance because only a sample was surveyed rather than the entire population.)
- Timeliness. Timeliness implies that the proxy is published regularly, preferably at least once a quarter. The market baskets are updated quarterly, and therefore, it is important for the underlying price proxies to be up-to-date, reflecting the most recent data available. We believe that using proxies that are published regularly (at least quarterly, whenever possible) helps to ensure that we are using the most recent data available to update the market basket. We strive to use publications that are disseminated frequently, because we believe that this is an optimal way to stay abreast of the most current data available.
- Availability. Availability means that the proxy is publicly available. We prefer that our proxies are publicly available because this will help ensure that our market basket updates are as transparent to the public as possible. In addition, this enables the public to be able to obtain the price proxy data on a regular basis.
- Relevance. Relevance means that the proxy is applicable and representative of the cost category weight to which it is applied. The CPIs, PPIs, and ECIs that we have selected meet these criteria. Therefore, we believe that they continue to be the best measure of price changes for the cost categories to which they would be applied.
Table 10 lists all price proxies that we proposed to use for the 2016-based IRF market basket. Below is a detailed explanation of the price proxies we proposed for each cost category weight. We did not receive any specific comments on our proposed price proxies for the 2016-based IRF market basket. Therefore, in this final rule, we are finalizing the price proxies as proposed.
a. Price Proxies for the Operating Portion of the 2016-Based IRF Market Basket
(1) Wages and Salaries
We proposed to continue to use the ECI for Wages and Salaries for All Civilian workers in Hospitals (BLS series code CIU1026220000000I) to measure the wage rate growth of this cost category. This is the same price proxy used in the 2012-based IRF market basket (80 FR 47060).
(2) Benefits
We proposed to continue to use the ECI for Total Benefits for All Civilian workers in Hospitals to measure price growth of this category. This ECI is calculated using the ECI for Total Compensation for All Civilian workers in Hospitals (BLS series code CIU1016220000000I) and the relative importance of wages and salaries within total compensation. This is the same price proxy used in the 2012-based IRF market basket (80 FR 47060).
(3) Electricity
We proposed to continue to use the PPI Commodity Index for Commercial Electric Power (BLS series code WPU0542) to measure the price growth of this cost category. This is the same price proxy used in the 2012-based IRF market basket (80 FR 47060).
(4) Fuel, Oil, and Gasoline
Similar to the 2012-based IRF market basket, for the 2016-based IRF market basket, we proposed to use a blend of the PPI for Petroleum Refineries and the PPI Commodity for Natural Gas. Our analysis of the Bureau of Economic Analysis' 2012 Benchmark Input-Output data (use table before redefinitions, purchaser's value for NAICS 622000 [Hospitals]), shows that Petroleum Refineries expenses account for approximately 90 percent and Natural Gas expenses account for approximately 10 percent of Hospitals' (NAICS 622000) total Fuel, Oil, and Gasoline expenses. Therefore, we proposed to use a blend of 90 percent of the PPI for Petroleum Refineries (BLS series code PCU324110324110) and 10 percent of the PPI Commodity Index for Natural Gas (BLS series code WPU0531) as the price proxy for this cost category. The 2012-based IRF market basket used a 70/30 blend of these price proxies, reflecting the 2007 I-O data (80 FR 47060). We believe that these two price proxies continue to be the most technically appropriate indices available to measure the price growth of the Fuel, Oil, and Gasoline cost category in the 2016-based IRF market basket.
(5) Professional Liability Insurance
We proposed to continue to use the CMS Hospital Professional Liability Index to measure changes in PLI premiums. To generate this index, we collect commercial insurance premiums for a fixed level of coverage while holding non-price factors constant (such as a change in the level of coverage). This is the same proxy used in the 2012-based IRF market basket (80 FR 47060).
(6) Pharmaceuticals
We proposed to continue to use the PPI for Pharmaceuticals for Human Use, Prescription (BLS series code WPUSI07003) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47060).
(7) Food: Direct Purchases
We proposed to continue to use the PPI for Processed Foods and Feeds (BLS series code WPU02) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47060).
(8) Food: Contract Purchases
We proposed to continue to use the CPI for Food Away From Home (BLS series code CUUR0000SEFV) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47060 through 47061).
(9) Chemicals
Similar to the 2012-based IRF market basket, we proposed to use a four part blended PPI as the proxy for the chemical cost category in the 2016-based IRF market basket. The proposed blend is composed of the PPI for Industrial Gas Manufacturing, Primary Products (BLS series code PCU325120325120P), the PPI for Other Basic Inorganic Chemical Manufacturing (BLS series code PCU32518-32518-), the PPI for Other Basic Organic Chemical Manufacturing (BLS series code PCU32519-32519-), and the PPI for Other Miscellaneous Chemical Product Manufacturing (BLS series code PCU325998325998). We note that the four part blended PPI used in the 2012-based IRF market basket is composed of the PPI for Industrial Gas Manufacturing (BLS series code PCU325120325120P), the PPI for Other Basic Inorganic Chemical Manufacturing (BLS series code PCU32518-32518-), the PPI for Other Basic Organic Chemical Manufacturing (BLS series code PCU32519-32519-), and the PPI for Soap and Cleaning Compound Manufacturing (BLS series code PCU32561-32561-). For the 2016-based IRF market basket, we proposed to derive the weights for the PPIs using the 2012 Benchmark I-O data. The 2012-based IRF market basket used the 2007 Benchmark I-O data to derive the weights for the four PPIs (80 FR 47061).
Table 8 shows the weights for each of the four PPIs used to create the proposed blended Chemical proxy for the 2016 IRF market basket compared to the 2012-based blended Chemical proxy.
(10) Medical Instruments
We proposed to continue to use a blend of two PPIs for the Medical Instruments cost category. The 2012 Benchmark Input-Output data shows an approximate 57/43 split between Surgical and Medical Instruments and Medical and Surgical Appliances and Supplies for this cost category. Therefore, we proposed a blend composed of 57 percent of the commodity-based PPI for Surgical and Medical Instruments (BLS series code WPU1562) and 43 percent of the commodity-based PPI for Medical and Surgical Appliances and Supplies (BLS series code WPU1563). The 2012-based IRF market basket used a 50/50 blend of these PPIs based on the 2007 Benchmark I-O data (80 FR 47061).
(11) Rubber and Plastics
We proposed to continue to use the PPI for Rubber and Plastic Products (BLS series code WPU07) to measure price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(12) Paper and Printing Products
We proposed to continue to use the PPI for Converted Paper and Paperboard Products (BLS series code WPU0915) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(13) Miscellaneous Products
We proposed to continue to use the PPI for Finished Goods Less Food and Energy (BLS series code WPUFD4131) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(14) Professional Fees: Labor-Related
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Professional and Related (BLS series code CIU2010000120000I) to measure the price growth of this category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(15) Administrative and Facilities Support Services
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Office and Administrative Support (BLS series code CIU2010000220000I) to measure the price growth of this category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(16) Installation, Maintenance, and Repair
We proposed to continue to use the ECI for Total Compensation for Civilian workers in Installation, Maintenance, and Repair (BLS series code CIU1010000430000I) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(17) All Other: Labor-Related Services
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Service Occupations (BLS series code CIU2010000300000I) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(18) Professional Fees: Nonlabor-Related
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Professional and Related (BLS series code CIU2010000120000I) to measure the price growth of this category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(19) Financial Services
We proposed to continue to use the ECI for Total Compensation for Private Industry workers in Financial Activities (BLS series code CIU201520A000000I) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(20) Telephone Services
We proposed to continue to use the CPI for Telephone Services (BLS series code CUUR0000SEED) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
(21) All Other: Nonlabor-Related Services
We proposed to continue to use the CPI for All Items Less Food and Energy (BLS series code CUUR0000SA0L1E) to measure the price growth of this cost category. This is the same proxy used in the 2012-based IRF market basket (80 FR 47061).
b. Price Proxies for the Capital Portion of the 2016-Based IRF Market Basket
(1) Capital Price Proxies Prior to Vintage Weighting
We proposed to continue to use the same price proxies for the capital-related cost categories in the 2016-based IRF market basket as were used in the 2012-based IRF market basket (80 FR 47062), which are provided in Table 10 and described below. Specifically, we proposed to proxy:
- Depreciation: Building and Fixed Equipment cost category by BEA's Chained Price Index for Nonresidential Construction for Hospitals and Special Care Facilities (BEA Table 5.4.4. Price Indexes for Private Fixed Investment in Structures by Type).
- Depreciation: Movable Equipment cost category by the PPI for Machinery and Equipment (BLS series code WPU11).
- Nonprofit Interest cost category by the average yield on domestic municipal bonds (Bond Buyer 20-bond index).
- For-profit Interest cost category by the average yield on Moody's Aaa bonds (Federal Reserve).
- Other Capital-Related cost category by the CPI-U for Rent of Primary Residence (BLS series code CUUS0000SEHA).
We believe these are the most appropriate proxies for IRF capital-related costs that meet our selection criteria of relevance, timeliness, availability, and reliability. We proposed to continue to vintage weight the capital price proxies for Depreciation and Interest to capture the long-term consumption of capital. This vintage weighting method is similar to the method used for the 2012-based IRF market basket (80 FR 47062) and is described below.
(2) Vintage Weights for Price Proxies
Because capital is acquired and paid for over time, capital-related expenses in any given year are determined by both past and present purchases of physical and financial capital. The vintage-weighted capital-related portion of the 2016-based IRF market basket is intended to capture the long-term consumption of capital, using vintage weights for depreciation (physical capital) and interest (financial capital). These vintage weights reflect the proportion of capital-related purchases attributable to each year of the expected life of building and fixed equipment, movable equipment, and interest. We proposed to use vintage weights to compute vintage-weighted price changes associated with depreciation and interest expenses.
Capital-related costs are inherently complicated and are determined by complex capital-related purchasing decisions, over time, based on such factors as interest rates and debt financing. In addition, capital is depreciated over time instead of being consumed in the same period it is purchased. By accounting for the vintage nature of capital, we are able to provide an accurate and stable annual measure of price changes. Annual non-vintage price changes for capital are unstable due to the volatility of interest rate changes, and therefore, do not reflect the actual annual price changes for IRF capital-related costs. The capital-related component of the 2016-based IRF market basket reflects the underlying stability of the capital-related acquisition process.
The methodology used to calculate the vintage weights for the 2016-based IRF market basket is the same as that used for the 2012-based IRF market basket (80 FR 47062 through 47063) with the only difference being the inclusion of more recent data. To calculate the vintage weights for depreciation and interest expenses, we first need a time series of capital-related purchases for building and fixed equipment and movable equipment. We found no single source that provides an appropriate time series of capital-related purchases by hospitals for all of the above components of capital purchases. The early Medicare cost reports did not have sufficient capital-related data to meet this need. Data we obtained from the American Hospital Association (AHA) do not include annual capital-related purchases. However, we are able to obtain data on total expenses back to 1963 from the AHA. Consequently, we proposed to use data from the AHA Panel Survey and the AHA Annual Survey to obtain a time series of total expenses for hospitals. We then proposed to use data from the AHA Panel Survey supplemented with the ratio of depreciation to total hospital expenses obtained from the Medicare cost reports to derive a trend of annual depreciation expenses for 1963 through 2016. We proposed to separate these depreciation expenses into annual amounts of building and fixed equipment depreciation and movable equipment depreciation as determined earlier. From these annual depreciation amounts, we derive annual end-of-year book values for building and fixed equipment and movable equipment using the expected life for each type of asset category. While data is not available that is specific to IRFs, we believe this information for all hospitals serves as a reasonable alternative for the pattern of depreciation for IRFs.
To continue to calculate the vintage weights for depreciation and interest expenses, we also need to account for the expected lives for Building and Fixed Equipment, Movable Equipment, and Interest for the 2016-based IRF market basket. We proposed to calculate the expected lives using MCR data from freestanding and hospital-based IRFs. The expected life of any asset can be determined by dividing the value of the asset (excluding fully depreciated assets) by its current year depreciation amount. This calculation yields the estimated expected life of an asset if the rates of depreciation were to continue at current year levels, assuming straight-line depreciation. We proposed to determine the expected life of building and fixed equipment separately for hospital-based IRFs and freestanding IRFs, and then weight these expected lives using the percent of total capital costs each provider type represents. We proposed to apply a similar method for movable equipment. Using these methods, we determined the average expected life of building and fixed equipment to be equal to 22 years, and the average expected life of movable equipment to be equal to 11 years. For the expected life of interest, we believe vintage weights for interest should represent the average expected life of building and fixed equipment because, based on previous research described in the FY 1997 IPPS final rule (61 FR 46198), the expected life of hospital debt instruments and the expected life of buildings and fixed equipment are similar. We note that for the 2012-based IRF market basket, the expected life of building and fixed equipment is 23 years, and the expected life of movable equipment is 11 years (80 FR 47062).
Multiplying these expected lives by the annual depreciation amounts results in annual year-end asset costs for building and fixed equipment and movable equipment. We then calculate a time series, beginning in 1964, of annual capital purchases by subtracting the previous year's asset costs from the current year's asset costs.
For the building and fixed equipment and movable equipment vintage weights, we proposed to use the real annual capital-related purchase amounts for each asset type to capture the actual amount of the physical acquisition, net of the effect of price inflation. These real annual capital-related purchase amounts are produced by deflating the nominal annual purchase amount by the associated price proxy as provided earlier in this final rule. For the interest vintage weights, we proposed to use the total nominal annual capital-related purchase amounts to capture the value of the debt instrument (including, but not limited to, mortgages and bonds). Using these capital-related purchase time series specific to each asset type, we proposed to calculate the vintage weights for building and fixed equipment, for movable equipment, and for interest.
The vintage weights for each asset type are deemed to represent the average purchase pattern of the asset over its expected life (in the case of building and fixed equipment and interest, 22 years, and in the case of movable equipment, 11 years). For each asset type, we used the time series of annual capital-related purchase amounts available from 2016 back to 1964. These data allow us to derive 32, 22-year periods of capital-related purchases for building and fixed equipment and interest, and 43, 11-year periods of capital-related purchases for movable equipment. For each 22-year period for building and fixed equipment and interest, or 11-year period for movable equipment, we calculate annual vintage weights by dividing the capital-related purchase amount in any given year by the total amount of purchases over the entire 22-year or 11-year period. This calculation is done for each year in the 22-year or 11-year period and for each of the periods for which we have data. We then calculate the average vintage weight for a given year of the expected life by taking the average of these vintage weights across the multiple periods of data.
We did not receive any specific public comments on our proposed calculation of the vintage weights for the 2016-based IRF market basket. Therefore, in this final rule, we are finalizing the vintage weights as proposed. The vintage weights for the capital-related portion of the 2016-based IRF market basket and the 2012-based IRF market basket are presented in Table 9.

The process of creating vintage-weighted price proxies requires applying the vintage weights to the price proxy index where the last applied vintage weight in Table 8 is applied to the most recent data point. We have provided on the CMS website an example of how the vintage weighting price proxies are calculated, using example vintage weights and example price indices. The example can be found at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketResearch.html in the zip file titled “Weight Calculations as described in the IPPS FY 2010 Proposed Rule.”
c. Summary of Price Proxies of the 2016-Based IRF Market Basket
Table 10 shows both the operating and capital price proxies for the 2016-based IRF market basket.

D. FY 2020 Market Basket Update and Productivity Adjustment
1. FY 2020 Market Basket Update
For FY 2020 (that is, beginning October 1, 2019 and ending September 30, 2020), we proposed to use the 2016-based IRF market basket increase factor described in section V.C. of the proposed rule to update the IRF PPS base payment rate. Consistent with historical practice, we proposed to estimate the market basket update for the IRF PPS based on IHS Global Inc.'s (IGI's) forecast using the most recent available data. IGI is a nationally-recognized economic and financial forecasting firm with which we contract to forecast the components of the market baskets and MFP. In the FY 2020 IRF PPS proposed rule (84 FR 17274), we proposed a market basket increase factor of 3.0 percent for FY 2020, which was based on IGI's first quarter 2019 forecast with historical data through fourth quarter 2018.
In the FY 2020 IRF PPS proposed rule, we also proposed that if more recent data were subsequently available (for example, a more recent estimate of the market basket and MFP adjustment), we would use such data to determine the FY 2020 update in the final rule. Incorporating more recent data, the projected 2016-based IRF market basket increase factor for FY 2020 is 2.9 percent, which is based on IGI's second quarter 2019 forecast with historical data through first quarter 2019.
We received several comments on our proposed market basket update and productivity adjustment, which are summarized below.
Comment: Commenters supported the proposal to update the market basket and MFP adjustment using the latest available data, and encouraged CMS to update these factors using the latest available data as part of the release of the FY 2020 IRF PPS final rule.
Response: We appreciate the commenters' support for updating the market basket and MFP adjustments using the latest available data.
Comment: A few commenters expressed concern about the lack of transparency of the market basket and MFP payment updates. The commenters stated that the IGI forecast appears to be procured specifically for the purpose of CMS updating the IRF market basket and productivity adjustment. The commenters also noted that it is concerning that CMS does not provide IGI's analyses or report to the public given the key role the market basket and productivity adjustment play in updating the payment system each year and that without such information stakeholders are unable to evaluate the accuracy of the update. The commenters also mentioned that the same comment was submitted in the FY 2019 rulemaking process but they do not believe that the response was adequate since the actual analysis or report used to create the forecasts was not provided (83 FR 38525). The commenters requested that CMS release an IGI report and analysis used to update the IRF market basket and standard payment conversion factor.
Response: IGI regularly produces and publishes a wide variety of forecasted series on a monthly or quarterly basis. These forecasts are derived using a framework of proprietary economic models that are created and updated regularly by IGI. IGI provides these forecasts to a wide array of clients in addition to CMS. We use a contractor for the price forecasts so that the forecasts are independent and reflect a complete economic forecasting model, a capability that we do not have. IGI has received multiple awards for their macroeconomic forecast accuracy of major economic indicators. We use IGI's price forecasts in all of the FFS market baskets used for payment updates and has used the forecasts produced by this company for many years.
We select approximately 30 individual price proxies as inputs to the IRF market basket calculation. The price series are discussed in detail as part of the rulemaking process. In order to derive a forecast of the IRF market basket index, we contract with IGI to procure the forecasts of these individual price proxies on a quarterly basis. We then combine these price proxies with the market basket base year cost weights to derive the levels of the IRF market basket. The data sources and methods used to derive these cost weights are discussed in detail as part of the rulemaking process.
As provided in our previous response to this comment in the FY 2019 IRF PPS final rule (83 FR 38525), the market basket update is derived using: (1) The market basket base year cost weights as finalized by CMS through rulemaking; and (2) the most up-to-date forecast of the price proxies used in the market basket as forecasted by IGI. Specifically, for each cost category in the market basket (for example, Wages and Salaries, Pharmaceuticals), the level of each of these price proxies are multiplied by the cost weight for that cost category. The sum of these products (that is, weights multiplied by proxied index levels) for all cost categories yields the composite index level in the market basket in a given year.
As acknowledged by the commenters, we provided a link from the CMS website to the top-line market basket updates. We also indicated that more detailed forecasts of the IRF market basket calculations are readily available by request by sending an email to CMSDNHS@cms.hhs.gov to request this information (83 FR 38525). Using these detailed data, the commenter would be able to replicate the levels of the IRF market basket update in the history and the forecast period. We encourage stakeholders to utilize these data, which we believe will address the commenters' concerns.
Incorporating more recent data, the projected 2016-based IRF market basket update for FY 2020 is 2.9 percent. After careful consideration of the comments, consistent with our historical practice of estimating market basket increases based on the best available data, we are finalizing a market basket increase factor of 2.9 percent for FY 2020. For comparison, the current 2012-based IRF market basket is also projected to increase by 2.9 percent in FY 2020 based on IGI's second quarter 2019 forecast.
Table 11 compares the 2016-based IRF market basket and the 2012-based IRF market basket percent changes.

2. Productivity Adjustment
According to section 1886(j)(3)(C)(i) of the Act, the Secretary shall establish an increase factor based on an appropriate percentage increase in a market basket of goods and services. As described in sections VI.C and VI.D.1. of this final rule, we are finalizing an estimate of the IRF PPS increase factor for FY 2020 based on the 2016-based IRF market basket. Section 1886(j)(3)(C)(ii) of the Act then requires that, after establishing the increase factor for a FY, the Secretary shall reduce such increase factor for FY 2012 and each subsequent FY, by the productivity adjustment described in section 1886(b)(3)(B)(xi)(II) of the Act. Section 1886(b)(3)(B)(xi)(II) of the Act sets forth the definition of this productivity adjustment. The statute defines the productivity adjustment to be equal to the 10-year moving average of changes in annual economy-wide private nonfarm business MFP (as projected by the Secretary for the 10-year period ending with the applicable FY, year, cost reporting period, or other annual period) (the “MFP adjustment”). The BLS publishes the official measure of private nonfarm business MFP. Please see http://www.bls.gov/mfp for the BLS historical published MFP data.
MFP is derived by subtracting the contribution of labor and capital input growth from output growth. The projections of the components of MFP are currently produced by IGI, a nationally recognized economic forecasting firm with which CMS contracts to forecast the components of the market basket and MFP. For more information on the productivity adjustment, we refer reader to the discussion in the FY 2016 IRF PPS final rule (80 FR 47065).
Using IGI's first quarter 2019 forecast, the proposed MFP adjustment for FY 2020 (the 10-year moving average of MFP for the period ending FY 2020) was 0.5 percent (84 FR 17274). Thus, in accordance with section 1886(j)(3)(C) of the Act, we proposed to base the FY 2020 market basket update, which is used to determine the applicable percentage increase for the IRF payments, on the most recent estimate of the 2016-based IRF market basket. We proposed to then reduce this percentage increase by the current estimate of the proposed MFP adjustment for FY 2020 of 0.5 percentage point (the 10-year moving average of MFP for the period ending FY 2020 based on IGI's first quarter 2019 forecast). Therefore, the proposed FY 2020 IRF update was 2.5 percent (3.0 percent market basket update, less 0.5 percentage point MFP adjustment). Furthermore, we proposed that if more recent data are subsequently available (for example, a more recent estimate of the market basket and MFP adjustment), we would use such data to determine the FY 2020 market basket update and MFP adjustment in the final rule.
We received a few comments on the application of the productivity adjustment, which are summarized below.
Comment: Commenters continue to be concerned about the application of the productivity adjustment to IRFs. One of the commenters stated that they understood CMS is bound by statute to reduce the market basket update by a productivity adjustment factor in accordance with the PPACA, but they believe that IRFs are unable to generate additional productivity gains at a pace matching the productivity of the economy at large on an ongoing, consistent basis. The commenter noted that the services provided in IRFs are labor-intensive and the services do not lend themselves to continuous productivity improvements. The commenter also noted that IRFs are bound by unchanging labor-intensive standards such as the 3-hour therapy rule and other regulatory requirements that reduce flexibility and restrict the pursuit of certain efficiencies. The commenter noted that continued application of a productivity adjustment to payments could results in decreased beneficiary access to IRF services. The commenter requested that CMS continue to monitor the impact that the multi-factor productivity adjustments have on the IRF sector, provide feedback to Congress as appropriate, and reduce the productivity adjustment. One commenter requested that, in addition to monitoring its effects on overall payments, CMS should evaluate whether IRFs are able to achieve the same level of productivity improvement as workers across the U.S. economy.
Response: We acknowledge the commenters' concerns regarding productivity growth at the economy-wide level and its application to IRFs. As the commenter acknowledges, section 1886(j)(3)(C)(ii)(I) of the Act requires the application of a productivity adjustment to the IRF PPS market basket increase factor.
We will continue to monitor the impact of the payment updates, including the effects of the productivity adjustment, on IRF finances, as well as beneficiary access to care.
We note that each year, MedPAC makes an annual update recommendation to Congress based on a variety of measures related to payment adequacy, including a detailed margin analysis and analysis of beneficiary access to care for IRF services. For FY 2020, MedPAC recommended that Congress reduce the IRF PPS base rate by 5 percent and found that beneficiary access to care was not a concern. The “March 2019 Report to the Congress: Medicare Payment Policy”, chapter 10 is publicly available at http://www.medpac.gov/-documents-/reports.
We would be very interested in better understanding IRF-specific productivity; however, the data elements required to estimate IRF specific multi-factor productivity are not produced at the level of detail that would allow this analysis. We have estimated hospital-sector multi-factor productivity and have published the findings on the CMS website at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/Downloads/ProductivityMemo2016.pdf.
After careful consideration of comments, we are incorporating more recent data to determine the market basket update and MFP adjustment for FY 2020. Using IGI's second quarter 2019 forecast, the current estimate of the MFP adjustment for FY 2020 (the 10-year moving average of MFP for the period ending FY 2020) is 0.4 percent. Thus, in accordance with section 1886(j)(3)(C) of the Act, we are finalizing a FY 2020 market basket update of 2.9 percent. We then reduce this percentage increase by the most recent estimate of the MFP adjustment for FY 2020 of 0.4 percentage point (the 10-year moving average of MFP for the period ending FY 2020 based on IGI's second quarter 2019 forecast). Therefore, the final FY 2020 IRF productivity-adjusted market basket update is equal to 2.5 percent (2.9 percent market basket update, less 0.4 percentage point MFP adjustment).
For FY 2020, the Medicare Payment Advisory Commission (MedPAC) recommends that a decrease of 5 percent be applied to IRF PPS payment rates. As discussed, and in accordance with section 1886(j)(3)(C) of the Act, we are finalizing an update to IRF PPS payment rates for FY 2020 by a productivity-adjusted market basket increase factor of 2.5 percent, as section 1886(j)(3)(C) of the Act does not provide the Secretary with the authority to apply a different update factor to IRF PPS payment rates for FY 2020.
Comment: One commenter (MedPAC) stated that they understand that CMS is required to implement the statutory update of market basket less productivity adjustment, but that their analysis of beneficiary access to rehabilitative services, the supply of providers, and aggregate IRF Medicare margins, which have been above 11 percent since 2012, indicates that the Congress should reduce the IRF payment rate by 5 percent for FY 2020.
Response: We appreciate MedPAC's interest in the IRF increase factor. However, we are required to update IRF PPS payments by the market basket reduced by the productivity adjustment, as directed by section 1886(j)(3)(C) of the Act.
E. Labor-Related Share for FY 2020
Section 1886(j)(6) of the Act specifies that the Secretary is to adjust the proportion (as estimated by the Secretary from time to time) of rehabilitation facilities' costs which are attributable to wages and wage-related costs, of the prospective payment rates computed under section 1886(j)(3) of the Act for area differences in wage levels by a factor (established by the Secretary) reflecting the relative hospital wage level in the geographic area of the rehabilitation facility compared to the national average wage level for such facilities. The labor-related share is determined by identifying the national average proportion of total costs that are related to, influenced by, or vary with the local labor market. We proposed to continue to classify a cost category as labor-related if the costs are labor-intensive and vary with the local labor market. As stated in the FY 2016 IRF PPS final rule (80 FR 47068), the labor-related share was defined as the sum of the relative importance of Wages and Salaries, Employee Benefits, Professional Fees: Labor-related Services, Administrative and Facilities Support Services, Installation, Maintenance, and Repair, All Other: Labor-related Services, and a portion of the Capital Costs from the 2012-based IRF market basket.
Based on our definition of the labor-related share and the cost categories in the 2016-based IRF market basket, we proposed to include in the labor-related share for FY 2020 the sum of the FY 2020 relative importance of Wages and Salaries, Employee Benefits, Professional Fees: Labor-related, Administrative and Facilities Support Services, Installation, Maintenance, and Repair, All Other: Labor-related Services, and a portion of the Capital-Related cost weight from the 2016-based IRF market basket.
Similar to the 2012-based IRF market basket (80 FR 47067), the 2016-based IRF market basket includes two cost categories for nonmedical Professional Fees (including, but not limited to, expenses for legal, accounting, and engineering services). These are Professional Fees: Labor-related and Professional Fees: Nonlabor-related. For the 2016-based IRF market basket, we proposed to estimate the labor-related percentage of non-medical professional fees (and assign these expenses to the Professional Fees: Labor-related services cost category) based on the same method that was used to determine the labor-related percentage of professional fees in the 2012-based IRF market basket.
As was done in the 2012-based IRF market basket (80 FR 47067), we proposed to determine the proportion of legal, accounting and auditing, engineering, and management consulting services that meet our definition of labor-related services based on a survey of hospitals conducted by us in 2008, a discussion of which can be found in the FY 2010 IPPS/LTCH PPS final rule (74 FR 43850 through 43856). Based on the weighted results of the survey, we determined that hospitals purchase, on average, the following portions of contracted professional services outside of their local labor market:
- 34 percent of accounting and auditing services.
- 30 percent of engineering services.
- 33 percent of legal services.
- 42 percent of management consulting services.
We proposed to apply each of these percentages to the respective Benchmark I-O cost category underlying the professional fees cost category to determine the Professional Fees: Nonlabor-related costs. The Professional Fees: Labor-related costs were determined to be the difference between the total costs for each Benchmark I-O category and the Professional Fees: Nonlabor-related costs. This is the same methodology that we used to separate the 2012-based IRF market basket professional fees category into Professional Fees: Labor-related and Professional Fees: Nonlabor-related cost categories (80 FR 47067).
In the 2016-based IRF market basket, nonmedical professional fees that are subject to allocation based on these survey results represent 4.4 percent of total costs (and are limited to those fees related to Accounting & Auditing, Legal, Engineering, and Management Consulting services). Based on our survey results, we proposed to apportion 2.8 percentage points of the 4.4 percentage point figure into the Professional Fees: Labor-related share cost category and designate the remaining 1.6 percentage point into the Professional Fees: Nonlabor-related cost category.
In addition to the professional services listed, for the 2016-based IRF market basket, we proposed to allocate a proportion of the Home Office Contract Labor cost weight, calculated using the Medicare cost reports as stated above, into the Professional Fees: Labor-related and Professional Fees: Nonlabor-related cost categories. We proposed to classify these expenses as labor-related and nonlabor-related as many facilities are not located in the same geographic area as their home office, and therefore, do not meet our definition for the labor-related share that requires the services to be purchased in the local labor market. For the 2012-based IRF market basket, we used the BEA I-O expense data for NAICS 55, Management of Companies and Enterprises, to estimate the Home Office Contract Labor cost weight (80 FR 47067). We then allocated these expenses into the Professional Fess: Labor-related and Professional Fees: Nonlabor-related cost categories.
Similar to the 2012-based IRF market basket, we proposed for the 2016-based IRF market basket to use the Medicare cost reports for both freestanding IRF providers and hospital-based IRF providers to determine the home office labor-related percentages. The MCR requires a hospital to report information regarding their home office provider. For the 2016-based IRF market basket, we proposed to start with the sample of IRF providers that passed the top 1 percent trim used to derive the Home Office Contract Labor cost weight as described in section VI.B. of this final rule. For both freestanding and hospital-based providers, we proposed to multiply each provider's Home Office Contract Labor cost weight (calculated using data from the total facility) by Medicare allowable total costs. This results in an amount of Medicare allowable home office compensation costs for each IRF. Using information on the Medicare cost report, we then compare the location of the IRF with the location of the IRF's home office. We proposed to classify an IRF with a home office located in their respective local labor market if the IRF and its home office are located in the same Metropolitan Statistical Area. We then calculate the proportion of Medicare allowable home office compensation costs that these IRFs represent of total Medicare allowable home office compensation costs. We proposed to multiply this percentage (42 percent) by the Home Office Contract Labor cost weight (3.7 percent) to determine the proportion of costs that should be allocated to the labor-related share. Therefore, we allocated 1.6 percentage points of the Home Office Contract Labor cost weight (3.7 percent times 42 percent) to the Professional Fees: Labor-related cost weight and 2.1 percentage points of the Home Office Contract Labor cost weight to the Professional Fees: Nonlabor-related cost weight (3.7 percent times 58 percent). For the 2012-based IRF market basket, we used a similar methodology but we relied on provider counts rather than home office/related organization contract labor compensation costs to determine the labor-related percentage (80 FR 47067).
In summary, we apportioned 2.8 percentage points of the non-medical professional fees and 1.6 percentage points of the home office/related organization contract labor cost weights into the Professional Fees: Labor-related cost category. This amount was added to the portion of professional fees that was identified to be labor-related using the I-O data such as contracted advertising and marketing costs (approximately 0.6 percentage point of total costs) resulting in a Professional Fees: Labor-related cost weight of 5.0 percent.
We received several comments on the proposed labor-related share, which are summarized below.
Comment: A few commenters noted that the cost weight for Home Office Contract Labor costs is 3.7 percent of all IRFs' costs and influences changes in other payment areas, such as the total labor-related share. The commenters stated that they believe the proposed changes to the methodology are responsible, at least in large part, to the notable proposed increase of approximately 2 percent of the labor-related share. Some of the commenters also stated that the increase in the labor-related share will adversely impact rural IRFs and IRFs with a wage index below 1.0.
Response: The labor-related share for IRFs is derived from the relative importance of the labor-related cost categories. The relative importance for FY 2020 reflects the different rates of price change for each of the individual cost categories between the base year and FY 2020. For the FY 2020 final rule, as proposed, the final labor-related share for FY 2020 is based on a more recent forecast of the 2016-based IRF market basket. Using the more recent forecast, the total difference between the FY 2020 labor-related share using the 2016-based IRF market basket and 2012-based IRF market basket is 2.0 percentage points (72.7 percent using 2016-based IRF market basket and 70.7 percent using 2012-based IRF market basket). This difference can be separated into two primary components: (1) Revision to the base year cost weights (1.4 percentage points); and (2) revision to starting point of calculation of relative importance (base year) from 2012 to 2016 (0.6 percentage point). Of the 1.4-percentage points difference in the base year cost weights, just 0.2 percentage point is attributable to deriving the Home Office Contract Labor cost weight using the MCR data rather than the I-O data; the remainder is due to the increase in Compensation and Capital cost weights (calculated using the MCR data) and the incorporation of the 2012 Benchmark I-O data.
The impact of using the MCR data to calculate the Home Office Contract Labor cost weight is minimal because it also lowers the residual “All Other” cost weight from 25.8 percent (using the I-O data to calculate the Home Office Contract Labor cost weight) to 22.2 percent (using the MCR data to calculate the Home Office Contract labor cost weight). The lower residual “All Other” cost weight then leads to relatively lower cost weights for Administrative and Business Support Services, Installation, Maintenance and Repair Services, and All Other: Labor-related Services (which are calculated using the Benchmark I-O data), each of which is also reflected in the labor-related share.
After careful consideration of comments, in this final rule, we are finalizing the 2016-based IRF market basket labor-related share cost weights as proposed.
As stated previously, we proposed to include in the labor-related share the sum of the relative importance of Wages and Salaries, Employee Benefits, Professional Fees: Labor-Related, Administrative and Facilities Support Services, Installation, Maintenance, and Repair, All Other: Labor-related Services, and a portion of the Capital-Related cost weight from the 2016-based IRF market basket. The relative importance reflects the different rates of price change for these cost categories between the base year (2016) and FY 2020. Based on IGI's 2nd quarter 2019 forecast for the 2016-based IRF market basket, the sum of the FY 2020 relative importance for Wages and Salaries, Employee Benefits, Professional Fees: Labor-related, Administrative and Facilities Support Services, Installation Maintenance & Repair Services, and All Other: Labor-related Services is 68.7 percent. The portion of Capital costs that are influenced by the local labor market is estimated to be 46 percent, which is the same percentage applied to the 2012-based IRF market basket (80 FR 47068). Since the relative importance for Capital is 8.6 percent of the 2016-based IRF market basket in FY 2020, we took 46 percent of 8.6 percent to determine the labor-related share of Capital for FY 2020 of 4.0 percent. Therefore, we are finalizing a total labor-related share for FY 2020 of 72.7 percent (the sum of 68.7 percent for the operating costs and 4.0 percent for the labor-related share of Capital).
Table 12 shows the FY 2020 labor-related share using the final 2016-based IRF market basket relative importance and the FY 2019 labor-related share which was based on the 2012-based IRF market basket relative importance.

F. Update to the IRF Wage Index To Use Concurrent IPPS Wage Index Beginning With FY 2020
1. Background
Section 1886(j)(6) of the Act requires the Secretary to adjust the proportion of rehabilitation facilities' costs attributable to wages and wage-related costs (as estimated by the Secretary from time to time) by a factor (established by the Secretary) reflecting the relative hospital wage level in the geographic area of the rehabilitation facility compared to the national average wage level for those facilities. The Secretary is required to update the IRF PPS wage index on the basis of information available to the Secretary on the wages and wage-related costs to furnish rehabilitation services. Any adjustment or updates made under section 1886(j)(6) of the Act for a FY are made in a budget-neutral manner.
2. Update to the IRF Wage Index To Use Concurrent IPPS Wage Index Beginning with FY 2020
When the IRF PPS was implemented in the FY 2002 IRF PPS final rule (66 FR 41358), we finalized the use of the FY IPPS wage data in the creation of an IRF wage index. We believed that a wage index based on FY IPPS wage data was the best proxy and most appropriate wage index to use in adjusting payments to IRFs, since both IPPS hospitals and IRFs compete in the same labor markets. For this reason, we believed, and continue to believe, that the wage data of IPPS hospitals accurately captures the relationship of wages and wage-related costs of IRFs in an area as compared with the national average. Therefore, in the FY 2002 IRF PPS final rule, we finalized use of the FY 1997 IPPS wage data to develop the wage index for the IRF PPS, as that was the most recent final data available.
For all subsequent years in which the IRF PPS wage index has been updated, we have continued to use the most recent final IPPS data available, which has led us to use the pre-floor, pre-reclassified FY IPPS wage index values from the prior fiscal year.
In the FY 2018 IRF PPS proposed rule (82 FR 20742 through 20743), we included a request for information (RFI) to solicit comments from stakeholders requesting information on CMS flexibilities and efficiencies. The purpose of the RFI was to receive feedback regarding ways in which we could reduce burden for hospitals and physicians, improve quality of care, decrease costs and ensure that patients receive the best care. We received comments from IRF industry associations, state and national hospital associations, industry groups, representing hospitals, and individual IRF providers in response to the solicitation. One of the responses we received to the RFI suggested that there is concern among IRF stakeholders about the different wage index data used in the different post-acute care (PAC) settings. For the IRF PPS, we use a 1-year lag of the pre-floor, pre-reclassified FY IPPS wage index, meaning that for the IRF PPS for FY 2019, we finalized use of the FY 2018 IPPS wage index (83 FR 38527). However, we base the wage indexes for the SNF PPS and the LTCH PPS on the concurrent IPPS wage index ((83 FR 39172 through 39178) and (83 FR 41731), respectively).
As we look towards a more unified PACpayment system, we believe that standardizing the wage index data across PAC settings is necessary. Therefore, we proposed to change the IRF wage index methodology to align with other PAC settings. Specifically, we proposed changing from our established policy of using the pre-floor, pre-reclassified FY IPPS wage index (that is, for FY 2020 we proposed to use the concurrent FY 2020 pre-floor, pre-reclassified IPPS wage index under the IRF PPS). This proposed change would use the concurrent IPPS pre-floor, pre-reclassified wage index for the IRF wage index beginning with FY 2020 and continuing for all subsequent years. Thus, for the FY 2020 IRF wage index, we proposed to use the FY 2020 pre-floor, pre-reclassified IPPS wage index, which is based on data submitted for hospital cost reporting periods beginning in FY 2016. We proposed to implement these revisions in a budget neutral manner. For more information on the distributional impacts of this proposal, we refer readers to the FY 2020 IRF PPS proposed rule (84 FR 17278).
Using the current pre-floor, pre-reclassified FY IPPS wage index would result in the most up-to-date wage data being the basis for the IRF wage index. It would also result in more consistency and equity in the wage index methodology used by Medicare.
We received 7 comments on this proposal to align the data timeframes with that of the IPPS by using the FY 2020 pre-floor, pre-reclassified FY IPPS wage index as the basis for the FY 2020 IRF wage index, which are summarized below.
Comment: All of the commenters supported CMS' proposal to use the FY 2020 pre-floor, pre-reclassified FY IPPS wage index for the FY 2020 IRF wage index. Commenters agreed that the proposed change to use the concurrent FY IPPS wage index data would align the wage index data across PAC settings and move in the direction of unified PAC payment. A few commenters recommended that CMS adopt other wage index policies for IRFs that apply to or have been proposed for IPPS hospitals, such as geographic reclassifications, suggesting that this would increase consistency and alignment across settings.
Response: We appreciate the commenter's support for the proposal. We agree that finalizing this proposal is necessary as we move towards a more unified PAC payment system. We plan to monitor the use of the concurrent FY IPPS wage index data before we consider any other potential wage index policy changes.
After careful consideration of the comments we received, we are finalizing our proposal to align the data timeframes with that of the IPPS by using the concurrent pre-floor, pre-reclassified IPPS wage index for the IRF wage index beginning with FY 2020 and continuing for all subsequent years. Thus, we will use the FY 2020 pre-floor, pre-reclassified IPPS wage index as the basis for the FY 2020 IRF wage index (that is, for all IRF discharges beginning on or after October 1, 2019). We will implement these revisions in a budget neutral manner. We refer readers to Table 20 in section XIII.C of this final rule for more information on the distributional effects of this change.
3. Wage Adjustment for FY 2020 Using Concurrent IPPS Wage Index Labor Market Area Definitions and the
Due to our proposal to use the concurrent IPPS wage index beginning with FY 2020, for FY 2020, we proposed using the policy and methodologies described in section VI. of this final rule related to the labor market area definitions and the wage index methodology for areas with wage data. Thus, we proposed using the CBSA labor market area definitions and the FY 2020 pre-reclassification and pre-floor IPPS wage index data. In accordance with section 1886(d)(3)(E) of the Act, the FY 2020 pre-reclassification and pre-floor IPPS wage index is based on data submitted for hospital cost reporting periods beginning on or after October 1, 2015 and before October 1, 2016 (that is, FY 2016 cost report data).
The labor market designations made by the OMB include some geographic areas where there are no hospitals and, thus, no hospital wage index data on which to base the calculation of the IRF PPS wage index. We proposed to continue to use the same methodology discussed in the FY 2008 IRF PPS final rule (72 FR 44299) to address those geographic areas where there are no hospitals and, thus, no hospital wage index data on which to base the calculation for the FY 2020 IRF PPS wage index.
We received one comment on this proposal, which is summarized below.
Comment: One commenter requested that, until a new wage index system is implemented, CMS should establish a smoothing variable to be applied to the current IRF wage index to reduce the fluctuations IRFs experience annually.
Response: Under section 1886(j)(6) of the Act, we adjust IRF PPS rates to account for differences in area wage levels. Any perceived volatility in the wage index is predicated upon volatility in actual wages in that area and reflects real differences in area wage levels. As we believe that the application of a smoothing variable would make the wage index values less reflective of the area wage levels, we do not believe it would be appropriate to implement such a change to the IRF wage index policy.
After careful consideration of the comments we received, we are finalizing our proposal to use the policy and methodologies described in section VI. of this final rule related to the labor market area definitions and the wage index methodology for areas with wage data. Thus, we are finalizing the use of the CBSA labor market area definitions and the FY 2020 pre-reclassification and pre-floor IPPS wage index data. We are finalizing the continued use of the same methodology discussed in the FY 2008 IRF PPS final rule (72 FR 44299) to address those geographic areas where there are no hospitals and, thus, no hospital wage index data on which to base the calculation for the FY 2020 IRF PPS wage index.
4. Core-Based Statistical Areas (CBSAs) for the FY 2020 IRF Wage Index
The wage index used for the IRF PPS is calculated using the pre-reclassification and pre-floor IPPS wage index data and is assigned to the IRF on the basis of the labor market area in which the IRF is geographically located. IRF labor market areas are delineated based on the CBSAs established by the OMB. The current CBSA delineations (which were implemented for the IRF PPS beginning with FY 2016) are based on revised OMB delineations issued on February 28, 2013, in OMB Bulletin No. 13-01. OMB Bulletin No. 13-01 established revised delineations for Metropolitan Statistical Areas, Micropolitan Statistical Areas, and Combined Statistical Areas in the United States and Puerto Rico based on the 2010 Census, and provided guidance on the use of the delineations of these statistical areas using standards published in the June 28, 2010 Federal Register (75 FR 37246 through 37252). We refer readers to the FY 2016 IRF PPS final rule (80 FR 47068 through 47076) for a full discussion of our implementation of the OMB labor market area delineations beginning with the FY 2016 wage index.
Generally, OMB issues major revisions to statistical areas every 10 years, based on the results of the decennial census. However, OMB occasionally issues minor updates and revisions to statistical areas in the years between the decennial censuses. On July 15, 2015, OMB issued OMB Bulletin No. 15-01, which provides minor updates to and supersedes OMB Bulletin No. 13-01 that was issued on February 28, 2013. The attachment to OMB Bulletin No. 15-01 provides detailed information on the update to statistical areas since February 28, 2013. The updates provided in OMB Bulletin No. 15-01 are based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2012 and July 1, 2013.
In the FY 2018 IRF PPS final rule (82 FR 36250 through 36251), we adopted the updates set forth in OMB Bulletin No. 15-01 effective October 1, 2017, beginning with the FY 2018 IRF wage index. For a complete discussion of the adoption of the updates set forth in OMB Bulletin No. 15-01, we refer readers to the FY 2018 IRF PPS final rule. In the FY 2019 IRF PPS final rule (83 FR 38527), we continued to use the OMB delineations that were adopted beginning with FY 2016 to calculate the area wage indexes, with updates set forth in OMB Bulletin No. 15-01 that we adopted beginning with the FY 2018 wage index.
On August 15, 2017, OMB issued OMB Bulletin No. 17-01, which provided updates to and superseded OMB Bulletin No. 15-01 that was issued on July 15, 2015. The attachments to OMB Bulletin No. 17-01 provide detailed information on the update to statistical areas since July 15, 2015, and are based on the application of the 2010 Standards for Delineating Metropolitan and Micropolitan Statistical Areas to Census Bureau population estimates for July 1, 2014 and July 1, 2015. In OMB Bulletin No. 17-01, OMB announced that one Micropolitan Statistical Area now qualifies as a Metropolitan Statistical Area. The new urban CBSA is as follows:
- Twin Falls, Idaho (CBSA 46300). This CBSA is comprised of the principal city of Twin Falls, Idaho in Jerome County, Idaho and Twin Falls County, Idaho.
The OMB bulletin is available on the OMB website at https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/bulletins/2017/b-17-01.pdf.
As we indicated in the FY 2019 IRF PPS final rule (83 FR 38528), we believe that it is important for the IRF PPS to use the latest labor market area delineations available as soon as is reasonably possible to maintain a more accurate and up-to-date payment system that reflects the reality of population shifts and labor market conditions. As discussed in the FY 2019 IPPS and LTCH PPS final rule (83 FR 20591), these updated labor market area definitions were implemented under the IPPS beginning on October 1, 2018. Therefore, we proposed to implement these revisions for the IRF PPS beginning October 1, 2019, consistent with our historical practice of modeling IRF PPS adoption of the labor market area delineations after IPPS adoption of these delineations.
We received 2 comments on this proposal, which are summarized below.
Comment: Commenters expressed concern that the IRF wage index values published in the FY 2020 IRF PPS proposed rule were not consistent with the values published in the FY 2020 IPPS proposed rule wage index public use file. These commenters suggested that CMS examine these wage index values and correct them if we find that they are in error prior to finalizing the use of the concurrent IPPS wage index data for the IRF PPS.
Response: We identified a slight error in the proposed rule wage index values after the FY 2020 IRF PPS proposed rule was published. A programming error caused the data for all providers in a single county to be included twice, which affected the national average hourly rate, and therefore, affected nearly all wage index values. We have corrected the programming logic so this error cannot occur again. We also standardized our procedures for rounding, to ensure consistency. The correction to the proposed rule wage index data was not completed until after the comment period closed on June 17, 2019. This final rule reflects the corrected and updated wage index data.
We are finalizing and implementing these revisions for the IRF PPS beginning October 1, 2019, consistent with our historical practice of modeling IRF PPS adoption of the labor market area delineations after IPPS adoption of these delineations.
5. Wage Adjustment
The FY 2020 wage index tables (which, as discussed in section VI.F above, we base on the FY 2020 pre-reclassified, pre-floor FY 2020 IPPS wage index) are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRF-Rules-and-Related-Files.html. Table A is for urban areas, and Table B is for rural areas.
To calculate the wage-adjusted facility payment for the payment rates set forth in this final rule, we would multiply the unadjusted federal payment rate for IRFs by the FY 2020 labor-related share based on the 2016-based IRF market basket (72.7 percent) to determine the labor-related portion of the standard payment amount. A full discussion of the calculation of the labor-related share is located in section VI.E of this final rule. We would then multiply the labor-related portion by the applicable IRF wage index from the tables in the addendum to this final rule. These tables are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRF-Rules-and-Related-Files.html. Adjustments or updates to the IRF wage index made under section 1886(j)(6) of the Act must be made in a budget-neutral manner. We proposed to calculate a budget-neutral wage adjustment factor as established in the FY 2004 IRF PPS final rule (68 FR 45689), codified at § 412.624(e)(1), as described in the steps below. We proposed to use the listed steps to ensure that the FY 2020 IRF standard payment conversion factor reflects the updates to the IRF wage index (based on the FY 2020 IPPS wage index) and the labor-related share in a budget-neutral manner:
Step 1. Determine the total amount of the estimated FY 2019 IRF PPS payments, using the FY 2019 standard payment conversion factor and the labor-related share and the wage indexes from FY 2019 (as published in the FY 2019 IRF PPS final rule (83 FR 38514)).
Step 2. Calculate the total amount of estimated IRF PPS payments using the FY 2020 standard payment conversion factor and the FY 2020 labor-related share and CBSA urban and rural wage indexes.
Step 3. Divide the amount calculated in step 1 by the amount calculated in step 2. The resulting quotient is the FY 2020 budget-neutral wage adjustment factor of 1.0076.
Step 4. Apply the FY 2020 budget-neutral wage adjustment factor from step 3 to the FY 2020 IRF PPS standard payment conversion factor after the application of the increase factor to determine the FY 2020 standard payment conversion factor.
We note that we have updated our data between the FY 2020 IRF PPS proposed and final rules to ensure that we use the most recent available data in calculating IRF PPS payments. This updated data includes a more complete set of claims for FY 2018 and updated wage index data. Based on our analysis using this updated data, we now estimate a budget-neutral wage adjustment factor of 1.0031 for FY 2020.
We discuss the calculation of the standard payment conversion factor for FY 2020 in section VI.H. of this final rule.
We invited public comments on this proposal. However, we did not receive any comments on the proposed methodology for calculating the budget-neutral wage adjustment factor.
As we did not receive any comments on the proposed methodology for calculating the budget-neutral wage adjustment factor, we are finalizing this policy as proposed for FY 2020.
G. Wage Index Comment Solicitation
Historically, we have calculated the IRF wage index values using unadjusted wage index values from another provider setting. Stakeholders have frequently commented on certain aspects of the IRF wage index values and their impact on payments. Therefore, we solicited public comments in the FY 2020 IRF PPS proposed rule (84 FR 17280) on concerns stakeholders may have regarding the wage index used to adjust IRF payments and suggestions for possible updates and improvements to the geographic adjustment of IRF payments.
We appreciate the commenters' responses to this solicitation and will take them into consideration for possible future policy development.
H. Description of the IRF Standard Payment Conversion Factor and Payment Rates for FY 2020
To calculate the standard payment conversion factor for FY 2020, as illustrated in Table 13, we begin by applying the increase factor for FY 2020, as adjusted in accordance with sections 1886(j)(3)(C) of the Act, to the standard payment conversion factor for FY 2019 ($16,021). Applying the 2.5 percent increase factor for FY 2020 to the standard payment conversion factor for FY 2019 of $16,021 yields a standard payment amount of $16,422. Then, we apply the budget neutrality factor for the FY 2020 wage index and labor-related share of 1.0031, which results in a standard payment amount of $16,472. We next apply the budget neutrality factor for the revised CMGs and CMG relative weights of 1.0010, which results in the standard payment conversion factor of $16,489 for FY 2020.

We received one comment on the proposed FY 2020 standard payment conversion factor, which is summarized below.
Comment: One commenter stated that the proposed rate update fails to cover the cost of medical inflation or payment reductions due to sequestration. As a result, this commenter expressed concern that their hospitals' financial viability and their ability to care for their patients will be threatened.
Response: We appreciate this commenter's concerns. However, we note that the IRF PPS payment rates are updated annually by an increase factor that reflects changes over time in the prices of an appropriate mix of goods and services included in the covered IRF services, as required by section 1886(j)(3)(C) of the Act.
After careful consideration of the comment we received, we are finalizing the IRF standard payment conversion factor of $16,489 for FY 2020.
After the application of the CMG relative weights described in section IV. of this final rule to the FY 2020 standard payment conversion factor ($16,489), the resulting unadjusted IRF prospective payment rates for FY 2020 are shown in Table 14.


H. Example of the Methodology for Adjusting the Prospective Payment Rates
Table 15 illustrates the methodology for adjusting the prospective payments (as described in section VI. of this final rule). The following examples are based on two hypothetical Medicare beneficiaries, both classified into CMG 0104 (without comorbidities). The unadjusted prospective payment rate for CMG 0104 (without comorbidities) appears in Table 14.
Example: One beneficiary is in Facility A, an IRF located in rural Spencer County, Indiana, and another beneficiary is in Facility B, an IRF located in urban Harrison County, Indiana. Facility A, a rural non-teaching hospital has a Disproportionate Share Hospital (DSH) percentage of 5 percent (which would result in a LIP adjustment of 1.0156), a wage index of 0.8319, and a rural adjustment of 14.9 percent. Facility B, an urban teaching hospital, has a DSH percentage of 15 percent (which would result in a LIP adjustment of 1.0454 percent), a wage index of 0.8844, and a teaching status adjustment of 0.0784.
To calculate each IRF's labor and non-labor portion of the prospective payment, we begin by taking the unadjusted prospective payment rate for CMG 0104 (without comorbidities) from Table 14. Then, we multiply the labor-related share for FY 2020 (72.7 percent) described in section VI.E. of this final rule by the unadjusted prospective payment rate. To determine the non-labor portion of the prospective payment rate, we subtract the labor portion of the federal payment from the unadjusted prospective payment.
To compute the wage-adjusted prospective payment, we multiply the labor portion of the federal payment by the appropriate wage index located in Tables A and B. These tables are available on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRF-Rules-and-Related-Files.html.
The resulting figure is the wage-adjusted labor amount. Next, we compute the wage-adjusted federal payment by adding the wage-adjusted labor amount to the non-labor portion of the federal payment.
Adjusting the wage-adjusted federal payment by the facility-level adjustments involves several steps. First, we take the wage-adjusted prospective payment and multiply it by the appropriate rural and LIP adjustments (if applicable). Second, to determine the appropriate amount of additional payment for the teaching status adjustment (if applicable), we multiply the teaching status adjustment (0.0784, in this example) by the wage-adjusted and rural-adjusted amount (if applicable). Finally, we add the additional teaching status payments (if applicable) to the wage, rural, and LIP-adjusted prospective payment rates. Table 15 illustrates the components of the adjusted payment calculation.

Thus, the adjusted payment for Facility A would be $28,327.82, and the adjusted payment for Facility B would be $28,467.16.
VII. Update to Payments for High-Cost Outliers Under the IRF PPS for FY 2020
A. Update to the Outlier Threshold Amount for FY 2020
Section 1886(j)(4) of the Act provides the Secretary with the authority to make payments in addition to the basic IRF prospective payments for cases incurring extraordinarily high costs. A case qualifies for an outlier payment if the estimated cost of the case exceeds the adjusted outlier threshold. We calculate the adjusted outlier threshold by adding the IRF PPS payment for the case (that is, the CMG payment adjusted by all of the relevant facility-level adjustments) and the adjusted threshold amount (also adjusted by all of the relevant facility-level adjustments). Then, we calculate the estimated cost of a case by multiplying the IRF's overall CCR by the Medicare allowable covered charge. If the estimated cost of the case is higher than the adjusted outlier threshold, we make an outlier payment for the case equal to 80 percent of the difference between the estimated cost of the case and the outlier threshold.
In the FY 2002 IRF PPS final rule (66 FR 41362 through 41363), we discussed our rationale for setting the outlier threshold amount for the IRF PPS so that estimated outlier payments would equal 3 percent of total estimated payments. For the 2002 IRF PPS final rule, we analyzed various outlier policies using 3, 4, and 5 percent of the total estimated payments, and we concluded that an outlier policy set at 3 percent of total estimated payments would optimize the extent to which we could reduce the financial risk to IRFs of caring for high-cost patients, while still providing for adequate payments for all other (non-high cost outlier) cases.
Subsequently, we updated the IRF outlier threshold amount in the FYs 2006 through 2019 IRF PPS final rules and the FY 2011 and FY 2013 notices (70 FR 47880, 71 FR 48354, 72 FR 44284, 73 FR 46370, 74 FR 39762, 75 FR 42836, 76 FR 47836, 76 FR 59256, 77 FR 44618, 78 FR 47860, 79 FR 45872, 80 FR 47036, 81 FR 52056, 82 FR 36238, and 83 FR 38514, respectively) to maintain estimated outlier payments at 3 percent of total estimated payments. We also stated in the FY 2009 final rule (73 FR 46370 at 46385) that we would continue to analyze the estimated outlier payments for subsequent years and adjust the outlier threshold amount as appropriate to maintain the 3 percent target.
To update the IRF outlier threshold amount for FY 2020, we proposed to use FY 2018 claims data and the same methodology that we used to set the initial outlier threshold amount in the FY 2002 IRF PPS final rule (66 FR 41316 and 41362 through 41363), which is also the same methodology that we used to update the outlier threshold amounts for FYs 2006 through 2019. The outlier threshold is calculated by simulating aggregate payments and using an iterative process to determine a threshold that results in outlier payments being equal to 3 percent of total payments under the simulation. To determine the outlier threshold for FY 2020, we estimate the amount of FY 2020 IRF PPS aggregate and outlier payments using the most recent claims available (FY 2018) and the FY 2020 standard payment conversion factor, labor-related share, and wage indexes, incorporating any applicable budget-neutrality adjustment factors. The outlier threshold is adjusted either up or down in this simulation until the estimated outlier payments equal 3 percent of the estimated aggregate payments. Based on an analysis of the preliminary data used for the proposed rule, we estimated that IRF outlier payments as a percentage of total estimated payments would be approximately 3.2 percent in FY 2019. Therefore, we proposed to update the outlier threshold amount from $9,402 for FY 2019 to $9,935 for FY 2020 to maintain estimated outlier payments at approximately 3 percent of total estimated aggregate IRF payments for FY 2020.
We note that, as we typically do, we updated our data between the FY 2020 IRF PPS proposed and final rules to ensure that we use the most recent available data in calculating IRF PPS payments. This updated data includes a more complete set of claims for FY 2018. Based on our analysis using this updated data, we now estimate that IRF outlier payments as a percentage of total estimated payments are approximately 3.0 percent in FY 2019. Although our analysis shows that we achieved our goal to have estimated outlier payments equal 3.0 percent of total estimated aggregate IRF payments for FY 2019, we still need to adjust the IRF outlier threshold to reflect changes in estimated costs and payments for IRFs in FY 2020. That is, as discussed in section VI. of this final rule, we are finalizing our proposal to increase IRF PPS payment rates by 2.5 percent, in accordance with section 1886(j)(3)(C) of the Act to account for changes over time in the prices of an appropriate mix of goods and services included in the covered IRF services. Similarly, we estimate costs for IRFs in FY 2020 are expected to increase to account for changes over time in the prices of goods and services included in the covered IRF services. Therefore, we will update the outlier threshold amount from $9,402 for FY 2019 to $9,300 for FY 2020 to account for the increases in IRF PPS payments and estimated costs and to maintain estimated outlier payments at approximately 3 percent of total estimated aggregate IRF payments for FY 2020.
We received three comments on the proposed update to the FY 2020 outlier threshold, which are summarized below.
Comment: Commenters suggested that historical outlier reconciliation dollars should be included in the calculation of the fixed loss threshold under the IRF PPS.
Response: As we did not propose a change to the methodology used to establish an outlier threshold for IRF PPS payments, these comments are outside the scope of this rule. However, we will continue to monitor our IRF outlier policies to ensure that they continue to compensate IRFs appropriately for treating unusually high-cost patients and do not limit access to care for patients who are likely to require unusually high-cost care.
Comment: A few commenters suggested that CMS consider implementing a cap on the amount of outlier payments an individual IRF can receive under the IRF PPS. One commenter was supportive of maintaining estimated payments for outlier payments at approximately 3 percent while other commenters expressed concern with maintaining the 3 percent target and suggested reducing the outlier pool below 3 percent.
Response: As we did not propose to implement a cap on the amount of outlier payments an individual IRF can receive under the IRF PPS, these comments are outside the scope of this rule. However, we note that any future consideration given to imposing a limit on outlier payments would have to carefully analyze and take into consideration the effect on access to IRF care for certain high-cost populations.
As most recently discussed in the FY 2019 IRF PPS final rule (83 FR 38532), we analyzed various outlier policies using 3, 4, and 5 percent of the total estimated payments for the FY 2002 IRF PPS final rule, and we concluded that an outlier policy set at 3 percent of total estimated payments would optimize the extent to which we could reduce the financial risk to IRFs of caring for high-cost patients, while still providing for adequate payments for all other (non-high cost outlier) cases. We continue to believe that the outlier policy of 3 percent of total estimated aggregate payments accomplishes this objective. We refer readers to the FY 2002 IRF PPS final rule (66 FR 41316, 41362 through 41363) for more information regarding the rationale for setting the outlier threshold amount for the IRF PPS so that estimated outlier payments would equal 3 percent of total estimated payments.
Comment: One commenter requested that CMS update the outlier threshold amount in the final rule using the latest available data.
Response: We agree that we should use the most recent data available to calculate the outlier threshold. Therefore, as previously stated, we updated the data used to calculate the outlier threshold between the FY 2020 IRF PPS proposed and final rules.
Having carefully considered the public comments received and also taking into account the most recent available data, we are finalizing the outlier threshold amount of $9,300 to maintain estimated outlier payments at approximately 3 percent of total estimated aggregate IRF payments for FY 2020.
B. Update to the IRF Cost-to-Charge Ratio Ceiling and Urban/Rural Averages for FY 2020
Cost-to-charge ratios are used to adjust charges from Medicare claims to costs and are computed annually from facility-specific data obtained from Medicare cost reports. IRF specific cost-to-charge ratios are used in the development of the CMG relative weights and the calculation of outlier payments under the IRF prospective payment system. In accordance with the methodology stated in the FY 2004 IRF PPS final rule (68 FR 45674, 45692 through 45694), we proposed to apply a ceiling to IRFs' CCRs. Using the methodology described in that final rule, we proposed to update the national urban and rural CCRs for IRFs, as well as the national CCR ceiling for FY 2020, based on analysis of the most recent data that is available. We apply the national urban and rural CCRs in the following situations:
- New IRFs that have not yet submitted their first Medicare cost report.
- IRFs whose overall CCR is in excess of the national CCR ceiling for FY 2020, as discussed below in this section.
- Other IRFs for which accurate data to calculate an overall CCR are not available.
Specifically, for FY 2020, we proposed to estimate a national average CCR of 0.500 for rural IRFs, which we calculated by taking an average of the CCRs for all rural IRFs using their most recently submitted cost report data. Similarly, we proposed to estimate a national average CCR of 0.406 for urban IRFs, which we calculated by taking an average of the CCRs for all urban IRFs using their most recently submitted cost report data. We apply weights to both of these averages using the IRFs' estimated costs, meaning that the CCRs of IRFs with higher total costs factor more heavily into the averages than the CCRs of IRFs with lower total costs. For this final rule, we have used the most recent available cost report data (FY 2017). This includes all IRFs whose cost reporting periods begin on or after October 1, 2016, and before October 1, 2017. If, for any IRF, the FY 2017 cost report was missing or had an “as submitted” status, we used data from a previous fiscal year's (that is, FY 2004 through FY 2016) settled cost report for that IRF. We do not use cost report data from before FY 2004 for any IRF because changes in IRF utilization since FY 2004 resulting from the 60 percent rule and IRF medical review activities suggest that these older data do not adequately reflect the current cost of care. Using updated FY 2017 cost report data for this final rule, we estimate a national average CCR of 0.500 for rural IRFs, and a national average CCR of 0.405 for urban IRFs.
In accordance with past practice, we proposed to set the national CCR ceiling at 3 standard deviations above the mean CCR. Using this method, we proposed a national CCR ceiling of 1.31 for FY 2020. This means that, if an individual IRF's CCR were to exceed this ceiling of 1.31 for FY 2020, we would replace the IRF's CCR with the appropriate proposed national average CCR (either rural or urban, depending on the geographic location of the IRF). We calculated the proposed national CCR ceiling by:
Step 1. Taking the national average CCR (weighted by each IRF's total costs, as previously discussed) of all IRFs for which we have sufficient cost report data (both rural and urban IRFs combined).
Step 2. Estimating the standard deviation of the national average CCR computed in step 1.
Step 3. Multiplying the standard deviation of the national average CCR computed in step 2 by a factor of 3 to compute a statistically significant reliable ceiling.
Step 4. Adding the result from step 3 to the national average CCR of all IRFs for which we have sufficient cost report data, from step 1.
Using the updated FY 2017 cost report data for this final rule, we estimate a national average CCR ceiling of 1.31, using the same methodology.
We did not receive comments on the proposed update to the IRF CCR ceiling and the urban/rural averages for FY 2020.
As we did not receive any comments on the proposed update to the IRF CCR ceiling and the urban/rural averages for FY 2020, we are finalizing the national average urban CCR at 0.405, the national average rural CCR at 0.500, and the national average CCR ceiling at 1.31 for FY 2020.
VIII. Amendments to § 412.622 To Clarify the Definition of a Rehabilitation Physician
Under § 412.622(a)(3)(iv), a rehabilitation physician is defined as “a licensed physician with specialized training and experience in inpatient rehabilitation.” The term rehabilitation physician is used in several other places in § 412.622, with corresponding references to § 412.622(a)(3)(iv). The definition at § 412.622(a)(3)(iv) does not specify the level or type of training and experience required for a licensed physician to be designated as a rehabilitation physician because we believe that the IRFs are in the best position to make this determination for purposes of § 412.622.
Therefore, we proposed to amend the definition of a rehabilitation physician to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF (84 FR 17284 through 17285). For clarity, we also proposed to remove this definition from § 412.622(a)(3)(iv) and move it to a new paragraph (§ 412.622(c)). We also proposed to make corresponding technical corrections elsewhere in § 412.622(a)(3)(iv), (a)(4)(i)(A), (a)(4)(iii)(A), and (a)(5)(i) to remove the references to § 412.622(a)(3)(iv) in those paragraphs, so as to reflect the new location of the definition.
We received 1,163 comments on the proposal to clarify the definition of a rehabilitation physician, to move the definition from § 412.622(a)(3)(iv) to § 412.622(c), and to make corresponding technical corrections elsewhere in § 412.622 to remove references to the current location of the definition in § 412.622(a)(3)(iv). The majority of these comments consisted of form letters, in which we received multiple copies of two types of identically-worded letters that had been signed and submitted by different individuals. The comments we received on this are summarized below.
Comment: Many of the commenters noted appreciation and support for the proposal to amend the definition of a rehabilitation physician to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. One commenter stated that while board-certified physiatrists play a crucial caregiver and leadership role in rehabilitation hospitals, they are not alone in doing so. Physicians representing other specialties can and do also display the leadership and caregiving skills and experience that clearly qualify them as a rehabilitation physician. One commenter indicated that CMS' proposal is consistent with CMS' previously stated position from 2010. Some commenters also stated that clarifying the regulation would reduce the number of claims denials by promoting a shared understanding of the requirements between IRFs and Medicare contractors.
Response: We appreciate the commenters' support and agree that this clarification in our regulations supports our longstanding position that the responsibility is, and always has been, on the IRF to ensure that the rehabilitation physician(s) who are making the admission decisions and treating the patients have the necessary training and experience.
Comment: Many commenters stated that they do not support CMS' proposal and suggested that CMS not finalize the proposed amendments to § 412.622. These commenters requested that CMS delay any changes to current regulations until CMS and stakeholders can work together to develop a consensus approach for protecting the quality and integrity of IRF care. These commenters stated that they believe that allowing the IRF to determine whether an individual physician meets the regulatory standards for a rehabilitation physician could increase the risks that some IRFs will hire or contract with unqualified or underqualified physicians, reduce the quality of care that patients receive in IRFs, and reduce the value of physiatrists. These commenters also stated that reducing the value of physiatrists could also deter students from wanting to pursue this specialty in the future. Some commenters also indicated that CMS' proposal, if finalized, would undermine CMS' ability to engage in appropriate program integrity oversight by not reviewing an IRF's decision to hire a particular physician to fill a rehabilitation physician role.
Response: While we appreciate and share the commenters' desire to ensure that Medicare beneficiaries in IRFs receive the highest-quality care from trained and qualified physicians, we do not believe that merely clarifying our existing policy would reduce quality of care. The regulation will continue to require a rehabilitation physician to be a licensed physician with specialized training and experience in inpatient rehabilitation. We are not lowering these requirements. However, we continue to believe that we need to clarify our existing policy that the IRF makes the determination as to whether a given physician qualifies as a rehabilitation physician in order to eliminate any unnecessary uncertainty on this issue. Over the past year, we have received questions regarding how this provision can be enforced, and we believe that this clarification will promote a shared understanding of how we intend the enforcement to occur. We expect that IRFs will continue to ensure that the rehabilitation physicians treating patients in their facilities have the necessary training and experience in inpatient rehabilitation. To this end, we will continue to work with stakeholders to refine Medicare's IRF payment policies in the future so that they support IRFs in providing the highest quality care to beneficiaries.
After careful consideration of the comments we received, we are finalizing our proposal to amend the definition of a rehabilitation physician to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. However, based on the stakeholder feedback, we will continue to assess whether future refinements to this policy may be needed.
For clarity, we are also removing this definition from § 412.622(a)(3)(iv) and moving it to a new paragraph (§ 412.622(c)). We are also making corresponding technical corrections elsewhere in § 412.622(a)(3)(iv), (a)(4)(i)(A), (a)(4)(iii)(A), and (a)(5)(i) to remove the references to § 412.622(a)(3)(iv) in those paragraphs, so as to reflect the new location of the definition.
IX. Updates to the IRF Quality Reporting Program (QRP)
A. Background
The IRF QRP is authorized by section 1886(j)(7) of the Act, and it applies to freestanding IRFs, as well as inpatient rehabilitation units of hospitals or critical access hospitals (CAHs) paid by Medicare under the IRF PPS. Under the IRF QRP, the Secretary must reduce the annual increase factor for discharges occurring during such fiscal year by 2 percentage points for any IRF that does not submit data in accordance with the requirements established by the Secretary. For more information on the background and statutory authority for the IRF QRP, we refer readers to the FY 2012 IRF PPS final rule (76 FR 47873 through 47874), the CY 2013 Hospital Outpatient Prospective Payment System/Ambulatory Surgical Center (OPPS/ASC) Payment Systems and Quality Reporting Programs final rule (77 FR 68500 through 68503), the FY 2014 IRF PPS final rule (78 FR 47902), the FY 2015 IRF PPS final rule (79 FR 45908), the FY 2016 IRF PPS final rule (80 FR 47080 through 47083), the FY 2017 IRF PPS final rule (81 FR 52080 through 52081), the FY 2018 IRF PPS final rule (82 FR 36269 through 36270), and the FY 2019 IRF PPS final rule (83 FR 38555 through 38556).
While we did not solicit comments on previously finalized IRF QRP policies, we received comments, which are summarized below.
Comment: A few commenters stated that the IRF QRP compliance threshold of 95 percent for assessment-based items is too high given the number of data elements that have been added to the IRF-PAI, and requested that CMS lower it to 80 percent in alignment with other programs.
Response: We did not propose any changes to the compliance threshold, which has been codified at § 412.634(f). While these comments were out of scope for this rule, we will take these comments under consideration.
B. General Considerations Used for the Selection of Measures for the IRF QRP
For a detailed discussion of the considerations we use for the selection of IRF QRP quality, resource use, and other measures, we refer readers to the FY 2016 IRF PPS final rule (80 FR 47083 through 47084).
C. Quality Measures Currently Adopted for the FY 2021 IRF QRP
The IRF QRP currently has 15 measures for the FY 2020 program year, which are set out in Table 16.

While we did not solicit comments on currently adopted measures (with the exception of the Discharge to Community Measure discussed in section IX.D.3 of this rule and the policies regarding public display of the Drug Regimen Review Conducted With Follow-Up for Identified Issues—PAC IRF QRP in section IX.I of this rule), we received several comments.
Comment: A few commenters had suggestions for removing measures they believe were “topped out” according to the Hospital Inpatient Quality Reporting (IQR) Program definition (83 FR 20408) and did not demonstrate variation across facilities, including Application of Percent of Residents Experiencing One or More Falls with Major Injury (Long Stay) (NQF #0674) and Application of Percent of Long-Term Care Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That Addresses Function (NQF #2631), and Changes in Skin Integrity Post-Acute Care: Pressure Ulcer/Injury. One commenter had suggestions for improving the training manual for the Drug Regimen Review measure in terms of considered clinically significant medication issue.
Response: We did not propose any changes to these previously finalized measures, nor did we propose measure removals from the IRF QRP. We wish to clarify that the IRF QRP has not adopted the Hospital Inpatient Quality Reporting (IQR) definition of “topped out” in the measure removal criteria finalized for the IRF QRP at § 412.634(2). We also note that we do not automatically remove high performing measures, and wish to reiterate that such measures may be retained for other specified reasons. For example, a particular measure with high performance rates may be retained if the measure addresses a topic related to quality that is so significant that we do not want to risk a decline in quality that could result if we removed the measure, or if the measure addresses a topic that is statutorily required. We will continue to monitor and evaluate the data from all IRF QRP measures.
With regard to the commenter's suggestions about the Drug Regimen Review measure, we interpret that the commenter is requesting additional clarification for coding. We will take these comments into account as we develop training materials for the IRF QRP.
D. Adoption of Two New Quality Measures and Updated Specifications for a Third Quality Measure Beginning With the FY 2022 IRF QRP
In the FY 2020 IRF PPS proposed rule (84 FR 17286 through 17291), we proposed to adopt two process measures for the IRF QRP that would satisfy section 1899B(c)(1)(E)(ii) of the Act, which requires that the quality measures specified by the Secretary include measures with respect to the quality measure domain titled “Accurately communicating the existence of and providing for the transfer of health information and care preferences of an individual to the individual, family caregiver of the individual, and providers of services furnishing items and services to the individual when the individual transitions from a PAC provider to another applicable setting, including a different PAC provider, a hospital, a critical access hospital, or the home of the individual.” Given the length of this domain title, hereafter, we will refer to this quality measure domain as “Transfer of Health Information.”
The two measures we proposed to adopt are: (1) Transfer of Health Information to the Provider—Post-Acute Care (PAC); and (2) Transfer of Health Information to the Patient—Post-Acute Care (PAC). Both of these measures support our Meaningful Measures priority of promoting effective communication and coordination of care, specifically the Meaningful Measure area of the transfer of health information and interoperability.
In addition to the two measure proposals, we proposed to update the specifications for the Discharge to Community—Post Acute Care (PAC) IRF QRP measure to exclude baseline nursing facility (NF) residents from the measure.
We sought public comment on each of these proposals. These comments are summarized after each proposal below.
1. Transfer of Health Information to the Provider—Post-Acute Care (PAC) Measure
The Transfer of Health Information to the Provider—Post-Acute Care (PAC) Measure that we proposed to adopt beginning with the FY 2022 IRF QRP is a process-based measure that assesses whether or not a current reconciled medication list is given to the subsequent provider when a patient is discharged or transferred from his or her current PAC setting.
a. Background
In 2013, 22.3 percent of all acute hospital discharges were discharged to PAC settings, including 11 percent who were discharged to home under the care of a home health agency, and 9 percent who were discharged to SNFs.[2] The proportion of patients being discharged from an acute care hospital to a PAC setting was greater among beneficiaries enrolled in Medicare FFS. Among Medicare FFS patients discharged from an acute hospital, 42 percent went directly to PAC settings. Of that 42 percent, 20 percent were discharged to a SNF, 18 percent were discharged to a home health agency (HHA), 3 percent were discharged to an IRF, and 1 percent were discharged to an LTCH.[3] Of the Medicare FFS beneficiaries with an IRF stay in FYs 2016 and 2017, an estimated 10 percent were discharged or transferred to an acute care hospital, 51 percent discharged home with home health services, 16 percent discharged or transferred to a SNF, and one percent discharged or transferred to another PAC setting (for example, another IRF, a hospice, or an LTCH).[4]
The transfer and/or exchange of health information from one provider to another can be done verbally (for example, clinician-to-clinician communication in-person or by telephone), paper-based (for example, faxed or printed copies of records), and via electronic communication (for example, through a health information exchange network using an electronic health/medical record, and/or secure messaging). Health information, such as medication information, that is incomplete or missing increases the likelihood of a patient or resident safety risk, and is often life-threatening.5 6 7 8 9 10 Poor communication and coordination across health care settings contributes to patient complications, hospital readmissions, emergency department visits, and medication errors.[11 12 13 14 15 16 17 18 19 20] Communication has been cited as the third most frequent root cause in sentinel events, which The Joint Commission defines [21] as a patient safety event that results in death, permanent harm, or severe temporary harm. Failed or ineffective patient handoffs are estimated to play a role in 20 percent of serious preventable adverse events.[22] When care transitions are enhanced through care coordination activities, such as expedited patient information flow, these activities can reduce duplication of care services and costs of care, resolve conflicting care plans, and prevent medical errors.[23 24 25 26 27]
Care transitions across health care settings have been characterized as complex, costly, and potentially hazardous, and may increase the risk for multiple adverse outcomes.[28 29] The rising incidence of preventable adverse events, complications, and hospital readmissions have drawn attention to the importance of the timely transfer of health information and care preferences at the time of transition. Failures of care coordination, including poor communication of information, were estimated to cost the U.S. health care system between $25 billion and $45 billion in wasteful spending in 2011.[30] The communication of health information and patient care preferences is critical to ensuring safe and effective transitions from one health care setting to another.[31 32]
Patients in PAC settings often have complicated medication regimens and require efficient and effective communication and coordination of care between settings, including detailed transfer of medication information.[33 34 35] Individuals in PAC settings may be vulnerable to adverse health outcomes due to insufficient medication information on the part of their health care providers, and the higher likelihood for multiple comorbid chronic conditions, polypharmacy, and complicated transitions between care settings.[36 37] Preventable adverse drug events (ADEs) may occur after hospital discharge in a variety of settings including PAC.[38] A 2014 Office of Inspector General report found that 10 percent of Medicare patients in IRFs experienced adverse events, with most of those events being medication related. Over 45 percent of the adverse events and temporary harm events were clearly or likely preventable.[39] Medication errors and one-fifth of ADEs occur during transitions between settings, including admission to or discharge from a hospital to home or a PAC setting, or transfer between hospitals.[40 41]
Patients in PAC settings are often taking multiple medications. Consequently, PAC providers regularly are in the position of starting complex new medication regimens with little knowledge of the patients or their medication history upon admission. Furthermore, inter-facility communication barriers delay resolving medication discrepancies during transitions of care.[42] Medication discrepancies are common [43] and found to occur in 86 percent of all transitions, increasing the likelihood of ADEs.[44 45 46] Up to 90 percent of patients experience at least one medication discrepancy in the transition from hospital to home care, and discrepancies occur within all therapeutic classes of medications.[47 48]
Transfer of a medication list between providers is necessary for medication reconciliation interventions, which have been shown to be a cost-effective way to avoid ADEs by reducing errors 49 50 51especially when medications are reviewed by a pharmacist using electronic medical records.[52]
b. Stakeholder and Technical Expert Panel (TEP) Input
The proposed measure was developed after consideration of feedback we received from stakeholders and four TEPs convened by our contractors. Further, the proposed measure was developed after evaluation of data collected during two pilot tests we conducted in accordance with the CMS Measures Management System Blueprint.
Our measure development contractors constituted a TEP which met on September 27, 2016,[53] January 27, 2017,[54] and August 3, 2017 [55] to provide input on a prior version of this measure. Based on this input, we updated the measure concept in late 2017 to include the transfer of a specific component of health information—medication information. Our measure development contractors reconvened this TEP on April 20, 2018 for the purpose of obtaining expert input on the proposed measure, including the measure's reliability, components of face validity, and feasibility of being implemented across PAC settings. Overall, the TEP was supportive of the proposed measure, affirming that the measure provides an opportunity to improve the transfer of medication information. A summary of the April 20, 2018 TEP proceedings titled “Transfer of Health Information TEP Meeting 4—June 2018” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Our measure development contractors solicited stakeholder feedback on the proposed measure by requesting comment on the CMS Measures Management System Blueprint website, and accepted comments that were submitted from March 19, 2018 to May 3, 2018. The comments received noted overall support for the measure. Several commenters suggested ways to improve the measure, primarily related to what types of information should be included at transfer. We incorporated this input into development of the proposed measure. The summary report for the March 19 to May 3, 2018 public comment period titled “IMPACT Medication Profile Transferred Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
c. Pilot Testing
The proposed measure was tested between June and August 2018 in a pilot test that involved 24 PAC facilities/agencies, including five IRFs, six SNFs, six LTCHs, and seven HHAs. The 24 pilot sites submitted a total of 801 records. Analysis of agreement between coders within each participating facility (266 qualifying pairs) indicated a 93 percent agreement for this measure. Overall, pilot testing enabled us to verify its reliability, components of face validity, and feasibility of being implemented across PAC settings. Further, more than half of the sites that participated in the pilot test stated during the debriefing interviews that the measure could distinguish facilities or agencies with higher quality medication information transfer from those with lower quality medication information transfer at discharge. The pilot test summary report titled “Transfer of Health Information 2018 Pilot Test Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
d. Measure Applications Partnership (MAP) Review and Related Measures
We included the proposed measure in the IRF QRP section of the 2018 Measures Under Consideration (MUC) List. The MAP conditionally supported this measure pending NQF endorsement, noting that the measure can promote the transfer of important medication information. The MAP also suggested that we consider a measure that can be adapted to capture bi-directional information exchange, and recommended that the medication information transferred include important information about supplements and opioids. More information about the MAP's recommendations for this measure is available at http://www.qualityforum.org/Publications/2019/02/MAP_2019_Considerations_for_Implementing_Measures_Final_Report_-_PAC-LTC.aspx.
As part of the measure development and selection process, we also identified one NQF-endorsed quality measure similar to the proposed measure, titled Documentation of Current Medications in the Medical Record (NQF #0419, CMS eCQM ID: CMS68v8). This measure was adopted as one of the recommended adult core clinical quality measures for eligible professionals for the EHR Incentive Program beginning in 2014 and was also adopted under the Merit-based Incentive Payment System (MIPS) quality performance category beginning in 2017. The measure is calculated based on the percentage of visits for patients aged 18 years and older for which the eligible professional or eligible clinician attests to documenting a list of current medications using all resources immediately available on the date of the encounter.
The proposed Transfer of Health Information to the Provider—Post-Acute Care (PAC) measure addresses the transfer of information whereas the NQF-endorsed measure #0419 assesses the documentation of medications, but not the transfer of such information. This is important as the proposed measure assesses for the transfer of medication information for the proposed measure calculation. Further, the proposed measure utilizes standardized patient assessment data elements (SPADEs), which is a requirement for measures specified under the Transfer of Health Information measure domain under section 1899B(c)(1)(E) of the Act, whereas NQF #0419 does not.
After review of the NQF-endorsed measure, we determined that the proposed Transfer of Health Information to the Provider—Post-Acute Care (PAC) measure better addresses the Transfer of Health Information measure domain, which requires that at least some of the data used to calculate the measure be collected as standardized patient assessment data through the post-acute care assessment instruments. Section 1886(j)(7)(D)(i) of the Act requires that any measure specified by the Secretary be endorsed by the entity with a contract under section 1890(a) of the Act, which is currently the National Quality Form (NQF). However, when a feasible and practical measure has not been NQF endorsed for a specified area or medical topic determined appropriate by the Secretary, section 1886(j)(7)(D)(ii) of the Act allows the Secretary to specify a measure that is not NQF endorsed as long as due consideration is given to the measures that have been endorsed or adopted by a consensus organization identified by the Secretary. For the reasons discussed previously, we believe that there is currently no feasible NQF-endorsed measure that we could adopt under section 1886(j)(7)(D)(ii) of the Act. However, we note that we intend to submit the proposed measure to the NQF for consideration of endorsement when feasible.
e. Quality Measure Calculation
The proposed Transfer of Health Information to the Provider—Post-Acute Care (PAC) quality measure is calculated as the proportion of patient stays with a discharge assessment indicating that a current reconciled medication list was provided to the subsequent provider at the time of discharge. The proposed measure denominator is the total number of IRF patient stays ending in discharge to a subsequent provider, which is defined as a short-term general acute-care hospital, intermediate care (intellectual and developmental disabilities providers), home under care of an organized home health service organization or hospice, hospice in an institutional facility, a SNF, an LTCH, another IRF, an IPF, or a CAH. These health care providers were selected for inclusion in the denominator because they are identified as subsequent providers on the discharge destination item that is currently included on the IRF PAI. The proposed measure numerator is the number of IRF patient stays with an IRF-PAI discharge assessment indicating a current reconciled medication list was provided to the subsequent provider at the time of discharge. For additional technical information about this proposed measure, we refer readers to the document titled, “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. The data source for the proposed quality measure is the IRF-PAI assessment instrument for IRF patients.
For more information about the data submission requirements we proposed for this measure, we refer readers to section VIII.G.3. of this final rule.
Commenters submitted the following comments related to the proposed rule's discussion of the IRF QRP Quality Measure Proposals beginning with the FY 2022 IRF QRP. A discussion of these comments, along with our responses, appears below. We also address comments on the proposed Transfer of Health Information to the Patient—Post-Acute Care measure (discussed further in a subsequent section of this final rule) in this section because commenters frequently addressed both Transfer of Health Information measures together.
Response: We thank the commenters for their support of the Transfer of Health Information measures.
Comment: One commenter suggested that other providers, such as outpatient physical therapists, should be included in the definition of a subsequent provider for the Transfer of Health Information to the Provider—Post-Acute Care measure.
Response: We appreciate the suggestion to expand the Transfer of Health Information to the Provider—Post-Acute Care measure outcome to assess the transfer of health information to other providers such as outpatient physical therapists. We recognize that sharing medication information with outpatient providers is important, and will take into consideration additional providers in future measure modifications. Through our measure development and pilot testing we learned that outpatient providers cannot always be readily identified by the PAC provider. For this process measure, which serves as a building block for improving the transfer of medication information, we specified providers who will be involved in the care of the patient and medication management after discharge and can be readily identified through the discharge location item on the IRF-PAI. The clear delineation of the recipient of the medication list in the measure specifications will improve measure reliability and validity.
Comment: A commenter recommended that the Transfer of Health Information to the Provider—Post-Acute Care measure be expanded to include the transfer of information that would help prevent infections and facilitate appropriate infection prevention and control interventions during care transitions in addition to the medication information in the finalized measure.
Response: The Transfer of Health Information to the Provider—Post-Acute Care measure focuses on the transfer of a reconciled medication list. The measure was designed after input from TEPs, public comment, and other stakeholders that suggested the quality measures focus on the transfer of the most critical pieces of information to support patient safety and care coordination. However, we acknowledge that the transfer of many other forms of health information is important, and while the focus of this measure is on a reconciled medication list, we hope to expand our measures in the future.
Comment: Several commenters raised concerns about both of the Transfer of Health Information measures not being endorsed by the National Quality Forum (NQF). A few commenters requested that we consider delaying rollout of these two new measures until endorsed by NQF. A few commenters recommended that we only adopt measures that have NQF approval. One commenter was opposed to the measures because they have not been endorsed by NQF.
Response: While this measure is not currently NQF-endorsed, we recognize that the NQF endorsement process is an important part of measure development. As discussed in the FY 2020 IRF PPS proposed rule (84 FR 17286 through 17291), we believe the measures better address the Transfer of Health Information measure domain, which requires that at least some of the data used to calculate the measure be collected as standardized patient assessment data through the post-acute care assessment instruments, than any endorsed measures. While section 1886(j)(7)(D)(i) of the Act requires that any measure specified by the Secretary be endorsed by the entity with a contract under section 1890(a) of the Act, which is currently the National Quality Form (NQF), when a feasible and practical measure has not been NQF endorsed for a specified area or medical topic determined appropriate by the Secretary, section 1886(j)(7)(D)(ii) of the Act allows the Secretary to specify a measure that is not NQF endorsed as long as due consideration is given to the measures that has been endorsed or adopted by a consensus organization identified by the Secretary. We plan to submit the measure for NQF endorsement consideration as soon as feasible.
Comment: Several commenters stated that the Transfer of Health Information measures will add burden. Two commenters did not support the measures for this reason. One commenter stated that achieving high performance on the measures will add administrative burden. Another commenter stated that the measures will add burden with no added value. Another commenter stated that while there will be additional burden on IRFs to collect and report data for these new measures, the benefit to patients and the CMS program outweighs the additional burden on providers.
Response: We agree that the benefit to patients outweighs any additional burden on providers. We are also very mindful of burden that may occur from the collection and reporting of our measures, as supported by the Meaningful Measures and Patients over Paperwork initiatives. We emphasize that both measures are comprised of one item, and further, the activities associated with the measure align with existing requirements related to transferring information at the time of discharge to safeguard patients. Additionally, TEP feedback and pilot test found that the burden of reporting will not be significant. We believe that these measures will likely drive improvements in the transfer of medication information between providers and with patients, families, and caregivers.
Comment: One commenter stated that there will be no additional burden to IRFs, because providing medication information as part of discharge planning is a Condition of Participation requirement for Medicaid and Medicare, and the medication list can be generated from the electronic medical record.
Response: We believe that the Transfer of Health Information measures will not substantially increase burden because we understand that many hospitals already generate medication lists as a best practice.
Comment: We received comments related to the validity and reliability of both Transfer of Health Information measures. One commenter suggested that CMS should ensure accuracy of these measures. Other commenters suggested that additional testing is needed to ensure that these measures will be able to differentiate among IRF providers. Another commenter questioned if the measures would be topped out shortly after adoption, since medication reconciliation is already completed by facilities at discharge.
Response: Elements of validity and reliability were analyzed during pilot testing of these measures, with good results, including inter-rater reliability of at least 87 percent for all tested items. Pilot testing also indicated that there is room for improvement for IRFs and other settings, so we do not expect the measure to be topped out shortly after adoption. As we monitor the outcomes of these measures, we will ensure that reliability and validity of the measures meet acceptable standards.
Comment: Some commenters recommended ways in which the Transfer of Health Information measures specifications could be updated or changed. A few commenters suggested that the “not applicable” (NA) answer choice available in the home health version of the measure be made available in all settings, including IRFs. A few commenters also requested clarification about why patients discharged home under the care of an organized home health service or hospice would be captured in the denominators of both Transfer of Health information measures.
Response: We are appreciative of the measure modification suggestions and clarify why the response option of N/A was considered only for the HH version of this measure. The coding response N/A, or “not applicable” is used when the HHA was not made aware of the transfer in a timely manner and, therefore, the HHA is not able to provide the medication list at the time of transfer to the subsequent provider. For example, a HHA may not be immediately aware when a patient is taken to the emergency room. For facility settings, such as the IRF setting, where 24-hour care is being provided, the facility should always be aware and actively involved in the discharge of the patient, and therefore, able to provide the current reconciled medication list at the time of discharge. Therefore, we believed the coding option of “N/A” would not be useful in the facility-based measure as the facility is aware and involved in the discharge. We wish to note that while the N/A option is considered for the HHA version of the measure, the measure specifications indicate that these patients are not removed from the denominator. In addition, discharge to home under the care of an organized HHA or hospice is captured in the denominator of both the Transfer of Health Information to Provider and Transfer of Health Information to Patient measures because this type of discharge represents two opportunities to transfer the medication list. These measures aim to assure that each of these transfers is taking place. We refer readers to the measure specifications where updates or changes can be found and are available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Comment: One commenter suggested that the Transfer of Health Information measures should include a measure of the timeliness of the transfer. The commenter stated that, as currently specified, the measures give equal credit for information that is sent immediately and information sent days later.
Response: We appreciate the suggestions that CMS develop and adopt measures that assess for the timeliness of transfer. We agree that measure concepts of this type are important and would complement the measures that focus for whether information was transferred at the time the patient leaves the facility. We clarify that the measures do not give credit for when information was sent, whether immediately or days later. This is because there may be circumstances where information may not be sent at the immediate time of discharge. However, the measures do require that information be shared with the subsequent provider and/or the patient as close to the time of discharge as this is actionable, allows for shared decision making, and will increase coordinated care. We are not establishing a new standard of transfer at discharge; we are simply assessing if information was sent at the time a patient leaves the facility. As we move through future measure development work, we will consider a “timeliness” component for these measure concepts.
Comment: A commenter noted that although CMS provided guidelines regarding what should be included in the transfer of medication information, the data collection on this measure does not require that these guidelines be met. The commenter questioned if CMS intends to audit IRFs to ensure that the measure values are consistent with the information being shared.
Response: The Transfer of Health Information measures serve as a check to ensure that a reconciled medication list is provided as the patient changes care settings. Defining the completeness of that medication list is left to the discretion of the providers and patient who are coordinating this care. We interpret the comment about audits to be referring to data validation. While we do not have a data validation program in place at this time, we are exploring such a program akin to that of the hospital QRPs. For all measures and data collected for the IRF QRP, we monitor and evaluate our data to assess for coding patterns, errors, reliability, and soundness of the data. Through data monitoring, we are able to assess if measure outcomes are consistent with the information that is collected. We note that all data are subject to review and audit.
Comment: A few comments included concerns that the Transfer of Health Information measures are not indicative of provider quality and questioned the ability of the measures to improve patient outcomes. Two commenters did not support the measures for this reason. Commenters noted that the measures assess whether a medication list was transferred and not whether that medication list was accurate and received by the subsequent provider.
Response: The Transfer of Health Information to the Provider—Post-Acute Care and Transfer of Health Information to the Patient—Post-Acute Care measures are process measures designed to address and improve an important aspect of care quality. Lack of timely transfer of medication information at transitions has been demonstrated to lead to increased risk of adverse events, medication errors, and hospitalizations. In addition, public commenters and our TEP members identified many problems and gaps in the timely transfer of medication information at transitions. Process measures, such as these, are building blocks toward improved coordinated care and discharge planning, providing information that will improve shared decision making and coordination. Further, process measures hold a lot of value as they delineate negative and/or positive aspects of the health care process. These measures will capture the quality of the process of medication information transfer and, we believe, help to improve those processes. When developing future measures, we will take into consideration suggestions about measures that assess the accuracy of the medication list and whether it was received by the subsequent provider.
Comment: One commenter suggested that CMS work to identify interoperability solutions as a means of decreasing opportunities for errors by providing clinicians and patients secure access to the most up-to-date medication-related information. The commenter also suggests that if CMS is required by the IMPACT Act to adopt these measures, that we do so as an interim step, within a defined timeframe, while interoperability solutions are explored and tested.
Response: We agree with the comments on the importance of interoperability solutions to support health information transfer. CMS and ONC are focused on improving interoperability and the timely sharing of information between providers, patients, families and caregivers. We believe that PAC provider health information exchange supports the goals of high quality, personalized, efficient healthcare, care coordination, person-centered care, and supports real-time, data driven, clinical decision making. We are optimistic that this measure will encourage the electronic transfer of current and important medication information at transitions. These measures and related efforts may help accelerate interoperability solutions. The Transfer of Health Information measures assess the process of medication transfer, which can occur through both electronic and non-electronic means. We clarify that these measures are an interim step in improving coordinated care, and we also believe that other interoperable solutions should be explored. Finalizing these Transfer of Health measures will be a first step in measuring the transfer of this medication-related information.
After consideration of the public comments, we are finalizing our proposal to adopt the Transfer of Health Information to the Provider—Post Acute Care (PAC) measure, under section 1899B(c)(1)(E) of the Act, with data collection for discharges beginning October 1, 2020.
2. Transfer of Health Information to the Patient—Post-Acute Care (PAC) Measure
Beginning with the FY 2022 IRF QRP, we proposed to adopt the Transfer of Health Information to the Patient—Post Acute Care (PAC) measure, a measure that satisfies the IMPACT Act domain of Transfer of Health Information, with data collection for discharges beginning October 1, 2020. This process-based measure assesses whether or not a current reconciled medication list was provided to the patient, family, or caregiver when the patient was discharged from a PAC setting to a private home/apartment, a board and care home, assisted living, a group home, transitional living or home under care of an organized home health service organization, or a hospice.
a. Background
In 2013, 22.3 percent of all acute hospital discharges were discharged to PAC settings, including 11 percent who were discharged to home under the care of a home health agency.[56] Of the Medicare FFS beneficiaries with an IRF stay in FYs 2016 and 2017, an estimated 51 percent were discharged home with home health services, 21 percent were discharged home with self-care, and 0.5 percent were discharged with home hospice services.[57]
The communication of health information, such as a reconciled medication list, is critical to ensuring safe and effective patient transitions from health care settings to home and/or other community settings. Incomplete or missing health information, such as medication information, increases the likelihood of a patient safety risk, often life-threatening.[58 59 60 61 62] Individuals who use PAC care services are particularly vulnerable to adverse health outcomes due to their higher likelihood of having multiple comorbid chronic conditions, polypharmacy, and complicated transitions between care settings.[63 64] Upon discharge to home, individuals in PAC settings may be faced with numerous medication changes, new medication regimes, and follow-up details.[65 66 67] The efficient and effective communication and coordination of medication information may be critical to prevent potentially deadly adverse effects. When care coordination activities enhance care transitions, these activities can reduce duplication of care services and costs of care, resolve conflicting care plans, and prevent medical errors.[68 69]
Finally, the transfer of a patient's discharge medication information to the patient, family, or caregiver is common practice and supported by discharge planning requirements for participation in Medicare and Medicaid programs.[70] [71] Most PAC EHR systems generate a discharge medication list to promote patient participation in medication management, which has been shown to be potentially useful for improving patient outcomes and transitional care.[72]
b. Stakeholder and Technical Expert Panel (TEP) Input
The proposed measure was developed after consideration of feedback we received from stakeholders and four TEPs convened by our contractors. Further, the proposed measure was developed after evaluation of data collected during two pilot tests we conducted in accordance with the CMS Measures Management System Blueprint.
Our measure development contractors constituted a TEP which met on September 27, 2016,[73] January 27, 2017,[74] and August 3, 2017 [75] to provide input on a prior version of this measure. Based on this input, we updated the measure concept in late 2017 to include the transfer of a specific component of health information—medication information. Our measure development contractors reconvened this TEP on April 20, 2018 to seek expert input on the measure. Overall, the TEP members supported the proposed measure, affirming that the measure provides an opportunity to improve the transfer of medication information. Most of the TEP members believed that the measure could improve the transfer of medication information to patients, families, and caregivers. Several TEP members emphasized the importance of transferring information to patients and their caregivers in a clear manner using plain language. A summary of the April 20, 2018 TEP proceedings titled “Transfer of Health Information TEP Meeting 4—June 2018” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Our measure development contractors solicited stakeholder feedback on the proposed measure by requesting comment on the CMS Measures Management System Blueprint website, and accepted comments that were submitted from March 19, 2018 to May 3, 2018. Several commenters noted the importance of ensuring that the instruction provided to patients and caregivers is clear and understandable to promote transparent access to medical record information and meet the goals of the IMPACT Act. The summary report for the March 19 to May 3, 2018 public comment period titled “IMPACT—Medication Profile Transferred Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
c. Pilot Testing
Between June and August 2018, we held a pilot test involving 24 PAC facilities/agencies, including five IRFs, six SNFs, six LTCHs, and seven HHAs. The 24 pilot sites submitted a total of 801 assessments. Analysis of agreement between coders within each participating facility (241 qualifying pairs) indicated an 87 percent agreement for this measure. Overall, pilot testing enabled us to verify its reliability, components of face validity, and feasibility of being implemented across PAC settings. Further, more than half of the sites that participated in the pilot test stated, during debriefing interviews, that the measure could distinguish facilities or agencies with higher quality medication information transfer from those with lower quality medication information transfer at discharge. The pilot test summary report titled “Transfer of Health Information 2018 Pilot Test Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
d. Measure Applications Partnership (MAP) Review and Related Measures
We included the proposed measure in the IRF QRP section of the 2018 MUC list. The MAP conditionally supported this measure pending NQF endorsement, noting that the measure can promote the transfer of important medication information to the patient. The MAP recommended that providers transmit medication information to patients that is easy to understand because health literacy can impact a person's ability to take medication as directed. More information about the MAP's recommendations for this measure is available at http://www.qualityforum.org/Publications/2019/02/MAP_2019_Considerations_for_Implementing_Measures_Final_Report_-_PAC-LTC.aspx.
Section 1886(j)(7)(D)(i) of the Act, requires that any measure specified by the Secretary be endorsed by the entity with a contract under section 1890(a) of the Act, which is currently the NQF. However, when a feasible and practical measure has not been NQF endorsed for a specified area or medical topic determined appropriate by the Secretary, section 1886(j)(7)(D)(ii) of the Act allows the Secretary to specify a measure that is not NQF endorsed as long as due consideration is given to the measures that have been endorsed or adopted by a consensus organization identified by the Secretary. Therefore, in the absence of any NQF-endorsed measures that address the proposed Transfer of Health Information to the Patient—Post-Acute Care (PAC), which requires that at least some of the data used to calculate the measure be collected as standardized patient assessment data through PAC assessment instruments, we believe that there is currently no feasible NQF-endorsed measure that we could adopt under section 1886(j)(7)(D)(ii) of the Act. However, we note that we intend to submit the proposed measure to the NQF for consideration of endorsement when feasible.
e. Quality Measure Calculation
The calculation of the proposed Transfer of Health Information to the Patient—Post-Acute Care (PAC) measure would be based on the proportion of patient stays with a discharge assessment indicating that a current reconciled medication list was provided to the patient, family, or caregiver at the time of discharge.
The proposed measure denominator is the total number of IRF patient stays ending in discharge to a private home/apartment, a board and care home, assisted living, a group home, transitional living or home under care of an organized home health service organization, or a hospice. These locations were selected for inclusion in the denominator because they are identified as home locations on the discharge destination item that is currently included on the IRF-PAI. The proposed measure numerator is the number of IRF patient stays with an IRF-PAI discharge assessment indicating a current reconciled medication list was provided to the patient, family, or caregiver at the time of discharge. For technical information about this proposed measure, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. Data for the proposed quality measure would be calculated using data from the IRF-PAI assessment instrument for IRF patients.
For more information about the data submission requirements we proposed for this measure, we refer readers to section VIII.G.3. of this rule.
Commenters submitted the following comments related to the proposed rule's discussion of the IRF QRP Quality Measure Proposals Beginning with the FY 2022 IRF QRP. A discussion of these comments, along with our responses, appears below. We received many comments that addressed both of the Transfer of Health Information measures. Comments that applied to both measures are discussed above in IX.D.1 of this rule.
Comment: One commenter suggested that CMS use the field's experience with transferring information to patients and reporting on this measure to disseminate best practices about how to best convey the medication list and suggested this include formats and informational elements helpful to patients and families.
Response: We have interpreted “the field” to mean PAC providers. Facilities and clinicians should use clinical judgement to guide their practices around transferring information to patients and how to best convey the medication list, including identifying the best formats and informational elements. This may be determined by the patient's individualized needs in response to their medical condition. We do not determine clinical best practices standards and facilities are advised to refer to other sources, such as professional guidelines.
Comment: One commenter suggested that the Transfer of Health Information to the Patient—Post-Acute Care (PAC) Measure require transfer of the medication list to both the patient and family or caregiver.
Response: We agree there are times when it is appropriate for the IRF to provide the medication list to the patient and family and this decision should be based on clinical judgement. However, because it is not always necessary or appropriate to provide the medication list to both the patient and family, we are not requiring this for the measure.
After consideration of the public comments, we are finalizing our proposal to adopt the Transfer of Health Information to the Patient—Post Acute Care (PAC) measure, under section 1899B(c)(1)(E) of the Act, with data collection for discharges beginning October 1, 2020.
3. Update to the Discharge to Community—Post Acute Care (PAC) Inpatient Rehabilitation Facility (IRF) Quality Reporting Program (QRP) Measure
In the FY 2020 IRF PPS proposed rule (84 FR 17291), we proposed to update the specifications for the Discharge to Community—PAC IRF QRP measure to exclude baseline nursing facility (NF) residents from the measure. This measure reports an IRF's risk-standardized rate of Medicare FFS patients who are discharged to the community following an IRF stay, do not have an unplanned readmission to an acute care hospital or LTCH in the 31 days following discharge to community, and who remain alive during the 31 days following discharge to community. We adopted this measure in the FY 2017 IRF PPS final rule (81 FR 52095 through 52103).
In the FY 2017 IRF PPS final rule (81 FR 52099), we addressed public comments recommending exclusion of IRF patients who were baseline NF residents, as these patients lived in a NF prior to their IRF stay, as these patients may not be expected to return to the community following their IRF stay. In the FY 2018 IRF PPS final rule (82 FR 36285), we addressed public comments expressing support for a potential future modification of the measure that would exclude baseline NF residents; commenters stated that the exclusion would result in the measure more accurately portraying quality of care provided by IRFs, while controlling for factors outside of IRF control.
We assessed the impact of excluding baseline NF residents from the measure using CY 2015 and CY 2016 data, and found that this exclusion impacted both patient- and facility-level discharge to community rates. We defined baseline NF residents as IRF patients who had a long-term NF stay in the 180 days preceding their hospitalization and IRF stay, with no intervening community discharge between the NF stay and qualifying hospitalization for measure inclusion. Baseline NF residents represented 0.3 percent of the measure population after all measure exclusions were applied. Observed patient-level discharge to community rates were significantly lower for baseline NF residents (20.82 percent) compared with non-NF residents (64.52 percent). The national observed patient-level discharge to community rate was 64.41 percent when baseline NF residents were included in the measure, increasing to 64.52 percent when they were excluded from the measure. After excluding baseline NF residents, 26.9 percent of IRFs had an increase in their risk-standardized discharge to community rate that exceeded the increase in the national observed patient-level discharge to community rate.
Based on public comments received and our impact analysis, we proposed to exclude baseline NF residents from the Discharge to Community—PAC IRF QRP measure beginning with the FY 2020 IRF QRP, with baseline NF residents defined as IRF patients who had a long-term NF stay in the 180 days preceding their hospitalization and IRF stay, with no intervening community discharge between the NF stay and hospitalization.
For additional technical information regarding the Discharge to Community—PAC IRF QRP measure, including technical information about the proposed exclusion, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We sought public comment on this proposal and received several comments. A discussion of these comments, along with our responses, appears below.
Comment: Several commenters supported the proposed exclusion of baseline NF residents from the Discharge to Community—PAC IRF QRP measure. Commenters referred to their recommendation of this exclusion in prior years and appreciated CMS' willingness to consider and implement stakeholder feedback. One commenter stated they did not foresee any negative impacts of the exclusion. One commenter suggested that CMS instead consider other quality measures for NF residents, such as functional status measures, to determine whether residents receive the appropriate standard of care they need in a long-term NF stay.
Response: We thank the commenters for their support of the proposed exclusion of baseline nursing facility residents from this measure and for recommending other measures for consideration for baseline NF residents.
Comment: MedPAC did not support the proposed exclusion of baseline nursing facility residents from the Discharge to Community—PAC IRF QRP measure. They suggested that CMS instead expand their definition of “return to the community” to include baseline nursing home residents returning to the nursing home where they live, as this represents their home or community. MedPAC also stated that providers should be held accountable for the quality of care they provide for as much of their Medicare patient population as feasible.
Response: We agree that providers should be accountable for quality of care for as much of their Medicare population as feasible; we endeavor to do this as much as possible, only specifying exclusions we believe are necessary for measure validity. We also believe that monitoring quality of care and outcomes is important for all PAC patients, including baseline NF residents who return to a NF after their PAC stay. We publicly report several long-stay resident quality measures on Nursing Home Compare including measures of hospitalization and emergency department visits.
Community is traditionally understood as representing non-institutional settings by policy makers, providers, and other stakeholders. Including long-term care NF in the definition of community would confuse this long-standing concept of community and would misalign with CMS' definition of community in patient assessment instruments. We conceptualized this measure using the traditional definition of “community” and specified the measure as a discharge to community measure, rather than a discharge to baseline residence measure.
Baseline NF residents represent an inherently different patient population with not only a significantly lower likelihood of discharge to community settings, but also a higher likelihood of post-discharge readmissions and death compared with PAC patients who did not live in a NF at baseline. The inherent differences in patient characteristics and PAC processes and goals of care for baseline NF residents and non-NF residents are significant enough that we do not believe risk adjustment using a NF flag would provide adequate control. While we acknowledge that a return to nursing home for baseline NF residents represents a return to their home, this outcome does not align with our measure concept. Thus, we have chosen to exclude baseline NF residents from the measure.
Comment: One commenter suggested the definition of “long-term” NF stay in the proposed measure exclusion, requesting further clarification in the measure specifications.
Response: We have further clarified the definition of long-term NF stay in the final measure specifications, Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. A long-term NF stay is identified by the presence of a non-SNF PPS MDS assessment in the 180 days preceding the qualifying prior acute care admission and index SNF stay.
Comment: One commenter questioned whether the methodology for calculating confidence intervals for performance categories used in public display of the Discharge to Community—PAC measures has been updated.
Response: On May 31, 2019, we announced an update to the methodology used for calculating confidence intervals for provider assignment to performance categories for public display of the Discharge to Community—PAC measures. For more information, we refer readers to the “Fact Sheet for Discharge to Community Post-Acute Care Measures” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/LTCH-Quality-Reporting/Downloads/Fact-Sheet-for-Discharge-to-Community-Post-Acute-Care-Measures.pdf and the “FAQ for Discharge to Community Post-Acute Care Measures” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/LTCH-Quality-Reporting/Downloads/FAQ-for-Discharge-to-Community-Post-Acute-Care-Measures.pdf.
After consideration of the public comments, we are finalizing our proposal to exclude baseline NF residents from the Discharge to Community—PAC IRF QRP measure as proposed beginning with the FY 2020 IRF QRP.
E. IRF QRP Quality Measures, Measure Concepts, and Standardized Patient Assessment Data Elements Under Consideration for Future Years: Request for Information
We sought input on the importance, relevance, appropriateness, and applicability of each of the measures, standardized patient assessment data elements (SPADEs), and concepts under consideration listed in the Table 17 for future years in the IRF QRP.

While we will not be responding to specific comments submitted in response to this Request for Information, we intend to use this input to inform our future measure and SPADE development efforts.
We received several comments on this RFI, which are summarized below.
Comment: Several commenters supported the inclusion of all of the proposed measures and SPADEs listed in Table 17. One commenter agreed that the SPADE categories will provide a fuller picture of the patients in the IRF setting and could be used for creating and risk adjusting quality measures.
Many commenters supported the dementia SPADE, since dementia can affect a beneficiary's ability to participate in his or her care in the PAC setting, in addition to managing chronic conditions and medications after discharge. One commenter also agreed that regularly assessing cognitive function and mental health status presents opportunities for better care and quality of life.
One commenter did not support the cognitive complexity SPADEs, since there is no singular assessment tool designed to assess executive function and memory, and it would be overly burdensome for IRFs to conduct testing on every patient. The commenter recommended that CMS work with stakeholders to prioritize which patient conditions would benefit from a cognitive complexity assessment and screen for those cases.
Many commenters supported the caregiver status SPADE; one commenter stated that regular assessment of caregivers will result in better care for the beneficiary and quality of life for both individuals. Another commenter encouraged CMS to capture caregiver status, along with the caregiver's willingness and ability, and account for it in discharge disposition outcomes.
With regard to an opioids-based quality measure, providers had some concerns about unintended consequences of reporting of opioid use, including the over- or under-prescribing of opioids or limiting patients access to critical treatments for pain management.
Many commenters were supportive of SPADEs focused on bowel and bladder continence. One commenter noted that this is already collected on admission and did not support a bowel and bladder SPADE on discharge, citing that IRFs already communicate continence needs at discharge and this would be duplicative. A few commenters had concerns about the burden of future measures and SPADEs. One commenter recommended that prior to adding measures or data elements, CMS reassess and analyze all of the measures and data elements currently collected to limit administrative burden and create a meaningful set of measures and data elements. Another commenter supported utilization of data from the suggested measures and SPADEs and suggested using existing data sources, such a Medicare claims data. One commenter did not support any future SPADE concepts that were not required by the IMPACT Act. Another commenter suggested that CMS should explore beneficiary-matching methods with the Department of Veteran's Affairs to collect veteran status without additional IRF data collection burden.
Response: We appreciate the input provided by commenters. While we will not be responding to specific comments submitted in response to this Request for Information, we intend to use this input to inform our future measure and SPADE development efforts.
F. Standardized Patient Assessment Data Reporting Beginning With the FY 2022 IRF QRP
Section 1886(j)(7)(F)(ii) of the Act requires that, for FY 2019 and each subsequent fiscal year, IRFs must report standardized patient assessment data required under section 1899B(b)(1) of the Act. Section 1899B(a)(1)(C) of the Act requires, in part, the Secretary to modify the PAC assessment instruments in order for PAC providers, including IRFs, to submit SPADEs under the Medicare program. Section 1899B(b)(1)(A) of the Act requires PAC providers to submit SPADEs under applicable reporting provisions (which, for IRFs, is the IRF QRP) with respect to the admission and discharge of an individual (and more frequently as the Secretary deems appropriate), and section 1899B(b)(1)(B) of the Act defines standardized patient assessment data as data required for at least the quality measures described in section 1899B(c)(1) of the Act and that is with respect to the following categories: (1) Functional status, such as mobility and self-care at admission to a PAC provider and before discharge from a PAC provider; (2) cognitive function, such as ability to express ideas and to understand, and mental status, such as depression and dementia; (3) special services, treatments, and interventions, such as need for ventilator use, dialysis, chemotherapy, central line placement, and total parenteral nutrition; (4) medical conditions and comorbidities, such as diabetes, congestive heart failure, and pressure ulcers; (5) impairments, such as incontinence and an impaired ability to hear, see, or swallow; and (6) other categories deemed necessary and appropriate by the Secretary.
In the FY 2018 IRF PPS proposed rule (82 FR 20722 through 20739), we proposed to adopt SPADEs that would satisfy the first five categories. In the FY 2018 IRF PPS final rule (82 FR 36287 through 36289), we summarized comments that supported our adoption of SPADEs, including support for our broader standardization goal and support for the clinical usefulness of specific proposed SPADEs. However, we did not finalize the majority of our SPADE proposals in recognition of the concern raised by many commenters that we were moving too fast to adopt the SPADEs and modify our assessment instruments in light of all of the other requirements we were also adopting under the IMPACT Act at that time (82 FR 36292 through 36294). In addition, commenters noted that we should conduct further testing of the data elements we have proposed (82 FR 36288).
However, we finalized the adoption of SPADEs for two of the categories described in section 1899B(b)(1)(B) of the Act: (1) Functional status: Data elements currently reported by IRFs to calculate the measure Application of Percent of Long-Term Care Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That Addresses Function (NQF #2631); and (2) Medical conditions and comorbidities: The data elements used to calculate the pressure ulcer measures, Percent of Residents or Patients with Pressure Ulcers That Are New or Worsened (Short Stay) (NQF #0678) and the replacement measure, Changes in Skin Integrity Post-Acute Care: Pressure Ulcer/Injury. We stated that these data elements were important for care planning, known to be valid and reliable, and already being reported by IRFs for the calculation of quality measures.
Since we issued the FY 2018 IRF PPS final rule, IRFs have had an opportunity to familiarize themselves with other new reporting requirements that we have adopted under the IMPACT Act. We have also conducted further testing of the SPADEs, as described more fully below, and believe that this testing supports the use of the SPADEs in our PAC assessment instruments. Therefore, we proposed to adopt many of the same SPADEs that we previously proposed to adopt, along with other SPADEs.
We proposed that IRFs would be required to report these SPADEs beginning with the FY 2022 IRF QRP. If finalized as proposed, IRFs would be required to report these data with respect to admission and discharge for Medicare Part A and Medicare Advantage patients discharged between October 1, 2020, and December 31, 2020 for the FY 2022 IRF QRP. Beginning with the FY 2023 IRF QRP, we proposed that IRFs must report data with respect to Medicare Part A and Medicare Advantage admissions and discharges that occur during the subsequent calendar year (for example, CY 2021 for the FY 2023 IRF QRP, CY 2022 for the FY 2024 IRF QRP).
We also proposed that IRFs that submit the Hearing, Vision, Race, and Ethnicity SPADEs with respect to admission will be deemed to have submitted those SPADEs with respect to both admission and discharge, because it is unlikely that the assessment of those SPADEs at admission will differ from the assessment of the same SPADEs at discharge.
In selecting the proposed SPADEs below, we considered the burden of assessment-based data collection and aimed to minimize additional burden by evaluating whether any data that is currently collected through one or more PAC assessment instruments could be collected as SPADEs. In selecting the SPADEs below, we also took into consideration the following factors with respect to each data element:
(1) Overall clinical relevance;
(2) Interoperable exchange to facilitate care coordination during transitions in care;
(3) Ability to capture medical complexity and risk factors that can inform both payment and quality; and
(4) Scientific reliability and validity, general consensus agreement for its usability.
In identifying the SPADEs proposed below, we additionally drew on input from several sources, including TEPs held by our data element contractor, public input, and the results of a recent National Beta Test of candidate data elements conducted by our data element contractor (hereafter “National Beta Test”).
The National Beta Test collected data from 3,121 patients and residents across 143 PAC facilities (26 LTCHs, 60 SNFs, 22 IRFs, and 35 HHAs) from November 2017 to August 2018 to evaluate the feasibility, reliability, and validity of the candidate data elements across PAC settings. The 3,121 patients and residents with an admission assessment included 507 in LTCHs, 1,167 in SNFs, 794 in IRFs, and 653 in HHAs. The National Beta Test also gathered feedback on the candidate data elements from staff who administered the test protocol in order to understand usability and workflow of the candidate data elements. More information on the methods, analysis plan, and results for the National Beta Test can be found in the document titled, “Development and Evaluation of Candidate Standardized Patient Assessment Data Elements: Findings from the National Beta Test (Volume 2),” available in the document titled, “Development and Evaluation of Candidate Standardized Patient Assessment Data Elements: Findings from the National Beta Test (Volume 2),” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Further, to inform the proposed SPADEs, we took into account feedback from stakeholders, as well as from technical and clinical experts, including feedback on whether the candidate data elements would support the factors described above. Where relevant, we also took into account the results of the Post-Acute Care Payment Reform Demonstration (PAC PRD) that took place from 2006 to 2012.
Comment: Several commenters were supportive of the SPADE proposals. A commenter recognized that the proposed SPADEs may influence care, impact case mix and risk adjustment scores, and drive planning for future management. Other commenters supported the proposals to add the proposed SPADEs to the IRF-PAI, with one noting that many of the data elements are already collected and reported on, and the other stating that the items are important to describing current IRF patients and are applicable to determining patient acuity. Another commenter stated that data standardization as accomplished by the SPADEs will help facilitate appropriate payment reforms and appropriate quality measures.
Response: We thank the commenters for their support. We selected the proposed SPADEs in part because of the attributes that the commenters noted, such as their ability to describe IRF patients and to support future quality measurement.
Comment: Some commenters stated support but noted reservations. One commenter described the SPADEs as an appropriate start, but noted that the SPADEs cannot stand alone, and must be built upon in order to be useful for risk adjustment and quality measurement. Similarly, another commenter suggested CMS continue working with clinicians and researchers to ensure that the SPADEs are collecting valid, reliable, and useful data, and to continue to refine and explore new data elements for standardization.
Response: We agree with the commenter's statement that the SPADEs are an appropriate start for standardization, but we disagree that they cannot stand alone. While we intend to evaluate the SPADEs as they are submitted and explore additional opportunities for standardization, we also believe that the SPADEs as proposed represent an important core set of information about clinical status and patient characteristics and they will be useful for quality measurement. We will continue to explore the use of the SPADEs across our PAC setting, continuing our efforts to explore the feasibility, reliability, validity, and usability of the data elements in our measure models and QRPs. We would welcome continued input, recommendations, and feedback from stakeholders about ways to improve assessment and quality measurement for PAC providers, including ways that the SPADEs could be used in the IRF QRP. Input can be shared with CMS through our PAC Quality Initiatives email address PACQualityInitiative@cms.hhs.gov.
Comment: One commenter noted support for the goals of the IMPACT Act, but expressed concern about the scope and timing of proposed changes, including the SPADEs. The same commenter suggested that CMS share with the public a data use strategy and analysis plan for the SPADEs so that providers better understand how CMS will assess the potential usability of the SPADEs to support changes to payment and quality programs.
Response: We thank the commenter for the support and appreciate their concern about the proposed changes. We intend to monitor and evaluate SPADEs as they are submitted, and to continue to engage stakeholders around ways the SPADEs could be best used in the PAC quality programs. We will continue to communicate and collaborate with stakeholders by soliciting input on use of the SPADEs in the IRF QRP through future rulemaking.
Comment: One commenter was generally critical of the set of SPADEs proposed, stating they fail to adequately describe a patient's clinical situation with regard to their level of independence, including swallowing function, communication, and cognitive function.
Response: The proposed SPADEs were selected based on their overall clinical relevance to PAC providers, including IRFs, their ability to facilitate care coordination during transitions, their ability to capture medical complexity and risk factors, and their scientific reliability and validity. We have strived to balance the scope and level of detail of the data elements against the potential burden placed on patients and providers. At this time, SPADEs focused on impairments are limited to sensory impairments (that is, hearing and vision) and do not include swallowing. The patient's ability to communicate is also not captured with a SPADE, although we note that the IRF-PAI includes two data elements on communication: Expression of Ideas and Wants, and Understanding Verbal and Non-Verbal Content. However, in combination with other sections of the IRF-PAI that have been standardized across PAC providers, we believe the proposed SPADEs capture key clinical information (for example, cognitive function for patients who are able to communicate, as collected by the BIMS) and form an important foundation of standardized assessment on which to build.
Comment: One commenter described several concerns about the scope and implementation of the National Beta Test, including the representativeness of IRFs included in the sample, the share of total IRF patients included in the National Beta Test, the reported exclusion of patients with communication and cognitive impairments, and the exclusion of non-English speaking patients, and described how these concerns compromise their confidence in the findings of the National Beta Test.
Response: In a supplementary document to the proposed rule, we described key findings from the National Beta Test related to the proposed SPADEs. We also referred readers to an initial volume of the National Beta Test report that details the methodology of the field test (“Development and Evaluation of Candidate Standardized Patient Assessment Data Elements: Findings from the National Beta Test (Volume 2),” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html). Additional volumes of the National Beta Test report will be available in late 2019.
To address the commenter's specific concerns, we note that the National Beta Test was designed to generate valid and robust national SPADE performance estimates for each of the four PAC provider types, which required acceptable geographic diversity, sufficient sample size, and reasonable coverage of the range of clinical characteristics. To meet these requirements, the National Beta Test was carefully designed so that data could be collected from a wide range of environments, allowing for thorough evaluation of candidate SPADE performance in all PAC settings. The approach included a stratified random sample, to maximize generalizability, and subsequent analyses included extensive checks on the sampling design.
The commenter further implied that the small share of overall IRF admissions included in the Beta test is indicative of inadequate representativeness. The objective of the National Beta Test was to evaluate the performance of candidate SPADEs for cross-setting use. It is true that the proportion of IRFs may not reflect actual proportion in the United States, but our sampling design ensured that sufficient spread of IRFs across randomly selected markets, and adequate numbers to provide ample data with which to evaluate SPADE performance in IRFs relative to other settings.
The National Beta Test did not exclude non-communicative patients/residents; rather, it had two distinct samples, one of which focused on patients/residents who were able to communicate, and one of which focused on patient/residents who were not able to communicate. The assessment of non-communicative patients/residents differed primarily in that observational assessments were substituted for some interview assessments. Non-English-speaking patients were excluded from the National Beta Test due to feasibility constraints during the field test. Including limited English proficiency patients/residents in the sample would have required the Beta test facilities to engage or involve translators during the test assessments. We anticipated that this would have added undue complexity to what facilities/agencies were being requested to do, and would have undermined the ability of facility/agency staff to complete the requested number of assessments during the study period. Moreover, there is strong existing evidence for the feasibility of all clinical patient/resident interview SPADEs included in this final rule (BIMS [section IX.G.1 in this final rule], Pain Interference [section IX.G.3 in this final rule], PHQ [section IX.G.1 in this final rule]) when administered in other languages, either through standard PAC workflow, as tested and currently collected in the MDS 3.0, or through rigorous translation and testing, such as the PHQ. For all these reasons, we determined that the performance of translated versions of these patient/resident interview SPADEs did not need to be further evaluated. In addition, because their exclusion did not threaten our ability to achieve acceptable geographic diversity, sufficient sample size, and reasonable coverage of the range of PAC patient/resident clinical characteristics, the exclusion of limited English proficiency patients/residents was not considered a limitation to interpretation of the National Beta Test results.
Comment: Two commenters wanted CMS to share more information from the National Beta Test. One of the commenters remarked on the lack of information about clinical characteristics that has been shared with stakeholders, limiting their ability to draw conclusions about the data, and requested that CMS release the data from the National Beta Test to be analyzed by third parties. The other commenter noted that CMS has not shared quantitative results of the National Beta Test which has limited the ability of stakeholders to determine if these items will yield useful information for quality and/or payment purposes, and suggested CMS release additional information, such as response frequencies, and analysis from the field test to provide evidence of the validity and utility of the SPADEs for quality and payment.
Response: We shared both quantitative and qualitative findings from the National Beta Test with stakeholders at a public meeting on November 27, 2018. For each SPADE proposed in this rule within the clinical categories in the IMPACT Act, we provided information in the supplementary documents to the proposed rule (the document titled “Proposed Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html) on the feasibility and reliability based on findings from the National Beta Test.
We are in the process of writing the final report for the National Beta Test, which includes the clinical SPADEs in this rule as well as additional data elements. Volume 2 of that report (“Development and Evaluation of Candidate Standardized Patient Assessment Data Elements. Findings from the National Beta Test (Volume 2)”) was posted on CMS' website in March 2019. The other volumes will be available in late 2019. In addition, we are committed to making data available for researchers and the public to analyze, and to doing so in a way that protects the privacy of patients and providers who participated in the National Beta Test. We are in the process of creating research identifiable files that we anticipate will be available through a data use agreement sometime in 2019.
Comment: Many commenters expressed concerns with respect to the standardized patient assessment data proposals. Several commenters stated that the standardized patient assessment data reporting requirements will impose significant burden on providers, given the volume of new standardized patient assessment data elements, and corresponding sub-elements, that were proposed to be added to the IRF-PAI. One commenter noted that the addition of the proposed standardized patient assessment data elements would require an expanded timeline to implement to ensure necessary operational and workflow revisions.
Response: We acknowledge the additional burden that the SPADEs will impose on providers and patients. Our development and selection process for the SPADEs we are adopting in this final rule prioritized data elements that are essential to comprehensive patient care. We maintain that there will be significant benefit associated with each of the SPADEs to providers and patients, in that they are clinically useful (for example, for care planning), they support patient-centered care, and they will promote interoperability and data exchange between providers. During the SPADE development process, we were cognizant of the changes that providers will need to make to implement these additions to the IRF-PAI. In the last two rules (82 FR 36287 through 36289, 83 FR 38555), we provided information about goals, scope, and timeline for implementing SPADEs, as well as updated IRFs about ongoing development and testing of data elements through other public forums. We believe that IRFs have had an opportunity to familiarize themselves with other new reporting requirements that we have adopted under the IMPACT Act and prepare for additional changes.
Comment: Some commenters expressed concern that this additional burden was not justified because, in their view, there was limited or no evidence for the SPADEs to describe case mix, measure quality, or improve care. One of these commenters noted that CMS has provided evidence of validity, reliability, and feasibility through documents related to the National Beta Test, but stated that CMS has not provided any evidence that the proposed SPADEs have the “potential for improving quality” or “utility for describing case mix.”
Response: The clinical SPADEs proposed in this rule were the result of an extensive consensus vetting process in which experts and stakeholders were engaged through Technical Expert Panels, Special Open Door Forums, and posting of interim reports and other documents on the CMS website. Results of these activities provide evidence that experts and providers believe that the proposed SPADEs have the potential for measuring quality, for describing case mix, and improving care. We refer the commenter to the most recent TEP report: A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)”, which is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. In this report, we summarize the TEP's discussion of individual SPADEs in which they reflect on the clinical usefulness and importance of the SPADEs for describing patient acuity (case mix) and providing high-quality clinical care (improving quality). Therefore, we have provided evidence that the SPADEs have the potential for improving quality and utility for describing case mix.
Comment: One commenter believes that the expansion of the IRF-PAI assessment will prove to be intrusive and prove challenging for patients who are elderly, frail, in pain, or have cognitive deficits, causing the patients to lose focus, and thus, impact the accuracy of the data.
Response: We acknowledge that several SPADEs in this rule require the patient to be asked questions directly. We believe that direct patient assessment and patient-reported outcomes on these topics have benefits for providers and patients. These data elements support patient-centered care by soliciting the patient's perspective, and better information on a patient's status is expected to improve the care the patient receives.[76 77 78] The burden the patient-interview data elements place on patients is necessary for accurate assessment of the patient's status. Regarding the validity and performance of interview-based data elements, we note that many of these data elements (for example, the BIMS, PHQ, and Pain Interference data elements) are currently used in the MDS in SNFs. Evidence from that setting, as well as from the National Beta Test, demonstrates feasibility of these data elements for even very sick patients, such as many patients receiving care from IRFs.
Comment: Commenters also stated that the time burden (as in, “time-to-complete”) associated with the clinical SPADEs was underestimated, with some commenters noting that it did not account for clinician time to review charts and update treatment plans or that test conditions do not represent conditions of day-to-day operation. One commenter stated that the estimated time to complete reported in the National Beta Test was based only on the time needed to enter a value on a tablet and did not include the time to evaluate the patient on each item. Another commenter stated that because testing conditions focused on cognitively intact, English-speaking patients with no speech or language deficits, the estimates of impact to providers' time and resources is inadequate.
Response: We disagree with the commenters that the National Beta Test time-to-complete estimates are underestimates. Contrary to what one commenter noted, we wish to clarify that time-to-complete estimates from the National Beta Test included the time spent both to collect data, including the review of the medical record, if needed, and to enter the data elements into a tablet. We note that time-to-complete estimates were calculated using the data from Facility/Agency Staff only, and not Research Nurses, who completed more training and conducted more assessments overall than the Facility/Agency staff. This decision to calculate time-to-complete estimates from Facility/Agency Staff only supports our claim that the time-to-complete estimates are accurate reflections of the time the SPADEs will require when implemented by PAC providers in day-to-day operations. Contrary to another commenter's statement, we also wish to clarify that National Beta Test did exclude patients/residents who were not able to communicate in English, but did not categorically exclude patients with cognitive impairment or patients with speech or language deficits. Therefore, we believe that our estimates of time-to-complete capture the general population of IRF patients, including those with communication impairments.
Comment: Some commenters recommended changes to when and how SPADEs would be collected in order to reduce administrative burden. These recommendations included collecting data only at admission when answers are unlikely to change between admission and discharge, adopting a staged implementation or only a subset of the proposed data elements, and that CMS explore options for obtaining these data via claims or voluntary reporting only, particularly as many of the proposed SPADEs are not relevant to IRF patients.
Response: We appreciate the commenters' recommendations. To support data exchange between settings, and to support quality measurement, section 1899B(b)(1)(A) of the Act requires that the SPADEs be collected with respect to both admission and discharge. In the FY 2020 IRF PPS proposed rule (84 FR 17292), we proposed that IRFs that submit four SPADEs with respect to admission will be deemed to have submitted those SPADEs with respect to both admission and discharge, because we stated that it is unlikely that the assessment of those SPADEs at admission would differ from the assessment of the same SPADEs at discharge. We note that a patient's ability to hear or ability to see are more likely to change between admission and discharge than, for example, a patient's self-report of his or her race, ethnicity, preferred language, or need for interpreter services. The Hearing and Vision SPADEs are also different from the other SPADEs (that is, Race, Ethnicity, Preferred Language, and Interpreter Services) because evaluation of sensory status is a fundamental part of the ongoing nursing assessment conducted for IRF patients. Therefore, clinically significant changes that occur in a patient's hearing or vision status during the IRF stay would be captured as part of the clinical record and communicated to the next setting of care, as well as taken into account during discharge planning as a part of standard best practice.
After consideration of public comments discussed in sections IX.G.4 and IX.G.4.b in this final rule, we will deem IRFs that submit the Hearing, Vision, Race, Ethnicity, Preferred Language, and Interpreter Services SPADEs with respect to admission to have submitted with respect to both admission and discharge. We will take into consideration the recommendation to obtain patient data from claims data in future work.
Comment: A commenter recommended that CMS limit the number and type of data elements implemented in the coming year, continue ongoing dialogue with stakeholders, and develop and implement a process to assess the value of specific indicators for all patient types. Another commenter recommended that CMS conduct a thorough analysis of SPADEs currently collected to determine if any current data elements could be eliminated. One commenter believed that CMS should not finalize the implementation of the SPADEs until they evaluate alternative means of data collection (such as via billing/claims data), or measures to reduce burden (such as removal of duplicative data elements and elimination of data collection at discharge).
Response: We note that we adopted SPADEs in the last two rule cycles to support the adoption of the IRF Functional Outcomes Measures (Application of Percent of Long-Term Care Hospital Patients with an Admission and Discharge Functional Assessment and a Care Plan That Addresses Function (80 FR 47111); Change in Self-Care for Medical Rehabilitation Patients (80 FR 47117); Change in Mobility Score for Medical Rehabilitation Patients (80 FR 47118); Discharge Self-Care Score for Medical Rehabilitation Patients (80 FR 47119); Discharge Mobility Score for Medical Rehabilitation Patients (80 FR 47120)) and drug regimen review (Drug Regimen Review Conducted with Follow-Up for Identified Issues (81 FR 52111)). We have also communicated about the SPADE development work with stakeholders over the last 2 years through SODFs held on June 20, 2017, September 28, 2017, December 12, 2017, March 28, 2018, June 19, 2018, and July 25, 2018, and at a public meeting of stakeholders on November 27, 2018. Therefore, our implementation to date has been incremental while we have strived to keep stakeholders apprised as to the status of ongoing SPADE development. We have also conducted a large-scale test of feasibility and reliability—the National Beta Test, described in the proposed rule (84 FR 17293)—which, along with the consensus vetting activities described in the proposals for each SPADE, provide evidence of the value of the SPADEs for patients across PAC settings, including IRF patients. We will monitor and conduct analysis on the SPADEs as they are submitted in order to identify any problems and to identify any unnecessary burden or duplication.
Comment: One commenter recommended that CMS focus on providing funding and administrative support to allow improvements and standardization to the electronic medical record to allow effective interoperability across all post-acute sites.
Response: We appreciate the commenter's recommendation. At this time, funding for electronic medical record adoption and support is not currently authorized for PAC providers.
Final decisions on the SPADEs are given below, following more detailed comments on each SPADE proposal.
G. Standardized Patient Assessment Data by Category
1. Cognitive Function and Mental Status Data
A number of underlying conditions, including dementia, stroke, traumatic brain injury, side effects of medication, metabolic and/or endocrine imbalances, delirium, and depression, can affect cognitive function and mental status in PAC patient and resident populations.[79] The assessment of cognitive function and mental status by PAC providers is important because of the high percentage of patients and residents with these conditions,[80] and because these assessments provide opportunity for improving quality of care.
Symptoms of dementia may improve with pharmacotherapy, occupational therapy, or physical activity,[81 82 83] and promising treatments for severe traumatic brain injury are currently being tested.[84] For older patients and residents diagnosed with depression, treatment options to reduce symptoms and improve quality of life include antidepressant medication and psychotherapy,[85 86 87 88] and targeted services, such as therapeutic recreation, exercise, and restorative nursing, to increase opportunities for psychosocial interaction.[89]
In alignment with our Meaningful Measures Initiative, accurate assessment of cognitive function and mental status of patients and residents in PAC is expected to make care safer by reducing harm caused in the delivery of care; promote effective prevention and treatment of chronic disease; strengthen person and family engagement as partners in their care; and promote effective communication and coordination of care. For example, standardized assessment of cognitive function and mental status of patients and residents in PAC will support establishing a baseline for identifying changes in cognitive function and mental status (for example, delirium), anticipating the patient's or resident's ability to understand and participate in treatments during a PAC stay, ensuring patient and resident safety (for example, risk of falls), and identifying appropriate support needs at the time of discharge or transfer. Standardized patient assessment data elements will enable or support clinical decision-making and early clinical intervention; person-centered, high quality care through facilitating better care continuity and coordination; better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable standardized patient assessment data elements assessing cognitive function and mental status are needed to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events.
The data elements related to cognitive function and mental status were first proposed as standardized patient assessment data elements in the FY 2018 IRF PPS proposed rule (82 FR 20723 through 20726). In response to our proposals, a few commenters noted that the proposed data elements did not capture some dimensions of cognitive function and mental status, such as functional cognition, communication, attention, concentration, and agitation. One commenter also suggested that other cognitive assessments should be considered for standardization. Another commenter stated support for the standardized assessment of cognitive function and mental status, because it could support appropriate use of skilled therapy for beneficiaries with degenerative conditions, such as dementia, and appropriate use of medications for behavioral and psychological symptoms of dementia.
We sought comment on our proposals to collect as standardized patient assessment data the following data with respect to cognitive function and mental status.
Commenters submitted the following comments related to the proposed rule's discussion of the cognitive function and mental status data elements.
Comment: A few commenters were supportive of the proposal to adopt the BIMS, CAM, and PHQ-2 to 9 as SPADEs on the topic of cognitive function and mental status. One commenter agreed that standardizing cognitive assessments will allow providers to identify changes in status, support clinical decision-making, and improve care continuity and interventions.
Response: We thank the commenters for their support. We selected the Cognitive Function and Mental Status data elements for proposal as standardized data in part because of the attributes that the commenters noted.
Comment: A few commenters noted limitations of these SPADEs to fully assess all areas of cognition and mental status, particularly mild to moderate cognitive impairment, and performance deficits that may be related to cognitive impairment. Some commenters suggested CMS continue exploring assessment tools on the topic of cognition and to include a more comprehensive assessment of cognitive function for use in PAC settings, noting that highly vulnerable patients with a mild cognitive impairment cannot be readily identified through the current SPADEs.
Response: We have strived to balance the scope and level of detail of the data elements against the potential burden placed on patients and providers. In our past work, we evaluated the potential of several different cognition assessments for use as standardized data elements in PAC settings. We ultimately decided on the BIMS, CAM, and PHQ-2 to 9 data elements in our proposal as a starting point. We would welcome continued input, recommendations, and feedback from stakeholders about additional data elements for standardization, which can be shared with CMS through our PAC Quality Initiatives email address: PACQualityInitiative@cms.hhs.gov.
Comment: A commenter stated that cognitive assessment should be individualized, rather than standardized, and performed as determined by patient needs.
Response: We believe that the standardized assessment of cognitive function is essential to achieving the goals of the IMPACT Act. We also wish to clarify that the proposed SPADEs are not intended to replace comprehensive clinical evaluation and in no way preclude providers from conducting further patient evaluation or assessments in their settings as they believe are necessary and useful.
Comment: Regarding future use of these data elements, one commenter recommended that CMS monitor the use of the cognition and mental status SPADEs as risk adjustors and make appropriate adjustments to methodology as needed.
Response: We intend to monitor data submitted via the proposed SPADEs and will consider these uses in the future. We will also continue to review recommendation and feedback from stakeholders regarding data elements that would both satisfy the categories listed in the IMPACT Act and provide meaningful data.
Final decisions on the SPADEs are given below, following more detailed comments on each SPADE proposal.
• Brief Interview for Mental Status (BIMS)
In the FY 2020 IRF PPS proposed rule (84 FR 17294 through 17295), we proposed that the data elements that comprise the BIMS meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act.
As described in the FY 2018 IRF PPS Proposed Rule (82 FR 20723 through 20724), dementia and cognitive impairment are associated with long-term functional dependence and, consequently, poor quality of life and increased healthcare costs and mortality.[90] This makes assessment of mental status and early detection of cognitive decline or impairment critical in the PAC setting. The intensity of routine nursing care is higher for patients and residents with cognitive impairment than those without, and dementia is a significant variable in predicting readmission after discharge to the community from PAC providers.[91]
The BIMS is a performance-based cognitive assessment screening tool that assesses repetition, recall with and without prompting, and temporal orientation. The data elements that make up the BIMS are seven questions on the repetition of three words, temporal orientation, and recall that result in a cognitive function score. The BIMS was developed to be a brief, objective screening tool, with a focus on learning and memory. As a brief screener, the BIMS was not designed to diagnose dementia or cognitive impairment, but rather to be a relatively quick and easy to score assessment that could identify cognitively impaired patients, as well as those who may be at risk for cognitive decline and require further assessment. It is currently in use in two of the PAC assessments: The MDS used by SNFs and the IRF-PAI used by IRFs. For more information on the BIMS, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The data elements that comprise the BIMS were first proposed as standardized patient assessment data elements in the FY 2018 IRF PPS proposed rule (82 FR 20723 through 20724). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016, noted support for use of the BIMS, noting that it is reliable, feasible to use across settings, and will provide useful information about patients and residents. We also stated that the data collected through the BIMS will provide a clearer picture of patient or resident complexity, help with the care planning process, and be useful during care transitions and when coordinating across providers. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the use of the BIMS, especially in its capacity to inform care transitions, but other commenters were critical, noting the limitations of the BIMS to assess mild cognitive impairment and “functional” cognition, and that the BIMS cannot be completed by patients and residents who are unable to communicate. They also stated that other cognitive assessments available in the public domain should be considered for standardization. One commenter suggested that CMS require use of the BIMS with respect to discharge, as well as admission.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the BIMS was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the BIMS to be feasible and reliable for use with PAC patients and residents. More information about the performance of the BIMS in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements and the TEP supported the assessment of patient or resident cognitive status with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums (SODFs) and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Some commenters also expressed concern that the BIMS, if used alone, may not be sensitive enough to capture the range of cognitive impairments, including mild cognitive impairment. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We understand the concerns raised by stakeholders that BIMS, if used alone, may not be sensitive enough to capture the range of cognitive impairments, including functional cognition and MCI, but note that the purpose of the BIMS data elements as SPADEs is to screen for cognitive impairment in a broad population. We also acknowledge that further cognitive tests may be required based on a patient's condition and will take this feedback into consideration in the development of future standardized patient assessment data elements. However, taking together the importance of assessing for cognitive status, stakeholder input, and strong test results, we proposed that the BIMS data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act and to adopt the BIMS data elements as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the BIMS data elements.
Comment: One commenter supported the collection of BIMS at both admission and discharge and believes it will result in more complete data and better care.
Response: We thank the commenter for the support of the BIMS data element.
Comment: One commenter stated that the BIMS fails to detect mild cognitive impairment, differentiate cognitive impairment from a language impairment, link impairment to functional limitation, or identify issues with problem solving and executive function. This commenter recommended use of the Development of Outpatient Therapy Payment Alternatives (DOTPA) items for PAC, as well as a screener targeting functional cognition. Another commenter also recommended CMS identify a better cognitive assessment and not to move forward with the proposal.
Response: We recognize that the BIMS assesses components of cognition and does not, alone, provide a comprehensive assessment of potential cognitive impairment. We clarify that any SPADE is intended as a minimum assessment and does not limit the ability of providers to conduct a more comprehensive assessment of cognition to identify the complexities or potential impacts of cognitive impairment that the commenter describes.
We evaluated the suitability of the DOTPA, as well as other screening tools that targeted functional cognition, by engaging our TEP, through “alpha” feasibility testing, and through soliciting input from stakeholders. At the second meeting of TEP in March 2017, members questioned the use of data elements that rely on assessor observation and judgment, such as DOTPA CARE tool items, and favored other assessments of cognition that required patient interview or patient actions. The TEP also discussed performance-based assessment of functional cognition. These are assessments that require patients to respond by completing a simulated task, such as ordering from a menu, or reading medication instructions and simulating the taking of medications, as required by the Performance Assessment of Self-Care Skills (PASS) items.
In Alpha 2 feasibility testing, which was conducted between April and July 2017, we included a subset of items from the DOTPA as well as the PASS. Findings of that test identified several limitations of the DOTPA items for use as SPADEs, such as relatively long to administer (5 to 7 minutes), especially in the LTCH setting. Assessors also indicated that these items had low relevance for SNF and LTCH patients. In addition, interrater reliability was highly variable among the DOTPA items, both overall and across settings, with some items showing very low agreement (as low as 0.34) and others showing excellent agreement (as high as 0.81). Similarly, findings of the Alpha 2 feasibility test identified several limitations of the PASS for use as SPADEs. The PASS was relatively time-intensive to administer (also 5 to 7 minutes), many patients in HHAs and IRFs needed assistance completing the PASS tasks, and missing data were prevalent. Unlike the DOTPA items, interrater reliability was consistently high overall for PASS (ranging from 0.78 to 0.92), but the high reliability was not deemed to outweigh fundamental feasibility concerns related to administration challenges. A summary report for the Alpha 2 feasibility testing titled “Development and Maintenance of Standardized Cross Setting Patient Assessment Data for Post-Acute Care: Summary Report of Findings from Alpha 2 Pilot Testing” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Alpha-2-SPADE-Pilot-Summary-Document.pdf.
Feedback was obtained on the DOTPA and other assessments of functional cognition through a call for input that was open from April 26, 2017 to June 26, 2017. While we received support for the DOTPA, PASS, and other assessments of functional cognition, commenters also raised concerns about the reliability of the DOTPA, given that it is based on staff evaluation, and the feasibility of the PASS, given that the simulated medication task requires props, such as a medication bottle with printed label and pill box, which may not be accessible in all settings. A summary report for the April 26 to June 26, 2017 public comment period titled “Public Comment Summary Report 2” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Public-Comment-Summary-Report_Standardized-Patient-Assessment-Data-Element-Work_PC2_Jan-2018.pdf.
Based on the input from our TEP, results of alpha feasibility testing, and input from stakeholders, we decided to propose the BIMS for standardization at this time due to the body of research literature supporting its feasibility and validity, its relative brevity, and its existing use in the MDS and IRF-PAI.
Comment: A few commenters noted that BIMS is currently collected by IRFs and has not been demonstrated to predict costs or differentiate case-mix and believes that CMS has not provided any evidence that the BIMS is capable of being utilized for quality purposes to support the collection of these data elements at discharge. Another commenter stated that CMS has not provided quantitative evidence that the BIMS data elements are capable of measuring provider performance for quality or of differentiating case-mix for payment.
Response: We reiterate that the purpose of standardizing data elements, in accordance with the IMPACT Act, is to support care planning, clinical decision support, inform case-mix and quality measurement, support care transitions, and enable interoperable data exchange and data sharing between PAC settings. Before being identified as a SPADE, the BIMS underwent an extensive consensus vetting process in which experts and stakeholders were engaged through TEPs, SODFs, and posting of interim reports and other documents on the CMS.gov website. A summary of the most recent TEP meeting (September 17, 2018) titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. Results of these activities provide evidence that experts and providers believe that the BIMS data elements have the potential for measuring quality, describing case mix, and improving care.
Comment: A commenter believes that assessing BIMS at discharge would not be clinically useful and would not contribute to improved patient care or outcomes. The commenter noted that assessing BIMS at discharge was not evaluated during the National Beta Test, and objected to the BIMS being proposed for use at discharge.
Response: We maintain that a standardized cognitive assessment using the BIMS is clinically useful and has the potential to improve patient care and outcomes. The commenter stated that the BIMS was not administered at discharge in the National Beta Test. However, the BIMS was in fact assessed at both admission and discharge in the National Beta Test. Moreover, to support data exchange between settings, and to support quality measurement, the IMPACT Act requires that the SPADEs be collected with respect to both admission and discharge. After careful consideration of the public comments we received, we are finalizing our proposal to adopt the BIMS as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Confusion Assessment Method (CAM)
In the FY 2020 IRF PPS proposed rule (84 FR 17295), we proposed that the data elements that comprise the Confusion Assessment Method (CAM) meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20724), the CAM was developed to identify the signs and symptoms of delirium. It results in a score that suggests whether a patient or resident should be assigned a diagnosis of delirium. Because patients and residents with multiple comorbidities receive services from PAC providers, it is important to assess delirium, which is associated with a high mortality rate and prolonged duration of stay in hospitalized older adults.[92] Assessing these signs and symptoms of delirium is clinically relevant for care planning by PAC providers.
The CAM is a patient assessment that screens for overall cognitive impairment, as well as distinguishes delirium or reversible confusion from other types of cognitive impairment. The CAM is currently in use in two of the PAC assessments: A four-item version of the CAM is used in the MDS in SNFs; and a six-item version of the CAM is used in the LTCH CARE Data Set (LCDS) in LTCHs. We proposed the four-item version of the CAM that assesses acute change in mental status, inattention, disorganized thinking, and altered level of consciousness. For more information on the CAM, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The data elements that comprise the CAM were first proposed as standardized patient assessment data elements in the FY 2018 IRF PPS proposed rule (82 FR 20724). In that proposed rule, we stated that the proposal was informed by public input we received on the CAM through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 noted support for use of the CAM, noting that it would provide important information for care planning and care coordination, and therefore, contribute to quality improvement. We also stated that those commenters had noted the CAM is particularly helpful in distinguishing delirium and reversible confusion from other types of cognitive impairment. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, one commenter supported use of the CAM for standardized patient assessment data. However, some commenters expressed concerns that the CAM data elements assess: The presence of behavioral symptoms, but not the cause; the possibility of a false positive for delirium due to patient cognitive or communication impairments; and the lack of specificity of the assessment specifications. In addition, other commenters noted that the CAM is not necessary because: Delirium is easily diagnosed without a tool; the CAM and BIMS assessments are redundant; and some CAM response options are not meaningful.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the CAM was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the CAM to be feasible and reliable for use with PAC patients and residents. More information about the performance of the CAM in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although they did not specifically discuss the CAM data elements, the TEP supported the assessment of patient or resident cognitive status with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for delirium, stakeholder input, and strong test results, we proposed that the CAM data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act and to adopt the CAM data elements as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the proposed CAM data elements.
Comment: A few commenters stated that the CAM would be redundant with other cognitive assessments, such as BIMS. One commenter stated that delirium would be assessed prior to discharge from the acute care setting, making the assessment of delirium at admission to the IRF redundant. Another commenter stated that concerns about burden outweighed the value that the CAM might have for some populations, and noted that daily physician visits and daily assessments of patients by the interdisciplinary team were sufficient to assess cognitive needs.
Response: The CAM specifically screens for change in mental status, inattention, disorganized thinking and altered level of consciousness, which can indicate symptoms of delirium. These symptoms are not assessed by other cognitive assessments in the IRF-PAI. We believe the assessment of delirium at admission and discharge is important to informing patient care. Delirium occurs in up to half of patients/residents receiving PAC services,[93] and signs and symptoms of delirium are associated with poor functional recovery,[94] re-hospitalization, and mortality.[95] Because the majority of delirium episodes are transient,[96] we would not expect assessment of delirium prior to discharge from the acute care setting to capture all cases of delirium in PAC, as there may be an acute change in mental status from the patient's baseline or fluctuations in the patient's behaviors that are identified after PAC admission.
Comment: Several commenters noted doubts about the usefulness of the CAM. One commenter was unsure if CAM will identify differences in cognitive status or measure changes during the stay resulting from therapeutic interventions. A few commenters stated that the CAM would not provide information that would be useful clinically, that it was not specific enough or too narrowly focused, and that it should not be required at discharge. Another commenter suggested that CMS not include the CAM as SPADE because they believe delirium is clinically apparent, and therefore, doubt that a standardized assessment of delirium will contribute to improving patient care or outcomes. Another commenter expressed concern that the CAM data elements would not identify cognitive needs that would impact quality in therapeutic intervention across facilities.
Response: As with any brief screening tool, we believe that the CAM has value as a universal assessment to identify patients in need of further clinical evaluation. Delirium occurs in up to 50 percent of patients/residents in PAC [97] and is associated with poor outcomes.[98 99] Hyperactive delirium—the type of delirium that manifests with agitation—makes up only a quarter of delirium cases.[100 101] Delirium more commonly manifests as hypoactive, or “quiet” delirium,[102] suggesting that brief, universal screening is appropriate. Moreover, because there are treatments for delirium that can be developed based on medication review, physical examination, laboratory tests, and evaluation of environmental factors,103we believe that screening for delirium would support care planning and care transitions for these patients.
Comment: A few commenters believe the CAM would be difficult to administer and raised concerns about the training that staff would receive in order to ensure that administration is consistent and valid.
Response: We appreciate the commenters' recommendation to provide clear training for administering the CAM, and will take it into consideration as we revise the current training for the IRF-PAI. We intend to reinforce assessment tips and item rationale through training, open door forums, and future rulemaking efforts.
Comment: One commenter disagreed that delirium assesses a dimension of cognitive function.
Response: The CAM data elements were proposed to meet the definition of the standardized patient assessment data with respect to cognitive function and mental status. Section 1899B(b)(1)(B)(ii) of the Act specifies that PAC providers shall be required to submit standardized patient assessment data for the category of cognitive function, such as the ability to express ideas and to understand, and mental status, such as depression and dementia. A recent deterioration in cognitive function or present and fluctuating behaviors of inattention, disorganized thinking, or altered level of consciousness may indicate delirium.[104] Delirium can also be misdiagnosed as dementia.[105]
Comment: A commenter stated that CMS has not provided quantitative evidence that the CAM data elements are capable of measuring provider performance for quality or of differentiating case-mix for payment.
Response: The clinical SPADEs proposed in this rule, including CAM, were the result of an extensive consensus vetting process. Over the past several years, we have engaged experts and a wide range of stakeholders through TEPs, Special Open Door Forums, and documents made available on the CMS.gov website. A summary of the most recent TEP meeting (September 17, 2018) titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. Results of these activities provide evidence that experts and providers believe that the proposed SPADEs, including the CAM data elements, have the potential for measuring quality, describing case mix, and improving care.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the CAM as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Patient Health Questionnaire-2 to 9 (PHQ-2 to 9)
In the FY 2020 IRF PPS proposed rule (84 FR 17296 through 17297), we proposed that the Patient Health Questionnaire-2 to 9 (PHQ-2 to 9) data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act. The proposed data elements are based on the PHQ-2 mood interview, which focuses on only the two cardinal symptoms of depression, and the longer PHQ-9 mood interview, which assesses presence and frequency of nine signs and symptoms of depression. The name of the data element, the PHQ-2 to 9, refers to an embedded skip pattern that transitions patients with a threshold level of symptoms in the PHQ-2 to the longer assessment of the PHQ-9. The skip pattern is described further below. As described in the FY 2018 IRF PPS proposed rule (82 FR 20725 through 20726), depression is a common and under-recognized mental health condition. Assessments of depression help PAC providers better understand the needs of their patients and residents by: Prompting further evaluation after establishing a diagnosis of depression; elucidating the patient's or resident's ability to participate in therapies for conditions other than depression during their stay; and identifying appropriate ongoing treatment and support needs at the time of discharge.
The proposed PHQ-2 to 9 is based on the PHQ-9 mood interview. The PHQ-2 consists of questions about only the first two symptoms addressed in the PHQ-9: Depressed mood and anhedonia (inability to pleasure), which are the cardinal symptoms of depression. The PHQ-2 has performed well as both a screening tool for identifying depression, to assess depression severity, and to monitor patient mood over time.[106 107] If a patient demonstrates signs of depressed mood and anhedonia under the PHQ-2, then the patient is administered the lengthier PHQ-9. This skip pattern (also referred to as a gateway) is designed to reduce the length of the interview assessment for patients who fail to report the cardinal symptoms of depression. The design of the PHQ-2 to 9 reduces the burden that would be associated with requiring the full PHQ-9, while ensuring that patients and residents with indications of depressive symptoms based on the PHQ-2 receive the longer assessment.
Components of the proposed data elements are currently used in the OASIS for HHAs (PHQ-2) and the MDS for SNFs (PHQ-9). For more information on the PHQ-2 to 9, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We proposed the PHQ-2 data elements as SPADEs in the FY 2018 IRF proposed rule (82 FR 20725 through 20726). In that proposed rule, we stated that the proposal was informed by input we received from the TEP convened by our data element contractor on April 6 and 7, 2016. The TEP members particularly noted that the brevity of the PHQ-2 made it feasible to administer with low burden for both assessors and PAC patients or residents. A summary of the April 6 and 7, 2016 TEP meeting titled “SPADE Technical Expert Panel Summary (First Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The rule proposal was also informed by public input that we received through a call for input published on the CMS Measures Management System Blueprint website. Input was submitted from August 12 to September 12, 2016 on three versions of the PHQ depression screener: The PHQ-2; the PHQ-9; and the PHQ-2 to 9 with the skip pattern design. Many commenters were supportive of the standardized assessment of mood in PAC settings, given the role that depression plays in well-being. Several commenters noted support for an approach that would use PHQ-2 as a gateway to the longer PHQ-9 while still potentially reducing burden on most patients and residents, as well as test administrators, and ensuring the administration of the PHQ-9, which exhibits higher specificity,[108] for patients and residents who showed signs and symptoms of depression on the PHQ-2. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal to use the PHQ-2 in the FY 2018 IRF PPS proposed rule (82 FR 20725 through 20726), we received comments agreeing to the importance of a standardized assessment of depression in patients and residents receiving PAC services. Commenters also raised concerns about the ability of the PHQ-2 to correctly identify all patients and residents with signs and symptoms of depression. One commenter supported using the PHQ-2 as a gateway assessment and conducting a more thorough evaluation of depression symptoms with the PHQ-9 if the PHQ-2 is positive. Another commenter expressed concern that standardized assessment of signs and symptoms of depression via the PHQ-2 is not appropriate in the IRF setting, as patients may have recently experienced acute illness or injury, and routine screening may lead to overprescribing of antidepressant medications. Another commenter expressed concern about potential conflicts between the results of screening assessments and documented diagnoses based on the expertise of physicians and other clinicians. In response to these comments, we carried out additional testing, and we provide our findings below.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the PHQ-2 to 9 was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the PHQ-2 to 9 to be feasible and reliable for use with PAC patients and residents. More information about the performance of the PHQ-2 to 9 in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the PHQ-2 to 9. The TEP was supportive of the PHQ-2 to 9 data element set as a screener for signs and symptoms of depression. The TEP's discussion noted that symptoms evaluated by the full PHQ-9 (for example, concentration, sleep, appetite) had relevance to care planning and the overall well-being of the patient or resident, but that the gateway approach of the PHQ-2 to 9 would be appropriate as a depression screening assessment, as it depends on the well-validated PHQ-2 and focuses on the cardinal symptoms of depression. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our on-going SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for depression, stakeholder input, and test results, we proposed that the PHQ-2 to 9 data elements meet the definition of standardized patient assessment data with respect to cognitive function and mental status under section 1899B(b)(1)(B)(ii) of the Act and to adopt the PHQ-2 to 9 data elements as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the PHQ-2 to 9 data elements.
Comment: Some commenters supported the inclusion of the PHQ-2 to 9. One of these commenters was particularly supportive of the use of the 2-item gateway in the PHQ-2 to 9 approach to improve efficiency.
Response: We thank the commenters for their support of the PHQ-2 to 9, including the gateway approach as a way to decrease burden for providers and patients.
Comment: One commenter was unsure if PHQ-2 to 9 will identify differences in cognitive status or measure changes during the stay resulting from therapeutic interventions. Another commenter expressed concern that the PHQ-2 to 9 data elements would not identify cognitive needs that would impact quality in therapeutic intervention across facilities.
Response: As with any brief screening tool, we believe that the PHQ-2 to 9 has value as a universal assessment to identify patients in need of further clinical evaluation. We believe that applying a brief, standardized assessment of depression across PAC settings, including IRFs, will improve detection based on the PHQ-2 to 9 interview. A universal depression screening is expected to improve patient outcomes by increasing the likelihood that depression will be identified and treated in IRF patients. The proposal of the PHQ-2 to 9 was the result of an extensive consensus vetting process in which experts and stakeholders were engaged through TEPs, SODFs, and posting of interim reports and other documents on CMS.gov. These experts and stakeholders were supportive of the clinical usefulness of the PHQ-2 to 9 assessment. A summary of the most recent TEP meeting (September 17, 2018) titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Comment: A few commenters raised concerns about administration of the PHQ-2 to 9 to IRF patients. One commenter noted that patients in acute rehabilitation may have limited attention and working memory that affects their ability to complete the PHQ-2 to 9. Another commenter noted doubts that PHQ-9 is a good tool for IRFs because of the likelihood of false positives, given patients who are adjusting to recent injuries, surgeries, conditions, and various disabilities. Rather, the commenter believes that assessment by rehabilitation psychologists, who have specialty training in working with rehabilitation populations, would provide a comprehensive evaluation and informed treatment plan. Another commenter expressed concerns about the use of the PHQ in short-stay IRF patients, suggesting that being assessed for depression, especially if assessed multiple times, will affect the patient's perception of how they should be experiencing their situation.
Response: We recognize the challenges faced by patients receiving care from IRF providers. We believe that the PHQ-2 to 9 is the most accurate and appropriate depression screening for the PAC population, including patients in IRFs, and that assessing for depression is necessary for high-quality clinical care. As stated in our proposal above, the PHQ-2 has performed well as a screening tool for identifying depression, to assess depression severity, and to monitor patient mood over time.[109] [110] Additionally, the PHQ-2 and PHQ-9 instruments have been validated in primary care populations against a gold standard diagnostic interview.[111] We believe this prior validation research generalizes to the IRF population. We also note that, regardless of the LOS of patients, the timeframe over which they may have been experiencing signs and symptoms of depression, and the types of circumstances that have led to their IRF stay, it is the responsibility of the IRF to deliver high quality care for all the symptoms or conditions a patient may have. The expectation that the episode of care will be short does not exempt an IRF from screening and treating patients for the full range of physical and mental health problems. Similarly, if a patient self-reports a significant number of depressive symptoms, we do not believe that they should be considered to be a “false positive” because of, for example, a recent trauma or acute care stay. As a screening tool, the PHQ-2 to 9 is intended to capture likely depression to have those patients referred for further evaluation, which will ascertain if their condition is consistent with the full diagnostic criteria for a major depressive disorder. Moreover, standardized screening for the signs and symptoms of depression with the PHQ-2 to 9 does not preclude or provide a substitute for assessment by rehabilitation psychologist or other clinicians, as deemed appropriate by a patient's care team.
Comment: Several commenters cited concerns related to the findings from the National Beta Test related to the PHQ-2 to 9, namely, that testing found it to be burdensome for staff and patients and the wording difficult to understand.
Response: We acknowledge that some assessors in the National Beta Test noted concerns regarding the burden of the PHQ-2 to 9 for staff and patients and that the wording of some items was challenging for patients to understand. In the National Beta Test, the PHQ-2 to 9 was one of a collection of mood assessments, meaning that assessors and patients completed additional questions about depressed mood and well-being immediately before and after the PHQ-2 to 9. We believe that the perception of burden of the PHQ-2 to 9 was in part due to the larger mood assessment section included in the National Beta Test. Despite the burden and administration challenges noted by National Beta Test assessors, assessors generally appreciated the clinical utility and relevance of the PHQ-2 to 9 and noted the importance of standardizing the assessment of depressive symptoms.
Comment: Additional concerns about administration focused on the patient interview format of the PHQ-2 to 9. Some commenters raised concerns about administering the PHQ-2 to 9 to patients with severe cognitive deficits, prior mental health issues, or non-communicative conditions. One commenter suggested that CMS develop exemptions from repeated screenings for short stay patients, and for patients whose medical or cognitive status make it inappropriate to administer the PHQ-2 to 9. Another commenter suggested that the PHQ-2 to 9 have an option to be self-administered by the patient via a patient-friendly paper and pencil layout, which would reduce time burden placed on assessors.
Response: We appreciate commenters' concerns that administering the PHQ-2 to 9 to patients whose medical or cognitive status make it inappropriate to administer. The guidance for completing the data elements will include instructions that if the patient is rarely or never understood verbally, in writing, or using another method, the PHQ-2 to 9 interview will not be completed and the assessor code the responses to the first two items (Little interest or pleasure in doing things; Feeling down, depressed, or hopeless) as 9 (no response). We will take the suggestion to explore the possibility for patient self-administration of the PHQ-2 to 9 into consideration in future SPADE development work.
Comment: One commenter noted confusion about how depression relates to cognitive function.
Response: Section 1899(b)(1)(B)(ii) of the Act specifies the category of “cognitive function, such as ability to express ideas and to understand, and mental status, such as depression and dementia.” We proposed the PHQ-2 to 9 data elements to meet the definition of the standardized patient assessment data with respect to cognitive function and mental status, particularly the “mental status” topic within that category.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the PHQ-2 to 9 data elements as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
2. Special Services, Treatments, and Interventions Data
Special services, treatments, and interventions performed in PAC can have a major effect on an individual's health status, self-image, and quality of life. The assessment of these special services, treatments, and interventions in PAC is important to ensure the continuing appropriateness of care for the patients and residents receiving them, and to support care transitions from one PAC provider to another, an acute care hospital, or discharge. In alignment with our Meaningful Measures Initiative, accurate assessment of special services, treatments, and interventions of patients and residents served by PAC providers is expected to make care safer by reducing harm caused in the delivery of care; promote effective prevention and treatment of chronic disease; strengthen person and family engagement as partners in their care; and promote effective communication and coordination of care.
For example, standardized assessment of special services, treatments, and interventions used in PAC can promote patient and resident safety through appropriate care planning (for example, mitigating risks such as infection or pulmonary embolism associated with central intravenous access), and identifying life-sustaining treatments that must be continued, such as mechanical ventilation, dialysis, suctioning, and chemotherapy, at the time of discharge or transfer. Standardized assessment of these data elements will enable or support: Clinical decision-making and early clinical intervention; person-centered, high quality care through, for example, facilitating better care continuity and coordination; better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable data elements assessing special services, treatments, and interventions are needed to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events.
A TEP convened by our data element contractor provided input on the proposed data elements for special services, treatments, and interventions. In a meeting held on January 5 and 6, 2017, this TEP found that these data elements are appropriate for standardization because they would provide useful clinical information to inform care planning and care coordination. The TEP affirmed that assessment of these services and interventions is standard clinical practice, and that the collection of these data by means of a list and checkbox format would conform with common workflow for PAC providers. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Comments on the category of special services, treatments, and interventions were also submitted by stakeholders during the FY 2018 IRF PPS proposed rule (82 FR 20726 through 20736) public comment period. One commenter supported adding the SPADEs for special services, treatments, and interventions. Others stated labor costs and staff burden would increase for data collection. The Medicare Payment Advisory Commission (MedPAC) suggested that a few other high-cost services, such as cardiac monitoring and specialty bed/surfaces, may warrant consideration for inclusion in future collection efforts. One commenter believes that the low frequency of the special services, treatments, and interventions in the IRF setting makes them not worth assessing for patients given the cost of data collection and reporting. A few commenters noted that many of these data elements should be obtainable from administrative data (that is, coding and Medicare claims), and therefore, assessing them through patient record review would be duplicated effort.
Information on data element performance in the National Beta Test, which collected data between November 2017 and August 2018, is reported within each data element proposal below. Clinical staff who participated in the National Beta Test supported these data elements because of their importance in conveying patient or resident significant health care needs, complexity, and progress. However, clinical staff also noted that, despite the simple “check box” format of these data element, they sometimes needed to consult multiple information sources to determine a patient's or resident's treatments.
We sought comment on our proposals to collect as standardized patient assessment data the following data with respect to special services, treatments, and interventions.
Commenters submitted the following comments related to the proposed rule's discussion of special services, treatments, and interventions data elements.
Comment: One commenter was supportive of collecting these data elements, noting that collection will help to better inform CMS and IRF providers on the severity and needs of patients in this setting.
Response: We thank the commenter for the support of these items. We selected the Special Services, Treatments, and Interventions data elements for proposal as standardized data in part because of the attributes noted.
Comment: Some commenters were concerned about the reliability of the Special Services, Treatments, and Interventions data elements, noting that the results of the National Beta Test indicated that these data elements had a low interrater reliability kappa statistic relative to other data elements in the test.
Response: In the category of Special Services, Treatments, and Interventions, for SPADEs where kappas could be calculated, 1 data element and 2 sub-elements demonstrated overall reliabilities in the moderate range (0.41-0.60) and only 1 sub-element demonstrated an overall reliability in the slight/poor range (0.00-0.20). These overall reliabilities were as follows: 0.60 for the Therapeutic Diet data element; 0.55 for the “Continuous” sub-element of Oxygen Therapy; 0.46 for the “Other” sub-element of IV Medications; and 0.13 for the “Anticoagulant” sub-element of IV Medications. However, the overall reliabilities for all other data elements and sub-elements where kappas could be calculated were substantial/good or excellent/almost perfect. When looking at percent agreement—an alternative measure of interrater agreement—values of overall percent agreement for all Special Services, Treatments, and Interventions SPADEs and sub-elements ranged from 80 to 100 percent.
Comment: Commenters also noted concern around the burden of completing these data elements, in particular because of their low frequency of occurrence in IRF settings. To reduce burden around collection of this information, commenters recommended that CMS explore obtaining this data via claims. Additionally, one commenter added that if these data elements are finalized, they should be collected at discharge only, to reduce administrative burden.
Response: We appreciate the commenters' concern for burden on clinical staff due to completing assessments with respect to both admission and discharge. We believe that assessment of various special services, treatments, and interventions received by patients in the IRF setting will provide important information for care planning and resource use in IRFs. The assessments of the special services, treatments, and interventions with multiple responses are formatted as a “check all that apply” format. Therefore, when treatments do not apply—as the commenters note, this is the case for many IRF patients—the assessor need only check one row for “None of the Above.” We will take under consideration the commenters' recommendation to explore the feasibility of collecting information on special services, treatments, and interventions through claims-based data. Regarding the recommendation to collect these SPADEs at discharge only, we state that it is clinically appropriate and important to the ultimate usefulness of these SPADEs that they are collected with respect to both admission and discharge. For example, for patients coming from acute care or from the community, the admission assessment establishes a baseline for the IRF stay. For all patients, the admission assessment ensures that each patient is systematically assessed for a broad range of health and well-being issues, which we expect to inform care planning.
Comment: One commenter expressed concern that the Special Services, Treatments, and Interventions data elements assess the presence or absence of something rather than the clinical rationale or patient outcomes. This commenter stressed the importance of bringing this assessment to “the next level” in order to determine impact of these treatments on patients' outcomes.
Response: We agree with commenter's concern that recording the presence or absence of certain treatments is only a first step in characterizing the complexity that is often the cause of a patient's receipt of special services, treatments, and interventions. We clarify that all the SPADEs we proposed were intended as a minimum assessment and do not limit the ability of providers to conduct a more comprehensive evaluation of a patient's situation to identify the potential impacts on outcomes that the commenter describes.
Comment: One commenter noted that the item numbering in the Special Services, Treatments, and Interventions data elements is extremely confusing and needs to be reworked.
Response: Several patient assessment tools have traditionally combined letters and numbers, along with labels, to distinguish between data elements. The proposed data elements in the Special Services, Treatments, and Interventions section follow the conventions established by CMS. However, we will take this feedback into consideration in our evaluation and refinement of patient assessment instruments.
Final decisions on the SPADEs are given below, following more detailed comments on each SPADE proposal.
• Cancer Treatment: Chemotherapy (IV, Oral, Other)
In the FY 2020 IRF PPS proposed rule (84 FR 17297 through 17299), we proposed that the Chemotherapy (IV, Oral, Other) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20726 through 20727), chemotherapy is a type of cancer treatment that uses drugs to destroy cancer cells. It is sometimes used when a patient has a malignancy (cancer), which is a serious, often life-threatening or life-limiting condition. Both intravenous (IV) and oral chemotherapy have serious side effects, including nausea/vomiting, extreme fatigue, risk of infection due to a suppressed immune system, anemia, and an increased risk of bleeding due to low platelet counts. Oral chemotherapy can be as potent as chemotherapy given by IV and can be significantly more convenient and less resource-intensive to administer. Because of the toxicity of these agents, special care must be exercised in handling and transporting chemotherapy drugs. IV chemotherapy is administered either peripherally, or more commonly, given via an indwelling central line, which raises the risk of bloodstream infections. Given the significant burden of malignancy, the resource intensity of administering chemotherapy, and the side effects and potential complications of these highly-toxic medications, assessing the receipt of chemotherapy is important in the PAC setting for care planning and determining resource use. The need for chemotherapy predicts resource intensity, both because of the complexity of administering these potent, toxic drug combinations under specific protocols, and because of what the need for chemotherapy signals about the patient's underlying medical condition. Furthermore, the resource intensity of IV chemotherapy is higher than for oral chemotherapy, as the protocols for administration and the care of the central line (if present) for IV chemotherapy require significant resources.
The Chemotherapy (IV, Oral, Other) data element consists of a principal data element (Chemotherapy) and three response option sub-elements: IV chemotherapy, which is generally resource-intensive; Oral chemotherapy, which is less invasive and generally requires less intensive administration protocols; and a third category, Other, provided to enable the capture of other less common chemotherapeutic approaches. This third category is potentially associated with higher risks and is more resource intensive due to delivery by other routes (for example, intraventricular or intrathecal). If the assessor indicates that the patient is receiving chemotherapy on the principal Chemotherapy data element, the assessor would then indicate by which route or routes (for example, IV, Oral, Other) the chemotherapy is administered.
A single Chemotherapy data element that does not include the proposed three sub-elements is currently in use in the MDS in SNFs. For more information on the Chemotherapy (IV, Oral, Other) data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Chemotherapy data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20726 through 20727). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 noted support for the IV Chemotherapy data element and suggested it be included as standardized patient assessment data. We also stated that those commenters had noted that assessing the use of chemotherapy services is relevant to share across the care continuum to facilitate care coordination and care transitions and noted the validity of the data element. Commenters also noted the importance of capturing all types of chemotherapy, regardless of route, and stated that collecting data only on patients and residents who received chemotherapy by IV would limit the usefulness of this standardized data element. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Chemotherapy data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Chemotherapy data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Chemotherapy data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Chemotherapy data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP members did not specifically discuss the Chemotherapy data element, the TEP members supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for chemotherapy, stakeholder input, and strong test results, we proposed that the Chemotherapy (IV, Oral, Other) data element with a principal data element and three sub-elements meet the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Chemotherapy (IV, Oral, Other) data element as standardized patient assessment data for use in the IRF QRP.
A commenter submitted the following comment related to the proposed rule's discussion of the Chemotherapy data element.
Comment: One commenter agreed that it is important to know if a patient is receiving chemotherapy for cancer and the method of administration, but also expressed concern about the lack of an association with a patient outcome. This commenter noted that implications of chemotherapy for patients needing speech-language pathology services include chemotherapy-related cognitive impairment, dysphagia, and speech- and voice-related deficits.
Response: We appreciate the commenter's concern. We agree with the commenter that chemotherapy can create related treatment needs for patients, such as the examples noted by the commenter. However, we believe that it is not feasible for SPADEs to capture all of a patient's needs related to any given treatment, and we maintain that the Special Services, Treatments, and Interventions SPADEs provide a common foundation of clinical assessment, which can be built on by the individual provider or a patient's care team.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Chemotherapy (IV, Oral, Other) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Cancer Treatment: Radiation
In the FY 2020 IRF PPS proposed rule (84 FR 17299), we proposed that the Radiation data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20727 through 20728), radiation is a type of cancer treatment that uses high-energy radioactivity to stop cancer by damaging cancer cell DNA, but it can also damage normal cells. Radiation is an important therapy for particular types of cancer, and the resource utilization is high, with frequent radiation sessions required, often daily for a period of several weeks. Assessing whether a patient or resident is receiving radiation therapy is important to determine resource utilization because PAC patients and residents will need to be transported to and from radiation treatments, and monitored and treated for side effects after receiving this intervention. Therefore, assessing the receipt of radiation therapy, which would compete with other care processes given the time burden, would be important for care planning and care coordination by PAC providers.
The proposed data element consists of the single Radiation data element. The Radiation data element is currently in use in the MDS in SNFs. For more information on the Radiation data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Radiation data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20727 through 20728). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 noted support for the Radiation data element, noting its importance and clinical usefulness for patients and residents in PAC settings, due to the side effects and consequences of radiation treatment on patients and residents that need to be considered in care planning and care transitions, the feasibility of the item, and the potential for it to improve quality. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Radiation data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Radiation data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Radiation data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Radiation data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP members did not specifically discuss the Radiation data element, the TEP members supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for radiation, stakeholder input, and strong test results, we proposed that the Radiation data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Radiation data element as standardized patient assessment data for use in the IRF QRP.
A commenter submitted the following comment related to the proposed rule's discussion of the Radiation data element.
Comment: One commenter expressed concern that the Radiation data element assesses whether a patient is receiving radiation for cancer treatment, but does not identify the rationale for and outcomes associated with radiation. The commenter noted that implications of radiation for patients needing speech-language pathology services include reduced head and neck range of motion due to radiation or severe fibrosis, scar bands, and reconstructive surgery complications and that these can impact both communication and swallowing abilities.
Response: We appreciate the commenter's concern. We agree with the commenter that radiation can create related treatment needs for patients, such as the examples noted by the commenter. However, we believe that it is not feasible for SPADEs to capture all of a patient's needs related to any given treatment, and we maintain that the Special Services, Treatments, and Interventions SPADEs provide a common foundation of clinical assessment, which can be built on by the individual provider or a patient's care team.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Radiation data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Respiratory Treatment: Oxygen Therapy (Intermittent, Continuous, High-concentration Oxygen Delivery System)
In the FY 2020 IRF PPS proposed rule (84 FR 17299 through 17300), we proposed that the Oxygen Therapy (Intermittent, Continuous, High-concentration Oxygen Delivery System) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20728), we proposed a similar data element related to oxygen therapy. Oxygen therapy provides a patient or resident with extra oxygen when medical conditions such as chronic obstructive pulmonary disease, pneumonia, or severe asthma prevent the patient or resident from getting enough oxygen from breathing. Oxygen administration is a resource-intensive intervention, as it requires specialized equipment such as a source of oxygen, delivery systems (for example, oxygen concentrator, liquid oxygen containers, and high-pressure systems), the patient interface (for example, nasal cannula or mask), and other accessories (for example, regulators, filters, tubing). The data element proposed here captures patient or resident use of three types of oxygen therapy (intermittent, continuous, and high-concentration oxygen delivery system), which reflects the intensity of care needed, including the level of monitoring and bedside care required. Assessing the receipt of this service is important for care planning and resource use for PAC providers.
The proposed data element, Oxygen Therapy, consists of the principal Oxygen Therapy data element and three response option sub-elements: Continuous (whether the oxygen was delivered continuously, typically defined as > =14 hours per day); Intermittent; or High-concentration Oxygen Delivery System. Based on public comments and input from expert advisors about the importance and clinical usefulness of documenting the extent of oxygen use, we added a third sub-element, high-concentration oxygen delivery system, to the sub-elements, which previously included only intermittent and continuous. If the assessor indicates that the patient is receiving oxygen therapy on the principal oxygen therapy data element, the assessor then would indicate the type of oxygen the patient receives (for example, Intermittent, Continuous, High-concentration oxygen delivery system).
These three proposed sub-elements were developed based on similar data elements that assess oxygen therapy, currently in use in the MDS in SNFs (“Oxygen Therapy”), previously used in the OASIS (“Oxygen (intermittent or continuous)”), and a data element tested in the PAC PRD that focused on intensive oxygen therapy (“High O2 Concentration Delivery System with FiO2 > 40 percent”). For more information on the proposed Oxygen Therapy (Continuous, Intermittent, High-concentration oxygen delivery system) data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Oxygen Therapy (Intermittent, Continuous) data element was first proposed as standardized patient assessment data in the FY 2018 IRF PPS proposed rule (82 FR 20728). In that proposed rule, we stated that the proposal was informed by input we received on the single data element, Oxygen (inclusive of intermittent and continuous oxygen use), through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016, noted the importance of the Oxygen data element, noting feasibility of this item in PAC, and the relevance of it to facilitating care coordination and supporting care transitions, but suggesting that the extent of oxygen use be documented. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Oxygen Therapy (Intermittent, Continuous) data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Oxygen Therapy data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Oxygen Therapy data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Oxygen Therapy data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Oxygen Therapy data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing oxygen therapy, stakeholder input, and strong test results, we proposed that the Oxygen Therapy (Intermittent, Continuous, High-concentration Oxygen Delivery System) data element with a principal data element and three sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Oxygen Therapy (Intermittent, Continuous, High-concentration Oxygen Delivery System) data element as standardized patient assessment data for use in the IRF QRP.
We invited public comment on this proposal. While we received support from some commenters on the Special Services, Treatments, and Interventions section (IX.G.2 in this final rule) and its proposals as a whole (section IX.F in this final rule), we did not receive any specific comments on the Oxygen Therapy (Intermittent, Continuous, High-concentration Oxygen Delivery System) data element in particular.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Oxygen Therapy (Intermittent, Continuous, High-Concentration Oxygen Delivery System) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Respiratory Treatment: Suctioning (Scheduled, as Needed)
In the FY 2020 IRF PPS proposed rule (84 FR 17300 through 17302), we proposed that the Suctioning (Scheduled, As needed) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20728 through 20729), suctioning is a process used to clear secretions from the airway when a person cannot clear those secretions on his or her own. It is done by aspirating secretions through a catheter connected to a suction source. Types of suctioning include oropharyngeal and nasopharyngeal suctioning, nasotracheal suctioning, and suctioning through an artificial airway such as a tracheostomy tube. Oropharyngeal and nasopharyngeal suctioning are a key part of many patients' or residents' care plans, both to prevent the accumulation of secretions than can lead to aspiration pneumonias (a common condition in patients and residents with inadequate gag reflexes), and to relieve obstructions from mucus plugging during an acute or chronic respiratory infection, which often lead to desaturations and increased respiratory effort. Suctioning can be done on a scheduled basis if the patient is judged to clinically benefit from regular interventions, or can be done as needed when secretions become so prominent that gurgling or choking is noted, or a sudden desaturation occurs from a mucus plug. As suctioning is generally performed by a care provider rather than independently, this intervention can be quite resource intensive if it occurs every hour, for example, rather than once a shift. It also signifies an underlying medical condition that prevents the patient from clearing his/her secretions effectively (such as after a stroke, or during an acute respiratory infection). Generally, suctioning is necessary to ensure that the airway is clear of secretions which can inhibit successful oxygenation of the individual. The intent of suctioning is to maintain a patent airway, the loss of which can lead to death or complications associated with hypoxia.
The Suctioning (Scheduled, As needed) data element consists of a principal data element, and two sub-elements: Scheduled and As needed. These sub-elements capture two types of suctioning. Scheduled indicates suctioning based on a specific frequency, such as every hour. As needed means suctioning only when indicated. If the assessor indicates that the patient is receiving suctioning on the principal Suctioning data element, the assessor would then indicate the frequency (for example, Scheduled, As needed). The proposed data element is based on an item currently in use in the MDS in SNFs which does not include our proposed two sub-elements, as well as data elements tested in the PAC PRD that focused on the frequency of suctioning required for patients and residents with tracheostomies (“Trach Tube with Suctioning: Specify most intensive frequency of suctioning during stay [Every __hours]”). For more information on the Suctioning data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Suctioning data element was first proposed as standardized patient assessment data elements in the FY 2018 IRF PPS proposed rule (82 FR 20728 through 20729). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 noted support for the Suctioning data element. The input noted the feasibility of this item in PAC, and the relevance of this data element to facilitating care coordination and supporting care transitions.
We also stated that those commenters had suggested that we examine the frequency of suctioning to better understand the use of staff time, the impact on a patient or resident's capacity to speak and swallow, and intensity of care required. Based on these comments, we decided to add two sub-elements (Scheduled and As needed) to the suctioning element. The proposed Suctioning data element includes both the principal Suctioning data element that is included on the MDS in SNFs and two sub-elements, Scheduled and As needed. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Suctioning data element. Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Suctioning data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Suctioning data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Suctioning data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Suctioning data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicited additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for suctioning, stakeholder input, and strong test results, we proposed that the Suctioning (Scheduled, As needed) data element with a principal data element and two sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Suctioning (Scheduled, As needed) data element as standardized patient assessment data for use in the IRF QRP.
A commenter submitted the following comment related to the proposed rule's discussion of the Suctioning data element.
Comment: One commenter requested that this data element also assess the frequency of suctioning, as it can impact resource utilization and potential medication changes in the plan of care.
Response: We appreciate the commenter's feedback that the response options for this data element may not fully capture impacts to resource utilization and care plans. The Suctioning data element does include sub-elements to identify if suctioning is performed on a “Scheduled” or “As Needed” basis, but it does not directly assess the frequency of suctioning by, for example, asking an assessor to specify how often suctioning is scheduled. As finalized, this data element differentiates between patients who only occasionally need suctioning, and patients for whom assessment of suctioning needs is a frequent and routine part of the care (that is, where suctioning is performed on a schedule according to physician instructions). In our work to identify standardized data elements, we have strived to balance the scope and level of detail of the data elements against the potential burden placed on patients and providers. However, we clarify that any SPADE is intended as a minimum assessment and does not limit the ability of providers to conduct a more comprehensive evaluation of a patient's situation to identify the potential impacts on outcomes that the commenter describes.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Suctioning (Scheduled, As needed) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Respiratory Treatment: Tracheostomy Care
In the FY 2020 IRF PPS proposed rule (84 FR 17302), we proposed that the Tracheostomy Care data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20729 through 20730), a tracheostomy provides an air passage to help a patient or resident breathe when the usual route for breathing is obstructed or impaired. Generally, in all of these cases, suctioning is necessary to ensure that the tracheostomy is clear of secretions, which can inhibit successful oxygenation of the individual. Often, individuals with tracheostomies are also receiving supplemental oxygenation. The presence of a tracheostomy, albeit permanent or temporary, warrants careful monitoring and immediate intervention if the tracheostomy becomes occluded or if the device used becomes dislodged. While in rare cases the presence of a tracheostomy is not associated with increased care demands (and in some of those instances, the care of the ostomy is performed by the patient) in general the presence of such as device is associated with increased patient risk, and clinical care services will necessarily include close monitoring to ensure that no life-threatening events occur as a result of the tracheostomy. In addition, tracheostomy care, which primarily consists of cleansing, dressing changes, and replacement of the tracheostomy cannula (tube), is a critical part of the care plan. Regular cleansing is important to prevent infection, such as pneumonia, and to prevent any occlusions with which there are risks for inadequate oxygenation.
The proposed data element consists of the single Tracheostomy Care data element. The proposed data element is currently in use in the MDS in SNFs (“Tracheostomy care”). For more information on the Tracheostomy Care data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Tracheostomy Care data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20729 through 20730). In that proposed rule, we stated that the proposal was informed by input we received on the Tracheostomy Care data element through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 noted support for this data element, noting the feasibility of this item in PAC, and the relevance of this data element to facilitating care coordination and supporting care transitions. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Tracheostomy Care data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Tracheostomy Care data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Tracheostomy Care data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Tracheostomy Care data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Tracheostomy Care data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for tracheostomy care, stakeholder input, and strong test results, we proposed that the Tracheostomy Care data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Tracheostomy Care data element as standardized patient assessment data for use in the IRF QRP.
We invited public comment on this proposal. While we received support from some commenters on Special Services, Treatments, and Interventions as a whole (section IX.G.2 in this final rule), we did not receive any specific comments on Tracheostomy Care data element.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Tracheostomy Care data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Respiratory Treatment: Non-Invasive Mechanical Ventilator (BiPAP, CPAP)
In the FY 2020 IRF PPS proposed rule (84 FR 17303), we proposed that the Non-invasive Mechanical Ventilator (Bilevel Positive Airway Pressure [BiPAP], Continuous Positive Airway Pressure [CPAP]) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20730), BiPAP and CPAP are respiratory support devices that prevent the airways from closing by delivering slightly pressurized air via electronic cycling throughout the breathing cycle (BiPAP) or through a mask continuously (CPAP). Assessment of non-invasive mechanical ventilation is important in care planning, as both CPAP and BiPAP are resource-intensive (although less so than invasive mechanical ventilation) and signify underlying medical conditions about the patient or resident who requires the use of this intervention. Particularly when used in settings of acute illness or progressive respiratory decline, additional staff (for example, respiratory therapists) are required to monitor and adjust the CPAP and BiPAP settings and the patient or resident may require more nursing resources.
The proposed data element, Non-invasive Mechanical Ventilator (BiPAP, CPAP), consists of the principal Non-invasive Mechanical Ventilator data element and two response option sub-elements: BiPAP and CPAP. If the assessor indicates that the patient is receiving non-invasive mechanical ventilation on the principal Non-invasive Mechanical Ventilator data element, the assessor would then indicate which type (for example, BiPAP, CPAP). Data elements that assess non-invasive mechanical ventilation are currently included on LCDS for the LTCH setting (“Non-invasive Ventilator (BiPAP, CPAP)”), and the MDS for the SNF setting (“Non-invasive Mechanical Ventilator (BiPAP/CPAP)”). For more information on the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Non-invasive Mechanical Ventilator data element was first proposed as standardized patient assessment data elements in the FY 2018 IRF PPS proposed rule (82 FR 20730). In that proposed rule, we stated that the proposal was informed by input we received through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 on a single data element, BiPAP/CPAP, that captures equivalent clinical information but uses a different label than the data element currently used in the MDS in SNFs and LCDS, noted support for this data element, noting the feasibility of these items in PAC, and the relevance of this data element for facilitating care coordination and supporting care transitions. In addition, we also stated that some commenters supported separating out BiPAP and CPAP as distinct sub-elements, as they are therapies used for different types of patients and residents. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general. One commenter noted appreciation of the revisions to the Non-invasive Mechanical Ventilator data element in response to comments submitted during a public input period held from August 12 to September 12, 2016.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Non-invasive Mechanical Ventilator data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Non-invasive Mechanical Ventilator data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Non-invasive Mechanical Ventilator data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Non-invasive Mechanical Ventilator data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for non-invasive mechanical ventilation, stakeholder input, and strong test results, we proposed that the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element with a principal data element and two sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element as standardized patient assessment data for use in the IRF QRP.
We invited public comment on this proposal. While we received support from some commenters on Special Services, Treatments, and Interventions as a whole (section IX.G.2 in this final rule), we did not receive any specific comments on the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Non-invasive Mechanical Ventilator (BiPAP, CPAP) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Respiratory Treatment: Invasive Mechanical Ventilator
In the FY 2020 IRF PPS proposed rule (84 FR 17304), we proposed that the Invasive Mechanical Ventilator data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20730 through 20731), invasive mechanical ventilation includes ventilators and respirators that ventilate the patient through a tube that extends via the oral airway into the pulmonary region or through a surgical opening directly into the trachea. Thus, assessment of invasive mechanical ventilation is important in care planning and risk mitigation. Ventilation in this manner is a resource-intensive therapy associated with life-threatening conditions without which the patient or resident would not survive. However, ventilator use has inherent risks requiring close monitoring. Failure to adequately care for the patient or resident who is ventilator dependent can lead to iatrogenic events such as death, pneumonia, and sepsis. Mechanical ventilation further signifies the complexity of the patient's underlying medical or surgical condition. Of note, invasive mechanical ventilation is associated with high daily and aggregate costs.[112]
The proposed data element, Invasive Mechanical Ventilator, consists of a single data element. Data elements that capture invasive mechanical ventilation are currently in use in the MDS in SNFs and LCDS in LTCHs. For more information on the Invasive Mechanical Ventilator data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Invasive Mechanical Ventilator data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20730 through 20731). In that proposed rule, we stated that the proposal was informed by input we received on data elements that assess invasive ventilator use and weaning status that were tested in the PAC PRD (“Ventilator—Weaning” and “Ventilator—Non-Weaning”) through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016, noted support for this data element, highlighting the importance of this information in supporting care coordination and care transitions. We also stated that some commenters had expressed concern about the appropriateness for standardization given: The prevalence of ventilator weaning across PAC providers; the timing of administration; how weaning is defined; and how weaning status in particular relates to quality of care. These public comments guided our decision to propose a single data element focused on current use of invasive mechanical ventilation only, which does not attempt to capture weaning status. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” we received is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general. Two commenters noted their appreciation of the revisions to the Invasive Mechanical Ventilator data element in response to comments submitted during a public input period held from August 12 to September 12, 2016.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Invasive Mechanical Ventilator data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Invasive Mechanical Ventilator data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Invasive Mechanical Ventilator data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data element. Although the TEP did not specifically discuss the Invasive Mechanical Ventilator data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for invasive mechanical ventilation, stakeholder input, and strong test results, we proposed that the Invasive Mechanical Ventilator data element that assesses the use of an invasive mechanical ventilator meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Invasive Mechanical Ventilator data element as standardized patient assessment data for use in the IRF QRP.
A commenter submitted the following comment related to the proposed rule's discussion of the Invasive Mechanical Ventilator data element.
Comment: One commenter noted disappointment over seeing that the SPADE for invasive mechanical ventilator only assesses whether or not a patient is on a mechanical ventilator. The commenter suggested CMS consider collecting data to track functional outcomes related to progress towards independence in communication and swallowing.
Response: We have attempted to balance the scope and level of detail of the data elements against the potential burden placed on patients and providers. We believe that assessing the use of an invasive mechanical ventilator will be a useful point of information to inform care planning and further assessment, such as related to functional outcomes, as the commenter suggests.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Invasive Mechanical Ventilator data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Intravenous (IV) Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other)
In the FY 2020 IRF PPS proposed rule (84 FR 17305 through 17306), we proposed that the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20731 through 20732), when we proposed a similar data element related to IV medications, IV medications are solutions of a specific medication (for example, antibiotics, anticoagulants) administered directly into the venous circulation via a syringe or intravenous catheter. IV medications are administered via intravenous push, single, intermittent, or continuous infusion through a catheter placed into the vein. Further, IV medications are more resource intensive to administer than oral medications, and signify a higher patient complexity (and often higher severity of illness).
The clinical indications for each of the sub-elements of the IV Medications data element (Antibiotics, Anticoagulants, Vasoactive Medications, and Other) are very different. IV antibiotics are used for severe infections when the bioavailability of the oral form of the medication would be inadequate to kill the pathogen or an oral form of the medication does not exist. IV anticoagulants refer to anti-clotting medications (that is, “blood thinners”). IV anticoagulants are commonly used for hospitalized patients who have deep venous thrombosis, pulmonary embolism, or myocardial infarction, as well as those undergoing interventional cardiac procedures. Vasoactive medications refer to the IV administration of vasoactive drugs, including vasopressors, vasodilators, and continuous medication for pulmonary edema, which increase or decrease blood pressure or heart rate. The indications, risks, and benefits of each of these classes of IV medications are distinct, making it important to assess each separately in PAC. Knowing whether or not patients and residents are receiving IV medication and the type of medication provided by each PAC provider will improve quality of care.
The IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, and Other) data element we proposed consists of a principal data element (IV Medications) and four response option sub-elements: Antibiotics, Anticoagulants, Vasoactive Medications, and Other. The Vasoactive Medications sub-element was not proposed in the FY 2018 IRF PPS proposed rule (82 FR 20731 through 20732). We added the Vasoactive Medications sub-element to our proposal in order to harmonize the proposed IV Mediciations element with the data currently collected in the LCDS.
If the assessor indicates that the patient is receiving IV medications on the principal IV Medications data element, the assessor would then indicate which types of medications (for example, Antibiotics, Anticoagulants, Vasoactive Medications, Other). An IV Medications data element is currently in use on the MDS in SNFs and there is a related data element in OASIS that collects information on Intravenous and Infusion Therapies. For more information on the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
An IV Medications data element was first proposed as standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20731 through 20732). In that proposed rule, we stated that the proposal was informed by input we received on Vasoactive Medications through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 supported this data element with one noting the importance of this data element in supporting care transitions. We also stated that those commenters had criticized the need for collecting specifically Vasoactive Medications, giving feedback that the data element was too narrowly focused. In addition, public comment received indicated that the clinical significance of vasoactive medications administration alone was not high enough in PAC to merit mandated assessment, noting that related and more useful information could be captured in an item that assessed all IV medication use. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the IV Medications data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the IV Medications data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the IV Medications data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the IV Medications data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the IV Medications data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for IV medications, stakeholder input, and strong test results, we proposed that the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element with a principal data element and four sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the IV Medications data elements.
Comment: One commenter noted that the IV Medications data elements seem redundant of the proposed High-Risk Drug Classes: Use and Indication data elements.
Response: We wish to clarify that the IV Medications data element collects information on medications received by IV only, with sub-elements specific to antibiotics, anticoagulants, and vasoactive medications only. In contrast, the High Risk Drug Classes: Use and Indication data element collects information on medications received by any route, only for six specific drug classes, and collects information on the presence of an indication. We believe the overlap between these SPADEs is minimal, as it would only occur when a medication in a high-risk drug class is delivered by IV. Additionally, in this case, the High-Risk Drug Classes: Use and Indication data element would assess the presence of an indication in the patient's medical record, which the IV Medications data element does not do.
Comment: Commenters were concerned about the performance of the IV Medications data element in the National Beta Test, noting that its reliability was only fair to good and poor for the anticoagulation sub-element.
Response: The kappa for the overarching IV Medications data element was 0.70 across settings, which falls in the range of “substantial/good” agreement. The IV Medications sub-element that had a “slight/poor” reliability (in the range of 0.00-0.20) was the IV Anticoagulants sub-element (kappa = 0.13). The Other IV Medications sub-element had “moderate” reliability (kappa = 0.46). Consultation with assessors suggested that the low kappa for the IV Anticoagulants sub-element was likely due to inconsistent interpretation of the coding instructions. Having identified the likely source of the relatively lower interrater reliability, we are confident that with proper training of IRFs on how to report the data elements, the reliability of these sub-elements will be improved.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the IV Medications (Antibiotics, Anticoagulants, Vasoactive Medications, Other) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Transfusions
In the FY 2020 IRF PPS proposed rule (84 FR 17306), we proposed that the Transfusions data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20732), transfusion refers to introducing blood or blood products into the circulatory system of a person. Blood transfusions are based on specific protocols, with multiple safety checks and monitoring required during and after the infusion in case of adverse events. Coordination with the provider's blood bank is necessary, as well as documentation by clinical staff to ensure compliance with regulatory requirements. In addition, the need for transfusions signifies underlying patient complexity that is likely to require care coordination and patient monitoring, and impacts planning for transitions of care, as transfusions are not performed by all PAC providers.
The proposed data element consists of the single Transfusions data element. A data element on transfusion is currently in use in the MDS in SNFs (“Transfusions”) and a data element tested in the PAC PRD (“Blood Transfusions”) was found feasible for use in each of the four PAC settings. For more information on the Transfusions data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Transfusions data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20732). In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Transfusions data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Transfusions data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Transfusions data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Transfusions data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Transfusions data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for transfusions, stakeholder input, and strong test results, we proposed that the Transfusions data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Transfusions data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the Transfusions data element.
Comment: One commenter applauded CMS for including the Transfusions data element, noting that it will provide information on care planning, clinical decision making, patient safety, care transitions, and resource use in IRFs and will contribute to higher quality and coordinated care for patients who rely on these life-saving treatments.
Response: We thank the commenter for their support. We selected the Transfusions data element for proposal as standardized data in part because of the attributes that the commenter noted.
Comment: One commenter was concerned that IRFs will not have the resources needed to provide patients with access to blood transfusions and requested that CMS consider whether payments to IRFs are adequate to cover the cost of this resource intensive, specialized service.
Response: We wish to clarify that this item is finalized only to collect information on the complexity of the patient and resources the patient requires. At this time, this item will not be used for any payment purposes, and thus we are not able to comment on cost of this service. We wish to clarify that this SPADE is not intended to measure the ability of an IRF to provide in-house transfusions, only to capture the services a given patient may be receiving. Further, for patients who require services related to blood transfusions, information collected by this data element is a part of common clinical workflow, and thus, we believe that burden on resource intensity would not be affected by the standardization of this data element.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Transfusions data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Dialysis (Hemodialysis, Peritoneal Dialysis)
In the FY 2020 IRF PPS proposed rule (84 FR 17306 through 17307), we proposed that the Dialysis (Hemodialysis, Peritoneal dialysis) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20732 through 20733), dialysis is a treatment primarily used to provide replacement for lost kidney function. Both forms of dialysis (hemodialysis and peritoneal dialysis) are resource intensive, not only during the actual dialysis process but before, during, and following. Patients and residents who need and undergo dialysis procedures are at high risk for physiologic and hemodynamic instability from fluid shifts and electrolyte disturbances, as well as infections that can lead to sepsis. Further, patients or residents receiving hemodialysis are often transported to a different facility, or at a minimum, to a different location in the same facility for treatment. Close monitoring for fluid shifts, blood pressure abnormalities, and other adverse effects is required prior to, during, and following each dialysis session. Nursing staff typically perform peritoneal dialysis at the bedside, and as with hemodialysis, close monitoring is required.
The proposed data element, Dialysis (Hemodialysis, Peritoneal dialysis) consists of the principal Dialysis data element and two response option sub-elements: Hemodialysis and Peritoneal dialysis. If the assessor indicates that the patient is receiving dialysis on the principal Dialysis data element, the assessor would then indicate which type (Hemodialysis or Peritoneal dialysis). The principal Dialysis data element is currently included on the MDS in SNFs and the LCDS for LTCHs and assesses the overall use of dialysis.
As the result public feedback described below, in the proposed rule, we proposed a data element that includes the principal Dialysis data element and two sub-elements (Hemodialysis and Peritoneal dialysis). For more information on the Dialysis data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Dialysis data element was first proposed as standardized patient assessment data in the FY 2018 IRF PPS proposed rule (82 FR 20732 through 20733). In that proposed rule, we stated that the proposal was informed by input we received on a singular Hemodialysis data element through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 supported the assessment of hemodialysis and recommended that the data element be expanded to include peritoneal dialysis. We also stated that those commenters had supported the singular Hemodialysis data element, noting the relevance of this information for sharing across the care continuum to facilitate care coordination and care transitions, the potential for this data element to be used to improve quality, and the feasibility for use in PAC. In addition, we received comments that the item would be useful in improving patient and resident transitions of care. We also noted that several commenters had stated that peritoneal dialysis should be included in a standardized data element on dialysis and recommended collecting information on peritoneal dialysis in addition to hemodialysis. The rationale for including peritoneal dialysis from commenters included the fact that patients and residents receiving peritoneal dialysis will have different needs at post-acute discharge compared to those receiving hemodialysis or not having any dialysis. Based on these comments, the Hemodialysis data element was expanded to include a principal Dialysis data element and two sub-elements, Hemodialysis and Peritoneal dialysis. We proposed the version of the Dialysis element that includes two types of dialysis. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received comments in support of the special services, treatments, and interventions data elements in general. One commenter noted that they appreciated the revisions to the Dialysis data element in response to comments submitted during a public input period held from August 12 to September 12, 2016.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Dialysis data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Dialysis data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Dialysis data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although they did not specifically discuss the Dialysis data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for dialysis, stakeholder input, and strong test results, we proposed that the Dialysis (Hemodialysis, Peritoneal dialysis) data element with a principal data element and two sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Dialysis (Hemodialysis, Peritoneal dialysis) data element as standardized patient assessment data for use in the IRF QRP.
We invited public comment on this proposal. While we received support from some commenters on this Special Services, Treatments, and Interventions as a whole (section IX.G.2 in this final rule), we did not receive any specific comments on the Dialysis (Hemodialysis, Peritoneal dialysis) data element.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Dialysis (Hemodialysis, Peritoneal dialysis) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Intravenous (IV) Access (Peripheral IV, Midline, Central Line)
In the FY 2020 IRF PPS proposed rule (84 FR 17307 through 17308), we proposed that the IV Access (Peripheral IV, Midline, Central line) data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20733 through 20734), patients or residents with central lines, including those peripherally inserted or who have subcutaneous central line “port” access, always require vigilant nursing care to keep patency of the lines and ensure that such invasive lines remain free from any potentially life-threatening events such as infection, air embolism, or bleeding from an open lumen. Clinically complex patients and residents are likely to be receiving medications or nutrition intravenously. The sub-elements included in the IV Access data elements distinguish between peripheral access and different types of central access. The rationale for distinguishing between a peripheral IV and central IV access is that central lines confer higher risks associated with life-threatening events such as pulmonary embolism, infection, and bleeding.
The proposed data element, IV Access (Peripheral IV, Midline, Central line), consists of the principal IV Access data element and three response option sub-elements: Peripheral IV, Midline, and Central line. The proposed IV Access data element is not currently included on any of the PAC assessment instruments. For more information on the IV Access data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The IV Access data element was first proposed as standardized patient assessment data elements in the FY 2018 IRF PPS proposed rule (82 FR 20733 through 20734). In that proposed rule, we stated that the proposal was informed by input we received on one of the PAC PRD data elements, Central Line Management, through a call for input published on the CMS Measures Management System Blueprint website. A central line is a type of IV access. Input submitted from August 12 to September 12, 2016 supported the assessment of central line management and recommended that the data element be broadened to also include other types of IV access. Several commenters noted feasibility and importance for facilitating care coordination and care transitions. However, a few commenters recommended that the definition of this data element be broadened to include peripherally inserted central catheters (“PICC lines”) and midline IVs. Based on public comment feedback and in consultation with expert input, described below, we created an overarching IV Access data element with sub-elements for other types of IV access in addition to central lines (that is, peripheral IV and midline). This expanded version of IV Access is the data element being proposed. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general. One commenter noted appreciation of the revisions to the IV Access data element in response to comments submitted during a public input period held from August 12 to September 12, 2016.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the IV Access data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the IV Access data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the IV Access data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the IV Access data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for IV access, stakeholder input, and strong test results, we proposed that the IV access (Peripheral IV, Midline, Central line) data element with a principal data element and three sub-elements meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the IV Access (Peripheral IV, Midline, Central line) data element as standardized patient assessment data for use in the IRF QRP.
We invited public comment on this proposal. While we received support from some commenters on this Special Services, Treatments, and Interventions as a whole (section IX.G.2 in this final rule), we did not receive any specific comments on the IV Access (Peripheral IV, Midline, Central line) data element.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the IV Access (Peripheral IV, Midline, Central line) data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Nutritional Approach: Parenteral/IV Feeding
In the FY 2020 IRF PPS proposed rule (84 FR 17308 through 17309), we proposed that the Parenteral/IV Feeding data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20734), parenteral nutrition/IV feeding refers to a patient or resident being fed intravenously using an infusion pump, bypassing the usual process of eating and digestion. The need for IV/parenteral feeding indicates a clinical complexity that prevents the patient or resident from meeting his or her nutritional needs enterally, and is more resource intensive than other forms of nutrition, as it often requires monitoring of blood chemistries and the maintenance of a central line. Therefore, assessing a patient's or resident's need for parenteral feeding is important for care planning and resource use. In addition to the risks associated with central and peripheral intravenous access, total parenteral nutrition is associated with significant risks, such as air embolism and sepsis.
The proposed data element consists of the single Parenteral/IV Feeding data element. The proposed Parenteral/IV Feeding data element is currently in use in the MDS in SNFs, and equivalent or related data elements are in use in the LCDS, IRF-PAI, and OASIS. We proposed to rename the existing Tube/Parenteral feeding item in the IRF-PAI to be the Parenteral/IV Feeding data element. For more information on the Parenteral/IV Feeding data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Parenteral/IV Feeding data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20734). In that proposed rule, we stated that the proposal was informed by input we received on Total Parenteral Nutrition (an item with nearly the same meaning as the proposed data element, but with the label used in the PAC PRD), through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 supported this data element, noting its relevance to facilitating care coordination and supporting care transitions. After the public comment period, the Total Parenteral Nutrition data element was renamed Parenteral/IV Feeding, to be consistent with how this data element is referred to in the MDS in SNFs. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Parenteral/IV Feeding data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Parenteral/IV Feeding data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Parenteral/IV Feeding data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Parenteral/IV Feeding data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Parenteral/IV Feeding data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for parenteral/IV feeding, stakeholder input, and strong test results, we proposed that the Parenteral/IV Feeding data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Parenteral/IV Feeding data element as standardized patient assessment data for use in the IRF QRP.
A commenter submitted the following comment related to the proposed rule's discussion of the Parenteral/IV Feeding data element.
Comment: One commenter was supportive of collecting this data element, but noted that it should not be a substitute for capturing information related to swallowing which reflects additional patient complexity and resource use.
Response: We thank the commenter for their support and appreciate the concerns raised. We agree that the Parenteral/IV Feeding SPADE should not be used as a substitute for an assessment of a patient's swallowing function. The proposed SPADEs are not intended to replace comprehensive clinical evaluation and in no way preclude providers from conducting further patient evaluation or assessments in their settings as they believe are necessary and useful. We agree that information related to swallowing can capture patient complexity. However, we also note that Parenteral/IV Feeding data element captures a different construct than an evaluation of swallowing. That is, the Parenteral/IV Feeding data element captures a patient's need to receive calories and nutrients intravenously, while an assessment of swallowing would capture a patient's functional ability to safely consume food/liquids orally for digestion in their gastrointestinal tract.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Parenteral/IV Feeding data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Nutritional Approach: Feeding Tube
In the FY 2020 IRF PPS proposed rule (84 FR 17309 through 17310), we proposed that the Feeding Tube data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20734 through 20735), the majority of patients admitted to acute care hospitals experience deterioration of their nutritional status during their hospital stay, making assessment of nutritional status and method of feeding if unable to eat orally very important in PAC. A feeding tube can be inserted through the nose or the skin on the abdomen to deliver liquid nutrition into the stomach or small intestine. Feeding tubes are resource intensive, and therefore, are important to assess for care planning and resource use. Patients with severe malnutrition are at higher risk for a variety of complications.[113] In PAC settings, there are a variety of reasons that patients and residents may not be able to eat orally (including clinical or cognitive status).
The proposed data element consists of the single Feeding Tube data element. The Feeding Tube data element is currently included in the MDS for SNFs, and in the OASIS for HHAs, where it is labeled Enteral Nutrition. A related data element, collected in the IRF-PAI for IRFs (Tube/Parenteral Feeding), assesses use of both feeding tubes and parenteral nutrition. We proposed to rename the existing Tube/Parenteral feeding item in the IRF-PAI to the Feeding Tube data element. For more information on the Feeding Tube data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Feeding Tube data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20734 through 20735). In that proposed rule, we stated that the proposal was informed by input we received on an Enteral Nutrition data element (the Enteral Nutrition data item is the same as the data element we proposed, but is used in the OASIS under a different name) through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 supported the data element, noting the importance of assessing enteral nutrition status for facilitating care coordination and care transitions. After the public comment period, the Enteral Nutrition data element used in public comment was renamed Feeding Tube, indicating the presence of an assistive device. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general. In addition, a commenter recommended that the term “enteral feeding” be used instead of “feeding tube”.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Feeding Tube data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Feeding Tube data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Feeding Tube data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Feeding Tube data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for feeding tubes, stakeholder input, and strong test results, we proposed that the Feeding Tube data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Feeding Tube data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the Feeding Tube data element.
Comment: One commenter noted that in addition to identifying if the patient is on a feeding tube or not, it would be important to assess the patient's progression towards oral feeding within this data element, as this impacts the tube feeding regimen.
Response: We agree that progression to oral feeding is important for care planning and transfer. At this time, we are finalizing a singular Feeding Tube SPADE, which assesses the nutritional approach only and does not capture the patient's prognosis with regard to oral feeding. We wish to clarify that the proposed SPADEs are not intended to replace comprehensive clinical evaluation and in no way preclude providers from conducting further patient evaluation or assessments in their settings as they believe are necessary and useful. We will take this recommendation into consideration in future work on standardized data elements.
Comment: One commenter noted that this data element should designate between percutaneous endoscopic gastrostomy (PEG) tube and nasogastric (NG) tube because the different routes of access have different levels of resource requirements.
Response: We appreciate the commenter's suggestion, but we have decided to maintain the singular Feeding Tube SPADE. We agree that different routes of access may have different levels of resource requirements. However, we do not believe collecting this level of information about nutritional therapies via a SPADE would be significantly more clinically useful or supportive of care transitions than the singular Feeding Tube SPADE. However, we will take this suggestion into consideration in future refinement of the clinical SPADEs.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Feeding Tube data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Nutritional Approach: Mechanically Altered Diet
In the FY 2020 IRF PPS proposed rule (84 FR 17310 through 17311), we proposed that the Mechanically Altered Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20735 through 20736), the Mechanically Altered Diet data element refers to food that has been altered to make it easier for the patient or resident to chew and swallow, and this type of diet is used for patients and residents who have difficulty performing these functions. Patients with severe malnutrition are at higher risk for a variety of complications.[114]
In PAC settings, there are a variety of reasons that patients and residents may have impairments related to oral feedings, including clinical or cognitive status. The provision of a mechanically altered diet may be resource intensive, and can signal difficulties associated with swallowing/eating safety, including dysphagia. In other cases, it signifies the type of altered food source, such as ground or puree that will enable the safe and thorough ingestion of nutritional substances and ensure safe and adequate delivery of nourishment to the patient. Often, patients and residents on mechanically altered diets also require additional nursing support, such as individual feeding or direct observation, to ensure the safe consumption of the food product. Therefore, assessing whether a patient or resident requires a mechanically altered diet is important for care planning and resource identification.
The proposed data element consists of the single Mechanically Altered Diet data element. The proposed data element is currently included on the MDS for SNFs. A related data element (“Modified food consistency/supervision”) is currently included on the IRF-PAI for IRFs. Another related data element is included in the OASIS for HHAs that collects information about independent eating that requires “a liquid, pureed or ground meat diet.” We proposed to replace the existing Modified food consistency/supervision data element in the IRF-PAI to the Mechanically Altered Diet data element. For more information on the Mechanically Altered Diet data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Mechanically Altered Diet data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20735 through 20736). In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general; no additional comments were received that were specific to the Mechanically Altered Diet data element.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Mechanically Altered Diet data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Mechanically Altered Diet data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Mechanically Altered Diet data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Mechanically Altered Diet data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for mechanically altered diet, stakeholder input, and strong test results, we proposed that the Mechanically Altered Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Mechanically Altered Diet data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the Mechanically Altered Diet data element.
Comment: Commenters were concerned about the performance of this data element in the National Beta Test, noting that its reliability was only moderate in IRF settings.
Response: We provided supplementary information with the proposed rule on the reliability of the SPADEs, described by the kappa statistic and by the “percent agreement” between assessor, another measure of reliability that is in some cases more accurate than the kappa statistic, depending on the underlying distribution. (The document titled “Proposed Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html). In this document, we stated that the interrater reliability for Mechanically Altered Diet data element, as measured by kappa, was “substantial/good” across the four PAC provider types (LTCH, SNF, HHA, and IRF) in which it was tested (kappa = 0.65) and “moderate” in the IRF setting (kappa = 0.53). However, percent agreement for the data element was 93 percent across all PAC settings in the National Beta Test (that is, HHA, IRF, LTCH, and SNF) and 89 percent in the IRF setting. That is, when assessing if patients required a mechanically altered diet, the facility staff and the external research nurse agreed 89 percent of the time for IRF patients.
Comment: One commenter was concerned that the Mechanically Altered Diet data element does not capture clinical complexity and does not provide any insight into resource allocation because it only measures whether the patient needs a mechanically altered diet and not, for example, the extent of help a patient needs in consuming his or her meal.
Response: We believe that assessing patients' needs for mechanically altered diets captures one piece of information about resource intensity. That is, patients with this special nutritional requirement may require additional nutritional planning services, special meals, and staff to ensure that meals are prepared and served in the way the patient needs. Additional factors that would affect resource allocation, such as those noted by the commenter, are not captured by this data element. We have attempted to balance the scope and level of detail of the data elements against the potential burden placed on providers who must complete the assessment. We will take this suggestion into consideration in future refinement of the clinical SPADEs.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Mechanically Altered Diet data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Nutritional Approach: Therapeutic Diet
In the FY 2020 IRF PPS proposed rule (84 FR 17311 through 17312), we proposed that the Therapeutic Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20736), a therapeutic diet refers to meals planned to increase, decrease, or eliminate specific foods or nutrients in a patient's or resident's diet, such as a low-salt diet, for the purpose of treating a medical condition. The use of therapeutic diets among patients and residents in PAC provides insight on the clinical complexity of these patients and residents and their multiple comorbidities. Therapeutic diets are less resource intensive from the bedside nursing perspective, but do signify one or more underlying clinical conditions that preclude the patient from eating a regular diet. The communication among PAC providers about whether a patient is receiving a particular therapeutic diet is critical to ensure safe transitions of care.
The proposed data element consists of the single Therapeutic Diet data element. This data element is currently in use in the MDS in SNFs. For more information on the Therapeutic Diet data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Therapeutic Diet data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20736). In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of the special services, treatments, and interventions data elements in general. One commenter recommended that the definition of Therapeutic Diet be aligned with the Academy of Nutrition and Dietetics' definition and that “medically altered diet” be added to the list of nutritional approaches.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Therapeutic Diet data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Therapeutic Diet data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Therapeutic Diet data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. Although the TEP did not specifically discuss the Therapeutic Diet data element, the TEP supported the assessment of the special services, treatments, and interventions included in the National Beta Test with respect to both admission and discharge. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for therapeutic diet, stakeholder input, and strong test results, we proposed that the Therapeutic Diet data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the Therapeutic Diet data element as standardized patient assessment data for use in the IRF QRP.
We invited public comment on this proposal. While we received support from some commenters on Special Services, Treatments, and Interventions as a whole (section IX.G.2 in this final rule), we did not receive any specific comments on the Therapeutic Diet data element.
After careful consideration of the public comments we received on the category of Special Services, Treatments, and Interventions, we are finalizing our proposal to adopt the Therapeutic Diet data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• High-Risk Drug Classes: Use and Indication
In the FY 2020 IRF PPS proposed rule (84 FR 17312 through 17314), we proposed that the High-Risk Drug Classes: Use and Indication data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act.
Most patients and residents receiving PAC services depend on short- and long-term medications to manage their medical conditions. However, as a treatment, medications are not without risk; medications are, in fact, a leading cause of adverse events. A study by the U.S. Department of Health and Human Services found that 31 percent of adverse events that occurred in 2008 among hospitalized Medicare beneficiaries were related to medication.[115] Moreover, changes in a patient's condition, medications, and transitions between care settings put patients at risk of medication errors and adverse drug events (ADEs). ADEs may be caused by medication errors such as drug omissions, errors in dosage, and errors in dosing frequency.[116]
ADEs are known to occur across different types of healthcare settings. For example, the incidence of ADEs in the outpatient setting has been estimated at 1.15 ADEs per 100 person-months,[117] while the rate of ADEs in the long-term care setting is approximately 9.80 ADEs per 100 resident-months.[118] In the hospital setting, the incidence has been estimated at 15 ADEs per 100 admissions.[119] In addition, approximately half of all hospital-related medication errors and 20 percent of ADEs occur during transitions within, admission to, transfer to, or discharge from a hospital.[120] [121] [122] ADEs are more common among older adults, who make up most patients receiving PAC services. The rate of emergency department visits for ADEs is three times higher among adults 65 years of age and older compared to that among those younger than age 65.[123]
Understanding the types of medication a patient is taking, and the reason for its use, are key facets of a patient's treatment with respect to medication. Some classes of drugs are associated with more risk than others.[124] We proposed one High-Risk Drug Class data element with six sub-elements. The response options that correspond to the six medication classes are: Anticoagulants, antiplatelets, hypoglycemics (including insulin), opioids, antipsychotics, and antibiotics. These drug classes are high-risk due to the adverse effects that may result from use. In particular, bleeding risk is associated with anticoagulants and antiplatelets; [125] [126] fluid retention, heart failure, and lactic acidosis are associated with hypoglycemics; [127] misuse is associated with opioids; [128] fractures and strokes are associated with antipsychotics; [129] [130] and various adverse events, such as central nervous systems effects and gastrointestinal intolerance, are associated with antimicrobials,[131] the larger category of medications that include antibiotics. Moreover, some medications in five of the six drug classes included in this data element are included in the 2019 Updated Beers Criteria® list as potentially inappropriate medications for use in older adults.[132] Finally, although a complete medication list should record several important attributes of each medication (for example, dosage, route, stop date), recording an indication for the drug is of crucial importance.[133]
The High-Risk Drug Classes: Use and Indication data element requires an assessor to record whether or not a patient is taking any medications within the six drug classes. The six response options for this data element are high-risk drug classes with particular relevance to PAC patients and residents, as identified by our data element contractor. The six data element response options are Anticoagulants, Antiplatelets, Hypoglycemics, Opioids, Antipsychotics, and Antibiotics. For each drug class, the assessor is required to indicate if the patient is taking any medications within the class, and, for drug classes in which medications were being taken, whether indications for all drugs in the class are noted in the medical record. For example, for the response option Anticoagulants, if the assessor indicates that the patient has received anticoagulant medication, the assessor would then indicate if an indication is recorded in the medication record for the anticoagulant(s).
The High-Risk Drug Classes: Use and Indication data element that is being proposed as a SPADE was developed as part of a larger set of data elements to assess medication reconciliation, the process of obtaining a patient's multiple medication lists and reconciling any discrepancies. For more information on the High-Risk Drug Classes: Use and Indication data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We sought public input on the relevance of conducting assessments on medication reconciliation and specifically on the proposed High-Risk Drug Classes: Use and Indication data element. Our data element contractor presented data elements related to medication reconciliation to the TEP convened on April 6 and 7, 2016. The TEP supported a focus on high-risk drugs, because of higher potential for harm to patients and residents, and were in favor of a data element to capture whether or not indications for medications were recorded in the medical record. A summary of the April 6 and 7, 2016 TEP meeting titled “SPADE Technical Expert Panel Summary (First Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html. Medication reconciliation data elements were also discussed at a second TEP meeting on January 5 and 6, 2017, convened by our data element contractor. At this meeting, the TEP agreed about the importance of evaluating the medication reconciliation process, but disagreed about how this could be accomplished through standardized assessment. The TEP also disagreed about the usability and appropriateness of using the Beers Criteria to identify high-risk medications.[134] A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also solicited public input on data elements related to medication reconciliation during a public input period from April 26 to June 26, 2017. Several commenters noted support for the medication reconciliation data elements that were put on display, noting the importance of medication reconciliation in preventing medication errors and stated that the items seemed feasible and clinically useful. A few commenters were critical of the choice of 10 drug classes posted during that comment period, stating that ADEs are not limited to high-risk drugs, and raised issues related to training assessors to correctly complete a valid assessment of medication reconciliation. A summary report for the April 26 to June 26, 2017 public comment period titled “SPADE May-June 2017 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The High-Risk Drug Classes: Use and Indication data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the High-Risk Drug Classes: Use and Indication data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the High-Risk Drug Classes: Use and Indication data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018, for the purpose of soliciting input on the proposed standardized patient assessment data elements. The TEP acknowledged the challenges of assessing medication safety, but were supportive of some of the data elements focused on medication reconciliation that were tested in the National Beta Test. The TEP was especially supportive of the focus on the six high-risk drug classes and using these classes to assess whether the indication for a drug is recorded. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. These activities provided updates on the field-testing work and solicited feedback on data elements considered for standardization, including the High-Risk Drug Classes: Use and Indication data element. One stakeholder group was critical of the six drug classes included as response options in the High-Risk Drug Classes: Use and Indication data element, noting that potentially risky medications (for example, muscle relaxants) are not included in this list; that there may be important differences between drugs within classes (for example, more recent versus older style antidepressants); and that drug allergy information is not captured. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, one commenter questioned whether the time to complete the High-Risk Drug Classes: Use and Indication data element would differ across settings. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing high-risk drugs and for whether or not indications are noted for high-risk drugs, stakeholder input, and strong test results, we proposed that the High-Risk Drug Classes: Use and Indication data element meets the definition of standardized patient assessment data with respect to special services, treatments, and interventions under section 1899B(b)(1)(B)(iii) of the Act and to adopt the High-Risk Drug Classes: Use and Indication data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the High-Risk Drug Classes: Use and Indication data element.
Comment: Some commenters noted that the proposed High-Risk Drug Classes: Use and Indication data elements are redundant of the existing standards in the Hospital Conditions of Participation (CoPs) and that requiring the collection of these data elements would be duplicative, unnecessary, and at odds with the Meaningful Measures framework.
Response: We disagree that assessing the extent to which medications from certain drug classes are being taken and the extent to which indications are recorded for medications in these classes is redundant with the existing CoPs. The CoPs provide guidance on clinical practice, while the proposed SPADEs attempt to collect information about individual patients in order to understand clinical acuity and to populate a core set of information that can be exchanged with the patient across care transitions.
Comment: Commenters noted that because adverse drug events (ADEs) are not limited to high-risk drugs, this data element has limited utility.
Response: We acknowledge that not all ADEs are associated with “high-risk” drugs, and we also note that medications in the named drug classes are mostly used in a safe manner. Prescribed high-risk medications are defined as a “proximate factor” to preventable ADEs by the Joint Commission.[135] However, the Joint Commission's conceptual model of preventable ADEs also includes provider, patient, health care system, organization, and technical factors, all of which present many opportunities for disrupting preventable ADEs. We have decided to focus on a selection of drug classes that are commonly used by older adults and are related to ADEs which are clinically significant, preventable, and measurable. Anticoagulants, antibiotics, and diabetic agents have been implicated in an estimated 46.9 percent (95 percent CI, 44.2 percent-49.7 percent) of emergency department visits for adverse drug events.[136] Among older adults (aged ≥65 years), three drug classes (anticoagulants, diabetic agents, and opioid analgesics) have been implicated in an estimated 59.9 percent (95 percent CI, 56.8 percent-62.9 percent) of ED visits for adverse drug events.[137] Further, antipsychotic medications have been identified as a drug class for which there is a need for increased outreach and educational efforts to reduce use among older adults.
Comment: One commenter was concerned with the addition of the High-Risk Drug Classes: Use and Indication data elements, noting that providers should be granted clinical judgment to effectively treat patients without CMS monitoring of medications used for treatment.
Response: The proposed SPADEs attempt to collect information about individual patients to understand clinical acuity and to populate a core set of information that can be exchanged with the patient across care transitions. The intent of these data elements is not to monitor prescribing practices, but rather to assess the extent to which indications are noted for medications in certain drug classes.
Comment: A few commenters noted that the High-Risk Drug Class: Use and Indication data elements seemed redundant with other SPADEs (that is, IV Medications) and measures (that is, Provision of Current Reconciled Medication List to Subsequent Provider at Discharge), or duplicative of existing standards in the Hospital CoPs related to procurement, preparation, and administration of drugs, which creates unnecessary burden.
Response: The High-Risk Drugs: Use and Indications data element captures unique information compared to the other SPADEs and measures to which the commenters referred. With regard to the reference to the measure Provision of Current Reconciled Medication List to Subsequent Provider at Discharge, we wish to clarify that the High-Risk Drug Classes: Use and Indication data elements capture medications taken by any route and focuses on a select set of drug classes, not the act of communicating a complete medication list. To the extent that the activities captured by the High-Risk Drugs: Use and Indications data element are already being performed by providers as part of the Hospital CoPs, we believe that reporting of this data elements should be easily integrated into existing workflow.
Comment: One commenter noted that medication indications are typically documented in narrative notes by the medical staff and would therefore be difficult to collect.
Response: We maintain that collecting information on the presence of indications in the medical record is clinically important information that can inform care planning and support care transitions. It is the responsibility of IRF providers to record patient data in a way that is useful and appropriate to meet clinical and administrative needs. It is possible that the adoption of this SPADE and related reporting requirement will promote a more efficient method for documenting the clinical indication for each medication.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the High-Risk Drug Classes: Use and Indication data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
3. Medical Condition and Comorbidity Data
Assessing medical conditions and comorbidities is critically important for care planning and safety for patients and residents receiving PAC services, and the standardized assessment of selected medical conditions and comorbidities across PAC providers is important for managing care transitions and understanding medical complexity.
In this section we discuss our proposals for data elements related to the medical condition of pain as standardized patient assessment data. Appropriate pain management begins with a standardized assessment, and thereafter establishing and implementing an overall plan of care that is person-centered, multi-modal, and includes the treatment team and the patient. Assessing and documenting the effect of pain on sleep, participation in therapy, and other activities may provide information on undiagnosed conditions and comorbidities and the level of care required, and do so more objectively than subjective numerical scores. With that, we assess that taken separately and together, these proposed data elements are essential for care planning, consistency across transitions of care, and identifying medical complexities including undiagnosed conditions. We also conclude that it is the standard of care to always consider the risks and benefits associated with a personalized care plan, including the risks of any pharmacological therapy, especially opioids.[138] We also conclude that in addition to assessing and appropriately treating pain through the optimum mix of pharmacologic, non-pharmacologic, and alternative therapies, while being cognizant of current prescribing guidelines, clinicians in partnership with patients are best able to mitigate factors that contribute to the current opioid crisis.[139] [140] [141]
In alignment with our Meaningful Measures Initiative, accurate assessment of medical conditions and comorbidities of patients and residents in PAC is expected to make care safer by reducing harm caused in the delivery of care; promote effective prevention and treatment of chronic disease; strengthen person and family engagement as partners in their care; and promote effective communication and coordination of care. The SPADEs will enable or support: Clinical decision-making and early clinical intervention; person-centered, high quality care through: facilitating better care continuity and coordination; better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable data elements assessing medical conditions and comorbidities are needed to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events.
We sought comment that applies specifically to the standardized patient assessment data for the category of medical conditions and co-morbidities. We did not receive any comments on the category of medical conditions and co-morbidities.
Final decisions on the SPADEs are given below, following more detailed comments on each SPADE proposal.
• Pain Interference (Pain Effect on Sleep, Pain Interference With Therapy Activities, and Pain Interference With Day-to-Day Activities)
In acknowledgement of the opioid crisis, we specifically sought comment on whether or not we should add these pain items in light of those concerns. Commenters were asked to address to what extent the collection of the SPADEs described below through patient queries might encourage providers to prescribe opioids.
In the FY 2020 IRF PPS proposed rule (84 FR 17314 through 17316), we proposed that a set of three data elements on the topic of Pain Interference (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) meet the definition of standardized patient assessment data with respect to medical condition and comorbidity data under section 1899B(b)(1)(B)(iv) of the Act.
The practice of pain management began to undergo significant changes in the 1990s because the inadequate, non-standardized, non-evidence-based assessment and treatment of pain became a public health issue.[142] In pain management, a critical part of providing comprehensive care is performance of a thorough initial evaluation, including assessment of both the medical and any biopsychosocial factors causing or contributing to the pain, with a treatment plan to address the causes of pain and to manage pain that persists over time.[143] Quality pain management, based on current guidelines and evidence-based practices, can minimize unnecessary opioid prescribing both by offering alternatives or supplemental treatment to opioids and by clearly stating when they may be appropriate, and how to utilize risk-benefit analysis for opioid and non-opioid treatment modalities.[144]
Pain is not a surprising symptom in PAC patients and residents, where healing, recovery, and rehabilitation often require regaining mobility and other functions after an acute event. Standardized assessment of pain that interferes with function is an important first step towards appropriate pain management in PAC settings. The National Pain Strategy called for refined assessment items on the topic of pain, and describes the need for these improved measures to be implemented in PAC assessments.[145] Further, the focus on pain interference, as opposed to pain intensity or pain frequency, was supported by the TEP convened by our data element contractor as an appropriate and actionable metric for assessing pain. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We appreciate the important concerns related to the misuse and overuse of opioids in the treatment of pain and to that end we note that in the proposed rule we have also proposed a SPADE that assess for the use of, as well as importantly the indication for the use of, high-risk drugs, including opioids. Further, in the FY 2017 IRF PPS final rule (81 FR 52111) we adopted the Drug Regimen Review Conducted With Follow-Up for Identified Issues—Post Acute Care (PAC) IRF QRP measure which assesses whether PAC providers were responsive to potential or actual clinically significant medication issue(s), which includes issues associated with use and misuse of opioids for pain management, when such issues were identified.
We also note that the proposed SPADE related to pain assessment are not associated with any particular approach to management. Since the use of opioids is associated with serious complications, particularly in the elderly,[146] [147] [148] an array of successful non-pharmacologic and non-opioid approaches to pain management may be considered. PAC providers have historically used a range of pain management strategies, including non-steroidal anti-inflammatory drugs, ice, transcutaneous electrical nerve stimulation (TENS) therapy, supportive devices, acupuncture, and the like. In addition, non-pharmacological interventions for pain management include, but are not limited to, biofeedback, application of heat/cold, massage, physical therapy, stretching and strengthening exercises, chiropractic, electrical stimulation, radiotherapy, and ultrasound.[149] [150] [151]
We believe that standardized assessment of pain interference will support PAC clinicians in applying best-practices in pain management for chronic and acute pain, consistent with current clinical guidelines. For example, the standardized assessment of both opioids and pain interference would support providers in successfully tapering the dosage regimens in patients/residents who arrive in the PAC setting with long-term opioid use off of opioids onto non-pharmacologic treatments and non-opioid medications, as recommended by the Society for Post-Acute and Long-Term Care Medicine,[152] and consistent with HHS's 5-Point Strategy To Combat the Opioid Crisis [153] which includes “Better Pain Management.”
The Pain Interference data elements consist of three data elements: Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities. Pain Effect on Sleep assesses the frequency with which pain affects a resident's sleep. Pain Interference with Therapy Activities assesses the frequency with which pain interferes with a resident's ability to participate in therapies. The Pain Interference with Day-to-Day Activities assesses the extent to which pain interferes with a resident's ability to participate in day-to-day activities excluding therapy.
A similar data element on the effect of pain on activities is currently included in the OASIS. A similar data element on the effect on sleep is currently included in the MDS instrument. For more information on the Pain Interference data elements, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We sought public input on the relevance of conducting assessments on pain and specifically on the larger set of Pain Interview data elements included in the National Beta Test. The proposed data elements were supported by comments from the TEP meeting held by our data element contractor on April 7 to 8, 2016. The TEP affirmed the feasibility and clinical utility of pain as a concept in a standardized assessment. The TEP agreed that data elements on pain interference with ability to participate in therapies versus other activities should be addressed. Further, during a more recent convening of the same TEP on September 17, 2018, the TEP supported the interview-based pain data elements included in the National Beta Test. The TEP members were particularly supportive of the items that focused on how pain interferes with activities (that is, Pain Interference data elements), because understanding the extent to which pain interferes with function would enable clinicians to determine the need for appropriate pain treatment. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We held a public input period in 2016 to solicit feedback on the standardization of pain and several other items that were under development in prior efforts. From the prior public comment period, we included several pain data elements (Pain Effect on Sleep; Pain Interference—Therapy Activities; Pain Interference—Other Activities) in a second call for public input, open from April 26 to June 26, 2017. The items we sought comment on were modified from all stakeholder and test efforts. Commenters provided general comments about pain assessment in general in addition to feedback on the specific pain items. A few commenters shared their support for assessing pain, the potential for pain assessment to improve the quality of care, and for the validity and reliability of the data elements. Commenters affirmed that the item of pain and the effect on sleep would be suitable for PAC settings. Commenters' main concerns included redundancy with existing data elements, feasibility and utility for cross-setting use, and the applicability of interview-based items to patients and residents with cognitive or communication impairments, and deficits. A summary report for the April 26 to June 26, 2017 public comment period titled “SPADE May-June 2017 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Pain Interference data elements were included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Pain Interference data elements to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Pain Interference data elements in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on September 17, 2018 for the purpose of soliciting input on the standardized patient assessment data elements. The TEP supported the interview-based pain data elements included in the National Beta Test. The TEP members were particularly supportive of the items that focused on how pain interferes with activities (that is, Pain Interference data elements), because understanding the extent to which pain interferes with function would enable clinicians to determine the need for pain treatment. A summary of the September 17, 2018 TEP meeting titled “SPADE Technical Expert Panel Summary (Third Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our on-going SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, one commenter noted strong support for the Pain data elements and was encouraged by the fact that this portion of the assessment goes beyond merely measuring the presence of pain. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Taking together the importance of assessing for the effect of pain on function, stakeholder input, and strong test results, we proposed that the three Pain Interference data elements (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) meet the definition of standardized patient assessment data with respect to medical conditions and comorbidities under section 1899B(b)(1)(B)(iv) of the Act and to adopt the Pain Interference data elements (Pain Effect on Sleep; Pain Interference with Therapy Activities; and Pain Interference with Day-to-Day Activities) as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to our proposal to adopt the Pain Interference (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) data elements.
Comment: A few commenters noted support for the Pain Interference data element, noting that the data element will provide a useful and more accurate assessment of a patient's ability to function, and that understanding the impact of pain on therapy and other activities, including sleep, can improve the quality of care, which in turn will support providers in their ability to provide effective pain management services.
Response: We thank the commenters for their support of the Pain Interference data element.
Comment: A commenter noted that the proposed Pain Interference SPADEs document pain frequency, but stated that it is important to identify both pain frequency and pain intensity.
Response: We wish to clarify, the Pain Interference interview data elements question the patient on the frequency with which pain interferes with sleep, therapy, or non-therapy activities. These data elements therefore combine the concepts of frequency and intensity, with the measure of intensity being interference with the named activities. Self-reported measures of pain intensity are often criticized for being infeasible to standardize. In these data elements, we use interference with activities as an alternative to inquiring about intensity.
Comment: A commenter expressed concerns about the suitability of the Pain Interference data elements for use in patients with cognitive and communication deficits and recommended CMS consider the use of non-verbal means to allow patients to respond to SPADEs related to pain.
Response: We appreciate the commenter's concern surrounding pain assessment with patients with cognitive and communication deficits. The Pain Interference interview SPADEs require that a patient be able to communicate, whether verbally, in writing, or using another method; assessors may use non-verbal means to administer the questions (for example, providing the questions and response in writing for a patient with severe hearing impairment). Patients who are unable to communicate by any means would not be required to complete the Pain Interference interview SPADEs. However, evidence suggests that pain presence can be reliably assessed in non-communicative patients through structural observational protocols. To that end, we tested observational pain presence elements in the National Beta Test, but have chosen not to propose those data elements as SPADEs at this time. We will take the commenter's concern into consideration as the SPADEs are monitored and refined in the future.
Comment: A commenter expressed concerns about how CMS might use these data elements, noting particular concern that collection of these data elements may inappropriately translate into an assessment of quality, and that data collection on this topic could create incentives that directly or indirectly interfere with treatment decisions.
Response: We appreciate the commenter's concern related to wanting to understand how we will use the SPADEs in the future. We intend to continue to communicate and collaborate with stakeholders about how the SPADEs will be used in the IRF QRP, as those plans are developed, by soliciting input during the development process and establishing use of the SPADEs in payment and quality programs through future rulemaking.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Pain Interference (Pain Effect on Sleep, Pain Interference with Therapy Activities, and Pain Interference with Day-to-Day Activities) data elements as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
4. Impairment Data
Hearing and vision impairments are conditions that, if unaddressed, affect activities of daily living, communication, physical functioning, rehabilitation outcomes, and overall quality of life. Sensory limitations can lead to confusion in new settings, increase isolation, contribute to mood disorders, and impede accurate assessment of other medical conditions. Failure to appropriately assess, accommodate, and treat these conditions increases the likelihood that patients and residents will require more intensive and prolonged treatment. Onset of these conditions can be gradual, so individualized assessment with accurate screening tools and follow-up evaluations are essential to determining which patients and residents need hearing- or vision-specific medical attention or assistive devices and accommodations, including auxiliary aids and/or services, and to ensure that person-directed care plans are developed to accommodate a patient's or resident's needs. Accurate diagnosis and management of hearing or vision impairment would likely improve rehabilitation outcomes and care transitions, including transition from institutional-based care to the community. Accurate assessment of hearing and vision impairment would be expected to lead to appropriate treatment, accommodations, including the provision of auxiliary aids and services during the stay, and ensure that patients and residents continue to have their vision and hearing needs met when they leave the facility.
In alignment with our Meaningful Measures Initiative, we expect accurate and individualized assessment, treatment, and accommodation of hearing and vision impairments of patients and residents in PAC to make care safer by reducing harm caused in the delivery of care; promote effective prevention and treatment of chronic disease; strengthen person and family engagement as partners in their care; and promote effective communication and coordination of care. For example, standardized assessment of hearing and vision impairments used in PAC will support ensuring patient safety (for example, risk of falls), identifying accommodations needed during the stay, and appropriate support needs at the time of discharge or transfer. Standardized assessment of these data elements will: Enable or support clinical decision-making and early clinical intervention; person-centered, high quality care (for example, facilitating better care continuity and coordination); better data exchange and interoperability between settings; and longitudinal outcome analysis. Therefore, reliable data elements assessing hearing and vision impairments are needed to initiate a management program that can optimize a patient's or resident's prognosis and reduce the possibility of adverse events.
Comments on the category of impairments were also submitted by stakeholders during the FY 2018 IRF PPS proposed rule (82 FR 20737 through 20739) public comment period. A commenter stated hearing and vision assessments should be administered at the beginning of the assessment process to provide evidence about any sensory deficits that may affect the patient's ability to participate in the assessment and to allow the assessor to offer an assistive device.
We sought comment on our proposals to collect as standardized patient assessment data the following data with respect to impairments. We did not receive any comments on the category of impairments.
Final decisions on the SPADEs are given below, following more detailed comments on each SPADE proposal.
• Hearing
In the FY 2020 IRF PPS proposed rule (84 FR 17317 through 17318), we proposed that the Hearing data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20737 through 20738), accurate assessment of hearing impairment is important in the PAC setting for care planning and resource use. Hearing impairment has been associated with lower quality of life, including poorer physical, mental, social functioning, and emotional health.[154 155] Treatment and accommodation of hearing impairment led to improved health outcomes including, but not limited to, quality of life.[156] For example, hearing loss in elderly individuals has been associated with depression and cognitive impairment,[157 158 159] higher rates of incident cognitive impairment and cognitive decline,[160] and less time in occupational therapy.[161] Accurate assessment of hearing impairment is important in the PAC setting for care planning and defining resource use.
The proposed data element consists of the single Hearing data element. This data consists of one question that assesses level of hearing impairment. This data element is currently in use in the MDS in SNFs. For more information on the Hearing data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Hearing data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20737 through 20738). In that proposed rule, we stated that the proposal was informed by input we received on the PAC PRD form of the data element (“Ability to Hear”) through a call for input published on the CMS Measures Management System Blueprint website. Input submitted from August 12 to September 12, 2016 recommended that hearing, vision, and communication assessments be administered at the beginning of patient assessment process. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received public comments in support of adopting the Hearing data element for standardized cross-setting use, noting that it would help address the needs of patient and residents with disabilities and that failing to identify impairments during the initial assessment can result in inaccurate diagnoses of impaired language or cognition and can invalidate other information obtained from patient assessment.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Hearing data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Hearing data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Hearing data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on January 5 and 6, 2017 for the purpose of soliciting input on all the SPADEs, including the Hearing data element. The TEP affirmed the importance of standardized assessment of hearing impairment in PAC patients and residents. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held Special Open Door Forums and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, a commenter noted support for the Hearing data element and suggested administration at the beginning of the patient assessment to maximize utility. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Due to the relatively stable nature of hearing impairment, it is unlikely that a patient's score on this assessment would change between the start and end of the IRF stay. Therefore, we proposed that IRFs that submit the Hearing data element with respect to admission will be deemed to have submitted with respect to both admission and discharge.
Taking together the importance of assessing for hearing, stakeholder input, and strong test results, we proposed that the Hearing data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act and to adopt the Hearing data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to our proposal for the Hearing data element.
Comment: A few commenters supported the collection of information on hearing impairment. One of these commenters also suggested that CMS consider how hearing impairment impacts a patient's ability to respond to the assessment tool in general.
Response: We thank the commenters for their support of the Hearing data element. We intend to reinforce assessment tips and item rationale through training, open door forums, and future rulemaking efforts.
In the existing guidance manual for the IRF-PAI, we offer tips for administration that direct assessors to take appropriate steps to accommodate sensory and communication impairments when conducting the assessment.
Comment: Some commenters expressed concern that severely impaired hearing occurs infrequently in IRF patients, thereby limiting the utility of the data collected.
Response: The Hearing SPADE consists of one data element completed by the assessor based primarily on interacting with the patient and reviewing the medical record. Given the low burden of reporting the Hearing data element, and despite severe hearing impairment occurring in a small proportion of IRF patients, we believe it is important to systematically assess for hearing impairment in order to improve clinical care and care transitions.
Comment: One commenter recommended adding “unable to assess” as a response option, which the commenter believes would be the appropriate choice if the patient is comatose or is unable to effectively answer questions related to an assessment of their hearing.
Response: We appreciate the commenter's recommendation. The assessment of hearing is completed based on observing the patient during assessment, patient interactions with others, reviewing medical record documentation, and consulting with patient's family and other staff, in addition to interviewing the patient, so it can be completed when the patient is unable to effectively answer questions related to an assessment of their hearing.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Hearing data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
• Vision
In the FY 2020 IRF PPS proposed rule (84 FR 17318 through 17319), we proposed that the Vision data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act.
As described in the FY 2018 IRF PPS proposed rule (82 FR 20738 through 20739), evaluation of an individual's ability to see is important for assessing for risks such as falls and provides opportunities for improvement through treatment and the provision of accommodations, including auxiliary aids and services, which can safeguard patients and residents and improve their overall quality of life. Further, vision impairment is often a treatable risk factor associated with adverse events and poor quality of life. For example, individuals with visual impairment are more likely to experience falls and hip fracture, have less mobility, and report depressive symptoms.[162 163 164 165 166 167 168] Individualized initial screening can lead to life-improving interventions such as accommodations, including the provision of auxiliary aids and services, during the stay and/or treatments that can improve vision and prevent or slow further vision loss.
In addition, vision impairment is often a treatable risk factor associated with adverse events which can be prevented and accommodated during the stay. Accurate assessment of vision impairment is important in the IRF setting for care planning and defining resource use.
The proposed data element consists of the single Vision data element (Ability To See in Adequate Light) that consists of one question with five response categories. The Vision data element that we proposed for standardization was tested as part of the development of the MDS and is currently in use in that assessment in SNFs. Similar data elements, but with different wording and fewer response option categories, are in use in the OASIS. For more information on the Vision data element, we refer readers to the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
The Vision data element was first proposed as a standardized patient assessment data element in the FY 2018 IRF PPS proposed rule (82 FR 20738 through 20739).
In that proposed rule, we stated that the proposal was informed by input we received on the Ability to See in Adequate Light data element (version tested in the PAC PRD with three response categories) through a call for input published on the CMS Measures Management System Blueprint website. Although the data element in public comment differed from the proposed data element, input submitted from August 12 to September 12, 2016 supported assessing vision in PAC settings and the useful information a vision data element would provide.
We also stated that commenters had noted that the Ability to See item would provide important information that would facilitate care coordination and care planning, and consequently improve the quality of care. Other commenters suggested it would be helpful as an indicator of resource use and noted that the item would provide useful information about the abilities of patients and residents to care for themselves. Additional commenters noted that the item could feasibly be implemented across PAC providers and that its kappa scores from the PAC PRD support its validity. Some commenters noted a preference for MDS version of the Vision data element in SNFs over the form put forward in public comment, citing the widespread use of this data element. A summary report for the August 12 to September 12, 2016 public comment period titled “SPADE August 2016 Public Comment Summary Report” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In response to our proposal in the FY 2018 IRF PPS proposed rule, we received a comment supporting having a standardized patient assessment data element for vision across PAC settings, but it stated the proposed data element captures only basic information for risk adjustment, and more detailed information would need to be collected to use it as an outcome measure.
Subsequent to receiving comments on the FY 2018 IRF PPS rule, the Vision data element was included in the National Beta Test of candidate data elements conducted by our data element contractor from November 2017 to August 2018. Results of this test found the Vision data element to be feasible and reliable for use with PAC patients and residents. More information about the performance of the Vision data element in the National Beta Test can be found in the document titled “Final Specifications for IRF QRP Quality Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In addition, our data element contractor convened a TEP on January 5 and 6, 2017 for the purpose of soliciting input on all the SPADEs including the Vision data element. The TEP affirmed the importance of standardized assessment of vision impairment in PAC patients and residents. A summary of the January 5 and 6, 2017 TEP meeting titled “SPADE Technical Expert Panel Summary (Second Convening)” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We also held SODFs and small-group discussions with PAC providers and other stakeholders in 2018 for the purpose of updating the public about our ongoing SPADE development efforts. Finally, on November 27, 2018, our data element contractor hosted a public meeting of stakeholders to present the results of the National Beta Test and solicit additional comments. General input on the testing and item development process and concerns about burden were received from stakeholders during this meeting and via email through February 1, 2019. Additionally, a commenter noted support for the Vision data element and suggested administration at the beginning of the patient assessment to maximize utility. A summary of the public input received from the November 27, 2018 stakeholder meeting titled “Input on Standardized Patient Assessment Data Elements (SPADEs) Received After November 27, 2018 Stakeholder Meeting” is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
Due to the relatively stable nature of vision impairment, it is unlikely that a patient's score on this assessment would change between the start and end of the IRF stay. Therefore, we proposed that IRFs that submit the Vision data element with respect to admission will be deemed to have submitted with respect to both admissions and discharge.
Taking together the importance of assessing for vision, stakeholder input, and strong test results, we proposed that the Vision data element meets the definition of standardized patient assessment data with respect to impairments under section 1899B(b)(1)(B)(v) of the Act and to adopt the Vision data element as standardized patient assessment data for use in the IRF QRP.
Commenters submitted the following comments related to the proposed rule's discussion of the Vision data element.
Comment: A few commenters supported the collection of information on vision impairment. One of the commenters noted that the collection of information on vision impairment would support the identification and appropriate treatment of vision problems, which they stated were prevalent and undertreated.
Response: We thank the commenters for their support.
Comment: One commenter recommended that a doctor of optometry should play a lead role in conducting vision assessments, and that vision assessments done by other clinicians should also obtain the patient's own assessment of his or her vision, such as used by the Centers for Disease Control and Prevention (CDC) Behavioral Risk Factors Surveillance System survey, which questions patients “Do you have serious difficulty seeing, even when wearing glasses?” This commenter expressed concerns about the proposed SPADE being subjective and risks of mis-categorizing patients.
Response: We appreciate the commenter's recommendation about how to assess for vision impairment. We do not require that a certain type of clinician complete assessments; the SPADEs have been developed so that any clinician who is trained in the administration of the assessment will be able to administer it correctly. The proposed item relies on the assessor's evaluation of the patient's vision, which has the advantage of reducing burden placed on the patient. We will take the recommendation to use patient-reported vision impairment assessment into consideration in the development of future assessments.
Comment: Some commenters expressed concern that severely impaired vision occurs infrequently in IRF patients, thereby limiting the utility of the data collected.
Response: The Vision SPADE consists of one data element completed by the assessor based primarily on interacting with the patient and reviewing the medical record. Given the low burden of the Vision data element, and despite severe vision impairment occurring in a small proportion of IRF patients, we believe it is important to systematically assess for vision impairment in order to improve clinical care and care transitions.
Comment: A commenter recommended that CMS require a vision assessment at discharge, noting that vision impairment could be related to challenges in medication management and compliance with written follow-up instructions for care.
Response: We appreciate the commenter's feedback. We agree that adequate vision—or the accommodations and assistive technology needed to compensate for vision impairment—is important to patient safety in the community, in part for the reasons the commenter mentions. In the FY 2020 IRF PPS proposed rule (84 FR 17292), we proposed that IRFs that submitted the Vision SPADE with respect to admission will be deemed to have submitted with respect to both admission and discharge; we stated that it is unlikely that the assessment of this SPADEs at admission would differ from the assessment at discharge. Vision assessment, collected via the Vision SPADE with respect to admission, will provide information that will support the patient's care while in the IRF. Out of consideration for the burden of data collection, and with an understanding that significant clinical changes to a patient's vision will be documented in the medical record as part of routine clinical practice, we are finalizing our proposal that IRFs that submit the Vision SPADE with respect to admission will be deemed to have submitted with respect to both admission and discharge. We note that during the discharge planning process, it is incumbent on IRF providers to make reasonable assurances that the patient's needs will be met in the next care setting, including in the home.
Comment: One commenter recommended adding “unable to assess” as a response option, which the commenter believes would be the appropriate choice if the patient is comatose or is unable to effectively answer questions related to an assessment of their vision.
Response: We appreciate the commenter's recommendation. However, the assessment of vision is completed based on consulting with patient's family and other staff, observing the patient including requesting the patient to read text or examine pictures or numbers in addition to interviewing the patient about their vision abilities. These other sources/methods can be used to complete the assessment of vision when the patient is unable to effectively answer questions related to an assessment of their vision.
After careful consideration of the public comments we received, we are finalizing our proposal to adopt the Vision data element as standardized patient assessment data beginning with the FY 2022 IRF QRP as proposed.
4. New Category: Social Determinants of Health
a. Social Determinants of Health Data Collection To Inform Measures and Other Purposes
Section 2(d)(2)(A) of the IMPACT Act requires CMS to assess appropriate adjustments to quality measures, resource measures and other measures, and to assess and implement appropriate adjustments to payment under Medicare, based on those measures, after taking into account studies conducted by ASPE on social risk factors (described below) and other information, and based on an individual's health status and other factors. Paragraph (C) of section 2(d)(2) of the IMPACT Act further requires the Secretary to carry out periodic analyses, at least every 3 years, based on the factors referred to paragraph (A) so as to monitor changes in possible relationships. Paragraph (B) of section 2(d)(2) of the IMPACT Act requires CMS to collect or otherwise obtain access to data necessary to carry out the requirement of the paragraph (both assessing adjustments described above in such paragraph (A) and for periodic analyses in such paragraph (C)). Accordingly we proposed to use our authority under paragraph (B) of section 2(d)(2) of the IMPACT Act to establish a new data source for information to meet the requirements of paragraphs (A) and (C) of section 2(d)(2) of the IMPACT Act. In this rule, we proposed to collect and access data about social determinants of health (SDOH) in order to perform CMS' responsibilities under paragraphs (A) and (C) of section 2(d)(2) of the IMPACT Act, as explained in more detail below. Social determinants of health, also known as social risk factors, or health-related social needs, are the socioeconomic, cultural and environmental circumstances in which individuals live that impact their health. We proposed to collect information on seven proposed SDOH SPADE data elements relating to race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation; a detailed discussion of each of the proposed SDOH data elements is found in section VII.G.5.b. of this rule.
We also proposed to use the assessment instrument for the IRF QRP, the IRF-PAI, described as a PAC assessment instrument under section 1899B(a)(2)(B) of the Act, to collect these data via an existing data collection mechanism. We believe this approach will provide CMS with access to data with respect to the requirements of section 2(d)(2) of the IMPACT Act, while minimizing the reporting burden on PAC health care providers by relying on a data reporting mechanism already used and an existing system to which PAC health care providers are already accustomed.
The IMPACT Act includes several requirements applicable to the Secretary, in addition to those imposing new data reporting obligations on certain PAC providers as discussed in IX.G.4.b. of this final rule. Paragraphs (A) and (B) of sections 2(d)(1) of the IMPACT Act require the Secretary, acting through the Office of the Assistant Secretary for Planning and Evaluation (ASPE), to conduct two studies that examine the effect of risk factors, including individuals' socioeconomic status, on quality, resource use and other measures under the Medicare program. The first ASPE study was completed in December 2016 and is discussed below, and the second study is to be completed in the fall of 2019. We recognize that ASPE, in its studies, is considering a broader range of social risk factors than the SDOH data elements in this proposal, and address both PAC and non-PAC settings. We acknowledge that other data elements may be useful to understand, and that some of those elements may be of particular interest in non-PAC settings. For example, for beneficiaries receiving care in the community, as opposed to an in-patient facility, housing stability and food insecurity may be more relevant. We will continue to take into account the findings from both of ASPE's reports in future policy making.
One of the ASPE's first actions under the IMPACT Act was to commission the National Academies of Sciences, Engineering, and Medicine (NASEM) to define and conceptualize socioeconomic status for the purposes of ASPE's two studies under section 2(d)(1) of the IMPACT Act. The NASEM convened a panel of experts in the field and conducted an extensive literature review. Based on the information collected, the 2016 NASEM panel report titled, “Accounting for Social Risk Factors in Medicare Payment: Identifying Social Risk Factors”, concluded that the best way to assess how social processes and social relationships influence key health-related outcomes in Medicare beneficiaries is through a framework of social risk factors instead of socioeconomic status. Social risk factors discussed in the NASEM report include socioeconomic position, race, ethnicity, gender, social context, and community context. These factors are discussed at length in chapter 2 of the NASEM report, titled “Social Risk Factors.” [169] Consequently NASEM framed the results of its report in terms of “social risk factors” rather than “socioeconomic status” or “sociodemographic status.” The full text of the “Social Risk Factors” NASEM report is available for reading on the website at https://www.nap.edu/read/21858/chapter/1.
Each of the data elements we proposed to collect and access under our authority under section 2(d)(2)(B) of the IMPACT Act is identified in the 2016 NASEM report as a social risk factor that has been shown to impact care use, cost and outcomes for Medicare beneficiaries. CMS uses the term social determinants of health (SDOH) to denote social risk factors, which is consistent with the objectives of Healthy People 2020.[170]
ASPE issued its first Report to Congress, titled “Social Risk Factors and Performance Under Medicare's Value-Based Purchasing Programs,” under section 2(d)(1)(A) of the IMPACT Act on December 21, 2016.[171] Using NASEM's social risk factors framework, ASPE focused on the following social risk factors, in addition to disability: (1) Dual enrollment in Medicare and Medicaid as a marker for low income; (2) residence in a low-income area; (3) Black race; (4) Hispanic ethnicity; and (5) residence in a rural area. ASPE acknowledged that the social risk factors examined in its report were limited due to data availability. The report also noted that the data necessary to meaningfully attempt to reduce disparities and identify and reward improved outcomes for beneficiaries with social risk factors have not been collected consistently on a national level in PAC settings. Where these data have been collected, the collection frequently involves lengthy questionnaires. More information on the Report to Congress on Social Risk Factors and Performance under Medicare's Value-Based Purchasing Programs, including the full report, is available on the website at https://aspe.hhs.gov/social-risk-factors-and-medicares-value-based-purchasing-programs-reports.
Section 2(d)(2) of the IMPACT Act relates to CMS activities and imposes several responsibilities on the Secretary relating to quality, resource use, and other measures under Medicare. As mentioned previously, under paragraph (A) of section 2(d)(2) of the IMPACT Act, the Secretary is required, on an ongoing basis, taking into account the ASPE studies and other information, and based on an individual's health status and other factors, to assess appropriate adjustments to quality, resource use, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. Section 2(d)(2)(A)(i) of the IMPACT Act applies to measures adopted under sections (c) and (d) of section 1899B of the Act and to other measures under Medicare. However, CMS' ability to perform these analyses, and assess and make appropriate adjustments is hindered by limits of existing data collections on SDOH data elements for Medicare beneficiaries. In its first study in 2016, in discussing the second study, ASPE noted that information relating to many of the specific factors listed in the IMPACT Act, such as health literacy, limited English proficiency, and Medicare beneficiary activation, are not available in Medicare data.
Paragraph 2(d)(2)(A) of the IMPACT Act specifically requires the Secretary to take the studies and considerations from ASPE's reports to Congress, as well as other information as appropriate, into account in assessing and implementing adjustments to measures and related payments based on measures in Medicare. The results of the ASPE's first study demonstrated that Medicare beneficiaries with social risk factors tended to have worse outcomes on many quality measures, and providers who treated a disproportionate share of beneficiaries with social risk factors tended to have worse performance on quality measures. As a result of these findings, ASPE suggested a three-pronged strategy to guide the development of value-based payment programs under which all Medicare beneficiaries receive the highest quality healthcare services possible. The three components of this strategy are to: (1) Measure and report quality of care for beneficiaries with social risk factors; (2) set high, fair quality standards for care provided to all beneficiaries; and (3) reward and support better outcomes for beneficiaries with social risk factors. In discussing how measuring and reporting quality for beneficiaries with social risk factors can be applied to Medicare quality payment programs, the report offered nine considerations across the three-pronged strategy, including enhancing data collection and developing statistical techniques to allow measurement and reporting of performance for beneficiaries with social risk factors on key quality and resource use measures.
Congress, in section 2(d)(2)(B) of the IMPACT Act, required the Secretary to collect or otherwise obtain access to the data necessary to carry out the provisions of paragraph (2) of section 2(d) of the IMPACT Act through both new and existing data sources. Taking into consideration NASEM's conceptual framework for social risk factors discussed above, ASPE's study, and considerations under section 2(d)(1)(A) of the IMPACT Act, as well as the current data constraints of ASPE's first study and its suggested considerations, we proposed to collect and access data about SDOH under section 2(d)(2) of the IMPACT Act. Our collection and use of the SDOH data described in section IX.G.4.b. of this final rule, under section 2(d)(2) of the IMPACT Act would be independent of our proposal below (in section IX.G.4.b. of this final rule) and our authority to require submission of that data for use as SPADE under section 1899B(a)(1)(B) of the Act.
Accessing standardized data relating to the SDOH data elements on a national level is necessary to permit CMS to conduct periodic analyses, to assess appropriate adjustments to quality measures, resource use measures, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. We agree with ASPE's observations, in the value-based purchasing context, that the ability to measure and track quality, outcomes, and costs for beneficiaries with social risk factors over time is critical as policymakers and providers seek to reduce disparities and improve care for these groups. Collecting the data as proposed will provide the basis for our periodic analyses of the relationship between an individual's health status and other factors and quality, resource use, and other measures, as required by section 2(d)(2) of the IMPACT Act, and to assess appropriate adjustments. These data will also permit us to develop the statistical tools necessary to maximize the value of Medicare data, reduce costs and improve the quality of care for all beneficiaries. Collecting and accessing SDOH data in this way also supports the three-part strategy put forth in the first ASPE report, specifically ASPE's consideration to enhance data collection and develop statistical techniques to allow measurement and reporting of performance for beneficiaries with social risk factors on key quality and resource use measures.
For the reasons discussed above, we proposed under section 2(d)(2) of the IMPACT Act, to collect the data on the following SDOH: (1) Race, as described in section VII.G.4.b.(1) of this rule; (2) Ethnicity, as described in section VII.G.4.b.(1) of this rule; (3) Preferred Language, as described in section VII.G.4.b.(2) of this rule; (4) Interpreter Services, as described in section VII.G.4.b.(2) of this rule; (5) Health Literacy, as described in section VII.G.4.b.(3) of this rule; (6) Transportation, as described in section VII.G.4.b.(4) of this rule; and (7) Social Isolation, as described in section VII.G.4.b.(5) of this rule. These data elements are discussed in more detail below in section VII.G.4.b of this rule. A detailed discussion of the comments we received, along with our responses is included in each section.
b. Standardized Patient Assessment Data
Section 1899B(b)(1)(B)(vi) of the Act authorizes the Secretary to collect SPADEs with respect to other categories deemed necessary and appropriate. Below we proposed to create a Social Determinants of Health SPADE category under section 1899B(b)(1)(B)(vi) of the Act. In addition to collecting SDOH data for the purposes outlined above under section 2(d)(2)(B), we also proposed to collect as SPADE these same data elements (race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation) under section 1899B(b)(1)(B)(vi) of the Act. We believe that this proposed new category of Social Determinants of Health will inform provider understanding of individual patient risk factors and treatment preferences, facilitate coordinated care and care planning, and improve patient outcomes. We proposed to deem this category necessary and appropriate, for the purposes of SPADE, because using common standards and definitions for PAC data elements is important in ensuring interoperable exchange of longitudinal information between PAC providers and other providers to facilitate coordinated care, continuity in care planning, and the discharge planning process from PAC settings.
All of the Social Determinants of Health data elements we proposed under section 1899B(b)(1)(B)(vi) of the Act have the capacity to take into account treatment preferences and care goals of patients, and to inform our understanding of patient complexity and risk factors that may affect care outcomes. While acknowledging the existence and importance of additional social determinants of health, we proposed to assess some of the factors relevant for patients receiving PAC that PAC settings are in a position to impact through the provision of services and supports, such as connecting patients with identified needs with transportation programs, certified interpreters, or social support programs.
We proposed to adopt the following seven data elements as SPADE under the proposed Social Determinants of Health category: Race, ethnicity, preferred language, interpreter services, health literacy, transportation, and social isolation. To select these data elements, we reviewed the research literature, a number of validated assessment tools and frameworks for addressing SDOH currently in use (for example, Health Leads,[172] NASEM, Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE), and ICD-10), and we engaged in discussions with stakeholders. We also prioritized balancing the reporting burden for PAC providers with our policy objective to collect SPADEs that will inform care planning and coordination and quality improvement across care settings. Furthermore, incorporating SDOH data elements into care planning has the potential to reduce readmissions and help beneficiaries achieve and maintain their health goals.
We also considered feedback received during a listening session that we held on December 13, 2018. The purpose of the listening session was to solicit feedback from health systems, research organizations, advocacy organizations and state agencies and other members of the public on collecting patient-level data on SDOH across care settings, including consideration of race, ethnicity, spoken language, health literacy, social isolation, transportation, sex, gender identity, and sexual orientation. We also gave participants an option to submit written comments. A full summary of the listening session, titled “Listening Session on Social Determinants of Health Data Elements: Summary of Findings,” includes a list of participating stakeholders and their affiliations, and is available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
We solicited comment on these proposals.
Commenters submitted the following comments related to the proposed rule's discussion of SDOH SPADEs. A discussion of these comments, along with our responses, appears below.
Comment: One commenter supported the incorporation of SDOH in the IRF QRP, in the interest of promoting access and assuring high-quality care for all beneficiaries. The commenter also encouraged CMS to be mindful of meaningful data collection and the potential impact for data overload. Since SDOH have impacts far beyond the post‐acute care setting, the commenter cautioned data collection that cannot be readily gathered, shared, or replicated beyond the PAC setting.
The commenter also encouraged CMS to consider leveraging data points collected during primary care visits by using social risk factor data captured during those encounters. They pointed out that the ability to have a hospital's or physician's EHR also collect, capture, and exchange segments of this information is powerful. The commenter recommended that CMS take a holistic view of SDOH across the care continuum so that all care settings may gather, collect or leverage this data efficiently and in way that maximizes its impact.
Response: We agree that collecting SDOH data elements can be useful in identifying and addressing health disparities. We also agree that CMS should be mindful that data elements selected are useful. The proposed SDOH SPADEs are aligned with SDOH identified in the 2016 NASEM report, which was commissioned by ASPE. Regarding the commenter's suggestion that CMS consider how it can align existing and future SDOH data collection to minimize burden on providers, we agree that it is important to minimize duplication of effort and will take this under advisement for future policy development.
Comment: One commenter recommended that CMS consider admission assessment for certain SPADEs as also fulfilling the discharge assessment requirement. The commenter supported the inclusion of the SDOH SPADEs and recommended that CMS require these items be assessed at some point during the patient's stay instead of during the admission assessment time window. The commenter recommended that any SDOH SPADES finalized should be assessed at any point during the stay.
Response: We disagree with the commenters regarding SDOH SPADES should be assessed at any point during the stay. Each of the SDOH SPADE data elements will assist with care planning when the patient is admitted. It is important for providers to identify a patient's needs in order to better inform the patient's care decisions made during and after the stay, including a patient's unique risk factors and treatment preferences.
Comment: Commenters were generally in favor of the concept of collecting SDOH data elements and provided that, if implemented appropriately, the data could be useful in identifying and addressing health care disparities, as well as refining the risk adjustment of outcome measures. However, some of the commenters suggested CMS not to finalize the proposed policy until CMS can address important issues around the potential future uses of these elements and the requirements around data collection for certain elements. The commenters provided that CMS did not state explicitly in the rule whether it anticipates the SDOH SPADEs will be used in adjusting measures and believe that the IMPACT Act's requirements make it likely the SPADEs will be considered for use in future adjustments. The commenters recommended CMS to be circumspect and transparent in its approaches to incorporating the data elements proposed in payment and quality adjustments, such as by collecting stakeholder feedback before implementing any adjustments.
Response: We appreciate the commenters for recognizing that collecting SDOH data elements can be useful in identifying and address health disparities. We intend to use this data to assess the impact that the social determinants of health have on health outcomes. We will continue to work with stakeholders to promote transparency and support providers who serve vulnerable populations, promote high quality care, and refine and further implement SDOH SPADE. We appreciate the comment on collecting stakeholder feedback before implementing any adjustments to measures based on the SDOH SPADE. Collection of this data will help us in identifying potential disparities, conducting analyses, and assessing whether any adjustments are needed. Any future policy development based on this data would be done transparently, and involve solicitation of stakeholder feedback through the notice and comment rulemaking process as appropriate.
Comment: Several commenters recommended that CMS include disability status as a SDOH that contributes to overall patient access to care, health status, outcomes, and many other determinants of health since it is already included in some Medicare risk adjustment. The commenters stated that ASPE's report to Congress entitled “Social Risk Factors and Performance Under Medicare's Value-Based Purchasing Programs” reported that disability is an independent predictor of poor mental and physical health outcomes and that individuals with disabilities may receive lower-quality preventive care.
Response: We appreciate the comments and suggestions provided by the commenters. We agree that it is important to understand and meet the needs of patients with disabilities. While disability is not being currently assessed through the SPADE, it is comprehensively assessed as part of existing protocols around care plans and health goals. However, as we continue to evaluate SDOH SPADEs, we will keep commenters' feedback in mind and may consider these suggestions in future rulemaking.
Comment: One commenter supported CMS's proposal to collect SDOH data within SPADEs but was concerned that all of these new elements may be burdensome. The commenter recommended that CMS require data collection on race, ethnicity, preferred language, and interpreter services, and make data collection on health literacy, transportation, and social isolation voluntary for now and have the requirement phased into future rulemaking. The commenter noted that this would give IRFs an opportunity to adjust to the new data collection methods, while signaling their importance as entities that are currently collecting information on SDOH are experiencing various workflow, privacy, and other challenges. The commenter recommended that CMS consider including the collection of housing status in the future as individuals with unmet housing needs, such as homelessness or substandard housing, have higher health care costs and can be at risk for readmissions.
Response: We thank the commenter for their comment. As discussed above, section 2(d)(2)(B) of the IMPACT Act requires the Secretary to collect or otherwise obtain access to the data necessary to carry out the provisions of paragraph (2) of section 2(d) of the IMPACT Act through both new and existing data sources. Accessing standardized data relating to the SDOH data elements on a national level is necessary to permit CMS to conduct periodic analyses, to assess appropriate adjustments to quality measures, resource use measures, and other measures, and to assess and implement appropriate adjustments to Medicare payments based on those measures. Collecting the data as proposed will provide the basis for our periodic analyses of the relationship between an individual's health status and other factors and quality, resource use, and other measures, as required by section 2(d)(2) of the IMPACT Act, and to assess appropriate adjustments. Regarding the suggestion that CMS consider a housing status SPADE data element in future rulemaking efforts, we appreciate this feedback and will consider this suggestion in future rulemaking efforts on SPADE SDOH data elements.
(1) Race and Ethnicity
The persistence of racial and ethnic disparities in health and health care is widely documented, including in PAC settings.[173 174 175 176 177] Despite the trend toward overall improvements in quality of care and health outcomes, the Agency for Healthcare Research and Quality, in its National Healthcare Quality and Disparities Reports, consistently indicates that racial and ethnic disparities persist, even after controlling for factors such as income, geography, and insurance.[178] For example, racial and ethnic minorities tend to have higher rates of infant mortality, diabetes and other chronic conditions, and visits to the emergency department, and lower rates of having a usual source of care and receiving immunizations such as the flu vaccine.[179] Studies have also shown that African Americans are significantly more likely than white Americans to die prematurely from heart disease and stroke.[180] However, our ability to identify and address racial and ethnic health disparities has historically been constrained by data limitations, particularly for smaller populations groups such as Asians, American Indians and Alaska Natives, and Native Hawaiians and other Pacific Islanders.[181]
The ability to improve understanding of and address racial and ethnic disparities in PAC outcomes requires the availability of better data. There is currently a Race and Ethnicity data element, collected in the MDS, LCDS, IRF-PAI, and OASIS, that consists of a single question, which aligns with the 1997 Office of Management and Budget (OMB) minimum data standards for federal data collection efforts.[182] The 1997 OMB Standard lists five minimum categories of race: (1) American Indian or Alaska Native; (2) Asian; (3) Black or African American; (4) Native Hawaiian or Other Pacific Islander; (5) and White. The 1997 OMB Standard also lists two minimum categories of ethnicity: (1) Hispanic or Latino; and (2) Not Hispanic or Latino. The 2011 HHS Data Standards requires a two-question format when self-identification is used to collect data on race and ethnicity. Large federal surveys such as the National Health Interview Survey, Behavioral Risk Factor Surveillance System, and the National Survey on Drug Use and Health, have implemented the 2011 HHS race and ethnicity data standards. CMS has similarly updated the Medicare Current Beneficiary Survey, Medicare Health Outcomes Survey, and the Health Insurance Marketplace Application for Health Coverage with the 2011 HHS data standards. More information about the HHS Race and Ethnicity Data Standards are available on the website at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=54.
We proposed to revise the current Race and Ethnicity data element for purposes of this proposal to conform to the 2011 HHS Data Standards for person-level data collection, while also meeting the 1997 OMB minimum data standards for race and ethnicity. Rather than one data element that assesses both race and ethnicity, we proposed two separate data elements: One for Race and one for Ethnicity, that would conform with the 2011 HHS Data Standards and the 1997 OMB Standard. In accordance with the 2011 HHS Data Standards a two-question format would be used for the proposed race and ethnicity data elements.
The proposed Race data element asks, “What is your race? We proposed to include fourteen response options under the race data element: (1) White; (2) Black or African American; (3) American Indian or Alaska Native; (4) Asian Indian; (5) Chinese; (6) Filipino; (7) Japanese; (8) Korean; (9) Vietnamese; (10) Other Asian; (11) Native Hawaiian; (12) Guamanian or Chamorro; (13) Samoan; and (14) Other Pacific Islander.
The proposed Ethnicity data element asks, “Are you Hispanic, Latino/a, or Spanish origin?” We proposed to include five response options under the ethnicity data element: (1) Not of Hispanic, Latino/a, or Spanish origin; (2) Mexican, Mexican American, Chicano/a; (3) Puerto Rican; (4) Cuban; and (5) Another Hispanic, Latino, or Spanish Origin. We are including the addition of “of” to the Ethnicity data element to read, “Are you of Hispanic, Latino/a, or Spanish origin?”
We believe that the two proposed data elements for race and ethnicity conform to the 2011 HHS Data Standards for person-level data collection, while also meeting the 1997 OMB minimum data standards for race and ethnicity, because under those standards, more detailed information on population groups can be collected if those additional categories can be aggregated into the OMB minimum standard set of categories.
In addition, we received stakeholder feedback during the December 13, 2018 SDOH listening session on the importance of improving response options for race and ethnicity as a component of health care assessments and for monitoring disparities. Some stakeholders emphasized the importance of allowing for self-identification of race and ethnicity for more categories than are included in the 2011 HHS Standard to better reflect state and local diversity, while acknowledging the burden of coding an open-ended health care assessment question across different settings.
We believe that the proposed modified race and ethnicity data elements more accurately reflect the diversity of the U.S. population than the current race/ethnicity data element included in MDS, LCDS, IRF-PAI, and OASIS.[183 184 185 186] We believe, and research consistently shows, that improving how race and ethnicity data are collected is an important first step in improving quality of care and health outcomes. Addressing disparities in access to care, quality of care, and health outcomes for Medicare beneficiaries begins with identifying and analyzing how SDOH, such as race and ethnicity, align with disparities in these areas.[187] Standardizing self-reported data collection for race and ethnicity allows for the equal comparison of data across multiple healthcare entities.[188] By collecting and analyzing these data, CMS and other healthcare entities will be able to identify challenges and monitor progress. The growing diversity of the U.S. population and knowledge of racial and ethnic disparities within and across population groups supports the collection of more granular data beyond the 1997 OMB minimum standard for reporting categories. The 2011 HHS race and ethnicity data standard includes additional detail that may be used by PAC providers to target quality improvement efforts for racial and ethnic groups experiencing disparate outcomes. For more information on the Race and Ethnicity data elements, we refer readers to the document titled “Final Specifications for IRF QRP Measures and Standardized Patient Assessment Data Elements,” available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of race and ethnicity data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Race and Ethnicity data elements described above as SPADEs with respect to the proposed Social Determinants of Health category.
Specifically, we proposed to replace the current Race/Ethnicity data element with the proposed Race and Ethnicity data elements on the IRF-PAI. We also proposed that IRFs that submit the Race and Ethnicity data elements with respect to admission will be considered to have submitted with respect to discharge as well, because it is unlikely that the results of these assessment findings will change between the start and end of the IRF stay, making the information submitted with respect to a patient's admission the same with respect to a patient's discharge.
We solicited comment on these proposals.
Commenters submitted the following comments related to the proposed rule's discussion of the Race and Ethnicity SPADEs. A discussion of these comments, along with our responses, appears below.
Comment: Some commenters noted that the response options for race do not align with those used in other government data, such as the U.S. Census or the Office of Management and Budget (OMB). The commenters also stated these responses are not consistent with the recommendations made in the 2009 Institute of Medicine report. The commenters pointed out that IOM report recommended using broader OMB race categories and granular ethnicities chosen from a national standard set that can be “rolled up” into the broader categories. The commenters stated that it is unclear how CMS chose the 14 response options under the race data element and the five options under the ethnicity element and worried that these response options would add to the confusion that already may exist for patients about what terms like “race” and “ethnicity” mean for the purposes of health care data collection. The commenters also noted that CMS should confer directly with experts on the issue to ensure patient assessments are collecting the right data in the right way before these SDOH SPADEs are finalized.
Response: The proposed Race and Ethnicity categories align with and are rolled up into the 1997 OMB minimum data standards and conforming with the 2011 HHS Data Standards as described in the implementation guidance titled “U.S. Department of Health and Human Services Implementation Guidance on Data Collection Standards for Race, Ethnicity, Sex, Primary Language, and Disability Status” at https://aspe.hhs.gov/basic-report/hhs-implementation-guidance-data-collection-standards-race-ethnicity-sex-primary-language-and-disability-status. As stated in the proposed rule, the 14 race categories and the 5 ethnicity categories conform with the 2011 HHS Data Standards for person-level data collection, which were developed in fulfillment of section 4302 of the Affordable Care Act that required the Secretary of HHS to establish data collection standards for race, ethnicity, sex, primary language, and disability status. Through the HHS Data Council, which is the principal, senior internal Departmental forum and advisory body to the Secretary on health and human services data policy and coordinates HHS data collection and analysis activities, the Section 4302 Standards Workgroup was formed. The Workgroup included representatives from HHS, the OMB, and the Census Bureau. The Workgroup examined current federal data collection standards, adequacy of prior testing, and quality of the data produced in prior surveys; consulted with statistical agencies and programs; reviewed OMB data collection standards and the Institute of Medicine (IOM) Report Race, Ethnicity, and Language Data Collection: Standardization for Health Care Quality Improvement; sought input from national experts; and built on its members' experience with collecting and analyzing demographic data. As a result of this Workgroup, a set of data collection standards were developed, and then published for public comment. This set of data collection standards is referred to as the 2011 HHS Data Standards.[189] As described in the implementation guidance provided above, the categories of race and ethnicity under the 2011 HHS Data Standards allow for more detailed information to be collected and the additional categories under the 2011 HHS Data Standards can be aggregated into the OMB minimum standards set of categories.
As noted in the FY 2020 IRF PPS proposed rule (84 FR 17321 through 17323), we conferred with experts by conducting a listening session regarding the proposed SDOH data elements regarding the importance of improving response options for race and ethnicity as a component of health care assessments and for monitoring disparities. Some stakeholders emphasized the importance of allowing for self-identification of race and ethnicity for more categories than are included in the 2011 HHS Data Standards to better reflect state and local diversity.
Comment: A commenter recommended that CMS consider the implications of having PAC providers collect Race and Ethnicity codes that vary from the Race and Ethnicity codes collected by other healthcare providers, specifically acute-care hospitals. The commenter noted that unless all care providers are expected to utilize the uniform 2011 HHS Data Standards, the consistency and accuracy of race and ethnicity data across settings will likely be unreliable and problematic. Another commenter provided that the proposed list of response options for Race may not include all races that should be reflected, for example, Native African and Middle Eastern. In addition, the item should include “check all that apply” to ensure accurate and complete data collection. The commenter encouraged CMS to refine the list of response options for Race and provide a rationale for the final list of response options.
Response: We thank the commenter and agree that it is important to collect race and ethnicity data in a consistent way. The race and ethnicity categories that were proposed align with the 2011 HHS Data Standards and are rolled up into the 1997 OMB minimum data standards, which can be found at https://aspe.hhs.gov/basic-report/hhs-implementation-guidance-data-collection-standards-race-ethnicity-sex-primary-language-and-disability-status. For example, the 1997 OMB minimum data standard for Hispanic is the roll up category for the following response options on the 2011 HHS Data Standards: Mexican, Mexican American, Chicano/a; Puerto Rican; Cuban; another Hispanic, Latino, or Spanish origin. However, we will take the comment under advisement for future consideration. We also note that the option for “check all that apply” is available for providers to choose from the list of response options.
Comment: A commenter supported the opportunities to better account for SDOH in the diagnosis and treatment of patients but is concerned by the specificity of several of the seven proposed element for data collection for example, collection of race by Japanese, Chinese, Korean, etc. The commenter's concern is with the added burden in collecting the level of specificity outlined, and the commenter requested that CMS provide more detailed guidance in the final rule regarding how this information should be collected and shared in compliance with HIPAA. Further, the commenter asked that the agency outlines its expectations for how this newly collected information will be used by Medicare for payment and public reporting.
Response: For the Race and Ethnicity SPADE, this data should be completed based on the response of the patient. It is important to ask the patient to select the category or categories that most closely correspond to their race and ethnicity. Respondents should be offered the option of selecting one or more race and ethnicity categories. Observer identification or medical record documentation may not be used.
The SDOH data elements that will be collected will assist with care coordination and with evaluating the impact of disparities. With respect to how the data will be used for payment and public reporting, any potential future use of the data for these purposes would be done through future rulemaking.
SDOH data elements should be treated the same as other data collected on the assessment tool. As to any specific HIPAA questions, we appreciate the commenter's commitment to compliance with the HIPAA requirements, but note that the Office for Civil Rights (OCR) is tasked with implementing and enforcing HIPAA, not CMS. Commenters should consult appropriate counsel in instances in which they are unsure of their HIPAA status, or the permissibility of a disclosure under the HIPAA Privacy Rule. In doing so, commenters may wish to consult 45 CFR 164.103 (definition of “required by law”) and § 164.512(a) (allowing “required by law” disclosures).
(2) Preferred Language and Interpreter Services
More than 64 million Americans speak a language other than English at home, and nearly 40 million of those individuals have limited English proficiency (LEP).[190] Individuals with LEP have been shown to receive worse care and have poorer health outcomes, including higher readmission rates.[191 192 193] Communication with individuals with LEP is an important component of high quality health care, which starts by understanding the population in need of language services. Unaddressed language barriers between a patient and provider care team negatively affects the ability to identify and address individual medical and non-medical care needs, to convey and understand clinical information, as well as discharge and follow up instructions, all of which are necessary for providing high quality care. Understanding the communication assistance needs of patients with LEP, including individuals who are Deaf or hard of hearing, is critical for ensuring good outcomes.
Presently, the preferred language of patients and residents and need for interpreter services are assessed in two PAC assessment tools. The LCDS and the MDS use the same two data elements to assess preferred language and whether a patient or resident needs or wants an interpreter to communicate with health care staff. The MDS initially implemented preferred language and interpreter services data elements to assess the needs of SNF residents and patients and inform care planning. For alignment purposes, the LCDS later adopted the same data elements for LTCHs. The 2009 NASEM (formerly Institute of Medicine) report on standardizing data for health care quality improvement emphasizes that language and communication needs should be assessed as a standard part of health care delivery and quality improvement strategies.[194]
In developing our proposal for a standardized language data element across PAC settings, we considered the current preferred language and interpreter services data elements that are in LCDS and MDS. We also considered the 2011 HHS Primary Language Data Standard and peer-reviewed research. The current preferred language data element in LCDS and MDS asks, “What is your preferred language?” Because the preferred language data element is open-ended, the patient or resident is able to identify their preferred language, including American Sign Language (ASL). Finally, we considered the recommendations from the 2009 NASEM (formerly Institute of Medicine) report, “Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement.” In it, the committee recommended that organizations evaluating a patient's language and communication needs for health care purposes, should collect data on the preferred spoken language and on an individual's assessment of his/her level of English proficiency.
A second language data element in LCDS and MDS asks, “Do you want or need an interpreter to communicate with a doctor or health care staff?” and includes yes or no response options. In contrast, the 2011 HHS Primary Language Data Standard recommends either a single question to assess how well someone speaks English or, if more granular information is needed, a two-part question to assess whether a language other than English is spoken at home and if so, identify that language. However, neither option allows for a direct assessment of a patient's or resident's preferred spoken or written language nor whether they want or need interpreter services for communication with a doctor or care team, both of which are an important part of assessing patient/resident needs and the care planning process. More information about the HHS Data Standard for Primary Language is available on the website at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=54.
Research consistently recommends collecting information about an individual's preferred spoken language and evaluating those responses for purposes of determining language access needs in health care.[195] However, using “preferred spoken language” as the metric does not adequately account for people whose preferred language is ASL, which would necessitate adopting an additional data element to identify visual language. The need to improve the assessment of language preferences and communication needs across PAC settings should be balanced with the burden associated with data collection on the provider and patient. Therefore we proposed to retain the Preferred Language and Interpreter Services data elements currently in use on the MDS and LCDS on the IRF-PAI.
In addition, we received feedback during the December 13, 2018 listening session on the importance of evaluating and acting on language preferences early to facilitate communication and allowing for patient self-identification of preferred language. Although the discussion about language was focused on preferred spoken language, there was general consensus among participants that stated language preferences may or may not accurately indicate the need for interpreter services, which supports collecting and evaluating data to determine language preference, as well as the need for interpreter services. An alternate suggestion was made to inquire about preferred language specifically for discussing health or health care needs. While this suggestion does allow for ASL as a response option, we do not have data indicating how useful this question might be for assessing the desired information and thus we are not including this question in our proposal.
Improving how preferred language and need for interpreter services data are collected is an important component of improving quality by helping PAC providers and other providers understand patient needs and develop plans to address them. For more information on the Preferred Language and Interpreter Services data elements, we refer readers to the document titled “Final Specifications for IRF QRP Measures and Standardized Patient Assessment Data Elements,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of language data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Preferred Language and Interpreter Services data elements currently used on the MDS and LCDS, and described above, as SPADEs with respect to the Social Determinants of Health category. We proposed to add the current Preferred Language and Interpreter Services data elements from the MDS and LCDS to the IRF-PAI.
We solicited comment on these proposals.
Commenters submitted the following comments related to the proposed rule's discussion of Preferred Language and Interpreter Services SPADEs. A discussion of these comments, along with our responses, appears below.
Comment: Some commenters noted that, if finalized, IRFs should only need to submit data on the race and ethnicity SPADEs with respect to admission and would not need to collect and report again at discharge, as it is unlikely that patient status for these elements will change. The commenters believe that a patient's preferred language and need for an interpreter also are unlikely to change between admission and discharge; thus, the commenter urged CMS to require collection of these SDOH SPADEs with respect to admission only.
Response: We thank the commenters for the comment. With regard to the submission of the Preferred Language SPADE and the Interpreter Services SPADE, we agree with the commenters that it is unlikely that the assessment of Preferred Language and Interpreter Services at admission would differ from assessment at discharge. As discussed in previous response for Vision and Hearing, we believe that the submission of preferred language and the need for an interpreter is similar to the submission of Race, Ethnicity, Hearing, and Vision SPADES.
We account for this change to the Collection of Information requirements for the IRF QRP in XIV.C of this final rule. Based on the comments received, and for the reasons discussed, we are finalizing that the Preferred Language and Interpreter Services SPADEs be collected as proposed with the modification that we will deem IRFs that submit these two SPADEs with respect to admission to have submitted with respect to both admission and discharge.
(3) Health Literacy
The Department of Health and Human Services defines health literacy as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.” [196] Similar to language barriers, low health literacy can interfere with communication between the provider and patient and the ability for patients or their caregivers to understand and follow treatment plans, including medication management. Poor health literacy is linked to lower levels of knowledge about health, worse health outcomes, and the receipt of fewer preventive services, but higher medical costs and rates of emergency department use.[197]
Health literacy is prioritized by Healthy People 2020 as an SDOH.[198] Healthy People 2020 is a long-term, evidence-based effort led by the Department of Health and Human Services that aims to identify nationwide health improvement priorities and improve the health of all Americans. Although not designated as a social risk factor in NASEM's 2016 report on accounting for social risk factors in Medicare payment, the NASEM noted that health literacy is impacted by other social risk factors and can affect access to care, as well as quality of care and health outcomes.[199] Assessing for health literacy across PAC settings would facilitate better care coordination and discharge planning. A significant challenge in assessing the health literacy of individuals is avoiding excessive burden on patients and health care providers. The majority of existing, validated health literacy assessment tools use multiple screening items, generally with no fewer than four, which would make them burdensome if adopted in MDS, LCDS, IRF-PAI, and OASIS. The Single Item Literacy Screener (SILS) question questions, “How often do you need to have someone help you when you read instructions, pamphlets, or other written material from your doctor or pharmacy?” Possible response options are: (1) Never; (2) Rarely; (3) Sometimes; (4) Often; and (5) Always. The SILS question, which assesses reading ability, (a primary component of health literacy), tested reasonably well against the 36 item Short Test of Functional Health Literacy in Adults (S-TOFHLA), a thoroughly vetted and widely adopted health literacy test, in assessing the likelihood of low health literacy in an adult sample from primary care practices participating in the Vermont Diabetes Information System.[200 201] The S-TOFHLA is a more complex assessment instrument developed using actual hospital related materials such as prescription bottle labels and appointment slips, and often considered the instrument of choice for a detailed evaluation of health literacy.[202] Furthermore, the S-TOFHLA instrument is proprietary and subject to purchase for individual entities or users.[203] Given that SILS is publicly available, shorter and easier to administer than the full health literacy screen, and research found that a positive result on the SILS demonstrates an increased likelihood that an individual has low health literacy, we proposed to use the single-item reading question for health literacy in the standardized data collection across PAC settings. We believe that use of this data element will provide sufficient information about the health literacy of IRF patients to facilitate appropriate care planning, care coordination, and interoperable data exchange across PAC settings.
In addition, we received feedback during the December 13, 2018 SDOH listening session on the importance of recognizing health literacy as more than understanding written materials and filling out forms, as it is also important to evaluate whether patients understand their conditions. However, the NASEM recently recommended that health care providers implement health literacy universal precautions instead of taking steps to ensure care is provided at an appropriate literacy level based on individualized assessment of health literacy.[204] Given the dearth of Medicare data on health literacy and gaps in addressing health literacy in practice, we recommend the addition of a health literacy data element.
The proposed Health Literacy data element is consistent with considerations raised by NASEM and other stakeholders and research on health literacy, which demonstrates an impact on health care use, cost, and outcomes.[205] For more information on the proposed Health Literacy data element, we refer readers to the document titled “Final Specifications for IRF QRP Measures and Standardized Patient Assessment Data Elements,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of health literacy data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt SILS question described above for the Health Literacy data element as SPADE under the Social Determinants of Health Category. We proposed to add the Health Literacy data element to the IRF-PAI.
We solicited comment on this proposals. A discussion of these comments, along with our responses, appears below.
Comment: Some commenters noted that, if finalized, IRFs should only need to submit data on the race and ethnicity SPADEs with respect to admission and would not need to collect and report again at discharge, as it is unlikely that patient status for these elements will change. The commenters believe that a patient's health literacy is unlikely to change between admission and discharge; thus, the commenter urged CMS to require collection of all SDOH SPADEs with respect to admission only.
Response: We disagree with the commenters that it is unlikely patient status for health literacy will change from admission to discharge. Unlike the Vision, Hearing, Race, Ethnicity, Preferred Language, and Interpreter Services SPADEs, we believe that the response to this data element may change from admission to discharge for some patients. Health literacy can impact a patient's ability to manage their conditions, and it something that should be taken into account when developing care plans. The collection of the Health Literacy SPADE at discharge is to support patients, whose circumstances may have changed over the duration of their admission, in having the appropriate supports post-discharge. Therefore, the health literacy data element should be collected at both admission and discharge given the impact this could have on health outcomes and care planning.
Comment: One commenter stated that the health literacy question could be improved to capture whether the patient can read, understand, and implement/respond to the information. In addition, the commenter stated that the question does not take into account whether a patient's need for help is due to limited vision, which is different from the purpose of the separate Vision Impairment data element. Another possible question the commenter suggested was “How often do you have difficulty?” The commenter suggested that a single construct may not be sufficient for this area, depending on the aspect of health literacy that CMS intends to identify.
Response: We thank the commenters for the comment on the health literacy data element. We agree that knowing whether a patient has a reading or comprehension challenge, or limited vision would be helpful. However, we specifically proposed data elements that have been tested. We were also mindful to try and limit the potential burden of asking additional questions related to health literacy. The SILS Health Literacy data element that we proposed performed well when tested, and it minimizes concerns related to burden by requiring one instead of multiple questions on health literacy.[206 207] If commenters have examples of SDOH questions that have been cognitively tested, we would welcome that feedback as we seek to refine SDOH SPADE data elements in future rulemaking.
(4) Transportation
Transportation barriers commonly affect access to necessary health care, causing missed appointments, delayed care, and unfilled prescriptions, all of which can have a negative impact on health outcomes.[208] Access to transportation for ongoing health care and medication access needs, particularly for those with chronic diseases, is essential to successful chronic disease management. Adopting a data element to collect and analyze information regarding transportation needs across PAC settings would facilitate the connection to programs that can address identified needs. We therefore proposed to adopt as SPADE a single transportation data element that is from the Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences (PRAPARE) assessment tool and currently part of the Accountable Health Communities (AHC) Screening Tool.
The proposed Transportation data element from the PRAPARE tool questions, “Has lack of transportation kept you from medical appointments, meetings, work, or from getting things needed for daily living?” The three response options are: (1) Yes, it has kept me from medical appointments or from getting my medications; (2) Yes, it has kept me from non-medical meetings, appointments, work, or from getting things that I need; and (3) No. The patient would be given the option to select all responses that apply. We proposed to use the transportation data element from the PRAPARE Tool, with permission from National Association of Community Health Centers (NACHC), after considering research on the importance of addressing transportation needs as a critical SDOH.[209]
The proposed data element is responsive to research on the importance of addressing transportation needs as a critical SDOH and would adopt the Transportation item from the PRAPARE tool.[210] This data element comes from the national PRAPARE social determinants of health assessment protocol, developed and owned by NACHC, in partnership with the Association of Asian Pacific Community Health Organization, the Oregon Primary Care Association, and the Institute for Alternative Futures. Similarly the Transportation data element used in the AHC Screening Tool was adapted from the PRAPARE tool. The AHC screening tool was implemented by the Center for Medicare and Medicaid Innovation's AHC Model and developed by a panel of interdisciplinary experts that looked at evidence-based ways to measure SDOH, including transportation. While the transportation access data element in the AHC screening tool serves the same purposes as our proposed SPADE collection about transportation barriers, the AHC tool has binary yes or no response options that do not differentiate between challenges for medical versus non-medical appointments and activities. We believe that this is an important nuance for informing PAC discharge planning to a community setting, as transportation needs for non-medical activities may differ than for medical activities and should be taken into account.[211] We believe that use of this data element will provide sufficient information about transportation barriers to medical and non-medical care for IRF patients to facilitate appropriate discharge planning and care coordination across PAC settings. As such, we proposed to adopt the Transportation data element from PRAPARE. More information about development of the PRAPARE tool is available on the website at https://protect2.fireeye.com/url?k=7cb6eb44-20e2f238-7cb6da7b-0cc47adc5fa2-1751cb986c8c2f8c&u=http://www.nachc.org/prapare.
In addition, we received stakeholder feedback during the December 13, 2018 SDOH listening session on the impact of transportation barriers on unmet care needs. While recognizing that there is no consensus in the field about whether providers should have responsibility for resolving patient transportation needs, discussion focused on the importance of assessing transportation barriers to facilitate connections with available community resources.
Adding a Transportation data element to the collection of SPADE would be an important step to identifying and addressing SDOH that impact health outcomes and patient experience for Medicare beneficiaries. For more information on the Transportation data element, we refer readers to the document titled “Final Specifications for IRF QRP Measures and Standardized Patient Assessment Data Elements,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of transportation data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Transportation data element described above as SPADE with respect to the proposed Social Determinants of Health category. If finalized as proposed, we would add the Transportation data element to the IRF-PAI.
We solicited comment on these proposals. A discussion of these comments, along with our responses, appears below.
Comment: One commenter supported the collection of data to capture the reason(s) transportation affects a patient's access to health care. The commenter appreciated the inclusion of these items on the IRF-PAI and encouraged exploration of quality measures in this area as transportation is an extremely important instrumental activity of daily living to effectively transition to the community.
Response: We thank the commenter and we will consider this feedback as we continue to improve and refine the SPADEs.
Comment: Some commenters noted that, if finalized, IRFs should only need to submit data on the race and ethnicity SPADEs with respect to admission and would not need to collect and report again at discharge, as it is unlikely that patient status for these elements will change. The commenters believe that a patient's access to transportation is unlikely to change between admission and discharge; thus, the commenter suggested CMS to require collection of all SDOH SPADEs with respect to admission only.
Response: We disagree with the commenters that stated that access to transportation will always be the same from admission to discharge. Unlike the Vision, Hearing, Race, Ethnicity, Preferred Language, and Interpreter Services SPADEs, we believe that the response to this data element is likely to change from admission to discharge for some patients. For example, a patient could lose a family member or caregiver between admission and discharge, which could impact his or her access to transportation and impact how the patient responds to the access to transportation SPADE data element. Therefore, we believe that the response to this SDOH data element is likely to change from admission to discharge for some patients and we proposed to collect this SPADE data element with respect to both admission and discharge.
As outlined in the FY 2020 IRF PPS proposed rule, multiple studies have demonstrated that access to transportation has an impact on the health of patients (84 FR 17325). Therefore, it is important for providers to be able to identify a patient's needs when the patient is admitted and when the patient is discharged in order to better inform the patient's care decisions made during and after the stay, including understanding the patient's unique risk factors and treatment preferences. Because of this, we are requiring that the Access to Transportation data element be assessed with respect to both admission and discharge.
(5) Social Isolation
Distinct from loneliness, social isolation refers to an actual or perceived lack of contact with other people, such as living alone or residing in a remote area.[212 213] Social isolation tends to increase with age, is a risk factor for physical and mental illness, and a predictor of mortality.[214 215 216] PAC providers are well-suited to design and implement programs to increase social engagement of patients, while also taking into account individual needs and preferences. Adopting a data element to collect and analyze information about social isolation in IRFs and across PAC settings would facilitate the identification of patients who are socially isolated and who may benefit from engagement efforts.
We proposed to adopt as SPADE a single social isolation data element that is currently part of the AHC Screening Tool. The AHC item was selected from the Patient-Reported Outcomes Measurement Information System (PROMIS®) Item Bank on Emotional Distress and questions, “How often do you feel lonely or isolated from those around you?” The five response options are: (1) Never; (2) Rarely; (3) Sometimes; (4) Often; and (5) Always.[217] The AHC Screening Tool was developed by a panel of interdisciplinary experts that looked at evidence-based ways to measure SDOH, including social isolation. More information about the AHC Screening Tool is available on the website at https://innovation.cms.gov/Files/worksheets/ahcm-screeningtool.pdf.
In addition, we received stakeholder feedback during the December 13, 2018 SDOH listening session on the value of receiving information on social isolation for purposes of care planning. Some stakeholders also recommended assessing social isolation as an SDOH as opposed to social support.
The proposed Social Isolation data element is consistent with NASEM considerations about social isolation as a function of social relationships that impacts health outcomes and increases mortality risk, as well as the current work of a NASEM committee examining how social isolation and loneliness impact health outcomes in adults 50 years and older. We believe that adding a Social Isolation data element would be an important component of better understanding patient complexity and the care goals of patients, thereby facilitating care coordination and continuity in care planning across PAC settings. For more information on the Social Isolation data element, we refer readers to the document titled “Final Specifications for IRF QRP Measures and Standardized Patient Assessment Data Elements,” available on the website at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/IMPACT-Act-of-2014/IMPACT-Act-Downloads-and-Videos.html.
In an effort to standardize the submission of social isolation data among IRFs, HHAs, SNFs and LTCHs, for the purposes outlined in section 1899B(a)(1)(B) of the Act, while minimizing the reporting burden, we proposed to adopt the Social Isolation data element described above as SPADE with respect to the proposed Social Determinants of Health category. We proposed to add the Social Isolation data element to the IRF-PAI.
We sought public comment on this proposal. A discussion of these comments, along with our responses, appears below.
Comment: Commenters agreed with CMS that SDOH data could provide Medicare with valuable information about the role that non-clinical factors play in PAC patient outcomes and that the addition of the SDOH SPADEs will facilitate communication between PAC settings and other health care providers. A commenter noted that common standards and definitions are important for interoperability and communication across providers and encouraged CMS to ensure that the SDOH elements collected in IRF settings are aligned with future proposed SDOH data collection requirements in other settings. One commenter stated that there is increasing attention on the critical role that social factors play in individual and population health and that addressing health-related social needs through enhanced clinical-community linkages can improve health outcomes and reduce costs. Another commenter was also pleased that CMS is looking at SDOH and believes it is a positive step toward identifying disparities in health care.
Response: We thank the commenters for the comments.
Comment: Some commenters noted that, if finalized, IRFs should only need to submit data on the race and ethnicity SPADEs with respect to admission and would not need to collect and report again at discharge, as it is unlikely that patient status for these elements will change. The commenters believe that a patient's response to social isolation is unlikely to change between admission and discharge; thus, the commenter suggested CMS to require collection of all SDOH SPADEs with respect to admission only.
Response: We disagree with the commenters that stated that the response to the Social Isolation data element will be the same from admission to discharge. Unlike the Vision, Hearing, Race, Ethnicity, Preferred Language, and Interpreter Services SPADEs, we believe that the response to this data element is likely to change from admission to discharge for some patients. For example, a patient could lose a family member or caregiver between admission and discharge, which could impact their response to the Social Isolation data element. Therefore, we proposed to collect this SPADE data element with respect to both admission and discharge. As outlined in the FY 2020 IRF PPS proposed rule, multiple studies have demonstrated that social isolation has an impact on the health of patients (84 FR 17325 through 17326). Therefore, it is important for providers to be able to identify a patient's needs when the patient is admitted and when the patient is discharged in order to better inform the patient's care decisions made during and after the stay, including understanding the patient's unique risk factors and treatment preferences. Because of this, we are requiring that the Social Isolation data element be assessed at both admission and discharge.
Comment: One commenter stated that the proposed question on social isolation may have a very different answer based on the time horizon considered by the beneficiary as beneficiaries who are newly admitted to an IRF may have experienced differing levels of social isolation over the preceding week due to interactions with health care providers, emergency providers, and friends or family visiting due to hospitalization. The commenter believes this question could be improved by adding a timeframe to the question. For example, “How often have you felt lonely or isolated from those around you in the past 6 months?”
Response: We thank the commenter for this comment. The Social Isolation data element assesses whether a patient has experienced social isolation in the past 6 months to a year. The social isolation question proposed is currently part of the Accountable Health Communities (AHC) Screening Tool. The AHC item was selected from the Patient-Reported Outcomes Measurement Information System (PROMIS®) Item Bank on Emotional Distress.
Comment: A commenter suggested that collecting SDOH SPADEs that have no clinical value, such as transportation and social isolation during an assigned period of either admission or discharge, is a significant concern. The commenter stated that at admission, the focus should be on assessing the patient's medical needs and plan of care, and at discharge, the focus shifts to patient's transition plan and caregiver education. As there are already multiple required assessments on the IRF-PAI, the SDOH SPADEs would add burden and recommended that any SDOH SPADEs finalized should be assessed at any point during the stay.
Response: We disagree with the commenters that the Social Isolation and Transportation data elements have no value. As proposed in the transportation and social isolation section, multiple studies have demonstrated that access to transportation and social isolation have an impact on the health of patients.[218 219] For example, access to transportation is important to medication access. Similarly, social isolation is a predictor of mortality. Therefore, it is important for providers to identify a patient's needs both at admission and discharge in order to better inform the patient's care decisions made during and after the stay, including a patient's unique risk factors and treatment preferences. To minimize burden, we proposed to collect this data element with respect to admission and discharge, rather than more frequently.
After consideration of the public comments, we are finalizing our proposals to collect SDOH data for the purposes of section 2(d)(2)(B) of the IMPACT Act and section 1899B(b)(1)(B)(vi) of the Act as follows. With regard to Race, Ethnicity, Health Literacy, Transportation, and Social Isolation, we are finalizing our proposals as proposed. In response to stakeholder comments, we are revising our proposed policies and finalizing that IRFs that submit the Preferred Language and Interpreter Services SPADEs with respect to admission will be deemed to have submitted with respect to both admission and discharge.
H. Form, Manner, and Timing of Data Submission Under the IRF QRP
1. Background
We refer readers to § 412.634(b) for information regarding the current policies for reporting IRF QRP data.
2. Update to the CMS System for Reporting Quality Measures and Standardized Patient Assessment Data and Associated Procedural Proposals
IRFs are currently required to submit IRF-PAI data to CMS using the Quality Improvement and Evaluation System (QIES) Assessment and Submission Processing (ASAP) system. We will be migrating to a new internet Quality Improvement and Evaluation System (iQIES) that will enable real-time upgrades, and we proposed to designate that system as the data submission system for the IRF QRP beginning October 1, 2019. We proposed to revise § 412.634(a)(1) by replacing “Certification and Survey Provider Enhanced Reports (CASPER)” with “CMS designated data submission”. We proposed to revise § 412.634(d)(1) by replacing the reference to “Quality Improvement and Evaluation System Assessment Submission and Processing (QIES ASAP) system” with “CMS designated data submission system”. We proposed to revise § 412.634(d)(5) by replacing reference to the “QIES ASAP” with “CMS designated data submission”. We proposed to revise § 412.634(f)(1) by replacing “QIES” with “CMS designated data submission system”. In addition, we proposed to notify the public of any future changes to the CMS designated system using subregulatory mechanisms, such as website postings, listserv messaging, and webinars.
We invited public comment on our proposals.
Comment: One commenter supported this proposal and recommended that CMS begin educating and preparing IRFs for the transition as soon as possible.
Response: We thank the commenter for their support and appreciate the importance of educating for this transition. Information regarding the transition to iQIES and instructions for onboarding has been provided to IRFs and will be ongoing. Training resources are currently available on You-Tube at https://go.cms.gov/iQIES_Training and additional help content for users is available within iQIES. Ongoing technical support via email is also available at help@QTSO.com.
After consideration of the public comments, we are finalizing our proposal to revise § 412.634(a)(1), § 412.634(d)(1), § 412.634(d)(5), and § 412.634(f)(1) as proposed. We are also finalizing our proposal to notify the public of any future changes to the CMS designated system using subregulatory mechanisms, such as website postings, listserv messaging, and webinars.
3. Schedule for Reporting the Transfer of Health Information Quality Measures Beginning With the FY 2022 IRF QRP
As discussed in section VIII.D. of this final rule, we proposed to adopt the Transfer of Health Information to the Provider—Post-Acute Care (PAC) and Transfer of Health Information to the Patient—Post-Acute Care (PAC) quality measures beginning with the FY 2022 IRF QRP. We also proposed that IRFs would report the data on those measures using the IRF-PAI. IRFs would be required to collect data on both measures for Medicare Part A and Medicare Advantage patients beginning with patients discharged on or after October 1, 2020. We refer readers to the FY 2018 IRF PPS final rule (82 FR 36291 through 36292) for the data collection and submission timeframes that we finalized for the IRF QRP.
We sought public comment on this proposal and did not receive any comments.
We are finalizing our proposal that IRFs report the data on Transfer of Health Information to the Provider—Post-Acute Care (PAC) and Transfer of Health Information to the Patient—Post-Acute Care (PAC) quality measures using the IRF-PAI as proposed. IRFs will be required to collect data on both measures for Medicare Part A and Medicare Advantage patients beginning with patients discharged on or after October 1, 2020.
4. Schedule for Reporting Standardized Patient Assessment Data Elements Beginning With the FY 2022 IRF QRP
As discussed in section IV.F. of the proposed rule, we proposed to adopt SPADEs beginning with the FY 2022 IRF QRP. We proposed that IRFs would report the data using the IRF-PAI. Similar to the proposed schedule for reporting the Transfer of Health Information to the Provider—Post-Acute Care (PAC) and Transfer of Health Information to the Patient—Post-Acute Care (PAC) quality measures, IRFs would be required to collect the SPADEs for all Medicare Part A and Medicare Advantage patients discharged on or after October 1, 2020, at both admission and discharge. IRFs that submit data with respect to admission for the Hearing, Vision, Race, and Ethnicity SPADEs would be considered to have submitted data with respect to discharges. We refer readers to the FY 2018 IRF PPS final rule (82 FR 36291 through 36292) for the data collection and submission timeframes that we finalized for the IRF QRP.
We sought public comment on this proposal and did not receive any comments.
We are finalizing our proposal that IRFs must submit the SPADEs for all Medicare Part A and Medicare Advantage patients discharged on or after October 1, 2020, with respect to both admission and discharge, using the IRF-PAI. IRFs that submit data with respect to admission for the Hearing, Vision, Preferred Language, Interpreter Services, Race, and Ethnicity SPADEs will be considered to have submitted data with respect to discharges.
5. Data Reporting on Patients for the IRF Quality Reporting Program Beginning With the FY 2022 IRF QRP
We received public input suggesting that the quality measures used in the IRF QRP should be calculated using data collected from all IRF patients, regardless of the patients' payer. This input was provided to us via comments requested about quality measure development on the CMS Measures Management System Blueprint website,[220] as well as through comments we received from stakeholders via our IRF QRP mailbox, and feedback received from the NQF-convened MAP as part of their recommendations on Coordination Strategy for Post-Acute Care and Long-Term Care Performance Measurement.[221] Further, in the FY 2018 IRF PPS proposed rule (82 FR 20740), we sought input on expanding the reporting of quality measures to include all patients, regardless of payer, so as to ensure that the IRF QRP makes publicly available information regarding the quality of the services furnished to the IRF population as a whole, rather than just those patients who have Medicare.
In response to that request for public input, several commenters, including MedPAC, submitted comments stating that they would be supportive of an effort to collect data specified under the IRF QRP from all IRF patients regardless of their payer. Many commenters noted that this would not be overly burdensome, as most of their organizations' members currently complete the IRF-PAI on all patients, regardless of their payer. A few commenters had concerns, including recommending that CMS continue to align the patient assessment instruments across PAC settings and whether the use of the data would outweigh any additional reporting burden. For a more detailed discussion, we refer readers to the FY 2018 IRF final rule (82 FR 36292). We have taken these concerns under consideration in proposing this policy.
Further, given that we do not have access to other payer claims, we believe that the most accurate representation of the quality provided in IRFs would be best conveyed using data collected via the IRF-PAI on all IRF patients, regardless of payer, for the purposes of the IRF QRP. Medicare is the primary payer for approximately 60 percent of IRF patients.[222]
We also believe that data reporting on standardized patient assessment data elements using IRF-PAI should include all IRF patients for the same reasons for collecting data on all residents for the IRF QRP's quality measures: To promote higher quality and more efficient health care for Medicare beneficiaries and all patients receiving IRF services, for example through the exchange of information and longitudinal analysis of the data. With that, we believe that collecting quality measure and standardized patient assessment data via the IRF-PAI on all IRF patients ensures that quality care is provided for Medicare beneficiaries, and patients receiving IRF services as a whole. While we appreciate that collecting quality data on all patients regardless of payer may create additional burden, we also note that the effort to separate out Medicare beneficiaries from other patients is also burdensome.
Collecting data on all IRF patients will provide us with the most robust, accurate reflection of the quality of care delivered to Medicare beneficiaries as compared with non-Medicare patients and residents, and we intend to display the calculation of this data on IRF Compare in the future. Accordingly, we proposed that IRFs collect data on all IRF patients to ensure that all patients, regardless of their payer, are receiving the same care and that provider metrics measure performance across the spectrum of patients.
Therefore, to meet the quality reporting requirements for IRFs for the FY 2022 payment determination and each subsequent year, we proposed to expand the reporting of IRF-PAI data used for the IRF QRP to include data on all patients, regardless of their payer, beginning with patients discharged on or after October 1, 2020 for the FY 2022 IRF QRP and the IRF-PAI V4.0, effective October 1, 2020.
We sought public comment on this proposal and received several comments, which are discussed below.
Comment: Many commenters, including MedPAC, supported the proposal to expand the reporting of quality measures to all patients regardless of payer, agreeing that quality care should be a goal for all patients. Several commenters agreed that most providers already complete an IRF-PAI for all patients. MedPAC also cautioned that any future Medicare payment adjustments related to performance should be based only on outcomes for Medicare beneficiaries. One commenter stated that this approach is consistent with other quality programs and offers consumers a fuller picture of quality of care. One commenter recommended including quality data about all payers on IRF Compare, and another commenter supported the proposal but suggested CMS to allow adequate time to review and validate data before it is made public and allow data on IRF Compare to be analyzed by payer.
Response: We thank commenters for their support and appreciate suggestions for implementing this policy.
Comment: A few commenters requested additional details about how this proposal would be implemented. One commenter suggested that CMS verify comprehensive data submission on all patients to avoid “cherry-picking” patients. A few commenters recommended that CMS delay this proposal and study how this additional data affects quality measure performance.
Response: We appreciate the commenters' request for more details regarding the implementation of this proposal, how data submission will be verified to avoid cherry-picking, and how this data will affect quality measure performance. We acknowledge the commenters' concerns about the proposal's implementation timeline and the request to delay the proposal; however instead of delaying, we plan to use the comments received during this rulemaking cycle to bring a new all-payer policy proposal in the future. Therefore, after consideration of the public comments we received on these issues, we have decided that at this time, we will not finalize this proposal. We agree that it would be useful to assess further how to best implement the collection of data for all payers for the IRF QRP.
Comment: Many commenters had concerns about the burden of collecting quality data on all patients regardless of payer, citing that it contradicted the Patients over Paperwork initiative. One commenter suggested that CMS make this requirement voluntary and to conduct an analysis on the administrative burden on IRFs. Another commenter suggested that the Collection of Information section should contain an estimate of burden required for this reporting.
Response: We do not believe that that the intent of this policy contradicts the Patients over Paperwork initiative, which aims to simplify the documentation required for our programs. However, the all payer proposal would have imposed a new reporting burden on IRFs. We are sensitive to the issue of burden associated with data collection and acknowledge the commenters' concerns about the additional burden required to collect quality data on all patients. Although we believe that the reporting of all-payer data under the IRF QRP would add value to the program and provide a more accurate representation of the quality provided by IRFs, we believe we need to better quantify the new reporting burden on IRFs from this proposal for stakeholders to submit comments. Therefore, after consideration of the public comments, we received on these issues, we have decided that at this time, we will not finalize this proposal. We agree that this burden should be accounted for and we will estimate this burden in future rulemaking.
Comment: One commenter questioned whether IRFs support this proposal. Another commenter was concerned that this proposal would add complexity to CMS' administration of the IRF QRP compliance determination process. One commenter was concerned that quality data would be skewed because younger, non-Medicare patients have more room for improvement compared to older patients.
Response: We do not believe this will add complexity to the IRF QRP compliance determination process, since adding more patients will not change the overall process that we follow with regard to determining compliance. With regard to IRF support for this proposal, we sought input on this topic in the FY 2018 IRF PPS proposed rule (82 FR 20740) and we received several supportive comments. With regard to the commenter's concerns that quality data would be skewed because younger non-Medicare patients have more room for improvement, we note that risk adjustment is currently used for many quality measures, including measures that focus on improvement, such as the functional outcome measures. We take patient characteristics, such as age, into consideration when developing measures, and these are included as risk adjustors for the functional outcome measures.
Comment: Several commenters did not support the proposal, citing concerns about patient privacy. Some commenters suggested that collecting quality data from non-Medicare beneficiaries would be a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) since it is not required for reimbursement purposes. Another commenter was concerned that CMS' collection of, and possible disclosing of, sensitive health information from non-Medicare patients without consent may violate the Privacy Act of 1974, the E-Government Act of 2002, and other state level privacy acts. The commenter suggests amending § 412.608(a) to require the clinician at the IRF to provide the Privacy Act Statement and other information to non-Medicare patients.
Other commenters questioned how CMS would keep this non-Medicare data secure and were concerned that CMS could work with other payers to de-identify this data. A few commenters recommended informing non-Medicare beneficiaries of this reporting and to use only de-identified data. A few commenters requested more details from CMS about the scope of data collection, including non-quality information on the IRF-PAI.
Response: We appreciate the commenters' concerns but disagree that this proposal is a violation of HIPAA, Privacy Act of 1974, and e-Government Act of 2002. IRF-PAI data is collected under an existing system of records notice (66 FR 56682). Any disclosure of the data will be made in accordance with the Privacy Act and those routine uses outlined in the SORN. Medicare patients are currently given a Privacy Act Statement and would be given to every patient under the IRF QRP. Section 208 of the e-Government Act of 2002 requires federal agencies to perform Privacy Impact Assessments when acquiring or developing new information technology or making substantial changes to existing information technology that involves the collection maintenance, or dissemination of information in identifiable form. Because we are not acquiring or developing new information technology, or making substantial changes to existing information technology under this proposal, we disagree that this policy violates the e-Government Act.
With regard to questions about how CMS would keep data non-Medicare data secure, we safeguard the IRF-PAI data in a secure data system. The system limits data access to authorized users and monitors such users to ensure against unauthorized data access or disclosures. This system conforms to all applicable federal laws and regulations as well as federal government, Department of Health & Human Services (HHS), and CMS policies and standards as they relate to information security and data privacy. The applicable laws and regulations include, but are not limited to: The Privacy Act of 1974; the Federal Information Security Management Act of 2002; the Computer Fraud and Abuse Act of 1986; the Health Insurance Portability and Accountability Act of 1996; the E-Government Act of 2002; the Clinger-Cohen Act of 1996; the Medicare Modernization Act of 2003; and the corresponding implementing regulations. With regard to the scope of data collection, IRFs would be required to submit quality measure and standardized patient assessment data elements required by the IRF QRP. After consideration of the public comments we received on these issues, we have decided that at this time, we will not finalize this proposal. We appreciate concerns raised by providers and will take them into consideration for future rulemaking.
Comment: One commenter questioned whether CMS has the statutory authority to require IRFs to submit IRF-PAI data for the IRF QRP for all patients, regardless of payer, citing that it is inconsistent with section 1886(j)(2)(D) of the Act because data from non-Medicare IRF patients are not “necessary” for administering the IRF PPS. The commenter further noted that § 412.604(c) currently requires IRFs to complete an IRF-PAI for all Medicare Part A and Part C patients that an IRF admits or discharges and does not address reporting for non-Medicare patients.
Response: We believe that we generally have authority to collect all payer data for the IRF QRP under section 1886(j)(7) of the Act. We also note that with respect to the data submitted in accordance with section 1886(j)(7)(F) of the Act, the statute expressly requires that data on quality measures specified under section 1899B(c)(1) of the Act be submitted using the IRF PAI, to the extent possible, and that SPADE required under section 1899B(b)(1) of the Act be submitted using the IRF PAI. No all payer data collected for the IRF QRP would be used for purposes of administering the IRF PPS.
We appreciate the support offered by some commenters for our proposal to collect data on all IRF patients regardless of payer so as to ensure that the IRF QRP makes publicly available information regarding the quality of the services furnished to Medicare beneficiaries, as well as to the IRF population as a whole. However, we also acknowledge the concerns raised by some commenters with respect to the administrative challenges of implementing all payer data collection, the need to account for the burden related to this policy, as well as the need for us to provide further detail and training to IRFs. We continue to believe that the collection of quality data to include all patients would help to ensure that Medicare patients receive the same quality of care as other patients who are treated by IRFs.
Therefore, after careful consideration of the public comments we received, we will not finalize the proposal to expand the reporting of IRF quality data to include all patients, regardless of payer, at this time. We plan to use the comments we received on this proposal to help inform a future all payer proposal.
I. Policies Regarding Public Display of Measure Data for the IRF QRP
Section 1886(j)(7)(E) of the Act requires the Secretary to establish procedures for making the IRF QRP data available to the public after ensuring that IRFs have the opportunity to review their data prior to public display. Measure data are currently displayed on the Inpatient Rehabilitation Facility Compare website, an interactive web tool that assists individuals by providing information on IRF quality of care. For more information on IRF Compare, we refer readers to the website at https://www.medicare.gov/inpatientrehabilitationfacilitycompare/. For a more detailed discussion about our policies regarding public display of IRF QRP measure data and procedures for the opportunity to review and correct data and information, we refer readers to the FY 2017 IRF PPS final rule (81 FR 52125 through 52131).
In the proposed rule, we proposed to begin publicly displaying data for the Drug Regimen Review Conducted With Follow-Up for Identified Issues—PAC IRF QRP measure beginning CY 2020 or as soon as technically feasible. We finalized the Drug Regimen Review Conducted With Follow-Up for Identified Issues—PAC IRF QRP measure in the FY 2017 IRF PPS final rule (81 FR 52111 through 52116).
Data collection for this assessment-based measure began with patients discharged on or after October 1, 2018. We proposed to display data based on four rolling quarters, initially using discharges from January 1, 2019 through December 31, 2019 (Quarter 1 2019 through Quarter 4 2019). To ensure the statistical reliability of the data, we proposed that we would not publicly report an IRF's performance on the measure if the IRF had fewer than 20 eligible cases in any four consecutive rolling quarters. IRFs that have fewer than 20 eligible cases would be distinguished with a footnote that states, “The number of cases/patient stays is too small to publicly report.”
We sought public comment on these proposals and received several, which are summarized below.
Comment: Several commenters supported the proposal to begin publicly displaying data for the Drug Regimen Review Conducted With Follow-Up for Identified Issues—PAC IRF QRP measure in CY 2020 or as soon as technically feasible, including the exception for IRFs with fewer than 20 eligible cases. One commenter clarified that its support is contingent on the measure not utilizing performance categories.
Response: We appreciate the commenter's support.
After consideration of the public comments, we are finalizing our proposal to begin publicly displaying data for the Drug Regimen Review Conducted With Follow-Up for Identified Issues—PAC IRF QRP measure beginning CY 2020 or as soon as technically feasible.
J. Removal of the List of Compliant IRFs
In the FY 2016 IRF PPS final rule (80 FR 47125 through 47127), we finalized that we would publish a list of IRFs that successfully met the reporting requirements for the applicable payment determination on the IRF QRP website and update the list on an annual basis. We have received feedback from stakeholders that this list offers minimal benefit. Although the posting of successful providers was the final step in the applicable payment determination process, it does not provide new information or clarification to the providers regarding their annual payment update status. Therefore, we proposed that we will no longer publish a list of compliant IRFs on the IRF QRP website, effective beginning with the FY 2020 payment determination.
We sought public comment on this proposal and received several comments.
Comment: One commenter supported this proposal, but suggested that CMS make this information available to stakeholders upon request in the interest of transparency.
Response: We thank commenters for their support. At this time, we do not plan to make the list of compliant IRFs available upon request, in alignment with other QRPs that do not provide this list. We believe stakeholders can find sufficient quality information about IRFs on the IRF compare website.
Comment: Several commenters did not support the proposal removal of the list of compliant IRFs. One commenter agreed that the list was not relevant to IRF providers in reviewing their own compliance status, but stated that it could be of interest to patients and other IRFs. Other commenters recommended posting the list because it is helpful for large health systems to quickly determine which hospitals are compliant. One commenter further suggested that the list continue to be posted in a standardized manner across the various QRPs to improve transparency.
Response: We acknowledge commenters' concerns about removing the requirement to post the list of compliant IRFs. Patients and consumers can still find information about IRF quality on the IRF Compare website. We do not believe that removing this list will have a negative impact for IRFs, since the list does not give any new information to IRF providers or health providers about their own compliance status. We also note that other QRPs do not require posting of a list of compliant facilities.
After consideration of the comments, we are finalizing our proposal and will no longer publish a list of compliant IRFs on the IRF QRP website, beginning with the FY 2020 payment determination.
K. Method for Applying the Reduction to the FY 2020 IRF Increase Factor for IRFs That Fail To Meet the Quality Reporting Requirements
As previously noted, section 1886(j)(7)(A)(i) of the Act requires the application of a 2-percentage point reduction of the applicable market basket increase factor for payments for discharges occurring during such fiscal year for IRFs that fail to comply with the quality data submission requirements.
We proposed to apply a 2-percentage point reduction to the applicable FY 2020 proposed market basket increase factor in calculating an adjusted FY 2020 proposed standard payment conversion factor to apply to payments for only those IRFs that failed to comply with the data submission requirements. As previously noted, application of the 2-percentage point reduction may result in an update that is less than 0.0 for a fiscal year and in payment rates for a fiscal year being less than such payment rates for the preceding fiscal year. Also, reporting-based reductions to the market basket increase factor will not be cumulative; they will only apply for the FY involved.
We invited public comment on the proposed method for applying the reduction to the FY 2020 IRF increase factor for IRFs that fail to meet the quality reporting requirements, which are summarized below.
Comment: Some commenters suggested that CMS provide flexibility in its application of the IRF QRP payment penalty for IRFs who make a good-faith effort to comply and submit quality reporting data.
Response: We interpret the commenter's suggestion that we take into consideration case by case exceptions and apply leniency for providers have attempted but failed to submit their quality reporting data for the IRF QRP. We are unable to provide flexibility with respect to the 2 percent payment penalty; as noted previously, section 1886(j)(7) of the Act requires the Secretary to reduce the annual increase factor for IRFs that fail to comply with the quality data submission requirements. While we did not seek comment on flexibilities on which the penalty is applied, we note that we have provided flexibility where the failure of the IRF to comply with the requirements of the IRF QRP stemmed from circumstances beyond its control. For example, we have finalized policies that grant exceptions or extensions for IRFs if we determine that a systemic problem with one of our data collection systems affected the ability of IRFs to submit data (79 FR 45920). We have also adopted policies (78 FR 47920) that allow us to grant exemptions or extensions to an IRF if it has experienced an extraordinary circumstance beyond its control. In addition, we set the reporting compliance threshold at 95 percent rather than at 100 percent to data to for account for the rare instances when assessment data collection and submission maybe impossible, such as when patients have been discharged emergently, or against medical advice.
Table 18 shows the calculation of the adjusted FY 2020 standard payment conversion factor that will be used to compute IRF PPS payment rates for any IRF that failed to meet the quality reporting requirements for the applicable reporting period.

After consideration of the comments, we are finalizing our proposal to apply a 2-percentage point reduction to the applicable FY 2020 proposed market basket increase factor in calculating an adjusted FY 2020 proposed standard payment conversion factor to apply to payments for only those IRFs that failed to comply with the data submission requirements.
X. Miscellaneous Comments
We received several comments that were outside the scope of the FY 2020 IRF PPS proposed rule. Specifically, we received comments regarding the processes for updating the IRF facility-level adjustment factors and the transparency of these updates, the application of a cost-of-living adjustment for IRFs located in Alaska and Hawaii, the need for CMS education and instruction on the appropriate IGC/ICD coding on the IRF-PAI, re-evaluating and phasing out the 60 percent rule as criteria for IRF admission, and federal funding for universal health care. We thank commenters for bringing these issues to our attention, and we will take these comments into consideration for potential policy refinements.
XI. Provisions of the Final Regulations
In this final rule, we are adopting the provisions set forth in the FY 2020 IRF PPS proposed rule (84 FR 17244).
Specifically:
- We will adopt an unweighted motor score to assign patients to CMGs, the removal of one item from the score, and revisions to the CMGs beginning on October 1, 2019, based on analysis of 2 years of data (FYs 2017 and 2018) using the Quality Indicator items in the IRF-PAI. This includes revisions to the CMG relative weights and average LOS values for FY 2020, in a budget neutral manner, as discussed in section IV. of this final rule.
- We will rebase and revise the IRF market basket to reflect a 2016 base year rather than the current 2012 base year as discussed in section VI. of this FY 2020 IRF PPS final rule.
- We will update the IRF PPS payment rates for FY 2020 by the market basket increase factor, based upon the most current data available, with a productivity adjustment required by section 1886(j)(3)(C)(ii)(I) of the Act, as described in section VI. of this final rule.
- We will update to the IRF wage index to use the concurrent FY IPPS wage index and the FY 2020 labor-related share in a budget-neutral manner, as described in section VI. of this final rule.
- The facility-level adjustments will remain frozen at the FY 2014 levels for FY 2015 and all subsequent years, as discussed in section V. of this final rule.
- We will calculate the final IRF standard payment conversion factor for FY 2020, as discussed in section VI. of this final rule.
- We will update the outlier threshold amount for FY 2020, as discussed in section VII. of this final rule.
- We will update the CCR ceiling and urban/rural average CCRs for FY 2020, as discussed in section VII. of this final rule.
- We will amend the regulations at § 412.622 to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF, as discussed in section VIII. of this final rule.
- We will adopt updates requirements to the IRF QRP, as discussed in section IX. of this final rule.
XII. Collection of Information Requirements
A. Statutory Requirement for Solicitation of Comments
Under the Paperwork Reduction Act of 1995 (PRA), we are required to provide 60-day notice in the Federal Register and solicit public comment before a collection of information requirement is submitted to the OMB for review and approval. To fairly evaluate whether an information collection should be approved by OMB, section 3506(c)(2)(A) of the PRA requires that we solicit comment on the following issues:
- The need for the information collection and its usefulness in carrying out the proper functions of our agency.
- The accuracy of our estimate of the information collection burden.
- The quality, utility, and clarity of the information to be collected.
- Recommendations to minimize the information collection burden on the affected public, including automated collection techniques.
This final rule makes reference to associated information collections that are not discussed in the regulation text contained in this document.
B. Collection of Information Requirements for Updates Related to the IRF QRP
An IRF that does not meet the requirements of the IRF QRP for a fiscal year will receive a 2 percentage point reduction to its otherwise applicable annual increase factor for that fiscal year. Information is not currently available to determine the precise number of IRFs that will receive less than the full annual increase factor for FY 2020 due to non-compliance with the requirements of the IRF QRP.
We believe that the burden associated with the IRF QRP is the time and effort associated with complying with the requirements of the IRF QRP. As of July 15, 2019, there are approximately 1,122 IRFs reporting quality data to CMS. For the purposes of calculating the costs associated with the collection of information requirements, we obtained mean hourly wages for these staff from the U.S. Bureau of Labor Statistics' May 2018 National Occupational Employment and Wage Estimates (http://www.bls.gov/oes/current/oes_nat.htm). To account for overhead and fringe benefits, we have doubled the hourly wage. These amounts are detailed in Table 19.

As discussed in section VIII.D. of this final rule, we are adopting two new measures, (1) Transfer of Health Information to the Provider—Post-Acute Care (PAC); and (2) Transfer of Health Information to the Patient—Post-Acute Care (PAC), beginning with the FY 2022 IRF QRP. As a result, the estimated burden and cost for IRFs for complying with requirements of the FY 2022 IRF QRP will increase. Specifically, we believe that there will be a 1.2 minute addition in clinical staff time to report data per patient stay. We estimate 411,622 discharges from 1,122 IRFs annually. This equates to an increase of 8,232 hours in burden for all IRFs (0.02 hours per assessment × 411,622 discharges). Given 0.7 minutes of RN time at $70.72 per hour and 0.5 minutes of LVN time at $43.96 per hour, we estimate that the total cost will be increased by $437 per IRF annually, or $490,314 for all IRFs annually. This increase in burden will be accounted for in the information collection under OMB control number (0938-0842), which expires December 31, 2021.
In addition, we are finalizing our proposal to add the standardized patient assessment data elements described in section VIII.F of this final rule beginning with the FY 2022 IRF QRP. As a result, the estimated burden and cost for IRFs for complying with requirements of the FY 2022 IRF QRP will be increased. Specifically, we believe that there will be an addition of 7.8 minutes on admission, and 10.95 minutes on discharge, for a total of 18.8 minutes of additional clinical staff time to report data per patient stay. Note that this is a decrease from the proposed 11.1 minutes at discharge because of the changes in section XIII.G.4.2 of this final rule. We estimate 411,622 discharges from 1,122 IRFs annually. This equates to an increase of 122,995 hours in burden for all IRFs (0.3 hours per assessment × 409,982 discharges). Given 11.3 minutes of RN time at $70.72 per hour and 7.5 minutes of LVN time at $43.96 per hour, we estimate that the total cost will be increased by $6,902 per IRF annually, or $7,744,044 for all IRFs. This increase in burden will be accounted for in the information collection under OMB control number (0938-0842), which expires December 31, 2021.
In summary, the newly adopted IRF QRP quality measures and standardized patient assessment data elements will result in a burden addition of $7,339 per IRF annually, and $8,234,450 for all IRFs annually.
XIII. Regulatory Impact Analysis
A. Statement of Need
This final rule updates the IRF prospective payment rates for FY 2020 as required under section 1886(j)(3)(C) of the Act. It responds to section 1886(j)(5) of the Act, which requires the Secretary to publish in the Federal Register on or before the August 1 that precedes the start of each fiscal year, the classification and weighting factors for the IRF PPS's CMGs, and a description of the methodology and data used in computing the prospective payment rates for that fiscal year.
This final rule also implements sections 1886(j)(3)(C) of the Act. Section 1886(j)(3)(C)(ii)(I) of the Act requires the Secretary to apply a MFP adjustment to the market basket increase factor. The productivity adjustment applies to FYs from 2012 forward.
Furthermore, this final rule also adopts policy changes under the statutory discretion afforded to the Secretary under section 1886(j)(7) of the Act. Specifically, we are rebasing and revising the IRF market basket to reflect a 2016 base year rather than the current 2012 base year, revising the CMGs, making a technical correction to the regulatory language to indicate that the determination of whether a treating physician has specialized training and experience in inpatient rehabilitation is made by the IRF and updating regulatory language related to IRF QRP data collection.
B. Overall Impact
We have examined the impacts of this rule as required by Executive Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563 on Improving Regulation and Regulatory Review (January 18, 2011), the Regulatory Flexibility Act (RFA) (September 19, 1980, Pub. L. 96-354), section 1102(b) of the Act, section 202 of the Unfunded Mandates Reform Act of 1995 (March 22, 1995; Pub. L. 104-4), Executive Order 13132 on Federalism (August 4, 1999), the Congressional Review Act (5 U.S.C. 804(2) and Executive Order 13771 on Reducing Regulation and Controlling Regulatory Costs (January 30, 2017).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of available regulatory alternatives and, if regulation is necessary, to select regulatory approaches that maximize net benefits (including potential economic, environmental, public health and safety effects, distributive impacts, and equity). Section 3(f) of Executive Order 12866 defines a “significant regulatory action” as an action that is likely to result in a rule: (1) Having an annual effect on the economy of $100 million or more in any 1 year, or adversely and materially affecting a sector of the economy, productivity, competition, jobs, the environment, public health or safety, or state, local or tribal governments or communities (also referred to as “economically significant”); (2) creating a serious inconsistency or otherwise interfering with an action taken or planned by another agency; (3) materially altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and obligations of recipients thereof; or (4) raising novel legal or policy issues arising out of legal mandates, the President's priorities, or the principles set forth in the Executive Order.
A regulatory impact analysis (RIA) must be prepared for major rules with economically significant effects ($100 million or more in any 1 year). We estimate the total impact of the policy updates described in this final rule by comparing the estimated payments in FY 2020 with those in FY 2019. This analysis results in an estimated $210 million increase for FY 2020 IRF PPS payments. Additionally we estimate that costs associated with the proposals to update the reporting requirements under the IRF QRP result in an estimated $8.2 million addition in costs in FY 2020 for IRFs. We estimate that this rulemaking is “economically significant” as measured by the $100 million threshold, and hence also a major rule under the Congressional Review Act. Also, the rule has been reviewed by OMB. Accordingly, we have prepared a Regulatory Impact Analysis that, to the best of our ability, presents the costs and benefits of the rulemaking.
C. Anticipated Effects
1. Effects on IRFs
The RFA requires agencies to analyze options for regulatory relief of small entities, if a rule has a significant impact on a substantial number of small entities. For purposes of the RFA, small entities include small businesses, nonprofit organizations, and small governmental jurisdictions. Most IRFs and most other providers and suppliers are small entities, either by having revenues of $7.5 million to $38.5 million or less in any 1 year depending on industry classification, or by being nonprofit organizations that are not dominant in their markets. (For details, see the Small Business Administration's final rule that set forth size standards for health care industries, at 65 FR 69432 at http://www.sba.gov/sites/default/files/files/Size_Standards_Table.pdf, effective March 26, 2012 and updated on February 26, 2016.) Because we lack data on individual hospital receipts, we cannot determine the number of small proprietary IRFs or the proportion of IRFs' revenue that is derived from Medicare payments. Therefore, we assume that all IRFs (an approximate total of 1,120 IRFs, of which approximately 55 percent are nonprofit facilities) are considered small entities and that Medicare payment constitutes the majority of their revenues. The HHS generally uses a revenue impact of 3 to 5 percent as a significance threshold under the RFA. As shown in Table 20, we estimate that the net revenue impact of this final rule on all IRFs is to increase estimated payments by approximately 2.5 percent. The rates and policies set forth in this final rule will not have a significant impact (not greater than 3 percent) on a substantial number of small entities. Medicare Administrative Contractors are not considered to be small entities. Individuals and states are not included in the definition of a small entity.
In addition, section 1102(b) of the Act requires us to prepare a regulatory impact analysis if a rule may have a significant impact on the operations of a substantial number of small rural hospitals. This analysis must conform to the provisions of section 604 of the RFA. For purposes of section 1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a Metropolitan Statistical Area and has fewer than 100 beds. As discussed in detail below in this section, the rates and policies set forth in this final rule will not have a significant impact (not greater than 3 percent) on a substantial number of rural hospitals based on the data of the 136 rural units and 11 rural hospitals in our database of 1,122 IRFs for which data were available.
Section 202 of the Unfunded Mandates Reform Act of 1995 (Pub. L. 104-04, enacted March 22, 1995) (UMRA) also requires that agencies assess anticipated costs and benefits before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995 dollars, updated annually for inflation. In 2019, that threshold is approximately $154 million. This final rule does not mandate any requirements for State, local, or tribal governments, or for the private sector.
Executive Order 13132 establishes certain requirements that an agency must meet when it issues a proposed rule (and subsequent final rule) that imposes substantial direct requirement costs on state and local governments, preempts state law, or otherwise has federalism implications. As stated, this final rule will not have a substantial effect on state and local governments, preempt state law, or otherwise have a federalism implication.
Executive Order 13771, titled Reducing Regulation and Controlling Regulatory Costs, was issued on January 30, 2017 and requires that the costs associated with significant new regulations “shall, to the extent permitted by law, be offset by the elimination of existing costs associated with at least two prior regulations.” This final rule is considered an E.O. 13771 regulatory action. We estimate that this rule would generate $6.18 million in annualized cost, discounted at 7 percent relative to year 2016, over a perpetual time horizon. Details on the estimated costs of this rule can be found in the preceding analyses.
2. Detailed Economic Analysis
This final rule updates to the IRF PPS rates contained in the FY 2019 IRF PPS final rule (83 FR 38514). Specifically, this final rule updates the CMG relative weights and average LOS values, the wage index, and the outlier threshold for high-cost cases. This final rule applies a MFP adjustment to the FY 2020 IRF market basket increase factor in accordance with section 1886(j)(3)(C)(ii)(I) of the Act. Further, this final rule rebases and revises the IRF market basket to reflect a 2016 base year rather than the current 2012 base year, revises the CMGs based on FYs 2017 and 2018 data and amends the regulatory language to clarify that the determination of whether a treating physician has specialized training and experience in inpatient rehabilitation is made by the IRF.
We estimate that the impact of the changes and updates described in this final rule will be a net estimated increase of $210 million in payments to IRF providers. This estimate does not include the implementation of the required 2 percentage point reduction of the market basket increase factor for any IRF that fails to meet the IRF quality reporting requirements (as discussed in section IX.K. of this final rule). The impact analysis in Table 20 of this final rule represents the projected effects of the updates to IRF PPS payments for FY 2020 compared with the estimated IRF PPS payments in FY 2019. We determine the effects by estimating payments while holding all other payment variables constant. We use the best data available, but we do not attempt to predict behavioral responses to these changes, and we do not make adjustments for future changes in such variables as number of discharges or case-mix.
We note that certain events may combine to limit the scope or accuracy of our impact analysis, because such an analysis is future-oriented and, thus, susceptible to forecasting errors because of other changes in the forecasted impact time period. Some examples could be legislative changes made by the Congress to the Medicare program that would impact program funding, or changes specifically related to IRFs. Although some of these changes may not necessarily be specific to the IRF PPS, the nature of the Medicare program is such that the changes may interact, and the complexity of the interaction of these changes could make it difficult to predict accurately the full scope of the impact upon IRFs.
In updating the rates for FY 2020, we are adopting standard annual revisions described in this final rule (for example, the update to the wage and market basket indexes used to adjust the federal rates). We are also implementing a productivity adjustment to the FY 2020 IRF market basket increase factor in accordance with section 1886(j)(3)(C)(ii)(I) of the Act. We estimate the total increase in payments to IRFs in FY 2020, relative to FY 2019, will be approximately $210 million.
This estimate is derived from the application of the FY 2020 IRF market basket increase factor, as reduced by a productivity adjustment in accordance with section 1886(j)(3)(C)(ii)(I) of the Act, which yields an estimated increase in aggregate payments to IRFs of $210 million. Outlier payments are estimated to remain at 3 percent in FY 2020. Therefore, we estimate that these updates will result in a net increase in estimated payments of $210 million from FY 2019 to FY 2020.
The effects of the updates that impact IRF PPS payment rates are shown in Table 20. The following updates that affect the IRF PPS payment rates are discussed separately below:
- The effects of the update to the outlier threshold amount, from approximately 3.0 percent to 3.0 percent of total estimated payments for FY 2020, consistent with section 1886(j)(4) of the Act.
- The effects of the annual market basket update (using the IRF market basket) to IRF PPS payment rates, as required by sections 1886(j)(3)(A)(i) and (j)(3)(C) of the Act, including a productivity adjustment in accordance with section 1886(j)(3)(C)(i)(I) of the Act.
- The effects of applying the budget-neutral labor-related share and wage index adjustment, as required under section 1886(j)(6) of the Act.
- The effects of the budget-neutral changes to the CMGs, relative weights and average LOS values, under the authority of section 1886(j)(2)(C)(i) of the Act.
- The total change in estimated payments based on the FY 2020 payment changes relative to the estimated FY 2019 payments.
3. Description of Table 20
Table 20 shows the overall impact on the 1,122 IRFs included in the analysis.
The next 12 rows of Table 20 contain IRFs categorized according to their geographic location, designation as either a freestanding hospital or a unit of a hospital, and by type of ownership; all urban, which is further divided into urban units of a hospital, urban freestanding hospitals, and by type of ownership; and all rural, which is further divided into rural units of a hospital, rural freestanding hospitals, and by type of ownership. There are 975 IRFs located in urban areas included in our analysis. Among these, there are 697 IRF units of hospitals located in urban areas and 278 freestanding IRF hospitals located in urban areas. There are 147 IRFs located in rural areas included in our analysis. Among these, there are 136 IRF units of hospitals located in rural areas and 11 freestanding IRF hospitals located in rural areas. There are 393 for-profit IRFs. Among these, there are 357 IRFs in urban areas and 36 IRFs in rural areas. There are 616 non-profit IRFs. Among these, there are 526 urban IRFs and 90 rural IRFs. There are 113 government-owned IRFs. Among these, there are 92 urban IRFs and 21 rural IRFs.
The remaining four parts of Table 20 show IRFs grouped by their geographic location within a region, by teaching status, and by DSH PP. First, IRFs located in urban areas are categorized for their location within a particular one of the nine Census geographic regions. Second, IRFs located in rural areas are categorized for their location within a particular one of the nine Census geographic regions. In some cases, especially for rural IRFs located in the New England, Mountain, and Pacific regions, the number of IRFs represented is small. IRFs are then grouped by teaching status, including non-teaching IRFs, IRFs with an intern and resident to average daily census (ADC) ratio less than 10 percent, IRFs with an intern and resident to ADC ratio greater than or equal to 10 percent and less than or equal to 19 percent, and IRFs with an intern and resident to ADC ratio greater than 19 percent. Finally, IRFs are grouped by DSH PP, including IRFs with zero DSH PP, IRFs with a DSH PP less than 5 percent, IRFs with a DSH PP between 5 and less than 10 percent, IRFs with a DSH PP between 10 and 20 percent, and IRFs with a DSH PP greater than 20 percent.
The estimated impacts of each policy described in this rule to the facility categories listed are shown in the columns of Table 20. The description of each column is as follows:
- Column (1) shows the facility classification categories.
- Column (2) shows the number of IRFs in each category in our FY 2020 analysis file.
- Column (3) shows the number of cases in each category in our FY 2020 analysis file.
- Column (4) shows the estimated effect of the adjustment to the outlier threshold amount.
- Column (5) shows the estimated effect of the update to the IRF labor-related share and wage index, in a budget-neutral manner.
- Column (6) shows the estimated effect of the update to the CMGs, relative weights, and average LOS values, in a budget-neutral manner.
- Column (7) compares our estimates of the payments per discharge, incorporating all of the policies reflected in this final rule for FY 2020 to our estimates of payments per discharge in FY 2019.
The average estimated increase for all IRFs is approximately 2.5 percent. This estimated net increase includes the effects of the IRF market basket increase factor for FY 2020 of 2.9 percent, reduced by a productivity adjustment of 0.4 percentage point in accordance with section 1886(j)(3)(C)(ii)(I) of the Act. There is no change in estimated IRF outlier payments from the update to the outlier threshold amount. Since we are making the updates to the IRF wage index and the CMG relative weights in a budget-neutral manner, they will not be expected to affect total estimated IRF payments in the aggregate. However, as described in more detail in each section, they will be expected to affect the estimated distribution of payments among providers.

4. Impact of the Update to the Outlier Threshold Amount
The estimated effects of the update to the outlier threshold adjustment are presented in column 4 of Table 20. In the FY 2019 IRF PPS final rule (83 FR 38531 through 38532), we used FY 2017 IRF claims data (the best, most complete data available at that time) to set the outlier threshold amount for FY 2019 so that estimated outlier payments would equal 3 percent of total estimated payments for FY 2019.
For the FY 2020 IRF PPS proposed rule (84 FR 17244), we used preliminary FY 2018 IRF claims data, and, based on that preliminary analysis, we estimated that IRF outlier payments as a percentage of total estimated IRF payments would be 3.2 percent in FY 2019. As we typically do between the proposed and final rules each year, we updated our FY 2018 IRF claims data to ensure that we are using the most recent available data in setting IRF payments. Therefore, based on updated analysis of the most recent IRF claims data for this final rule, we now estimate that IRF outlier payments as a percentage of total IRF payments as 3.0 in FY 2019. Thus, we are adjusting the outlier threshold amount in this final rule to maintain total estimated outlier payments equal to 3 percent of total estimated payments in FY 2020.
The impact of this outlier adjustment update (as shown in column 4 of Table 20) is to maintain estimated overall payments to IRFs at 3 percent.
5. Impact of the CBSA Wage Index and Labor-Related Share
In column 5 of Table 20, we present the effects of the budget-neutral update of the wage index and labor-related share. The changes to the wage index and the labor-related share are discussed together because the wage index is applied to the labor-related share portion of payments, so the changes in the two have a combined effect on payments to providers. As discussed in section VI.E. of this final rule, we are updating the labor-related share from 70.5 percent in FY 2019 to 72.7 percent in FY 2020.
6. Impact of the Update to the CMG Relative Weights and Average LOS Values
In column 6 of Table 20, we present the effects of the budget-neutral update of the CMGs, relative weights and average LOS values. In the aggregate, we do not estimate that these updates will affect overall estimated payments of IRFs. However, we do expect these updates to have small distributional effects.
7. Effects of the Requirements for the IRF QRP for FY 2020
In accordance with section 1886(j)(7)(A) of the Act, the Secretary must reduce by 2 percentage points the market basket increase factor otherwise applicable to an IRF for a fiscal year if the IRF does not comply with the requirements of the IRF QRP for that fiscal year. In section VIII.J of this final rule, we discuss the method for applying the 2 percentage point reduction to IRFs that fail to meet the IRF QRP requirements.
As discussed in section VIII.D. of this final rule, we are finalizing our proposal to add two measures to the IRF QRP: (1) Transfer of Health Information to the Provider—Post-Acute Care (PAC); and (2) Transfer of Health Information to the Patient—Post-Acute Care (PAC), beginning with the FY 2022 IRF QRP. We are also finalizing our proposal to add standardized patient assessment data elements, as discussed in section IV.G of this final rule. We describe the estimated burden and cost reductions for both of these measures in section VIII.C of this final rule. In summary, the changes to the IRF QRP will result in a burden addition of $7,339 per IRF annually, and $8,234,450 for all IRFs annually.
We intend to continue to closely monitor the effects of the IRF QRP on IRFs and to help perpetuate successful reporting outcomes through ongoing stakeholder education, national trainings, IRF announcements, website postings, CMS Open Door Forums, and general and technical help desks.
8. Effects of the Amending § 412.622(a)(3)(iv) To Clarify the Definition of a Rehabilitation Physician
As discussed in section VIII. of this final rule, we are amending § 412.622(a)(3)(iv) to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. We do not expect this to have any effect on the quality of care that beneficiaries receive in IRFs because we continue to require that the rehabilitation physicians caring for patients in IRFs be licensed physicians with specialized training and experience in inpatient rehabilitation. We expect IRFs to continue ensuring that the rehabilitation physicians meet these requirements. Although we do not currently collect data from IRFs on the physicians specialties that are providing care to patients in IRFs, we do not expect this to change as a result of the amendments we are making to § 412.622(a)(3)(iv). However, we will continue to monitor the quality of care beneficiaries receive in IRFs, and will initiate appropriate actions through future rulemaking if we observe any declines in quality of care in IRFs.
As this is merely clarifying our existing policy regarding the definition of a rehabilitation physician in § 412.622(a)(3)(iv), we do not expect this to result in any financial impacts for the Medicare contractors, IRFs, other providers, or for the Medicare program. However, we expect that this clarification may ease some administrative burden for IRFs and for Medicare contractors by making it easier for IRF providers to document their decisions regarding the licensed physicians in their facilities that meet the regulatory definition of a rehabilitation physician and for the Medicare contractors to continue to accept the IRFs' decisions in this regard. We are unable at this time to quantify how much administrative burden may have existed because of the previous ambiguity surrounding the definition of a rehabilitation physician, but we are hopeful that this clarification will alleviate any administrative burden that might have existed before.
We expect this clarification to enhance Medicare's program integrity efforts in this area by eliminating uncertainty surrounding the definition of a rehabilitation physician.
D. Alternatives Considered
The following is a discussion of the alternatives considered for the IRF PPS updates contained in this final rule.
Section 1886(j)(3)(C) of the Act requires the Secretary to update the IRF PPS payment rates by an increase factor that reflects changes over time in the prices of an appropriate mix of goods and services included in the covered IRF services.
We are adopting a market basket increase factor for FY 2020 that is based on a rebased and revised market basket reflecting a 2016 base year. We considered the alternative of continuing to use the IRF market basket without rebasing to determine the market basket increase factor for FY 2020. However, we typically rebase and revise the market baskets for the various PPS every 4 to 5 years so that the cost weights and price proxies reflect more recent data. Therefore, we believe it is more technically appropriate to use a 2016-based IRF market basket since it allows for the FY 2020 market basket increase factor to reflect a more up-to-date cost structure experienced by IRFs.
As noted previously in this final rule, section 1886(j)(3)(C)(ii)(I) of the Act requires the Secretary to apply a productivity adjustment to the market basket increase factor for FY 2020. Thus, in accordance with section 1886(j)(3)(C) of the Act, we are updating the IRF prospective payments in this final rule by 2.5 percent (which equals the 2.9 percent estimated IRF market basket increase factor for FY 2020 reduced by a 0.4 percentage point productivity adjustment as determined under section 1886(b)(3)(B)(xi)(II) of the Act (as required by section 1886(j)(3)(C)(ii)(I) of the Act)).
As we finalized in the FY 2019 IRF PPS final rule (83 FR 38514) use of the Quality Indicators items in determining payment and the associated CMG and CMG relative weight revisions using 2 years of data (FYs 2017 and 2018) beginning with FY 2020, we did not consider any alternative to proposing these changes.
However, we did consider whether or not to apply a weighting methodology to the IRF motor score that was finalized in the FY 2019 IRF PPS final rule (83 FR 38514) to assign patients to CMGs beginning in FY 2020. As described in the FY 2020 IRF PPS proposed rule (84 FR 17244, 17249 through 17260), we explored the use of a weighted motor score, as requested by stakeholders. Our analysis showed that weighting the motor score would improve the accuracy of payments under the IRF PPS. The improved accuracy combined with the requests from stakeholders to explore a weighted methodology led us to propose to use a weighted motor score to assign patients to CMGs beginning on October 1, 2019. However, in light of the many concerned stakeholder comments on the FY 2020 IRF PPS proposed rule that requested that we go back to an unweighted motor score methodology until we can more fully analyze a weighted motor score, the fact that the improvement in accuracy using the weighted motor score is small, and the greater simplicity achieved through the use of an unweighted motor score, we are finalizing an unweighted motor score, in which each of the 18 items have a weight of 1, beginning October 1, 2019. We will continue to analyze weighted motor score approaches and will consider possible revisions to the motor score for future rulemaking.
We considered not removing the item GG0170A1 Roll left and right from the composition of the motor score. However, this item was found to be very collinear with other items in the motor score and did not behave as expected in the models. Therefore, we believe it is appropriate to remove this item from the construction of the motor score.
We considered updating facility-level adjustment factors for FY 2020. However, as discussed in more detail in the FY 2015 final rule (79 FR 45872), we believe that freezing the facility-level adjustments at FY 2014 levels for FY 2015 and all subsequent years (unless and until the data indicate that they need to be further updated) will allow us an opportunity to monitor the effects of the substantial changes to the adjustment factors for FY 2014, and will allow IRFs time to adjust to the previous changes.
We considered not updating the IRF wage index to use the concurrent fiscal year's IPPS wage index and instead continuing to use a 1-year lag of the IPPS wage index. However, we believe that updating the IRF wage index based on the concurrent fiscal year's IPPS wage index will better align the data across acute and PAC settings in support of our efforts to move toward more unified Medicare payments across PAC settings.
We considered maintaining the existing outlier threshold amount for FY 2020. However, the outlier threshold must be adjusted to reflect changes in estimated costs and payments for IRFs in FY 2020. Consequently, we are adjusting the outlier threshold amount in this final rule to maintain total outlier payments equal to 3 percent of aggregate estimated payments in FY 2020.
We considered not amending § 412.622(a)(3)(iv) to clarify that the determination as to whether a physician qualifies as a rehabilitation physician (that is, a licensed physician with specialized training and experience in inpatient rehabilitation) is made by the IRF. Instead, we considered addressing this issue through subregulatory means, such as issuing guidance to the Medicare contractors. However, we believe that it is important to clarify this definition in regulation to ensure that IRF providers and Medicare contractors have a shared understanding of these regulatory requirements and to make certain that there is no room for further ambiguity on this point.
In addition, we considered addressing this issue by amending § 412.622(a)(3)(iv) to add further specificity to the definition of a rehabilitation physician. However, we did not take this approach because we continue to believe that the IRFs are in the best position to make the determination as to which licensed physicians meet the requirements for purposes of § 412.622, and we did not want to inadvertently affect access to IRF care for beneficiaries. However, we will continue to monitor this policy and engage with stakeholders to determine if further specificity of these requirements may be warranted in the future.
E. Regulatory Review Costs
If regulations impose administrative costs on private entities, such as the time needed to read and interpret this final rule, we should estimate the cost associated with regulatory review. Due to the uncertainty involved with accurately quantifying the number of entities that will review the rule, we assume that the total number of unique commenters on the FY 2020 IRF PPS proposed rule will be the number of reviewers of this final rule. We acknowledge that this assumption may understate or overstate the costs of reviewing this final rule. It is possible that not all commenters reviewed the FY 2020 IRF PPS proposed rule in detail, and it is also possible that some reviewers chose not to comment on the proposed rule. For these reasons we thought that the number of past commenters would be a fair estimate of the number of reviewers of this final rule.
We also recognize that different types of entities are in many cases affected by mutually exclusive sections of this final rule, and therefore, for the purposes of our estimate we assume that each reviewer reads approximately 50 percent of the rule. We sought comments on this assumption.
Using the wage information from the BLS for medical and health service managers (Code 11-9111), we estimate that the cost of reviewing this rule is $107.38 per hour, including overhead and fringe benefits (https://www.bls.gov/oes/current/oes_nat.htm). Assuming an average reading speed, we estimate that it would take approximately 2 hours for the staff to review half of this final rule. For each IRF that reviews the rule, the estimated cost is $218.72 (2 hours × $109.36). Therefore, we estimate that the total cost of reviewing this regulation is $274,931.04 ($218.72 × 1,257 reviewers).
We received one comment on the proposed methodology for estimating the total cost of reviewing this regulation which is summarized below.
Comment: One commenter suggested that CMS should take into consideration the number of times the proposed rule has been downloaded in estimating the cost of reviewing this regulation.
Response: The regulatory review cost is an estimate that makes several assumptions such as average reading speed and number of the people who read the document, etc. For more than 2 years, we have used the number of comments received as a proxy for the number of staff members who review the document. This assumption is well accepted by the general public. The number of comments received is a more reasonable proxy than the number of downloads since those who provide comments must actually read the rule, as those that download the rule may not read the rule.
F. Accounting Statement and Table
As required by OMB Circular A-4 (available at http://www.whitehouse.gov/sites/default/files/omb/assets/omb/circulars/a004/a-4.pdf), in Table 21, we have prepared an accounting statement showing the classification of the expenditures associated with the provisions of this final rule. Table 21 provides our best estimate of the increase in Medicare payments under the IRF PPS as a result of the updates presented in this final rule based on the data for 1,122 IRFs in our database. In addition, Table 21 presents the costs associated with the new IRF QRP requirements for FY 2020.

G. Conclusion
Overall, the estimated payments per discharge for IRFs in FY 2020 are projected to increase by 2.5 percent, compared with the estimated payments in FY 2019, as reflected in column 7 of Table 20.
IRF payments per discharge are estimated to increase by 2.4 percent in urban areas and 4.4 percent in rural areas, compared with estimated FY 2019 payments. Payments per discharge to rehabilitation units are estimated to increase 5.0 percent in urban areas and 5.7 percent in rural areas. Payments per discharge to freestanding rehabilitation hospitals are estimated to increase 0.2 percent in urban areas and decrease 2.1 percent in rural areas.
Overall, IRFs are estimated to experience a net increase in payments as a result of the policies in this final rule. The largest payment increase is estimated to be a 6.8 percent increase for rural government IRFs and rural IRFs located in the West South Central region. The analysis above, together with the remainder of this preamble, provides a Regulatory Impact Analysis.
In accordance with the provisions of Executive Order 12866, this regulation was reviewed by the Office of Management and Budget.
List of Subjects in 42 CFR Part 412
- Administrative practice and procedure
- Health facilities
- Medicare
- Puerto Rico
- Reporting and recordkeeping requirements
For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services amends 42 CFR chapter IV as set forth below:
PART 412—PROSPECTIVE PAYMENT SYSTEMS FOR INPATIENT HOSPITAL SERVICES
1. The authority citation for part 412 is revised to read as follows:
2. Section 412.622 is amended by revising paragraphs (a)(3)(iv), (a)(4)(i)(A), (a)(4)(iii)(A), and (a)(5)(i) and adding paragraph (c) to read as follows:
(a) * * *
(3) * * *
(iv) Requires physician supervision by a rehabilitation physician. The requirement for medical supervision means that the rehabilitation physician must conduct face-to-face visits with the patient at least 3 days per week throughout the patient's stay in the IRF to assess the patient both medically and functionally, as well as to modify the course of treatment as needed to maximize the patient's capacity to benefit from the rehabilitation process. The post-admission physician evaluation described in paragraph (a)(4)(ii) of this section may count as one of the face-to-face visits.
(4) * * *
(i) * * *
(A) It is conducted by a licensed or certified clinician(s) designated by a rehabilitation physician within the 48 hours immediately preceding the IRF admission. A preadmission screening that includes all of the required elements, but that is conducted more than 48 hours immediately preceding the IRF admission, will be accepted as long as an update is conducted in person or by telephone to update the patient's medical and functional status within the 48 hours immediately preceding the IRF admission and is documented in the patient's medical record.
(iii) * * *
(A) It is developed by a rehabilitation physician with input from the interdisciplinary team within 4 days of the patient's admission to the IRF.
(5) * * *
(i) The team meetings are led by a rehabilitation physician and further consist of a registered nurse with specialized training or experience in rehabilitation; a social worker or case manager (or both); and a licensed or certified therapist from each therapy discipline involved in treating the patient. All team members must have current knowledge of the patient's medical and functional status. The rehabilitation physician may lead the interdisciplinary team meeting remotely via a mode of communication such as video or telephone conferencing.
(c) Definitions. As used in this section—
Rehabilitation physician means a licensed physician who is determined by the IRF to have specialized training and experience in inpatient rehabilitation.
3. Section 412.634 is amended by revising paragraphs (a)(1), (d)(1) and (5), and (f)(1) to read as follows:
(a) * * *
(1) For the FY 2018 payment determination and subsequent years, an IRF must begin reporting data under the IRF QRP requirements no later than the first day of the calendar quarter subsequent to 30 days after the date on its CMS Certification Number (CCN) notification letter, which designates the IRF as operating in the CMS designated data submission system.
(d) * * *
(1) IRFs that do not meet the requirement in paragraph (b) of this section for a program year will receive a written notification of non-compliance through at least one of the following methods: The CMS designated data submission system, the United States Postal Service, or via an email from the Medicare Administrative Contractor (MAC).
(5) CMS will notify IRFs, in writing, of its final decision regarding any reconsideration request through at least one of the following methods: CMS designated data submission system, the United States Postal Service, or via an email from the Medicare Administrative Contractor (MAC).
(f) * * *
(1) IRFs must meet or exceed two separate data completeness thresholds: One threshold set at 95 percent for completion of required quality measures data and standardized patient assessment data collected using the IRF-PAI submitted through the CMS designated data submission system; and a second threshold set at 100 percent for measures data collected and submitted using the CDC NHSN.
Dated: July 23, 2019.
Seema Verma,
Administrator, Centers for Medicare & Medicaid Services.
Dated: July 25, 2019.
Alex M. Azar II,
Secretary, Department of Health and Human Services.
Footnotes
1. http://www.bea.gov/papers/pdf/IOmanual_092906.pdf.
Back to Citation2. Tian, W. “An all-payer view of hospital discharge to post-acute care,” May 2016. Available at https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp.
Back to Citation3. Ibid.
Back to Citation4. RTI International analysis of Medicare claims data for index stays in IRF 2016/2017. (RTI program reference: MM150).
5. Kwan, J.L., Lo, L., Sampson, M., & Shojania, K.G., “Medication reconciliation during transitions of care as a patient safety strategy: a systematic review,” Annals of Internal Medicine, 2013, Vol. 158(5), pp. 397-403.
6. Boockvar, K.S., Blum, S., Kugler, A., Livote, E., Mergenhagen, K.A., Nebeker, J.R., & Yeh, J., “Effect of admission medication reconciliation on adverse drug events from admission medication changes,” Archives of Internal Medicine, 2011, Vol. 171(9), pp. 860-861.
7. Bell, C.M., Brener, S.S., Gunraj, N., Huo, C., Bierman, A.S., Scales, D.C., & Urbach, D.R., “Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases,” JAMA, 2011, Vol. 306(8), pp. 840-847.
8. Basey, A.J., Krska, J., Kennedy, T.D., & Mackridge, A.J., “Prescribing errors on admission to hospital and their potential impact: a mixed-methods study,” BMJ Quality & Safety, 2014, Vol. 23(1), pp. 17-25.
9. Desai, R., Williams, C.E., Greene, S.B., Pierson, S., & Hansen, R.A., “Medication errors during patient transitions into nursing homes: characteristics and association with patient harm,” The American Journal of Geriatric Pharmacotherapy, 2011, Vol. 9(6), pp. 413-422.
10. Boling, P.A., “Care transitions and home health care,” Clinical Geriatric Medicine, 2009, Vol.25(1), pp. 135-48.
11. Barnsteiner, J.H., “Medication Reconciliation: Transfer of medication information across settings—keeping it free from error,” The American Journal of Nursing, 2005, Vol. 105(3), pp. 31-36.
12. Arbaje, A.I., Kansagara, D.L., Salanitro, A.H., Englander, H.L., Kripalani, S., Jencks, S.F., & Lindquist, L.A., “Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs,” Journal of General Internal Medicine, 2014, Vol. 29(6), pp. 932-939.
13. Jencks, S.F., Williams, M.V., & Coleman, E.A., “Rehospitalizations among patients in the Medicare fee-for-service program,” New England Journal of Medicine, 2009, Vol. 360(14), pp. 1418-1428.
14. Institute of Medicine. “Preventing medication errors: quality chasm series,” Washington, DC: The National Academies Press 2007. Available at https://www.nap.edu/read/11623/chapter/1.
Back to Citation15. Kitson, N.A., Price, M., Lau, F.Y., & Showler, G., “Developing a medication communication framework across continuums of care using the Circle of Care Modeling approach,” BMC Health Services Research, 2013, Vol. 13(1), pp. 1-10.
16. Mor, V., Intrator, O., Feng, Z., & Grabowski, D.C., “The revolving door of rehospitalization from skilled nursing facilities,” Health Affairs, 2010, Vol. 29(1), pp. 57-64.
17. Institute of Medicine. “Preventing medication errors: quality chasm series,” Washington, DC: The National Academies Press 2007. Available at https://www.nap.edu/read/11623/chapter/1.
18. Kitson, N.A., Price, M., Lau, F.Y., & Showler, G., “Developing a medication communication framework across continuums of care using the Circle of Care Modeling approach,” BMC Health Services Research, 2013, Vol. 13(1), pp. 1-10.
19. Forster, A.J., Murff, H.J., Peterson, J.F., Gandhi, T.K., & Bates, D.W., “The incidence and severity of adverse events affecting patients after discharge from the hospital.” Annals of Internal Medicine, 2003,138(3), pp. 161-167.
20. King, B.J., Gilmore-Bykovskyi, A.L., Roiland, R.A., Polnaszek, B.E., Bowers, B.J., & Kind, A.J. “The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study,” Journal of the American Geriatrics Society, 2013, Vol. 61(7), 1095-1102.
Back to Citation21. The Joint Commission, “Sentinel Event Policy” available at https://www.jointcommission.org/sentinel_event_policy_and_procedures/.
Back to Citation22. The Joint Commission. “Sentinel Event Data Root Causes by Event Type 2004-2015.” 2016. Available at https://www.jointcommission.org/assets/1/23/jconline_Mar_2_2016.pdf.
Back to Citation23. Mor, V., Intrator, O., Feng, Z., & Grabowski, D.C., “The revolving door of rehospitalization from skilled nursing facilities,” Health Affairs, 2010, Vol. 29(1), pp. 57-64.
24. Institute of Medicine, “Preventing medication errors: quality chasm series,” Washington, DC: The National Academies Press, 2007. Available at https://www.nap.edu/read/11623/chapter/1.
25. Starmer, A.J., Sectish, T.C., Simon, D.W., Keohane, C., McSweeney, M.E., Chung, E.Y., Yoon, C.S., Lipsitz, S.R., Wassner, A.J., Harper, M.B., & Landrigan, C.P., “Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle,” JAMA, 2013, Vol. 310(21), pp. 2262-2270.
26. Pronovost, P., M.M.E. Johns, S. Palmer, R.C. Bono, D.B. Fridsma, A. Gettinger, J. Goldman, W. Johnson, M. Karney, C. Samitt, R.D. Sriram, A. Zenooz, and Y.C. Wang, Editors. Procuring Interoperability: Achieving High-Quality, Connected, and Person-Centered Care. Washington, DC, 2018 National Academy of Medicine. Available at https://nam.edu/wp-content/uploads/2018/10/Procuring-Interoperability_web.pdf.
27. Balaban RB, Weissman JS, Samuel PA, & Woolhandler, S., “Redefining and redesigning hospital discharge to enhance patient care: a randomized controlled study,” J Gen Intern Med, 2008, Vol. 23(8), pp. 1228-33.
Back to Citation28. Arbaje, A.I., Kansagara, D.L., Salanitro, A.H., Englander, H.L., Kripalani, S., Jencks, S.F., & Lindquist, L.A., “Regardless of age: incorporating principles from geriatric medicine to improve care transitions for patients with complex needs,” Journal of General Internal Medicine, 2014, Vol 29(6), pp. 932-939.
29. Simmons, S., Schnelle, J., Slagle, J., Sathe, N.A., Stevenson, D., Carlo, M., & McPheeters, M.L., “Resident safety practices in nursing home settings.” Technical Brief No. 24 (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2015-00003-I.) AHRQ Publication No. 16-EHC022-EF. Rockville, MD: Agency for Healthcare Research and Quality. May 2016. Available at https://www.ncbi.nlm.nih.gov/books/NBK384624/.
Back to Citation30. Berwick, D.M. & Hackbarth, A.D. “Eliminating Waste in US Health Care,” JAMA, 2012, Vol. 307(14), pp.1513-1516.
Back to Citation31. McDonald, K.M., Sundaram, V., Bravata, D.M., Lewis, R., Lin, N., Kraft, S.A. & Owens, D.K. Care Coordination. Vol. 7 of: Shojania K.G., McDonald K.M., Wachter R.M., Owens D.K., editors. “Closing the quality gap: A critical analysis of quality improvement strategies.” Technical Review 9 (Prepared by the Stanford University-UCSF Evidence-based Practice Center under contract 290-02-0017). AHRQ Publication No. 04(07)-0051-7. Rockville, MD: Agency for Healthcare Research and Quality. June 2006. Available at https://www.ncbi.nlm.nih.gov/books/NBK44015/.
32. Lattimer, C., “When it comes to transitions in patient care, effective communication can make all the difference,” Generations, 2011, Vol. 35(1), pp. 69-72.
Back to Citation33. Starmer A.J, Spector N.D., Srivastava R., West, D.C., Rosenbluth, G., Allen, A.D., Noble, E.L., & Landrigen, C.P., “Changes in medical errors after implementation of a handoff program,” N Engl J Med, 2014, Vol. 37(1), pp. 1803-1812.
34. Kruse, C.S. Marquez, G., Nelson, D., & Polomares, O., “The use of health information exchange to augment patient handoff in long-term care: a systematic review,” Applied Clinical Informatics, 2018, Vol. 9(4), pp. 752-771.
35. Brody, A.A., Gibson, B., Tresner-Kirsch, D., Kramer, H., Thraen, I., Coarr, M.E., & Rupper, R., “High prevalence of medication discrepancies between home health referrals and Centers for Medicare and Medicaid Services home health certification and plan of care and their potential to affect safety of vulnerable elderly adults,” Journal of the American Geriatrics Society, 2016, Vol. 64(11), pp. e166-e170.
Back to Citation36. Chhabra, P.T., Rattinger, G.B., Dutcher, S.K., Hare, M.E., Parsons, K., L., & Zuckerman, I.H., “Medication reconciliation during the transition to and from long-term care settings: a systematic review,” Res Social Adm Pharm, 2012, Vol. 8(1), pp. 60-75.
37. Levinson, D.R., & General, I., “Adverse events in skilled nursing facilities: national incidence among Medicare beneficiaries.” Washington, DC: U.S. Department of Health and Human Services, Office of the Inspector General, February 2014. Available at https://oig.hhs.gov/oei/reports/oei-06-11-00370.pdf.
Back to Citation38. Battles J., Azam I., Grady M., & Reback K., “Advances in patient safety and medical liability,” AHRQ Publication No. 17-0017-EF. Rockville, MD: Agency for Healthcare Research and Quality, August 2017. Available at https://www.ahrq.gov/sites/default/files/publications/files/advances-complete_3.pdf.
Back to Citation39. Health and Human Services Office of Inspector General. Adverse Events in Rehabilitation Hospitals: National Incidence Among Medicare Beneficiaries. (OEI-06-14-00110). 2018. Available at https://oig.hhs.gov/oei/reports/oei-06-14-00110.asp.
Back to Citation40. Barnsteiner, J.H., “Medication Reconciliation: Transfer of medication information across settings—keeping it free from error,” The American Journal of Nursing, 2005, Vol. 105(3), pp. 31-36.
41. Gleason, K.M., Groszek, J.M., Sullivan, C., Rooney, D., Barnard, C., Noskin, G.A., “Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients,” American Journal of Health System Pharmacy, 2004, Vol. 61(16), pp. 1689-1694.
Back to Citation42. Patterson M., Foust J.B., Bollinger, S., Coleman, C., Nguyen, D., “Inter-facility communication barriers delay resolving medication discrepancies during transitions of care,” Research in Social & Administrative Pharmacy (2018), doi: 10.1016/j.sapharm.2018.05.124.
Back to Citation43. Manias, E., Annaikis, N., Considine, J., Weerasuriya, R., & Kusljic, S. “Patient-, medication- and environment-related factors affecting medication discrepancies in older patients,” Collegian, 2017, Vol. 24, pp. 571-577.
Back to Citation44. Tjia, J., Bonner, A., Briesacher, B.A., McGee, S., Terrill, E., Miller, K., “Medication discrepancies upon hospital to skilled nursing facility transitions,” J Gen Intern Med, 2009, Vol. 24(5), pp. 630-635.
45. Sinvani, L.D., Beizer, J., Akerman, M., Pekmezaris, R., Nouryan, C., Lutsky, L., Cal, C., Dlugacz, Y., Masick, K., Wolf-Klein, G.,”Medication reconciliation in continuum of care transitions: a moving target,” J Am Med Dir Assoc, 2013, Vol. 14(9), 668-672.
46. Coleman E.A., Parry C., Chalmers S., & Min, S.J., “The Care Transitions Intervention: results of a randomized controlled trial,” Arch Intern Med, 2006, Vol. 166, pp. 1822-28.
Back to Citation47. Corbett C.L., Setter S.M., Neumiller J.J., & Wood, l.D., “Nurse identified hospital to home medication discrepancies: implications for improving transitional care,” Geriatr Nurs, 2011, Vol. 31(3), pp. 188-96.
48. Setter S.M., Corbett C.F., Neumiller J.J., Gates, B.J., Sclar, D.A., & Sonnett, T.E., “Effectiveness of a pharmacist-nurse intervention on resolving medication discrepancies in older patients transitioning from hospital to home care: impact of a pharmacy/nursing intervention,” Am J Health Syst Pharm, 2009, Vol. 66, pp. 2027-31.
Back to Citation49. Boockvar, K.S., Blum, S., Kugler, A., Livote, E., Mergenhagen, K.A., Nebeker, J.R., & Yeh, J., “Effect of admission medication reconciliation on adverse drug events from admission medication changes,” Archives of Internal Medicine, 2011, Vol. 171(9), pp. 860-861.
50. Kwan, J.L., Lo, L., Sampson, M., & Shojania, K.G., “Medication reconciliation during transitions of care as a patient safety strategy: a systematic review,” Annals of Internal Medicine, 2013, Vol. 158(5), pp. 397-403.
51. Chhabra, P.T., Rattinger, G.B., Dutcher, S.K., Hare, M.E., Parsons, K., L., & Zuckerman, I.H., “Medication reconciliation during the transition to and from long-term care settings: a systematic review,” Res Social Adm Pharm, 2012, Vol. 8(1), pp. 60-75.
Back to Citation52. Agrawal A, Wu WY. “Reducing medication errors and improving systems reliability using an electronic medication reconciliation system,” The Joint Commission Journal on Quality and Patient Safety, 2009, Vol. 35(2), pp. 106-114.
Back to Citation53. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-Information-TEP_Summary_Report_Final-June-2017.pdf.
Back to Citation54. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-Information-TEP-Meetings-2-3-Summary-Report_Final_Feb2018.pdf.
Back to Citation55. Ibid.
Back to Citation56. Tian, W. “An all-payer view of hospital discharge to postacute care,” May 2016. Available at https://www.hcup-us.ahrq.gov/reports/statbriefs/sb205-Hospital-Discharge-Postacute-Care.jsp.
Back to Citation57. RTI International analysis of Medicare claims data for index stays in IRF 2016/2017. (RTI program reference: MM150).
Back to Citation58. Kwan, J.L., Lo, L., Sampson, M., & Shojania, K.G., “Medication reconciliation during transitions of care as a patient safety strategy: A systematic review,” Annals of Internal Medicine, 2013, Vol. 158(5), pp. 397-403.
59. Boockvar, K.S., Blum, S., Kugler, A., Livote, E., Mergenhagen, K.A., Nebeker, J.R., & Yeh, J., “Effect of admission medication reconciliation on adverse drug events from admission medication changes,” Archives of Internal Medicine, 2011, Vol. 171(9), pp. 860-861.
60. Bell, C.M., Brener, S.S., Gunraj, N., Huo, C., Bierman, A.S., Scales, D.C., & Urbach, D.R., “Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases,” JAMA, 2011, Vol. 306(8), pp. 840-847.
61. Basey, A.J., Krska, J., Kennedy, T.D., & Mackridge, A.J., “Prescribing errors on admission to hospital and their potential impact: A mixed-methods study,” BMJ Quality & Safety, 2014, Vol. 23(1), pp. 17-25.
62. Desai, R., Williams, C.E., Greene, S.B., Pierson, S., & Hansen, R.A., “Medication errors during patient transitions into nursing homes: Characteristics and association with patient harm,” The American Journal of Geriatric Pharmacotherapy, 2011, Vol. 9(6), pp. 413-422.
Back to Citation63. Brody, A.A., Gibson, B., Tresner-Kirsch, D., Kramer, H., Thraen, I., Coarr, M.E., & Rupper, R. “High prevalence of medication discrepancies between home health referrals and Centers for Medicare and Medicaid Services home health certification and plan of care and their potential to affect safety of vulnerable elderly adults,” Journal of the American Geriatrics Society, 2016, Vol. 64(11), pp. e166-e170.
64. Chhabra, P.T., Rattinger, G.B., Dutcher, S.K., Hare, M.E., Parsons, K., L., & Zuckerman, I.H., “Medication reconciliation during the transition to and from long-term care settings: A systematic review,” Res Social Adm Pharm, 2012, Vol. 8(1), pp. 60-75.
Back to Citation65. Brody, A.A., Gibson, B., Tresner-Kirsch, D., Kramer, H., Thraen, I., Coarr, M.E., & Rupper, R. “High prevalence of medication discrepancies between home health referrals and Centers for Medicare and Medicaid Services home health certification and plan of care and their potential to affect safety of vulnerable elderly adults,” Journal of the American Geriatrics Society, 2016, Vol. 64(11), pp. e166-e170.
66. Bell, C.M., Brener, S.S., Gunraj, N., Huo, C., Bierman, A.S., Scales, D.C., & Urbach, D.R., “Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases,” JAMA, 2011, Vol. 306(8), pp. 840-847.
67. Sheehan, O.C., Kharrazi, H., Carl, K.J., Leff, B., Wolff, J.L., Roth, D.L., Gabbard, J., & Boyd, C.M., “Helping older adults improve their medication experience (HOME) by addressing medication regimen complexity in home healthcare,” Home Healthcare Now. 2018, Vol. 36(1) pp. 10-19.
Back to Citation68. Mor, V., Intrator, O., Feng, Z., & Grabowski, D. C., “The revolving door of rehospitalization from skilled nursing facilities,” Health Affairs, 2010, Vol. 29(1), pp. 57-64.
69. Starmer, A.J., Sectish, T.C., Simon, D.W., Keohane, C., McSweeney, M.E., Chung, E.Y., Yoon, C.S., Lipsitz, S.R., Wassner, A.J., Harper, M.B., & Landrigan, C.P., “Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle,” JAMA, 2013, Vol. 310(21), pp. 2262-2270.
Back to Citation70. CMS, “Revision to state operations manual (SOM), Hospital Appendix A—Interpretive Guidelines for 42 CFR 482.43, Discharge Planning” May 17, 2013. Available at https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-13-32.pdf.
71. The State Operations Manual Guidance to Surveyors for Long Term Care Facilities (Guidance § 483.21(c)(1) Rev. 11-22-17) for discharge planning process. Available at https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf.
Back to Citation72. Toles, M., Colon-Emeric, C., Naylor, M.D., Asafu-Adjei, J., Hanson, L.C., “Connect-home: Transitional care of skilled nursing facility patients and their caregivers,” Am Geriatr Soc., 2017, Vol. 65(10), pp. 2322-2328.
Back to Citation73. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-Information-TEP_Summary_Report_Final-June-2017.pdf.
Back to Citation74. Technical Expert Panel Summary Report: Development of two quality measures to satisfy the Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) Domain of Transfer of health Information and Care Preferences When an Individual Transitions to Skilled Nursing Facilities (SNFs), Inpatient Rehabilitation Facilities (IRFs), Long Term Care Hospitals (LTCHs) and Home Health Agencies (HHAs). Available at https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Transfer-of-Health-Information-TEP-Meetings-2-3-Summary-Report_Final_Feb2018.pdf.
Back to Citation75. Ibid.
Back to Citation76. Boyce MB, Browne JP, Greenhalgh J The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research BMJ Quality & Safety 2014;23:508-518.
77. Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organizations in an oncologic setting. BMC Health Services Research 2013;13:211.
78. Marshall, S., Haywood, K. and Fitzpatrick, R. (2006), Impact of patient‐reported outcome measures on routine practice: A structured review. Journal of Evaluation in Clinical Practice, 12: 559-568. doi:10.1111/j.1365-2753.2006.00650.x.
Back to Citation79. National Institute on Aging. (2014). Assessing Cognitive Impairment in Older Patients. A Quick Guide for Primary Care Physicians. Retrieved from https://www.nia.nih.gov/alzheimers/publication/assessing-cognitive-impairment-older-patients.
Back to Citation80. Gage B., Morley M., Smith L., et al. (2012). Post-Acute Care Payment Reform Demonstration (Final report, Volume 4 of 4). Research Triangle Park, NC: RTI International.
Back to Citation81. Casey D.A., Antimisiaris D., O'Brien J. (2010). Drugs for Alzheimer's Disease: Are They Effective? Pharmacology & Therapeutics, 35, 208-11.
82. Graff M.J., Vernooij-Dassen M.J., Thijssen M., Dekker J., Hoefnagels W.H., Rikkert M.G.O. (2006). Community Based Occupational Therapy for Patients with Dementia and their Care Givers: Randomised Controlled Trial. BMJ, 333(7580): 1196.
83. Bherer L., Erickson K.I., Liu-Ambrose T. (2013). A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. Journal of Aging Research, 657508.
Back to Citation84. Giacino J.T., Whyte J., Bagiella E., et al. (2012). Placebo-controlled trial of amantadine for severe traumatic brain injury. New England Journal of Medicine, 366(9), 819-826.
Back to Citation85. Alexopoulos G.S., Katz I.R., Reynolds C.F. 3rd, Carpenter D., Docherty J.P., Ross R.W. (2001). Pharmacotherapy of depression in older patients: A summary of the expert consensus guidelines. Journal of Psychiatric Practice, 7(6), 361-376.
86. Arean P.A., Cook B.L. (2002). Psychotherapy and combined psychotherapy/pharmacotherapy for late life depression. Biological Psychiatry, 52(3), 293-303.
87. Hollon S.D., Jarrett R.B., Nierenberg A.A., Thase M.E., Trivedi M., Rush A.J. (2005). Psychotherapy and medication in the treatment of adult and geriatric depression: Which monotherapy or combined treatment? Journal of Clinical Psychiatry, 66(4), 455-468.
88. Wagenaar D, Colenda CC, Kreft M, Sawade J, Gardiner J, Poverejan E. (2003). Treating depression in nursing homes: Practice guidelines in the real world. J Am Osteopath Assoc. 103(10), 465-469.
Back to Citation89. Crespy SD, Van Haitsma K, Kleban M, Hann CJ. Reducing Depressive Symptoms in Nursing Home Residents: Evaluation of the Pennsylvania Depression Collaborative Quality Improvement Program. J Healthc Qual. 2016. Vol. 38, No. 6, pp. e76-e88.
Back to Citation90. Agüero-Torres, H., Fratiglioni, L., Guo, Z., Viitanen, M., von Strauss, E., & Winblad, B. (1998). “Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study.” Am J of Public Health 88(10): 1452-1456.
Back to Citation91. RTI International. Proposed Measure Specifications for Measures Proposed in the FY 2017 IRF QRP NPRM. Research Triangle Park, NC. 2016.
Back to Citation92. Fick, D.M., Steis, M.R., Waller, J.L., & Inouye, S.K. (2013). “Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults.” J of Hospital Med 8(9): 500-505.
Back to Citation93. Dan K. Kiely et al., “Characteristics Associated with Delirium Persistence Among Newly Admitted Post-Acute Facility Patients,” Journals of Gerontology: Series A (Biological Sciences and Medical Sciences), Vol. 59, No. 4, April 2004; Edward R. Marcantonio et al., “Delirium Symptoms in Post-Acute Care: Prevalent, Persistent, and Associated with Poor Functional Recovery,” Journal of the American Geriatrics Society, Vol. 51, No. 1, January 2003.
Back to Citation94. Marcantonio, Edward R., Samuel E. Simon, Margaret A. Bergmann, Richard N. Jones, Katharine M. Murphy, and John N. Morris, “Delirium Symptoms in Post-Acute Care: Prevalent, Persistent, and Associated with Poor Functional Recovery,” Journal of the American Geriatrics Society, Vol. 51, No. 1, January 2003, pp. 4-9.
Back to Citation95. Edward R. Marcantonio et al., Outcomes of Older People Admitted to Postacute Facilities with Delirium,” Journal of the American Geratrics Society, Vol. 53, No. 6, June 2005.
Back to Citation96. Cole MG, Ciampi A, Belzile E, Zhong L. Persistent delirium in older hospital patients: A systematic review of frequency and prognosis. Age Ageing 2009;38:19-26.
Back to Citation97. Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci 2007;62:174-179.
Back to Citation98. Marcantonio, Edward R., Samuel E. Simon, Margaret A. Bergmann, Richard N. Jones, Katharine M. Murphy, and John N. Morris, “Delirium Symptoms in Post-Acute Care: Prevalent, Persistent, and Associated with Poor Functional Recovery,” Journal of the American Geriatrics Society, Vol. 51, No. 1, January 2003, pp. 4-9.
99. Edward R. Marcantonio et al., Outcomes of Older People Admitted to Postacute Facilities with Delirium,” Journal of the American Geratrics Society, Vol. 53, No. 6, June 2005.
Back to Citation100. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-922.
101. Marcantonio ER. In the clinic: Delirium. Ann Intern Med 2011;154:ITC6-1-ITC6-1.
Back to Citation102. Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: Patterns, prevalence, and prognosis. Psychosomatics 2009;50:248-254.
Back to Citation103. Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017 Oct 12;377(15):1456-1466.
Back to Citation104. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990 Dec 15;113(12):941-8.
Back to Citation105. Marcantonio ER. Delirium in Hospitalized Older Adults. N Engl J Med. 2017 Oct 12;377(15):1456-1466.
Back to Citation106. Li, C., Friedman, B., Conwell, Y., & Fiscella, K. (2007). “Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people.” J of the A Geriatrics Society, 55(4): 596-602.
107. Löwe, B., Kroenke, K., & Gräfe, K. (2005). “Detecting and monitoring depression with a two-item questionnaire (PHQ-2).” J of Psychosomatic Research, 58(2): 163-171.
Back to Citation108. Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Annals of family medicine. 2010;8(4):348-53. doi: 10.1370/afm.1139 pmid:20644190; PubMed Central PMCID: PMC2906530.
Back to Citation109. Li, C., Friedman, B., Conwell, Y., & Fiscella, K. (2007). “Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people.” J of the A Geriatrics Society, 55(4): 596-602.
110. Löwe, B., Kroenke, K., & Gräfe, K. (2005). “Detecting and monitoring depression with a two-item questionnaire (PHQ-2).” J of Psychosomatic Research, 58(2): 163-171.
Back to Citation111. Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Annals of family medicine. 2010;8(4):348-353.
Back to Citation112. Wunsch, H., Linde-Zwirble, W.T., Angus, D.C., Hartman, M.E., Milbrandt, E.B., & Kahn, J.M. (2010). “The epidemiology of mechanical ventilation use in the United States.” Critical Care Med 38(10): 1947-1953.
Back to Citation113. Dempsey, D.T., Mullen, J.L., & Buzby, G.P. (1988). “The link between nutritional status and clinical outcome: can nutritional intervention modify it?” Am J of Clinical Nutrition, 47(2): 352-356.
Back to Citation114. Dempsey, D.T., Mullen, J.L., & Buzby, G.P. (1988). “The link between nutritional status and clinical outcome: can nutritional intervention modify it?” Am J of Clinical Nutrition, 47(2): 352-356.
Back to Citation115. U.S. Department of Health and Human Services. Office of Inspector General. Daniel R. Levinson. Adverse Events in Hospitals: National Incidence Among Medicare Beneficiaries. OEI-06-09-00090. November 2010.
Back to Citation116. Boockvar KS, Liu S, Goldstein N, Nebeker J, Siu A, Fried T. Prescribing discrepancies likely to cause adverse drug events after patient transfer. Qual Saf Health Care. 2009;18(1):32-6.
Back to Citation117. Gandhi TK, Seger AC, Overhage JM, et al. Outpatient adverse drug events identified by screening electronic health records. J Patient Saf 2010;6:91-6.doi:10.1097/PTS.0b013e3181dcae06.
Back to Citation118. Gurwitz JH, Field TS, Judge J, Rochon P, Harrold LR, Cadoret C, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med. 2005; 118(3):251±8. Epub 2005/03/05. https://doi.org/10.1016/j.amjmed.2004.09.018 PMID: 15745723.
Back to Citation119. Hug BL, Witkowski DJ, Sox CM, Keohane CA, Seger DL, Yoon C, Matheny ME, Bates DW. Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int. 2009; 76:1192-1198. [PubMed: 19759525].
Back to Citation120. Barnsteiner JH. Medication reconciliation: transfer of medication information across settings-keeping it free from error. J Infus Nurs. 2005;28(2 Suppl):31-36.
121. Rozich J, Roger, R. Medication safety: one organization's approach to the challenge. Journal of Clinical Outcomes Management. 2001(8):27-34.
122. Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm. 2004;61(16):1689-1695.
Back to Citation123. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. doi: 10.1001/jama.2016.16201.
Back to Citation124. Ibid.
Back to Citation125. Shoeb M, Fang MC. Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis. 2013;35(3):312-319. doi: 10.1007/s11239-013-0899-7.
126. Melkonian M, Jarzebowski W, Pautas E. Bleeding risk of antiplatelet drugs compared with oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta‐analysis. J Thromb Haemost. 2017;15:1500-1510. DOI: 10.1111/jth.13697.
Back to Citation127. Hamnvik OP, McMahon GT. Balancing Risk and Benefit with Oral Hypoglycemic Drugs. The Mount Sinai journal of medicine, New York. 2009; 76:234-243.
Back to Citation128. Naples JG, Gellad WF, Hanlon JT. The Role of Opioid Analgesics in Geriatric Pain Management. Clin Geriatr Med. 2016;32(4):725-735.
Back to Citation129. Rigler SK, Shireman TI, Cook-Wiens GJ, Ellerbeck EF, Whittle JC, Mehr DR, Mahnken JD. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc. 2013; 61(5):715-722. [PubMed: 23590366].
130. Wang S, Linkletter C, Dore D et al. Age, antipsychotics, and the risk of ischemic stroke in the Veterans Health Administration. Stroke 2012;43:28-31. doi:10.1161/STROKEAHA.111.617191.
Back to Citation131. Faulkner CM, Cox HL, Williamson JC. Unique aspects of antimicrobial use in older adults. Clin Infect Dis. 2005;40(7):997-1004.
Back to Citation132. American Geriatrics Society 2019 Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2019; 00:1-21.
Back to Citation133. Li Y, Salmasian H, Harpaz R, Chase H, Friedman C. Determining the reasons for medication prescriptions in the EHR using knowledge and natural language processing. AMIA Annu Symp Proc. 2011; 2011:768-76.
Back to Citation134. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society. Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015; 63:2227-2246.
Back to Citation135. Chang A, Schyve PM, Croteau RJ, O'Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: A standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105.
Back to Citation136. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA 2016;316(2):2115-2125.
Back to Citation137. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA 2016;316(2):2115-2125.
Back to Citation138. Department of Health and Human Services: Pain Management Best Practices Inter-Agency Task Force. Draft Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. Accessed April 1, 2019. https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf.
Back to Citation139. Department of Health and Human Services: Pain Management Best Practices Inter-Agency Task Force. Draft Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. Accessed April 1, 2019. https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf.
140. Fishman SM, Carr DB, Hogans B, et al. Scope and Nature of Pain- and Analgesia-Related Content of the United States Medical Licensing Examination (USMLE). Pain Med Malden Mass. 2018;19(3):449-459. doi:10.1093/pm/pnx336.
141. Fishman SM, Young HM, Lucas Arwood E, et al. Core competencies for pain management: results of an interprofessional consensus summit. Pain Med Malden Mass. 2013;14(7):971-981. doi:10.1111/pme.12107.
Back to Citation142. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press (US); 2011. http://www.ncbi.nlm.nih.gov/books/NBK91497/.
Back to Citation143. Department of Health and Human Services: Pain Management Best Practices Inter-Agency Task Force. Draft Report on Pain Management Best Practices: Updates, Gaps, Inconsistencies, and Recommendations. Accessed April 1, 2019. https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf.
Back to Citation144. National Academies. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington DC: National Academies of Sciences, Engineering, and Medicine.; 2017.
Back to Citation145. National Pain Strategy: A Comprehensive Population-Health Level Strategy for Pain. https://iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf.
Back to Citation146. Chau, D. L., Walker, V., Pai, L., & Cho, L. M. (2008). Opiates and elderly: use and side effects. Clinical interventions in aging, 3 (2), 273-8.
147. Fine, P. G. (2009). Chronic Pain Management in Older Adults: Special Considerations. Journal of Pain and Symptom Management, 38(2): S4-S14.
148. Solomon, D. H., Rassen, J. A., Glynn, R. J., Garneau, K., Levin, R., Lee, J., & Schneeweiss, S. (2010). Archives Internal Medicine, 170(22):1979-1986.
Back to Citation149. Byrd L. Managing chronic pain in older adults: a long-term care perspective. Annals of Long-Term Care: Clinical Care and Aging. 2013;21(12):34-40.
150. Kligler, B., Bair, M.J., Banerjea, R. et al. (2018). Clinical Policy Recommendations from the VHA State-of-the-Art Conference on Non-Pharmacological Approaches to Chronic Musculoskeletal Pain. Journal of General Internal Medicine, 33 (Suppl 1): 16. https://doi.org/10.1007/s11606-018-4323-z.
151. Chou, R., Deyo, R., Friedly, J., et al. (2017). Nonpharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. An nals of Internal Medicine, 166 (7):493-505.
Back to Citation152. Society for Post-Acute and Long-Term Care Medicine (AMDA). (2018). Opioids in Nursing Homes: Position Statement. https://paltc.org/opioids%20in%20nursing%20homes.
Back to Citation153. https://www.hhs.gov/opioids/about-the-epidemic/hhs-response/index.html.
Back to Citation154. Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661-668.
155. Hawkins K, Bottone FG, Jr., Ozminkowski RJ, et al. The prevalence of hearing impairment and its burden on the quality of life among adults with Medicare Supplement Insurance. Qual Life Res. 2012;21(7):1135-1147.
Back to Citation156. Horn KL, McMahon NB, McMahon DC, Lewis JS, Barker M, Gherini S. Functional use of the Nucleus 22-channel cochlear implant in the elderly. The Laryngoscope. 1991;101(3):284-288.
Back to Citation157. Sprinzl GM, Riechelmann H. Current trends in treating hearing loss in elderly people: a review of the technology and treatment options—a mini-review. Gerontology. 2010;56(3):351-358.
158. Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing Loss Prevalence and Risk Factors Among Older Adults in the United States. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(5):582-590.
159. Hawkins K, Bottone FG, Jr., Ozminkowski RJ, et al. The prevalence of hearing impairment and its burden on the quality of life among adults with Medicare Supplement Insurance. Qual Life Res. 2012;21(7):1135-1147.
Back to Citation160. Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing Loss and Incident Dementia. Arch Neurol. 2011;68(2):214-220.
Back to Citation161. Cimarolli VR, Jung S. Intensity of Occupational Therapy Utilization in Nursing Home Residents: The Role of Sensory Impairments. J Am Med Dir Assoc. 2016;17(10):939-942.
Back to Citation162. Colon-Emeric CS, Biggs DP, Schenck AP, Lyles KW. Risk factors for hip fracture in skilled nursing facilities: Who should be evaluated? Osteoporos Int. 2003;14(6):484-489.
163. Freeman EE, Munoz B, Rubin G, West SK. Visual field loss increases the risk of falls in older adults: The Salisbury eye evaluation. Invest Ophthalmol Vis Sci. 2007;48(10):4445-4450.
164. Keepnews D, Capitman JA, Rosati RJ. Measuring patient-level clinical outcomes of home health care. J Nurs Scholarsh. 2004;36(1):79-85.
165. Nguyen HT, Black SA, Ray LA, Espino DV, Markides KS. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002;57(3):M181-185.
166. Prager AJ, Liebmann JM, Cioffi GA, Blumberg DM. Self-reported Function, Health Resource Use, and Total Health Care Costs Among Medicare Beneficiaries With Glaucoma. JAMA ophthalmology. 2016;134(4):357-365.
167. Rovner BW, Ganguli M. Depression and disability associated with impaired vision: The MoVies Project. J Am Geriatr Soc. 1998;46(5):617-619.
168. Tinetti ME, Ginter SF. The nursing home life-space diameter. A measure of extent and frequency of mobility among nursing home residents. J Am Geriatr Soc. 1990;38(12):1311-1315.
Back to Citation169. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Chapter 2. Washington, DC: The National Academies Press.
Back to Citation170. Social Determinants of Health. Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. (February 2019).
Back to Citation171. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. 2016. Report to Congress: Social Risk Factors and Performance Under Medicare's Value-Based Payment Programs. Washington, DC.
Back to Citation172. Health Leads. Available at https://healthleadsusa.org/.
Back to Citation173. 2017 National Healthcare Quality and Disparities Report. Rockville, MD: Agency for Healthcare Research and Quality; September 2018. AHRQ Pub. No. 18-0033-EF.
174. Fiscella, K. and Sanders, M.R. Racial and Ethnic Disparities in the Quality of Health Care. (2016). Annual Review of Public Health. 37:375-394.
175. 2018 National Impact Assessment of the Centers for Medicare & Medicaid Services (CMS) Quality Measures Reports. Baltimore, MD: U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services; February 28, 2018.
176. Smedley, B.D., Stith, A.Y., & Nelson, A.R. (2003). Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC, National Academy Press.
177. Chase, J., Huang, L. and Russell, D. (2017). Racial/ethnic disparities in disability outcomes among post-acute home care patients. J of Aging and Health. 30(9):1406-1426.
Back to Citation178. National Healthcare Quality and Disparities Reports. (December 2018). Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/research/findings/nhqrdr/index.html.
Back to Citation179. National Center for Health Statistics. Health, United States, 2017: With special feature on mortality. Hyattsville, Maryland. 2018.
Back to Citation180. HHS. Heart disease and African Americans. 2016b. (October 24, 2016). http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=19.
Back to Citation181. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States; Baciu A, Negussie Y, Geller A, et al., editors. Communities in Action: Pathways to Health Equity. Washington (DC): National Academies Press (US); 2017 Jan 11. 2, The State of Health Disparities in the United States. Available at https://www.ncbi.nlm.nih.gov/books/NBK425844/.
Back to Citation182. “Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity (Notice of Decision)”. Federal Register 62:210 (October 30, 1997) pp. 58782-58790. Available at https://www.govinfo.gov/content/pkg/FR-1997-10-30/pdf/97-28653.pdf.
Back to Citation183. Penman-Aguilar, A., Talih, M., Huang, D., Moonesinghe, R., Bouye, K., Beckles, G. (2016). Measurement of Health Disparities, Health Inequities, and Social Determinants of Health to Support the Advancement of Health Equity. J Public Health Manag Pract. 22 Suppl 1: S33-42.
184. Ramos, R., Davis, J.L., Ross, T., Grant, C.G., Green, B.L. (2012). Measuring health disparities and health inequities: Do you have REGAL data? Qual Manag Health Care. 21(3):176-87.
185. IOM (Institute of Medicine). 2009. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: The National Academies Press.
186. “Revision of Standards for Maintaining, Collecting, and Presenting Federal Data on Race and Ethnicity: Proposals From Federal Interagency Working Group (Notice and Request for Comments).” Federal Register 82: 39 (March 1, 2017) p. 12242.
Back to Citation187. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States; Baciu A, Negussie Y, Geller A, et al., editors. Communities in Action: Pathways to Health Equity. Washington (DC): National Academies Press (US); 2017 Jan 11. 2, The State of Health Disparities in the United States. Available at https://www.ncbi.nlm.nih.gov/books/NBK425844/.
Back to Citation188. IOM (Institute of Medicine). 2009. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: The National Academies Press.
Back to Citation189. HHS Data Standards. Available at https://aspe.hhs.gov/basic-report/hhs-implementation-guidance-data-collection-standards-race-ethnicity-sex-primary-language-and-disability-status.
Back to Citation190. U.S. Census Bureau, 2013-2017 American Community Survey 5-Year Estimates.
Back to Citation191. Karliner LS, Kim SE, Meltzer DO, Auerbach AD. Influence of language barriers on outcomes of hospital care for general medicine inpatients. J Hosp Med. 2010 May-Jun;5(5):276-82. doi: 10.1002/jhm.658.
192. Kim EJ, Kim T, Paasche-Orlow MK, et al. Disparities in Hypertension Associated with Limited English Proficiency. J Gen Intern Med. 2017 Jun;32(6):632-639. doi: 10.1007/s11606-017-3999-9.
193. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Washington, DC: The National Academies Press.
Back to Citation194. IOM (Institute of Medicine). 2009. Race, Ethnicity, and Language Data: Standardization for Health Care Quality Improvement. Washington, DC: The National Academies Press.
Back to Citation195. Guerino, P. and James, C. Race, Ethnicity, and Language Preference in the Health Insurance Marketplaces 2017 Open Enrollment Period. Centers for Medicare & Medicaid Services, Office of Minority Health. Data Highlight: Volume 7—April 2017. Available at https://www.cms.gov/About-CMS/Agency-Information/OMH/Downloads/Data-Highlight-Race-Ethnicity-and-Language-Preference-Marketplace.pdf.
Back to Citation196. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National action plan to improve health literacy. Washington (DC): Author; 2010.
Back to Citation197. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Washington, DC: The National Academies Press.
Back to Citation198. Social Determinants of Health. Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. (February 2019).
Back to Citation199. U.S. Department of Health & Human Services, Office of the Assistant Secretary for Planning and Evaluation. Report to Congress: Social Risk Factors and Performance Under Medicare's Value-Based Purchasing Programs. Available at https://aspe.hhs.gov/pdf-report/report-congress-social-risk-factors-and-performance-under-medicares-value-based-purchasing-programs. Washington, DC: 2016.
Back to Citation200. Morris, N.S., MacLean, C.D., Chew, L.D., & Littenberg, B. (2006). The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC family practice, 7, 21. doi:10.1186/1471-2296-7-21.
201. Brice, J.H., Foster, M.B., Principe, S., Moss, C., Shofer, F.S., Falk, R.J., Ferris, M.E., DeWalt, D.A. (2013). Single-item or two-item literacy screener to predict the S-TOFHLA among adult hemodialysis patients. Patient Educ Couns. 94(1):71-5.
Back to Citation202. University of Miami, School of Nursing & Health Studies, Center of Excellence for Health Disparities Research. Test of Functional Health Literacy in Adults (TOFHLA). (March 2019). Available at https://elcentro.sonhs.miami.edu/research/measures-library/tofhla/index.html.
Back to Citation203. Nurss, J.R., Parker, R.M., Williams, M.V., &Baker, D.W. David W. (2001). TOFHLA. Peppercorn Books & Press. Available at http://www.peppercornbooks.com/catalog/information.php?info_id=5.
Back to Citation204. Hudson, S., Rikard, R.V., Staiculescu, I. & Edison, K. (2017). Improving health and the bottom line: The case for health literacy. In Building the case for health literacy: Proceedings of a workshop. Washington, DC: The National Academies Press.
Back to Citation205. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for Social Risk Factors in Medicare Payment: Identifying Social Risk Factors. Washington, DC: The National Academies Press.
Back to Citation206. Morris, N.S., MacLean, C.D., Chew, L.D., & Littenberg, B. (2006). The Single Item Literacy Screener: Evaluation of a brief instrument to identify limited reading ability. BMC family practice, 7, 21. doi:10.1186/1471-2296-7-21.
207. Brice, J.H., Foster, M.B., Principe, S., Moss, C., Shofer, F.S., Falk, R.J., Ferris, M.E., DeWalt, D.A. (2013). Single-item or two-item literacy screener to predict the S-TOFHLA among adult hemodialysis patients. Patient Educ Couns. 94(1):71-5.
Back to Citation208. Syed, S.T., Gerber, B.S., and Sharp, L.K. (2013). Traveling Towards Disease: Transportation Barriers to Health Care Access. J Community Health. 38(5): 976-993.
Back to Citation209. Health Research & Educational Trust. (2017, November). Social determinants of health series: Transportation and the role of hospitals. Chicago, IL. Available at www.aha.org/transportation.www.aha.org/transportation.
Back to Citation210. Health Research & Educational Trust. (2017, November). Social determinants of health series: Transportation and the role of hospitals. Chicago, IL. Available at www.aha.org/transportation.
Back to Citation211. Northwestern University. (2017). PROMIS Item Bank v. 1.0—Emotional Distress—Anger—Short Form 1.
Back to Citation212. Tomaka, J., Thompson, S., and Palacios, R. (2006). The Relation of Social Isolation, Loneliness, and Social Support to Disease Outcomes Among the Elderly. J of Aging and Health. 18(3): 359-384.
213. Social Connectedness and Engagement Technology for Long-Term and Post-Acute Care: A Primer and Provider Selection Guide. (2019). Leading Age. Available at https://www.leadingage.org/white-papers/social-connectedness-and-engagement-technology-long-term-and-post-acute-care-primer-and#1.1.
Back to Citation214. Landeiro, F., Barrows, P., Nuttall Musson, E., Gray, A.M., and Leal, J. (2017). Reducing Social Loneliness in Older People: A Systematic Review Protocol. BMJ Open. 7(5): e013778.
215. Ong, A.D., Uchino, B.N., and Wethington, E. (2016). Loneliness and Health in Older Adults: A Mini-Review and Synthesis. Gerontology. 62:443-449.
216. Leigh-Hunt, N., Bagguley, D., Bash, K., Turner, V., Turnbull, S., Valtorta, N., and Caan, W. (2017). An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 152:157-171.
Back to Citation217. Northwestern University. (2017). PROMIS Item Bank v. 1.0—Emotional Distress—Anger—Short Form 1.
Back to Citation218. Syed, S.T., Gerber, B.S., and Sharp, L.K. (2013). Traveling Towards Disease: Transportation Barriers to Health Care Access. J Community Health. 38(5): 976-993.
219. Leigh-Hunt, N., Bagguley, D., Bash, K., Turner, V., Turnbull, S., Valtorta, N., and Caan, W. (2017). An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health. 152:157-171.
Back to Citation220. Public Comment Summary Report Posting for Transfer of Health Information and Care Preferences. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Post-Acute-Care-Quality-Initiatives/Downloads/Development-of-Cross-Setting-Transfer-of-Health-Information-Quality-Meas.pdf.
Back to Citation221. MAP Coordination Strategy for Post-Acute Care and Long-Term Care Performance Measurement. Feb 2012. http://www.qualityforum.org/Publications/2012/02/MAP_Coordination_Strategy_for_Post-Acute_Care_and_Long-Term_Care_Performance_Measurement.aspx.
Back to Citation222. National Academies of Sciences, Engineering, and Medicine. 2016. Accounting for social risk factors in Medicare payment: Identifying social risk factors. Washington, DC: The National Acadiemies Press.
Back to CitationBILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
BILLING CODE 4120-01-P
BILLING CODE 4120-01-C
[FR Doc. 2019-16603 Filed 7-31-19; 4:15 pm]
BILLING CODE 4120-01-P
